Abstract
Objective
To characterize the demographic and clinical features of pediatric severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) syndromes and identify admission variables predictive of disease severity.
Study design
We conducted a multicenter, retrospective, and prospective study of pediatric patients hospitalized with acute SARS-CoV-2 infections and multisystem inflammatory syndrome in children (MIS-C) at 8 sites in New York, New Jersey, and Connecticut.
Results
We identified 281 hospitalized patients with SARS-CoV-2 infections and divided them into 3 groups based on clinical features. Overall, 143 (51%) had respiratory disease, 69 (25%) had MIS-C, and 69 (25%) had other manifestations including gastrointestinal illness or fever. Patients with MIS-C were more likely to identify as non-Hispanic black compared with patients with respiratory disease (35% vs 18%, P = .02). Seven patients (2%) died and 114 (41%) were admitted to the intensive care unit. In multivariable analyses, obesity (OR 3.39, 95% CI 1.26-9.10, P = .02) and hypoxia on admission (OR 4.01; 95% CI 1.14-14.15; P = .03) were predictive of severe respiratory disease. Lower absolute lymphocyte count (OR 8.33 per unit decrease in 109 cells/L, 95% CI 2.32-33.33, P = .001) and greater C-reactive protein (OR 1.06 per unit increase in mg/dL, 95% CI 1.01-1.12, P = .017) were predictive of severe MIS-C. Race/ethnicity or socioeconomic status were not predictive of disease severity.
Conclusions
We identified variables at the time of hospitalization that may help predict the development of severe SARS-CoV-2 disease manifestations in children and youth. These variables may have implications for future prognostic tools that inform hospital admission and clinical management.
Keywords: COVID-19, biomarkers
Abbreviations: ALC, Absolute lymphocyte count; COVID-19, Coronavirus disease 2019; CRP, C-reactive protein; ICU, Intensive care unit; MIS-C, Multisystem inflammatory syndrome in children; PCR, Polymerase chain reaction; SARS-CoV-2, Severe acute respiratory syndrome coronavirus 2; SES, Socioeconomic status
The disease associated with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), coronavirus disease 2019 (COVID-19), predominantly affects adults, but case series have documented that SARS-CoV-2 also can cause severe disease in children and youth.1, 2, 3 Manifestations of SARS-CoV-2 in children include a respiratory disease similar to what is described in adults, as well as a syndrome that was first described and appears to be more common in children, designated as multisystem inflammatory syndrome in children (MIS-C).1, 2, 3, 4, 5, 6, 7
Although there is a growing body of research about COVID-19 in adults,8, 9, 10 much is still unknown about the effects of this virus in the pediatric population. Most studies of COVID-19 in the pediatric population have been single-center, limited by size, or have focused on only one clinical manifestation. Thus, we still have a relatively limited understanding of the full spectrum of disease in children and youth.1, 2, 3 , 5 , 6 , 11 Furthermore, although it has been reported that COVID-19 disproportionately affects minorities and people of lower socioeconomic status (SES), this observation has not yet been examined formally in a large pediatric dataset of hospitalized patients.12, 13, 14 Given the uncertainly around natural history and prognosis, there is a need to define the spectrum of disease and develop prognostic tools to identify children at risk for clinical decompensation.
As an important first step to addressing these gaps, this study aims to characterize the clinical course and outcomes of children and youth hospitalized with SARS-CoV-2 infections and identify patient characteristics associated with an increased risk of becoming critically ill during hospitalization.
Methods
Study Design and Data Collection
We conducted a retrospective and prospective multicenter cohort study of hospitalized pediatric patients, defined as children and youth ≤22 years of age, with laboratory-confirmed SARS-CoV-2 infection or MIS-C, at 8 pediatric centers in New York, New Jersey, and Connecticut, an early epicenter of the pandemic in the US. Data were collected retrospectively for patients admitted up until April 12, 2020, and prospectively thereafter for patients site. The hospitals, further described in Table I (available at www.jpeds.com),15 comprise the Tri-State Pediatric COVID-19 Consortium. Cases of patients in this study have been published previously at least in part (Appendix; available at www.jpeds.com).2 , 3 , 11 , 16, 17, 18, 19, 20, 21, 22, 23, 24
Cases were identified at each participating site using active, hospital-based surveillance for COVID-19 and MIS-C using standardized inclusion criteria and case definitions. Patients were included if they were hospitalized between March 1, 2020, and May 22, 2020 for any reason, were ≤22 years of age at the time of admission, and had a positive diagnostic test for SARS-CoV-2 using reverse-transcriptase polymerase chain reaction (PCR) to detect the viral RNA, or met the MIS-C case definition as set by Centers for Disease Control and Prevention,25 which includes age <21 years, reported or documented fever for more than 24 hours, laboratory markers of inflammation, multisystem (≥2) organ involvement, positive SARS-CoV-2 testing (PCR or serology) or exposure to a COVID-19 case within 4 weeks of symptom onset, and no plausible alternative diagnoses. Each site identified cases retrospectively and prospectively by reviewing COVID-19 test results (laboratory-based surveillance) and inpatient admission logs (MIS-C syndromic surveillance). Neonates who tested positive on the first day of life and pregnant patients were excluded. In addition, patients who were admitted to the hospital with unrelated illnesses such as trauma, scheduled chemotherapy, or psychiatric disease and were found to be incidentally positive for SARS-CoV-2 by admission PCR screening also were excluded.
We reviewed the electronic medical records for demographic, clinical, laboratory, radiographic, and hospitalization outcome data from admission to discharge. Investigators at each site used a standardized data collection form and transmitted data to a central database using a Web-based data capture program hosted at Albert Einstein College of Medicine (Bronx, New York). The study was approved by each center's institutional review board, and each site was exempted from obtaining informed consent.
Study Definitions
Respiratory COVID-19 was defined based on the World Health Organization criteria and included patients having any of the following clinical features: cough, dyspnea, tachypnea, oxygen requirement, or imaging suggestive of pneumonia.26 Each MIS-C case was independently reviewed to ensure it met Centers for Disease Control and Prevention criteria for MIS-C. Patients who did not meet the criteria for either respiratory COVID-19 or MIS-C were classified based on their primary reason for hospitalization. For the comparative analysis, non-respiratory, non-MIS-C SARS-CoV-2 infected hospitalized patients were broadly classified as “other.” Severe disease was defined as an intensive care unit (ICU) admission ≥48 hours.
Self-reported race and ethnicity were categorized into Hispanic, non-Hispanic white, non-Hispanic black, and non-Hispanic other. Patients were categorized having obesity if they had a body mass index or weight-for-age (if <2 years old) that was ≥95th percentile for age and sex.27 Patients were considered medically complex if they had comorbidities that required multiple services, were technology-dependent, were considered medically fragile (eg, cancer), or had a severe disability (eg, intellectual disability).28 , 29 Admission vital signs and relevant laboratory test results were described using age-adjusted standard values.30 Study definitions are detailed in Table II (available at www.jpeds.com).31, 32, 33 Socioeconomic measures at the ZIP code level were used to generate a composite index and proxy measure of individual-level SES. The selected area-level measures were based on previously described and validated instruments using data from the American Community Survey (2014–2018)15 , 31 and are further described in Table II. Patients were considered as low SES if they resided in a ZIP code that was ≥1 SD below the mean area-level SES index.
Statistical Analyses
Descriptive statistics were used to summarize the clinical and sociodemographic characteristics of all patients for whom data were available. The clinical syndromes were collapsed into 3 groups: respiratory, MIS-C, and other. Differences in the distribution of the measured variables were compared using Fisher exact and Kruskal–Wallis tests for categorical and continuous variables, respectively. Results were adjusted for multiple comparisons with Tukey test.
Multivariable logistic regression models were fit for both respiratory and MIS-C groups separately to identify patient characteristics on admission that were predictive of developing severe disease. Potential predictor variables included clinical manifestations, body mass index, comorbidities, laboratory results, insurance status, race/ethnicity, age, sex, and SES. Categories of predictor variables with sparse data were combined before inclusion in the multivariable models. Specifically, insurance status was examined as Medicare/Medicaid vs all other types of payers, and race/ethnicity was examined as Hispanic, Non-Hispanic black, and non-Hispanic white or non-Hispanic other. Only 3 patients with respiratory classification and 6 patients with MIS-C were classified as non-Hispanic other.
For the severity models, aORs were adjusted for hospital site using a fixed-effects approach given concerns about the performance and lack of convergence of random-effects models when the number of sites (clusters) is small.34 For the multivariable analyses, the initial model included variables with P < .25 in bivariate analyses, in addition to age and race, which were deemed a priori to be important clinically (Model 1). An additional model also was fit using a stepwise backward selection strategy in which only those variables with P < .05 were retained (Model 2). Missing data rates in the predictor variables ranged from 0% to 16% and were handled using both list-wise deletion (ie, available data) and multiple imputation using chained equations. C-reactive protein (CRP) level was not considered for inclusion in the respiratory-specific model due to a high rate of missing data (28%) and potential bias from non-random missingness because CRP likely was measured more frequently in patients with severe disease.
No sample-size calculations were performed a priori. All tests of statistical significance were 2-sided, and P < .05 was defined as statistically significant. Statistical analyses were performed using both Stata (version 15, StataCorp) and SAS (version 9.4, SAS Institute Inc) statistical software.
Results
Demographics and Clinical Characteristics
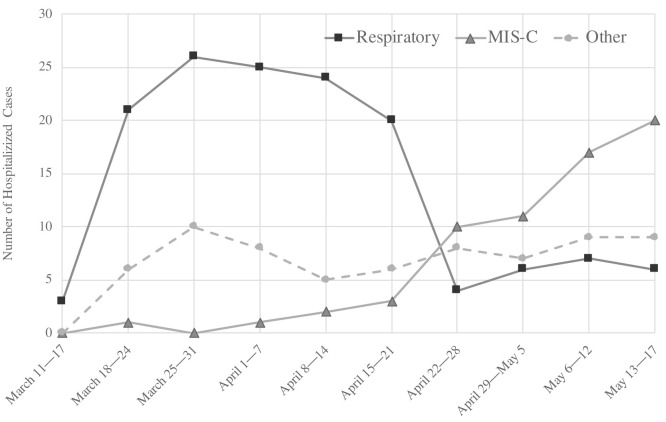
We identified 315 hospitalized pediatric patients with laboratory-confirmed SARS-CoV-2 infection or MIS-C during the peak 3-month period of the pandemic in the Tri-State area. We excluded 34 patients from the final cohort for analysis because review of the medical record indicated that they were hospitalized for unrelated problems deemed unlikely to be etiologically related to SARS-CoV-2, leaving a final cohort of 281 patients (Table II). The majority of the cases came from hospitals in New York (192/281 [68%]) followed by New Jersey (68/281 [24%]) and Connecticut (21/281 [7%]) (Table I). The New York hospitals were located in Brooklyn, Bronx, and Suffolk counties, which were among the hardest impacted areas. Hospitalizations for respiratory disease peaked during the third week of the study period and began to decline 3 weeks later, as MIS-C cases began to increase (Figure 1 ). Hospitalizations for other syndromes peaked during the fourth week of the pandemic and remained relatively constant throughout the study period.
Figure 1.
Hospitalized cases by syndrome during the study period.
