Abstract
Objective
To quantify the density of the macular microvasculature and the area of the foveal avascular zone (FAZ) in patients recovered from coronavirus disease 2019 (COVID-19) using optical coherence tomography angiography (OCTA) analysis.
Methods
In a comparative cross-sectional, observational study, patients recovered from COVID-19 were included in this study. All included subjects exhibited a reverse transcription-polymerase chain reaction—confirmed diagnosis of COVID-19. Spectral domain macular OCTA was performed at least 2 weeks after recovery from systemic COVID-19. Vessel density (VD) of the superficial (SCP) and deep retinal capillary plexus (DCP) and the area of the FAZ were measured in COVID-19 recovered patients versus age-matched normal controls.
Results
Thirty-one recovered COVID-19 patients and 23 healthy normal controls were studied. Mean quality scan index was 7.64 ± 0.66 in the COVID cases and 8.34 ± 0.71 in the normal controls (p = 0.001). Mean SCP VD and DCP VD of the COVID cohort were significantly lower than the SCP VD and DCP VD of the control group in the foveal and parafoveal regions. FAZ area was greater in the COVID cohort, but this difference was not statistically significant. In addition, in the COVID cohort, VD of the SCP was lower in patients with a history of COVID-19 hospitalization versus those without such a history, but this did not reach statistical significance.
Conclusions
Patients recovered from COVID-19 displayed alterations in the retinal microvasculature, including a significantly lower VD in the SCP and DCP. Patients with coronavirus infection may be at risk of retinal vascular complications.
Résumé
Objectif
Quantifier la densité de la microvasculature maculaire et l'aire de la zone fovéale avasculaire (ZFA) après une infection à coronavirus 2019 (COVID-19) grâce à l'angiographie-tomographie par cohérence optique (OCT-A, pour optical coherence tomography angiography).
Méthodes
Une étude d'observation comparative transversale a porté sur des patients qui ont contracté la COVID-19. Tous les sujets inclus avaient eu un diagnostic de COVID-19 confirmé par test RT-PCR (reverse transcription-polymerase chain reaction). Une OCT-A en domaine spectral de la macula a été réalisée au moins 2 semaines après la disparition des symptômes généraux de la COVID-19. La densité vasculaire (DV) des plexus capillaires superficiel (PCS) et profond (PCP) de la rétine, de même que l'aire de la ZFA, ont été mesurées chez les patients qui se sont remis de la COVID-19 et comparées à celles de sujets témoins en bonne santé et appariés pour l’âge.
Résultats
Ainsi, 31 patients qui se sont remis de la COVID-19 et 23 témoins en bonne santé ont été étudiés. L'indice moyen de qualité des images se chiffrait à 7,64 ± 0,66 dans le groupe COVID et à 8,34 ± 0,71 dans le groupe témoin (p = 0,001). La DV moyenne du PCS et du PCP dans le groupe COVID était significativement inférieure à celle du groupe témoin dans les régions fovéale et parafovéale. L'aire de la ZFA était plus grande dans le groupe COVID, mais la différence n’était pas significative sur le plan statistique. De même, la DV du PCS et du PCP des patients du groupe COVID était moindre chez ceux qui ont dû être hospitalisés en raison de la COVID-19 que chez ceux qui n'ont pas été hospitalisés, bien que la différence n'ait pas atteint le seuil de signification statistique.
Conclusion
Les patients qui se sont rétablis après un diagnostic de COVID-19 ont présenté des anomalies de la microvasculature rétinienne, dont une DV significativement réduite dans le PCS et le PCP. Les patients qui sont infectés par le coronavirus sont donc exposés à un risque de complications vasculaires rétiniennes.
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is a highly pathogenic human coronavirus, which can cause serious life-threatening respiratory illness, namely, severe pneumonia,1 and even multiorgan failure.2 , 3 Despite the growing body of knowledge about various clinical presentations and fatal consequences of coronavirus disease 2019 (COVID-19), reports regarding ocular manifestations are uncommon.4 , 5
Virus replication starts after binding to epithelial cells in the upper respiratory tract with subsequent propagation and migration down the respiratory tract triggering the innate immune response. Angiotensin-converting enzyme (ACE) 2 has been identified as the main receptor for SARS-CoV26 and its receptors are present in cell membranes of type II alveolar cells in the lung and enterocytes of the small intestine, and also in the arterial and venous endothelial cells and arterial smooth muscle cells of most organs.7 8 ACE and ACE2 have been found in the choroid and in different cell types of the retina, including Müller cells, ganglion cells, retinal vascular endothelial cells, and photoreceptor cells.9
Reports on the ocular manifestations of COVID-19 mostly describe anterior segment disorders, including conjunctival congestion, chemosis, and conjunctivitis.10 , 11 Reports of the retinal findings are rare. One group from Brazil12 described various retinal complications of COVID-19, but the validity of this study has been called into question.13
Optical coherence tomography angiography (OCTA) can provide depth-resolved imaging of blood flow in the retina and choroid with microvascular detail that exceeds the capability of other forms of imaging.14 This study evaluated patients previously infected with coronavirus, using OCTA analysis to assess the retinal microvasculature. The objective of this study was to measure the vessel density (VD) of the retinal capillary plexuses and the area of the foveal avascular zone versus an age-matched normal control group.
