Highlights
-
•
Angiotensin converting enzyme (ACE) genotypes may influence COVID-19 mortality.
-
•
II genotype frequency was significantly associated with decreased mortality.
-
•
Association between increased mortality and DD genotype frequency was not detected.
Keywords: Viral pneumonia, Genotype, Epidemiology, Mortality
Abstract
Background
Angiotensin converting enzyme (ACE) genotypes are known to be associated with development of acute respiratory distress syndrome (ARDS) and resultant mortality. In the present study, we examined the association between distribution frequency of ACE genotypes and COVID-19 mortality.
Methods
We undertook an ecological study to examine the association between ACE genotypes and COVID-19 mortality across 25 countries to represent different geographical regions of the world. The population frequencies of ACE genotypes were drawn from previously published reports and data on COVID-19-related mortality were extracted from ‘Worldometer’. Multivariable analyses were also undertaken adjusting for age (median age), sex (percentage of females) and the number of COVID-19 tests undertaken. Associations between genotypes deletion/deletion (DD) and insertion/insertion (II) prevalence and COVID-19-related mortality (per million people per day since the first diagnosed case) were evaluated.
Results
The frequency of II genotype is highest in east Asian countries and lower among the European and African countries. An inverse geographical distribution frequency was noted for DD genotype. Increasing II genotype frequency was significantly associated with decreased COVID-19 mortality rates (adjusted incident rate ratio [IRR] 0.3, 95% confidence interval [CI]: 0.002–0.7, p = 0.03). However, no association was found between DD genotype frequency and COVID-19 mortality rates (adjusted IRR 4.3, 95% CI: 0.5–41.2, p = 0.2).
Conclusions
Distribution frequency of ACE insertion/insertion (II) genotype may have a significant influence on COVID-19 mortality. This information has potential utility for resource planning at a systemic level, as well as for clinical management.
Introduction
The coronavirus disease 2019 (COVID-19), caused by SARS-CoV2 virus, has evolved rapidly into a pandemic, with total confirmed cases of nearly 9 million, and more than 460,000 deaths worldwide as of 22 June 2020.1 The COVID-19 fatality rates vary greatly across countries, and have been attributed to multiple factors including availability of healthcare resources and mitigation strategies, including border closures, social distancing, mass screening, contact tracing and quarantine.2 , 3
The clinical presentation of COVID-19 ranges from mild symptoms to potentially fatal respiratory failure due to development of adult respiratory distress syndrome (ARDS). The renin-angiotensin-aldosterone system (RAAS) has been implicated in the pathogenesis of COVID-19.4 Moreover, the role of angiotensin converting enzymes 1 and 2 (ACE and ACE2) in the development of ARDS is well established.5 The coronavirus utilises membrane bound ACE2 receptors for entry into alveolar epithelial cells. This disrupts counter regulatory mechanisms between ACE and ACE2, leading to endothelial dysfunction and activation of severe maladaptive immune responses, the hallmark of ARDS.5 , 6
The ACE gene is known to consist of two variant alleles; insertion (I) and deletion (D) polymorphisms.7 The allelic distribution of the ACE gene within a population follows the Hardy-Weinberg equilibrium, with three distinct genotypes: II, ID, and DD.8 Diseases such as stroke and diabetic nephropathy have been shown to be associated with the DD genotype.9 , 10 Additionally, previous studies on patients with sepsis (not due to coronavirus) demonstrated an association between DD genotype and development of ARDS resulting in higher mortality risk11 On the other hand, the II genotype is associated with decreased mortality from ARDS.12 The ACE genotype distributions vary across geographical regions, with frequency of DD genotype being lowest among east Asian populations and highest among Caucasian and African populations.8
Recent studies demonstrate that infection with COVID-19 may be associated with I/D polymorphisms, with increasing D allele frequency correlating to a reduction in prevalence but increase in mortality from COVID-19 infection.13 , 14 Currently, it remains unclear whether the observed variations in COVID-19 mortality among countries can be explained by variations in ACE polymorphisms, specifically in relation to the frequency of the II and DD genotypes. Hence, we undertook an ecological study to examine the association between the distribution frequency of ACE genotypes and COVID-19 mortality in selected countries representing different geographical regions of the world.