The baseline characteristics of the overall cohort are detailed in Table III . Patients were predominantly male (170/281 [60%]) with a median age of 10 years (IQR 1-17). The majority of patients were either Hispanic (125/245 [51%]) or non-Hispanic black (57/245 [23%]). Most of the patients had public insurance through Medicaid or Medicare (188/281 [67%]). Obesity and asthma were the most commonly reported preexisting comorbidities (85/250 [34%] and 40 of 281 [14%], respectively). Overall, 21% (59/281) of cases were considered medically complex.
Table III.
Baseline characteristics of patients hospitalized with SARS-CoV-2
| Characteristics | Total, N = 281 | Clinical group |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Respiratory, N = 143 | MIS-C, N = 69 | Other, N = 69 | P value | ||||||
| Age, y | |||||||||
| Median age, y (IQR) | 10 | (1-17) | 14 | (3-19) | 7 | (3-11) | 7 | (0-16) | <.001 |
| Sex | |||||||||
| Male | 170/281 | (60.5%) | 87/143 | (60.8%) | 42/69 | (60.9%) | 41/69 | (59.4%) | .99 |
| Race/ethnicity | |||||||||
| Hispanic | 125/245 | (51.0%) | 70/120 | (58.3%) | 27/65 | (41.5%) | 28/60 | (46.7%) | .040 |
| Non-Hispanic black | 57/245 | (23.3%) | 21/120 | (17.5%) | 23/65 | (35.4%) | 13/60 | (21.7%) | |
| Non-Hispanic white | 49/245 | (20.0%) | 25/120 | (20.8%) | 9/65 | (13.8%) | 15/60 | (25.0%) | |
| Non-Hispanic other | 14/245 | (5.7%) | 4/120 | (3.3%) | 6/65 | (9.2%) | 4/60 | (6.7%) | |
| Insurance | |||||||||
| Private | 72/281 | (25.6%) | 32/143 | (22.4%) | 28/69 | (40.6%) | 12/69 | (17.4%) | .039 |
| Medicaid/Medicare | 188/281 | (66.9%) | 97/143 | (67.8%) | 38/69 | (55.1%) | 53/69 | (76.8%) | |
| Uninsured/self-pay | 5/281 | (1.8%) | 3/143 | (2.1%) | 1/69 | (1.4%) | 1/69 | (1.4%) | |
| Other/unknown | 16/281 | (5.7%) | 11/143 | (7.7%) | 2/69 | (2.9%) | 3/69 | (4.3%) | |
| SES by ZIP code | |||||||||
| Low SES∗ | 87/281 | (31.0%) | 47/143 | (32.9%) | 21/69 | (30.4%) | 19/69 | (27.5%) | .75 |
| Coexisting conditions | |||||||||
| Obesity∗ | 85/250 | (34.0%) | 62/134 | (46.3%) | 18/64 | (28.1%) | 5/52 | (9.6%) | <.001 |
| Respiratory∗ | 49/281 | (17.4%) | 39/143 | (27.3%) | 6/69 | (8.7%) | 4/69 | (5.8%) | <.001 |
| Neurologic∗ | 23/281 | (8.2%) | 22/143 | (15.4%) | 0/69 | (0.0%) | 1/69 | (1.4%) | <.001 |
| Immunosuppressed∗ | 16/281 | (5.7%) | 13/143 | (9.1%) | 1/69 | (1.4%) | 2/69 | (2.9%) | .052 |
| Diabetes∗ | 11/281 | (3.9%) | 8/143 | (5.6%) | 0/69 | (0.0%) | 3/69 | (4.3%) | .14 |
| Sickle cell | 9/281 | (3.2%) | 7/143 | (4.9%) | 2/69 | (2.9%) | 0/69 | (0.0%) | .21 |
| Cardiovascular∗ | 18/281 | (6.4%) | 12/143 | (8.4%) | 2/69 | (2.9%) | 4/69 | (5.8%) | .30 |
| Gastrointestinal∗ | 10/281 | (3.6%) | 10/143 | (7.0%) | 0/69 | (0.0%) | 0/69 | (0.0%) | .005 |
| History of smoking∗ | 13/228 | (5.7%) | 10/116 | (8.6%) | 0/52 | (0.0%) | 3/60 | (5.0%) | .069 |
| Medical complexity∗ | 59/281 | (21.0%) | 45/143 | (31.5%) | 5/69 | (7.2%) | 9/69 | (13.0%) | <.001 |
| COVID-19 testing | |||||||||
| Only RT-PCR+ | 204/281 | (72.6%) | 133/143 | (93.0%) | 10/69 | (14.5%) | 61/69 | (88.4%) | <.001 |
| Only IgG+ | 44/281 | (15.7%) | 3/143 | (2.1%) | 36/69 | (52.2%) | 5/69 | (7.2%) | |
| Both PCR+ and IgG+ | 20/281 | (7.1%) | 7/143 | (4.9%) | 10/69 | (14.5%) | 3/69 | (4.3%) | |
| Only exposure | 13/281 | (4.6%) | 0/143 | (0.0%) | 13/69 | (18.8%) | 0/69 | (0.0%) | |
| Vital signs on admission | |||||||||
| O2 saturation <90% | 16/281 | (5.7%) | 16/143 | (11.2%) | 0/69 | (0.0%) | 0/69 | (0.0%) | <.001 |
| Tachypnea for age∗ | 66/281 | (23.5%) | 47/143 | (32.9%) | 18/69 | (26.1%) | 1/69 | (1.4%) | <.001 |
| Admission laboratory test results, median (IQR) | |||||||||
| Hemoglobin, g/dL | 12.1, n = 270 | (10.8-13.8) | 12.7, n = 137 | (10.9-14.5) | 11.4, n = 69 | (10.4-12.2) | 12.6, n = 64 | (11.5-13.95) | <.001 |
| WBC, × 109/L | 9.0, n = 272 | (6.2-14.2) | 8.5, n = 138 | (5.6-12.6) | 9.8, n = 69 | (7.4-14.6) | 9.8, n = 65 | (6.4-14.9) | .051 |
| Absolute neutrophil count, × 109/L | 5.8, n = 269 | (3.3-9.6) | 5.1, n = 137 | (2.7-8.9) | 7.6, n = 69 | (5.6-11.1) | 5.1, n = 63 | (2.6-9.8) | <.001 |
| Absolute lymphocyte count, × 109/L | 1.6, n = 269 | (0.9-2.7) | 1.5, n = 137 | (0.9-2.65) | 1.3, n = 69 | (0.8-1.9) | 2.1, n = 63 | (1.4-3.4) | <.001 |
| Platelets, × 109/L | 231, n = 270 | (164-346) | 233, n = 137 | (178-342) | 164, n = 69 | (112-287) | 301 n = 64 | (215-412) | <.001 |
| Alanine aminotransferase, U/L | 29.0, n = 247 | (18-55) | 31.5, n = 128 | (18.5-58) | 38.0, n = 67 | (22-64) | 20.0, n = 52 | (16-26) | <.001 |
| CRP, mg/dL | 7.8, n = 207 | (1.73-27.2) | 4.5, n = 104 | (1.0-14.5) | 25.7, n = 67 | (10-38.1) | 3.5, n = 36 | (0.5-7.9) | <.001 |
| Coinfections | |||||||||
| Viral infections | 12/281 | (4.3%) | 6/143 | (4.2%) | 3/69 | (4.3%) | 3/69 | (4.3%) | 1.00 |
| Chest radiograph findings | |||||||||
| Bilateral infiltrates | 71/215 | (33.0%) | 63/143 | (49.2%) | 8/69 | (12.7%) | 0/24 | (0.0%) | <.001 |
RT, reverse transcriptase; WBC, white blood cell.
Data are presented as n/total (%) for categorical measures and median (IQR) for continuous measures. Pairwise comparison between groups are shown in Table VI. Continuous variables are compared using ANOVA or Kruskal–Wallis based on normality test, categorical variables are compared using Fisher exact tests.
See definitions in Table II.
Among the 281 patients, 143 (51%) presented with respiratory disease, 69 (25%) with MIS-C, and 69 (25%) with one of the other acute SARS-CoV-2–related clinical syndromes or conditions. The “other” group included 32 patients with gastrointestinal symptoms, 21 febrile infants, 6 with neurologic disease, 6 with diabetic ketoacidosis, and 4 patients hospitalized for other indications listed in Table IV (available at www.jpeds.com). Additional details on the clinical features and outcomes of patients in this “other” group are shown in Table V (available at www.jpeds.com).
Differences in Demographics and Clinical Characteristics by Clinical Syndrome
The distribution of baseline patient characteristics varied significantly between the different clinical syndromes (Table III). Patients with respiratory disease were older than those with MIS-C (median age: 14 [IQR 3-19] vs 7 [IQR 3-11] years, respectively, P < .001). Compared with respiratory COVID-19, patients with MIS-C identified their race/ethnicity more commonly as non-Hispanic black (difference = 18%, 95% CI 2%-33%, P = .02). Notably, the prevalence of obesity was 18% greater (95% CI 2%-34%, P = .02) in the respiratory disease group compared with MIS-C. Similarly, the prevalence of medical complexity was 24% greater (95% CI 10%-38%, P < .001) in the respiratory disease group compared with patients with MIS-C. Differences in other baseline characteristics by syndrome are shown in Table VI (available at www.jpeds.com).
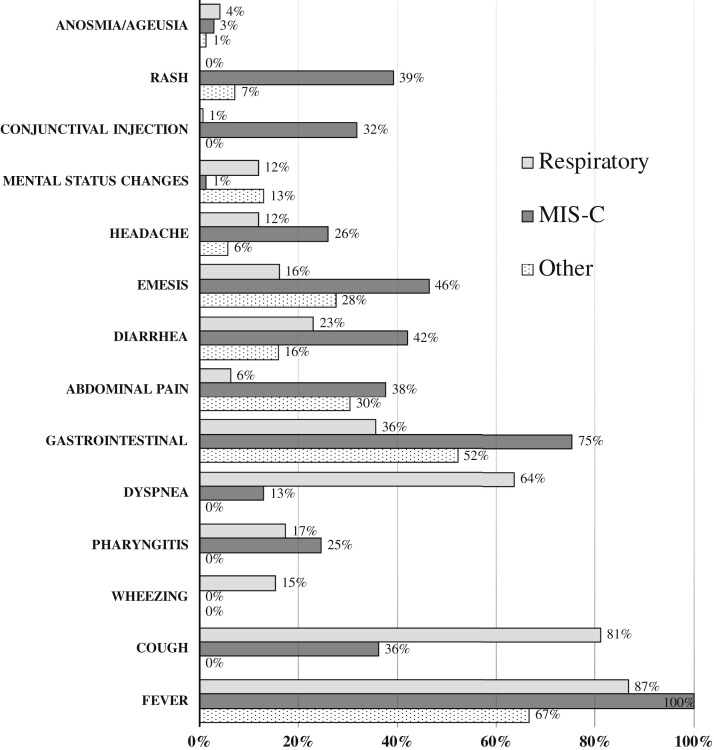
The most common signs and symptoms on admission are shown in Figure 2 (available at www.jpeds.com). Most patients had a fever (235/281 [84%]), and 58% (162/281) reported a respiratory symptom (ie, cough, wheezing, pharyngitis, or dyspnea). Anosmia and ageusia were reported in few patients (9/281 [3%]). Gastrointestinal symptoms, such as emesis, diarrhea, or abdominal pain, were common, particularly among children with MIS-C (52/69 [75%]). Several patients with MIS-C also manifested a rash (27/69 [39%]) and/or conjunctival injection (22/69 [32%]), which was rarely reported among the other clinical phenotypes.