Materials and Methods
Study Participants
A cross-sectional study was conducted at the Imam Reza Hospital, Mashhad University of Medical Sciences (MUMS), Mashhad, Iran. Patients with a definite history of COVID-19, confirmed by a positive test result with real-time, reverse transcription-polymerase chain reaction of a nasopharyngeal swab sample, and with a history of recovery from the systemic symptoms for at least 2 weeks, were included. Detailed ocular and systemic histories were obtained from each subject. Patients recruited for this study were all doctors and nurses from the Imam Reza Hospital who had recovered from COVID-19 and all volunteered to undergo ophthalmological examination and OCTA analysis for the purpose of this research investigation.
Exclusion criteria included any history of refractive or intraocular surgery. Any patients who admitted to a history of diabetes mellitus, auto-immune disease, current pregnancy, breastfeeding, or migraine were also excluded. Additional exclusion criteria included absolute spherical refractive error greater than 5 diopters and cylindrical refractive error more than 2 diopters. Patients with evidence of glaucoma or clinically apparent retinal disease were also not enrolled. Any evidence of ocular media opacity preventing high-quality imaging or reduced OCTA scan quality (i.e., quality scan index less than 7/10) were also excluded from the analysis. Any subjects with best-corrected visual acuity less than 20/20 were also not included in the protocol.
The age-matched control cohort comprised normal individuals: nurses and physicians from the MUMS who were imaged on the same OCTA machine at the Imam Reza Hospital in 2019 as part of a prior study to build a local OCTA normative database.
Complete history regarding the patients’ symptoms, disease course, and hospitalization were recorded. Oxygen saturation at the time of examination was measured by a portable pulse oximeter (Nonin 7500 Pulse Oximeter, Nonin Medical Inc, Plymouth, Minn) and refraction was evaluated using a KR-1 Auto Kerato-Refractometer (Topcon Medical Systems, Inc, Tokyo, Japan).
Image Acquisition and Analysis
All OCTA scans were performed with the AngioVue (RTVue XR Avanti, Optovue, Fremont, Calif; Software Version 2018.0.0.14) system with an A scan rate of 70,000 scans per second, a light source of 840 nm, and a band width of 45 nm. Each B scan was repeated for image decorrelation and macular cubes were performed in the horizontal and vertical orthogonal directions. All measurements were acquired using the automated default segmentation with the preset settings for the superficial retinal capillary plexus (SCP) and the deep retinal capillary plexus (DCP). The AngioRetina 3 × 3 mm scan (304 lines × 304 A-scans) protocol with AngioVue 3D Projection Artifact Removal was used.
All images were centred on the fovea and displayed a quality scan index of at least 7/10. All images with quality scan index less than 7 were excluded. All images in the study were carefully reviewed by the first author (M.A.) and the senior authors (V.S., D.S.) to ensure sufficient quality and resolution, and any images with motion artefact significant enough to interfere with vessel density analysis as determined by both the first author (M.A.) and senior authors (V.S., D.S.) were also excluded.
For the 3 × 3 mm scans, measurements of the fovea avascular zone (FAZ, including FAZ area, the perimeter circumference of the FAZ [PERIM], and foveal vessel density [FD]) and of the vascular density (VD) of the fovea and parafovea at the level of the SCP and DCP were recorded from the AngioAnalytic report. Parafovea was defined as a ring around the fovea with diameter of 3 mm. All the images were checked for segmentation errors by 2 retina specialists (M.A. and M.M.).
Statistical Analysis
The normal distribution of variables was examined using the Shapiro-Wilk test, and normality plots and homogeneity of variances were ascertained by Levene's test. Based on data distribution and type, the independent-samples t test, paired t tests, Mann-Whitney U test, or Pearson correlation test were used for comparisons. For categorical variables, χ2 test and Fisher's exact test were used. Multivariate linear regression analysis and multivariate logistic regression analysis were performed to address the effect of potential confounders on continuous and nominal dependent variables, respectively. For variables without normal distribution, the variable was logarithm transformed and then entered into the regression model. Control of concomitant continuous independent variables (covariates) was performed using multivariate analysis of covariance (MANCOVA). After performing MANCOVA, revised p values were added to tables and multivariate analysis results were reported. The level of statistical significance was set at 0.05. All statistical analyses were performed using the SPSS program for Windows, version 20 (IBM SPSS Statistics, IBM Corporation, Chicago, Ill).