Methods
Genotype frequency data
Data on the distribution of ACE genotypes were collected for 25 countries representing a diverse cross-section of geographical regions of the world (Table 1 ). The data were drawn from systematic reviews and meta analyses published in the past 15 years (since 2005) examining ACE genotypes and risk of cardiovascular, respiratory, diabetes, renal and stroke diseases.8 , 9 , 15, 16, 17, 18, 19, 20, 21, 22 Published studies in English which contained the largest cohort of patients were selected to represent each country. For countries where ACE genotype data were not available from systematic reviews and meta-analyses, further searching was conducted through PubMed and Google Scholar, using search terms ‘ACE polymorphisms + (country name)’ or ‘ACE genotype + (country name)’. The full list of citations for included studies can be found in Supplementary Materials. Selected studies for each country included genotype distribution data for healthy controls or general population and diseased populations, except for France, where data were not available for the general population. The combined data for healthy and diseased populations were taken as the overall genotype frequency of that particular country.
Table 1.
Adjusted death rates from COVID-19 and II and DD genotype distribution of 25 included countries.
| Region | Country | First case (date) | Tests/1million | Total Deaths | Death/1 million/day | II Genotype% | DD Genotype% | Genotype data source* |
|---|---|---|---|---|---|---|---|---|
| Asia | China | 31-Dec-19 | Not available | 4634 | 0.14 | 40.6 | 19.0 | Yan 2005 |
| Asia | South Korea | 20-Jan-20 | 19,860 | 273 | 0.17 | 36.9 | 15.5 | Um 2003 |
| Asia | Japan | 24-Jan-20 | 2486 | 916 | 0.26 | 42.1 | 13.0 | Mannami 2001 |
| Asia | Singapore | 24-Jan-20 | 69,824 | 25 | 0.12 | 47.9 | 8.4 | Lau 2002 |
| Asia | India | 30-Jan-20 | 3381 | 7207 | 0.12 | 30.8 | 24.8 | Pulla 2010 |
| Central Asia | Iran | 19-Feb-20 | 12,916 | 8281 | 14.08 | 26.1 | 20.4 | Nikzamir 2008 |
| Central Asia | Pakistan | 26-Feb-20 | 3095 | 2002 | 0.48 | 23.0 | 22.3 | Hussain 2018 |
| Middle East | Saudi Arabia | 2-Mar-20 | 27,525 | 712 | 1.7 | 30.4 | 22.5 | Al-Saikhan 2017 |
| Europe | UK | 31-Jan-20 | 82,212 | 40,542 | 17.6 | 20.6 | 31.8 | Steeds 2001 |
| Europe | Italy | 31-Jan-20 | 70,070 | 33,899 | 24.4 | 4.1 | 58.5 | Santovito 2019 |
| Europe | Spain | 1-Feb-20 | 86,918 | 27,136 | 19.4 | 16.8 | 37.6 | Alvarez 1999 |
| Europe | France | 24-Jan-20 | 21,213 | 29,155 | 12.4 | 17.6 | 35.6 | Hadjadj 2008 |
| Europe | Germany | 27-Jan-20 | 51,906 | 8776 | 3.1 | 26.5 | 26.8 | Pabst 2009 |
| Europe | Sweden | 1-Feb-20 | 27,279 | 4659 | 13.6 | 24.0 | 29.3 | Zettergren 2017 |
| Europe | Denmark | 27-Feb-20 | 121,964 | 589 | 8.5 | 22.7 | 26.0 | Bladbjerg 1999 |
| Europe | Russia | 1-Feb-20 | 87,173 | 5859 | 0.89 | 24.3 | 32.0 | Fomicheva 2000 |
| Europe | Turkey | 10-Mar-20 | 27,728 | 4692 | 6.95 | 18.5 | 40.5 | Serdaroglu 2005 |
| Australasia | Australia (Non-indigenous) | 25-Jan-20 | 63,510 | 102 | 0.09 | 19.8 | 32.2 | Lester 1999 |
| North America | US (All races) | 23-Jan-20 | 64,325 | 112,469 | 8.7 | 21.2 | 27.6 | Ned 2012 |