Figure 2.
Signs and symptoms on admission by clinical syndrome.
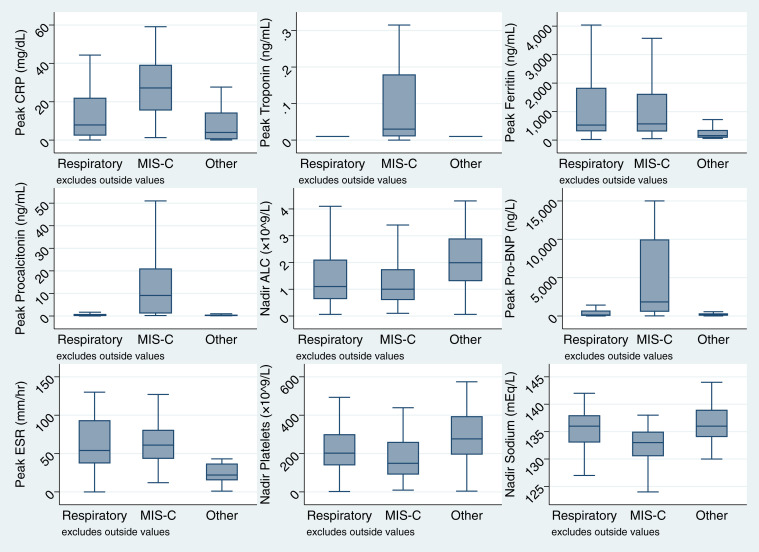
Notable admission laboratory test results are shown in Table III and Table VI. Considering the entire cohort, admission CRP often was elevated (median 7.8 [IQR 1.7-27.2]) and absolute lymphocyte count (ALC) frequently was decreased (median 1.6 [0.9-2.7]). The highest CRP and lowest ALC were found in the patients with MIS-C (median 25.7 [IQR 10.0-38.1] and 1.3 [IQR: 0.8-1.9], respectively). Other peak and nadir results are shown in Figure 3 (available at www.jpeds.com). Peak procalcitonin was greater in patients with MIS-C compared with respiratory illness (median 9.1 [IQR 1.1-21.1]) vs 0.2 [IQR 0.1-0.9], P < .001).
Figure 3.
Peak and nadir laboratory results by syndrome. ESR, erythrocyte sedimentation rate; Pro-BNP, pro-B-type natriuretic peptide.
Almost one-half of the patients with respiratory symptoms had a chest radiograph with bilateral infiltrates on admission (63/143 [44%]), and 18% (25/143) required invasive mechanical ventilation (Table VII ). Conversely, 64% (40/63) of the patients with MIS-C had a normal chest radiograph, and only 4% (3/69) were placed on a ventilator during hospitalization. Medical therapy varied considerably both between and within groups (Table VII). Patients with MIS-C were commonly prescribed methylprednisolone (32/69 [46%]) and/or intravenous immunoglobulin (41/69 [59%]), which were used infrequently in patients with respiratory disease (39/143 [27%] and 3/143 [2%], respectively). Remdesivir was given to 18% (26/143) of patients with respiratory disease and in 7% (5/69) of patients with MIS-C.
Table VII.
Clinical characteristics during hospital admission
| Clinical measures | Clinical subgroups |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Total, N = 281 |
Respiratory, N = 143 | MIS-C, N = 69 |
Other, N = 69 |
P value | |||||
| Maximum respiratory support | |||||||||
| Ambient air | 169/281 | (60.1%) | 60/143 | (42.0%) | 43/69 | (62.3%) | 66/69 | (95.7%) | <.001 |
| Noninvasive respiratory support | |||||||||
| Low-flow nasal cannula | 42/281 | (14.9%) | 29/143 | (20.3%) | 11/69 | (15.9%) | 2/69 | (2.9%) | .001 |
| High-flow nasal cannula | 24/281 | (8.5%) | 16/143 | (11.2%) | 8/69 | (11.6%) | 0/69 | (0.0%) | .004 |
| Noninvasive positive-pressure ventilation | 8/281 | (2.8%) | 5/143 | (3.5%) | 3/69 | (4.3%) | 0/69 | (0.0%) | .24 |
| Invasive mechanical ventilation | 29/281 | (10.3%) | 25/143 | (17.5%) | 3/69 | (4.3%) | 1/69 | (1.4%) | <.001 |
| Medical therapy | |||||||||
| Hydroxychloroquine | 50/281 | (17.8%) | 49/143 | (34.3%) | 0/69 | (0.0%) | 1/69 | (1.4%) | <.001 |
| Remdesivir | 31/281 | (11.0%) | 26/143 | (18.2%) | 5/69 | (7.2%) | 0/69 | (0.0%) | <.001 |
| Methylprednisolone | 72/281 | (25.6%) | 39/143 | (27.3%) | 32/69 | (46.4%) | 1/69 | (1.4%) | <.001 |
| Interleukin inhibitor | 23/281 | (8.2%) | 10/143 | (7.0%) | 13/69 | (18.8%) | 0/69 | (0.0%) | <.001 |
| Azithromycin | 38/281 | (13.5%) | 34/143 | (23.8%) | 4/69 | (5.8%) | 0/69 | (0.0%) | <.001 |
| Convalescent plasma | 4/281 | (1.4%) | 3/143 | (2.1%) | 1/69 | (1.4%) | 0/69 | (0.0%) | .81 |
| Intravenous immunoglobulin | 47/281 | (16.7%) | 3/143 | (2.1%) | 41/69 | (59.4%) | 3/69 | (4.3%) | <.001 |
| Empiric antibiotics (excluding azithromycin) | 178/281 | (63.3%) | 93/143 | (65.0%) | 47/69 | (68.1%) | 38/69 | (55.1%) | .23 |
| Anticoagulant therapy | 98/281 | (34.9%) | 55/143 | (38.5%) | 41/69 | (59.4%) | 2/69 | (2.9%) | <.001 |
| Complications | |||||||||
| Acute respiratory distress syndrome∗ | 27/281 | (9.6%) | 24/143 | (16.8%) | 3/69 | (4.3%) | 0/69 | (0.0%) | <.001 |
| Acute kidney injury∗ | 37/281 | (13.2%) | 15/143 | (10.5%) | 17/69 | (24.6%) | 5/69 | (7.2%) | .008 |
| Carditis∗ | 20/281 | (7.1%) | 3/143 | (2.1%) | 17/69 | (24.6%) | 0/69 | (0.0%) | <.001 |
| Shock∗ | 26/281 | (9.3%) | 2/143 | (1.4%) | 24/69 | (34.8%) | 0/69 | (0.0%) | <.001 |
| Thrombotic event∗ | 12/281 | (4.3%) | 11/143 | (7.7%) | 1/69 | (1.4%) | 0/69 | (0.0%) | .014 |
| Bacteremia | 12/281 | (4.3%) | 10/143 | (7.0%) | 2/69 | (2.9%) | 0/69 | (0.0%) | .050 |
| Urinary tract infection | 10/281 | (3.6%) | 9/143 | (6.3%) | 0/69 | (0.0%) | 1/69 | (1.4%) | .037 |
| Outcomes | |||||||||
| Discharged home | 267/281 | (95.0%) | 133/143 | (93.0%) | 66/69 | (95.7%) | 68/69 | (98.6%) | .21 |
| Hospital length of stay, median d (IQR)† | 4 | (2-8) | 5 | (2-10) | 6 | (3-8) | 2 | (2-4) | <.001 |
| Required ICU stay | 114/281 | (40.6%) | 64/143 | (44.8%) | 44/69 | (63.8%) | 6/69 | (8.7%) | <.001 |
| ICU length of stay, median d (IQR)‡ | 5 | (2-10) | 6 | (2-17) | 4 | (2-7) | 2 | (1-3) | <.001 |
Data are presented as median (IQR) for continuous measures and n/total (%) for categorical measures.
P values estimated using Fisher exact and Kruskal–Wallis tests for categorical and continuous variables, respectively.
See definitions in Table II.
Hospital length of stay excluding patients who were transferred to another facility.
ICU length of stay excluding patients who did not spend time in ICU.
The 2 most common complications in the respiratory disease cohort were acute respiratory distress syndrome (24/143 [17%]) and acute kidney injury (15/143 [11%]). In contrast, cardiovascular complications such as shock (24/69 [35%]) and cardiac dysfunction (17/69 [25%]) were common in MIS-C. Although 12 patients with MIS-C (17%) had depressed ejection fractions on echocardiogram, none had a coronary artery aneurysm (z-score dilation ≥2.5) identified during the study period.
Outcome and Predictors of Disease Severity
Nearly all patients (267/281 [95%]) recovered from their illness and were discharged home by the end of the study (Table VIII; available at www.jpeds.com), with a median length of hospitalization of 4 days (IQR 2-8). Overall, 114 of 281 (41%) of the patients were admitted to the ICU; 16 were admitted for <48 hours. Patients with MIS-C were more likely than those with respiratory disease to be admitted to the ICU (44/69 [64%] vs 64/143 [45%], P < .001), but all deaths (7/315 [2%]) occurred in the respiratory group. Four of the patients who died were considered medically complex; 2 had asthma as their only prior medical problem, and 1 had no previous medical conditions. Additional details on the deceased patients are provided in Table IX (available at www.jpeds.com).
Overall, 40% (56/141) of patients with respiratory disease, 56% (38/68) with MIS-C, and 6% (4/69) with “other” phenotypes met our case definition of severe disease (ie, ≥48 hours in the ICU). In multivariable analyses of the respiratory group (Table IX), younger age (aOR 1.09 per 1-year decrease, 95% CI 1.02-1.16), obesity (aOR 3.39, 95% CI 1.26-9.10), increasing white blood cell count (aOR 1.11 per unit increase in 109/L, 95% CI 1.03-1.20), hypoxia (aOR 4.01, 95% CI 1.14-14.15), and bilateral infiltrates on chest radiograph (aOR 3.69, 95% CI 1.46-9.32) at admission were independent predictors of severe disease (model 2); race adjusted results were similar (model 1). In the MIS-C group (Table X ), only lower ALC (aOR 8.33 per unit decrease in 109 cells/L, 95% CI 2.32- 33.33) and increasing CRP (aOR 1.06 per unit increase in mg/dL, 95% CI 1.01-1.12) at admission were independent predictors of severity (model 2). Insurance status, race/ethnicity, and SES were not significantly predictive of respiratory or MIS-C disease severity. Three patients were excluded from the analysis of disease severity (1 respiratory patient who died on the day of admission, 1 patient who was previously chronically ventilator-dependent and discharged on the same day of admission, and 1 patient with MIS-C transferred to another facility on the day of admission). Results after conducting multiple imputation for missing data were similar to those estimated using available data (Table XI; available at www.jpeds.com).
Table X.
