Editor—Coronavirus disease 2019 (COVID-19) has affected 31.1 million people worldwide and caused more than 1.2 million deaths as of November 2020.1 The majority of critical care patients with COVID-19 pneumonia fulfil the Berlin definition of acute respiratory distress syndrome (ARDS) requiring invasive mechanical ventilation.2 Despite standard ARDS interventions with high positive end-expiratory pressure, prone positioning and lung protective ventilation, some patients remain profoundly hypoxaemic and hypercapnic.2 Inhaled nitric oxide (iNO) has been a rescue strategy used previously in ARDS, where it increases the PaO2/FiO2 ratio and reduces physiologic dead space fraction at 24 h, but without improving mortality or ICU length of stay.3 A dual role for iNO has been proposed in COVID-19 as an anti-viral agent and as a pulmonary vasodilator, especially given the ‘pulmonary vascular’ phenotype increasingly apparent in this disease.4 , 5
Whether patients with COVID-19 are ‘responders’ to iNO, and factors that predict potential responsiveness remain unknown. A recent publication in this journal6 described an observational series of 16 patients with COVID-19-related ARDS, in whom iNO was used as a rescue therapy for refractory hypoxaemia. The authors report a lesser improvement in the PaO2/FiO2 ratio after iNO initiation than in a historical (non-COVID-19) ARDS comparison cohort from their institution. They were unable to delineate any biochemical or physiological characteristics predictive of a positive response to iNO in COVID-19 ARDS patients. For comparison, we present our experience at one of the five Severe Acute Respiratory Failure centres in the UK. We report the effects of iNO in a population of patients with COVID-19-induced ARDS with refractory hypoxaemia and evaluate the predictors of iNO responsiveness in these patients.
ICU patients admitted to a tertiary respiratory failure centre in the UK receiving iNO with at least moderate ARDS (PaO2/FiO2 ratio <26.7 mm Hg/3.56 kPa)7 between March 2020 and May 2020 were included in this observational study. Data were collected on patient characteristics, respiratory physiology, baseline blood tests, and the use of ARDS interventions during the study period. Oxygenation and carbon dioxide clearance were evaluated before and in the first 24 h after initiating iNO. Measurements were taken as an average of three values at each time point from stored ventilator data to calculate PaO2/FiO2 ratio, oxygenation index (OI),8 and dead space fraction based on the Engelhoff modification of the Bohr equation.9 In patients who received prone positioning, follow-up measurements were taken in the same patient position. Responders to iNO were defined as those exhibiting an increase in PaO2/FiO2 ratio of >1.33 kPa at 24 h.10 Change in PaO2/FiO2 ratio, OI, and dead space fraction at 3 and 5 days, and in the 24 h before and after stopping iNO were also examined. Outcomes including length of stay and 30 day mortality were analysed.
Data are presented as mean (standard deviation) or median (inter-quartile range). For statistical analysis, we used GraphPad Prism (version 5; GraphPad, San Diego, CA, USA). Ethical approval for analysis of retrospective data was in place (A-CLUE 285452, IRAS Reference: 285452) through the Royal Brompton and Harefield Research Ethics Committee. All patients lacked capacity, and the need for individual informed consent was waived for retrospective analysis of data collected prospectively for routine care, without breach of privacy or anonymity.
Thirty-five consecutive patients (20% female) were included, with a mean age of 57.6 (8.1) yr and mean BMI of 30.8 (5.3) kg m−2, with 32 (91.4%) patients undergoing proning at the time of initiation of nitric oxide. Patients who were not being proned had right ventricular (RV) failure (one patient), recent cardiac arrest (one patient), or morbid obesity (one patient). Two of these patients required proning at a later date. Patients were treated with 20 ppm iNO for an average of 146.4 (80.8) h, with the exception of one patient treated with 40 ppm.
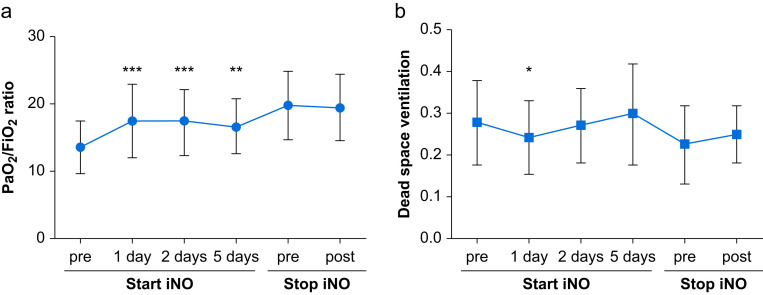
Within 24 h of iNO initiation, there was a significant increase in the PaO2/FiO2 ratio from baseline (13.6 [3.9] vs 17.4 [5.5] kPa, P<0.001) (Fig. 1 a), a reduction in OI (20.6 [15.2–24.0] vs. 14.4 [11.9–20.8], P<0.001) and a reduction in dead space fraction (0.28 [0.10] vs 0.24 [0.09], P=0.038) (Fig. 1b). The change in PaO2/FiO2 ratio and OI (data not shown), but not in dead space fraction, was preserved in those who survived to 48 h and 5 days. Compared with the immediate values before weaning, when stopping iNO there was no significant change in the PaO2/FiO2 ratio or dead space fraction (Fig. 1a and b).