Ethical Considerations
The study protocol adhered to the tenets of the Declaration of Helsinki. All participants provided written informed consent before enrollment, and the ethical aspects of the study were approved by the Regional Committee on Medical Ethics at Mashhad University of Medical Sciences, Mashhad, Iran (IR.MUMS.REC.1399.104).
Results
Thirty-one recovered COVID-19 patients (14 females, 45.2%) with a mean age of 40.4 ± 9.2 years and 23 healthy normal controls (9 females, 39.1%) with a mean age of 36.6 ± 7.1 years were enrolled in the study after exclusion criteria were applied. Age (p = 0.115) and sex (p = 0.658) were not significantly different between the 2 groups.
In total, 3 cases were excluded from the analysis. Two patients were excluded in the COVID group because the quality scan score was less than 7 and because scans displayed significant motion artefact. An additional case was excluded from the normal cohort to a quality scan score less than 7.
All patients were symptom-free for at least 2 weeks. Medical history was otherwise unremarkable for all of the patients and controls, except for 2. None of the COVID patients or controls endorsed a history of diabetes mellitus, and only 2 cases in the COVID group disclosed a history of hypertension that was well controlled with medications or diet. Except for these 2 subjects, the other patients did not provide any history of chronic drug consumption.
For all subjects, only the data of the eye with better image quality were used for analysis. Nine patients (29%) endorsed a history of hospitalization for COVID-19. O2 saturation was in the normal range (94%–99%) in these patients and was not different between the hospitalized and nonhospitalized patients (p = 0.616). None of the hospitalized patients required invasive ventilation. None of the patients received steroid as these agents were not yet introduced into the COVID-19 treatment protocol. Face mask oxygen supplementation was used in 6 of 9 hospitalized patients. Although visual acuity was 20/20 in all COVID cases and normal controls at examination, 2 of the hospitalized patients (22.2%) and 4 of the nonhospitalized patients (18.1%) admitted to a history of blurred vision during their symptomatic period that subsequently resolved.
Mean quality scan index was 7.64 ± 0.66 in the COVID cases and 8.34 ± 0.71 in the normal controls (p = 0.001). None of the images included in the final analysis displayed segmentation error.
The 3 × 3 mm mean whole-image SCP VD in the COVID-19 group (44.98 ± 4.16) was significantly lower than the mean SCP VD in the normal control group (48.36 ± 2.24) (p = 0.001) (Table 1 ). The 3 × 3 mm mean whole-image DCP VD in the COVID-19 cohort (49.74 ± 3.39) was also significantly lower than the mean DCP VD of the control cohort (53.03 ± 3.29) (p = 0.001) (Table 1).
Table 1.
Vascular density of the superficial and deep retinal capillary plexuses in the foveal and parafoveal regions in recovered patients with COVID-19 versus normal controls
| Normal Eyes, Mean ± SD (Range) | COVID-19 Patients’ Eyes, Mean ± SD (Range) | P Value* (Compared with Normal) | P Value* (Compared with Normal) with Multivariate Analysis | |
|---|---|---|---|---|
| Whole-image SCP VD | 48.36 ± 2.24 (42.70–52.60) | 44.98 ± 4.16 (29.30–51.80) | 0.001 | 0.073 |
| Superior-hemi SCP VD | 48.32 ± 2.28 (43.30–52.40) | 44.85 ± 4.12 (30.20–52.30) | 0.001 | 0.048 |
| Inferior-hemi SCP VD | 48.41 ± 2.39 (42–52.90) | 45.10 ± 4.26 (28.40–51.30) | 0.002 | 0.101 |
| Fovea SCP VD | 21.10 ± 5.35 (12.70–31.40) | 16.70 ± 5.35 (4.20–29.20) | 0.004 | 0.003 |
| Parafovea SCP VD | 51.23 ± 2.56 (45.80–55.60) | 47.91 ± 4.46 (31.70–55.40) | 0.002 | 0.160 |
| Parafoveal superior-hemi SCP VD | 51.18 ± 2.63 (45.80–55.60) | 47.86 ± 4.43 (32.80–55.70) | 0.002 | 0.154 |
