Abstract
Recent studies showed that comorbidities such as diabetes, hypertension and obesity contribute to severe and worse outcomes of coronavirus disease 2019 (COVID-19), suggesting that metabolic syndrome and its components are associated with severity of COVID-19. Here, I systematically reviewed a possible association of metabolic syndrome with the susceptibility to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection and severity of COVID-19 by literature search. A population-based study and UK Biobank studies showed that patients with metabolic syndrome is highly susceptible to SARS-CoV-2 infection. Recent meta-analyses showed that metabolic syndrome is significantly associated with the development of severe COVID-19. Angiotensin-converting enzyme (ACE) 2 is the cellular entry receptor of SARS-CoV-2. Enhanced ACE2 expression, pre-existing endothelial dysfunction and procoagulant state induced by adipocytokines dysregulation in metabolic syndrome may play a crucial role for the development of severe COVID-19.
Keywords: Adipocytokines, COVID-19, Cytokines, Metabolic syndrome, Endothelial dysfunction
Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-Cov-2), which causes coronavirus disease 2019 (COVID-19) has reached a pandemic level. In a retrospective cohort study of COVID-19, comorbidities such as diabetes and hypertension were present in nearly half of patients [1]. The odds of in-hospital death were significantly higher in patients with diabetes and hypertension [1]. Among 383 COVID-19 Chinese patients, after adjusting for other risk factors, obesity was associated with about 2.4-fold higher odds for developing severe pneumonia, compared to patients with normal weight [2]. The components of metabolic syndrome seem to be associated with severe COVID-19. Here, I will describe a possible association of metabolic syndrome and its components with the susceptibility to SARS-CoV-2 infection and severity of COVID-19.
Metabolic Syndrome and Susceptibility to SARS-CoV-2 Infection
Ghoneim et al performed a population-based study to determine the relationship of metabolic syndrome and its components with the risk of COVID-19 [3]. They reviewed data from a large commercial database that aggregates electronic health records from 26 large nationwide healthcare systems. Out of 61.4 million active adult patients in the database, 8,885 (0.01%) had documented COVID-19. The cumulative incidence of COVID-19 was higher in patients with metabolic syndrome (odds ratio (OR), 7.00; 95% confidence interval (CI), 6.11 - 8.01). The adjusted OR of having COVID-19 was higher in patients with hypertension (OR, 2.53; 95% CI, 2.40 - 2.68), obesity (OR, 2.20; 95% CI, 2.10 - 2.32), diabetes (OR, 1.41; 95% CI, 1.33 - 1.48), hyperlipidemia (OR, 1.70; 95% CI, 1.56 - 1.74). This study showed that patients with metabolic syndrome and/or its each component is highly susceptible to SARS-CoV-2 infection.
Scalsky et al examined the effect of body mass index (BMI), lipid profiles, diabetes on the risk of testing positive for SARS-CoV-2 among 9,005 UK Biobank (UKBB) participants tested for SARS-CoV-2 from March 16 through June 29, 2020 [4]. BMI, type 2 diabetes and hemoglobin A1c (HbA1c) were associated with increased SARS-CoV-2 infection risk (P < 0.05) while high-density lipoprotein (HDL) and apolipoprotein A were associated with decreased SARS-CoV-2 infection risk (P < 0.001) [4]. This study also indicated a significant association of metabolic syndrome components such as obesity, diabetes and dyslipidemia with the susceptibility to SARS-CoV-2 infection.
Leong et al performed an inverse-variance weighted averages of variant-specific causal estimates for susceptibility, defined as people who tested positive for COVID-19 vs. population controls, and performed the analysis for BMI using effect estimates from UKBB [5]. Genetically increased BMI was causally associated with testing positive for COVID-19 (OR, 1.08; 95% CI, 1.03 - 1.13). Genetic evidence supported BMI as a causal risk factor for COVID-19 susceptibility.
