Abstract
Jatropha curcas L. belongs to Euphorbiaceae family, and it synthesizes flavonoid and diterpene compounds that have showed antioxidant, anti-inflammatory, anticancer, antiviral, antimicrobial, antifungal and insecticide activity. Seeds of this plant accumulate phorbol esters, which are tigliane type diterpenes, reported as toxic and, depending on its concentration, toxic and non-toxic varieties has been identified. The aim of this work was to characterize the chemical profile of the extracts from seeds, leaves and callus of both varieties (toxic and non-toxic) of Jatropha curcas, to verify the presence of important compounds in dedifferentiated cells and consider the possibility of using these cultures for the massive production of metabolites. Callus induction was obtained using NAA (1.5 mg L−1) and BAP (1.5 mg L−1) after 21 d for both varieties. Thin layer chromatography analysis showed differences in compounds accumulation in callus from non-toxic variety throughout the time of culture, diterpenes showed an increase along the time, in contrast with flavonoids which decreased. Based on the results obtained through microQTOF-QII spectrometer it is suggested a higher accumulation of phorbol esters, derived from 12-deoxy-16-hydroxy-phorbol (m/z 365 [M+H]+), in callus of 38 d than those of 14 d culture, from both varieties. Unlike flavonoids accumulation, the MS chromatograms analysis allowed to suggest lower accumulation of flavonoids as the culture time progresses, in callus from both varieties. The presence of six glycosylated flavonoids is also suggested in leaf and callus extracts derived from both varieties (toxic and non-toxic), including: apigenin 6-C-α-L-arabinopyranosyl-8-C-β-D-xylopyranoside (m/z 535 [M+H]+), apigenin 4′-O-rhamnoside (m/z 417 [M+H]+), vitexin (m/z 433 [M+H]+), vitexin 4′-O-glucoside-2″-O-rhamnoside (m/z 741 [M+H]+), vicenin-2 (m/z 595 [M+H]+), and vicenin-2,6″-O-glucoside (m/z 757 [M+H]+).
Keywords: Jatropha curcas, micrOTOF Q-II, Callus, Phorbol esters, Glycosilated flavonoids
Introduction
Jatropha curcas L. (Euphorbiaceae) is a multipurpose plant native to Mesoamerica, and it is important because of its usefulness as raw material in biofuels production (Salvador-Figueroa et al., 2015) as well as in veterinary and human traditional medicine (Zhang et al., 2017). Several compounds with different biological activities have been isolated from different species of Jatropha (Ferreira-Rodrigues et al., 2016; Katagi et al., 2016). The identification of biologically active compounds extracted from different organs of this plant has been reported (Prasad, Izam & Khan, 2012; Sharma, Dhamija & Parashar, 2012). Isolated compounds or whole plant extracts have been studied because of their potential pharmacological activity (Cocan et al., 2018). Biological effects of J. curcas include antibacterial (Rampadarath, Puchooa & Jeewon, 2016), cytotoxic (Katagi et al., 2016), anti-inflammatory (Salim et al., 2018), and antifungal effects (Abdelgader, Suleiman & Ali, 2019; Srinivasan, Palanisamy & Mulpuri, 2019). Most research on J. curcas have been done with toxic varieties; toxicity is referred to phorbol esters content in seeds.
In Mexico, Brazil and India, non-toxic varieties of this species have been identified with very low or non-detectable levels of phorbol esters (PEs) in seeds (Laviola et al., 2010; Martínez-Herrera, Chel-Guerrero & Martínez-Ayala, 2004; Kumar, Anand & Reddy, 2011). PEs are known as Jatropha factors because each one of them has the same nucleus diterpene moiety, namely, 12-deoxy-16-hydroxy-phorbol (DHP) which is coupled to unstables intramolecular diterpenes (named C1–C6 factors) (Hirota et al., 2017).
Plants are the most successful source of chemical compounds, and their potential mode of action makes them an alternative phytomedicinal drug, since several natural products have shown benefits against human diseases (Aye et al., 2019). Several compounds are tissue-specific accumulated, and are usually structurally complex (Armaly et al., 2015). Therefore, it is necessary the use of chemical analysis techniques to isolate and identify the extracted plant metabolites (Hernandez & Sarlah, 2019). There are a few cases where the use of the plant cell culture of Jatropha curcas has allowed the production of bioactive compounds (Alvero-Bascos & Ungson, 2012; Mahalakshmi, Eganathan & Parida, 2013; Nassar et al., 2013; Zaragoza-Martínez et al., 2016); the study of the culture at different stages of toxic and non-toxic varieties generates the opportunity to design biotechnological models for production of bioactive compounds i.e., terpenoids, alkaloids, flavonoids (Abdelgadir & Van Staden, 2013; Sabandar et al., 2013), providing opportunities for new drugs discovery.
Secondary metabolites are generally in complex matrices at very low concentrations in plant organs, and lower in dedifferentiated cells. These compounds have a wide range of polarities; therefore, it is necessary the use of solvents with different polarity to obtain the extracts (Chemat et al., 2019). The aim of this work was to characterize the chemical profile of the extracts from callus of both varieties (toxic and non-toxic) of Jatropha curcas, through the cell culture, to verify the presence of important compounds in dedifferentiated cells and consider the possibility of using these cultures for the massive production of bioactive compounds.
Materials & Methods
Plant material
Seeds and young leaves of Jatropha curcas were collected. Non-toxic variety samples from Centro de Desarrollo de Productos Bióticos-IPN, Yautepec, Morelos, México (18°53′09″N, 99°03′38″W). The toxic variety samples were collected from Campo Experimental Zacatepec, Instituto Nacional de Investigaciones Agrícolas y Pecuarias (INIFAP), Zacatepec, Morelos, México (18°39′23″N, 99°11′28″W).
To induce cell dedifferentiation, two different explants were surface-sterilized according to Vanegas-Espinoza et al. (2002). Leaf blade of approximately 0.25 cm2 and petiole of approximately three mm in length were cultured in MS medium (Murashige & Skoog, 1962) supplemented with sucrose (30 g L−1), phytagel (3 g L−1) (Sigma-Aldrich®). Since there are no reports of the induction of dedifferentiated cells in the varieties analyzed in this study, the combinations of three concentrations (0.0, 1.5 and 3.0 mg L−1) of naphthaleneacetic acid (NAA) and 6-benzyl-aminopurine (BAP) were evaluated according to Verma (2013), pH was adjusted to 5.7, media were sterilized at 121 °C for 15 min. Ten explants per Petri dish with 3 repetitions per treatment were incubated at 25 ± 2 °C, photoperiod of 16 h light/8 h darkness for 35 d (Kumar et al., 2015). Explants dedifferentiation was recorded every seven days using a stereoscopic microscope (Nikon, model SMZ 1500, Japan). In order to observe differences in accumulation of compounds during callus development, completely dedifferentiated cells were cultured under the above described conditions for 38 d, samples were taken on days 0, 2 and every 4 d thereafter.
Fresh washed leaves were indoors dried at 25 ± 2 °C during 3 weeks. Seeds without tegument and callus, were oven dried at 50 °C for 48 h, dried samples were ground with a mortar and sieved through a mesh size 53 µm.