| Parafoveal inferior-hemi SCP VD | 51.30 ± 2.75 (44.90–55.70) | 47.98 ± 4.62 (30.50–55.40) | 0.004 | 0.188 |
| Parafoveal temporal SCP VD | 49.38 ± 2.35 (43.90–54) | 46.68 ± 4.49 (29.70–54) | 0.011 | 0.271 |
| Parafoveal superior SCP VD | 52.19 ± 3.32 (45.80–57.70) | 48.39 ± 4.62 (33.40–56.30) | 0.001 | 0.099 |
| Parafoveal nasal SCP VD | 50.14 ± 3.05 (42–55.30) | 47.47 ± 4.81 (31.70–55.60) | 0.024 | 0.755 |
| Parafoveal inferior SCP VD | 53.36 ± 2.83 (46.50–57) | 49.13 ± 4.60 (31.90–56.40) | <0.001 | 0.027 |
| Whole-image DCP VD | 53.03 ± 3.29 (43.50–57.50) | 49.74 ± 3.39 (43.40–55.70) | 0.001 | 0.044 |
| Superior-hemi DCP VD | 53.48 ± 3.47 (43.70–58.20) | 49.62 ± 3.39 (42.80–54.90) | <0.001 | 0.011 |
| Inferior-hemi DCP VD | 52.54 ± 3.25 (43.20–57.20) | 49.85 ± 3.53 (43.90–56.40) | 0.006 | 0.164 |
| Fovea DCP VD | 37.61 ± 4.92 (29.40–48.60) | 32.63 ± 6.50 (14.10–45.40) | 0.003 | 0.002 |
| Parafoveal DCP VD | 54.62 ± 3.33 (45.20–58.80) | 52.12 ± 3.53 (45.80–58.50) | 0.011 | 0.263 |
| Parafoveal superior-hemi DCP VD | 55.11 ± 3.47 (45.20–59.80) | 52.06 ± 3.51 (46.30–58.20) | 0.003 | 0.089 |
| Parafoveal inferior-hemi DCP VD | 54.12 ± 3.33 45.30–59.70) | 52.19 ± 3.68 (45.20–58.80) | 0.053 | 0.642 |
| Parafoveal temporal DCP VD | 54.06 ± 3.50 (43.30–58.70) | 52.49 ± 3.35 (45.30–57.80) | 0.103 | 0.789 |
| Parafoveal superior DCP VD | 55.04 ± 3.67 (44.90–59.50) | 51.42 ± 4.09 (43.90–58.60) | 0.002 | 0.062 |
| Parafoveal nasal DCP VD | 55.15 ± 3.29 (46.90–59.60) | 52.72 ± 3.57 (46.50–59.80) | 0.014 | 0.270 |
| Parafoveal inferior DCP VD | 54.20 ± 3.38 (45.90–60.90) | 51.84 ± 3.99 (44.40–59.20) | 0.026 | 0.379 |
SCP, superficial retinal capillary plexus; DCP, deep retinal capillary plexus; VD, vascular density; NA, not available.
* Statistically significant P values are in Bold.
The mean FAZ area in the COVID cohort (0.27 ± 0.11) was not significantly greater (p = 0.191) than the mean FAZ area in the control cohort (0.24 ± 0.08) (Table 2 ).
Table 2.
Measurements of the FAZ (including FAZ area, PERIM, and FD) in recovered patients with COVID-19 versus normal controls
| Normal Eyes, Mean ± SD (Range) | COVID-19 Patients’ Eyes, Mean ± SD (Range) | P Value (Compared with Normal | P Value* (Compared with Normal) with Multivariate Analysis | |
|---|---|---|---|---|
| FAZ | 0.24 ± 0.08 (0.07–0.35) | 0.27 ± 0.11 (0.07–0.57) | 0.191 | 0.025 |
| PERIM | 1.91 ± 0.37 (1.14–2.42) | 2.07 ± 0.40 (1.30–3.01) | 0.126 | 0.016 |
| FD | 51.59 ± 3.38 (40.49–55.08) | 50.23 ± 4 (40.65–56.60) | 0.197 | 0.556 |
FAZ, fovea avascular zone; PERIM, perimeter circumference of the FAZ; FD, foveal vessel density.
* Statistically significant P values are in Bold.
We applied a one-sample MANCOVA to identify the effects of scan quality on SCP and DCP VD comparisons between cases and controls, and considered the quality scan index a covariate of interest. After MANCOVA analysis, statistical significance in the comparison of the various SCP and DCP VD datasets versus normal controls was maintained in several VD comparisons, whereas others lost statistical significance. Of note, fovea SCP and DCP VD and superior hemisphere SCP and DCP VD and whole-image DCP VD all maintained statistically significant lower VD values versus controls after MANCOVA. Interestingly, FAZ area and FAZ PERIM (perimeter circumference of the FAZ) were significantly greater after MANCOVA versus normal controls (p values of 0.025 and 0.016, respectively).
Of note, mean SCP VD and DCP VD and FAZ area were not statistically significantly different in the hospitalized group compared with the nonhospitalized COVID-19 group (Tables 3 and 4 ) although the mean SCP VD was consistently lower in the hospitalized patients.
Table 3.
