Abstract
The ability to estimate age and determine the growth status of free-ranging dugongs (Dugong dugon) is vital to providing insight into the basic biology of this endangered species. Currently, age estimation in dugong carcasses relies on counting dentin growth layer groups (GLGs) in tusks, but a disadvantage is they need to be intact. We explored whether measures of telomere length could be used as an alternative approach to age estimation in dugongs given that in other species, telomere length and age are inversely related. In this study, relative telomere length (rTL) was measured by qPCR in skin samples from 24 dugongs of varying ages determined by counts of GLGs. In addition, relationships between age by GLG counts and body weight and length and were examined. Our findings indicate that age estimated by GLGs was negatively correlated with telomere length using the logistic formula with a rate of telomere attrition of approximately 0.036 rTL/year between the ages of 5–20 years. By comparison, both body weight and length were positively correlated with GLG-based age, with growth rates of ~8.8 kg/year for weight and ~3.58 cm/year for length, respectively. After that, growth rates slowed substantially and then plateaued. The results suggest that physical maturity in dugongs occurs at 20 years of age and that measures of rTL might serve as a tool for age estimation in dugongs, living and deceased.
Keywords: Age, Growth, Telomere, Tusk, Senescence, Sirenia
Introduction
The dugong (Dugong dugon) is one of four species belonging to the Order Sirenia, Family Dugongidae. It is distinct compared to other marine mammals in being almost entirely herbivorous. In Thailand, dugongs are found along the Andaman Sea and Gulf of Thailand coastlines. Of the estimated 200–250 dugongs in Thailand, about 70% reside in the waters around the island of Koh Libong. Dugongs are listed as Vulnerable on the IUCN Red List (Marsh & Sobtzick, 2019), with threats primarily related to anthropogenic activities such as coastal development, habitat degradation, pollution, fishing nets, boat strikes and poaching. There are five laws in Thailand that can protect dugongs and seagrass (their main food source): (i) Fisheries Act, B.E. 2490 (1947); (ii) National Park Act, B.E. 2504 (1961); (iii) Export and Import of Goods Act, B.E. 2522 (1979); (iv) Wildlife Preservation and Protection Act, B.E. 2535 (1992); and (v) the Convention on International Trade in Endangered Species (CITES) (Adulyanukosol, Ellen & Potchana, 2010). All laws prohibit the killing, taking, possessing and trading of dugongs or their body parts in Thailand, while CITES bans these activities internationally. Still, dugong populations continue to decline (Marsh & Sobtzick, 2019), including in Thailand.
Dugongs have a life span of around 60–70 years (Marsh, 1995) and reach sexual maturity at ~10–12 years of age (Whittemore et al., 2019); however, age at physical maturity is unknown. Dugongs with unknown birth dates have been aged by analysis of dentition (Lanyon & Sanson, 2006). They have six pairs of cheekteeth that move forward with age. The dental formula for adult dugongs is 1/0, 0/0, 3/3, 3/3 (Marsh, 1980), which means they have one incisors, no canines, three premolars and three molars on each side of the upper jaw, and three premolars, and three molars on the lower jaw (Marsh, 1980; Myers, 2002). Premolars erupt at birth and fall out or are partially resorbed, while molars progressively erupt posteriorly during growth (Lanyon & Sanson, 2006; Mitchell, 1973). Two pairs of upper incisors are formed; the anterior pair is vestigial, nonfunctional and deciduous, while the posterior pair is permanent and forms the tusks. The tusks erupt later, primarily in males, but occasionally in females (Marsh, 1980). The tusks are the only teeth present throughout life, and so have been used for age estimation (Marsh, 1980; Mitchell, 1976; Read, Hohn & Lockyer, 2018) through counts of dentin growth layer groups (GLGs). The technique was first described by Scheffer in 1970 (Scheffer, 1970) and is now broadly accepted for age estimation of dugongs (Adulyanukosol, Amorena & Miyazaki, 1998; Kasuya & Nishiwaki, 1978; Marsh, 1980; Mitchell, 1976; Mitchell, 1978; Read, Hohn & Lockyer, 2018; Scheffer, 1970). There is a seasonal deposition of dentin, resulting in one GLG per year. The technique is applied widely to age estimates of other marine mammal species, including manatees (Trichechus manatus) (Marmontel et al., 1996), gray seals (Halichoerus grypus), walruses (Odobenus rosmarus), killer whales (Orcinus orca), California lion seals (Zalophus californianus) (Rust et al., 2019) and bottlenose dolphins (Tursiops truncatus) (Read, Hohn & Lockyer, 2018). Most species estimated for age by GLG in mineralized tissues (bones and teeth) were free-ranging animals with unknown age, contributing to trouble in how reliable this technique is. However, a few studies revealed the accuracy of age estimation by counting GLG in tooth dentin of sea lion as 71–79% (Rust et al., 2019) and in manatee bone as approximately R2 of 0.97 between known age and estimated age (Marmontel et al., 1996). Apart from teeth, there are other dense connective tissues that can be used for age estimation; for example, ear plugs in baleen whales, (Lockyer, 1984), periotic bones in manatees (Marmontel et al., 1996), and tympanic bullae in bowhead whales (Balaena mysticetus) (Sensor et al., 2018) and minke whales (Balaenoptera acutorostrata) (Sukhovskaya et al., 1985).