Very recently, I studied the correlation of SARS-CoV-2 infection and the prevalence of overweight/obesity and mean BMI in the Top 50 SARS-CoV-2 endemic countries [6]. The prevalence of overweight and obesity, and mean BMI was significantly and positively correlated with the number of SARS-CoV-2 infected patients, showing a significant association between overweight/obesity and the susceptibility to SARS-CoV-2 infection by using the world-wide epidemiological data.
Angiotensin-converting enzyme (ACE) 2 is the cellular entry receptor of SARS-CoV-2 [7]. Increased ACE2 expression in chronic obstructive pulmonary disease (COPD) patients who are overweight compared to those not-overweight was observed [8], indicating that SARS-CoV-2 is more likely to enter into the human body in obese people as compared with non-obese people. Enhanced expression of ACE2 in metabolic syndrome induced by obesity may explain partly a significant association between metabolic syndrome and the susceptibility to SARS-CoV-2 infection.
Metabolic Syndrome and Severity of COVID-19
I searched the prevalence of diabetes and hypertension in all COVID-19 patients, severe and non-severe COVID-19 patients by using PubMed [9], and I found 12 studies. Six studies showed a statistically significantly higher prevalence of diabetes in severe patients than in non-severe patients [9]. In the meta-analysis, the prevalence of diabetes in severe patients was significantly higher than that in non-severe patients (OR, 3.52; 95% CI: 2.65 - 4.67). Five of 12 studies showed a statistically significantly higher prevalence of hypertension in severe patients than in non-severe patients [9]. In the meta-analysis, the prevalence of hypertension in severe patients was significantly higher than non-severe patients (OR, 2.69; 95% CI, 2.16 - 3.34). Further, I studied the prevalence of cardiovascular disease (CVD) in severe and non-severe COVID-19 patients. Four of 11 studies showed a statistically significantly higher prevalence of CVD in severe patients than in non-severe patients [9]. In the meta-analysis, the prevalence of CVD in severe patients was significantly higher than that in non-severe patients (OR, 5.37; 95% CI, 3.73 - 7.74). Our study demonstrated that diabetes, hypertension which are components of metabolic syndrome and CVD which is induced by metabolic syndrome are associated with the development of severe COVID-19.
A recent meta-analysis on the association between obesity and severe COVID-19 showed that COVID-19 patients with obesity were more severe and have a worse outcome than those without obesity (OR, 2.31; 95% CI, 1.3 - 4.12) [10]. Hariyanto et al systematically searched all articles published on COVID-19 and dyslipidemia by using PubMed [11]. A total of seven studies with a total of 6,922 patients were included in their analysis, and this meta-analysis showed that dyslipidemia is associated with severe COVID-19 (relative risk (RR), 1.39; 95% CI, 1.03 - 1.87; P = 0.03). Obesity which is the principal cause for metabolic syndrome and dyslipidemia which is the component of metabolic syndrome were also associated with the development of severe COVID-19.
Recently, I suggested the potential risks for severe COVID-19 in obesity before SARS-CoV-2 infection and the mechanisms for adiposity-mediated exacerbation of COVID-19 [12]. Expression of ACE2 is enhanced in obesity, which makes it possible that SARS-CoV-2 is more likely to enter into the human body in obese people as compared with non-obese people. The greater expression of ACE2 may induce more SARS-CoV-2 entry into the human body, which may increase cytokines release and result in exacerbation of COVID-19. ACE2 is present in lung alveolar epithelial cells, small intestinal epithelial cells, vascular endothelial cells and adipose tissue, and its expression is increased due to obesity [13]. The greater expression of ACE2 may also expand the distribution of SARS-CoV-2 in human body, inducing severe COVID-19. However, further studies are warranted to elucidate the association between enhanced ACE2 expression and severity of COVID-19.