Vascular density of the superficial and deep retinal capillary plexuses in the foveal and parafoveal regions in hospitalized and nonhospitalized patients with COVID-19
| Nonhospitalized, Mean ± SD (Range), N = 22 | Hospitalized, Mean ± SD (Range), N = 9 | P value | |
|---|---|---|---|
| Whole-image SCP VD | 45.177 ± 4.35 (29.30–51.80) | 44.51 ± 3.85 (38.30–51.50) | 0.693 |
| Superior-hemi SCP VD | 45 ± 4.24 (30.20–52.30) | 44.50 ± 4.05 (38.10–52.30) | 0.763 |
| Inferior-hemi SCP VD | 45.34 ± 4.55 (28.40–51.30) | 44.50 ± 3.65 (38.50–50.60) | 0.625 |
| Fovea SCP VD | 16.09 ± 4.54 (6.10–25.30) | 18.22 ± 7.05 (4.20–29.20) | 0.323 |
| Parafovea SCP VD | 48.13 ± 4.67 (31.70–55.40) | 47.40 ± 4.13 (40.70–55) | 0.686 |
| Parafoveal superior-hemi SCP VD | 47.98 ± 4.55 (32.80–55.40) | 47.56 ± 4.35 (41–55.70) | 0.817 |
| Parafoveal inferior-hemi SCP VD | 48.29 ± 4.91 (30.50–55.40) | 47.23 ± 3.96 (40.40–54.20) | 0.572 |
| Parafoveal temporal SCP VD | 46.77 ± 4.74 (29.70–53.60) | 46.45 ± 4.08 (40.30–54) | 0.860 |
| Parafoveal superior SCP VD | 48.58 ± 4.83 (33.40–56.30) | 47.92 ± 4.28 (41.90–55.30) | 0.725 |
| Parafoveal nasal SCP VD | 47.64 ± 4.89 (31.70–55.20) | 47.05 ± 4.86 (38.5–55.60) | 0.764 |
| Parafoveal inferior SCP VD | 49.54 ± 4.86 (31.90–56.40) | 48.12 ± 3.95 (41.80–54.90) | 0.444 |
| Whole-image DCP VD | 49.70 ± 3.60 (43.40–55.30) | 49.85 ± 3.03 (45.70–55.70) | 0.910 |
| Superior-hemi DCP VD | 49.59 ± 3.65 (42.80–54.90) | 49.71 ± 2.86 (46.20–54.90) | 0.930 |
| Inferior-hemi DCP VD | 49.79 ± 3.69 (43.90–55.60) | 50.29 ± 3.30 (45.20–56.40) | 0.884 |
| Fovea DCP VD | 32.07 ± 5.11 (18.10–40.20) | 34.10 ± 9.32 (14.10–45.40) | 0.463 |
| Parafoveal DCP VD | 52.16 ± 3.61 (45.80–58.50) | 52.02 ± 3.54 (46.60–58) | 0.919 |
| Parafoveal superior-hemi DCP VD | 52.16 ± 3.64 (46.30–58.20) | 51.82 ± 3.38 (46.70–57.50) | 0.809 |
| Parafoveal inferior-hemi DCP VD | 52.18 ± 3.74 (45.20–58.80) | 52.21 ± 3.75 (46.50–58.50) | 0.987 |
| Parafoveal temporal DCP VD | 52.41 ± 3.54 (45.30–57.80) | 52.68 ± 3.02 (48.80–56.80) | 0.843 |
| Parafoveal superior DCP VD | 51.53 ± 4.19 (43.90–58.20) | 51.16 ± 4.06 (45.30–58.60) | 0.824 |
| Parafoveal nasal DCP VD | 52.93 ± 3.81 (46.50–59.80) | 52.22 ± 3.08 (46.70–57.40) | 0.622 |
| Parafoveal inferior DCP VD | 51.79 ± 3.91 (44.40–58.30) | 51.97 ± 4.43 (45.60–59.20) | 0.911 |
SCP, superficial retinal capillary plexus; DCP, deep retinal capillary plexus; VD, vascular density.
Table 4.
Measurements of the FAZ (including FAZ area, PERIM, and FD) in hospitalized and nonhospitalized patients with COVID-19
| Nonhospitalized, Mean ± SD (Range), N = 22 | Hospitalized, Mean ± SD (Range), N = 9 | P value | |
|---|---|---|---|
| FAZ | 0.29 ± 0.08 (0.17–0.53) | 0.25 ± 0.16 (0.07–0.57) | 0.394 |
| PERIM | 2.13 ± 0.30 (1.70–2.84) | 1.93 ± 0.58 (1.30–3.01) | 0.343 |
| FD | 50.89 ± 3.05 (44.20–56.60) | 48.62 ± 5.62 (40.65–56.50) | 0.279 |
FAZ, fovea avascular zone; PERIM, perimeter circumference of the FAZ; FD, foveal vessel density.