| North America | Mexico | 28-Feb-20 | 2609 | 13,699 | 5.3 | 37.7 | 15.6 | Isordia-Salas 2019 |
| South America | Brazil | 25-Feb-20 | 4704 | 37,312 | 10.3 | 24.2 | 21.3 | Pinheiro 2019 |
| Africa | Nigeria | 28-Feb-20 | 373 | 364 | 0.06 | 12.0 | 41.8 | Kooffreh 2014 |
| Africa | Tunisia | 2-Mar-20 | 4684 | 49 | 0.19 | 11.8 | 64.7 | Baroudi 2009 |
| Africa | Egypt | 15-Feb-20 | 1319 | 1237 | 0.43 | 4.8 | 40.0 | Settin 2015 |
| Africa | South Africa | 5-Mar-20 | 15,513 | 989 | 1.29 | 10.5 | 45.4 | Aung 2018 |
Genotype frequency data reflect the overall prevalence (of both cases and controls) in each included study. Where cases or control groups data are missing, and no alternative source of genotype data exists, the available data is taken as the overall genotype data. Complete list of citations and details of included studies can be found in Supplementary Materials.
COVID-19 data
COVID-19 data as of 8 June 2020 were extracted from ‘Worldometer’, a website owned by Dadax providing daily updated coronavirus data since 15 February 2020.23 For each of the selected countries, we extracted data pertaining to the number of confirmed COVID-19 cases, date of the first reported case, number of deaths and number of tests. COVID-19 related death was defined as per Worldometer's definition of cumulative number of deaths among detected cases (https://www.worldometers.info/coronavirus/about/). For countries in which the first case was reported prior to 15 February 2020, date of the first reported COVID-19 case was obtained from the ‘Our World in Data’ website, which presents updated figures provided by the European Centre for Disease Prevention and Control (ECDC).24 The United Nations population database was used to collect information regarding population, median age and gender distribution.25
Statistical analysis
The outcome of interest was COVID-19-related deaths per million people per day since the first reported case.
We first plotted this outcome (natural log transformed) against percentage of population with DD and II genotypes. We then used multivariable generalised linear models (GLMs) to determine its association with percentage of population with DD and II genotypes, with adjustment for median age of the population, percentage of females within the population and number of diagnostic tests per million people. The models excluded China because data regarding the number of tests conducted in this country could not be sourced. We considered percentage of population with DD and II genotypes as a continuous variable in the primary analyses, and as a categorical variable comprising four quartiles in secondary analyses, with the first (lowest) quartile as the reference category.
Results
The ACE genotype distribution and adjusted mortality rates for each country are shown in Table 1. Overall, the DD genotype frequency was lowest in east Asian countries and highest in African countries. Among European countries, Italy displayed the high prevalence of the DD genotype, at 58%. The II genotype frequency was highest in east Asian countries. Geographical variations in genotype distribution were also noted when genotype frequency data were drawn from sub-populations with existing disease (Table 1).