Given that angiotensin receptor blockers (ARBs) and ACE inhibitors (ACEIs) upregulated ACE2 expression in animal studies, the concern might arise regarding whether ARBs and ACEIs would increase the morbidity and mortality of COVID-19 [14]. Recently, two meta-analyses on ARBs and ACEIs and outcomes in patients with COVID-19 were performed. Lo et al searched PubMed and CINAHL databases as well as pre-print servers for studies investigating usage of ACEIs/ARBs in patients with COVID-19 compared to a control group of COVID-19 patients without ACEI/ARB use [15]. Twenty-one studies were included in the meta-analysis. For mortality with ACEI/ARB use, the pooled OR was 1.29 (95% CI, 0.89 - 1.87; P = 0.18), and the pooled OR for COVID-19 severity was 0.94 (95% CI, 0.59 - 1.50; P = 0.81). In combining both mortality and severe disease outcomes, the pooled OR was 1.09 (95% CI, 0.80 - 1.48; P = 0.58). They concluded that the use of ACEI/ARB was not associated with increased mortality or severe COVID-19. In the meta-analysis by Grover et al, a search was conducted on PubMed, Google Scholar, EMBASE, and various preprint servers for studies comparing clinical outcomes and mortality in COVID-19 patients on ACEIs and/or ARBs [16]. A total of 16 studies were included for the meta-analysis. In a pooled analysis of four studies, there was a statistically non-significant association of ACEI/ARB use with lower odds of developing severe disease vs. non-users (OR, 0.81; 95% CI, 0.41 - 1.58; P = 0.53). In a pooled analysis of six studies, there was a statistically non-significant association of ACEI/ARB use with lower odds of mortality as compared with non-users (OR, 0.86; 95% CI, 0.53 - 1.41; P = 0.55). They concluded that ACEIs and ARBs should be continued in COVID-19 patients, reinforcing the recommendations made by several medical societies.
It has been proposed that the increase of secretion of interleukin-6 (IL-6) and tumor necrosis factor-alpha (TNF-α) by adipose tissue in obesity-induced insulin resistance, could underlie the associations of insulin resistance with endothelial dysfunction and coagulopathy [17]. Insulin resistance is associated with elevated concentrations of fibrinolytic inhibitor plasminogen activator inhibitor-1 (PAI-1), and increased expression and secretion of PAI-1 by hepatocytes and endothelial cells are induced by insulin, triglyceride-rich lipoproteins [17]. Von Willebrand factor (VWF) is also elevated in insulin-resistant states, suggesting the existence of insulin-resistance-induced endothelial dysfunction [17]. Elevation of inflammatory cytokines, endothelial dysfunction and procoagulant state already exist in obese people even before SARS-CoV-2 infection. SARS-CoV-2 infection may enhance elevation of inflammatory cytokines which leads to cytokine storm, and induce endothelial injury and thrombosis.
As shown in Figure 1 [10-12], recent meta-analyses showed that obesity, dyslipidemia, hypertension and diabetes, which are the components of metabolic syndrome, are strongly involved in the aggravation of COVID-19. In addition, my meta-analysis showed that CVD, which is caused by metabolic syndrome, is also involved in the aggravation of COVID-19.
Figure 1.