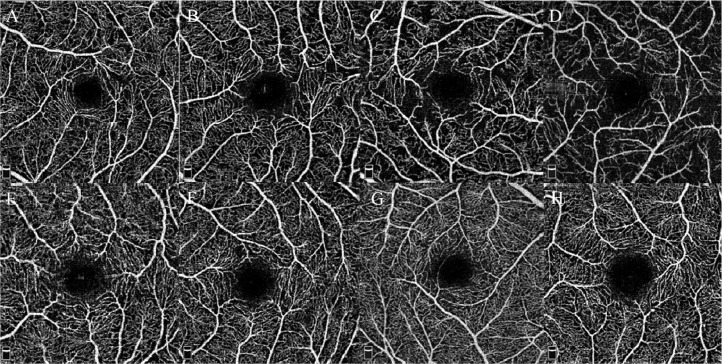
All scans were qualitatively evaluated for evidence of frank microvascular abnormalities, and 4 COVID cases did demonstrate apparent flow deficit, especially in the SCP. No frank microvascular abnormalities such as microaneuysms or beading were detected (Fig. 1 ). Note that structural OCT was evaluated in all cases and failed to display any abnormalities.
Fig. 1.
En-face optical coherence tomography angiograms segmented at the level of the superficial retinal capillary plexus from 4 recovered COVID-19 patients (A–D) versus 4 age-matched normal controls (E–H). Note the remarkable flow deficits present in the en-face angiograms from the COVID cases.
Discussion
In the present study, OCTA was performed to compare the VD of the retinal capillary microvasculature in a relatively young cohort of recovered COVID-19 patients versus age-matched normal controls. The course of COVID-19 was relatively mild, with a minority of patients requiring hospitalization. Mean macular SCP VD and DCP VD were significantly reduced in the COVID cohort versus the age-matched controls. FAZ area was also greater in the COVID group, but this did not reach statistical significance. Qualitative analysis identified apparent flow deficits in 4 cases although frank microvascular abnormalities were not detected. This analysis did not detect any significant differences in SCP and DCP VD and FAZ area in the hospitalized versus the nonhospitalized cohorts.
In a recent case series, Marinho and colleagues reported cotton-wool spots and microhemorrhages in 4 patients suggestive of an inner retinal ischemic process.12 The authors also reported abnormalities with OCT imaging, but a subsequent editorial letter indicated that these findings represented normal vascular landmarks.13 Savastano et al identified reduced perfusion density of the radial peripapillary capillary plexus in recovered COVID-19 patients versus age-matched controls using OCTA analysis.15 A systematic analysis comparing retinal capillary abnormalities in COVID patients versus age-matched normal controls has not yet been performed to our knowledge.
In an autopsy study, Casagrande and associates detected SARS-CoV-2 viral ribonucleic acid in the retina of patients who had died owing to COVID-19.16 Given the presence of ACE2 receptors in various layers of the retina and choroid, pathoanatomical abnormalities in these ocular tissues may be expected. Reports of microvascular injury and thrombosis in patients with severe COVID-19 infection would appear to highlight the importance of evaluating retinal vascular involvement with this disease.17
In this study, the SCP VD and DCP VD were significantly reduced versus age-matched controls. Although the FAZ area and circumference were also numerically increased in these comparative analyses, the differences were not statistically significant. This may be because the study was underpowered to detect small differences. OCTA analysis can be an invaluable tool in the detection of retinal vascular disease in systemic disorders like diabetes before the onset of clinically evident retinopathy. This technology may detect microvascular abnormalities such as microaneurysms, venous beading, enlargement of the FAZ, and capillary nonperfusion in diabetic eyes without any clinical signs of retinopathy.17, 18, 19 OCTA microvascular abnormalities and quantitative flow deficit analysis have been closely correlated with the clinical stage of retinopathy, and therefore OCTA can provide a biomarker of retinal disease in patients with systemic disease such as diabetes.20 OCTA may provide similar benefits in patients with other systemic disorders such as COVID-19 as the VD reductions in this study are comparable to those in patients with diabetes.
The causes of the retinal capillary alterations detected in this study are unclear. Although direct coronavirus infection of the retina is possible, secondary effects of inflammation cannot be excluded. Exacerbation of underlying systemic diseases is unlikely given the young age of the cohort analyzed and the absence of pre-existing systemic disorders, although 2 cases did endorse a history of medically controlled hypertension. Although our cohort of 31 cases with inactive disease may not be representative of the much larger population of infected COVID patients worldwide, it is interesting to note that retinal capillary alterations were detected in our study even though the affected cohort was young without pre-existing systemic illness, indicating that potentially more serious retinal complications may develop in higher-risk COVID subjects.