Logistic regression models of severe disease
| Predictors | Bivariate |
Multivariable |
||||
|---|---|---|---|---|---|---|
| Model 1 (N = 106) |
Model 2 (N = 127) |
|||||
| OR (95% CI) | P value | aOR (95% CI) | P value | aOR (95% CI) | P value | |
| Respiratory illness: 56 events, N = 141 | ||||||
| Age (per 1-year decrease) | 1.02 (0.98-1.08) | .29 | 1.09 (1.01-1.18) | .02 | 1.09 (1.02-1.16) | .01 |
| BMI∗ (per unit increase in kg/m2) | 1.01 (0.98-1.04) | .49 | ||||
| Obesity∗ (reference = no obesity) | 1.78 (0.85-3.73) | .12 | 3.66 (1.14-11.78) | .03 | 3.39 (1.26-9.10) | .02 |
| Days of illness before admission | 1.02 (0.94-1.11) | .64 | ||||
| Admission WBC (per unit increase in 109/L) | 1.07 (1.01-1.14) | .03 | 1.11 (1.01-1.21) | .03 | 1.11 (1.03-1.20) | .007 |
| Admission absolute lymphocyte count (per unit decrease in U/L) | 0.92 (0.79-1.08) | .31 | ||||
| Admission CRP level (per unit increase in mg/dL) | 1.02 (1.00-1.04) | .09 | ||||
| Hispanic (reference = non-Hispanic white/other) | 1.29 (0.49-3.37) | .61 | 0.88 (0.26-2.98) | .84 | ||
| Non-Hispanic black (reference = non-Hispanic white/other) | 1.77 (0.50-6.20) | .37 | 1.65 (0.31-8.87) | .56 | ||
| Medicare/Medicaid (reference = private/other) | 0.95 (0.45-2.04) | .9 | ||||
| Oxygen saturation <90% (reference = no) | 3.55 (1.13-11.20) | .03 | 4.25 (1.10-16.49) | .04 | 4.01 (1.14-14.15) | .03 |
| Male (reference = female) | 0.91 (0.45-1.85) | .79 | ||||
| Medical complexity∗ (reference = no) | 1.99 (0.94-4.21) | .07 | 1.51 (0.51-4.42) | .45 | ||
| Bilateral infiltrates on radiograph (reference = no) | 3.14 (1.51-6.53) | .002 | 3.88 (1.36-11.08) | .01 | 3.69 (1.46-9.32) | .006 |
| Low SES∗ (reference ≥–1) |
0.86 (0.31-2.38) |
.77 |
||||
| Predictor |
Bivariate |
Multivariable |
||||
| Model 1 (N = 60) |
Model 2 (N = 66) |
|||||
| OR (95% CI) |
P value |
aOR (95% CI) |
P value |
aOR (95% CI) |
P value |
|
| MIS-C: 38 events, N = 68 | ||||||
| Age (per 1-year decrease) | 0.83 (0.74-0.93) | .002 | 1.00 (0.84-1.19) | .98 | ||
| BMI∗ (per unit increase in kg/m2) | 1.02 (0.92-1.14) | .69 | ||||
| Obesity∗ (reference = no obesity) | 1.16 (0.35-3.91) | .81 | ||||
| Days of illness before admission | 0.98 (0.77-1.25) | .87 | ||||
| WBC (per unit increase in 109/L) | 0.95 (0.88-1.04) | .29 | ||||
| Admission absolute lymphocyte count (per unit decrease in U/L) | 5.88 (2.13-16.67) | <.001 | 12.50 (1.85-100.0) | .009 | 8.33 (2.32-33.33) | .001 |
| Admission CRP level (per unit increase in mg/dL) | 1.04 (1.00-1.07) | .03 | 1.05 (0.98-1.12) | .14 | 1.06 (1.01-1.12) | .02 |
| Hispanic (reference = Non-Hispanic white/other) | 1.09 (0.26-4.55) | .9 | 2.54 (0.23-28.23) | .45 | ||
| Non-Hispanic black (reference = non-Hispanic white/other) | 4.05 (0.87-18.93) | .07 | 3.09 (0.22, 42.80) | .4 | ||
| Medicare/Medicaid (reference = private/other) | 0.39 (0.14-1.12) | .08 | 0.21 (0.04-1.15) | .07 | ||
| Male (reference = female) | 1.66 (0.60-4.60) | .33 | ||||
| Medical complexity∗ (reference = no) | 0.51 (0.08-3.37) | .49 | ||||
| Bilateral infiltrates on radiograph (reference = no) | 6.59 (0.73-59.81) | .09 | ||||
| Low SES∗ (reference ≥–1) | 0.78 (0.23-2.62) | .69 | ||||
BMI, body mass index.
P values for OR and aOR estimated using logistic regression. All estimates are adjusted for site with fixed effects. Excluded 2 Respiratory cases (one who was chronically ventilator-dependent and discharged on hospital day 1, and one who died on the day of admission) and 1 MIS-C case (transferred on the day of admission). Model 1 includes variables with P < .25 in bivariate analysis, age and race. Model 2 includes only variables with P < .05 using stepwise backward selection strategy. CRP level excluded in multivariable Respiratory models because 28% missing and data not missing at random. Bilateral infiltrates on radiograph not included in multivariable MIS-C due to limited number of MIS-C patients with this condition (N = 7).
See definitions in Table II.
In a separate analysis, laboratory variables measured during hospitalization (as opposed to at admission) also were compared between clinical syndromes. Compared with non-severe cases, both severe respiratory disease and MIS-C were significantly associated with a greater peak CRP, procalcitonin, or troponin level, and a lower nadir ALC, platelet count, or serum sodium level (Table XII; available at www.jpeds.com). Although 78% (45/58) of patients with MIS-C had an elevated B-type natriuretic peptide, peak B-type natriuretic peptide did not differ significantly by severity status.
Discussion
This multicenter cohort study describes a spectrum of clinical manifestations of SARS-CoV-2 in children and youth admitted to hospitals that serve racially and ethnically diverse regions of New York, New Jersey, and Connecticut. Although the populations served by the hospitals vary in terms of sociodemographic diversity, with several having a predominantly non-Hispanic white population, nearly all sites reported that the majority of patients with SARS-CoV-2 were Hispanic and/or black. Notably, patients with MIS-C were more likely to be non-Hispanic black. Previous MIS-C case series also have shown that black children represent a significant percentage of MIS-C cases in the US, ranging from 25-40%.2 , 3 , 23 These data reinforce the notion that minorities bear a disproportionate burden of disease.
There also have been reports that communities with a high proportion of lower-income individuals are experiencing a disproportionately greater rate of COVID-19.35 , 36 Consistent with these reports, we found that 31% of patients hospitalized with SARS-CoV-2 were of low SES. Poverty is associated with poor health outcomes and greater rates of pediatric ICU admissions in general.37 Importantly, however, children of lower SES in our study were not more likely to have severe outcomes following hospitalization.
Several features differentiated the various SARS-CoV-2 infection phenotypes. More than one-half of the patients with respiratory disease (similar to COVID-19 in adults) were older than 13 years on admission. Similar to what has been reported for adults, children and youth with respiratory COVID-19 commonly had obesity, pre-existing pulmonary and neurologic disease, as well as medical complexity. In contrast, patients with MIS-C often had no comorbidities, and more than one-half were younger than 7 years of age. Although patients with MIS-C often were critically ill, requiring vasopressor and immunomodulatory therapy, their hospitalization outcomes generally were excellent.
There is little evidence-based guidance available to aid clinicians in the management of children and youth with acute COVID-19 or MIS-C. Predicting clinical decompensation has important ramifications in terms of resource use, hospital admission, and patient management. In terms of severity, close to one-third of the hospitalized patients in our cohort spent ≥48 hours in the ICU, and 2% (7/281) died. Patients with respiratory COVID-19 were more likely to develop severe disease if, on admission, they had either an elevated white blood cell count, hypoxia, bilateral infiltrates on chest radiograph, were of younger age, or were obese. The association between weight and severe respiratory COVID-19 is consistent with the adult literature; however, the mechanisms of this association require further study.38 For MIS-C, only admission laboratory values of CRP and absolute lymphocyte count were predictive of severity. This study builds on the growing body of evidence showing that mortality in hospitalized pediatric patients is low compared with adults.8 , 9 However, it highlights that the young population is not universally spared from morbidity and that even previously healthy children and youth can develop severe disease requiring supportive therapy.39 , 40
We found a wide array of clinical manifestations in children and youth hospitalized with SARS-CoV-2. Although most of the clinical manifestations in children also have been reported in adults, the frequency of some differ considerably. For example, gastrointestinal symptoms such as abdominal pain, emesis, and diarrhea occur in less than one-quarter of hospitalized adults, yet up to one-half of the patients in our cohort reported 1 of these symptoms.41 Ocular and dermatologic findings also are reported rarely in adults yet were observed in 32% and 39% of MIS-C cases, respectively. We also found that SARS-CoV-2 can be an incidental finding in a substantial number of hospitalized pediatric patients. As testing became more accessible and routine for all hospital admissions in late March, we documented a steady rate of incidental SARS-CoV-2 infections (ie, not plausibly etiologically related). This observation has implications for infection control policies and for monitoring community prevalence of infection. Although data are limited, studies show that children can have high viral loads even when asymptomatic or affected with mild disease, and some studies suggest that they can spread disease,42 making it important to screen hospitalized children to both limit potential transmission and track community prevalence of the virus.
Our study has limitations. The study population included patients within the Tri-State area but did not include patients hospitalized in all of the New York City boroughs and may not be generalizable to other geographic regions. Decisions to admit to the hospital and ICU may have varied by location. To date, children have been excluded from randomized controlled trials of antiviral drugs such as remdesivir43 , 44 and there have been no controlled studies on the optimal treatment of MIS-C. Therefore, approaches to treatment, and as a consequence, the clinical outcomes of the patients in this study, may have varied across sites.
Acknowledgments
We thank each of the COVID-19 treatment teams and healthcare providers at the sites involved in the care of patients with COVID-19 for their work and dedication to patient care.
Footnotes
M.D.C. serves on the Editorial Board for The Journal of Pediatrics and is a member of the United States Preventive Services Task Force. This manuscript does not necessarily reflect the views of the United States Preventive Services Task Force. The other authors declare no conflicts of interest.
Appendix
Table I.
Characteristics of participating hospitals
| Hospitals | Study admissions, n (%) | Pediatric ED visits, no. in 2019 | Population estimates: Race and ethnicity in hospital county∗ (%)† |
Race and ethnicity of reported SARS-CoV-2 infections by hospital (%)† |
||||
|---|---|---|---|---|---|---|---|---|
| Hispanic | Black | White | Hispanic | Black | White | |||
| New York | ||||||||
| Children's Hospital at Montefiore‡ | 107 (38%) | 51 284 | 56% | 44% | 45% | 52% | 22% | 5% |
| Maimonides Children's Hospital§ | 31 (11%) | 32 006 | 19% | 34% | 50% | 16% | 13% | 55% |
| Stony Brook Children's Hospital¶ | 26 (9%) | 21 653 | 20% | 9% | 84% | 50% | 12% | 42% |
| Kings County Hospital Center§ | 18 (6%) | 37 000 | 19% | 34% | 50% | 0% | 78% | 0% |
| The Children's Hospital at State University of New York (SUNY) Downstate§ | 10 (4%) | 18 000 | 19% | 34% | 50% | 0% | 90% | 0% |
| New Jersey | ||||||||
| Joseph M. Sanzari Children's Hospital∗∗ | 51 (18%) | 38 000 | 21% | 7% | 73% | 65% | 8% | 47% |
| K. Hovnanian Children's Hospital†† | 17 (6%) | 20 300 | 11% | 8% | 85% | 29% | 6% | 64% |
| Connecticut | ||||||||
| Yale New Haven Children's Hospital‡‡ | 21 (7%) | 32 500 | 19% | 15% | 77% | 62% | 10% | 38% |
ED, emergency department.
