Abstract
Adolescents with diabetes have a higher prevalence of depression compared with their peers. The American Diabetes Association recommends routine mental health screening for youth with diabetes. This screening is often conducted through accessible and free depression screeners, such as the nine-item Patient Health Questionnaire (PHQ-9). Although the PHQ-9 has been validated for use in adolescents and with other medical conditions, it has yet to be validated for use in pediatric diabetes. This study evaluated adolescents’ depression symptom endorsement through retrospective review of PHQ-9 screening and semi-structured interviews with a mental health provider in a multidisciplinary diabetes clinic (patients with type 1 or type 2 diabetes). Adolescent participants (n = 96) screened during one to three separate visits (n = 148) endorsed some depressive symptoms in 56% of visits (n = 84) and moderate to severe symptoms in 6% of visits on the PHQ-9. Approximately 95% of study participants did not meet the clinic cutoff for further evaluation, but greater rates of depression were endorsed in youth with type 1 diabetes. Low mood was endorsed at a higher rate during a semi-structured interview with embedded mental health providers than on the PHQ-9. Symptoms specific to low mood, including anhedonia, sleep disturbance, concentration disturbance, motor disturbance, and thoughts of death/self-harm, were more frequently endorsed on the PHQ-9 than during the interview. Although the PHQ-9 is a good screening tool, the availability of mental health providers in diabetes clinics is important to address specific endorsed symptoms and place them in perspective based on specialized training. Until more definitive research is available on the sensitivity and specificity of this measure in this population and setting, a two-part screening approach that includes both the screening questionnaire and a brief semi-structured interview is warranted.
Pediatric diabetes is one of the most common chronic childhood conditions in the United States, with a prevalence rate of 1.93 cases per 1,000 children and an estimated 18,000 new cases per year in youth <20 years of age (1). The daily burden of diabetes management is significant, placing patients at higher risk for behavioral, mood, and other psychological concerns. Youth with diabetes are at increased risk for depression (2) and exhibit depressive symptoms at higher rates than their healthy peers (3). When depressive symptoms are present in youth with diabetes, they are often associated with lower engagement around diabetes care, increased glycemic variability, higher A1C, lower frequency of blood glucose monitoring, and more frequent visits to the emergency department (3–6). Given the high prevalence of depressive symptoms among youth with diabetes, as well as the negative correlation between depressive symptoms and overall glycemic stability, it is important for medical providers to screen pediatric patients for depressive symptoms and provide access to appropriate mental health supports.
There is growing recognition of the importance of incorporating mental health providers into the multidisciplinary treatment of pediatric diabetes to support patients, families, and the diabetes treatment team in recognizing and managing mental health concerns (7,8). Existing literature indicates that, although many pediatric patients exhibit depressive symptoms, most are not receiving routine mental health care (9). In the American Diabetes Association (ADA) Standards of Medical Care in Diabetes—2020, routine depression screenings are recommended for all patients with diabetes as well as providing evidence-based referral and treatment with qualified mental health providers for patients who screen positive on these measures (8). However, significant challenges exist, including difficulty obtaining funding for such services, challenges with fee-for-service models, and time (10,11). Recent investigations have focused on demonstrating the feasibility of screening for depressive symptoms during routine clinical care (9). One potential means of efficiently and effectively identifying depressive symptoms in youth with diabetes is the nine-item Patient Health Questionnaire (PHQ-9) modified for adolescents, a valid measure of both the presence and severity of depressive symptoms in people ≥11 years of age (12,13). This questionnaire also has been proven useful in screening for suicidality in adolescents and adults during routine clinic visits (14). The PHQ-9 is free and easily accessible in the public domain and places minimal burden on patients and clinic flow. However, although it is commonly used across a number of medical settings and populations, it has not been specifically validated within the diabetes population.
This retrospective chart review aimed to examine the endorsement of depressive symptoms in a pediatric multidisciplinary diabetes clinic and to determine the consistency between an objective pencil-and-paper measure (the PHQ-9) and a semi-structured clinical psychology interview in assessing for depression symptoms in adolescents with diabetes.
Research Design and Methods
Participants and Procedure
Participants included adolescents aged 12–18 years with type 1 or type 2 diabetes who were seen at a multidisciplinary diabetes clinic at an academic, university-affiliated tertiary care center between September 2017 and October 2018. Inclusion criteria were: 1) patient was seen in pediatric diabetes clinic by psychology and/or medicine staff, 2) patient was offered and completed a PHQ-9 during his or her clinic visit, and 3) patient participated in a semi-structured interview with psychology care providers about mental health and mood symptoms. Patients were excluded if they were in state custody or if the family declined either the PHQ-9 or a patient semi-structured interview.