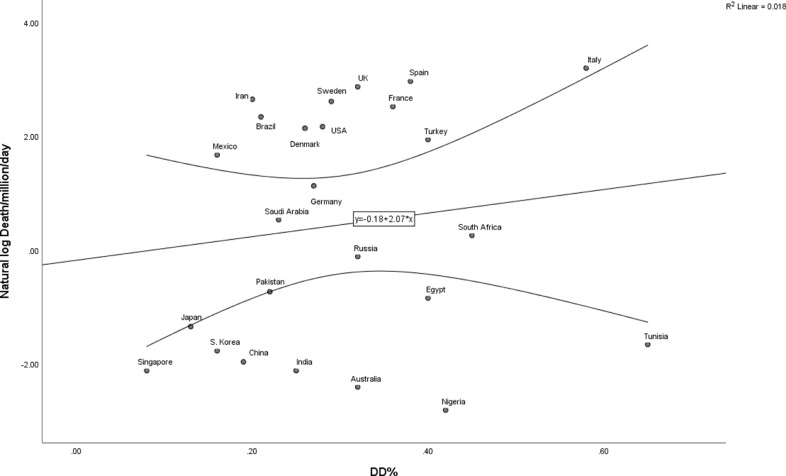
Fig. 1 A and B show the correlation between COVID-19-related deaths per million people per day since the first reported case (natural log transformed) and genotype frequencies. A positive relationship was noted between frequency of the DD genotype and COVID-19 mortality, whereas a negative relationship was observed for frequency of the II genotype.
Fig. 1.
A. Mortality rate (per million per day, natural log transformed) and DD genotype frequency
B. Mortality rate (per million per day, natural log transformed) and II genotype frequency
Legend: curved lines represent 95% confidence intervals.
Table 2 displays incident rate ratios drawn from the GLMs, with and without adjustment for median age, proportion of females and diagnostic tests per million people. Frequency of the II genotype was significantly negatively associated with COVD-19-related mortality rates (incident rate ratio [IRR] 0.02, 95% confidence interval [CI]: 0.0004–0.6, p = 0.03), while frequency of the DD genotype did not show any significant correlation (IRR 10.3, 95% CI: 0.4–243.4, p = 0.15).
Table 2.
Incident rate ratios and adjusted incident rate ratios for mortality according to DD and II genotypes.
|
IRR (95% CI) | |||||
|---|---|---|---|---|---|
| ACE genotypes | Overall | Q1 | Q2 | Q3 | Q4 |
| DD | 10.3 (0.4–243.4), p = 0.15 | 1.0 (ref) | 1.4 (0.4–5.0), p = 0.6 | 5.1 (1.8–14.3), p = 0.002* | 1.6 (0.3–8.8), p = 0.57 |
| II | 0.02 (0.0004–0.6), p = 0.03* | 1.0 (ref) | 0.8 (0.2–2.8), p = 0.76 | 0.7 (0.2–2.2), p = 0.5 | 0.1 (0.02–0.6), p = 0.014* |
|
Adjusted IRR (95% CI) | |||||
|---|---|---|---|---|---|
| ACE genotypes | Overall | Q1 | Q2 | Q3 | Q4 |
| DD | 4.3 (0.5–41.2), p = 0.2 | 1.0 (ref) | 0.7 (0.2–3.3), p = 0.7 | 2.0 (0.5–8.2), p = 0.3 | 1.2 (0.3–4.7), p = 0.8 |
| II | 0.3 (0.002–0.7), p = 0.03* | 1.0 (ref) | 1.1 (0.4–3.1), p = 0.9 | 0.6 (0.2–1.7), p = 0.3 | 0.2 (0.02–1.6), p = 0.1 |
CI: confidence interval; IRR: incident rate ratio; Q1: first quartile; Q2: second quartile; Q3: third quartile; Q4: fourth quartile; ref: reference.
IRR was adjusted for median age, sex (% female) and number of tests per million for each country.
Statistical significance.
Discussion
COVID-19 has severely challenged governments and healthcare systems worldwide. Variation in mortality does not seem to be adequately explained by differences in country-specific mitigation strategies alone. Our study suggests that differences in COVID-19 mortality may be partially explained by differing frequencies of ACE genotypes, in particular, II genotype.
