Abstract
Since about the turn of the millennium, octocorals have been increasing in abundance on Caribbean reefs. The mechanisms underlying this trend have not been resolved, but the emergent species assemblage appears to be more resilient than the scleractinians they are replacing. The sea fan Gorgonia ventalina is an iconic species in the contemporary octocoral fauna, and here its population dynamics are described from St. John, US Virgin Islands, from 2013 to 2019. Mean densities of G. ventalina at Yawzi Point (9-m depth) varied from 1.4–1.5 colonies m−2, and their mean heights from 24–30 cm; nearby at Tektite (14-m depth), they varied from 0.6–0.8 colonies m−2 and from 25–33 cm. These reefs were impacted by two Category 5 hurricanes in 2017, but neither the density of G. ventalina, the density of their recruits (< 5-cm tall), nor the height of colonies, differed among years, although growth was depressed after the hurricanes. Nevertheless, at Tektite, colony height trended upwards over time, in part because colonies 10.1–20 cm tall were reduced in abundance after the hurricanes. These trends were sustained without density-associated effects mediating recruitment or self-thinning of adults. The dynamics of G. ventalina over seven years reveals the high resilience of this species that will contribute to the persistence of octocorals as a dominant state on Caribbean reefs.
Keywords: Octocorals, Gorgonians, Caribbean, Coral reef, Virgin Islands
Introduction
Tropical coral reefs are undergoing profound changes in community structure as scleractinians are dying (Jackson et al., 2014; Edmunds & Lasker, 2016; Hughes et al., 2018), thus transitioning alternative taxa into functional dominance (Norström et al., 2009). These changes are being accompanied by modifications in the ecological processes structuring reef communities (Jouffray et al., 2015; Hughes et al., 2018), as well as the goods and services they provide (Wild et al., 2011), for instance, through depressed community calcification (Eyre et al., 2018), and impaired capacity to protect shorelines from erosion (Elliff & Silva, 2017). These trends highlight the need to study the ecology of emerging tropical reef communities, and on shallow Caribbean reefs, one front of this effort focuses on octocorals (Ruzicka et al., 2013; Lasker et al., 2020).
Octocorals have long been recognized as important on Caribbean reefs (Cary, 1914; Kinzie, 1973), but over the last 20 years, their importance has been accentuated by regional increases in their abundance (Ruzicka et al., 2013; Lenz et al., 2015; Lasker et al., 2020). The causes of this trend remain unclear, but rapid vertical growth (Borgstein, Beltrán & Prada, 2020) is advantageous in allowing many octocorals to escape the risks of competition with spatially aggressive benthic taxa. Competition with macroalgae, for example, is detrimental for small scleractinians (Box & Mumby, 2007), whereas arborescent octocorals can quickly extend above macroalgae from a small site of attachment (Jackson, 1979; Borgstein, Beltrán & Prada, 2020). The positive demographic implications of rapid growth are augmented by recruitment, which is modest relative to scleractinians when octocoral recruits are considered as colonies ≤ 10 cm tall (Lasker et al., 2020), but can be high when measured as polyps on settlement tiles evaluated over days-weeks (Wells et al., in review). Together, these features are likely to be important in supporting greater ecological resilience of octocorals relative to scleractinians (Tsounis & Edmunds, 2017), and promoting octocoral persistence to form structurally dominant communities on contemporary reefs (Lasker et al., 2020).
Central to understanding the mechanisms through which octocorals have increased in abundance on Caribbean reefs is their demography. However, after decades of neglect, demographic data are available for only a few octocorals (e.g., Lasker, 1991; Yoshioka, 1994; Bruno et al., 2011). These data reveal situations under which octocorals are more resistant to environmental challenges than scleractinians, such as when reefs are affected by high temperature (Goulet et al., 2017), and during recovery following storms (Tsounis & Edmunds, 2017; Lasker et al., 2020). In warm seawater, octocorals are less likely than scleractinians to bleach (Prada, Weil & Yoshioka, 2010; Goulet et al., 2017), and during severe wave events, octocorals tend to be lost from the reef through failure of the substratum to which they are attached, rather than breakage of the colony (Kinzie, 1973; Birkeland, 1974). Addressing mechanisms promoting octocoral success has benefitted from the analogy with terrestrial forests (Rossi et al., 2017), which has motivated consideration of the emergent consequences of dense stands of octocorals. These include creating a unique sub-canopy flow regime (Guizien & Ghisalberti, 2016), modified recruitment (Privitera-Johnson, Lenz & Edmunds, 2015), and a context in which self-thinning (sensu Yoda et al., 1963) might play a role in regulating population size (Edmunds & Lasker, 2019).
Sea fans in the genus Gorgonia are iconic on Caribbean reefs and have been studied for decades. Early work focused on colony orientation relative to seawater flow (Wainwright & Dillon, 1969), predation by the gastropod Cyphoma gibbosum (Birkeland & Gregory, 1975), natural products chemistry (Ciereszko & Karns, 1973), and population biology (Birkeland, 1974). Arguably, however, Gorgonia spp. is best known through its infection with a disease that first was reported in 1984 from Costa Rica (Guzmán & Cortés, 1984), and later attributed to the fungus, Aspergillus sydowii (Smith et al., 1996). Aspergillosus has negatively affected populations of G. ventalina and G. flabellum throughout the Caribbean (Kim & Rypien, 2016), and has motivated one of the most comprehensive demographic analyses of any octocoral (Bruno et al., 2011).
The present study describes the population dynamics of Gorgonia ventalina from 2013–2019 at two sites on the south shore of St. John, US Virgin Islands, and uses the results to explore population stability over a period punctuated by severe storms. Stands of G. ventalina at Yawzi Point (9-m depth) and Tektite (14-m depth) are ∼650 m apart, and occur as dense aggregates on a framework dominated by the scleractinian Orbicella annularis (Edmunds, 2015). Since G. ventalina reproduces through gonochoric spawning (Petes et al., 2003) with pelagic larvae that disperse over <2 km (Andras, Rypien & Harvell, 2012), the two stands are likely to function as a single population. The octocoral communities at these sites are less well developed than those found a few hundred meters away (Tsounis et al., 2018; Lasker et al., 2020), and are characterized by high relative abundance of G. ventalina (i.e., 61–66% of colonies were G. ventalina in 2019). With high densities of one species, it is easier to make inferences regarding the demographic properties mediating population dynamics, because intraspecific interactions are likely to be more important than interspecific interactions.
The main objective of this study was to test for changes in the G. ventalina stands over 7 years that included two Category 5 hurricanes in September 2017 (Edmunds, 2019a). Using the results from the main objective as a context, changes in the stands of G. ventalina were explored for signs that they conformed to the changes expected from underlying density-dependent mechanisms (recruitment and self-thinning (Yoda et al., 1963; Westoby, 1984)) that can regulate population sizes of plants and animals (Westoby, 1984; Fréchette & Lefaivre, 1995). These mechanisms appear to modulate the population size of some octocorals (Privitera-Johnson, Lenz & Edmunds, 2015; Linares et al., 2008; Edmunds & Lasker, 2019), but exploring for population regulation in species assemblages is problematic, especially for self-thinning (White, 1981). By studying G. ventalina at two sites in St. John, it was possible to reduce these effects and use this system to explore: (1) the association between recruitment and density of adult colonies (i.e., density-dependent recruitment), and (2) the association between biomass (using colony height as a proxy) and density (i.e., self-thinning). Interpretation of the trends revealed by these analyses is subject to several caveats, notably that the association between abundance and other variables cannot demonstrate density-dependence (i.e., cause and effect), and the experimental design has limitations. These include the range of densities of G. ventalina that were sampled, and the necessity of pooling results among years to explore density-associated affects. These caveats are addressed in the discussion.