Participant information was accessed from a preexisting clinical services database. Additional retrospective chart reviews were completed to obtain demographic information and psychology interview content for up to four visits per patient within the 1-year timeframe. Data from hard copies of the PHQ-9 completed at the time of visits were reviewed and entered into the database. The use of the clinical database for this program evaluation study and all study procedures were approved by the hospital’s institutional review board.
Clinic Structure
The multidisciplinary clinic was composed of pediatric endocrinologists, nurses, dietitians, on-call social workers, and psychology providers (i.e., a licensed psychologist and supervised postdoctoral fellows). Patients met with the diabetes nurse educator and pediatric endocrinologist at their appointments. Nutrition and psychology services were consultative and provided within the diabetes clinic appointment. Psychology consultation services were elective; patients were referred based on medical provider recommendations and parents’ willingness to consent to services.
Depression Screening
Adolescents ≥11 years of age were given a PHQ-9 modified for adolescents (12) at every diabetes clinic visit. The PHQ-9 is a self-administered screening questionnaire containing nine questions about symptoms of depression experienced during the past 2 weeks (e.g., fatigue, concentration, depressive complaints, and thoughts of death). Items have four possible responses (“not at all,” “various days,” “more than half the days,” and “almost every day”), with corresponding numeric scores of 0–3; total scores range from 0 to 27. Scores are classified into five categories: no/minimal depression (score 0–4), mild depression (score 5–9), moderate depression (score 10–14), moderately severe depression (score 15–19), and severe depression (score 20–27). The questionnaire is based on the criteria for diagnosing major depressive disorder in the fourth edition of the Diagnostic and Statistical Manual of Mental Disorders (15). Research indicates that the optimal cutoff for maximizing sensitivity and specificity in detecting youth who meet the criteria for major depressive disorder is a score ≥11.
Nursing staff and medical providers were responsible for scoring and interpreting the PHQ-9 data and determining the need for psychology consultation by following a scoring flowsheet and triage protocol created by the consulting psychology providers. The nurses and medical providers received training on scoring and triage by a licensed psychologist with expertise in treating children and adolescents with diabetes. Figure 1 outlines the clinic protocol for triaging PHQ-9 scores.
FIGURE 1.
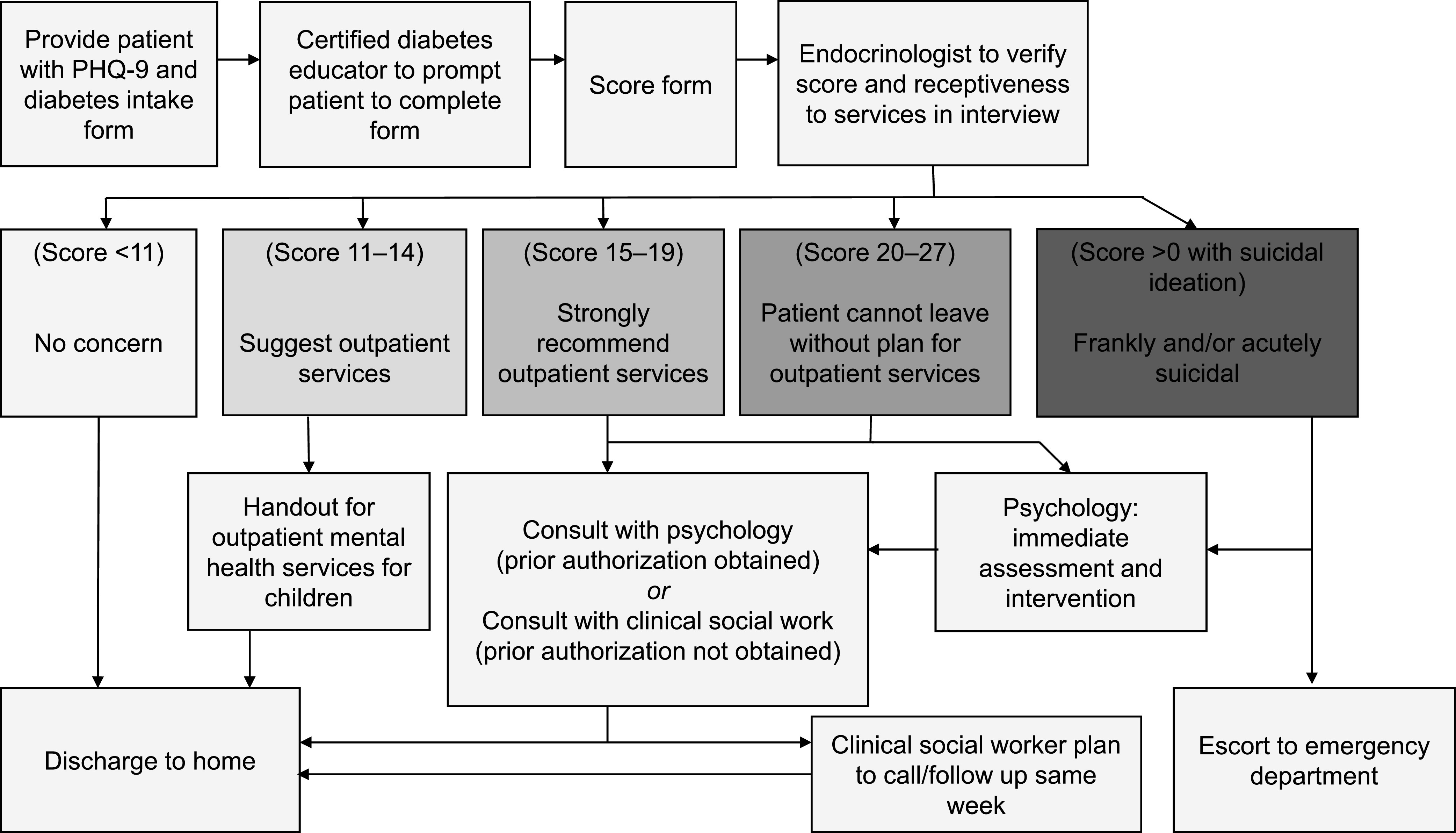
PHQ-9 triage protocol.
Psychology Semi-Structured Interview