Based on US Census data for the county of the pediatric hospital.15
For these estimates, Hispanic patients could be of any race, so they are also included in applicable race categories. Other races were not included.
Children's Hospital at Montefiore, Bronx County.
Maimonides Children's Hospital, Kings County.
Stony Brook Children's Hospital, Suffolk County.
Joseph M. Sanzari Children's Hospital, Bergen County.
K. Hovnanian Children's Hospital, Monmouth County.
Yale New Haven Children's Hospital, New Haven County.
Table II.
Study definitions
| Variables | Definition |
|---|---|
| SARS-CoV-2 infection | Respiratory: any 1 of the following reported or documented clinical features: cough, dyspnea, tachypnea, increased oxygen requirement, or imaging suggestive of pneumonia. MIS-C: aged <21 years, with fever for >24 h, laboratory markers of inflammation, multisystem organ involvement, positive for SARS-CoV-2 testing or exposure to a suspected or confirmed COVID-19 case within 4 weeks of symptom onset, and no plausible alternative diagnoses. Other: Patients who did not meet the criteria for either respiratory COVID-19 or MIS-C. |
| Excluded cases | Patients with incidental SARS-CoV-2 included those hospitalized for psychiatric diseases (n = 9), trauma (n = 7), cancer treatment (n = 2), gastrostomy tube malfunction (n = 1), skin and soft-tissue infection (n = 8), urinary tract infection (n = 3), bacteremia (n = 2), otorrhea (n = 1), and Epstein–Barr virus infection (n = 1). |
| Severe disease | Previous definitions for severe disease include any admission to the ICU, need for supplemental oxygen or invasive mechanical ventilation and, for MIS-C, vasopressor support.1,3,5 We defined severe disease as ≥48 h in the ICU. With the exception of 1 patient who was transferred on the day of admission, 1 patient who was chronically ventilator-dependent and discharged on hospital day 1, and 1 patient who died on the day of admission, this definition included all children who were ventilated during hospitalization and/or required vasopressors. There were 16 patients (6%) who were admitted to the ICU for <48 h and none of these required mechanical ventilation or vasopressors. |
| Multisystem organ involvement | Two or more of the following:
|
| Thrombocytopenia | Defined as platelets count of <150 000/μL |
| Anemia for age | Defined as hemoglobin <10 g/dL if age <1 y, otherwise hemoglobin <9 g/dL |
| Lymphopenia | Defined as an absolute lymphocyte count of <1200/μL |
| Acute respiratory distress syndrome | Based on the Berlin definition45 or a physician diagnosis |
| Acute kidney injury | Defined as: increase in serum creatinine to 1.5 times baseline/age-appropriate standard32 |
| Carditis | Defined as patients with a physician-diagnosed myocarditis or cardiomyopathy |
| Shock | Defined as requiring vasopressors |
| Thrombotic event | Defined as patients with 1 of the following: deep vein thrombus, pulmonary embolus, intracranial thrombus, or atrial thrombus |
| Obesity | Defined as a body mass index or weight-for-age (if <2 years old) ≥95th percentile for age and sex |
| Other coexisting conditions |
|
| Immunosuppressed | Defined as patients with immunosuppressive conditions or actively receiving immunosuppressant drugs |
| History of smoking | Defined as self-reported history of smoking (cigarettes or marijuana) or vaping |
| Medical complexity | Any 1 of the following: multiple comorbidities that require multiple services, technology-dependence, medical fragility (eg, cancer, congenital heart disease), or severe disability (eg, intellectual disability). |
| COVID-19 exposure | Given the high incidence of COVID-19 infection in the Tri-State area during the study period, all patients who met criteria for MIS-C were considered exposed. |
| Fever | Defined as having subjective fever or measured temperature of ≥38.0°C by any method |
| Hypoxia | Defined as oxygen saturation <90% |
| Tachypnea for age | Refers to a presenting RR per minute above the 95th percentile for age33 as follows: age <1 mo and presenting RR > 60; age 1-12 mo presenting RR > 50; age 1-4 y presenting RR > 40; age 4-12 y presenting RR > 30; age >12 y presenting RR > 25 |
| SES index | Using data from the American Community Survey (2014-2018), we calculated a socioeconomic score index based on patient home addresses as previously described and validated.31 The index includes data from each patient's home zip code using census-derived ZIP code tabulation areas and combines six variables to form a SES score for each geographic area. The variables are the percentage of (1) adults with less than a high school education; (2) families with income below the federal poverty level; (3) households receiving public assistance; (4) female-headed households with children; (5) male unemployment; and (6) median household income. These measures were standardized to a mean of 0 and a standard deviation of 1, with positive values associated with a greater socioeconomic status. The variables were then summed and re-standardized to a mean of 0 and a SD of 1. |
RR, respiratory rate.
Table IV.
Primary reasons for admission in children and youth with other clinical syndromes
| Descriptions | n |
|---|---|
| Gastrointestinal | 32 |
| Rule out appendicitis | 17 |
| Gastroenteritis | 7 |
| Gastrointestinal bleed | 3 |
| Appendicitis complication | 1 |
| Ileitis | 1 |
| Intussusception | 1 |
| Rule out pyloric stenosis | 1 |
| Rule out cholangitis | 1 |
| Febrile infant | 21 |
| Neurologic | 6 |
| Seizures | 4 |
| Weakness/lethargy | 1 |
| Irritability | 1 |
| Endocrine | 7 |
| Diabetic ketoacidosis | 6 |
| New-onset hyperglycemia | 1 |
| Hematology/oncology | 3 |
| Hemolytic anemia | 1 |
| Thrombocytopenia | 1 |
| Fever in patient with cancer | 1 |
Table V.
Baseline characteristics of patients hospitalized with other SARS-CoV-2 infection (not classified as respiratory or MIS-C)
| Characteristics | Other clinical subgroups, N = 69 |
P value | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Gastrointestinal | Febrile infant | Neurologic | Diabetes | Hematology or oncology | |||||||
| Total | N = 32 | N = 21 | N = 6 | N = 7 | N = 3 | ||||||
| Age, y | |||||||||||
| Median age (IQR) | 12 | (6-17) | 0 | (0-0) | 3 | (1-11) | 15 | (13-18) | 11 | (1-18) | <.001 |
| Sex | |||||||||||
| Male | 20/32 | (62%) | 11/21 | (52%) | 5/6 | (83%) | 3/7 | (43%) | 2/3 | (67%) | .61 |
| Race/ethnicity | |||||||||||
| Hispanic | 17/30 | (57%) | 7/17 | (41%) | 1/5 | (20%) | 2/6 | (33%) | 1/2 | (50%) | .21 |
| Non-Hispanic black | 7/30 | (23%) | 2/17 | (12%) | 1/5 | (20%) | 3/6 | (50%) | 0/2 | (0%) | |
| Non-Hispanic white | 5/30 | (17%) | 6/17 | (35%) | 3/5 | (60%) | 1/6 | (17%) | 0/2 | (0%) | |
| Non-Hispanic other | 1/30 | (3%) | 2/17 | (12%) | 0/5 | (0%) | 0/6 | (0%) | 1/2 | (50%) | |
| Insurance | |||||||||||
| Private | 8/32 | (25%) | 2/21 | (10%) | 0/6 | (0%) | 2/7 | (29%) | 0/3 | (0%) | .73 |
| Medicaid/Medicare | 22/32 | (69%) | 17/21 | (81%) | 6/6 | (100%) | 5/7 | (71%) | 3/3 | (100%) | |
| Uninsured/self-pay | 1/32 | (3%) | 0/21 | (0%) | 0/6 | (0%) | 0/7 | (0%) | 0/3 | (0%) | |
| Other/unknown | 1/32 | (3%) | 2/21 | (10%) | 0/6 | (0%) | 0/7 | (0%) | 0/3 | (0%) | |
| SES by ZIP code | |||||||||||
| Low SES∗ | 9/32 | (28%) | 4/21 | (19%) | 2/6 | (33%) | 4/7 | (57%) | 0/3 | (0%) | .32 |
| Coexisting conditions | |||||||||||
| Obesity∗ | 3/30 | (10%) | 1/8 | (12%) | 0/4 | (0%) | 0/7 | (0%) | 1/3 | (33%) | .52 |
| Respiratory∗ | 2/32 | (6%) | 0/21 | (0%) | 0/6 | (0%) | 2/7 | (29%) | 0/3 | (0%) | .16 |
| Neurologic∗ | 0/32 | (0%) | 0/21 | (0%) | 1/6 | (17%) | 0/7 | (0%) | 0/3 | (0%) | .13 |
| Immunosuppressed∗ | 1/32 | (3%) | 0/21 | (0%) | 0/6 | (0%) | 0/7 | (0%) | 1/3 | (33%) | .12 |
| Diabetes∗ | 1/32 | (3%) | 0/21 | (0%) | 1/6 | (17%) | 1/7 | (14%) | 0/3 | (0%) | .19 |
| Cardiovascular∗ | 3/32 | (9%) | 1/21 | (5%) | 0/6 | (0%) | 0/7 | (0%) | 0/3 | (0%) | 1.00 |
| History of smoking∗ | 3/27 | (11%) | 0/21 | (0%) | 0/6 | (0%) | 0/4 | (0%) | 0/2 | (0%) | .52 |
| Medical complexity∗ | 5/32 | (16%) | 1/21 | (5%) | 1/6 | (17%) | 1/7 | (14%) | 1/3 | (33%) | .38 |
| COVID-19 testing | |||||||||||
| Only RT-PCR+ | 27/32 | (84%) | 20/21 | (95%) | 5/6 | (83%) | 6/7 | (86%) | 3/3 | (100%) | .65 |
| Only IgG+ | 3/32 | (9%) | 1/21 | (5%) | 0/6 | (0%) | 1/7 | (14%) | 0/3 | (0%) | |
| Both PCR+ and IgG+ | 2/32 | (6%) | 0/21 | (0%) | 1/6 | (17%) | 0/7 | (0%) | 0/3 | (0%) | |
| Admission laboratories, median (IQR) | |||||||||||
| Hemoglobin, g/dL | 13, N = 31 | (12-14) | 13, N = 19 | (11-14) | 12, N = 6 | (12-12) | 14, N = 5 | (14-15) | 11, N = 3 | (6-12) | .017 |
| WBC, × 109/L | 11, N = 32 | (7-16) | 9, N = 19 | (6-13) | 9, N = 6 | (5-10) | 10, N = 5 | (9-14) | 4, N = 3 | (1-12) | .27 |
| Absolute neutrophil count, × 109/L | 7, N = 31 | (4-13) | 3, N = 18 | (2-5) | 4, N = 6 | (2-5) | 7, N = 5 | (6-11) | 3, N = 3 | (1-3) | .004 |
| Absolute lymphocyte count, × 109/L | 2, N = 31 | (1-2) | 3, N = 18 | (2-5) | 3, N = 6 | (3-3) | 2, N = 5 | (2-2) | 1, N = 3 | (0-7) | .010 |
| Platelets, × 109/L | 244, N = 31 | (213-411) | 341, N = 19 | (275-421) | 300, N = 6 | (204-416) | 334, N = 5 | (305-345) | 88, N = 3 | (4-160) | .036 |
| Alanine aminotransferase, U/L | 19, N = 26 | (15-24) | 22, N = 14 | (18-26) | 17, N = 4 | (12-21) | 20, N = 5 | (17-26) | 55, N = 3 | (23-70) | .15 |
| CRP, mg/dL | 7, N = 21 | (2-17) | 0, N = 10 | (0-1) | 10, N = 2 | (5-14) | 2, N = 2 | (0-4) | 1, N = 1 | (1-1) | .073 |
| Coinfections | |||||||||||
| Viral infections | 1/32 | (3%) | 0/21 | (0%) | 1/6 | (17%) | 1/7 | (14%) | 0/3 | (0%) | .19 |
RT-PCR, reverse transcription polymerase chain reaction; WBC, white blood cell.