One problem with counts of GLGs for age estimation is that they are not accurate for animals with incomplete tusks (Adulyanukosol, Amorena & Miyazaki, 1998; Read, Hohn & Lockyer, 2018). For that reason, measurement of telomere length to estimate age may be an alternative approach. Telomeres are composed of repeating nucleotides at the ends of chromosomes and important for maintaining chromosome integrity and protecting against DNA deterioration or damage (Fathi et al., 2019; Shay & Wright, 2000). They shorten with each cell division throughout the life of an organism (Fathi et al., 2019; Hewakapuge et al., 2008; Vleck, Haussmann & Vleck, 2003), and so measures of telomere length have been used to estimate age in humans (Homo sapiens) (Hewakapuge et al., 2008; Kaewkool et al., 2020; Karlsson et al., 2008) and other mammalian species, including mice (Mus musculus) (Callicott & Womack, 2006; Whittemore et al., 2019), dogs (Canis lupus familiaris) (Adulyanukosol, Amorena & Miyazaki, 1998; Fick et al., 2012; McKevitt et al., 2002), cats (Felis catus) (Mitchell, 1976), goats (Capra hircus) (Whittemore et al., 2019), Asian elephants (Elephas maximus) (Buddhachat et al., 2017; Whittemore et al., 2019), Australian sea lions (Neophoca cinerea) (Izzo et al., 2011), humpback whales (Megaptera novaeangliae) (Olsen et al., 2014) and bottlenose dolphins (Whittemore et al., 2019). Most of these studies used peripheral blood to determine telomere length and found generally negative relationships with age (R2 < 0.3), with large variation potentially due to extrinsic (nutrition, behavior and environment) or intrinsic (rate of telomere shortening, initial telomere length, metabolic rate and genetics) factors contributing to low or no significant relationships between age and telomere length measurements using qPCR (Buddhachat et al., 2017; Fick et al., 2012; Hewakapuge et al., 2008; Izzo et al., 2011; Kaewkool et al., 2020; Karlsson et al., 2008). However, in humpback whales, the telomere length measurement was significantly correlated to age with an R2 = 0.244 (Olsen et al., 2014). Recently, Whittemore et al. (2019), measured telomere length in a variety of species and found there was no correlation between initial telomere length and lifespan, but rather it was related to telomere attrition rate. In contrast, a previous study of Fick et al. (2012) demonstrated that average telomere length correlated to average of lifespan across different breeds of dogs. This might indicate that the variation in telomere length affects lifespan only within species due to the same genetic background, but not for between species.
An advantage of telomere length measurement over the GLG technique is it can be done on living or deceased animals, does not require intact carcasses or tissues, and presumably can be applied to a variety of tissue types, such as skin, muscle and leukocytes (Daniali et al., 2013; Dlouha et al., 2014). The purpose of this study was to measure telomere length in dugong skin samples and determine how it correlates with chronological age estimated by GLG counts in tusks. We also examined relationships between age and body weight and length. Our hypothesis is that age estimates by GLG counts will correlate negatively with telomere length, and positively with body length and body weight. The goal is to find alternative approaches for age estimation of dugongs, particularly in those with incomplete tusks, to aid in population demographic analyses and studies of species biology.
Materials and Methods
Animals
Whole tusks from 24 stranded dugong (Dugong dugon) carcasses (female = 16, male = 8) found along the Gulf of Thailand and Andaman Sea coastlines of Thailand (Fig. 1) were obtained from the Phuket Marine Biological Center (Phuket, Thailand). Skin tissue samples (2 × 2 cm) from these carcasses were obtained and preserved in 95% ethanol for DNA extraction. According to the Animals for Scientific Purposes Act, B.E. 2558 (2015), since a part of this study was performed on carcasses of stranded dugongs, no ethical approval was required, as confirmed by the Animal Ethics Committee, Faculty of Veterinary Medicine, Chiang Mai University (License number U1006312558).
Figure 1. Distribution of 24 carcasses of stranded dugongs found along the coastlines of the Gulf of Thailand and Andaman sea among nine provinces.
1 = Rayong (n = 2); 2 = Chonburi (n = 2); 3 = Chumphon (n = 1); 4 = Ranong (n = 1); 5 = Phangnga (n = 4); 6 = Phuket (n = 1); 7 = Krabi (n = 3); 8 = Trang (n = 9); 9 = Satun (n = 1). (Photo created in Microsoft Excel, version 16.36, 20041300, licensed to Chiang Mai University).
Measures of dentin GLGs
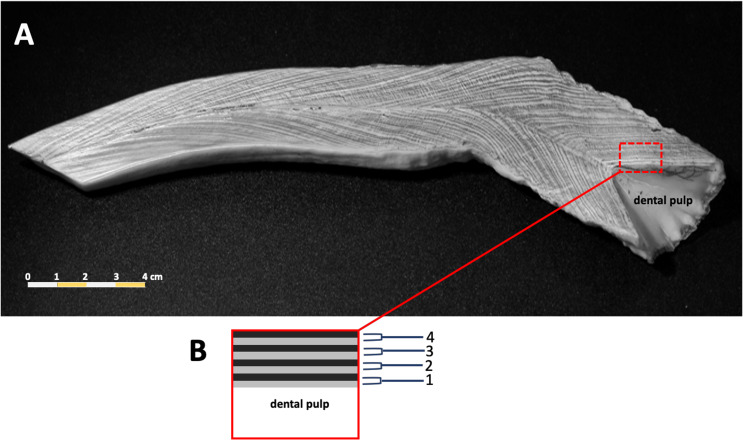
Counts of dentin GLGs followed previous protocols (Adulyanukosol, Amorena & Miyazaki, 1998; Mitchell, 1978), incomplete or worn tusks were excluded from this study. The tusks were sectioned longitudinally in the mid-sagittal plane using a Buehler Isomet low speed saw (Struers Minitom, Denmark), polished using progressively finer grades of sandpaper (150–1,200), and then etched in 10% formic acid for 1–3 h. Tusks were washed in tap water for 10 min to remove the etching reagent, followed by immersion in 100% acetone for 2–3 min to increase the clarity of the GLGs. Samples were rewashed in tap water for 12 h to remove the formic acid. The etched tusk was dried at room temperature for 1–2 days and then rubbed with laser toner powder (Canon, Thailand). The number of GLGs in each tusk was counted in triplicate (Fig. 2).
Figure 2. A representative photo of counted dentinal growth layer groups in the tusk of a 47+ year-old female dugong (A); each GLG was represented by one light and one dark band in the dental pulp from base to tip of the tusk (B).
DNA extraction and real-time PCR
Skin samples were extracted using genomic DNA extraction kits according to manufacturer’s instructions (DNeasy Blood & Tissue Kit, QIAGEN, Germany) at the Faculty of Veterinary Medicine, Chiang Mai University. DNA was measured qualitatively and quantitatively by agarose gel electrophoresis and spectrophotometry at A260, respectively, with an A260 of 1 = 50 ng/μl. Subsequently, dugong DNA (50 ng) was used for measurement of telomere length by qPCR (Buddhachat et al., 2017; Cawthon, 2002; Kaewkool et al., 2020). A reaction consisted of 1X SensiFAST™ SYBR® No-ROX Kit (Bioline, England) was used and contained telomere primer concentrations of 270 nM of tel 1 5′ GGTTTTTGAGGGTGAGGGTGAGGGTGAGGGTGAGGGT 3′, and 900 nM of tel 2 5′ TCCCGACTATCCCTATCCCTATCCCTATCTATCCCTA 3′ in a total volume of 10 μl. Additionally, the acidic ribosomal phosphoprotein 36B4 (36B4) was single copy gene used as a internal control to measure relative quantity of telomeric repeat. The sequence of primer of 36B4 was forward CAGAGTGAYGTGCAGCTGAT and reverse AAGCACTTCAGGGTTGTAGATGCTGCC and their concentration performed for DNA amplification was 400 nM of each primer.