Materials and Methods
The study focused on Gorgonia ventalina at Yawzi Point and Tektite within Great Lameshur Bay, and the research was completed under permits issued by the Virgin Islands National Park (number VIIS-2013-SCI-0015). These reefs have been monitored from 1987 to present (Edmunds, 2015; Edmunds, 2019a; Edmunds, 2019b), and the present analysis was superimposed on the same study areas. At each site, three permanent transects were installed in 1987, with each ∼10 m long, parallel to one another, and ∼5 m apart. Contiguous photoquadrats (1 × 1 m) have been recorded along each transect to quantify the cover of benthic taxa.
Starting in August 2013, colonies of G. ventalina ± 1 m of each transect were surveyed, with their sizes recorded as height. Each colony was mapped through Cartesian coordinates along each transect, and their height was recorded using a flexible tape measure (± 0.5 cm) as the linear distance from the holdfast to the highest distal portion of living tissue. Fans that were symmetrical or slightly imperfect in shape with small areas of mortality (Figs. 1C, 1D) were easily measured, but fans that were torn and were affected by partial mortality (i.e., categorized as “ragged” fans, Fig. 1E) posed challenges for measurement. The size of ragged fans was recorded as their greatest height, which overestimated their size relative to the amount of G. ventalina tissue. Field logistics prevented finer resolution of tissue area (e.g., through photography (e.g., Bruno et al., 2011)), but separate analyses of photoquadrats were used to evaluate the abundance of ragged colonies in each year.
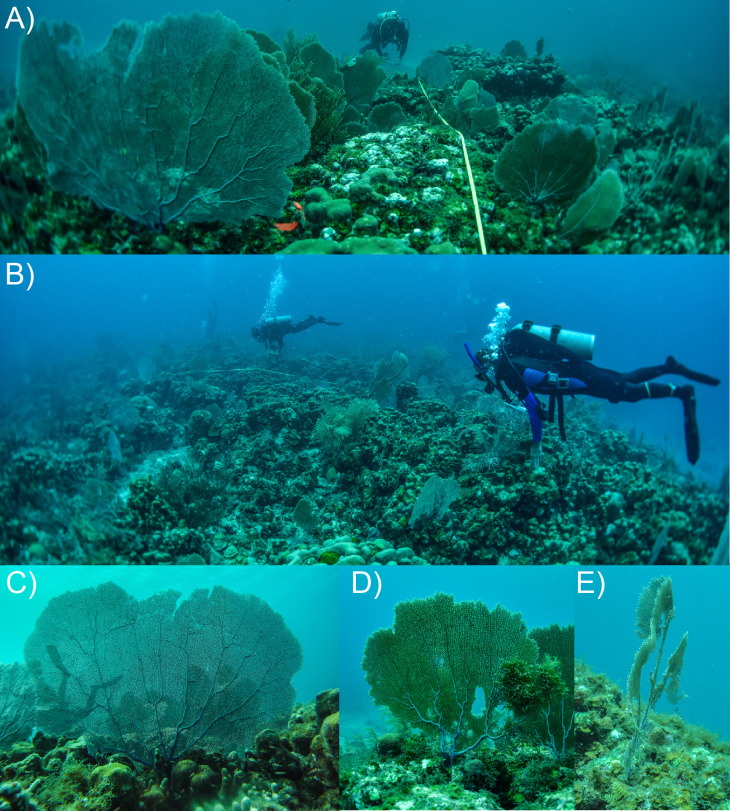
Figure 1. Study sites at Yawzi Point (9-m depth) (A) and Tektite (14-m depth) (B) in 2016.
Pictures (A, B) show high abundance of Gorgonia ventalina, with representative colonies below: (C) symmetric fan with no mortality, (D) asymmetric fan with small amounts of mortality, and (E) ragged fan with extensive partial mortality. Ragged fans accounted for 10–12% of fans over the 7-year study, but by year, they accounted for 0% (Yawzi Point in 2015) to 24% (Tektite in 2018) of fans. Photo credits: P.J. Edmunds.
The surveys of G. ventalina were repeated annually in August from 2013 to 2019, and on each occasion colonies were mapped and their sizes recorded as above. Sizes and Cartesian coordinates were compared between consecutive years to identify colonies that were evaluated in both years, colonies that had died between years (lost from the reef or reduced to a horny axis without tissue), or small colonies that recruited between years. To quantify the number of colonies that were ragged, photoquadrats along each line were used to evaluate the number of colonies that were: (a) symmetrical or asymmetrical with partial mortality, or (b) ragged (Figs. 1C–1D). Colonies were assigned to these categories in planar view, and were based on surveys of half the reef area compared to the areas surveyed for G. ventalina in situ (i.e., photoquadrats were recorded along one side of the transects).
Analyses
Mean densities of G. ventalina were compared among times using repeated measures ANOVA in which transects were repeatedly surveyed over time; a non-parametric Friedman test was used when statistical assumptions were not met. Mean colony sizes were compared over time using one-way ANOVA in which each colony was a replicate. The density of G. ventalina recruits was evaluated from colonies ≤ five cm tall (after Yoshioka, 1996), and their densities and heights were compared over time as described above. Colonies were considered to have left the recruiting size class when they were >five cm tall. The percentage distribution of colonies among sizes classes over time was evaluated using size (height) classes of 10 cm, with the largest class including colonies between 60 and 100 cm tall. The distribution of colonies among size classes was tested for variation among years using χ2 contingency tables. Growth was recorded as the change in height between consecutive years for colonies that were located in both years, and growth was compared over time using non-parametric Kruskal-Wallis (three or more groups) or Mann–Whitney U-Tests (two groups).
Density-associated effects were explored through analyses of the relationships between mean height and mean density for self-thinning, and between mean density of recruits and mean density of colonies > five cm tall, using transects as statistical replicates. Evidence for self-thinning would be revealed by an inverse relationship size and density, and a slope of the log size versus log density relationship of ∼1.5 (Westoby, 1984). Evidence of density-associated recruitment would be revealed by a linear relationship between the density of recruits and larger colonies, and a linear relationship (either positive or negative) between per-capita recruitment and density of colonies >five cm tall. Per capita recruitment was calculated by dividing the density of recruits by the density of colonies >five cm tall.
Results
The study reefs were dominated by dead colonies of Orbicella annularis (Yawzi Point), or live colonies of the same species (Tektite), and on these surfaces, Gorgonia ventalina were common, conspicuous, and often large (Fig. 1). Colonies at both sites were separated from one another on a scale of centimeters-to-a-meter, and while most colonies were symmetrical without mortality, or asymmetrical with small areas of partial mortality, some were ragged with little tissue and large tears and omissions in the fan surface (Figs. 1C–1D). Overall (pooled among years), 10% of colonies were ragged at Yawzi Point, and 12% at Tektite, and the mean percentage of ragged colonies increased from 7 ± 9% before the storms to 20 ± 1% after the storms at Yawzi Point, and from 10 ± 5% to 16 ± 12% at Tektite (± SD, n = 5 and 2, respectively).