Upon request from medical providers and consent from families, psychology providers completed a semi-structured clinical interview. Patients were referred to psychology providers for reasons including elevated PHQ-9 scores, routine assessment of diabetes burden, psychosocial stressors, or discrete intervention to support disease management. Interviews were conducted by either a licensed psychologist or supervised postdoctoral fellow with training in assessment and provision of psychology services to patients with diabetes. Providers met with patient and caregivers.
The semi-structured interview included behavioral observation, interim status (if indicated), and behavioral/mental health history. Semi-structured interviews were based on five domains: self-care/engagement in diabetes care, independence/shared responsibility of management tasks, adjustment/coping, mood, and behavior. The content of the interviews was established by psychologists and medical care providers based on relevant literature and feasibility of implementation in this specific clinic setting. Additionally, clinicians provided brief intervention, as well as recommendations, to adolescents and their caregivers based on presenting concerns.
Data Collection
This retrospective study was based on chart review and review of the clinical database. The demographic information collected included date of birth, sex, ethnicity, insurance type, type of diabetes diagnosis, date of diabetes diagnosis, mental health diagnoses in the medical record, additional medical diagnoses, and diabetes regimen (e.g., insulin treatment type). Patients’ age at diagnosis and up to four clinic visits documented during the 1-year timeframe of the study were then determined. Attainment of glycemic targets was assessed by point-of-care A1C as part of routine clinical care using the Afinion AS100 Analyzer, which has exhibited comparable laboratory precision (0.9–1.8% coefficient of variation) (16). Patients’ A1C results were retrieved from their electronic medical record (EMR).
Data Analysis
Descriptive statistics and frequencies were used to analyze and summarize participant demographics, PHQ-9 scores, and endorsement of depression symptoms during the semi-structured mental health interviews. Group differences on the measures and constructs described above between diabetes diagnosis, insurance, ethnicity, and sex were evaluated using χ2 tests. Results were reported in both longitudinal and cross-sectional analyses. As such, each participant may have a data series for up to four time points in one analysis and four separate visit data points in another analysis. These findings are specified by analyses below, as indicated. Correlations were run between A1C and PHQ-9 items. To allow for comparisons between interview question endorsement and PHQ-9 question endorsement, PHQ-9 responses were recoded such that a response of “not at all” on a question was coded as zero, and all other responses (i.e., “various days,” “more than half the days,” and “almost every day”) were coded as 1.