Ultrasound assisted extraction (UAE)
UAE was performed with an ultrasound bath Branson (2510R-MTH, CT, USA) with automatic control of time and temperature and ultrasound frequency of 40 kHz. 500 mg of grounded biomass dry weight (dw) were placed into a 50 mL borosilicate glass conical Erlenmeyer flask, then 20 mL of ethanol 80% (v/v) were added, and sonicated at 40 ± 5 °C during 30 min (Pandey et al., 2018; Dumitraşcu et al., 2019). During sonication flasks were suspended in the water without contact with the bottom of the ultrasonic bath, subsequently they were vortexed. Supernatant was filtered, concentrated to dryness at 25 ± 2 °C, and solubilized in 500 µL of HPLC grade MeOH (Sigma-Aldrich®) for chromatographic analysis (Saeed et al., 2006; Liu et al., 2013).
Phorbol esters (PEs) rich defatted extract
500 mg of dried sample were packed in a filter paper cartridge and defatted in a Soxhlet equipment with petroleum ether (60−80 °C) (Sigma-Aldrich®) for 4 h. Petroleum ether (Fermont®) extract was concentrated using rotary evaporator at 40 °C, 90 rpm, and 900 mbar. The methyl esters in the resulting oil, were extracted with MeOH, later filtered and concentrated to dryness at 25 ± 2 °C, then solubilized in 500 µL of HPLC grade MeOH for chromatographic analysis (Demissie & Lele, 2010).
Thin layer chromatography
Extracts were applied on normal phase silica plates (Merck Millipore, 60 F254, Germany). Chloroform-methanol (94:6 and 75:25) were used as mobile phase, reference standards were phorbol-12-myristate 13-acetate (PMA, Sigma-Aldrich®), quercetin, and vitexin (Sigma-Aldrich®), both plates were revealed with anisaldehyde (Kathiravan & Raman, 2010).
To analyze extracts obtained by sonication-ethanol 80% and Soxhlet-methanol a mobile phase consisting of chloroform-methanol (97:3) was used. The reference standard was PMA, and the plates were cerium sulfate-revealed, then observed at 366 nm, and white light. Retention factor (Rf) and color from the spots were compared with chromatographic terpenes profiles described by Reich & Schibli (2007).
MicrOTOF Q-II analysis
Electrospray ionization analysis (ESI) was performed using a micrOTOF-Q II mass spectrometer (Bruker Daltonics, Bremen, Germany) according to León-López et al. (2015). Samples were solubilized in 500 µL of HPLC grade MeOH and filtered with a syringe filter (nylon membrane, 0.45 µm, Agilent Technologies, Santa Clara, CA, USA). The molecular ions related to the extracts were analyzed in positive ion mode (ESI +). 20 µL of sample were directly injected into the evaporation chamber, capillary potential was −4.5 kV, gas temperature of 200 °C, drying gas flow of 4 L min−1 and nebulizer gas pressure of 0.4 Bar. Detection was performed at 50–3000 m/z. The predictive structures of the MS/MS partitioning profile were established utilizing the Competitive Fragmentation Modeling for Metabolite Identification (CFM-ID. Version 3.0, 2019) platform from Wishart-lab (http://cfmid3.wishartlab.com), which is referred to in the PubChem-NCBI site. Relative abundance was calculated according to Scigelova et al. (2011).
Results
Establishment of callus culture
Dedifferentiation cell was not observed in leaf blade explants. Petiole explants showed tissue dedifferentiation since seventh day of culture and complete process was evident at the day 21 (Fig. 1). Friable and light green callus was obtained on MS media added with both combinations: NAA (1.5 mg L−1), BAP (1.5 mg L−1), and NAA (3.0 mg L−1) and BAP (3.0 mg L−1).
Figure 1. Cell dedifferentiation of petiole explants from both toxic and non-toxic varieties of Jatropha curcas.
(A-D) Explants from non-toxic variety throughout dedifferentiation experiment (0, 7, 14, and 21 d, respectively), (E-H) Explants from toxic variety throughout dedifferentiation experiment (0, 7, 14, and 21 d, respectively). Both induced on MS culture medium added with NAA (1.5 mg.L-1) and BAP (1.5 mg.L-1).
Thin layer chromatography (TLC) analysis
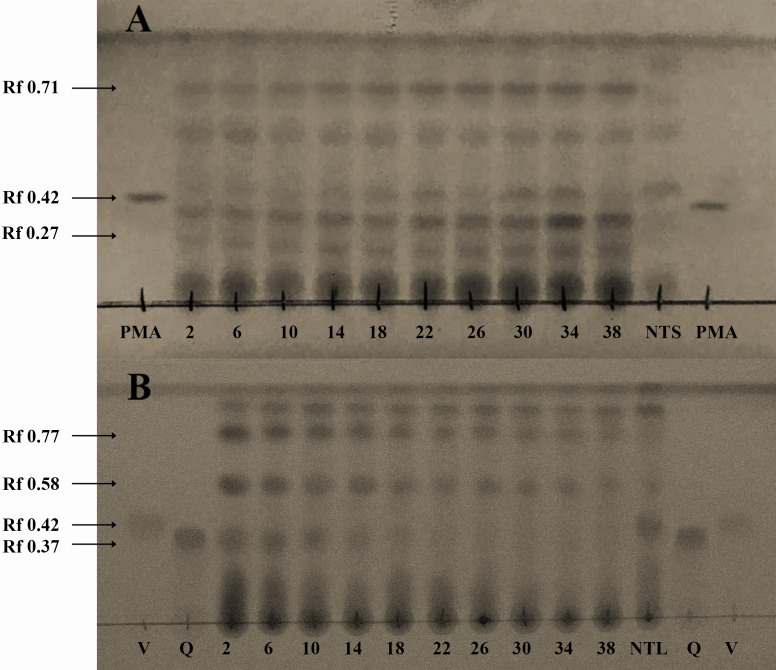
TLC showed differences in compounds accumulation during time culture (2, 6, 10, 14, 18, 22, 26, 30, 34 and 38 d). Regard diterpenes, spots with Rf of 0.71 and 0.27 showed higher intensity along this period (Fig. 2A), unlike flavonoids in which spots with Rf of 0.77 and 0.58, decreased throughout the same culture period (Fig. 2B). These results suggest that the accumulation of diterpenes and flavonoids was inversely related during callus development. To obtain diterpenes the Soxhlet-methanol extraction was more efficient than sonication-ethanol 80%. TLC analysis of extracts obtained by both methods evidenced differences in the size and intensity of spots in regard to: extraction method, variety (toxic and non-toxic), and plant material (seeds, leaves and callus) (Fig. S1).
Figure 2. Identification of both diterpenes-type (A), and flavonoids-type (B) compounds in seeds, leaves, and callus of Jatropha curcas, through thin layer chromatography.
Lanes from 2 to 38 correspond to extracts of callus of non-toxic variety throughout 38 d of culture, NTS= Non- toxic variety-seeds, PMA= Phorbol-12-myristate-13-acetate (Sigma) reference standard (Rf 0.42), V= Vitexin (Sigma) reference standard (Rf 0.42), and Q= Quercetin (Sigma) reference standard (Rf 0.37). A) The spots intensity increased throughout to culture time (Rfs 0.71, and 0.27), mobile phase chloroform-methanol (94:6). B) The spots intensity decreased throughout to culture time (Rfs 0.77, and 0.58), mobile phase chloroform-methanol (75:25). Plates were revealed with anisaldehyde.