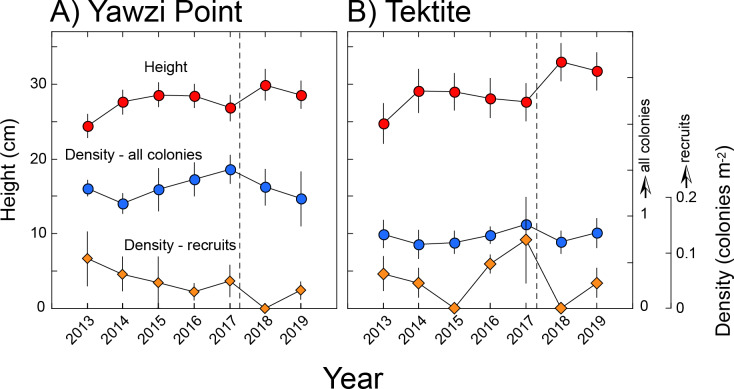
At Yawzi Point, mean (± SE, n = 3) densities ranged from 1.41 ± 0.11 colonies m−2 in 2014 to 1.51 ± 0.15 colonies m−2 in 2017, and mean (± SE) height varied from 24 ± 2 cm in 2013 (n = 84) to 30 ± 2 cm in 2018 (n = 54). Mean (± SE, n = 3) densities of recruits (colonies ≤ five cm) ranged from 0 colonies m−2 in 2018, to 0.09 ± 0.05 colonies m−2 in 2013 (Fig. 2). Differences over time were not significant for density of all colonies (RM ANOVA, F = 2.64, df = 5, 10, p = 0.140 [2018 excluded since only two transects were surveyed]), heights of all colonies (F = 1.101, df = 6, 548, p = 0.418), and density of juvenile colonies (χ2 = 1.044, df = 5, p = 0.959 [2018 excluded since only two transects were surveyed]). Mean density and height of colonies was not linearly related to time (F ≤ 4.205, df = 1, 5, p > 0.096), but the density of recruits declined (F = 3.006, df = 1, 5, p = 0.030).
Figure 2. Height and density of Gorgonia ventalina at Yawzi Point (A) and Tektite (B).
Mean ± SE shown with n = 42–97 for height, and n = 3 for density. Vertical dashed line shows the impacts of Hurricanes Irma and Maria.
At Tektite, mean (± SE, n = 3) densities ranged from 0.64 ± 0.17 colonies m−2 in 2018 to 0.81 ± 0.15 in 2013, and mean (± SE) height varied from 25 ± 3 cm in 2013 (n = 53) to 33 ± 3 cm (n = 42) in 2018; mean (± SE, n = 3) densities of recruits (colonies ≤ five cm) ranged from 0 colonies m−2 in 2015, to 0.12 ± 0.08 colonies m−2 in 2017 (Fig. 2). Differences over time were not significant for density of all colonies (RM ANOVA, F = 1.780, df = 6, 12, p = 0.186) heights of all colonies (F = 1.038, df = 6, 345, p = 0.400), and density of juvenile colonies (χ 2 = 8.953, df = 6, p = 0.176). Mean density of all colonies and recruits was not linearly related to time (F ≤ 0.430, df = 1, 5, p ≥ 0.541), but the height of colonies increased (F = 7.809, df = 1, 5, p = 0.038).
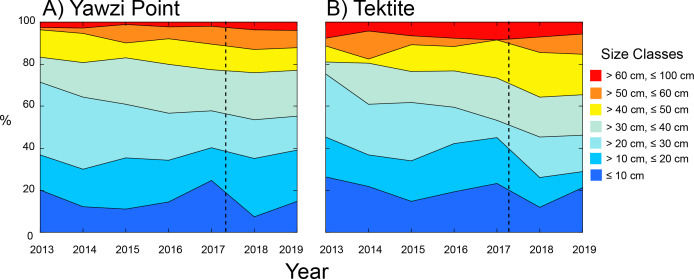
The distribution of colonies among size classes revealed that the majority (≥ 54%) was between 10.1 and 40 cm in height at both sites (Fig. 3). The distribution among size classes slightly varied among years with fewer of the smallest colonies (≤ 10 cm) following Hurricanes Irma and Maria, but the trend was not significant at Yawzi Point (χ 2 = 42.337, df = 36, p = 0.216) or Tektite (χ 2 = 43.594, df = 36, p = 0.180). When years were pooled into “before” and “after” hurricanes, the distribution of colonies among size classes still did vary between periods at Yawzi Point (χ 2 = 10.591, df = 6, p = 0.102), but it did at Tektite (χ 2 = 16.190, df = 6, p = 0.013). The variation in colony size class structure at Tektite reflected changes following the hurricanes causing an under-abundance of colonies between 10.1 and 20 cm high, and an over-abundance of colonies between 40.1 and 50 cm high (Fig. 3).
Figure 3. Percentage contribution of colonies of Gorgonia ventalina by size class to the populations at Yawzi Point (A) and Tektite (B) from 2013–2019.
n = 54–97 colonies y−1 at Yawzi Point, and n = 42–60 colonies y−1 at Tektite. Vertical dashed line shows the impacts of Hurricanes Irma and Maria.
G. ventalina for which growth was measured displayed null or positive increments over the year-long intervals, and this criterion was satisfied by 27–63 colonies year−1 at Yawzi Point, and 24–34 colonies year−1 at Tektite. At Yawzi Point, mean growth varied from 28 ± 5 mm y−1 (2018–2019, n = 30) to 42 ± 4 mm y−1 (2016–2017, n = 63), and at Tektite, from 30 ± 4 mm y−1 (2018–2019, n = 23) to 49 ± 4 mm y−1 (2015–2016, n = 28) (Fig. 4). Growth at Yawzi Point did not differ among years (H = 9.852, df = 5, p = 0.080), but it was 30% lower in the two years after the hurricanes (28 ± 4 mm y−1, n = 59) than in the four years before (40 ± 2 mm y−1, n = 218) (U = 7,625, df = 1, p = 0.030). Growth at Tektite did not differ among the six sampling years (H = 10.529, df = 5, p = 0.062), but was 23% lower in the two years after the hurricanes (33 ± 3 mm y−1, n = 51) than in the four years before (43 ± 2 mm y−1, n = 117) (U = 3,655, df = 1, p = 0.020).
Figure 4. Mean growth rate (±SE) of Gorgonia ventalina at Yawzi Point (n= 30–63) and Tektite (n= 23–31) from 2013–2019.
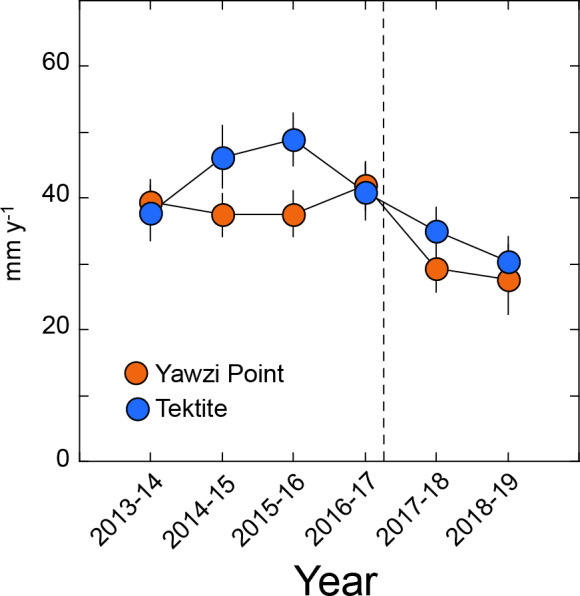
Values based on colonies that were measured in two consecutive years and either did not change in height, or increased in height (i.e., increments of ≥ 0 mm y−1). Vertical dashed line shows the impacts of Hurricanes Irma and Maria.