Results
Participant Characteristics
The overall study sample included 96 adolescents (50% male) aged 12–18 years at the time of their initial psychology screening (mean 14.73, SD 1.94). As seen in Table 1, the sample was primarily Caucasian (48%) or African American (41.7%). There were 295 visits, of which 290 had A1C values available in the records. Of these, 59.4% did not meet ADA glycemic target recommendations (<7.5%) (8). Additional information on diabetes diagnosis, insulin regimen, and insurance is outlined in Tables 1 and 2. Patients’ A1C levels were significantly correlated with PHQ-9 item 2 (i.e., “feeling down, depressed, or hopeless”; r = 0.294, P = 0.001). There were no correlations between any other single item or the overall PHQ-9 score and A1C.
TABLE 1.
Participant Characteristics
| Variable | Total n = 96 |
|---|---|
| Age, years* | 14.7 ± 1.94 |
| Ethnicity | |
| White | 48 (50) |
| Black | 40 (41.7) |
| Hispanic | 2 (2.1) |
| Other | 6 (6.3) |
| Sex | |
| Male | 48 (50) |
| Female | 48 (50) |
| Insurance | |
| Private | 46 (47.9) |
| Government-funded | 48 (50) |
| Both | 2 (2.1) |
| Diabetes diagnoses | |
| Type 1 diabetes | 78 (81.3) |
| Type 2 diabetes | 18 (18.8) |
| A1C, % | 9.33 ± 2.54 |
| Insulin treatment | |
| Pump | 43 (44.8) |
| Injection | 36 (37.5) |
| Metformin | 2 (2.1) |
| Multiple | 15 (15.6) |
| Mental health diagnosis in EMR | |
| None | 66 (68.8) |
| ADHD | 7 (7.3) |
| Mood or anxiety | 9 (9.4) |
| Stressor-related | 4 (4.2) |
| Externalizing | 3 (3.1) |
| Other | 5 (5.2) |
| Multiple | 2 (2.1) |
All data are n (%) except for age and A1C, which are mean ± SD. *At visit 1.
TABLE 2.
Interview and PHQ-9 Comparisons
| PHQ-9 Item | χ2 Statistic | Interview (n = 69) | PHQ-9 Score (n = 140) | |||||
|---|---|---|---|---|---|---|---|---|
| Yes | No | NA | Yes | No | NA | |||
| 1. Low mood*a | χ2(1) = 11.50, P <0.001 | 20 | 42 | 7 | 34 | 106 | 0 | |
| 2. Anhedonia*b | χ2(1) = 6.67, P = 0.010 | 5 | 42 | 22 | 38 | 100 | 2 | |
| 3. Sleep disturbance*b | χ2(1) = 5.23, P = 0.022 | 9 | 38 | 22 | 48 | 90 | 2 | |
| 4. Appetite disturbance | χ2(1) = 0.000, P = 1.00 | 6 | 39 | 24 | 31 | 108 | 1 | |
| 5. Fatigue | χ2(1) = 3.81, P = 0.125 | 5 | 41 | 23 | 53 | 87 | 0 | |
| 6. Guilt; low self-esteem | χ2(1) = 3.20, P = 0.084 | 1 | 43 | 25 | 25 | 115 | 0 | |
| 7. Concentration disturbance*b | χ2(1) = 6.86, P = 0.011 | 3 | 42 | 24 | 43 | 97 | 0 | |
| 8. Motor disturbance*b | χ2(1) = 15.00, P = 0.007 | 1 | 43 | 25 | 14 | 125 | 1 | |
| 9. Thoughts of death/self-harm*b | χ2(1) = 12.59, P = 0.002 | 2 | 51 | 15 | 7 | 132 | 1 | |
Significant at the P = 0.05 level. aGreater interview endorsement. bGreater PHQ-9 endorsement. NA, not applicable.