MicrOTOF Q-II and competitive fragmentation modeling for metabolite identification platform (CFM-ID)
Phorbol esters (PEs) analysis
Fragmentation profile analysis from seeds extract from both varieties showed several highs signals one of them with m/z of 365 [M+H]+ corresponding to 12-deoxy-16-hydroxy-phorbol (DHP), which is the fundamental structural core of the PEs. The MS/MS analysis of this molecular ion showed fragments with m/z of 295, 276, 234, 203, 185 and 127 [M+H]+ which is similar to the fragmentation profile of DHP presented in CFM-ID platform (Fig. 3), this suggests the identification of that molecular structure in all of the extracts obtained from seeds and callus of both toxic and non-toxic varieties. Based on signals intensities from 14 d and 38 d callus extracts from both varieties, it is suggested that the accumulation of DHP is time-dependent. Since, the corresponding signal was higher in callus of 38 d than in those of 14 d (Fig. 4). Furthermore, two signals with m/z of 547 and 591 [M+H]+ were observed, so it is proposed that they are related with the fragmentation profile of the signal with m/z of 711 [M+H]+ corresponding to any of the Jatropha factors (C1 or DHPB to C6) which nucleus structure is DHP (Wink et al., 2000; Haas, Sterk & Mittelbach, 2002) (Fig. S2). Table 1 shows the relative abundance of DHP molecular ion (m/z 365 [M+H]+) on 14 d and 38 d callus extracts from both varieties, evidencing the increment of these compounds through the callus development.
Figure 3. Spectrophotometrical analysis of phorbol esters in extracts of Jatropha curcas seeds.
MS/MS fragmentation profile of the molecular ion m/z 365 [M+H]+ related to 12-deoxy-16-hydroxy-phorbol, which is the structural core from Jatropha curcas-phorbol esters (referred as Jatropha factors). Predictive structures obtained through CFM-ID platform from each ionized fragment.
Figure 4. Mass spectra from callus extracts of J. curcas showing the relative intensity of the molecular ion m/z 365 [M+H]+ related to the structural core of the Jatropha-phorbol esters.
Callus extracts from toxic variety: (A) 14 d of culture; (B) 38 d of culture; non-toxic variety: (C) 14 d of culture, (D) 38 d of culture. The relative intensity from molecular ion m/z 365 [M+H]+ increased throughout culture time.
Table 1. Tentative compounds identified by ESI-MS in hydroalcoholic extracts from seeds, leaves, and callus of 14 and 38 d of culture from both toxic and non-toxic Jatropha curcas L. varieties.
| Compound type/ name | Elemental composition | Mass | Fragment ions in positive ion mode (m/z) | Plant material | Time of culture (d) | Variety | Relative abundance (%) |
|---|---|---|---|---|---|---|---|
| Phorbol | |||||||
| 12-deoxy-16-hydroxy-phorbol | C20H28O6 | 364.4 | 127, 185, 203, 234, 276, 295 | ||||
| Seeds | T | 64.70 | |||||
| NT | 21.05 | ||||||
| Callus | 14 | T | 14.28 | ||||
| NT | 8.69 | ||||||
| 38 | T | 30.00 | |||||
| NT | 25.00 | ||||||
| Glycosylated Flavonoids | |||||||
| Apigenin 6-C- α-L-arabinopyranosyl-8-C- β-D-xylopyranoside (m/z 535 [M+H]+), and Apigenin 4′-O-rhamnoside (m/z 417 [M+H]+) | C25H26O13 and C21H20O9 | 534.47 and 416.4 | 381 | ||||
| Leaves | T | 45.83 | |||||
| NT | 100 | ||||||
| Callus | 14 | T | 100 | ||||
| NT | 100 | ||||||
| 38 | T | 100 | |||||
| NT | 62.50 | ||||||
| Vitexin (m/z 433 [M+H]+) | C21H20O10 | 432.37 | 415 | ||||
| Leaves | T | 27.7 | |||||
| NT | 70.00 | ||||||
| Callus | 14 | T | 10.34 | ||||
| NT | 4.76 | ||||||
| 38 | T | 11.11 | |||||
| NT | <12.50 | ||||||
| Vitexin 4′-O-glucoside-2″-O-rhamnoside (m/z 741 [M+H]+) | C33H40O19 | 740.7 | 577 | ||||
| Leaves | T | 33.3 | |||||
| NT | 42.84 | ||||||
| Callus | 14 | T | 1.42 | ||||
| NT | 6.36 | ||||||
| 38 | T | 12.50 | |||||
| NT | 8.75 | ||||||
| Vicenin-2 (m/z 595 [M+H]+) | C27H30O15 | 594.5 | 503 | ||||
| Leaves | T | 25.00 | |||||
| NT | 29.16 | ||||||
| Callus | 14 | T | 7.14 | ||||
| NT | 4.00 | ||||||
| 38 | T | 6.25 | |||||
| NT | 3.75 | ||||||
| Vicenin-2,6″-O-glucoside (m/z 757 [M+H]+) | C33H40O20 | 756.7 | 757 | ||||
| Leaves | T | <9.09 | |||||
| NT | 8.00 | ||||||
| Callus | 14 | T | 1.42 | ||||
| NT | 1.73 | ||||||
| 38 | T | 2.22 | |||||
| NT | 1.25 | ||||||
Notes.
- T
- Toxic
- NT
- Non-toxic
Flavonoids analysis
On the other hand, the main group of compounds in Jatropha leaf extracts are flavonoids, among them the apigenin, nevertheless, it is important to refer that the natural condition of flavonoids in the plants is in glycosylated form. On this regard, another of the highest signals observed at the chromatograms was the m/z of 381 [M+H]+ ion, the MS-MS experiment of this signal and the proposed structures obtained by CFM-ID platform allowed to relate that molecular ion (m/z 381 [M+H]+) to the fragmentation profiles of apigenin 6-C-α-L-arabinopyranosyl-8-C- β-D-xylopyranoside, and of apigenin 4′-O-rhamnoside (Fig. 5). The fragmentation signals and their corresponding predictive structure were also related for vitexin (m/z 433 [M+H]+), vitexin 4′-O-glucoside-2″-O-rhamnoside (m/z 741 [M+H]+), vicenin-2 (m/z 595 [M+H]+), and vicenin-2,6″-O-glucoside m/z 757 [M+H]+ (Fig. S3). Table 1 shows the relative abundance of six tentatively identified compounds by relating their molecular ions on 14 d and 38 d callus extracts from both varieties. Inversely to observed on DHP related signal (m/z 365 [M+H]+), the intensity of the molecular ion related with glycosylated apigenin (m/z 381 [M+H]+) diminished (Fig. 6).
Figure 5. Fragmentation profile (MS/MS) of the molecular ion m/z 381 [M+H]+, observed in leaves extracts, and related to fragmentation of two glycosylated apigenin (apigenin (6-C- α-L-arabinopyranosyl-8-C-β-D-xylopyranoside m/z 535 [M+H]+, apigenin 4’-O-rhamnoside m/z 417 [M+H]+)).
(A) Fragmentation profile of the molecular ion m/z 381 [M+H]+; (B) Apigenin 6-C-α-L-arabinopyranosyl-8-C-β-D-xylopyranoside m/z 535 [M+H]+, and Apigenin 4’-O-rhamnoside m/z 417 [M+H]+; (C) structures predicted to each molecular ion (381, 355, 335, and 219 m/z), obtained from CFM-ID platform.
Figure 6. Mass spectra of callus extracts from both toxic and non-toxic varieties of J. curcas at 14 and 38 d culture, showing the relative intensity of the molecular ion m/z 381 [M+H]+ related to the fragmentation profile from two glycosylated apigenin.