Most COVID-19 deaths occur from ARDS.26 At the molecular level, the RAAS system underpins in the pathogenesis of ARDS in COVID-19 infection. High levels of ACE and ACE2 are found in the alveolar epithelial cells and serve as the key regulators of the RAAS system by maintaining pulmonary vascular and immunological homeostasis.5 Binding of SARS-CoV2 virus spike protein to ACE2 on the surface of epithelial cells leads to downregulation of its activity, thereby permitting unopposed action of ACE and angiotensin II. The consequent pulmonary endothelial cell dysfunction and immune activation results in the inflammatory exudate characterising the onset of ARDS.6 The different I/D polymorphisms alter the concentration of circulating and tissue ACE. In particular, deletion (DD) genotype is associated with higher serum levels of ACE compared to II or ID genotypes, and reduced tissue ACE2 expression, which may explain the reduced prevalence of COVID-19 infections but increasing propensity for a more severe disease.7 , 13
Previous studies have also highlighted the role of ACE in ARDS by demonstrating a direct correlation between measurable serum ACE levels and the course of ARDS.27 Additionally, ACE gene polymorphisms can determine the risk of ARDS and death from sepsis, with II genotype significantly favouring survival from ARDS at 28 days.11 , 12 ACE gene polymorphisms also serve as a risk factor for cardiovascular conditions, including hypertension, ischaemic heart disease, diabetes, renal disease and stroke.9 , 16 , 20 , 22 Recent large cohort studies demonstrated that patients with these comorbidities are also at a higher risk of death from COVID-19.26 , 28 All these findings support the hypothesis that ACE genotype can influence COVID-19 mortality.
There is significant interest in the potential effects of ACE inhibitors on the course of COVID-19.29 , 30 To date, no difference in mortality has been observed between COVID-19 patients taking and not taking ACE inhibitors.29 Furthermore, it is unknown how ACE genotypes affect binding of ACE inhibitors, while the capacity for these medications to affect SARS-CoV2 cellular entry remains unknown. Evaluation of the influence of ACE genotype and the pharmacokinetic properties of ACE inhibitors may be beneficial in the search for potential SARS-CoV2 therapies.
The main limitation of our study stems from use of data from only 25 countries, and some of the samples were over-represented by people with pre-existing diseases. However, the 25 countries are representative of their geographical regions in terms of ACE genotype profiles, as well as COVID-19 burden. The over-representation of people with pre-existing diseases for our ACE genotype data is mitigated by the fact that most COVID-19-related deaths occur among people with comorbidities.28 Additionally, genotype data in some countries, such as USA, Australia, South Africa and Singapore, may be represented by multiethnic populations. This limitation was overcome by analysis of country-specific mortality data, which included all ethnicities. Lastly, although we adjusted for some of the potential confounders in our analysis, many factors such as population density, accurate registries, case definitions and mitigation policies may further contribute to observed differences in COVID-19 mortality rates.
The findings from our study may partly explain the differential mortality observed across the world and are aligned with current understanding of SARS-CoV2 and ARDS pathophysiology. The role of ACE genotype as a prognostic factor in COVID-19 warrants further investigation. At a systemic level, knowledge of geographical variations in ACE genotype may allow for better prediction of the course of COVID-19, allowing safeguards to be implemented early, particularly in terms of healthcare resource allocation. At an individual patient level, risk stratification involving ACE genotype may inform more targeted clinical care, ultimately improving patient outcomes to reduce morbidity and mortality from the COVID-19 pandemic.
Declaration of Competing Interest
None to declare.
Acknowledgments
Acknowledgement
None.
Funding
None.
Contributors’ statement
AKA - conception, literature search, study design, data collection, data analysis, data interpretation, writing and final approval.
TA - conception, literature search, data collection, data analysis, data interpretation, writing and final approval.
BMT - literature search, data collection, data interpretation, writing and final approval.
CY - conception, data interpretation, writing and final approval.
RO - data analysis, data interpretation, writing and final approval.
KLC - conception, study design, data analysis, data interpretation, writing and final approval.
DL - conception, study design, data analysis, data interpretation, writing and final approval.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.jinf.2020.11.012.