This study has limitations. First, ascertainment bias may have been a factor during recruitment given that patients with more severe disease may be more likely to volunteer for the study. However, as previously mentioned, all patients in this analysis had fully recovered from their COVID infection and presented with 20/20 vision and no symptoms of vision loss. Second, our study included a relatively small sample size of over 30 patients and could be improved by a larger-scale OCTA analysis performed during the symptomatic phase of the disease. Longitudinal testing with repeat imaging at fixed intervals could provide valuable information regarding both the short- and long-term effects of COVID-19 on the retinal vascular system. Third, although this study strictly applied a quality scan threshold of greater than 7 for every case included in the final analysis, there was still a slightly greater scan quality in the normal controls versus the affected COVID cases. The quality of the scan can be a factor affecting the vessel density analysis.21 , 22 Although the ocular media was clear in all patients tested, ocular surface irregularities can degrade the quality scan index and dry eye has been associated with COVID infection,23 , 24 although none of the patients in this study complained of this problem. Given the overall high-quality score greater than 7 in the COVID cases and the margin of difference versus the controls of less than 1, the significant differences in VD between these 2 groups are likely owing to pathoanatomical factors. Moreover, after multivariable analysis using MANCOVA to control for the quality scan index difference, statistical significance was maintained with some key parameters, but not with all comparisons, indicating that although the quality of the scans can influence vessel density outcomes, the lower VD values in the COVID patients are likely real. Moreover, multivariable analysis indicated that the FAZ area and FAZ perimeter circumference were significantly greater in the COVID cohort. However, further validation of our findings will be essential to ensure that the microvascular reductions in COVID recovered eyes identified in this study are the result of true anatomical alterations and not the result of artefact such as a reduced quality scan or signal strength index. Finally, although we demonstrated statistically significant microvascular differences, the clinical relevance of this finding is unclear, as the patients were all asymptomatic with 20/20 vision at the time of this analysis, although some did note vision disturbances during the active infection. It is unclear if the findings in this specific subset of COVID recovered patients may be representative of the larger population of COVID patients worldwide. Our cohort of nurses and physicians may represent a nonrandom sample of the population. Nevertheless, the OCTA analysis of 31 patients with reverse transcription-polymerase chain reaction-confirmed COVID-19 positivity and the comparison with an age-matched normal control group, similarly imaged, is novel and may highlight the importance of continued vigilance for the detection of ocular and retinal complications secondary to COVID-19 as the pandemic evolves.
In conclusion, our study demonstrated significant retinal vascular alterations in patients with a history of COVID-19, including reduced vessel density in the SCP and DCP in the foveal and parafoveal regions. The potential involvement of the retina by COVID-19 warrants a further larger-scale study that may be more representative of the ever-growing population of patients infected with COVID-19 worldwide.
Acknowledgments
Footnotes and Disclosure
The authors would like to thank Abbas Saberi and Hojjat Salmani at Mashhad PERSIAN Cohort Center for their kind assistance with this research project. The authors would also like to thank Mr. Amir Reza Samad Zadeh for his support with personal protective equipment (PPE) for examinations. It is a pleasure for us to also acknowledge the kind support of Mrs. Mahbobeh Najafi.
The authors have no proprietary or commercial interest in any materials discussed in this article.
Supported by: The authors would like to acknowledge the financial support of the Vice-Chancellor of Research of Mashhad University of Medical Sciences for this research project (code: 990069). The funding organization had no role in the design or conduct of this research.