Fig 1.
(a) PaO2/FiO2 (kPa) in the 24 h before and at time points after starting iNO in patients with moderately severe ARDS and Covid-19 infection. There was no significant rebound worsening in the PaO2/FiO2 ratio in the 24 h after stopping iNO. (b) Dead space fraction, defined using Bohr equation with Engelhoff modification, in the 24 h before and at time points after starting iNO in patients with moderately severe ARDS and Covid-19 infection. There was no significant difference between pre stopping and post stopping iNO. Values are average of three measurements at each time point. ∗Denotes a significant difference between this time point and pre-starting iNO. ∗P<0.05; ∗∗P<0.01; ∗∗∗P<0.001. PaO2/FiO2, partial pressure of arterial oxygen/fraction of inspired oxygen; ARDS, acute respiratory distress syndrome; iNO, inhaled nitric oxide. 159×73 mm (300×300 DPI).
According to the pre-defined improvement in the PaO2/FiO2 ratio by at least 1.33 kPa, 23 patients [65.7%] responded to iNO at 24 h. Responders had a significantly lower baseline PaO2/FiO2 ratio (12.1 [2.8] vs. 16.3 [4.4] kPa, P<0.01) and higher baseline OI (21.6 [6.3] vs. 16.1 [5.2], P<0.01) than non-responders. Responders to iNO also had higher baseline brain natriuretic peptide (BNP; available in n=16) (187 [84–529] ng L−1 vs 43 [34–112] ng L−1, P=0.023). Baseline high sensitivity (hs)-troponin levels (available in n=33) taken before initiation of iNO were not different between the groups overall (Supplementary Table S1). Taking twice the upper limit of our hs-troponin assay (>27 ng L−1), patients in the non-responder group all fell below this cut-off, compared with higher hs-troponin levels in responders (Fisher's exact test P=0.015). Of the 35 patients studied, two later required extra-corporeal membrane oxygenation, both at 3 days after iNO initiation. The 30 day mortality after starting iNO was 17/35 (48.5%).
This is the third report of the use of iNO in patients with ARDS caused by COVID-19,6 , 11 and the largest cohort reported to date. In contrast to these previous reports, we observed that in patients with at least moderately severe ARDS, iNO at 20 ppm improved oxygenation (PaO2/FiO2 ratio and OI) and ventilatory efficiency (dead space fraction), with 65.7% of patients responding to iNO at 24 h. We were also able to differentiate responders from non-responders on the basis of higher levels of the cardiac biomarkers BNP and hs-troponin. More responders had severe ARDS (17/23 responders vs three/12 non-responders).
Several factors may account for differences between our findings and those of Longobardo and colleagues,6 who reported only minimal improvement in PaO2/FiO2 ratio with iNO, and did not identify any patient characteristics associated with response to therapy. We routinely collect hs-troponin on all COVID-19 patients admitted to our institution. We used a dose of 20 ppm in all patients except one (who received 40 ppm), whereas the Longobardo cohort received 10–20 ppm. Finally, our cohort was enriched for patients with right ventricular dysfunction or pulmonary hypertension, with 23/35 patients having one or both on transthoracic echocardiography before iNO initiation. The use of iNO is widespread in the management of oxygenation and pulmonary hypertension during lung or heart transplantation, and as a treatment for acute RV failure after cardiotomy and cardiopulmonary bypass.12 , 13 It is plausible that ARDS patients with these characteristics would be more likely to derive benefit from iNO therapy.
iNO has been shown to improve oxygenation and reduce dead space ventilation in ARDS.3 Our data support its use as a rescue therapy in COVID-19 ARDS refractory to standard management including proning. Whether iNO confers a mortality benefit in patients with COVID-19 pneumonia remains to be seen, but the potential disease-modifying effect4 make it an attractive intervention for further study. Reassuringly in this small cohort on a variety of other treatments, there was no evidence of rebound hypoxaemia or hypercapnia after cessation of iNO therapy.
These data suggest the iNO may be beneficial in those patients with more severe hypoxaemia with raised BNP and hs-troponin, likely suggestive of RV strain. It is also likely that these patients have a pulmonary vascular phenotype, increasingly recognised in COVID-19 pneumonia.5 This specific phenotypic response to iNO support the interplay between pulmonary vascular blood flow and right heart function in COVID-19 pneumonia, with RV dysfunction increasingly reported,14 and associated with radiological signs suggesting pulmonary microthrombosis in severe cases.15
iNO may be helpful in patients with COVID-19 with refractory hypoxaemia despite standard interventions, especially in those with raised BNP and troponin. BNP and hs-troponin may be useful biomarkers for phenotypic enrichment for future clinical trials of iNO in COVID-19 ARDS.