Appendix. Supplementary materials
References
- 1.Organization W.H. Vol. 154. 2020. Coronavirus disease 2019 (COVID-19): situation report. (Coronavirus disease 2019 (COVID-19): situation report). [Google Scholar]
- 2.Joint Task Force on Practice Parameters Executive summary of disease management of drug hypersensitivity: a practice parameter. Ann Allergy Asthma Immunol. 1999;83:665–700. [PubMed] [Google Scholar]
- 3.Remuzzi A., Remuzzi G. COVID-19 and Italy: what next? Lancet. 2020;395(10231):1225–1228. doi: 10.1016/S0140-6736(20)30627-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vaduganathan M., Vardeny O., Michel T., McMurray J.J., Pfeffer M.A., Solomon S.D. Renin–angiotensin–aldosterone system inhibitors in patients with Covid-19. N Engl J Med. 2020;382(17):1653–1659. doi: 10.1056/NEJMsr2005760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kuba K., Imai Y., Rao S., Gao H., Guo F., Guan B. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus–induced lung injury. Nat Med. 2005;11(8):875–879. doi: 10.1038/nm1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shi Y., Wang Y., Shao C., Huang J., Gan J., Huang X. COVID-19 infection: the perspectives on immune responses. Cell Death Differ. 2020;27(5):1451–1454. doi: 10.1038/s41418-020-0530-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rigat B., Hubert C., Alhenc-Gelas F., Cambien F., Corvol P., Soubrier F. An insertion/deletion polymorphism in the angiotensin I-converting enzyme gene accounting for half the variance of serum enzyme levels. J Clin Invest. 1990;86(4):1343–1346. doi: 10.1172/JCI114844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Saab Y., Gard P., Overall A. The geographic distribution of the ACE II genotype: a novel finding. Genet Res Camb. 2007;89(4):259–267. doi: 10.1017/S0016672307009019. [DOI] [PubMed] [Google Scholar]
- 9.Zhang Z., Xu G., Liu D., Fan X., Zhu W., Liu X. Angiotensin-converting enzyme insertion/deletion polymorphism contributes to ischemic stroke risk: a meta-analysis of 50 case-control studies. PLoS ONE. 2012;7(10):e46495. doi: 10.1371/journal.pone.0046495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marre M., Jeunemaitre X., Gallois Y., Rodier M., Chatellier G., Sert C. Contribution of genetic polymorphism in the renin-angiotensin system to the development of renal complications in insulin-dependent diabetes: genetique de la nephropathie diabetique (GENEDIAB) study group. J Clin Invest. 1997;99(7):1585–1595. doi: 10.1172/JCI119321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marshall R.P., Webb S., Bellingan G.J., Montgomery H.E., Chaudhari B., McAnulty R.J. Angiotensin converting enzyme insertion/deletion polymorphism is associated with susceptibility and outcome in acute respiratory distress syndrome. Am J Respir Crit Care Med. 2002;166(5):646–650. doi: 10.1164/rccm.2108086. [DOI] [PubMed] [Google Scholar]
- 12.Jerng J.-.S., Yu C.-.J., Wang H.-.C., Chen K.-.Y., Cheng S.-.L., Yang P.-.C. Polymorphism of the angiotensin-converting enzyme gene affects the outcome of acute respiratory distress syndrome. Crit Care Med. 2006;34(4):1001–1006. doi: 10.1097/01.CCM.0000206107.92476.39. [DOI] [PubMed] [Google Scholar]
- 13.Delanghe J.R., Speeckaert M.M., De Buyzere M.L. COVID-19 infections are also affected by human ACE1 D/I polymorphism. Clin Chem Lab Med. 2020;58(7):1125–1126. doi: 10.1515/cclm-2020-0425. [DOI] [PubMed] [Google Scholar]
- 14.Delanghe J.R., Speeckaert M.M., De Buyzere M.L. The host's angiotensin-converting enzyme polymorphism may explain epidemiological findings in COVID-19 infections. Clin Chim Acta. 2020;505:192–193. doi: 10.1016/j.cca.2020.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yako Y.Y., Guewo-Fokeng M., Balti E.V., Bouatia-Naji N., Matsha T.E., Sobngwi E. Genetic risk of type 2 diabetes in populations of the African continent: a systematic review and meta-analyses. Diabetes Res Clin Pract. 2016;114:136–150. doi: 10.1016/j.diabres.2016.01.003. [DOI] [PubMed] [Google Scholar]