Density-associated effects
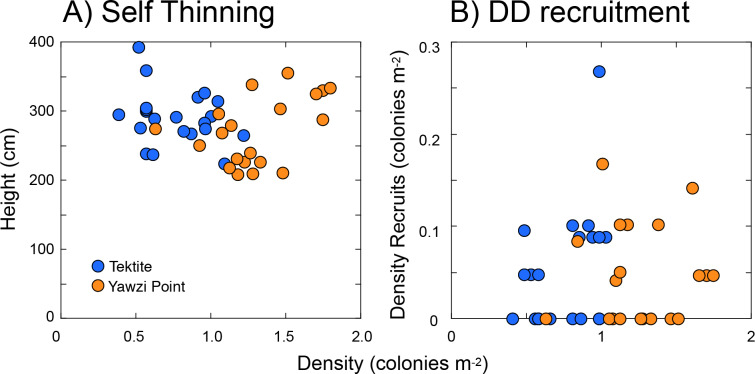
Two forms of density-associated effects were explored, one characterizing the relationship between the height and density of colonies (i.e., self-thinning), and the other between the density of recruits and colonies >five cm tall. Using the three transects at each site as replicates (and pooling between sites), the scatterplot of mean colony height and colony density (Fig. 5A) showed no relationship between the two on either linear (r = − 0.062, df = 39, p = 0.699 [Fig. 5A]) or logarithmic axes (r = − 0.151, df = 39, p = 0.346). Likewise, there was no relationship between the density of recruits and colonies >five cm tall (r = 0.113, df = 39, p = 0.481 [Fig. 5B]), or between per-capita recruitment and the density of colonies >five cm tall (r = − 0.126, df = 39, p = 0.432).
Figure 5. Scatter plots showing features of Gorgonia ventalina populations at Yawzi Point and Tektite as tests for density dependent effects.
(A) Height versus density as a test for self-thinning, and (B) density of recruits (≤ 5-cm tall) versus the density of larger colonies (> 5-cm tall) as a test for density dependent (DD) recruitment. Points represent one datum y−1 from 2013–2019. Neither height versus density (A), nor density of recruits versus density of larger colonies (B), are significantly associated (p ≥ 0.050).
Discussion
Through the lens of scleractinians, the last 40 years has been a sequence of disturbances serving as ecological ratchets promoting low coral cover (Birkeland, 2004; Birkeland, 2019), coral rarity (Edmunds, 2018), and coral extinction (Carpenter et al., 2008). These trends are striking in the Caribbean (Gardner et al., 2003; Jackson et al., 2014), where many reefs are unrecognizable compared to the 1960s (e.g., Goreau, 1959; Jackson et al., 2014; Dustan & Lang, 2019). On some reefs, however, arborescent octocorals have emerged as a dominant taxon (Ruzicka et al., 2013; Lenz et al., 2015) that contributes three-dimensional structure as flexible “forests” with a canopy of closely located branches (Rossi et al., 2017; Tsounis, Steele & Edmunds, 2020). The implications of this transition depend on the longevity of the new assemblages, some of which have been resilient to disturbances over decades (Tsounis & Edmunds, 2017), and have persisted through major hurricanes (Lasker et al., 2020). The present study shows that stands of Gorgonia ventalina on shallow reefs have maintained high densities over 7 years that included two large hurricanes in September 2017 (Edmunds, 2019a; Edmunds, 2019b). Bigger colonies were more abundant (and smaller colonies less abundant) after the storms than before, and growth rates declined following the storms, but these effects did not statistically affect density. They unfolded at mean densities of <1.8 colonies m−2, at which there was no evidence of density-dependence in the form of self-thinning or variable recruitment. As an iconic member of the octocoral forests that are becoming more prominent on Caribbean reefs, the resilience of G. ventalina to storms indicates that this species will play an important role on future reefs that are likely to have a greater abundance of octocorals.
The densities of G. ventalina at Yawzi Point and Tektite (0.7–1.5 colonies m−2) are high compared to nearby sites that have been surveyed from 2014–2019 (i.e., 0.4–1.3 colonies m−2) (Lasker, 2019), Jamaica (Discovery Bay) in the late 1960s (Gorgonia sp. 0.02–0.21 colonies m−2 in the lagoon, and 0–0.09 colonies m−2 at ∼10–15 m depth on the fore reef (Kinzie, 1973)), the Florida Keys from 1997–2003 (<8-m depth, 0.86–1.11 colonies m−2(Kim & Harvell, 2004)), and northwest Cuba from 2008–2015 (10-m depth, 0–2.10 colonies m−2, region-wide average 0.39 colonies m−2(Rey-Villiers et al., 2020)). All these densities are low compared to the values reported for G. ventalina from Puerto Rico in 1986 (7–11-m depth, 3.5–5.9 colonies m−2at two sites (Yoshioka & Yoshioka, 1989)), and Panama in 1971 (6.2 colonies m−2 (Birkeland, 1974)).
The densities of G. ventalina recruits at Yawzi Point and Tektite (0–0.12 colonies m−2) are low compared to previous studies, including from 7-m depth off Puerto Rico (0.25, 1.80 and 0.28 colonies m−2 in spring 1984, spring 1985, and spring 1988, respectively (Yoshioka, 1996)), and 2-m depth off Panama over 1971-1972 (0.15–0.40 colonies m−2 (Birkeland, 1974)). In the Florida Keys, G. ventalina recruited at <0.12 colonies m−2 prior to 2002 (colonies <10-cm tall at <8-m depth), but at 0.18–0.22 colonies m−2 from 2002–2003 (Kim & Harvell, 2004). As recruitment (colonies <4-cm tall) in a subset (30–50% of the same plots) of the present areas was 0.20–0.35 colonies m−2 in 2013 and 2014 (Privitera-Johnson, Lenz & Edmunds, 2015), spatial variation in recruitment of G. ventalina indicates caution is required in concluding that recruitment is low in St. John compared to other locations. The mean heights of G. ventalina at Yawzi Point and Tektite (24.4–40 cm) are comparable to heights previously reported from Puerto Rico (7–11-m depth, mean of 24.0–33.7 cm (Yoshioka & Yoshioka, 1991)), Panama (∼2 m depth, mean of 36–88 cm (Birkeland, 1974)), and the Florida Keys (≤ 8-m depth, median of 26–40 cm (Kim & Harvell, 2004)). Finally, the growth rates of G. ventalina in St. John (2.8–4.9 cm y−1) compare to growth rates of 1.9–2.3 cm y−1 in Puerto Rico from 1983–1988 (7–11 m depth (Yoshioka & Yoshioka, 1991)), 2.6 cm (240 d)−1 in Panama (∼2-m depth (Birkeland, 1974)), 3.9 cm y−1 in Puerto Rico from 2003–2006 (2–25-m depth (Borgstein, Beltrán & Prada, 2020)), and 4.9 ± 1.2 cm y−1 on the shallow reefs of Dry Tortugas (1910–1911 (Cary, 1914)). Together, the aforementioned contrasts indicate that the stands of G. ventalina at Yawzi Point and Tektite from 2013–2019 consisted of unusually high densities of averaged-sized colonies that grew at regionally-representative growth rates, but may not have been well supported by recruitment (i.e., colonies ≤ 5-cm tall).