(A and C) Extracts of J. curcas callus from J. curcas-toxic variety (14 and 38 d, respectively). (B and D) Extracts of J. curcas callus from non-toxic variety (14 and 38 d, respectively). The relative intensity from molecular ion m/z 381 [M+H]+ diminished throughout culture time.
Discussion
The highest callus induction (95.5%) was observed in petiole explants on MS medium added with NAA (3.0 mg L−1) and BAP (3.0 mg L−1), the second best result (87.7%) was obtained with NAA (1.5 mg L−1) and BAP (1.5 mg L−1), in contrast to reported by Nassar et al. (2013), who observed dedifferentiation with NAA and BAP at 0.5 mg L−1 of each one plant growth regulator. Explants dedifferentiation reported in this work was similar to reported by Kumar et al. (2015). The follow up of the explants dedifferentiation process, every 7 d showed callus formation on explants starting on the seventh day. Dedifferentiation began at the cutting sites as expected (Sujatha, Makkar & Becker, 2005; Nogueira et al., 2011; Ovando-Medina et al., 2016). The callus obtained was light green and friable, similar to reported by Hernández et al. (2015). It has been reported that high auxins concentrations could affect production and accumulation of secondary metabolites (Kim et al., 2007), hence, according to our results, it is suggested the use of the lowest effective concentration, 1.5 mg L−1 for both growth regulators. Muñoz-Valverde et al. (2003) concluded that BAP is determinant to induce callus formation in foliar explants of J. curcas. Likewise, Suárez & Salgado (2008) reported that the presence of NAA induce callus formation in Stevia rebaudiana, and this effect could be increased when adding BAP. On the other hand, Solange et al. (2002) determined that the use of NAA and BAP in equal proportion induces callus formation from leaf explants of Tridax procumbens. Coutiño-Cortés et al. (2013) reported the callus induction in J. curcas leaf explants at 10 d of culture, and total explant-cell dedifferentiation at 20 d using 2, 4-D, BAP and KIN, while in this work, petioles dedifferentiation started at 7 d and total explant-cell dedifferentiation was achieved at 21 d. These results support that synergy between NAA and BAP is essential to achieve a high dedifferentiation degree. Stable callus culture conditions for the two varieties of Jatropha curcas were established.
The PEs are responsible for the toxicity in the plant (Devappa, Makkar & Becker, 2011; Sabandar et al., 2013; Zhang et al., 2017). There are varieties of Jatropha curcas denominated as toxic and non-toxic (Makkar et al., 1997). The non-toxic varieties have PEs concentration lower than 0.86 mg/g of seed on dry basis (He et al., 2011). Martínez-Herrera et al. (2006) detected high levels of PEs in seed oil from the municipality of Coatzacoalcos, Veracruz, México, but did not detect PEs in seeds from the municipality of Yautepec, Morelos, México. This corroborates the differences between the seeds of the two varieties used in this study.
Regard, to TLC profile analysis, it has been reported that methanolic extraction from seed-oil facilitates separation and availability of methyl ester type compounds, mainly phorbol esters (PEs) (Demissie & Lele, 2010; Devappa, Bingham & Khanal, 2013). The detection by TLC of PEs in seed methanolic extracts from toxic and non-toxic J. curcas varieties was reported (Devappa, Makkar & Becker (2012), they reported higher spots intensity from toxic variety than from non-toxic, when plates were observed at 366 nm UV light, this result is similar to that observed in this work (Fig. S1). Makkar & Becker (2009) detected higher PEs accumulation in seeds than in leaves extracts. Similar results were obtained in this work, even with different method of extraction. Nevertheless, these results are different of that obtained by Martínez-Herrera, Chel-Guerrero & Martínez-Ayala (2004), because they reported 96% of PEs extraction through hydroalcoholic extraction, quantified by HPLC; while, in this work the intensity of the spots was higher on Soxhlet-methanol extracts than hydroalcoholic extraction (Fig. S1). Using TLC, differences between dedifferentiated cell extracts of both varieties of Jatropha curcas were evidenced.
On the other hand, Hirota et al. (2017) reported the identification of DHP as the fundamental structural core which is derived from 12-deoxy-16-hydroxy-phorbol-4′-[12′,14′-butadienyl]-6′-[16′,18′,20′-nonatrienyl]- bicycle [3.1.0] hexane-(13-O)-2′-[carboxylate]- (16-O)-3′- [8′-butenoic-10′]ate (DHPB or Jatropha factor C1), identified as DHPB-Na adduct m/z 733 [M+Na]+. Furthermore, DHPB m/z 711 [M+H]+ and DHP m/z of 365 [M+H]+ were also reported in J. curcas seeds (Wink et al., 2000). Even more so, Haas, Sterk & Mittelbach (2002) reported the identification of diterpenes named Jatropha factors C2 to C 6through ESI-MS m/z 711 [M+H]+ and of DHP (m/z of 365 [M+H] +). Furthermore, Nishshanka et al. (2016) identified six phorbol esters in J. curcas seeds by LC-MS, which have the same core (DHP), at the so named Jatropha factors (C1 to C6).
Regard to PEs identification by ESI-MS analysis, Verardo et al. (2019) identified six phorbol esters in J. curcas seeds with m/z of 711 [M+H]+, which have the same fundamental structural core (DHP) m/z of 365 [M+H]+ which is coupled to diterpenes of 24 carbon structures named Jatropha factors from C1 (DHPB) to C6. The relative intensity of the molecular ion m/z 365 [M+H] + was higher in seeds extracts from toxic variety, than in seed extracts from non-toxic variety (Data not shown). While in callus, the relative intensity is higher in toxic and non-toxic varieties callus of 38 d of culture (Figs. 4B, and 4D, than in toxic and non-toxic varieties callus of 14 d of culture (Figs. 4A, and 4C). These results could suggest the presence of PEs coupled to DHP in the samples analyzed and that their accumulation is differential in regard to the variety-derived cell culture and throughout the time of culture. These results suggest that their accumulation of DHP is time dependent. This ESI-MS analysis allowed to corroborate the results obtained by TLC (Fig. 2A). Nevertheless, the relative intensity of the signals observed in extracts from callus were lower than that obtained from seeds extracts as reported by Demissie & Lele (2010). By ESI-MS, differences in the relative intensity of the signal corresponding to DHP were observed between the callus extracts of both varieties, being higher in the toxic variety in addition to that in calluses at 38 d of culture, it was higher than in the 14 d.
By other hand, phenolic compounds are ubiquitously produced by plants (Kumar & Goel, 2019), the main role of phenols in plants is to protect them from biotic or abiotic stress (Pereira, 2016). These properties include antimicrobial, insecticidal, antiparasitic, antiviral, anti-ulcerogeBynic, cytotoxic, antioxidant, anti-hepatotoxic, anti-hypertensive and anti-inflammatory activities (Oskoueian et al., 2011; Papalia, Barreca & Panuccio, 2017). Flavonoids are recognized as polyphenols. Several of them have been identified in Jatropha genus, such as apigenin glycosides, vitexin, and isovitexin which have been considered as chemiotaxonomic compounds from the genus (Abdelgadir &Van Staden, 2013; Huang et al., 2014).
The tentative identification of glycosylates-flavonoids through microQTOF-QII has been already reported (Pezzini et al., 2019). In this regard Xie et al. (2003) reported the apigenin 6-C- α-L-arabinopyranosyl-8-C- β-D-xylopyranoside m/z 535 [M+H]+. Likewise, this result may be related to that obtained by Abd-Alla et al. (2009) who identified apigenin and its aglycone as majoritarian flavonoids in J. curcas leaves, as well as, that obtained by Reena, Nand & Sharma (2008) who reported to apigenin as major flavonoid in the same species. Those reports differ from that published by Papalia, Barreca & Panuccio (2017) who identified to vitexin and vicenin-2 as the majoritarian flavonoids.