The cycling profile for the telomere PCR was as follows: 40 cycles of 95 °C for 15 s and 65 °C for 2 min. For 36B4 as a single copy gene, 30 cycles of 95 °C for 15 s and 65.5 °C for 1 min were applied with an Eco Real-Time PCR System (Illumina, San Diego, CA, USA). After that, cycle threshold (Ct) values were acquired from qPCR and used to calculate the relative telomere length (rTL) derived from the following formula: 2−delta(ct(telomere)−ct(36B4 gene)). Each individual was done in triplicate for measuring rTL. The rate of telomere loss was obtained from the slope of the linear regression model (rTL/year).
Statistical analyses
A histogram for each parameter was created to determine the data distribution. To determine the variability of experimental error and biological error for rTL measurement using qPCR, coefficients of variation (CV; mean/standard deviation × 100) obtained from intra-individual (triplicate) and inter-individual within the same stage (premature and mature) presented the error caused by experiment and biological factor, respectively. Relationships among age, body weight, body length and rTL were determined by logistic, logarithm or linear regression models depending on the variable. A logistic model determined correlations between weight-age, length-age and rTL-age as either with or with and L (limiting value) or the curve’s maximum value. Weight-rTL and length-rTL were fitted by logarithm function as , and weight-length was explained by a linear regression model as . The model for age estimation by rTL measurement was determined for accuracy by R2 and precision by residual stand error based on the correlation of age estimated by GLG counts and by rTL using a linear regression model. Further, we created age cutoffs to analyze growth stages of immature and mature dugongs by coefficients of variation (CV) using 10-year periods between 5 and 60 years of age. After obtaining an age cutoff, dugong samples were divided into two classes: <20 or ≥20 years. A linear regression model was used to determine growth rates in body weight and length, as well as the rate of telomere attrition. In plotting a graph, all data points including the replicating data of each individual of rTL were used for creating the visualized graphs of their relationship. Differences between body weight, length and rTL between immature and mature dugongs were determined by Student’s t-tests. Data for dugongs <20 years old were available for males and females; however, males >20 years old were unavailable.
Results
Age determination by GLG counts
Chronological ages estimated for 24 tusks by counts of GLGs ranged from 5 to 69 years (Table 1) and were used to determine correlations between age, rTL and body morphometrics.
Table 1. Stranding information for the 24 dugongs in this study, and age estimates by counted dentinal growth layer groups from intact tusks.
| ID | Stranding information | Body information | Age* (year) | |||
|---|---|---|---|---|---|---|
| Date (M/D/Y) | Location | Sex | Length (cm) | Body (kg) | ||
| 307 | 24 January 2010 | Libong Island, Trang | Female | 277 | 358 | 54 |
| 300 | 5 October 2009 | Samran Beach, Trang | Female | 224 | 235 | 11 |
| 299 | 28 September 2009 | Libong Island, Trang | Female | 281 | 335 | 63 |
| 292 | 2/2009 | Ao Nang Beach, Krabi | Female | 282 | 305 | 41 |
| 291 | 1 February 2009 | Ko Sarai Island, Satun | Female | 263 | 317 | 67 |
| 285 | 26 July 2008 | Map Ta Phut, Rayong | Female | 200 | – | 24 |
| 283 | 9 April 2005 | Ko Lanta Yai Island, Krabi | Female | 300 | – | 38 |
| 272 | 30 August 2007 | Sattahip, Chonburi | Male | 245 | – | 13 |
| 258 | 12 August 2006 | Bang Sare, Chonburi | Female | 231 | 200 | 12 |
| 243 | 26 December 2004 | Ko Pa Yung Island, Phangnga | Male | 275 | 310 | 23 |
| 236 | 1 June 2004 | Ko Klang Island, Phangnga | Female | 250 | – | 69 |
| 234 | 22 April 2004 | Ko Yao Island, Phangnga | Female | 256.5 | 297 | 27 |
| 143 | 28 November 2001 | Libong Island, Trang | Female | 199.5 | 142 | 5 |
| 129 | 6 December 2000 | Khao Mai Kaeo, Trang | Female | 225 | 258 | 43 |
| 098 | 14 October 1998 | Kram, Rayong | Male | 214 | 180 | 16 |
| 084 | 3 August 1998 | Wichit, Phuket | Male | 219 | 184 | 8 |
| 078 | 19 May 1998 | Lamae, Chumphon | Female | 231 | 151 | 34 |
| 058 | 6 January 1997 | Libong Island, Trang | Male | 250 | 245 | 15 |
| 057 | 2 January 1997 | Libong Island, Trang | Female | 258 | 281 | 14 |
| 048 | 3 March 1996 | Suk Samran, Ranong | Female | 271 | 293 | 43 |
| 047 | 25 January 1996 | Ko Yao Island, Phangnga | Male | 221 | 143 | 6 |
| 038 | 21 June 1995 | Libong Island, Trang | Male | 257 | 250 | 14 |
| 036 | 31 March 1995 | Bo Hin, Trang | Female | 273 | 272 | 34 |
| 016 | 11 May 1993 | Muang Krabi, Krabi | Male | 254 | – | 16 |
Note:
Age estimated by counted dentinal growth layer groups in tusks.
Determination of variability of rTL measurement
In the present study, we tested the variability of rTL measurement using real-time PCR and if it was caused by differences in experimental design or biological factors as shown in Table 2. Our results revealed lower CVs obtained from intra-individual samples across both age groups compared to inter-individual values. These results suggest that although the experiment error had a relatively large variability, the biological factor (ages) appeared to be the main factor accounting for the variability in rTL.
Table 2. Intra- and inter-individual variability of rTL measurements by coefficient of variation (%CV) for premature and mature dugongs using real-time PCR.
| Stage | Intra-individual (%) | Inter-individual (%) |
|---|---|---|
| Premature (<20 years) | 38.97 | 47.02 |
| Mature (≥20 years) | 37.44 | 91.65 |
| Overall mean | 38.20 | 69.34 |
Correlation between age and GLG counts, rTL and body morphometrics
DNA was isolated from 24 dugong skin samples to measure telomere length by real-time PCR; however, there was positive amplification for only 14 specimens (56%). But all 24 dugong tusks were aged based on counts of GLGs. Using logistic regression, positive correlations were found between age estimated by GLG counts and both body weight and length, with limiting values of approximately 309 kg and 266 cm, respectively (Figs. 3A and 3B; Table 3). By contrast, the relationship between age by GLG counts and rTL fit a negative logistic curve, so increasing age was associated with decreasing telomere lengths (Fig. 3C; Table 3). Negative correlations between rTL and body weight and length were observed, with an adjusted R2 of 0.43 (p < 0.0001) and 0.51 (p < 0.0001), respectively, for the model logarithm function (Figs. 3D and 3E; Table 3). Body weight and length were positively correlated with an adjusted R2 of 0.78 (Fig. 3F; Table 3). In addition, we evaluated the performance of the model obtained from age determination by rTL using a linear regression to test the correlation between the predicted age from rTL and age from GLG counts, resulting in an R2 of 0.86 and residual standard error of 6.33 (Fig. S1).