From 2013 to 2019, the densities and sizes of G. ventalina at Yawzi Point and Tektite were similar among years, even though the study was interrupted by two Category 5 hurricanes in September 2017. Both storms passed ≤ 86 km from St. John with wind speeds of 266–298 km h−1, wave heights of 5.6–7.9 m (8 km from the study sites), and 11–17 cm of rain on the day of impact (Edmunds, 2019a). While damage underwater qualitatively was conspicuous (Edmunds, 2019a; Edmunds, 2019b; Gochfeld et al., 2020), the quantitative impacts were modest for scleractinians at 7–14-m depth (Edmunds, 2019a; Edmunds, 2019b), octocorals at 6–9-m depth (Lasker et al., 2020), and around St. Thomas, also for sponges (Gochfeld et al., 2020). Nevertheless, ∼5,852 Gorgonia spp. colonies were estimated to have washed onto the beach of Great Lameshur Bay in November 2017, whereas ∼61 were found in June 2017, and potentially the colonies in November 2017 came from the adjacent reefs. This inference is supported by the non-significant declines in density of G. ventalina between August 2017 and August 2018, representing a reduction of 0.20 colonies m−2 at Yawzi Point and 0.19 colonies m−2 at Tektite (Fig. 2). With fringing reefs ∼75 m wide along about half of the western coast of Cabritte Horn (690 m) and half the eastern coast of Yawzi Point (298 m), these declines in density of G. ventalina could have supplied ∼14,820 detached colonies, which is more than double what was estimated to be on the beach in Great Lameshur Bay in November 2017.
Previous studies of the response of Gorgonia spp. to storms have shown that colonies resist breakage, but are removed through failure of the substratum to which their holdfasts are attached (Kinzie, 1973; Birkeland, 1974; Yoshioka & Yoshioka, 1991; Zuluaga-Montero & Sabat, 2012). Without major disturbances, G. ventalina has high annual survivorship (∼92–95% (Birkeland, 1974); Yoshioka & Yoshioka, 1991), but over the last 25 years, mortality has been accentuated by the disease Aspergillosus, which killed 39% of sea fans (>20-cm tall) in the Florida Keys between 1997 and 2003 (Kim & Harvell, 2004). While Aspergillosus was seen on G. ventalina during the present study, the rarity of cases and the sustained population size (even with low recruitment) suggested this was not an ecologically important disease in this location between 2013 and 2019 (cf. Kim & Harvell, 2004). In St. John, Hurricanes Irma and Maria slightly reduced the density of G. ventalina, in part through the loss of large colonies, but mostly through the removal of small- (≤ 10-cm tall) and intermediate- (>10 and ≤ 40-cm tall) sized colonies. Presumably, these smaller colonies were weakly attached to the reef, and hence more readily removed by waves or sediment scour (Yoshioka & Yoshioka, 1987; Yoshioka, 2009). Since both recruitment and growth rates were depressed in the two years following the hurricanes, it probably was the removal of the smaller colonies that led to an increase in mean colony size. While the hurricanes also increased the number of ragged colonies in 2018 and 2019 versus the previous 5 years (20% versus 7% at Yawzi Point, and 16% versus 10% at Tektite, respectively), these events did not greatly alter colony height and, therefore, are unlikely to have mediated change in mean colony height at each site.
By focusing on sites where G. ventalina accounted for 61–66% of arborescent octocorals, tests for density-associated phenomena were less likely to be confounded by other species of octocorals. For self-thinning and recruitment, there was no evidence of density-association for these phenomena, although this outcome must be interpreted within the limitations of the study, including the range of densities that were evaluated, and the sample size employed (number of transects). Caution is also warranted in interpreting these trends because they were obtained by pooling results among years, which relies on the untested assumption that the demographic state variables were independent among years for common transects.
Self-thinning is well known in terrestrial forests (Westoby, 1981; Westoby, 1984), and it describes the reduction in density of even-aged trees that mediates an increase in size of the remaining trees, with this effect most strongly expressed at saturating biomass (Westoby, 1984; Weller, 1987). On a double logarithmic plot of size (i.e., biomass) against density, the slope commonly is considered to be -1.5 in the presence of self-thinning (White, 1981; Westoby, 1984). Density-dependent recruitment is well known in the marine environment (Caley et al., 1996; Hixon, Pacala & Sandin, 2002), but density-associated recruitment is only diagnostic of density-dependent recruitment if per capita recruitment covaries with adult density. While density-dependent larval supply is not possible in demographically open populations like those of mass spawning taxa, including G. ventalina, density-dependent settlement and recruitment could occur if larval delivery to the benthos is modified by flow effects within octocoral forests (Guizien & Ghisalberti, 2016), or if recruitment is modified by proximity to adults (Marhaver et al., 2013). Evidence of density-dependent effects were not detected in the present study, even though density-associated recruitment was reported from this locality for G. ventalina over 2013 and 2014 (Privitera-Johnson, Lenz & Edmunds, 2015), and Eunicea spp. in 3 of 4 years from 2014–2017 (Edmunds & Lasker, 2019), and signs of self-thinning were found for Eunicea spp. in shallow water (9-m depth) adjacent to Tektite (Edmunds & Lasker, 2019). Self-thinning also has been detected in the Mediterranean octocoral Paramuricea clavata (Linares et al., 2008).
The absence of support for self-thinning in Gorgonia ventalina is consistent with the mostly null results of testing for this effect in St. John (Edmunds & Lasker, 2019), but in the present analysis there is added weight to the outcome because the study system more closely approximated the monospecific, even-aged stands to which self-thinning is best applied (Weller, 1987; Norberg, 1988). Together, the weight of the evidence remains against self-thinning as an important ecological mechanism structuring octocoral forests on the shallow reefs of St. John (Edmunds & Lasker, 2019). Even though the present stands of G. ventalina occurred at high densities relative to most adjacent areas, the discrepancy in density versus what has been reported for this species (5.9–6.2 colonies m−2 (Birkeland, 1974; Privitera-Johnson, Lenz & Edmunds, 2015)) and all species combined (76.1 colonies m−2 (Yoshioka & Yoshioka, 1989)) leaves open the possibility that self-thinning may occur in a “crowded” octocoral community (Westoby, 1984; Weller, 1987).
For the positive density-associated recruitment detected for Gorgonia ventalina in St. John in 2013 and 2014, Privitera-Johnson, Lenz & Edmunds (2015) used results from surveys at the present sites augmented with 6–8 sites along the south coast. This collection of sites provided a range of densities with an upper value (5.9 colonies m−2) that was 3.3-folder greater than the highest value recorded in the present study (1.8 colonies m−2 along one transect at Yawzi Point in 2017), which raises the possibility that the null results for density-association of recruitment reflects the narrower range of densities of G. ventalina that were included in the present analysis. Although cause-and effect was not established in Privitera-Johnson, Lenz & Edmunds (2015), the positive density-association was hypothesized to result from several possibilities including self-recruitment, larval entrainment, enhanced fertilization, or enhanced post-settlement success. Quantitative manipulations of octocoral densities rarely have been completed, but two examples produced contradictory results: Birkeland (1974) removed 83% of the colonies of G. ventalina from a 20 m2 area in shallow water in Panama in 1971 (original density = 6.2 colonies m−2), and recruitment was elevated by ∼100%. In contrast, Yoshioka (1996) removed octocorals (all species) from eight small quadrats (0.5 m2, original densities 42-63 colonies m−2) at 7–11 m depth of Puerto Rico in 1987, and found recruitment was reduced by 15–94% over 1–5 months. Together, the present and previous results are consistent with the possibility that the relationship between recruitment and adult density in G. ventalina is driven by multiple phenomena, possibly with different mechanisms operating at different densities. For example, larval entrainment within canopies might function at low-to-medium densities, space limitation might operate at high densities, and enhanced fertilization might operate as extreme high densities.
In summary, the present results describe 7 years of population resilience for G. ventalina on the shallow reefs of St. John, even with respect to the effects of two Category 5 hurricanes. It is reasonable to infer, therefore, that G. ventalina will remain a persistent member of the octocoral forests that are emerging on present-day Caribbean reefs. While more work is required to address density-dependent demographic effects in this species, notably involving manipulative experiments and greater replication, the evidence presented here is equivocal with regards to the role of density-associated recruitment and self-thinning in modulating population size of G. ventalina.