Data are presented as median (IQR) for continuous measures, and n/total (%) for categorical measures. P values estimated using Fisher exact and Kruskal–Wallis tests for categorical and continuous variables, respectively.
See definitions in Table II.
Table VI.
Difference of baseline characteristics by clinical syndrome using the Tukey multiple comparison test
| Characteristics | MIS-C vs respiratory |
Other∗ vs respiratory |
Other vs MIS-C |
||||||
|---|---|---|---|---|---|---|---|---|---|
| RD | (95% CI) | P value | RD | 95% CI | P value | RD | 95% CI | P value | |
| Age, y | −4.38 | (–6.86, −1.90) | <.001 | −4.34 | (–6.84, −1.84) | <.001 | 0.04 | (–2.86, 2.94) | .92 |
| Sex | |||||||||
| Male | 0.01 | (–0.17, 0.17) | >.99 | −0.01 | (–0.18, 0.16 | .98 | −0.01 | (–0.21, 0.18) | .89 |
| Race/ethnicity | |||||||||
| Hispanic | −0.17 | (–0.35, 0.01) | .07 | −0.12 | (–0.3, 0.07) | .30 | 0.05 | (–0.16, 0.26) | .99 |
| Non-Hispanic black | 0.18 | (0.02, 0.33) | .02 | 0.04 | (–0.11, 0.2) | .80 | −0.14 | (–0.31, 0.04) | .31 |
| Non-Hispanic white | −0.07 | (–0.22, 0.08) | .51 | 0.04 | (–0.11, 0.19) | .79 | 0.11 | (–0.06, 0.28) | .07 |
| Non-Hispanic other | 0.06 | (–0.02, 0.14) | .21 | 0.03 | (–0.05, 0.12) | .64 | −0.03 | (–0.12, 0.07) | .57 |
| Insurance category | |||||||||
| Private | 0.18 | (0.03, 0.33) | .01 | −0.05 | (–0.2, 0.1) | .71 | −0.23 | (–0.4, −0.06) | .01 |
| Medicaid/Medicare | −0.13 | (–0.29, 0.03) | .15 | 0.09 | (–0.07, 0.25) | .39 | 0.22 | (0.03, 0.4) | .03 |
| Uninsured/self-pay | −0.01 | (–0.05, 0.04) | .93 | −0.01 | (–0.05, 0.04) | .94 | 0.01 | (–0.05, 0.05) | .97 |
| Other/unknown | −0.05 | (–0.13, 0.03) | .32 | −0.03 | (–0.11, 0.05) | .59 | 0.01 | (–0.08, 0.11) | .96 |
| SES by ZIP code | |||||||||
| Low SES∗ | −0.02 | (–0.18, 0.13) | .13 | −0.05 | (–0.21, 0.11) | .71 | −0.03 | (–0.22, 0.16) | .57 |
| Coexisting conditions | |||||||||
| Obesity∗ | −0.18 | (–0.34, −0.02) | .02 | −0.37 | (–0.54, −0.19) | <.001 | −0.19 | (–0.38, 0.01) | .10 |
| Respiratory∗ | −0.19 | (–0.31, −0.06) | <.001 | −0.21 | (–0.34, −0.09) | <.001 | −0.03 | (–0.18, 0.12) | .94 |
| Neurologic∗ | −0.15 | (–0.25, −0.06) | <.001 | −0.14 | (–0.23, −0.05) | <.001 | 0.01 | (–0.09, 0.12) | .62 |
| Immunosuppressed∗ | −0.08 | (–0.16, 0.00) | .06 | −0.06 | (–0.14, 0.02) | .16 | 0.01 | (–0.08, 0.11) | .78 |
| Diabetes∗ | −0.06 | (–0.12, 0.01) | .09 | −0.01 | (–0.08, 0.05) | .90 | 0.04 | (–0.03, 0.12) | .56 |
| Sickle cell | −0.02 | (–0.08, 0.04) | .04 | −0.05 | (–0.11, 0.01) | .14 | −0.03 | (–0.1, 0.04) | .76 |
| Cardiovascular∗ | −0.05 | (–0.14, 0.03) | .26 | −0.03 | (–0.11, 0.06) | .75 | 0.03 | (–0.07, 0.13) | .86 |
| Gastrointestinal∗ | −0.07 | (–0.13, −0.01) | .02 | −0.07 | (–0.13, −0.01) | .03 | 0.01 | (–0.07, 0.07) | .94 |
| History of smoking∗ | −0.09 | (–0.17, 0.00) | .06 | −0.04 | (–0.12, 0.05) | .59 | 0.05 | (–0.05, 0.15) | .50 |
| Medical complexity∗ | −0.24 | (–0.38, −0.10) | <.001 | −0.18 | (–0.32, −0.05) | <.001 | 0.06 | (–0.1, 0.22) | .18 |
| COVID-19 testing | |||||||||
| Only RT-PCR+ | −0.79 | (–0.89, −0.68) | <.001 | −0.05 | (–0.15, 0.06) | .55 | 0.74 | (0.62, 0.86) | <.001 |
| Only IgG+ | 0.50 | (0.40, 0.60) | <.001 | 0.05 | (–0.05, 0.15) | .47 | −0.45 | (–0.57, −0.33) | <.001 |
| Both PCR+ and IgG+ | 0.10 | (0.01, 0.18) | .02 | −0.01 | (–0.09, 0.08) | .99 | −0.1 | (–0.2, 0.01) | .02 |
| Only exposure | 0.19 | (0.12, 0.25) | <.01 | 0.01 | (–0.07, 0.07) | .99 | −0.19 | (–0.27, −0.11) | <.01 |
| Vital signs on admission | |||||||||
| O2 saturation of <90% | −0.11 | (–0.19, −0.04) | <.001 | −0.11 | (–0.19, −0.03) | <.001 | 0.01 | (–0.09, 0.09) | >.99 |
| Tachypnea for age∗ | −0.07 | (–0.02, 0.06) | .45 | −0.31 | (–0.45, −0.17) | <.001 | −0.25 | (–0.41, −0.08) | <.001 |
| Admission laboratories, median (IQR) | |||||||||
| Hemoglobin, g/dL | −1.18 | (–1.98, −0.39) | <.001 | −0.03 | (–0.88, 0.81) | .99 | 1.15 | (0.18, 2.12) | .01 |
| WBC, × 109/L | 1.45 | (–0.92, 3.82) | .32 | 1.17 | (–0.96, 3.31) | .40 | −0.28 | (−2.73, 2.18) | >.99 |
| Absolute neutrophil count, × 109/L | 2.43 | (0.69, 4.18) | <.001 | 0.23 | (−1.57, 2.03) | .95 | −2.2 | (−4.26, −0.14) | .01 |
| Absolute lymphocyte count, × 109/L | −0.48 | (–1.24, 0.28) | .29 | 0.53 | (–0.24, 1.30) | .24 | 1.01 | (0.13, 1.89) | .01 |
| Platelets, × 109/L | −47.5 | (–94.1, −0.99) | .04 | 53.1 | (6.64, 99.6) | .02 | 100.7 | (47.4, 154.0) | <.001 |
| Alanine aminotransferase, U/L | −1.45 | (–27.1, 24.2) | .99 | −10.5 | (–39.6, 18.5) | .67 | −9.08 | (–41.7, 23.5) | .48 |
| CRP, mg/dL | 26.3 | (10.8, 14.8) | <.001 | −8.32 | (–27.8, 11.2) | .57 | −34.6 | (–55.5, −13.8) | <.001 |
| Coinfections | |||||||||
| Viral infections | 0.01 | (–0.07, 0.07) | >.99 | 0.01 | (–0.07, 0.07) | .99 | 0.01 | (–0.08, 0.08) | .99 |
| Chest radiograph findings | |||||||||
| Bilateral infiltrates | −0.32 | (–0.45, −0.20) | <.001 | −0.49 | (–0.72, −0.27) | 0 | −0.13 | (–0.37, 0.12) | .11 |
Rate difference (RD) is the difference in means of each characteristic across clinical syndromes. Pairwise comparisons were computed for each combination of clinical syndrome. CIs and P values were adjusted to account for multiple comparisons using Tukey.
See definitions in Table II.
Table VIII.
Patient outcomes at conclusion of study
| Outcomes | n (%) |
|---|---|
| Discharged home | 267 (95%) |
| Transferred to inpatient rehabilitation | 3 (1%) |
| Another acute care hospital∗ | 4 (1%) |
| Death | 7 (2%) |
1 per parental request, 1 for cardiac surgery, 2 transferred to hospital for greater level of care.
Table IX.