Figure 3. Relationships of age estimation by GLG counts and rTL with body morphometrics.
(A) Age by GLGs and body weight; (B) age by GLGs and body length; (C) age by GLGs and rTL; (D) body weight and rTL; (E) body length and rTL; and (F) body length and body weight. Red dashed lines represent the limiting value of the logistic model. 95% confidence interval is indicated by gray stripe. Each individual was done in triplicates and all data were plotted in the graphs.
Table 3. The models fitted to relationships among body weight, body length, rTL and age estimated by GLG counts.
| Relationship (y~x) | Model used | β | c | Le | R2 | AIC | Residual standard error |
|---|---|---|---|---|---|---|---|
| Weight~age | Positive logistic modela | 0.14 | −0.81 | 309.33 | 0.77 | 181.24 | 32.61 |
| Length~age | MPositive logistic modela | 0.09 | 0.86 | 265.62 | 0.39 | 221.98 | 22.33 |
| rTL~age | Negative logistic modelb | −0.10 | −3.57 | −33.17 | 0.82 | −68.94 | 0.1005 |
| Weight~rTL | Logarithm modelc | 164.56 | −29.39 | – | 0.43* | 427.88 | 55.48 |
| Length~rTL | Logarithm modelc | 214.08 | −11.75 | – | 0.51* | 343.85 | 18.89 |
| Weight~length | Linear regression modeld | 161.33 | 0.34 | – | 0.78* | 448.32 | 11.93 |
Notes:
.
Y .
.
.
L represents the limiting value for logistic function.
p-Value < 0.0001.
Determining age at maturity
In general, the CV of dugongs <20 years was higher than that of those ≥20 years in age cutoffs (Figs. 4A and 4B). Differences in body weight and length CVs were noted in dugongs 20–30 years of age (Figs. 4A and 4B). By contrast, the pattern of CV for rTL was the opposite of that for the body indices, although the age cutoff based on a slow rate of telomere attrition was the same; thatis, 20–30 years (Fig. 4C). Taken together, age at physical maturity based on a plateau in growth occurs at an age of 20 years or older.
Figure 4. Determining age cutoffs for determining dugong maturity based on body indices and rTL in dugongs.
Coefficient of variation (CV) at different age cutoffs every 10 years from 0 to 60 years for (A) weight, (B) length and (C) rTL. Correlations are shown between age and (D) weight, (E) age and length and (F) age and rTL. Average (G) weight, (H) length and (I) rTL are shown for dugongs < or ≥20 years of age.
Based on those results, we used 20 years of age as a cutoff for separating dugongs into physically immature (<20 years) and mature (≥20 years) groups and found significant differences in body weight, body length and rTL between groups (p < 0.0001) (Figs. 4G–4I). A higher growth rate was observed for dugongs 5–20 years of age compared to those ≥20 years old (Figs. 4D and 4E). Body weight increased approximately 8.8 kg/year between 5 and 20 years of age, which was reduced to 1.65 kg/year after the age of 20 years (Fig. 4D; Table 4). Body length for the first 20 years increased at a rate of 3.58 cm/year, with no further changes after 20 years (adjusted R2 = 0.03, p = 0.1534). rTL decreased by a value of 0.036/year with an adjusted R2 = 0.56 (p < 0.0001) in immature dugongs, whereas in those ≥20 years of age, it decreased only about 0.001/year (Fig. 4F; Table 4).
Table 4. Description statistics of linear regression models for body weight, body length and rTL in dugongs < or ≥20 years of age.
| Parameter | Period | β | c | Adjusted R2 | p-Value |
|---|---|---|---|---|---|
| Weight | <20 years | 8.80 | 107.86 | 0.50 | <0.0001 |
| ≥20 years | 1.65 | 218.81 | 0.15 | 0.0187 | |
| Length | <20 years | 3.58 | 191.60 | 0.47 | <0.0001 |
| ≥20 years | 0.41 | 242.79 | 0.03 | 0.1534 | |
| rTL | <20 years | −0.036 | 0.74 | 0.56 | <0.0001 |
| ≥20 years | −0.001 | 0.07 | 0.39 | 0.0032 |
Sex differences in growth rates
The influence of sex on body indices and rTL was examined for immature dugongs only, because of an absence of data for mature males. There was a significant difference between females and males in body weight, but not body length (Figs. 5D and 5E). Yearly body weight and length increases in immature females (13.8 kg/year and 5.87 cm/year, respectively) were nearly double those observed in males (6.95 kg/year and 2.41 cm/year) (Figs. 5A and 5B). Both genders exhibited a reduction in telomere length of about 0.03 rTL/year, with the average rTL in males being higher than that in females (p = 0.02) (Figs. 5C and 5F).
Figure 5. Correlations of age with body indices and rTL, and overall averages for each parameter in female and male immature dugongs related to (A) body weight-age, (B) body length-age, (C) rTL-age, (D) average body weight, (E) average body length and (F) average.
Discussion
This is the first study to describe the use of telomere length measures for age estimation in dugongs and found a strong correlation between estimated age and telomere shortening. Previously, the only available method to age dugongs relied on counting GLGs in intact tusks of carcasses. The benefits of measuring rTL are the ability to use a variety of tissue types of both living and deceased animals, and that it does not require access to whole tusks. This study found that measurement of rTL via qPCR predicted dugong age using the negative logistic equation “” and it can be rearranged to to determine age of unknown individuals. This model gave a degree of correlation with age estimated by GLG counts with R2 of 0.86 and an uncertainty for age estimation as approximately ±6.33 years. A strong correlation also was found between body weight and length (R2 = 0.78) in these dugongs, while modest correlations were found between rTL and body weight (R2 = 0.43) and length (R2 = 0.51). Overall, results suggest that rTL is a robust method to estimate age in dugongs, better than body weight and length, and perhaps equal to using counts of tusk GCGs.