The results obtained by microQTOF-QII of the molecular ion m/z 381 [M+H]+ through the MS/MS experiment, and the predictive structures obtained through the CFM-ID platform allowed to suggest the relation of the structures from the molecular ion m/z 381 [M+H]+ with the fragmentation profile from apigenin 6-C- α-L-arabinopyranosyl-8-C- β-D-xylopyranoside m/z 535 [M+H]+, which was identified through ESI-MS in Viola yedoensis (Xie et al., 2003) and apigenin 4′-O-rhamnoside m/z 417 [M+H] +, which was identified in Olea europaea (Pieroni et al., 1996).
Based on the molecular ion, MS-MS fragmentation profile and the predictive structures obtained by CFM-ID platform, it is suggested the tentative identification of vitexin m/z of 433 [M+H]+, vicenin-2 m/z of 595 [M+H]+, and vitexin 4′-O-glucoside-2″-O-rhamnoside m/z of 741 [M+H]+ in leaves and callus from both varieties. These results are similar to obtained by Huang et al. (2014) who identified vitexin m/z of 433 [M+H]+ in J. curcas leaves. This flavonoid was also identified by ESI-MS in Parkinsonea aculeata m/z of 431 [M-H]− (Hassan, Abdelaziz & Al Yousef, 2019). In this work it is also suggested the tentative identification of vicenin-2,6″-O-glucoside m/z 757 [M+H]+ which has not been reported to Jatropha curcas, but to Stell aria holostea (Bouillant et al. 1984) (Fig. S2). By the MS-MS fragmentation profile, the identification of six glycosylated flavonoids is suggested, it was observed that relative intensities signals related to flavonoid related molecular ion m/z 381 [M+H]+ in callus of 14 d was higher than callus of 38 d, differences that were not observed between calluses of the different varieties.
Conclusions
Stable dedifferentiated cells culture from petiole explants of Jathopha curcas, were stablished from toxic and non-toxic varieties on MS medium added with NAA and BAP. Thin layer chromatography and mass spectrometry, suggest an inverse relationship between phorbol esters and flavonoids accumulation in callus throughout the time of culture. The tentative identification of diterpene type compounds such as 12-deoxy-16-hydroxy-phorbol and Jatropha factors by ESI-MS in seed and callus (14 and 38 d), as well as, the presence of six flavonoids glycosides in leaf and callus, in extracts from both toxic and non-toxic varieties of J. curcas, is suggested. Both of them, groups of compounds reported with bioactive activity with pharmaceutic/agroindistrial potential.
Supplemental Information
The extracts obtained with ethanol 80% - sonication are referred with numbers (1 8). The extracts obtained with Soxhlet methanol are referred with letters (A H). PMA: Phorbol-12-myristate-13-acetate Rf 0.22 (Sigma, PE reference standard). Toxic variety seed (1 and A), Non-toxic variety seed (2 and B), Toxic variety leaves (3 and C), Non-toxic variety leaves (4 and D), Toxic variety-callus 14 d (5 and E), Toxic variety-callus 38 d (6 and F), Non-toxic variety-callus 14 d (7 and G), Non-toxic variety-callus 38 d (8 and H). Mobile phase chloroform-methanol (97:3), cerium sulfate-revealed, observed at 366 nm UV light.
Toxic variety-callus 14 d extract (A), toxic variety-callus 38 d extract (B), non-toxic variety-callus 14 d extract (C), and non-toxic variety-callus 38 d extract (D).
It is included the predictive structure corresponding to vicenin-2,6”-O-Glucoside m/z 757 [M+H]+ which is not reported to, but it is to Stellaria holostea.
Acknowledgments
We recognize Centro de Nanociencias y Micro y Nanotecnologías (CNMN-IPN) for the experimental service with the micrOTOF-Q II spectrometer. IPN). We thank Dra. Silvia Evangelista Lozano from Centro de Desarrollo de Productos Bióticos (CEPROBI-IPN), for providing the non-toxic variety plant material, thanks also to Dr. Edwin Javier Barrios Gómez from Instituto Nacional de Investigaciones Forestales, Agrícolas y Pecuarias (INIFAP-Mor, Mexico), for facilitating the toxic variety plant material.
Funding Statement
This work was supported by Instituto Politécnico Nacional (IPN/COFAA/SIP-20195486). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Additional Information and Declarations
Competing Interests
The authors declare there are no competing interests.
Author Contributions
Gerardo Leyva-Padrón performed the experiments, prepared figures and/or tables, and approved the final draft.
Pablo Emilio Vanegas-Espinoza performed the experiments, analyzed the data, authored or reviewed drafts of the paper, and approved the final draft.
Alma Angélica Del Villar-Martínez conceived and designed the experiments, analyzed the data, authored or reviewed drafts of the paper, and approved the final draft.
Crescencio Bazaldua conceived and designed the experiments, analyzed the data, prepared figures and/or tables, authored or reviewed drafts of the paper, and approved the final draft.
Data Availability
The following information was supplied regarding data availability:
The raw data is available in the Supplemental Files.
References
- Abd-Alla et al. (2009).Abd-Alla HI, Moharram FA, Gaara AH, El-Safty MM. Phytoconstituents of Jatropha curcas L. leaves and their immunomodulatory activity on humoral and cell-mediated immune response in chicks. Zeitschrift für Naturforschung C. 2009;64(7–8):495–501. doi: 10.1515/znc-2009-7-805. [DOI] [PubMed] [Google Scholar]
- Abdelgader, Suleiman & Ali (2019).Abdelgader MGM, Suleiman EA, Ali SI. Study of Jatropha curcas as antifungal agent. International Journal of Current Medical and Pharmaceutical Research. 2019;5(5):4202–4210. doi: 10.24327/23956429.ijcmpr201905657. [DOI] [Google Scholar]
- Abdelgadir & Van Staden (2013).Abdelgadir HA, Van Staden J. Ethnobotany, ethnopharmacology and toxicity of Jatropha curcas L. (Euphorbiaceae): a review. South African Journal of Botany. 2013;88:204–218. doi: 10.1016/j.sajb.2013.07.021. [DOI] [Google Scholar]
- Alvero-Bascos & Ungson (2012).Alvero-Bascos EM, Ungson LB. Ultraviolet-B (UV-B) radiation as an elicitor of flavonoids production in callus cultures of jatropha (Jatropha curcas L.) Philippine Agricultural Scientist. 2012;95(4):335–343. [Google Scholar]
- Armaly et al. (2015).Armaly AM, DePorre YC, Groso EJ, Riehl PS, Schindler CS. Discovery of novel synthetic methodologies and reagents during natural product synthesis in the post-palytoxin era. Chemical Reviews. 2015;115(17):9232–9276. doi: 10.1021/acs.chemrev.5b00034. [DOI] [PubMed] [Google Scholar]