Narrative of deaths
| An 11-year-old male patient with intermittent asthma, asthma, seizure disorder, developmental delay, and a gastrostomy tube was admitted with a 3-day history of fever, cough, wheezing, dyspnea, and increased seizure frequency. He was started on high-flow nasal cannula, but by the first hospital day, he required intubation for mechanical ventilation. He was noted to have coinfection with rhinovirus as well as SARS-CoV-2. He received methylprednisolone on admission and was on a steroid taper throughout this hospitalization. A 10-day course of remdesivir and a single dose of anakinra were also given. He developed line-associated Enterococcus faecalis bacteremia and also was noted to have a right femoral deep-vein-thrombosis, for which he received antibiotics and anticoagulation, respectively. He died on hospital day 58. |
| An 11-year-old-male patient with metastatic osteosarcoma on palliative chemotherapy with baseline nasal-cannula oxygen requirement presented with dyspnea and cough of 1-day duration. He was immediately intubated and mechanically ventilated for respiratory failure per the family's request. Care was withdrawn approximately 1 week later, and the patient died from respiratory failure due to a combination of lung metastases and SARS-CoV-2 infection. |
| A 3-month-old female patient with pulmonary hypertension, large atrial septum defect, and a moderate patent ductus arteriosus was admitted with a 1-day history of cough, fever, and dyspnea. She was initially started on nasal-cannula; however, soon after admission, she developed tachypnea and desaturations and was subsequently intubated for mechanical ventilation. She received a 10-day course of remdesivir as well as intravenous immunoglobulin. She developed acute kidney injury, thrombocytopenia, and line-associated Enterococcus faecalis bacteremia. She remained mechanically ventilated and died on hospital day 30. |
| An 18-year-old female patient with morbid obesity, hypertension, and intermittent asthma presented with 8-day history of cough, fever, and dyspnea. She was immediately intubated and mechanically ventilated in the ICU. She had evidence of acute kidney injury and acute respiratory distress syndrome. She was started on hydroxychloroquine and azithromycin, but these were discontinued after 2 days. She received a 5-day course of methylprednisolone and a single dose of tocilizumab. However, she remained mechanically ventilated and died on hospital day 38. |
| A 20-year-old male patient with a medical history of intermittent asthma was admitted with respiratory distress. He had been ill for 21 days before hospital presentation with fever, cough, wheezing, myalgia, dyspnea, vomiting, fatigue, and neck swelling. He was immediately intubated and mechanically ventilated after arriving at the intensive care unit. He had evidence of thrombocytopenia, acute respiratory distress syndrome, and acute kidney injury. He received empiric antibiotic therapy, methylprednisolone, and convalescent plasma but died on hospital day 2. |
| A 5-month-old male patient with no medical history was admitted after he was found to be unresponsive and limp while at home. No proceeding symptoms or known exposure to COVID-19 was reported. On hospital presentation, he was immediately intubated and started on mechanical ventilation as well as epinephrine, norepinephrine, and vasopressin. In addition to the SARS-CoV-2 infection, he was found to have parainfluenza infection. He received empiric antibiotic therapy with ceftriaxone and vancomycin. Five days into his hospitalization, he developed severe thrombocytopenia and acute kidney injury. At that time, he was started on a 6-day course of hydroxychloroquine. One week into his hospitalization, he was noted to have a Staphylococcus epidermidis line-associated bacteremia as well as Stenotrophomonas maltophilia pneumonia. Despite these interventions, he remained mechanically ventilated. He was given convalescent plasma on hospital day 29. He died shortly after a 31-day hospitalization. |
| A 10-year-old male patient with a medical history of intermittent asthma was admitted with fever, cough, wheezing, and dyspnea for 7 days before presentation. The patient was noted to be in significant respiratory distress with hypoxia. He was admitted to the intensive care unit with acute respiratory distress syndrome and was mechanically ventilated and had left-sided chest tube placement. He had evidence of acute kidney injury with elevated creatinine. He received methylprednisolone and empiric antibiotic therapy with ceftaroline. He died on hospital day 2 due to respiratory distress. |
Table XI.
Sensitivity analysis: logistic regression models for severe outcome using multiple imputation for missing data
| Case description | Multivariable model 1 |
Multivariable model 2 |
||
|---|---|---|---|---|
| aOR (95% CI) | P value | aOR (95% CI) | P value | |
| Respiratory illness: 56 events, N = 141 | ||||
| Age (per 1-year decrease) | 1.09 (1.02-1.16) | .01 | 1.09 (1.02-1.15) | .01 |
| Obesity∗ (reference = no) | 3.48 (1.22- 9.96) | .02 | 2.84 (1.09-7.39) | .03 |
| Admission WBC count (per unit increase in 109/L) | 1.11 (1.02-1.21) | .01 | 1.10 (1.02-1.19) | .02 |
| Hispanic (reference = non-Hispanic white/other) | 0.93 (0.30-2.90) | .90 | ||
| Non-Hispanic black (reference = non-Hispanic white/other) | 1.64 (0.34-7.90) | .54 | ||
| Oxygen saturation <90% (reference = no) | 3.56 (0.98-12.95) | .05 | 4.08 (1.17-14.27) | .03 |
| Medical complexity∗ (reference = no) | 2.34 (0.94-5.81) | .07 | ||
| Bilateral infiltrates on radiograph (reference = no) |
4.05 (1.59-10.30) |
.003 |
3.88 (1.59-9.48) |
.003 |
| Multivariable model 1 |
||||
| aOR (95% CI) |
P value |
|||
| MIS-C: 38 events, N = 68 | ||||
| Age (per 1-year decrease) | 0.98 (0.84, 1.16) | .85 | ||
| Admission absolute lymphocyte count (per unit decrease in U/L) | 6.25 (1.41, 25.0) | .02 | ||
| Admission CRP level (per unit increase in mg/dL) | 1.03 (0.97, 1.09) | .33 | ||
| Hispanic (reference = non-Hispanic white/other) | 1.20 (0.14, 10.09) | .87 | ||
| Non-Hispanic black (reference = non-Hispanic white/other) | 2.86 (0.24, 33.71) | .40 | ||
| Medicaid/Medicare (reference = private/other) | 0.40 (0.09, 1.68) | .21 | ||
P values for aOR estimated using logistic regression. All estimates are adjusted for site with fixed effects.
For respiratory illness, excluded 2 respiratory cases (one who was chronically ventilator-dependent and discharged on hospital day 1, and one who died on the day of admission). Multiple imputation model included outcome, all predictor variables listed in Table X and hospital site. Results based on 40 imputed data sets.
For MIS-C, excluded 1 MIS-C case (transferred on the day of admission). Multiple imputation model included outcome, all predictor variables listed in Table X and hospital site. Results based on 40 imputed data sets. Model 1 includes variables with P < .25 in bivariate analysis, age and race. Model 2 had nearly complete data (2 missing values) so multiple imputation not performed.
See definitions in Table II.
Table XII.
Patient characteristics by disease severity status
| Clinical measures | Respiratory |
MIS-C |
Other |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Non-severe |
Severe |
P value∗ | Non-severe |
Severe |
P value∗ | Non-severe |
Severe |
P value∗ | |
| N = 85 | N = 56 | N = 30 | N = 38 | N = 65 | N = 4 | ||||
| Age, y | 17 (2-20) | 13 (3-16) | .12 | 3 (1-7) | 10 (6-13) | <.001 | 6 (0-16) | 11 (4-17) | .32 |
| Male | 53/85 (62%) | 33/56 (59%) | .73 | 17/30 (57%) | 25/38 (66%) | .46 | 38/65 (58%) | 3/4 (75%) | .64 |
| Race/ethnicity | |||||||||
| Hispanic | 44/70 (63%) | 26/48 (54%) | .37 | 15/28 (54%) | 12/36 (33%) | .09 | 26/56 (46%) | 2/4 (50%) | .25 |
| Non-Hispanic black | 9/70 (13%) | 11/48 (23%) | 6/28 (21%) | 17/36 (47%) | 11/56 (20%) | 2/4 (50%) | |||
| Non-Hispanic white/other | 17/70 (24%) | 11/48 (23%) | 7/28 (25%) | 7/36 (19%) | 19/56 (34%) | 0/4 (0%) | |||
| Insurance | |||||||||
| Medicaid/Medicare | 57/85 (67%) | 38/56 (68%) | >.99 | 21/30 (70%) | 17/38 (45%) | .05 | 50/65 (77%) | 3/4 (75%) | >.99 |
| SES by ZIP code | |||||||||
| Low SES† | 32/85 (38%) | 15/56 (27%) | .20 | 11/30 (37%) | 10/38 (26%) | .43 | 16/65 (25%) | 3/4 (75%) | .061 |
| Coexisting conditions | |||||||||
| Obesity† | 34/81 (42%) | 27/51 (53%) | .28 | 6/25 (24%) | 12/38 (32%) | .58 | 5/49 (10%) | 0/3 (0%) | >.99 |
| Medical complexity† | 22/85 (26%) | 22/56 (39%) | .099 | 3/30 (10%) | 2/38 (5%) | .65 | 8/65 (12%) | 1/4 (25%) | .44 |
| Vital signs on admission | |||||||||
| O2 saturation of <90% | 6/85 (7%) | 10/56 (18%) | .06 | 0/30 (0%) | 0/38 (0%) | – | 0/65 (0%) | 0/4 (0%) | – |
| Tachypnea for age† | 19/85 (22%) | 28/56 (50%) | <.001 | 8/30 (27%) | 10/38 (26%) | >.99 | 1/65 (2%) | 0/4 (0%) | >.99 |
| Days of illness before admission | 3 (1-7) | 4 (2-7) | .33 | 5 (3-6) | 5 (4-6) | .95 | 1 (1-3) | 2 (1-4) | .65 |
| Laboratories and imaging | |||||||||
| Peak CRP >25, mg/dL | 4/57 (7%) | 14/45 (31%) | .003 | 10/29 (34%) | 24/37 (65%) | .03 | 3/35 (9%) | 0/1 (0%) | >.99 |
| Peak procalcitonin >0.5, ng/mL | 8/40 (20%) | 16/31 (52%) | .01 | 13/18 (72%) | 29/30 (97%) | .02 | 5/15 (33%) | 0/1 (0%) | >.99 |
| Peak ESR >50, mm/h | 7/17 (41%) | 15/23 (65%) | .20 | 16/23 (70%) | 18/26 (69%) | >.99 | 2/13 (15%) | 0/1 (0%) | >.99 |
| Peak ferritin >500, ng/mL | 21/43 (49%) | 23/43 (53%) | .83 | 10/25 (40%) | 29/37 (78%) | .003 | 4/21 (19%) | 0/1 (0%) | >.99 |
| Peak troponin >0.01, ng/mL | 5/43 (12%) | 11/30 (37%) | .02 | 6/22 (27%) | 27/35 (77%) | <.001 | 2/17 (12%) | 1/2 (50%) | .30 |
| Peak BNP >500, ng/L | 5/31 (16%) | 10/24 (42%) | .07 | 16/22 (73%) | 29/36 (81%) | .53 | 3/12 (25%) | 0/1 (0%) | >.99 |
| Nadir absolute lymphocyte count <1.0, × 109/L | 29/76 (38%) | 31/54 (57%) | .03 | 7/29 (24%) | 27/38 (71%) | <.001 | 10/56 (18%) | 0/3 (0%) | >.99 |
| Nadir platelets <100, × 109/L | 4/77 (5%) | 14/54 (26%) | .001 | 3/30 (10%) | 19/38 (50%) | <.001 | 3/59 (5%) | 0/3 (0%) | >.99 |
| Nadir sodium <130, mEq/L | 4/78 (5%) | 11/55 (20%) | .01 | 3/29 (10%) | 13/38 (34%) | .04 | 4/58 (7%) | 1/4 (25%) | .29 |
| Viral coinfection | 2/85 (2%) | 4/56 (7%) | .21 | 0/30 (0%) | 2/38 (5%) | .50 | 2/65 (3%) | 1/4 (25%) | .17 |
BNP, B-type natriuretic peptide; ESR, erythrocyte sedimentation rate.
Data are presented as median (IQR) for continuous measures, and n/total (%) for categorical measures.
Continuous variables were compared using Wilcoxon rank-sum, categorical variables were compared using Fisher exact tests; Severity analysis excluded 1 MIS-C case (transferred on the day of admission) and 2 respiratory cases (one who was chronically ventilator-dependent and discharged on hospital day 1, and one who died on the day of admission).
See definitions in Table II.
Data Statement
Data sharing statement available at www.jpeds.com.