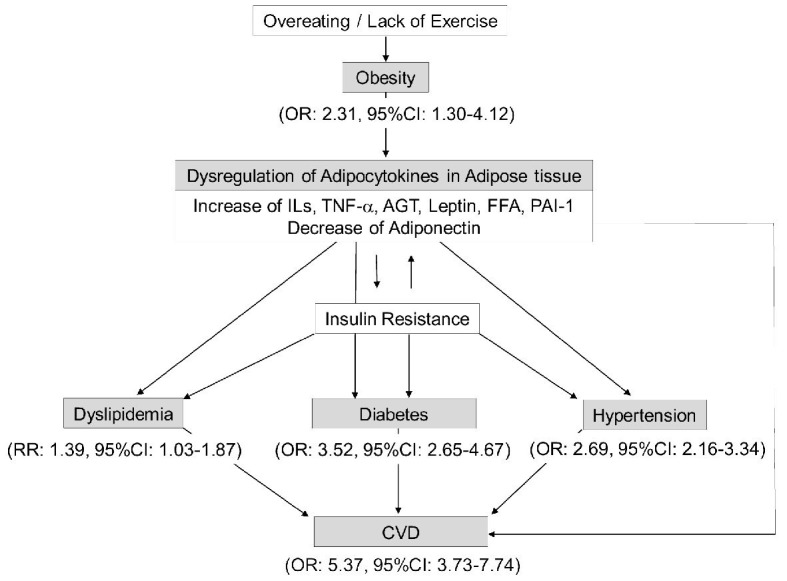
A significant association between metabolic syndrome and severity of COVID-19. OR (odds ratio) and RR (relative risk) indicate the risk for the development of severe COVID-19. AGT: angiotensinogen; CI: confidence interval; CVD: cardiovascular disease; FFA: free fatty acids; IL: interleukin; PAI-1: plasminogen activator inhibitor-1; TNF-α: tumor necrosis factor-alpha; COVID-19: coronavirus disease 2019.
Pre-existing endothelial dysfunction observed at early stage of atherosclerosis in patients with metabolic syndrome may play a crucial role for the development of severe COVID-19. Varga et al showed the presence of viral elements within endothelial cells and an accumulation of inflammatory cells, with evidence of endothelial and inflammatory cell death [18]. They suggested that COVID-19-endotheliitis could explain the systemic impaired microcirculatory function in different vascular beds and their clinical sequelae in patients with COVID-19 [18]. They proposed that the therapies to stabilize the endothelium while tackling viral replication could be particularly relevant for vulnerable patients with pre-existing endothelial dysfunction [18], supporting my hypothesis.
Systemic Severe Coagulopathic Vasculitis (SSCV) and Severe COVID-19
High levels of acute-phase proteins, very high levels of D-dimers (the marker for thrombosis), and absence of disseminated intravascular coagulation (DIC) have been observed in patients with severe COVID-19, suggesting the crosstalk between inflammation and coagulation in severe COVID-19 [19]. There appear to be high rates of venous thromboembolism and also, what has been poorly described before in acute lung injury, a high rate of pulmonary immune-thrombosis [19]. SSCV, thrombosis secondary to inflammation may play a crucial role in development of severe COVID-19 [12, 19].
Vascular endothelium influences not only the three classically interacting components of hemostasis: vessels, platelets and clotting and fibrinolytic systems of plasma, but also the natural sequelae: inflammation and tissue repair [20]. Under physiological conditions endothelium mediates vascular dilatation, prevents platelet adhesion and activation, blocks thrombin formation and mitigates fibrin deposition [20]. Endothelial injury due to inflammation induces completely opposing actions such as vasoconstriction, platelet and leukocyte activation and adhesion by upregulation of VWF and platelet activating factor (PAF), promotion of thrombin formation and coagulation and fibrin deposition at the vascular wall by upregulation of tissue factor (TF) and PAI-1 [20]. TNF-α expression has been reported to stimulate expression of TF, VWF and PAF, and release of PAI-1 [21].
Possible mechanisms for inflammatory cytokines-induced thrombosis were shown in Figure 2. PAF activates platelets, and TF and VWF are associated with elevation of coagulation and platelet adhesiveness, respectively. Further, PAI-1 decreases fibrinolysis. Inflammatory cytokines-induced vascular injury induces thrombosis via various cascades.
Figure 2.