- Aye et al. (2019).Aye MM, Aung HT, Sein MM, Armijos C. A review on the phytochemistry, medicinal properties and pharmacological activities of 15 selected Myanmar medicinal plants. Molecules. 2019;24(2):293. doi: 10.3390/molecules24020293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chemat et al. (2019).Chemat F, Abert-Vian M, Fabiano-Tixier AS, Strube J, Uhlenbrock L, Gunjevic V, Cravotto G. Green extraction of natural products. Origins, current status, and future challenges. TrAC Trends in Analytical Chemistry. 2019;118:248–263. doi: 10.1016/j.trac.2019.05.037. [DOI] [Google Scholar]
- Cocan et al. (2018).Cocan I, Alexa E, Danciu C, Radulov I, Galuscan A, Obistioiu D, Dehelean CA. Phytochemical screening and biological activity of Lamiaceae family plant extracts. Experimental and Therapeutic Medicine. 2018;15(2):1863–1870. doi: 10.3892/etm.2017.5640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coutiño-Cortés et al. (2013).Coutiño-Cortés AG, Ovando-Medina I, Adriano-Anaya ML, Salvador-Figueroa M, Ruiz-González S. Organogénesis de Jatropha curcas a partir de plantas adultas: Estudio de fitohormonas y factores fisicoquímicos. Quehacer Científico en Chiapas. 2013;8(2):1–9. [Google Scholar]
- Demissie & Lele (2010).Demissie AG, Lele SS. Bioassay-assisted identification of phorbol ester from Jatropha curcas (Linn.) tissue culture. International Journal of Pharma and Bio Sciences. 2010;1(3):1–7. [Google Scholar]
- Devappa, Bingham & Khanal (2013).Devappa RK, Bingham JP, Khanal SK. High performance liquid chromatography method for rapid quantification of phorbol esters in Jatropha curcas seed. Industrial Crops and Products. 2013;49:211–219. doi: 10.1016/j.indcrop.2013.04.044. [DOI] [Google Scholar]
- Devappa, Makkar & Becker (2011).Devappa RK, Makkar HP, Becker K. Jatropha diterpenes: a review. Journal of the American Oil Chemists’ Society. 2011;88(3):301–322. doi: 10.1007/s11746-010-1720-9. [DOI] [Google Scholar]
- Devappa, Makkar & Becker (2012).Devappa RK, Makkar HP, Becker K. Localisation of antinutrients and qualitative identification of toxic components in Jatropha curcas seed. Journal of the Science of Food and Agriculture. 2012;92(7):1519–1525. doi: 10.1002/jsfa.4736. [DOI] [PubMed] [Google Scholar]
- Dumitraşcu et al. (2019).Dumitraşcu L, Enachi E, Stänciuc N, Aprodu J. Optimization of ultrasound assisted extraction of phenolic compounds from cornelian cherry fruits using response surface methodology. CyTA-Journal of Food. 2019;17(1):814–823. doi: 10.1080/19476337.2019.1659418. [DOI] [Google Scholar]
- Ferreira-Rodrigues et al. (2016).Ferreira-Rodrigues SC, Rodrigues CM, Dos Santos MG, Gautuz JAA, Silva MG, Cogo JC, Oshima-Franco Y. Anti-inflammatory and antibothropic properties of Jatropha elliptica, a plant from brazilian cerrado biome. Advanced Pharmaceutical Bulletin. 2016;6(4):573–579. doi: 10.15171/apb.2016.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas, Sterk & Mittelbach (2002).Haas W, Sterk H, Mittelbach M. Novel 12-Deoxy-16-hydroxyphorbol diesters isolated from the seed oil of Jatropha curcas. Journal of Natural Products. 2002;65(10):1434–1440. doi: 10.1021/np020060d. [DOI] [PubMed] [Google Scholar]
- Hassan, Abdelaziz & Al Yousef (2019).Hassan WH, Abdelaziz S, Al Yousef HM. Chemical composition and biological activities of the aqueous araction of Parkinsonea aculeata L. growing in Saudi Arabia. Arabian Journal of Chemistry. 2019;12(3):377–387. doi: 10.1016/j.arabjc.2018.08.003. [DOI] [Google Scholar]
- He et al. (2011).He W, King AJ, Khan MA, Cuevas JA, Ramiaramanana D, Graham IA. Analysis of seed phorbol-ester and curcin content together with genetic diversity in multiple provenances of Jatropha curcas L. from Madagascar and Mexico. Plant Physiology and Biochemistry. 2011;49(10):1183–1190. doi: 10.1016/j.plaphy.2011.07.006. [DOI] [PubMed] [Google Scholar]
- Hernández et al. (2015).Hernández LR, Mendiola MAR, Castro CA, Gutiérrez-Miceli FA. Effect of plant growth regulators on fatty acids composition in Jatropha curcas L. callus culture. Journal of Oleo Science. 2015;64(3):325–330. doi: 10.5650/jos.ess14206. [DOI] [PubMed] [Google Scholar]
- Hernandez & Sarlah (2019).Hernandez LW, Sarlah D. Empowering synthesis of complex natural products. Chemistry–a European Journal. 2019;25(58):13248–13270. doi: 10.1002/chem.201901808. [DOI] [PubMed] [Google Scholar]
- Hirota et al. (2017).Hirota F, Maitree S, Anchalee R, Keisuke L, Pornngarm L. Sonthaya U, Masami S. Phorbol esters in seed oil of Jatropha curcas L. (saboodam in Thai) and their association with cancer prevention: from the initial investigation to the present topics. Journal of Cancer Research and Clinical Oncology. 2017;143:1359–1369. doi: 10.1007/s00432-017-2341-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang et al. (2014).Huang Q, Guo Y, Fu R, Peng T, Zhang Y, Chen F. Antioxidant activity of flavonoids from leaves of Jatropha curcas. Science Asia. 2014;40:193–197. doi: 10.2306/scienceasia1513-1874.2014.40.193. [DOI] [Google Scholar]
- Katagi et al. (2016).Katagi A, Sui L, Kamitori K, Suzuki T, Katayama T, Hossain A, Tokuda M. Inhibitory effect of isoamericanol A from Jatropha curcas seeds on the growth of MCF-7 human breast cancer cell line by G2/M cell cycle arrest. Heliyon. 2016;2(1):e00055. doi: 10.1016/j.heliyon.2015.e00055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kathiravan & Raman (2010).Kathiravan G, Raman VS. In vitro taxol production, by Pestalotiopsis breviseta-a first report. Fitoterapia. 2010;81(6):557–564. doi: 10.1016/j.fitote.2010.01.021. [DOI] [PubMed] [Google Scholar]
- Kim et al. (2007).Kim YS, Yeung EC, Hahn EJ, Paek KY. Combined effects of phytohormone, indole-3-butyric acid, and methyl jasmonate on root growth and ginsenoside production in adventitious root cultures of Panax ginseng CA Meyer. Biotechnology Letters. 2007;29(11):1789–1792. doi: 10.1007/s10529-007-9442-2. [DOI] [PubMed] [Google Scholar]
- Kumar, Anand & Reddy (2011).Kumar N, Anand KV, Reddy MP. In vitro regeneration from petiole explants of non-toxic Jatropha curcas. Industrial Crops and Products. 2011;33(1):146–151. doi: 10.1016/j.indcrop.2010.09.013. [DOI] [Google Scholar]
- Kumar & Goel (2019).Kumar N, Goel N. Phenolic acids: natural versatile molecules with promising therapeutic applications. Biotechnology Reports. 2019:e00370. doi: 10.1016/j.btre.2019.e00370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar et al. (2015).Kumar S, Kumar V, Sharma MK, Kumar N, Kumar A, Tomar KPS, Jaiswal N. Effects of different plant growth regulators on in vitro callus induction in physic nut (Jatropha curcas L.) Journal of Applied and Natural Science. 2015;7(1):30–37. doi: 10.31018/jans.v7i1.559. [DOI] [Google Scholar]