The rate of telomere attrition was age-related and more rapid at 0.0036/year in young dugongs aged 5–20 years compared to animals over 20 years of age, when it slowed to 0.001 rTL/year. Studies have shown associations between telomere attrition and age in a wide variety of species, including humans (Daniali et al., 2013) and zebra finches (Taeniopygia guttata) (Heidinger et al., 2012). In American alligators (Alligator mississippiensis), telomere length was negatively associated with body length as a proxy for age (Scott et al., 2006). In red-sided garter snakes (Thamnophis sirtalis parietalis), telomere length decreased with age in males, but not females (Rollings et al., 2017), perhaps as a physiological consequence of sexual dimorphism and energy investment differences in the mating system of this species. Typically, telomere length is measured in peripheral blood leucocytes; however, age-related changes have been documented in over a dozen tissue types, both in mice and humans (Scott et al., 2006). Differences in rTL variability in humans (Dlouha et al., 2014) and rates of stress-induced accelerated aging in mice (Baek et al., 2019) have been shown to be tissue-specific. In contrast, shortening of telomeres in human muscle, skin, fat and leukocytes occurred at equivalent rates, perhaps because stem cells replicate at similar rates among those tissues (Daniali et al., 2013). In the current study, we measured telomere length in skin cells, as these have been used to assess telomere attrition in other species, and shown to correlate with findings in peripheral leukocytes (Dlouha et al., 2014).
Long-lived animals generally exhibit a slower rate of telomere attrition compared to short-lived animals (Whittemore et al., 2019). For example, human (lifespan ~70 years) telomeres shorten at a rate of ~70 bp/year (Canela et al., 2007), while telomeres in mice (lifespan ~5 years) shorten at a rate of 7,000 bp/year (Vera et al., 2012). In other long-lived mammals, telomere shortening occurs at rates of ~110 bp/year in elephants (lifespan ~60 years) (Whittemore et al., 2019), ~770 bp/year in bottlenose dolphins (lifespan ~30–40 years) (Whittemore et al., 2019), ~110 bp/year in American flamingos (Phoenicopterus ruber) (lifespan ~40–60 years) (Whittemore et al., 2019) and ~210 bp/year in the griffon vulture (Gyps fulvus) (lifespan ~40 years) (Whittemore et al., 2019). Our study showed a high correlation between age and rTL, but it was not possible to calculate numbers of bp reduced per year because we relied on qPCR, a technique that determines a relative change between telomeres and internal gene control (Cawthon, 2002; Olsen et al., 2014), while other techniques like southern blot (Lincz et al., 2020) and quantitative fluorescence in situ hybridization (Canela et al., 2007; Whittemore et al., 2019) calculate actual numbers of base pairs. Still, our study corroborated a relationship between age and telomere shortening in the dugong, similar to other species.
There were significant correlations between age and both body weight and length, explained by positive sigmoid curves. In the first 20 years of life, the relationship between body weight and length was linear (R2 = 0.50 and 0.47, respectively) and then plateaued, suggesting the dugong is physically mature at about 20 years of age. In immature dugongs (<20 years), body weight increased approximately 8.8 kg/per, and then slowed to 1.65 kg/year in mature animals (≥20 years). Stronger relationships between body morphometrics and age were observed in females compared to males. In addition, in early life, females grew at rates nearly double those of males: 13.8 kg/year and 5.87 cm/year in females and 6.95 kg/year and 2.41 cm/year in males for body weight and length, respectively. A previous study in one pair of captive dugongs reported weight gains of about 42–45 kg/year during the first 10 years of life (Goto et al., 2004), which is higher than what we observed. Possible reasons could be that our study evaluated stranded carcasses, whereas that study was in captive dugongs. Confounding factors affecting growth rates of captive and wild animal can include differences in environmental quality (water, parasites, disease) and food sources. Variations in seagrass nutritional quality are evident (Fortes et al., 2018; Goto et al., 2004; Green & Short, 2003) and may differ from what is fed in captivity. Food consumption in captive dugongs has been reported to be 10–15 kg/day in a calf up to 23–26 kg/day in an adult (Goto et al., 2004). In nature, wild dugongs may not have access to as much seagrass or quantities of food available to captive dugongs. Density of dugongs in the habitat also may affect growth rates due to influences on food availability and habitat quality (Marsh, 1980; Nganvongpanit et al., 2017a, 2017b), which in turn could affect age of sexual maturity. Population differences in age at sexual maturity have been observed in wild dugongs. For example, those from Townsville, northern Queensland, Australia, mature at 10 years of age, compared to 18 years of age along the Papua New Guinean coast (Marsh, 1995). In dugongs along the Andaman sea and Gulf of Thailand coasts, the average mature body length and weight of males and females were 258 and 255 cm, and 250 and 251 kg, respectively (Adulyanukosol, Cherdsukjai & Boukaew, 2011). This was similar to maximum weights of 298 and 293 kg reported in captive male and female dugongs, respectively (Goto et al., 2004). Average lengths of 240–260 cm in adults (Burgess, Lanyon & Keeley, 2012; Marsh, 1980) fit with data based on our equation, where limiting values for body weight and length of dugongs in our study were approximately 309 kg and 266 cm, respectively. In the present study, the absolute age of each animal was unknown, so the standard practice of tusk GLGs counts was used for age estimation (Marsh, 1980; Nishiwaki & Marsh, 1985; Read, Hohn & Lockyer, 2018). Then that chronological age was correlated with body morphometric indices, finding significant relationships with both body weight and length.
There are some limitations to using GLG counts to estimate age in dugongs. One problem is related to poor tusk quality. Tusks are covered by the muzzle until puberty. In females, tusks are rarely observed past the muzzle, whereas in adult males they protrude past the muzzle after the eruption and then can become worn, often resembling the tip of a chisel. Thus, using the tips of tusks below the muzzle, GLG counts may not reveal a true chronological age (Adulyanukosol, Amorena & Miyazaki, 1998; Marsh, 1980). Quantifying GLGs also can be difficult because a clear separation between light and dark bands is not always evident, thus often requiring additional processing steps such as hematoxylin staining (Marsh, 1980). Moreover, dentin layer deposition can be affected by environmental factors, and so rates may vary from year to year (Adulyanukosol, Amorena & Miyazaki, 1998; Marsh, 1980; Nishiwaki & Marsh, 1985). For TL, the source of genomic DNA is a factor and telomeres can shorten at different rates depending on tissue type. For example, bone marrow, endothelium, iliac artery, kidney, lymphocyte, muscle cells, thyroid and dental pulp telomeres shorten at rates of 9, 47–147, 102, 14–60, 41, 24, 90 and 72 bp/year, respectively (Bekaert, De Meyer & Van Oostveldt, 2005; Takubo et al., 2010), while in other tissues (epidermis, bone, cartilage) telomeres do not shorten in relation to aging (Kaewkool et al., 2020). Thus, additional studies in dugongs are warranted to determine actual rates of telomere attrition across different tissue types.