References
- 1.Ahn DG, Shin HJ, Kim MH. Current status of epidemiology, diagnosis, therapeutics, and vaccines for novel coronavirus disease 2019 (COVID-19) J Microbiol Biotechnol. 2020;30:313–324. doi: 10.4014/jmb.2003.03011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gheblawi M, Wang K, Viveiros A. Angiotensin-converting enzyme 2: SARS-CoV-2 receptor and regulator of the renin-angiotensin system: celebrating the 20th anniversary of the discovery of ACE2. Circ Res. 2020;126:1456–1474. doi: 10.1161/CIRCRESAHA.120.317015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huang C, Wang Y, Li X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China [published correction appears in Lancet. 2020 Jan 30. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li JO, Lam DSC, Chen Y, Ting DSW. Novel coronavirus disease 2019 (COVID-19): the importance of recognising possible early ocular manifestation and using protective eyewear. Br J Ophthalmol. 2020;104:297–298. doi: 10.1136/bjophthalmol-2020-315994. [DOI] [PubMed] [Google Scholar]
- 5.Mungmungpuntipantip R, Wiwanitkit V. Ocular manifestation, eye protection, and COVID-19. Graefes Arch Clin Exp Ophthalmol. 2020;258:1339. doi: 10.1007/s00417-020-04662-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wan Y, Shang J, Graham R, Baric RS, Li F. Receptor recognition by the novel coronavirus from Wuhan: an analysis based on decade-long structural studies of SARS coronavirus. J Virol. 2020;94 doi: 10.1128/JVI.00127-20. e00127–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hamming I, Timens W, Bulthuis ML, Lely AT, Navis G, van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol. 2004;203:631–637. doi: 10.1002/path.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chamsi-Pasha MA, Shao Z, Tang WH. Angiotensin-converting enzyme 2 as a therapeutic target for heart failure. Curr Heart Fail Rep. 2014;11:58–63. doi: 10.1007/s11897-013-0178-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Choudhary R, Kapoor MS, Singh A, Bodakhe SH. Therapeutic targets of renin-angiotensin system in ocular disorders. J Curr Ophthalmol. 2016;29:7–16. doi: 10.1016/j.joco.2016.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu P, Duan F, Luo C. Characteristics of ocular findings of patients with coronavirus disease 2019 (COVID-19) in Hubei Province, China. JAMA Ophthalmol. 2020;138:575–578. doi: 10.1001/jamaophthalmol.2020.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abrishami M, Tohidinezhad F, Daneshvar R. Ocular manifestations of hospitalized patients with COVID-19 in northeast of Iran. Ocul Immunol Inflamm. 2020;28:739–744. doi: 10.1080/09273948.2020.1773868. [DOI] [PubMed] [Google Scholar]
- 12.Marinho PM, Marcos AAA, Romano AC, Nascimento H, Belfort R., Jr Retinal findings in patients with COVID-19. Lancet. 2020;395:1610. doi: 10.1016/S0140-6736(20)31014-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vavvas DG, Sarraf D, Sadda SR. Concerns about the interpretation of OCT and fundus findings in COVID-19 patients in recent Lancet publication. Eye (Lond) 2020 doi: 10.1038/s41433-020-1084-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Spaide RF, Fujimoto JG, Waheed NK, Sadda SR, Staurenghi G. Optical coherence tomography angiography. Prog Retin Eye Res. 2018;64:1–55. doi: 10.1016/j.preteyeres.2017.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Savastano A, Crincoli E, Savastano MC, Gemelli Against Covid-Post-Acute Care Study Group Peripapillary retinal vascular involvement in early post-COVID-19 patients. J Clin Med. 2020;9:E2895. doi: 10.3390/jcm9092895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Casagrande M, Fitzek A, Püschel K. Detection of SARS-CoV-2 in human retinal biopsies of deceased COVID-19 patients. Ocul Immunol Inflamm. 2020 doi: 10.1080/09273948.2020.1770301. [DOI] [PubMed] [Google Scholar]
- 17.Magro C, Mulvey JJ, Berlin D. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: a report of five cases. Transl Res. 2020;220:1–13. doi: 10.1016/j.trsl.2020.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.De Carlo TE, Chin AT, Bonini Filho MA. Detection of microvascular changes in eyes of patients with diabetes but not clinical diabetic retinopathy using optical coherence tomography angiography. Retina. 2015;35:2364–2370. doi: 10.1097/IAE.0000000000000882. [DOI] [PubMed] [Google Scholar]
- 19.Choi W, Waheed NK, Moult EM. Ultrahigh speed swept source optical coherence tomography angiography of retinal and choriocapillaris alterations in diabetic patients with and without retinopathy. Retina. 2017;37:11–21. doi: 10.1097/IAE.0000000000001250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Takase N, Nozaki M, Kato A. Enlargement of foveal avascular zone in diabetic eyes evaluated by en face optical coherence tomography angiography. Retina. 2015;35:2377–2383. doi: 10.1097/IAE.0000000000000849. [DOI] [PubMed] [Google Scholar]
- 21.Lim HB, Kim YW, Kim JM, Jo YJ, Kim JY. The importance of signal strength in quantitative assessment of retinal vessel density using optical coherence tomography angiography. Sci Rep. 2018;8:12897. doi: 10.1038/s41598-018-31321-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Czakó C, István L, Benyó F. The impact of deterministic signal loss on OCT angiography measurements. Transl Vis Sci Technol. 2020 doi: 10.1167/tvst.9.5.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hong N, Yu W, Xia J, Shen Y, Yap M, Han W. Evaluation of ocular symptoms and tropism of SARS-CoV-2 in patients confirmed with COVID-19. Acta Ophthalmol. 2020 doi: 10.1111/aos.14445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moshirfar M, West WB, Jr, Marx DP. Face mask-associated ocular irritation and dryness. Ophthalmol Ther. 2020;9:397–400. doi: 10.1007/s40123-020-00282-6. [DOI] [PMC free article] [PubMed] [Google Scholar]