Supplemental Information
The height of all colonies along three transects that were surveyed art Yawzi Point and Tektite from 2013 to 2019. They can be used to calculate mean density (using transects as replicates), mean heights (using colonies as replicates) and mean growth (using colonies that were relocated in consecutive years).
Acknowledgments
I thank the staff of the Virgin Islands Ecological Research Station for making my visits to St. John productive and enjoyable, S Prosterman for scuba support, and V Powell for local logistics. Multiple graduate students assisted with 7 years of research, and to all I am grateful. This is contribution number 313 of the CSUN Marine Biology program.
Funding Statement
This research was supported by the US National Science Foundation (OCE 13-32915, OCE 17-56678 and DEB 13-50146). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Additional Information and Declarations
Competing Interests
The author declares there are no competing interests.
Author Contributions
Peter J. Edmunds conceived and designed the experiments, performed the experiments, analyzed the data, prepared figures and/or tables, authored or reviewed drafts of the paper, and approved the final draft.
Field Study Permissions
The following information was supplied relating to field study approvals (i.e., approving body and any reference numbers):
Virgin Islands National Park approved the field study (VIIS-2013-SCI-0015).
Data Availability
The following information was supplied regarding data availability:
The raw data is available in the Supplemental File and at BCO-DMO (https://www.bco-dmo.org/): DOI: 10.26008/1912/bco-dmo.827851.1 and 10.26008/1912/bco-dmo.827897.1.
References
- Andras, Rypien & Harvell (2012).Andras JP, Rypien KL, Harvell CD. Range-wide population genetic structure of the Caribbean sea fan coral, Gorgonia ventalina. Molecular Ecology. 2012;22:56–73. doi: 10.1111/mec.12104. [DOI] [PubMed] [Google Scholar]
- Birkeland (1974).Birkeland C. The effects of wave action on the population dynamics of Gorgonia ventalina Linnaeus. Studies in Tropical Oceanography. 1974;12:115–126. [Google Scholar]
- Birkeland (2004).Birkeland C. Ratcheting down the coral reefs. Bioscience. 2004;54:1021–1027. doi: 10.1641/0006-3568(2004)054[1021:RDTCR]2.0.CO;2. [DOI] [Google Scholar]
- Birkeland (2019).Birkeland C. Global status of coral reefs: in combination, disturbances, and stressors become ratchets. In: Sheppard C, editor. World seas: an environmental evaluation. Academic Press; London: 2019. pp. 35–56. [DOI] [Google Scholar]
- Birkeland & Gregory (1975).Birkeland C, Gregory B. Foraging behavior and rates of feeding of the gastropod, Cyphoma gibbosum. Bulletin of the Natural History Museum of Los Angeles County. 1975;20:57–67. [Google Scholar]
- Borgstein, Beltrán & Prada (2020).Borgstein N, Beltrán DM, Prada C. Variable growth across species and life stages in Caribbean reef octocorals. Frontiers in Marine Science. 2020;7:1–11. doi: 10.3389/fmars.2020.00483. [DOI] [Google Scholar]
- Box & Mumby (2007).Box SJ, Mumby PJ. Effect of macroalgal competition on growth and survival of juvenile Caribbean corals. Marine Ecology Progress Series. 2007;342:139–149. doi: 10.3354/meps342139. [DOI] [Google Scholar]
- Bruno et al. (2011).Bruno JF, Ellner SP, Vu I, Kim K, Harvell CD. Impacts of aspergillosis on sea fan coral demography: modeling a moving target. Ecological Monographs. 2011;81:123–139. doi: 10.1890/09-1178.1. [DOI] [Google Scholar]
- Caley et al. (1996).Caley MJ, Carr MH, Hixon MA, Hughes TP, Jones GP, Menge BA. Recruitment and the local dynamics of open marine populations. Annual Review of Ecology and Systematics. 1996;27:477–500. doi: 10.1146/annurev.ecolsys.27.1.477. [DOI] [Google Scholar]
- Carpenter et al. (2008).Carpenter KE, Abrar M, Aeby G, Aronson RB, Banks S, Bruckner A, Chiriboga A, Cortés J, Delbeek C, DeVantier L, Edgar GJ, Edwards AJ, Fenner D, Guzmán HM, Hoeksema BW, Hodgson G, Johan O, Licuanan WY, Livingstone SR, Lovell ER, Moore JA, Obura DO, Ochavillo D, Polidoro BA, Precht WF, Quibilan MC, Reboton C, Richards ZT, Rogers AD, Sanciangco J, Sheppard A, Sheppard C, Smith J, Stuart S, Turak E, Veron JEN, Wallace C, Weil E, Wood E. One-third of reef-building corals face elevated extinction risk from climate change and local impacts. Science. 2008;321:560–563. doi: 10.1126/science.1159196. [DOI] [PubMed] [Google Scholar]
- Cary (1914).Cary LR. Contributions from the biological laboratories in Princeton University volume IV. Princeton University Publications; Princeton: 1914. Observations upon the growth-rate and ecology of gorgonians; pp. 79–92. [Google Scholar]
- Ciereszko & Karns (1973).Ciereszko LS, Karns TKB. Comparative biochemistry of coral reef coelenterates. In: Jones OA, Endean R, editors. Biology and geology of coral reefs volume II: biology 1. Academic Press; New York: 1973. pp. 183–204. [Google Scholar]
- Dustan & Lang (2019).Dustan P, Lang JC. Discovery Bay, Jamaica. In: Loya Y, Puglise KA, Bridge TCL, editors. Mesophotic coral ecosystems. Coral reefs of the world, vol 12. Cham: Springer; 2019. pp. 85–109. [DOI] [Google Scholar]
- Edmunds (2015).Edmunds PJ. A quarter-century demographic analysis of the Caribbean coral, Orbicella annularis, and projections of population size over the next century. Limnology and Oceanography. 2015;60:840–855. doi: 10.1002/lno.10075. [DOI] [Google Scholar]
- Edmunds (2018).Edmunds PJ. The hidden dynamics of low coral cover communities. Hydrobiologia. 2018;818:193–209. doi: 10.1007/s10750-018-3609-9. [DOI] [Google Scholar]
- Edmunds (2019a).Edmunds PJ. Three decades of degradation lead to diminished impacts of severe hurricanes on Caribbean reefs. Ecology. 2019a;100:e02587. doi: 10.1002/ecy.2587. [DOI] [PubMed] [Google Scholar]
- Edmunds (2019b).Edmunds PJ. The demography of hurricane effects on two coral populations differing in dynamics. Ecosphere. 2019b;10:e02836. [Google Scholar]
- Edmunds & Lasker (2016).Edmunds PJ, Lasker HR. Cryptic regime shift in benthic community structure on shallow reefs in St. John, US Virgin Islands. Marine Ecology Progress Series. 2016;559:1–12. doi: 10.3354/meps11900. [DOI] [Google Scholar]
- Edmunds & Lasker (2019).Edmunds PJ, Lasker HR. Regulation of population size of arborescent octocorals on shallow Caribbean reefs. Marine Ecology Progress Series. 2019;615:1–14. doi: 10.3354/meps12907. [DOI] [Google Scholar]