Conclusion
The dugong is a long-lived marine mammal with a low reproductive rate, and populations are threatened throughout their natural range (Adulyanukosol, Ellen & Potchana, 2010; Burgess, Lanyon & Keeley, 2012; Marsh, 1995). In particular, the incidence of stranding and deaths has dramatically increased, with deleterious effects on dugong population sustainability around the world (Marsh & Sobtzick, 2019; Sahri, Mustika & Murk, 2020). Being able to determine the age of individual dugongs, living or carcass, is important to understanding basic species biology, and also how factors in the environment affect survival and age-related changes in health, reproduction and welfare. We provide strong evidence that rTL can be used for estimating chronological age in this species and have established that physical maturity is reached at about 20 years of age. Thus, our findings offer an alternative approach for assessing age in dugongs that can be conducted using other tissue types and is not reliant on intact tusks, as is the case for GLG counts. This added flexibility makes this technique an important tool to aid in dugong conservation.
Supplemental Information
Acknowledgments
We dedicate the value of this research to Dr. Kanjana Adulyanukosol who passed away in 2015. As the initiator of the research projects on dugongs at the Phuket Marine Biological Center, Phuket, Thailand, she began to study dugongs in 1988 and worked tirelessly to bring awareness of dugongs and their conservation to Thailand and beyond. Since that time up to now, she has been one of the foremost dugong scientists in Thailand.
Funding Statement
This work was supported by the Excellence Center in Veterinary Bioscience, Chiang Mai University, Chiang Mai, Thailand. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Additional Information and Declarations
Competing Interests
Korakot Nganvongpanit is an Academic Editor for PeerJ.
Author Contributions
Phaothep Cherdsukjai conceived and designed the experiments, performed the experiments, prepared figures and/or tables, and approved the final draft.
Kittisak Buddhachat analyzed the data, prepared figures and/or tables, authored or reviewed drafts of the paper, and approved the final draft.
Janine Brown analyzed the data, authored or reviewed drafts of the paper, and approved the final draft.
Manthanee Kaewkool performed the experiments, prepared figures and/or tables, and approved the final draft.
Anocha Poommouang performed the experiments, prepared figures and/or tables, and approved the final draft.
Patcharaporn Kaewmong conceived and designed the experiments, authored or reviewed drafts of the paper, supplied all marine tusk and tooth samples and recorded data, and approved the final draft.
Kongkiat Kittiwattanawong conceived and designed the experiments, authored or reviewed drafts of the paper, supplied all marine tusk and tooth samples and recorded data, and approved the final draft.
Korakot Nganvongpanit conceived and designed the experiments, analyzed the data, prepared figures and/or tables, authored or reviewed drafts of the paper, research scholarship recipient, and approved the final draft.
Animal Ethics
The following information was supplied relating to ethical approvals (i.e., approving body and any reference numbers):
According to the Animals for Scientific Purposes Act, B.E. 2558 (2015), since a part of this experiment was performed on carcass of stranding dugong, no ethical approval was required for this study and confirmed by the Animal Ethics Committee, Faculty of Veterinary Medicine, Chiang Mai University (Licence number U1006312558).
Data Availability
The following information was supplied regarding data availability:
The raw measurements are available as a Supplemental File.
References
- Adulyanukosol, Amorena & Miyazaki (1998).Adulyanukosol K, Amorena M, Miyazaki N. Preliminary study on age determination of Dugong (Dugong dugon) in Thailand. The Fourth International Scientific Symposium Role of Ocean Sciences for Sustainable Development; Japan. 1998. pp. 369–377. [Google Scholar]
- Adulyanukosol, Cherdsukjai & Boukaew (2011).Adulyanukosol K, Cherdsukjai P, Boukaew P. Morphology and organ weight of dugongs (Dugong dugon) in Thai waters. Proceedings of the 6th International Symposium on SEASTAR2000 and Asian Bio-Logging Science (The 10th SEASTAR2000 Workshop) Graduate School of Informatics, Kyoto University; 2011. pp. 41–47. [Google Scholar]
- Adulyanukosol, Ellen & Potchana (2010).Adulyanukosol K, Ellen H, Potchana B. Cultural significance of dugong to Thai villagers: implications for conservation. 5th International Symposium on SEASTAR2000 and Asian Bio-Logging Science (The 9th SEASTAR2000 Workshop); Bangkok. Graduate School of Informatics, Kyoto University; 2010. pp. 43–49. [Google Scholar]
- Baek et al. (2019).Baek JH, Son H, Jeong YH, Park SW, Kim HJ. Chronological aging standard curves of telomere length and mitochondrial DNA copy number in twelve tissues of C57BL/6 male mouse. Cells. 2019;8(3):E247. doi: 10.3390/cells8030247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekaert, De Meyer & Van Oostveldt (2005).Bekaert S, De Meyer T, Van Oostveldt P. Telomere attrition as ageing biomarker. Anticancer Research. 2005;25:3011–3021. [PubMed] [Google Scholar]
- Buddhachat et al. (2017).Buddhachat K, Kriangwanich W, Kumoun I, Brown JL, Chailangkarn S, Somgird C, Thitaram C, Prasitwattanaseree S, Nganvongpanit K. Telomeric attrition with increasing age in short- (Chihuahua dog) and long- (Asian elephant) life span animals. Kafkas Universitesi Veteriner Fakultesi Dergisi. 2017;23:643–649. [Google Scholar]
- Burgess, Lanyon & Keeley (2012).Burgess EA, Lanyon JM, Keeley T. Testosterone and tusks: maturation and seasonal reproductive patterns of live, free-ranging male dugongs (Dugong dugon) in a subtropical population. Reproduction. 2012;143(5):683–697. doi: 10.1530/REP-11-0434. [DOI] [PubMed] [Google Scholar]