Depression Screening
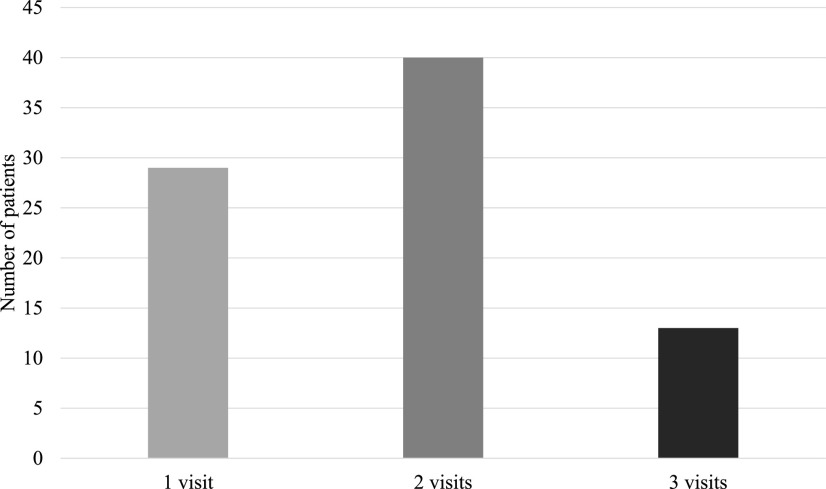
All 96 participants completed the PHQ-9 at least once. Some participants had one or more repeat PHQ-9 questionnaires (n = 53, 64.6%), yielding 148 visits with a completed PHQ-9 (Figure 2).
FIGURE 2.
PHQ-9 visit frequency.
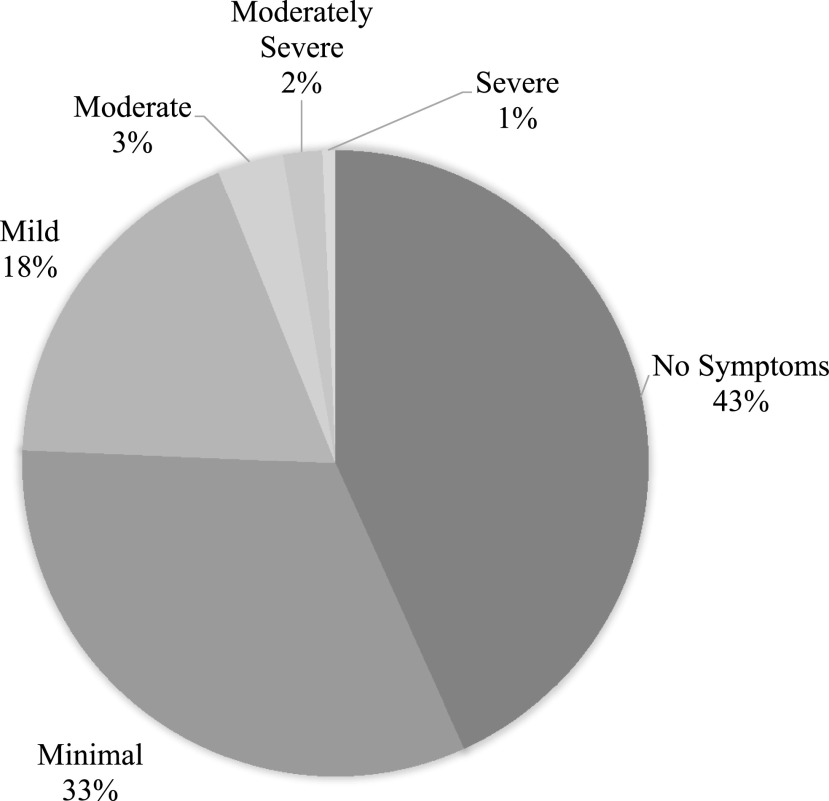
Patients endorsed some depressive symptoms in 56% of visits (84 of 148) (Figure 3). Scores ranged from 0 to 21 (mean 2.81, SD 3.89). Although the majority of youth (95.3%) had symptoms below the recommended cutoff score of 11, diabetes diagnosis was significantly associated with PHQ-9 scores, such that individuals with type 1 diabetes endorsed higher depression symptoms on the PHQ-9 than adolescents with type 2 diabetes [F(1, 146) = 16.094, P <0.001]. Figure 3 describes the number of participants at each level of symptom severity.
FIGURE 3.
Severity of patients’ depression symptoms as indicated by PHQ-9 scores.
Eleven of the participants were identified as high risk (seven had a total score of ≥11, and four endorsed suicidal ideation but did not score ≥11). Seven of the individuals had been referred to psychology services during their clinic visit to further evaluate their mood and conduct a risk assessment. These seven individuals were also provided with targeted recommendations, including follow-up with an outpatient provider (60%, who were already followed by a mental health professional) or referral for outpatient services (40%, who were referred for new mental health services). One patient was further evaluated by an on-call social worker because psychology services providers were not available in clinic that day. Two families declined to meet with a psychology service provider during the clinic visit, and one family was evaluated by a medical care provider when psychology personnel were unavailable. In these instances, medical care providers conducted the risk assessments. Of note, four of the 11 patients (36%) who scored ≥11 on the PHQ-9 had a diagnosis of a depressive disorder before the clinic visit. Diagnosis was determined based on the EMR. These diagnoses were entered into the medical record by medical providers based on family report. None of the identified patients required emergency department evaluation for acute suicidal ideation and plan development.