- Elliff & Silva (2017).Elliff CI, Silva I. Coral reefs as the first line of defense: shoreline protection in face of climate change. Marine Environmental Research. 2017;127:148–154. doi: 10.1016/j.marenvres.2017.03.007. [DOI] [PubMed] [Google Scholar]
- Eyre et al. (2018).Eyre BD, Cyronak T, Drupp P, De Carlo EH, Sachs JP, Andersson AJ. Coral reefs will transition to net dissolving before end of century. Science. 2018;359:908–911. doi: 10.1126/science.aao1118. [DOI] [PubMed] [Google Scholar]
- Fréchette & Lefaivre (1995).Fréchette M, Lefaivre D. On self-thinning in animals. Oikos. 1995;73:425–428. doi: 10.2307/3545971. [DOI] [Google Scholar]
- Gardner et al. (2003).Gardner TA, Côté IM, Gill JA, Grant A, Watkinson AR. Long-term region-wide declines in Caribbean corals. Science. 2003;301:958–960. doi: 10.1126/science.1086050. [DOI] [PubMed] [Google Scholar]
- Gochfeld et al. (2020).Gochfeld DJ, Olson JB, Chaves-Fonnegra A, Smith TB, Ennis RS, Brandt M. Impacts of hurricanes Irma and Maria on coral reef sponge communities in St. Thomas, U.S. Virgin Islands. Estuaries and Coasts. 2020;43:1235–1247. doi: 10.1007/s12237-020-00694-4. [DOI] [Google Scholar]
- Goreau (1959).Goreau TF. The ecology of Jamaican coral reefs I. species composition and zonation. Ecology. 1959;40:67–90. doi: 10.2307/1929924. [DOI] [Google Scholar]
- Goulet et al. (2017).Goulet TL, Shirur KP, Ramsby BD, Iglesias-Prieto R. The effects of elevated seawater temperatures on Caribbean gorgonian corals and their algal symbionts, Symbiodinium spp. PLOS ONE. 2017;12:e0171032. doi: 10.1371/journal.pone.0171032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guizien & Ghisalberti (2016).Guizien K, Ghisalberti M. Living in the canopy of the animal forest: physical and biogeochemical aspects. In: Rossi S, Bramanti L, Gori A, Orejas C, editors. Marine animal forests the ecology of benthic biodiversity hotspots. Springer International Publishing; Cham: 2016. pp. 507–528. [DOI] [Google Scholar]
- Guzmán & Cortés (1984).Guzmán HM, Cortés J. Mortandad de Gorgonia flabellum Linnaeus (Octocorallia: Gorgoniidae) en la Costa Caribe de Costa Rica. Revista de Biología Tropical. 1984;32:305–308. [Google Scholar]
- Hixon, Pacala & Sandin (2002).Hixon MA, Pacala SW, Sandin SA. Population regulation: historical context and contemporary challenges of open vs. closed systems. Ecology. 2002;83:1490–1508. doi: 10.1890/0012-9658(2002)083[1490:PRHCAC]2.0.CO;2. [DOI] [Google Scholar]
- Hughes et al. (2018).Hughes TP, Kerry JT, Baird AH, Connolly SR, Dietzel A, Eakin CM, Heron SF, Hoey AS, Hoogenboom MO, Liu G, McWilliam MJ, Pears RJ, Pratchett MS, Skirving WJ, Stella JS, Torda G. Global warming transforms coral reef assemblages. Nature. 2018;556:492–496. doi: 10.1038/s41586-018-0041-2. [DOI] [PubMed] [Google Scholar]
- Jackson (1979).Jackson JBC. Morphological strategies of sessile animals. In: Larwood G, Rosen BR, editors. Biology and systematics of colonial organisms. Academic Press; New York: 1979. pp. 499–555. [Google Scholar]
- Jackson et al. (2014).Jackson JBC, Donavan MK, Cramer KL, Lam VV, editors. Status and trends of Caribbean coral reefs: 1970–2012. Global Coral Reef Monitoring Network, IUNC; Gland: 2014. [Google Scholar]
- Jouffray et al. (2015).Jouffray J, Nyström M, Norström AV, Williams ID, Wedding LM, Kittinger JN, Williams GJ. Identifying multiple coral reef regimes and their drivers across the Hawaiian archipelago. Philosophical Transactions of the Royal Society B. 2015;370:20130268. doi: 10.1098/rstb.2013.0268. [DOI] [Google Scholar]
- Kim & Harvell (2004).Kim K, Harvell CD. The rise and fall of a six-year coral-fungal epizootic. The American Naturalist. 2004;164:S52–S63. doi: 10.1086/424609. [DOI] [PubMed] [Google Scholar]
- Kim & Rypien (2016).Kim K, Rypien K. Aspergillosis of Caribbean sea fan corals, Gorgonia spp. In: Woodley CM, Downs CA, Bruckner AW, Porter JW, Galloway SB, editors. Diseases of coral. John Wiley & Sons; Hoboken: 2016. pp. 236–241. [DOI] [Google Scholar]
- Kinzie (1973).Kinzie RA. The Zonation of the West Indian Gorgonians. Bulletin of Marine Science. 1973;23:93–155. [Google Scholar]
- Lasker (1991).Lasker HR. Population growth of a gorgonian coral: equilibrium and non-equilibrium sensitivity to changes in life history variables. Oecologia. 1991;86:503–509. doi: 10.1007/BF00318316. [DOI] [PubMed] [Google Scholar]
- Lasker (2019).Lasker HR. Dataset: octocoral colony heights measured during transect surveys at four sites on the south shore of St. John, US Virgin Islands in June 2018. Biological and Chemical Oceanography Data Management Office Data Sets. 2019 doi: 10.1575/1912/bco-dmo.765328.1. [DOI] [Google Scholar]
- Lasker et al. (2020).Lasker HR, Bramanti L, Tsounis G, Edmunds PJ. The rise of octocoral forests on Caribbean reefs. Advances in Marine Biology. 2020 doi: 10.1016/bs.amb.2020.08.009. [DOI] [PubMed] [Google Scholar]
- Lasker et al. (2020).Lasker HR, Martínez-Quintana Á, Bramanti L, Edmunds PJ. Resilience of octocoral forests to catastrophic storms. Scientific Reports. 2020;10:4286. doi: 10.1038/s41598-020-61238-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenz et al. (2015).Lenz EA, Bramanti L, Lasker HR, Edmunds PJ. Long-term variation of octocoral populations in St. John, US Virgin Islands. Coral Reefs. 2015;34:1099–1109. doi: 10.1007/s00338-015-1315-x. [DOI] [Google Scholar]
- Linares et al. (2008).Linares C, Coma R, Garrabou J, Díaz D, Zabala M. Size distribution, density and disturbance in two Mediterranean gorgonians: Paramuricea clavate and Eunicella singularis. Journal of Applied Ecology. 2008;45:688–699. doi: 10.1111/j.1365-2664.2007.01419.x. [DOI] [Google Scholar]
- Marhaver et al. (2013).Marhaver KL, Vermeij MJA, Rohwer F, Sandin SA. Janzen-Connell effects in a broadcast-spawning Caribbean coral: distance-dependent survival of larvae and settlers. Ecology. 2013;94:146–160. doi: 10.1890/12-0985.1. [DOI] [PubMed] [Google Scholar]
- Norberg (1988).Norberg RA. Theory of growth geometry of plants and self-thinning of plant populations: geometric similarity elastic similarity, and different growth modes of plant parts. The American Naturalist. 1988;131:220–256. doi: 10.1086/284787. [DOI] [Google Scholar]
- Norström et al. (2009).Norström AV, Nyström M, Lokrantz J, Folke C. Alternative states on coral reefs: beyond coral-macroalgal phase shifts. Marine Ecology Progress Series. 2009;376:295–306. doi: 10.3354/meps07815. [DOI] [Google Scholar]