- Laviola et al. (2010).Laviola BG, Rocha RB, Kobayashi AK, Rosado TB, Bhering LL. Genetic improvement of Jatropha for biodiesel production. Ceiba. 2010;51(1):1–10. doi: 10.5377/ceiba.v51i1.640. [DOI] [Google Scholar]
- León-López et al. (2015).León-López L, Márquez-Mota CC, Velázquez-Villegas LA, Gálvez-Mariscal A, Arrieta-Báez D, Dávila-Ortíz G, Torres N. Jatropha curcas protein concentrate stimulates insulin signaling, lipogenesis, protein synthesis and the PKCα pathway in rat liver. Plant Foods for Human Nutrition. 2015;70(3):351–356. doi: 10.1007/s11130-015-0502-9. [DOI] [PubMed] [Google Scholar]
- Liu et al. (2013).Liu B, Ma Y, Liu Y, Yang Z, Zhang L. Ultrasonic-assisted extraction and antioxidant activity of flavonoids from Adinandra nitida leaves. Tropical Journal of Pharmaceutical Research. 2013;12(6):1045–1051. doi: 10.4314/tjpr.v12i6.27. [DOI] [Google Scholar]
- Mahalakshmi, Eganathan & Parida (2013).Mahalakshmi R, Eganathan P, Parida AK. Salicylic acid elicitation on production of secondary metabolites by cell cultures of Jatropha curcas L. International Journal of Pharmacy and Pharmaceutical Science. 2013;5(4):655–659. [Google Scholar]
- Makkar & Becker (2009).Makkar HP, Becker K. Jatropha curcas, a promising crop for the generation of biodiesel and value-added coproducts. European Journal of Lipid Science and Technology. 2009;111(8):773–787. doi: 10.1002/ejlt.200800244. [DOI] [Google Scholar]
- Makkar et al. (1997).Makkar HPS, Becker K, Sporer F, Wink M. Studies on nutritive potential and toxic constituents of different provenances of Jatropha curcas. Journal of Agricultural and Food Chemistry. 1997;45(8):3152–3157. doi: 10.1021/jf970036j. [DOI] [Google Scholar]
- Martínez-Herrera, Chel-Guerrero & Martínez-Ayala (2004).Martínez-Herrera J, Chel-Guerrero L, Martínez-Ayala AL. The nutritional potential of Mexican piñon (Jatropha curcas). Toxic and antinutritional factors. Publication-European Association for Animal Production. 2004;110:185–188. doi: 10.3920/978-90-8686-524-6. [DOI] [Google Scholar]
- Martínez-Herrera et al. (2006).Martínez-Herrera J, Siddhuraju P, Francis G, Dávila-Ortíz G, Becker K. Chemical composition, toxic/antimetabolic constituents, and effects of different treatments on their levels, in four provenances of Jatropha curcas L. from Mexico. Food Chemistry. 2006;96(1):80–89. doi: 10.1016/j.foodchem.2005.01.059. [DOI] [Google Scholar]
- Murashige & Skoog (1962).Murashige T, Skoog F. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiologia Plantarum. 1962;15(3):473–497. doi: 10.1111/j.1399-3054.1962.tb08052.x. [DOI] [Google Scholar]
- Nassar et al. (2013).Nassar SA, El-Ahmady SH, Nassar AH, Al-Azizi MM. Studying the possible biotransformation of the cytotoxic diterpenoid paclitaxel using Jatropha curcas cell suspension culture. European Journal of Medicinal Plants. 2013;3(2):241–253. doi: 10.9734/EJMP/2013/2881. [DOI] [Google Scholar]
- Nishshanka et al. (2016).Nishshanka U, Jayasuriya H, Chattopadhaya C, Kijak PJ, Chu PS, Reimschuessel R, De Alwis HG. Screening for toxic phorbol esters in jerky pet treat products using LC–MS. Journal of Chromatography B. 2016;1020:90–95. doi: 10.1016/j.jchromb.2016.03.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nogueira et al. (2011).Nogueira AR, Soares AA, Ibrahim AB, Campos FA. Analysis of organogenic competence of cotyledons of Jatropha curcas and their in vitro histological behavior. African Journal of Biotechnology. 2011;10(54):11249–11258. doi: 10.5897/AJB11.978. [DOI] [Google Scholar]
- Oskoueian et al. (2011).Oskoueian E, Abdullah N, Ahmad S, Saad WZ, Omar AR, Ho YW. Bioactive compounds and biological activities of Jatropha curcas L. kernel meal extract. International Journal of Molecular Sciences. 2011;12(9):5955–5970. doi: 10.3390/ijms12095955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ovando-Medina et al. (2016).Ovando-Medina I, Pérez-Díaz LP, Ruiz-González S, Salvador-Figueroa M, Urbina-Reyes ME, Adriano-Anaya L. Production of cytotoxic compounds in dedifferentiated cells of Jatropha curcas L. (Euphorbiaceae) PeerJ. 2016;4:e2616. doi: 10.7717/peerj.2616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey et al. (2018).Pandey A, Belwal T, Sekar TC, Bhatt ID, Rawa RS. Optimization of ultrasonic-assisted (UAE) of phenolics and antioxidant compounds from rhizomes of Rheum moorcroftianum using response surface methodology (RSM) Industrial Crops and Products. 2018;119(1):28–225. doi: 10.1016/j.indcrop.2018.04.019. [DOI] [Google Scholar]
- Papalia, Barreca & Panuccio (2017).Papalia T, Barreca D, Panuccio MR. Assessment of antioxidant and cytoprotective potential of Jatropha (Jatropha curcas) grown in Southern Italy. International Journal of Molecular Sciences. 2017;18(3):660. doi: 10.3390/ijms18030660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira (2016).Pereira A. Plant abiotic stress challenges from the changing environment. Frontiers in Plant Science. 2016;7:1123. doi: 10.3389/fpls.2016.01123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pezzini et al. (2019).Pezzini V, Agostini F, Smiderle F, Touguinha L, Salvador M, Moura S. Grape juice by-products extracted by ultrasound and microwave-assisted with different solvents: a rich chemical composition. Food Science and Biotechnology. 2019;28(3):691–699. doi: 10.1007/s10068-018-0531-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pieroni et al. (1996).Pieroni A, Heimler D, Pieters L, Van Poel B, Vlietinck AJ. In vitro anti-complementary activity of flavonoids from oliva (Olea europaea L.) leaves. Pharmazie. 1996;51(10):765–767. [PubMed] [Google Scholar]
- Prasad, Izam & Khan (2012).Prasad DR, Izam A, Khan MMR. Jatropha curcas: plant of medical benefits. Journal of Medicinal Plants Research. 2012;6(14):2691–2699. doi: 10.5897/JMPR10.977. [DOI] [Google Scholar]
- Rampadarath, Puchooa & Jeewon (2016).Rampadarath S, Puchooa D, Jeewon R. Jatropha curcas L: phytochemical, antimicrobial and larvicidal properties. Asian Pacific Journal of Tropical Biomedicine. 2016;6(10):858–865. doi: 10.1016/j.apjtb.2016.01.019. [DOI] [Google Scholar]