Semi-Structured Interviews
Of the 96 total participants, 52 were referred for and completed a semi-structured interview with psychology service providers at least once. Some participants had repeat semi-structured interviews (n = 12, 17.4%), yielding 69 completed psychology visits (i.e., n = 40 for one visit, n = 8 for two visits, n = 3 for three visits, and n = 1 for four visits). No significant differences were found for completion of interviews across diabetes diagnosis [χ2 (2) = 1.798, P = 0.180], insurance type [χ2 (2) = 0.283, P = 0.868], ethnicity [χ2 (3) = 0.265, P = 0.966], or sex [χ2 (1) = 0.018, P = 0.894].
PHQ-9 Versus Semi-Structured Interviews
Depression screener items endorsed within semi-structured interviews and PHQ-9 are detailed in Table 2. χ2 Statistics were run only with participants for whom interview and PHQ-9 responses were available. Of the nine items, six had significantly different responses between the interview and PHQ-9. Notably, these differences were not consistently in the same direction, with some items endorsed at higher rates during the interview and others endorsed at higher rates on the PHQ-9. Specifically, low mood was endorsed at a higher rate during the interview than on the PHQ-9. Specific symptoms such as anhedonia, sleep disturbance, concentration disturbance, motor disturbance, and thoughts of death/self-harm were more frequently endorsed on the PHQ-9 than during the interview.
Discussion
This study was conducted with a group of adolescents attending a multidisciplinary outpatient pediatric diabetes clinic for routine care related to their disease management. Standard questionnaire-based screening for symptoms of depression was conducted regularly with this group at the time of their clinic visits. Comparisons between reports of depression symptoms on a questionnaire and in semi-structured interviews suggested differences in item endorsement. As a group, these adolescents endorsed some symptoms of depression on questionnaires during more than half of the visits sampled, but the vast majority of adolescents had symptoms below the clinically significant range. This finding is refreshing because, despite the burden of diabetes management among youth (e.g., monitoring blood glucose, administering insulin, and determining appropriate insulin doses based on dietary and physical factors), the majority of individuals in our sample were functioning well with respect to mood.
Notably, a few youths scored in the critical range for depression concerns and/or endorsed suicidal ideation. Furthermore, youth with type 1 diabetes reported significantly more depression symptoms in this sample than their counterparts with type 2 diabetes. These results are consistent with some of the literature that suggests increased risk of depression in pediatric patients with type 1 diabetes (3) and contradictory to other research that similarly compared people with type 1 diabetes to those with type 2 diabetes (2). The difference in findings between Lawrence et al. (2) and the current study regarding youth with type 1 or type 2 diabetes may be related to the differences in sociodemographic status of study samples. The majority of participants (67%) in that study were Caucasian, whereas the current study population had a larger proportion of racial/ethnic minority families. Indeed, when considering ethnicity, Lawrence et al. reported a higher prevalence of depressed mood in racial minority patients and those with lower household incomes.
In our sample, more than half of participants had an A1C above the current ADA recommended goal of 7.5%. Although not ideal, patients in the current study met clinical guidelines for A1C at greater rates than the national mean for adolescents (40.6 vs. 17%, respectively) (17). Our results suggest an association between severity of depression symptoms and A1C, specifically the endorsement of feeling down, depressed, or hopeless on the PHQ-9. This finding was consistent with previous research in which youth (≤25 years of age) with diabetes and depression had higher A1C than nondepressed control subjects (18). The rates of significant depression symptoms and suicidal ideation in the current study, as well as the relationship between low mood and metabolic control, highlight the importance of continued integrated screening and mental health services within diabetes clinics. These findings echo the needs addressed by current ADA guidelines for routine depression screening in pediatric diabetes (8). More broadly, it is also consistent with a Joint Commission sentinel event alert issued in 2016, which mandated that accredited health care facilities implement suicide screening in all health care settings (19).