- Callicott & Womack (2006).Callicott RJ, Womack JE. Real-time PCR assay for measurement of mouse telomeres. Comparative Medicine. 2006;56:17–22. [PubMed] [Google Scholar]
- Canela et al. (2007).Canela A, Vera E, Klatt P, Blasco MA. High-throughput telomere length quantification by FISH and its application to human population studies. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(13):5300–5305. doi: 10.1073/pnas.0609367104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cawthon (2002).Cawthon RM. Telomere measurement by quantitative PCR. Nucleic Acids Research. 2002;30(10):1–6. doi: 10.1093/nar/30.10.e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniali et al. (2013).Daniali L, Benetos A, Susser E, Kark JD, Labat C, Kimura M, Desai KK, Granick M, Aviv A. Telomeres shorten at equivalent rates in somatic tissues of adults. Nature Communications. 2013;4(1):1597. doi: 10.1038/ncomms2602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dlouha et al. (2014).Dlouha D, Maluskova J, Kralova Lesna I, Lanska V, Hubacek JA. Comparison of the relative telomere length measured in leukocytes and eleven different human tissues. Physiological Research. 2014;63:S343–S350. doi: 10.33549/physiolres.932856. [DOI] [PubMed] [Google Scholar]
- Fathi et al. (2019).Fathi E, Charoudeh HN, Sanaat Z, Farahzadi R. Telomere shortening as a hallmark of stem cell senescence. Stem Cell Investigation. 2019;6:7. doi: 10.21037/sci.2019.02.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fick et al. (2012).Fick LJ, Fick GH, Li Z, Cao E, Bao B, Heffelfinger D, Parker HG, Ostrander EA, Riabowol K. Telomere length correlates with life span of dog breeds. Cell Reports. 2012;2(6):1530–1536. doi: 10.1016/j.celrep.2012.11.021. [DOI] [PubMed] [Google Scholar]
- Fortes et al. (2018).Fortes MD, Ooi JLS, Tan YM, Prathep A, Bujang JS, Yaakub SM. Seagrass in Southeast Asia: a review of status and knowledge gaps, and a road map for conservation. Botanica Marina. 2018;61(3):269–288. doi: 10.1515/bot-2018-0008. [DOI] [Google Scholar]
- Goto et al. (2004).Goto M, Ito C, Yahaya MS, Wakamura K, Asano S, Wakai Y, Oka Y, Furuta M, Kataoka T. Effects of age, body size and season on food consumption and digestion of captive dugongs (Dugong dugon) Marine and Freshwater Behaviour and Physiology. 2004;37(2):89–97. doi: 10.1080/1023624042000199935. [DOI] [Google Scholar]
- Green & Short (2003).Green EP, Short FT. World atlas of seagresses. Berkelek: University of California Press; 2003. [Google Scholar]
- Heidinger et al. (2012).Heidinger BJ, Blount JD, Boner W, Griffiths K, Metcalfe NB, Monaghan P. Telomere length in early life predicts lifespan. Proceedings of the National Academy of Sciences of the United States of America. 2012;109(5):1743–1748. doi: 10.1073/pnas.1113306109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewakapuge et al. (2008).Hewakapuge S, van Oorschot RA, Lewandowski P, Baindur-Hudson S. Investigation of telomere lengths measurement by quantitative real-time PCR to predict age. Legal Medicine. 2008;10(5):236–242. doi: 10.1016/j.legalmed.2008.01.007. [DOI] [PubMed] [Google Scholar]
- Izzo et al. (2011).Izzo C, Hamer DJ, Bertozzi T, Donnellan SC, Gillanders BM. Telomere length and age in pinnipeds: the endangered Australian sea lion as a case study. Marine Mammal Science. 2011;27(4):841–851. doi: 10.1111/j.1748-7692.2010.00450.x. [DOI] [Google Scholar]
- Kaewkool et al. (2020).Kaewkool M, Kriangwanich W, Buddhachat K, Chomdej S, Mahakkanukrauh P, Nganvongpanit K. Age estimation by telomeric length using human epidermis, bone and cartilage samples was found to be ineffective. Chiang Mai University (CMU) Journal of Natural Sciences. 2020;19:1–10. [Google Scholar]
- Karlsson et al. (2008).Karlsson AO, Svensson A, Marklund A, Holmlund G. Estimating human age in forensic samples by analysis of telomere repeats. Forensic Science International: Genetics Supplement Series. 2008;1(1):569–571. doi: 10.1016/j.fsigss.2007.10.153. [DOI] [Google Scholar]
- Kasuya & Nishiwaki (1978).Kasuya T, Nishiwaki M. On the age characteristics and anatomy of the tusk of Dugong dugon. Scientific Reports the Whales Research Institute. 1978;30:301–311. [Google Scholar]
- Lanyon & Sanson (2006).Lanyon JM, Sanson GD. Degenerate dentition of the dugong (Dugong dugon), or why a grazer does not need teeth: morphology, occlusion and wear of mouthparts. Journal of Zoology. 2006;268(2):133–152. doi: 10.1111/j.1469-7998.2005.00004.x. [DOI] [Google Scholar]
- Lincz et al. (2020).Lincz LF, Scorgie FE, Garg MB, Gilbert J, Sakoff JA. A simplified method to calculate telomere length from Southern blot images of terminal restriction fragment lengths. Biotechniques. 2020;68(1):28–34. doi: 10.2144/btn-2019-0082. [DOI] [PubMed] [Google Scholar]
- Lockyer (1984).Lockyer C. Age determination by means of the ear plug in baleen whales. International Whaling Commission. 1984;34:692–696. [Google Scholar]
- Marmontel et al. (1996).Marmontel M, O’Shea TJ, Kochman HI, Humphrey SR. Age determination in manatees using growth-layer-group counts in bone. Marine Mammal Science. 1996;12(1):54–88. doi: 10.1111/j.1748-7692.1996.tb00305.x. [DOI] [Google Scholar]
- Marsh (1980).Marsh H. Age determination of the dugong (Dugong dugon (Muller)) in northern Australia and its biological implications. Report of the Meeting of the International Whaling Commission. 1980;30:181–201. [Google Scholar]
- Marsh (1995).Marsh H. The life history, pattern of breeding, and population dynamics of the dugong. National Biological Service: Information and Technology Report. 1995;1:75–83. [Google Scholar]
- Marsh & Sobtzick (2019).Marsh H, Sobtzick S. Dugong dugon (amended version of 2015 assessment) IUCN Red List of Threatened Species. 2019;2019:e.T6909A160756767. doi: 10.2305/IUCN.UK.2015-4.RLTS.T6909A160756767.en. [DOI] [Google Scholar]