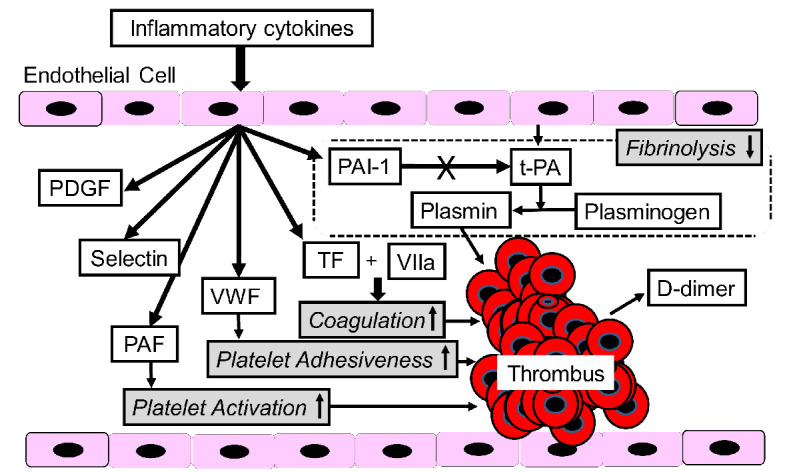
A mechanism for inflammatory cytokines-induced thrombosis formation. AGT: angiotensinogen; CI: confidence interval; FFA: free fatty acids; IL: interleukin; PAF: platelet-activating factor; PAI-1: plasminogen activator inhibitor-1; TF: tissue factor; t-PA: tissue-type plasminogen activator; PDGF: platelet-derived growth factor; VWF: von Willebrand factor.
Multivariable regression showed remarkably increasing odds of in-hospital death associated with D-dimer greater than 1 µg/mL (OR, 18.42; 95% CI, 2.64 - 128.55; P = 0.0033) on admission [1]. Significantly higher VWF, fibrinogen and D-dimer levels were observed in COVID-19 patients in intensive care unit (ICU) as compared with non-ICU patients [22].
Coagulopathies and thrombosis may be significantly associated with the development of severe COVID-19.
A Significant Association Between Dysregulation of Adipocytokines in Metabolic Syndrome and Severity of COVID-19
Accumulated visceral adipose tissue play an important role for the pathogenesis of metabolic syndrome by increased secretion of ILs, TNF-α, angiotensinogen (AGT), leptin, free fatty acids (FFA) and PAI-1, and by decreased secretion of adiponectin (Fig. 1).
ILs
In the meta-analysis of 23 studies involving 3,400 COVID-19 patients, IL-6 levels were lower in mild group (weighted mean difference (WMD), -24.49; 95% CI: -34.64 - -14.34; P < 0.001) but significantly increased in critically ill group (WMD, 30.66; 95% CI, 7.53 - 53.78; P = 0.009) [23]. A subgroup analysis comparing patients by survival, found an even higher IL-6 levels was observed in patients who died (WMD, 41.32; 95% CI, 28.15 - 54.49; P < 0.001).
The meta-analysis including a total number of 21 studies showed that IL-6 and IL-10 were strong discriminants for severe COVID-19 [24]. The meta-analysis including a total of 14 studies documenting the outcomes of 4,659 patients showed that those who died, compared with those who survived, differed on IL-6 (+4.6 ng/mL, 95% CI, 3.6 - 5.6; P < 0.00001) [25].
The meta-analysis including 50 studies with 7,865 patients showed a significant increase in IL-2, IL-4, IL-6, IL-8, IL-10, TNF-α and interferon-gamma (INF-γ) in the severe group compared to the non-severe group [26]. However, no significant differences were found in IL-1β and IL-17 between the two groups. Other eight meta-analyses suggested a significant association between an increase of IL-6 and severe COVID-19 [27-34]
TNF-α
Only one meta-analysis showed a significant increase in TNF-α (WMD, 0.24 pg/mL; 95% CI, 0.01 - 0.47; P < 0.001) in the severe group compared to the non-severe group [26].
AGT, leptin and adiponectin
There were no studies which showed a significant association of AGT, leptin and adiponectin levels with severe COVID-19.
FFA
To investigate metabolic effects of SARS-CoV-2 infection, Thomas et al evaluated serum metabolites of patients with COVID-19 (n = 33) as compared with COVID-19-negative controls [35]. All FA levels, except for nonanoic acid, were increased in all patients with COVID-19, independent of IL-6 levels.