A significant finding from this study is that youth endorsed depression symptoms differently when responding to a standardized questionnaire than when undergoing a semi-structured clinical interview by a mental health professional. Participants were more likely to endorse anhedonia, sleep disturbance, concentration disturbance, motor disturbance, and thoughts of death/self-harm on the PHQ-9, but they were more likely to endorse mood disturbance in a semi-structured interview.
These results suggest that the interview contributes in some significant way to the screening process in this context. For example, the interview may be a better approach for evaluating low mood, whereas the PHQ-9 provides more details about specific symptoms that may be associated with low mood, as well as thoughts of death and/or self-harm. Additionally, it is important to note that the interview assesses the impact of diabetes distress on patient functioning that is not captured by the PHQ-9. Existing research suggests that a model that considers emotional distress as a primary factor underlying diabetes-related distress is perhaps a more comprehensive approach for understanding and treating patients (20). Furthermore, it is likely that the rapport, validation, and support that can be conveyed during an interview provides a context that increases the probability of some types of symptom disclosure.
It is also important to consider that some symptoms included in a depression screening questionnaire are not specific to depression only. Some of these behavioral and somatic symptoms are also associated with other mental health issues (e.g., anxiety or attention-deficit/hyperactivity disorder [ADHD]) and medical conditions (e.g., decreased glycemic stability). Therefore, it is important to have a mental health provider present to consider symptom presentations critically and collaboratively with medical staff when developing diagnostic hypotheses and care plans.
Given the multiple barriers that exist with regard to embedding mental health providers in standard clinical care settings, medical providers should explore alternatives for accessing needed services. Options may include developing a network of community-based mental health providers who are available for outpatient consultation and referral or, as Majidi et al. (14) suggest, accessing mental health providers who are based in the hospital but not necessarily embedded in the clinic.
Given the constraints of this study and the existing body of literature on the discrepancies between depressive disorder and diabetes distress (20), it is not possible to say whether the PHQ-9 is sufficiently sensitive to crucial high-risk symptoms to be used alone by health care professionals in the clinic without the direct involvement of mental health professionals. This study represents a first look at this important question. Our results show that the PHQ-9 is a good screener for many of the symptoms of depression, but that mental health providers are better at identifying mood disturbances. These findings are also consistent with previous research suggesting that emotional distress be considered within the context of diabetes distress rather than as a distinct depressive disorder (20). It also highlights the importance of having mental health providers available in the clinic to address endorsed symptoms on the PHQ-9. Until more definitive research is available on the sensitivity and specificity of this measure in this population and setting, it would seem prudent to continue conducting a two-part screening approach using both the questionnaire and brief semi-structured interviews.
Limitations
These results are preliminary in light of the limitations of this uncontrolled clinical study. The use of a clinic sample, although relevant in many respects, is subject to sampling bias related to patient and family self-selection factors, lack of a standardized or validated semi-structured interview, and missing data resulting from time constraints and rates of appointment-keeping in an outpatient diabetes clinic. Although some of the participants were screened more than once during this 1-year timeframe, this study was cross-sectional and did not allow for trend analyses that might help differentiate temporary situational dysphoria from an emerging or ongoing depressive episode. This study also did not include a healthy control group or a comparison group with some other disease. Therefore, we are limited in our ability to discuss conditions that are unique to youth with diabetes. Furthermore, although the PHQ-9 is validated in many medical populations, diabetes is not one of them, and it would be important to complete a prospective study to evaluate its accuracy in pediatric diabetes.
Conclusion
The prevalence of depression in adolescents with diabetes remains high; therefore, screening for depression and identifying patients in need of treatment is important in diabetes care. Although the PHQ-9 is the most frequently used screening questionnaire, some depressive symptoms are better evaluated by a semi-structured interview with a mental health provider that provides important context such as disease progression, psychosocial stressors, and patient functioning. Thus, future research should investigate the most ideal screening method for this population and setting: questionnaire alone, semi-structured interview with a mental health provider, or a combination of the two.
Article Information
Duality of Interest
No potential conflicts of interest relevant to this article were reported.