- McKevitt et al. (2002).McKevitt TP, Nasir L, Devlin P, Argyle DJ. Telomere lengths in dogs decrease with increasing donor age. Journal of Nutrition. 2002;132(6):1–3. doi: 10.1093/jn/132.6.1604S. [DOI] [PubMed] [Google Scholar]
- Mitchell (1973).Mitchell J. Determination of relative age in the dugong, Dugong dugone (Muller) Biological Conservation. 1973;9(1):25–28. doi: 10.1016/0006-3207(76)90070-7. [DOI] [Google Scholar]
- Mitchell (1976).Mitchell J. Age determination in the dugong, Dugong dugon (Muller) Biological Conservation. 1976;9(1):25–28. doi: 10.1016/0006-3207(76)90070-7. [DOI] [Google Scholar]
- Mitchell (1978).Mitchell J. Incremental growth layers in the dentine of dugong incisors (Dugong dugon (Müller)) and their application to age determination. Zoological Journal of the Linnean Society. 1978;62(4):317–348. doi: 10.1111/j.1096-3642.1978.tb01043.x. [DOI] [Google Scholar]
- Myers (2002).Myers P. Dugongidae (on-line), animal diversity web. 2002. https://animaldiversity.org/accounts/Dugongidae/ [26 April 2020]. https://animaldiversity.org/accounts/Dugongidae/
- Nganvongpanit et al. (2017a).Nganvongpanit K, Buddhachat K, Kaewmong P, Cherdsukjai P, Kittiwatanawong K. What the skull and scapular morphology of the dugong (Dugong dugon) can tell us: sex, habitat and body length? Scientific Reports. 2017a;7(1):1964. doi: 10.1038/s41598-017-01899-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nganvongpanit et al. (2017b).Nganvongpanit K, Buddhachat K, Piboon P, Euppayo T, Kaewmong P, Cherdsukjai P, Kittiwatanawong K, Thitaram C. Elemental classification of the tusks of dugong (Dugong dugong) by HH-XRF analysis and comparison with other species. Scientific Reports. 2017b;7(1):46167. doi: 10.1038/srep46167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiwaki & Marsh (1985).Nishiwaki N, Marsh H. Dugong, Dugong dugon (Muller, 19776)—Handbook of marine mammals. London: Academic Press; 1985. pp. 1–31. [Google Scholar]
- Olsen et al. (2014).Olsen MT, Robbins J, Berube M, Rew MB, Palsboll PJ. Utility of telomere length measurments for age determination of humpback whales. Nammco Scientific Publications. 2014;10:1–17. [Google Scholar]
- Read, Hohn & Lockyer (2018).Read FL, Hohn AA, Lockyer CH. Age estimation methods in marine mammal: with special reference to monodontids. Nammco Scientific Publications. 2018;10:1–21. doi: 10.7557/3.4474. [DOI] [Google Scholar]
- Rollings et al. (2017).Rollings N, Uhrig EJ, Krohmer RW, Waye HL, Mason RT, Olsson M, Whittington CM, Friesen CR. Age-related sex differences in body condition and telomere dynamics of red-sided garter snakes. Proceedings of the Royal Society B: Biological Sciences. 2017;284(1852):20162146. doi: 10.1098/rspb.2016.2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rust et al. (2019).Rust LB, Danil K, Melin SR, Wilkerson B. Accuracy and precision of age determination using growth layer groups for California sea lions (Zalophus californianus) with known ages. Marine Mammal Science. 2019;35(4):1355–1368. doi: 10.1111/mms.12605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahri, Mustika & Murk (2020).Sahri A, Mustika PLK, Murk AJ. A critical review of marine mammal governance and protection in Indonesia. Marine Policy. 2020;117:103893. doi: 10.1016/j.marpol.2020.103893. [DOI] [Google Scholar]
- Scheffer (1970).Scheffer VB. Growth layers in a dugong tooth. Journal of Mammalogy. 1970;51(1):187–190. doi: 10.2307/1378561. [DOI] [Google Scholar]
- Scott et al. (2006).Scott NM, Haussmann MF, Elsey RM, Trosclair PL, Vleck CM. Telomere length shortens with body length in Alligator mississippiensis. Southeastern Naturalist. 2006;5(4):685–692. doi: 10.1656/1528-7092(2006)5[685:TLSWBL]2.0.CO;2. [DOI] [Google Scholar]
- Sensor et al. (2018).Sensor JD, George JC, Clementz MT, Lovano DM, Waugh DA, Givens GH, Suydam R, Stimmelmayr R, Thewissen JGM. Age estimation in bowhead whales using tympanic bulla histology and baleen isotopes. Marine Mammal Science. 2018;34(2):347–364. doi: 10.1111/mms.12476. [DOI] [Google Scholar]
- Shay & Wright (2000).Shay JW, Wright WE. Hayflick, his limit, and cellular ageing. Nature Reviews Molecular Cell Biology. 2000;1(1):72–76. doi: 10.1038/35036093. [DOI] [PubMed] [Google Scholar]
- Sukhovskaya et al. (1985).Sukhovskaya LI, Klevezal GA, Borisov VI, Lagerev SJ. Use of bone layers to determine age in minke whales. Acta Theriologica. 1985;30:275–286. doi: 10.4098/AT.arch.85-19. [DOI] [Google Scholar]
- Takubo et al. (2010).Takubo K, Aida J, Izumiyama-Shimomura N, Ishikawa N, Sawabe M, Kurabayashi R, Shiraishi H, Arai T, Nakamura K. Changes of telomere length with aging. Geriatrics & Gerontology International. 2010;10:S197–S206. doi: 10.1111/j.1447-0594.2010.00605.x. [DOI] [PubMed] [Google Scholar]
- Vera et al. (2012).Vera E, De Jesus BB, Foronda M, Flores JM, Blasco MA. The rate of increase of short telomeres predicts longevity in mammals. Cell Reports. 2012;2(4):732–737. doi: 10.1016/j.celrep.2012.08.023. [DOI] [PubMed] [Google Scholar]
- Vleck, Haussmann & Vleck (2003).Vleck CM, Haussmann MF, Vleck D. The natural history of telomeres: tools for aging animals and exploring the aging process. Experimental Gerontology. 2003;38(7):791–795. doi: 10.1016/S0531-5565(03)00110-4. [DOI] [PubMed] [Google Scholar]
- Whittemore et al. (2019).Whittemore K, Vera E, Martínez-Nevado E, Sanpera C, Blasco MA. Telomere shortening rate predicts species life span. Proceedings of the National Academy of Sciences of the United States of America. 2019;116(30):15122–15127. doi: 10.1073/pnas.1902452116. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The following information was supplied regarding data availability:
The raw measurements are available as a Supplemental File.