Supplementary Data
References
- 1.Shekerdemian L.S., Mahmood N.R., Wolfe K.K., Riggs B.J., Ross C.E., McKiernan C.A. Characteristics and outcomes of children with coronavirus disease 2019 (COVID-19) infection admitted to US and Canadian pediatric intensive care units. JAMA Pediatr. 2020;174:868–873. doi: 10.1001/jamapediatrics.2020.1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dufort E.M., Koumans E.H., Chow E.J., Rosenthal E.M., Muse A., Rowlands J. Multisystem inflammatory syndrome in children in New York State. N Engl J Med. 2020;383:347–358. doi: 10.1056/NEJMoa2021756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Feldstein L.R., Rose E.B., Horwitz S.M., Collins J.P., Newhams M.M., Son M.B.F. Multisystem inflammatory syndrome in U.S. children and adolescents. N Engl J Med. 2020;383:334–346. doi: 10.1056/NEJMoa2021680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dong Y., Mo X., Hu Y., Qi X., Jiang F., Jiang Z. Epidemiology of COVID-19 among children in China. Pediatrics. 2020;145:e20200702. doi: 10.1542/peds.2020-0702. [DOI] [PubMed] [Google Scholar]
- 5.Zachariah P., Johnson C.L., Halabi K.C., Ahn D., Sen A.I., Fischer A. Epidemiology, clinical features, and disease severity in patients with coronavirus disease 2019 (COVID-19) in a children's hospital in New York City, New York. JAMA Pediatr. 2020;174:e202430. doi: 10.1001/jamapediatrics.2020.2430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kainth M.K., Goenka P.K., Williamson K.A., Fishbein J.S., Subramony A., Schleien C. Early experience of COVID-19 in a US children' hospital. Pediatrics. 2020;146 doi: 10.1542/peds.2020-003186. e2020003186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Morris S.B., Schwartz N.G., Patel P., Abbo L., Beauchamps L., Balan S. Case series of multisystem inflammatory syndrome in adults associated with SARS-CoV-2 infection—United Kingdom and United States, March–August 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1450–1456. doi: 10.15585/mmwr.mm6940e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Richardson S., Hirsch J.S., Narasimhan M., Crawford J.M., McGinn T., Davidson K.W. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City Area. JAMA. 2020;323:2052–2059. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cummings M.J., Baldwin M.R., Abrams D., Jacobson S.D., Meyer B.J., Balough E.M. Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: a prospective cohort study. Lancet. 2020;395:1763–1770. doi: 10.1016/S0140-6736(20)31189-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Agha R., Kojaoghlanian T., Avner J.R. Initial observations of COVID-19 in US children. Hosp Pediatr. 2020;10:902–905. doi: 10.1542/hpeds.2020-000257. [DOI] [PubMed] [Google Scholar]
- 12.Azar K.M.J., Shen Z., Romanelli R.J., Lockhart S.H., Smits K., Robinson S. Disparities In outcomes among COVID-19 patients in a large health care system in California. Health Aff (Millwood) 2020;39:1253–1262. doi: 10.1377/hlthaff.2020.00598. [DOI] [PubMed] [Google Scholar]
- 13.Millett G.A., Jones A.T., Benkeser D., Baral S., Mercer L., Beyrer C. Assessing differential impacts of COVID-19 on black communities. Ann Epidemiol. 2020;47:37–44. doi: 10.1016/j.annepidem.2020.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lieberman-Cribbin W., Tuminello S., Flores R.M., Taioli E. Disparities in COVID-19 testing and positivity in New York City. Am J Prev Med. 2020;59:326–332. doi: 10.1016/j.amepre.2020.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.US Census Bureau American Community Survey 2013-2018. https://www.census.gov/newsroom/press-kits/2018/acs-5year.html Accessed June 15, 2020.
- 16.Chao J.Y., Derespina K.R., Herold B.C., Goldman D.L., Aldrich M., Weingarten J. Clinical characteristics and outcomes of hospitalized and critically ill children and adolescents with coronavirus disease 2019 (COVID-19) at a tertiary care medical center in New York City. J Pediatr. 2020;223:14–19. doi: 10.1016/j.jpeds.2020.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Derespina K.R., Kaushik S., Plichta A., Conway E.E., Jr., Bercow A., Choi J. Clinical manifestations and outcomes of critically ill children and adolescents with COVID-19 in New York City. J Pediatr. 2020;223:14–19. doi: 10.1016/j.jpeds.2020.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kaushik S., Aydin S.I., Derespina K.R., Bansal P.B., Kowalsky S., Trachtman R. Multisystem inflammatory syndrome in children associated with severe acute respiratory syndrome coronavirus 2 infection (MIS-C): a multi-institutional study from New York City. J Pediatr. 2020;224:24–29. doi: 10.1016/j.jpeds.2020.06.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Clouser K.N., Baer A., Bhavsar S., Gadhavi J., Li S., Schnall J. MIS-C after ARDS associated with SARS-CoV-2. Pediatr Infect Dis J. 2020;39:e363–e365. doi: 10.1097/INF.0000000000002879. [DOI] [PubMed] [Google Scholar]
- 20.Bhavsar S.M., Clouser K.N., Gadhavi J., Anene O., Kaur R., Lewis R. COVID-19 in pediatrics: characteristics of hospitalized children in New Jersey. Hospital Pediatr. 2020 doi: 10.1542/hpeds.2020-001719. In press. [DOI] [PubMed] [Google Scholar]
- 21.Perez A., Kogan-Liberman D., Sheflin-Findling S., Raizner A., Ahuja K.L., Ovchinsky N. Presentation of severe acute respiratory syndrome-coronavirus 2 infection as cholestatic jaundice in two healthy adolescents. J Pediatr. 2020;226:278–280. doi: 10.1016/j.jpeds.2020.07.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pierce C.A., Preston-Hurlburt P., Dai Y., Aschner C.B., Cheshenko N., Galen B. Immune responses to SARS-CoV-2 infection in hospitalized pediatric and adult patients. Sci Transl Med. 2020;12:eabd5487. doi: 10.1126/scitranslmed.abd5487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Godfred-Cato S., Bryant B., Leung J., Oster M.E., Conklin L., Abrams J. COVID-19-Associated Multisystem Inflammatory Syndrome in Children—United States, March-July 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1074–1080. doi: 10.15585/mmwr.mm6932e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bhavsar S.M., Agarwal S., Lewis R., Ganta A., Roshchina Y.S., Clouser K.N. COVID-19 infection associated with encephalitis in an adolescent. Neurol Clin Pract. 2020 doi: 10.1212/CPJ.0000000000000911. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Centers for Disease Control and Prevention Emergency preparedness and response: multisystem inflammatory syndrome in children (MIS-C) associated with coronavirus disease 2019 (COVID-19) https://www.cdc.gov/coronavirus/2019-nCoV/index.html?cid=EPR-homepage Accessed August 8, 2020.
- 26.World Health Organization Clinical management of COVID-19. https://www.who.int/publications/i/item/clinical-management-of-covid-19 Accessed July 10, 2020. [PubMed]
- 27.Centers for Disease Control and Prevention Defining Childhood Obesity. https://www.cdc.gov/obesity/childhood/defining.html Accessed July 10, 2020.
- 28.Cohen E., Kuo D.Z., Agrawal R., Berry J.G., Bhagat S.K., Simon T.D. Children with medical complexity: an emerging population for clinical and research initiatives. Pediatrics. 2011;127:529–538. doi: 10.1542/peds.2010-0910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kuo D.Z., Houtrow A.J., Council On Children With Disabilities Recognition and management of medical complexity. Pediatrics. 2016;138:e2016302. doi: 10.1542/peds.2016-3021. [DOI] [PubMed] [Google Scholar]
- 30.Hughes H., Kahl L.K. 21st ed. Elsevier; Philadelphia: 2018. The Harriet Lane handbook: a manual for pediatric house officers. [Google Scholar]
- 31.Martsolf G.R., Barrett M.L., Weiss A.J., Washington R., Steiner C.A., Mehrotra A. Impact of race/ethnicity and socioeconomic status on risk-adjusted readmission rates: implications for the hospital readmissions reduction program. Inquiry. 2016;53 doi: 10.2106/JBJS.15.00884. 0046958016667596. [DOI] [PubMed] [Google Scholar]
- 32.Kidney Disease Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group KDIGO Clinical Practice Guideline for Acute Kidney Injury. Kidney Int Suppl. 2012;2:1–138. [Google Scholar]
- 33.Bonafide C.P., Brady P.W., Keren R., Conway P.H., Marsolo K., Daymont C. Development of heart and respiratory rate percentile curves for hospitalized children. Pediatrics. 2013;131:e1150–e1157. doi: 10.1542/peds.2012-2443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McNeish D., Stapleton L.M. Modeling clustered data with very few clusters. Multivariate Behav Res. 2016;51:495–518. doi: 10.1080/00273171.2016.1167008. [DOI] [PubMed] [Google Scholar]
- 35.Hawkins D. Social Determinants of COVID-19 in Massachusetts, United States: an ecological study. J Prev Med Public Health. 2020;53:220–227. doi: 10.3961/jpmph.20.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Maroko A.R., Nash D., Pavilonis B.T. COVID-19 and inequity: a comparative spatial analysis of New York City and Chicago hot spots. J Urban Health. 2020;97:461–470. doi: 10.1007/s11524-020-00468-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Andrist E., Riley C.L., Brokamp C., Taylor S., Beck A.F. Neighborhood Poverty and pediatric intensive care use. Pediatrics. 2019;144 doi: 10.1542/peds.2019-0748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hajifathalian K., Kumar S., Newberry C., Shah S., Fortune B., Krisko T. Obesity is associated with worse outcomes in COVID-19: analysis of early data from New York City. Obesity (Silver Spring) 2020;28:1606–1612. doi: 10.1002/oby.22923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim L., Whitaker M., O'Halloran A., Kambhampati A., Chai S.J., Reingold A. Hospitalization rates and characteristics of children aged <18 years hospitalized with laboratory-confirmed COVID-19 - COVID-NET, 14 states, March 1-July 25, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1081–1088. doi: 10.15585/mmwr.mm6932e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gotzinger F., Santiago-Garcia B., Noguera-Julian A., Lanaspa M., Lancella L., Calo Carducci F.I. COVID-19 in children and adolescents in Europe: a multinational, multicentre cohort study. Lancet Child Adolesc Health. 2020;4:653–661. doi: 10.1016/S2352-4642(20)30177-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Goyal P., Choi J.J., Pinheiro L.C., Schenck E.J., Chen R., Jabri A. Clinical characteristics of Covid-19 in New York City. N Engl J Med. 2020;382:2372–2374. doi: 10.1056/NEJMc2010419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yonker L.M., Neilan A.M., Bartsch Y., Patel A.B., Regan J., Arya P. Pediatric SARS-CoV-2: clinical presentation, infectivity, and immune responses. J Pediatr. 2020;227:42–52. doi: 10.1016/j.jpeds.2020.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Beigel J.H., Tomashek K.M., Dodd L.E. Remdesivir for the Treatment of Covid-19—Preliminary Report. Reply. N Engl J Med. 2020;383 doi: 10.1056/NEJMc2022236. [DOI] [PubMed] [Google Scholar]
- 44.Wang Y., Zhang D., Du G., Du R., Zhao J., Jin Y. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2020;395:1569–1578. doi: 10.1016/S0140-6736(20)31022-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.ARDS Definition Task Force. Ranieri V.M., Rubenfeld G.D., Thompson B.T., Ferguson N.D., Caldwell E. Acute respiratory distress syndrome: the Berlin definition. JAMA. 2012;307:2526–2533. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.