Author Contributions
A.V., M.N., R.M.W., and L.C. conceived of the study. A.V., M.N., and L.C. performed the data collection. A.V. analyzed the data. All authors wrote and provided critical review of the manuscript and approved the final manuscript. L.C. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
References
- 1.Imperatore G, Mayer-Davis EJ, Orchard TJ, Zhong VW. Prevalence and incidence of type 1 diabetes among children and adults in the United States and comparison with non-US countries. In Diabetes in America. 3rd ed Cowie CC, Casagrande SS, Menke A, et al., Eds. Bethesda, MD, National Institutes of Health, 2018, p. 2-1–2.17 (NIH publ. no. 17-1468) [Google Scholar]
- 2.Lawrence JM, Standiford DA, Loots B, et al. ; SEARCH for Diabetes in Youth Study . Prevalence and correlates of depressed mood among youth with diabetes: the SEARCH for Diabetes in Youth study. Pediatrics 2006;117:1348–1358 [DOI] [PubMed] [Google Scholar]
- 3.Johnson B, Eiser C, Young V, Brierley S, Heller S. Prevalence of depression among young people with type 1 diabetes: a systematic review. Diabet Med 2013;30:199–208 [DOI] [PubMed] [Google Scholar]
- 4.Ducat L, Philipson LH, Anderson BJ. The mental health comorbidities of diabetes. JAMA 2014;312:691–692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baucom KJ, Queen TL, Wiebe DJ, et al. Depressive symptoms, daily stress, and adherence in late adolescents with type 1 diabetes. Health Psychol 2015;34:522–530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Corathers SD, Kichler J, Jones NH, et al. Improving depression screening for adolescents with type 1 diabetes. Pediatrics 2013;132:e1395–e1402 [DOI] [PubMed] [Google Scholar]
- 7.de Wit M, Pulgaron ER, Pattino-Fernandez AM, Delamater AM. Psychological support for children with diabetes: are the guidelines being met? J Clin Psychol Med Settings 2014;21:190–199 [DOI] [PubMed] [Google Scholar]
- 8.American Diabetes Association 13. Children and adolescents: Standards of Medical Care in Diabetes—2020. Diabetes Care 2020;43(Suppl. 1):S163–S182 [DOI] [PubMed] [Google Scholar]
- 9.Watson SE, Spurling SE, Fieldhouse AM, Montgomery VL, Wintergerst KA. Depression and anxiety screening in adolescents with diabetes. Clin Pediatr (Phila) 2020;59:445–449 [DOI] [PubMed] [Google Scholar]
- 10.Powell PW, Corathers SD, Raymond J, Streisand R. New approaches to providing individualized diabetes care in the 21st century. Curr Diabetes Rev 2015;11:222–230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guttmann-Bauman I, Thornton P, Adhikari S, et al. Pediatric endocrine society survey of diabetes practices in the United States: what is the current state? Pediatr Diabetes 2018;19:859–865 [DOI] [PubMed] [Google Scholar]
- 12.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med 2001;16:606–613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Richardson LP, McCauley E, Grossman DC, et al. Evaluation of the Patient Health Questionnaire-9 Item for detecting major depression among adolescents. Pediatrics 2010;126:1117–1123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Majidi S, O’Donnell HK, Stanek K, Youngkin E, Gomer T, Driscoll KA. Suicide risk assessment in youth and young adults with type 1 diabetes. Diabetes Care 2020;43:343–348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.American Psychiatric Association Diagnostic and Statistical Manual of Mental Disorders. 4th ed., text revision Washington, DC, American Psychiatric Publishing, 2000 [Google Scholar]
- 16.Arabadjief M, Nichols JH. Evaluation of the Afinion AS100 point-of-care analyzer for hemoglobin A1C. Point Care 2009;8:11–15 [Google Scholar]
- 17.Foster NC, Beck RW, Miller KM, et al. State of type 1 diabetes management and outcomes from the T1D Exchange in 2016–2018. Diabetes Technol Ther 2019;21:66–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Plener PL, Molz E, Berger G, et al. Depression, metabolic control, and antidepressant medication in young patients with type 1 diabetes. Pediatr Diabetes 2015;16:58–66 [DOI] [PubMed] [Google Scholar]
- 19.Joint Commission Sentinel event alert 56: detecting and treating suicide ideation in all settings. Available from https://www.jointcommission.org/en/resources/patient-safety-topics/sentinel-event/sentinel-event-alert-newsletters/sentinel-event-alert-56-detecting-and-treating-suicide-ideation-in-all-settings. Accessed 10 June 2020 [PubMed]
- 20.Fisher L, Gonzalez JS, Polonsky WH. The confusing tale of depression and distress in patients with diabetes: a call for greater clarity and precision. Diabet Med 2014;31:764–772 [DOI] [PMC free article] [PubMed] [Google Scholar]