PAI-1
Zuo et al found markedly elevated levels of tissue-type plasminogen activator (t-PA) and PAI-1 among patients hospitalized with COVID-19 [36]. Both factors demonstrated a strong correlation with neutrophil counts and markers of neutrophil activation, but not with D-dimer. High levels of t-PA and PAI-1 were associated with worse respiratory status.
Nougier et al evaluated fibrinolytic activity and thrombin generation in 78 COVID-19 patients [37]. Forty-eight patients admitted to ICU and 30 patients admitted to the internal medicine department were included in the study. They found a significantly higher level of PAI-1 (96.3 ± 35 ng/mL, P = 0.017 vs. non-ICU patients) in ICU patients than in non-ICU patients (76.8 ± 40 ng/mL).
Conclusions
Metabolic syndrome and its components are significantly associated with the susceptibility to SARS-CoV-2 infection and severity of COVID-19. Enhanced ACE2 expression, pre-existing endothelial dysfunction and procoagulant state induced by adipocytokines dysregulation in metabolic syndrome may play a crucial role for the development of severe COVID-19.
Acknowledgments
I thank the staffs of the Division of Research Support, National Center for Global Health and Medicine Kohnodai Hospital.
Financial Disclosure
Author has no financial disclosures to report.
Conflict of Interest
The author declares that he has no conflict of interest concerning this article.
Informed Consent
Not applicable.
Author Contributions
HY designed the research, and collected and analyzed data. HY wrote and approved the final paper.
Data Availability
The data supporting the findings of this study are available from the corresponding author upon reasonable request.
References
- 1.Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, Xiang J. et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cai Q, Chen F, Wang T, Luo F, Liu X, Wu Q, He Q. et al. Obesity and COVID-19 severity in a designated hospital in Shenzhen, China. Diabetes Care. 2020;43(7):1392–1398. doi: 10.2337/dc20-0576. [DOI] [PubMed] [Google Scholar]
- 3.Ghoneim S, Butt MU, Hamid O, Shah A, Asaad I. The incidence of COVID-19 in patients with metabolic syndrome and non-alcoholic steatohepatitis: A population-based study. Metabol Open. 2020;8:100057. doi: 10.1016/j.metop.2020.100057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Scalsky RJ, Desai K, Chen YJ, O'Connell JR, Perry JA, Hong CC. Baseline Cardiometabolic Profiles and SARS-CoV-2 Risk in the UK Biobank. medRxiv. 2020 doi: 10.1101/2020.07.25.20161091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Leong A, Cole J, Brenner LN, Meigs JB, Florez JC, Mercader JM. Cardiometabolic risk factors for COVID-19 susceptibility and severity: a mendelian randomization analysis. medRxiv. 2020. [DOI] [PMC free article] [PubMed]
- 6.Yanai H. Significant correlations of SARS-Cov2 infection with prevalence of overweight/obesity and mean body mass index in the SARS-Cov2 endemic countries. Cardiol Res. (in press) [DOI] [PMC free article] [PubMed]
- 7.Vaduganathan M, Vardeny O, Michel T, McMurray JJV, Pfeffer MA, Solomon SD. Renin-angiotensin-aldosterone system inhibitors in patients with COVID-19. N Engl J Med. 2020;382(17):1653–1659. doi: 10.1056/NEJMsr2005760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Higham A, Singh D. Increased ACE2 Expression in Bronchial Epithelium of COPD Patients who are Overweight. Obesity (Silver Spring) 2020;28(9):1586–1589. doi: 10.1002/oby.22907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yanai H. A significance of high prevalence of diabetes and hypertension in severe COVID-19 patients. J Clin Med Res. 2020;12(6):389–392. doi: 10.14740/jocmr4218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang J, Hu J, Zhu C. Obesity aggravates COVID-19: A systematic review and meta-analysis. J Med Virol. 