Author's contributions
Study concept and design: BG, BP, SL, SJW, LP
Critical revision for important intellectual content: BG, BP, SL, SJW, LP
Data acquisition: CB, AV, CM, MB
Data analysis and interpretation: BG, LP
Drafting of the article: BG, LP
All authors contributed to, revised, and approved the final manuscript.
Acknowledgements
The authors acknowledge all staff at the Royal Brompton and Harefield Hospital ICU, the RBH ICU Pharmacist (Christopher Remmington) and the Pulmonary Hypertension Pharmacists (Daisy Keegan and Deepa Lakhani).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bja.2020.11.006.
Declarations of interest
BP declares personal fees from GSK and grants from Mermaid Care A/C, ESICM, RBHT charity, European Commission, Academy of Medical Sciences and Imperial College London COVID fund. SJW declares grants and personal fees from Janssen and Bayer and personal fees from GSK and MSD. LP declares personal fees from Actelion. The other authors declare that they have no conflict of interest.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.World Health Organisation. Available from: https://www.who.int/emergencies/diseases/novel-coronavirus-2019; Accessed 25 Oct 2020.
- 2.Phua J., Weng L., Ling L. Intensive care management of coronavirus disease 2019 (COVID-19): challenges and recommendations. Lancet Respir Med. 2020;8:506–517. doi: 10.1016/S2213-2600(20)30161-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gebistorf F., Karam O., Wetterslev J. Inhaled nitric oxide for acute respiratory distress syndrome (ARDS) in children and adults. Cochrane Database Syst Rev. 2016;2016:CD002787. doi: 10.1002/14651858.CD002787.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hedenstierna G., Chen L., Hedenstierna M. Treatment of COVID-19 by inhaled NO to reduce shunt? Am J Respir Crit Care Med. 2020;202:618. doi: 10.1164/rccm.202004-0940LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Patel B.V., Arachchillage D.J., Ridge C.A. Pulmonary angiopathy in severe COVID-19: physiologic, imaging and hematologic observations. Am J Respir Crit Care Med. 2020;202:690–699. doi: 10.1164/rccm.202004-1412OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Longobardo A., Montanari C., Shulman R. Inhaled nitric oxide minimally improves oxygenation in COVID-19 related acute respiratory distress syndrome. Br J Anaesth. 2020 doi: 10.1016/j.bja.2020.10.011. Advance Access published on October 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Force A.D.T., Ranieri V.M., Rubenfeld G.D. Acute respiratory distress syndrome: the Berlin definition. JAMA. 2012;307:2526–2533. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 8.Trachsel D., McCrindle B.W., Nakagawa S. Oxygenation index predicts outcome in children with acute hypoxemic respiratory failure. Am J Respir Crit Care Med. 2005;172:206–211. doi: 10.1164/rccm.200405-625OC. [DOI] [PubMed] [Google Scholar]
- 9.Nuckton T.J., Alonso J.A., Kallet R.H. Pulmonary dead-space fraction as a risk factor for death in the acute respiratory distress syndrome. N Engl J Med. 2002;346:1281–1286. doi: 10.1056/NEJMoa012835. [DOI] [PubMed] [Google Scholar]
- 10.Charron C., Repesse X., Bouferrache K. PaCO2 and alveolar dead space are more relevant than PaO2/FiO2 ratio in monitoring the respiratory response to prone position in ARDS patients: a physiological study. Crit Care. 2011;15:R175. doi: 10.1186/cc10324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ferrari M., Santini A., Protti A. Inhaled nitric oxide in mechanically ventilated patients with COVID-19. J Crit Care. 2020;60:159–160. doi: 10.1016/j.jcrc.2020.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Benedetto M., Romano R., Baca G. Inhaled nitric oxide in cardiac surgery: evidence or tradition? Nitric Oxide. 2015;49:67–79. doi: 10.1016/j.niox.2015.06.002. [DOI] [PubMed] [Google Scholar]
- 13.Schmid E.R., Bürki C., Engel M.H. Inhaled nitric oxide versus intravenous vasodilators in severe pulmonary hypertension after cardiac surgery. Anesth Analg. 1999;89:1108–1115. [PubMed] [Google Scholar]
- 14.Argulian E., Sud K., Vogel B. Right ventricular dilation in hospitalized patients with COVID-19 infection. JACC Cardiovasc Imaging. 2020;13:2459–2461. doi: 10.1016/j.jcmg.2020.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ridge C.A., Desai S., Jeyin N. Dual energy computed tomography pulmonary angiography (DECTPA) depicts early vascular disturbances in severe COVID-19 pneumonia. Radiol Cardiothorac Imaging. 2020;2 doi: 10.1148/ryct.2020200428. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.