- Reena, Nand & Sharma (2008).Reena T, Nand KS, Sharma PB. Therapeutic biology of Jatropha curcas: a mini review. Current Pharmaceutical Biotechnology. 2008;9(4):315–324. doi: 10.2174/138920108785161505. [DOI] [PubMed] [Google Scholar]
- Reich & Schibli (2007).Reich E, Schibli A. High-performance thin-layer chromatography for the analysis of medicinal plants. Thieme Medical Publishers Inc.; New York, NY: 2007. [Google Scholar]
- Sabandar et al. (2013).Sabandar CW, Ahmat N, Jaafar FM, Sahidin I. Medicinal property, phytochemistry and pharmacology of several Jatropha species (Euphorbiaceae): a review. Phytochemistry. 2013;85:7–29. doi: 10.1016/j.phytochem.2012.10.009. [DOI] [PubMed] [Google Scholar]
- Saeed et al. (2006).Saeed MK, Deng Y, Perveen Z, Ahmad W, Dai R, Yu Y. Proceedings of the 2006 WSEAS international conference on cellular and molecular biology, biophysics and bioengineering. 2006. Optimal recovery of apigenin from Torreya grandis by extraction, fractionation and structure elucidation; pp. 32–38. [Google Scholar]
- Salim et al. (2018).Salim MN, Masyitha D, Harris A, Balqis U, Iskandar CD, Hambal M. Anti-inflammatory activity of Jatropha curcas Linn, latex in cream formulation on CD68 expression in mice skin wound. Veterinary World. 2018;11(2):99–103. doi: 10.14202/vetworld.2018.99-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvador-Figueroa et al. (2015).Salvador-Figueroa M, Magaña Ramos J, Vázquez-Ovando JA, Adriano-Anaya ML, Ovando-Medina I. Genetic diversity and structure of Jatropha curcas L. in its centre of origin. Plant Genetic Resources. 2015;13(1):9–17. doi: 10.1017/S1479262114000550. [DOI] [Google Scholar]
- Scigelova et al. (2011).Scigelova M, Hornshaw M, Giannakopoulos A, Makarov A. Fourier transform mass spectrometry. Molecular & Cellular Proteomics. 2011;10(7):M111.009431. doi: 10.1074/mcp.M111.009431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma, Dhamija & Parashar (2012).Sharma S, Dhamija HK, Parashar B. Jatropha curcas: a review. Asian Journal of Research in Pharmaceutical Science. 2012;2(3):107–111. [Google Scholar]
- Solange et al. (2002).Solange E, Pereira J, Ramalho A, Arbex N, Cardoso M, Alves O. Indução de calos em erva-de-touro (Tridax procumbens L.) utilizando diferentes reguladores de crescimento e tipos de explantes. Ciencia e Agrotecnologia. 2002;26(2):301–308. [Google Scholar]
- Srinivasan, Palanisamy & Mulpuri (2019).Srinivasan N, Palanisamy K, Mulpuri S. Jatropha, Challenges for a New Energy Crop. Singapore: Springer; 2019. Jatropha: phytochemistry, pharmacology, and toxicology; pp. 415–435. [DOI] [Google Scholar]
- Suárez & Salgado (2008).Suárez IE, Salgado JA. Propagación in vitro de Stevia rebaudiana Bert. (Asteraceae-Eupatorieae) a través de organogénesis. Temas Agrarios. 2008;13(1):40–48. [Google Scholar]
- Sujatha, Makkar & Becker (2005).Sujatha M, Makkar HPS, Becker K. Shoot bud proliferation from axillary nodes and leaf sections of non-toxic Jatropha curcas L. Plant Growth Regulation. 2005;47(1):83–90. doi: 10.1007/s10725-005-0859-0. [DOI] [Google Scholar]
- Muñoz-Valverde et al. (2003).Muñoz-Valverde J, Valerín-Berrocal K, Alvarenga-Venutolo S, Alán-Fonseca E. Cultivo in vitro de tempate (Jatropha curcas) Revista Tecnología en Marcha. 2003;16(4):53–59. [Google Scholar]
- Vanegas-Espinoza et al. (2002).Vanegas-Espinoza P, Cruz-Hernández A, Valverde ME, Paredes-López O. Plant regeneration via organogenesis in marigold. Plant Cell, Tissue and Organ Culture. 2002;69(3):279–283. doi: 10.1023/A:1015610011374. [DOI] [Google Scholar]
- Verardo et al. (2019).Verardo G, Baldini M, Ferfuia C, Gorassini A. Rapid and selective screening for toxic phorbol esters in Jatropha curcas seed oil using high-performance liquid chromatography-electrospray ionization-tandem mass spectrometry. Journal of Chromatography A. 2019;1597:63–75. doi: 10.1016/j.chroma.2019.03.015. [DOI] [PubMed] [Google Scholar]
- Verma (2013).Verma KC. Micropropagation study of Jatropha curcas for enhancing shoot induction frequency. International Journal of Agriculture, Environment and Biotechnology. 2013;6(2):217–222. [Google Scholar]
- Wink et al. (2000).Wink M, Grimm C, Koschmieder C, Sporer F, Bergeot O. Sequestration of phorbol esters by the aposematically coloured bug Pachycoris klugii (Heteroptera: Scutelleridae) feeding on Jatropha curcas (Euphorbiaceae) Chemoecology. 2000;10(4):179–184. doi: 10.1007/PL00001820. [DOI] [Google Scholar]
- Xie et al. (2003).Xie C, Veitch NC, Houghton PJ, Simmonds MSJ. Flavone C-glycosides from Viola yedoensis Makino. Chemical and Pharmaceutical Bulletin. 2003;51(10):1204–1207. doi: 10.1248/cpb.51.1204. [DOI] [PubMed] [Google Scholar]
- Zaragoza-Martínez et al. (2016).Zaragoza-Martínez F, Lucho-Constantino GG, Ponce-Noyola T, Esparza-García F, Poggi-Varaldo H, Cerda-García-Rojas CM, Trejo-Tapia G, Ramos-Valdivia AC. Jasmonic acid stimulates the oxidative response and triterpene production in Jatropha curcas cell suspension cultures through mevalonate as biosynthetic precursor. Plant Cell, Tissues and Organ Culture. 2016;127(1):47–56. doi: 10.1007/s11240-016-1028-z. [DOI] [Google Scholar]
- Zhang et al. (2017).Zhang Y, Yang Q, Li CH, Ding M, Lv X, Tao CH, Yu H, Chen F, Ying Xu Y. Curcin C, a novel type I ribosome-inactivating protein from the post-germinating cotyledons of Jatropha curcas. Amino Acids. 2017;49:1619–1631. doi: 10.1007/s00726-017-2456-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The extracts obtained with ethanol 80% - sonication are referred with numbers (1 8). The extracts obtained with Soxhlet methanol are referred with letters (A H). PMA: Phorbol-12-myristate-13-acetate Rf 0.22 (Sigma, PE reference standard). Toxic variety seed (1 and A), Non-toxic variety seed (2 and B), Toxic variety leaves (3 and C), Non-toxic variety leaves (4 and D), Toxic variety-callus 14 d (5 and E), Toxic variety-callus 38 d (6 and F), Non-toxic variety-callus 14 d (7 and G), Non-toxic variety-callus 38 d (8 and H). Mobile phase chloroform-methanol (97:3), cerium sulfate-revealed, observed at 366 nm UV light.
Toxic variety-callus 14 d extract (A), toxic variety-callus 38 d extract (B), non-toxic variety-callus 14 d extract (C), and non-toxic variety-callus 38 d extract (D).
It is included the predictive structure corresponding to vicenin-2,6”-O-Glucoside m/z 757 [M+H]+ which is not reported to, but it is to Stellaria holostea.
Data Availability Statement
The following information was supplied regarding data availability:
The raw data is available in the Supplemental Files.