2020 doi: 10.1002/jmv.26237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hariyanto TI, Kurniawan A. Dyslipidemia is associated with severe coronavirus disease 2019 (COVID-19) infection. Diabetes Metab Syndr. 2020;14(5):1463–1465. doi: 10.1016/j.dsx.2020.07.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yanai H. Adiposity is the crucial enhancer of COVID-19. Cardiol Res. 2020;11(5):353–354. doi: 10.14740/cr1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Engin AB, Engin ED, Engin A. Two important controversial risk factors in SARS-CoV-2 infection: obesity and smoking. Environ Toxicol Pharmacol. 2020;78:103411. doi: 10.1016/j.etap.2020.103411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kai H, Kai M. Interactions of coronaviruses with ACE2, angiotensin II, and RAS inhibitors-lessons from available evidence and insights into COVID-19. Hypertens Res. 2020;43(7):648–654. doi: 10.1038/s41440-020-0455-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lo KB, Bhargav R, Salacup G, Pelayo J, Albano J, McCullough PA, Rangaswami J. Angiotensin converting enzyme inhibitors and angiotensin II receptor blockers and outcomes in patients with COVID-19: a systematic review and meta-analysis. Expert Rev Cardiovasc Ther. 2020:1–12. doi: 10.1080/14779072.2020.1826308. [DOI] [PubMed] [Google Scholar]
- 16.Grover A, Oberoi M. A systematic review and meta-analysis to evaluate the clinical outcomes in COVID-19 patients on angiotensin-converting enzyme inhibitors or angiotensin receptor blockers. Eur Heart J Cardiovasc Pharmacother. 2020 doi: 10.1093/ehjcvp/pvaa064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yudkin JS. Abnormalities of coagulation and fibrinolysis in insulin resistance. Evidence for a common antecedent? Diabetes Care. 1999;22(Suppl 3):C25–30. [PubMed] [Google Scholar]
- 18.Varga Z, Flammer AJ, Steiger P, Haberecker M, Andermatt R, Zinkernagel AS, Mehra MR. et al. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395(10234):1417–1418. doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Levi M, Hunt BJ. Thrombosis and coagulopathy in COVID-19: An illustrated review. Res Pract Thromb Haemost. 2020;4(5):744–751. doi: 10.1002/rth2.12400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Becker BF, Heindl B, Kupatt C, Zahler S. Endothelial function and hemostasis. Z Kardiol. 2000;89(3):160–167. doi: 10.1007/PL00007320. [DOI] [PubMed] [Google Scholar]
- 21.Tuttolomondo A, Di Raimondo D, di Sciacca R, Pinto A, Licata G. Inflammatory cytokines in acute ischemic stroke. Curr Pharm Des. 2008;14(33):3574–3589. doi: 10.2174/138161208786848739. [DOI] [PubMed] [Google Scholar]
- 22.Panigada M, Bottino N, Tagliabue P, Grasselli G, Novembrino C, Chantarangkul V, Pesenti A. et al. Hypercoagulability of COVID-19 patients in intensive care unit: A report of thromboelastography findings and other parameters of hemostasis. J Thromb Haemost. 2020;18(7):1738–1742. doi: 10.1111/jth.14850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhu J, Pang J, Ji P, Zhong Z, Li H, Li B, Zhang J. Elevated interleukin-6 is associated with severity of COVID-19: a meta-analysis. J Med Virol. 2020 doi: 10.1002/jmv.26085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Henry BM, de Oliveira MHS, Benoit S, Plebani M, Lippi G. Hematologic, biochemical and immune biomarker abnormalities associated with severe illness and mortality in coronavirus disease 2019 (COVID-19): a meta-analysis. Clin Chem Lab Med. 2020;58(7):1021–1028. doi: 10.1515/cclm-2020-0369. [DOI] [PubMed] [Google Scholar]