- 16.Mengesha H.G., Petrucka P., Spence C., Tafesse T.B. Effects of angiotensin converting enzyme gene polymorphism on hypertension in Africa: a meta-analysis and systematic review. PLoS ONE. 2019;14(2) doi: 10.1371/journal.pone.0211054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Khodaeian M., Enayati S., Tabatabaei-Malazy O., Amoli M.M. Association between genetic variants and diabetes mellitus in Iranian populations: a systematic review of observational studies. J Diabetes Res. 2015;2015 doi: 10.1155/2015/585917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kang S.W., Kim S.K., Chung J.-.H., Jung H.-.J., Kim K.-.I., Kim J. Genetic polymorphism of angiotensin-converting enzyme and chronic obstructive pulmonary disease risk: an updated meta-analysis. Biomed Res Int. 2016;2016 doi: 10.1155/2016/7636123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ma Y., Tong X., Liu Y., Liu S., Xiong H., Fan H. ACE gene polymorphism is associated with COPD and COPD with pulmonary hypertension: a meta-analysis. Int J Chron Obstruct Pulmon Dis. 2018;13:2435–2446. doi: 10.2147/COPD.S168772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smyth L.J., Cañadas-Garre M., Cappa R.C., Maxwell A.P., McKnight A.J. Genetic associations between genes in the renin-angiotensin-aldosterone system and renal disease: a systematic review and meta-analysis. BMJ Open. 2019;9(4) doi: 10.1136/bmjopen-2018-026777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Niu W., Qi Y., Gao P., Zhu D. Association between angiotensin converting enzyme G2350A polymorphism and hypertension risk: a meta-analysis. J Renin Angiotensin Aldosterone Syst. 2011;12(1):8–14. doi: 10.1177/1470320310375859. [DOI] [PubMed] [Google Scholar]
- 22.YU Z.Y., CHEN L.S., ZHANG L.C., ZHOU T.B. Meta-analysis of the relationship between ACE I/D gene polymorphism and end-stage renal disease in patients with diabetic nephropathy. Nephrology. 2012;17(5):480–487. doi: 10.1111/j.1440-1797.2012.01592.x. [DOI] [PubMed] [Google Scholar]
- 23.Worldometers.info. Worldometer COVID-19 coronavirus pandemic [Internet] Dover, Delaware, U.S.A; 2020 Available from: https://www.worldometers.info/coronavirus/.
- 24.Roser M., Ritchie H., Ortiz-Ospina E., Hasell J.. Coronavirus disease (COVID-19) [Internet] Wales, UK: our world in data; 2020 Available from: https://ourworldindata.org/coronavirus.
- 25.World population prospects 2019 United Nations, Department of Economic and Social Affairs, population division2019. Available from: https://population.un.org/wpp/Download/Standard/Population/.
- 26.Richardson S.H.J., Narasimhan M., Crawford J.M., McGinn T., Davidson KWand the Northwell COVID-19 Research Consortium. Barnaby D.P. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020 doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fourrier F., Chopi C., Wallaert B., Mazurier C., Mangalahoyi J., Durocher A. Compared evolution of plasma fibronectin and angiotensin-converting enzyme levels in septic ARDS. Chest. 1985;87(2):191–195. doi: 10.1378/chest.87.2.191. [DOI] [PubMed] [Google Scholar]
- 28.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li J., Wang X., Chen J., Zhang H., Deng A. Association of renin-angiotensin system inhibitors with severity or risk of death in patients with hypertension hospitalized for coronavirus disease 2019 (COVID-19) infection in Wuhan, China. JAMA Cardiol. 2020;5(7):825–830. doi: 10.1001/jamacardio.2020.1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mancia G., Rea F., Ludergnani M., Apolone G., Corrao G. Renin–angiotensin–aldosterone system blockers and the risk of Covid-19. N Engl J Med. 2020;382:2431–2440. doi: 10.1056/NEJMoa2006923. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.