- Petes et al. (2003).Petes LE, Harvell CD, Peters EC, Webb MAH, Mullen KM. Pathogens compromise reproduction and induce melanization in Caribbean sea fans. Marine Ecology Progress Series. 2003;264:167–171. doi: 10.3354/meps264167. [DOI] [Google Scholar]
- Prada, Weil & Yoshioka (2010).Prada C, Weil E, Yoshioka PM. Octocoral bleaching during unusual thermal stress. Coral Reefs. 2010;29:41–45. doi: 10.1007/s00338-009-0547-z. [DOI] [Google Scholar]
- Privitera-Johnson, Lenz & Edmunds (2015).Privitera-Johnson K, Lenz EA, Edmunds PJ. Density-associated recruitment in octocoral communities in St. John, US Virgin Islands. Journal of Experimental Marine Biology and Ecology. 2015;473:103–109. doi: 10.1016/j.jembe.2015.08.006. [DOI] [Google Scholar]
- Rey-Villiers et al. (2020).Rey-Villiers N, Sánchez A, Caballero-Aragón, González-Díaz Spatio temporal variation in octocoral assemblages along water quality gradient in the northwestern region of Cuba. Marine Pollution Bulletin. 2020;153:110981. doi: 10.1016/j.marpolbul.2020.110981. [DOI] [PubMed] [Google Scholar]
- Rossi et al. (2017).Rossi S, Bramanti L, Gori A, Orejas C. Animal forests of the world: an overview. In: Rossi S, Bramanti L, Gori A, Orjas C, editors. Marine animal forests the ecology of benthic biodiversity hotspots. Springer International Publishing; Cham: 2017. pp. 1–28. [DOI] [Google Scholar]
- Ruzicka et al. (2013).Ruzicka RR, Colella MA, Porter JW, Morrison JM, Kidney JA, Brinkhuis V, Lunz KS, Macaulay KA, Bartlett LA, Meyers MK, Colee J. Temporal changes in benthic assemblages on Florida Keys reefs 11 years after the 1997/1998 El Niño. Marine Ecology Progress Series. 2013;489:125–141. doi: 10.3354/meps10427. [DOI] [Google Scholar]
- Smith et al. (1996).Smith GW, Ives LD, Nagelkerken IA, Ritchie KB. Caribbean sea-fan mortalities. Nature. 1996;383:487. doi: 10.1038/383487a0. [DOI] [Google Scholar]
- Tsounis & Edmunds (2017).Tsounis G, Edmunds PJ. Three decades of coral reef community dynamics in St. John, USVI: a contrast of scleractinians and octocorals. Ecosphere. 2017;8:eo1646. doi: 10.1002/ecs2.1646. [DOI] [Google Scholar]
- Tsounis et al. (2018).Tsounis G, Edmunds PJ, Bramanti L, Gambrel B, Lasker HR. Variability of size structure and species composition Caribbean octocoral communities under contrasting environmental conditions. Marine Biology. 2018;165:1–14. doi: 10.1007/s00227-018-32. [DOI] [Google Scholar]
- Tsounis, Steele & Edmunds (2020).Tsounis G, Steele MA, Edmunds PJ. Elevated feeding rates of fishes within octocoral canopies on Caribbean reefs. Coral Reefs. 2020;39:1299–1311. [Google Scholar]
- Wainwright & Dillon (1969).Wainwright SA, Dillon JR. On the Orientation of sea fans (genus Gorgonia) 1969;139:130–139. doi: 10.2307/1539674. [DOI] [Google Scholar]
- Weller (1987).Weller DE. A reevaluation of the -3/2 power rule of plant self-thinning. Ecological Monographs. 1987;57:23–43. doi: 10.2307/1942637. [DOI] [Google Scholar]
- Wells et al. (in review).Wells CD, Martinez-Quintana A, Tonra KJ, Lasker HR. Welcome to the jungle: algal turf negatively affects recruitment of a Caribbean octocoral. bioRxiv. doi: 10.1101/2020.05.27.119404. [DOI]
- Westoby (1981).Westoby M. The place of the self-thinning rule in population dynamics. The American Naturalist. 1981;118:581–587. doi: 10.1086/283853. [DOI] [Google Scholar]
- Westoby (1984).Westoby M. The self-thinning rule. Advances in Ecological Research. 1984;14:167–225. doi: 10.1016/S0065-2504(08)60171-3. [DOI] [Google Scholar]
- White (1981).White J. The allometric interpretation of the self-thinning rule. Journal of Theoretical Biology. 1981;89:475–500. doi: 10.1016/0022-5193(81)90363-5. [DOI] [Google Scholar]
- Wild et al. (2011).Wild C, Hoegh-Guldberg O, Naumann MS, Colombo-Pallotta MF, Ateweberhan M, Fitt WK, Iglesias-Prieto R, Palmer C, Bythell JC, Ortiz JC, Loya Y, VanWoesik R. Climate change impedes scleractinian corals as primary reef ecosystem engineers. Marine & Freshwater Research. 2011;62:205–215. doi: 10.1071/MF10254. [DOI] [Google Scholar]
- Yoda et al. (1963).Yoda K, Kira T, Ogawa H, Hozumi K. Self-thinning in overcrowded pure stands under cultivated and natural conditions. Journal of the Institute of Polytechnics, Osaka City University Series D Biology. 1963;14:107–129. [Google Scholar]
- Yoshioka (1994).Yoshioka PM. Size-specific life history pattern of shallow-water gorgonian. Journal of Experimental Marine Biology and Ecology. 1994;184:111–122. doi: 10.1016/0022-0981(94)90169-4. [DOI] [Google Scholar]
- Yoshioka (1996).Yoshioka PM. Variable recruitment and its effects on the population and community structure of shallow-water gorgonians. Bulletin of Marine Science. 1996;59:433–443. [Google Scholar]
- Yoshioka (2009).Yoshioka PM. Sediment transport and the distribution of shallow-water gorgonians. Caribbean Journal of Science. 2009;45:254–259. doi: 10.18475/cjos.v45i2.a12. [DOI] [Google Scholar]
- Yoshioka & Yoshioka (1987).Yoshioka PM, Yoshioka BB. Variable effects of Hurricane David on shallow water Gorgonians of Puerto Rico. Bulletin of Marine Science. 1987;40:132–144. [Google Scholar]
- Yoshioka & Yoshioka (1989).Yoshioka PM, Yoshioka BB. Effects of wave energy, topographic relief and sediment transport on the distribution of shallow-water gorgonians of Puerto Rico. Coral Reefs. 1989;8:145–152. doi: 10.1007/BF00338270. [DOI] [Google Scholar]
- Yoshioka & Yoshioka (1991).Yoshioka PM, Yoshioka BB. A comparison of the survivorship and growth of shallow-water gorgonian species of Puerto Rico. Marine Ecology Progress Series. 1991;69:253–260. doi: 10.3354/meps069253. [DOI] [Google Scholar]
- Zuluaga-Montero & Sabat (2012).Zuluaga-Montero A, Sabat AM. Spatial variability of disease incidence and mortality in the sea fan Gorgonia ventalina in Puerto Rico (Alcyonacea: Gorgoniidae) Revista de Biología Tropical. 2012;60:517–526. doi: 10.15517/rbt.v60i2.3910. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The height of all colonies along three transects that were surveyed art Yawzi Point and Tektite from 2013 to 2019. They can be used to calculate mean density (using transects as replicates), mean heights (using colonies as replicates) and mean growth (using colonies that were relocated in consecutive years).
Data Availability Statement
The following information was supplied regarding data availability:
The raw data is available in the Supplemental File and at BCO-DMO (https://www.bco-dmo.org/): DOI: 10.26008/1912/bco-dmo.827851.1 and 10.26008/1912/bco-dmo.827897.1.