- 25.Tian W, Jiang W, Yao J, Nicholson CJ, Li RH, Sigurslid HH, Wooster L. et al. Predictors of mortality in hospitalized COVID-19 patients: A systematic review and meta-analysis. J Med Virol. 2020 doi: 10.1002/jmv.26050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Akbari H, Tabrizi R, Lankarani KB, Aria H, Vakili S, Asadian F, Noroozi S. et al. The role of cytokine profile and lymphocyte subsets in the severity of coronavirus disease 2019 (COVID-19): A systematic review and meta-analysis. Life Sci. 2020;258:118167. doi: 10.1016/j.lfs.2020.118167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Aziz M, Fatima R, Assaly R. Elevated interleukin-6 and severe COVID-19: A meta-analysis. J Med Virol. 2020 doi: 10.1002/jmv.25948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang ZL, Hou YL, Li DT, Li FZ. Laboratory findings of COVID-19: a systematic review and meta-analysis. Scand J Clin Lab Invest. 2020;80(6):441–447. doi: 10.1080/00365513.2020.1768587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ghahramani S, Tabrizi R, Lankarani KB, Kashani SMA, Rezaei S, Zeidi N, Akbari M. et al. Laboratory features of severe vs. non-severe COVID-19 patients in Asian populations: a systematic review and meta-analysis. Eur J Med Res. 2020;25(1):30. doi: 10.1186/s40001-020-00432-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Elshazli RM, Toraih EA, Elgaml A, El-Mowafy M, El-Mesery M, Amin MN, Hussein MH. et al. Diagnostic and prognostic value of hematological and immunological markers in COVID-19 infection: A meta-analysis of 6320 patients. PLoS One. 2020;15(8):e0238160. doi: 10.1371/journal.pone.0238160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Coomes EA, Haghbayan H. Interleukin-6 in COVID-19: A systematic review and meta-analysis. Rev Med Virol. 2020:e2141. doi: 10.1002/rmv.2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mojtabavi H, Saghazadeh A, Rezaei N. Interleukin-6 and severe COVID-19: a systematic review and meta-analysis. Eur Cytokine Netw. 2020;31(2):44–49. doi: 10.1684/ecn.2020.0448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Borges do Nascimento IJ, von Groote TC, O'Mathuna DP, Abdulazeem HM, Henderson C, Jayarajah U, Weerasekara I. et al. Clinical, laboratory and radiological characteristics and outcomes of novel coronavirus (SARS-CoV-2) infection in humans: A systematic review and series of meta-analyses. PLoS One. 2020;15(9):e0239235. doi: 10.1371/journal.pone.0239235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moutchia J, Pokharel P, Kerri A, McGaw K, Uchai S, Nji M, Goodman M. Clinical laboratory parameters associated with severe or critical novel coronavirus disease 2019 (COVID-19): A systematic review and meta-analysis. PLoS One. 2020;15(10):e0239802. doi: 10.1371/journal.pone.0239802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thomas T, Stefanoni D, Reisz JA, Nemkov T, Bertolone L, Francis RO, Hudson KE. et al. COVID-19 infection alters kynurenine and fatty acid metabolism, correlating with IL-6 levels and renal status. JCI Insight. 2020;5(14) doi: 10.1172/jci.insight.140327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zuo Y, Warnock M, Harbaugh A, Yalavarthi S, Gockman K, Zuo M, Madison JA. et al. Plasma tissue plasminogen activator and plasminogen activator inhibitor-1 in hospitalized COVID-19 patients. medRxiv. 2020 doi: 10.1101/2020.08.29.20184358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nougier C, Benoit R, Simon M, Desmurs-Clavel H, Marcotte G, Argaud L, David JS. et al. Hypofibrinolytic state and high thrombin generation may play a major role in SARS-COV2 associated thrombosis. J Thromb Haemost. 2020 doi: 10.1111/jth.15016. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data supporting the findings of this study are available from the corresponding author upon reasonable request.


