Abstract
The Patient-centered HIV Care Model (PCHCM) integrated community-based pharmacists with medical providers and required sharing of patient clinical information and collaborative therapy-related action planning. We determined the proportions of participants with HIV and mental health conditions who were retained in care and the proportion virally suppressed, pre- and post-implementation. Overall, we found a relative 13% improvement in both retention (60% to 68% [p=0.009]) and viral suppression (79% to 90% [p<0.001]). Notable improvements were seen among persons triply diagnosed with HIV, mental illness and substance use (+36% [50% to 68%, p=0.036] and +32% [66% to 86%, p=0.001] in retention and viral suppression, respectively). There were no differences in the proportions of persons adherent to psychiatric medications, pre- to post-implementation, nor were there differences in the proportions of persons retained in care or virally suppressed by psychiatric medication adherence, post-implementation. PCHCM demonstrated that collaborations between community-based pharmacists and medical providers can improve HIV care continuum outcomes among persons with mental health conditions.
Keywords: HIV, mental disorders, retention in care, sustained virologic response, Patient-centered HIV Care Model
Abstract
Resumen
El modelo de atención para el VIH centrada en el paciente (PCHCM) integró a farmacéuticos de la comunidad y a proveedores médicos, y requirió compartir la información clínica del paciente y planificar medidas colaborativas relacionadas con la terapia. Determinamos las proporciones de participantes con VIH y afecciones de salud mental que permanecieron bajo atención médica y la proporción de quienes habían logrado la supresión viral, antes y después de la implementación. En líneas generales, hallamos una mejoría relativa del 13% tanto en la permanencia bajo atención médica (60% a 68% [p=0.009]) como en la supresión viral (79% a 90% [p<0.001]). Se observaron mejorías notables entre personas con diagnóstico triple de VIH, enfermedad mental y consumo de sustancias (+36% [50% a 68%, p=0.036] y +32 % [66% a 86%, p=0.001] en la permanencia bajo atención médica y la supresión viral, respectivamente). No hubo diferencias en las proporciones de personas con adhesión a los medicamentos siquiátricos, desde antes hasta después de la implementación, ni tampoco diferencias en la proporción de personas que permanecieron bajo atención médica o con supresión viral por su adhesión a los medicamentos siquiátricos, después de la implementación. El PCHCM demostró que las colaboraciones entre los farmacéuticos de la comunidad y los proveedores médicos pueden mejorar los resultados del proceso continuo de atención para el VIH entre personas con afecciones de salud mental.
Palabras clave: VIH, trastornos mentales, permanencia bajo atención médica, respuesta virológica sostenida, modelo de atención para el VIH centrada en el paciente
Introduction
Mental health disorders are prevalent among persons with HIV; an estimated 63% of persons with HIV have a mental health disorder compared to 31% of persons without HIV. (1, 2) Depression, in particular, is common among persons with HIV with an estimated prevalence of 20% to 40%. (3, 4)] Comorbid depression is associated with poorer outcomes along the HIV care continuum. Persons with HIV and depression are less likely to be retained in HIV care (5), adherent to antiretroviral therapy (ART)(6, 7), and virally suppressed. (8, 9) Also, depressive symptoms have shown a dose-response relationship to missed clinical appointments and virologic failure. (10)
Although most studies of HIV and mental illness focus on depression, the estimated prevalence of and association with other mental health conditions is high. For example, prevalence of generalized anxiety disorder and post-traumatic stress disorder is estimated at 16% and from 10% to 74%, compared with 2% and 8% among the general population, respectively. (11) Additionally, HIV has been associated with increased risk of schizophrenia and acute psychosis, particularly in the first few years after HIV diagnosis. (12) While the association of depression and HIV care continuum outcomes is well documented (5–10), the associations of care continuum outcomes and other mental health conditions (e.g., psychotic disorders, bipolar and other mood disorders) have shown mixed results. (7)
Potentially compounding the effect of mental illness on HIV outcomes is concomitant substance use. Like mental illness, the prevalence of substance use disorder among persons with HIV is high at an estimated 48%. (13) Further, the United States Substance Abuse and Mental Health Services Administration (SAMHSA) estimates that nearly one-quarter of persons with HIV, in the United States, need treatment for illicit drug or alcohol use. (14) Substance use or dependence is, by itself, associated with poor retention in HIV care and virologic failure (15, 16); these effects may be exacerbated by the co-occurrence of mental health disorders. In fact, several studies have shown that co-occurring psychosocial or “syndemic” factors (in particular, mental illness, alcohol and substance use, childhood sexual abuse, and intimate partner violence) increase risk of poor HIV viral suppression, poor retention in care, ART nonadherence, and mortality. (12, 17–22) Syndemic health problems occur when multiple health conditions act synergistically to contribute to excess disease burden in a population. (20)
Given the high prevalence of comorbid mental health conditions among persons with HIV and evidence that these conditions adversely affect health outcomes, interventions that improve outcomes among this population are necessary. As such, we used data from the Patient-centered HIV Care Model (PCHCM), a ten-site demonstration project, to conduct a sub-analysis of model outcomes. In previous analyses of project outcomes, the PCHCM was found to improve retention in HIV care and viral suppression among the entire participant cohort by a relative 13% and 15%, respectively. (23, 24) This sub-analysis sought to answer the following questions: 1) Does the model improve retention in care and viral suppression among persons with HIV and mental health conditions? 2) Does the model improve adherence to psychiatric medications? and 3) Do persons with HIV and mental health conditions, who adhere to their psychiatric medications, have better retention in care and viral suppression than those who are non-adherent or not on psychiatric medications?
Methods
Patient-centered HIV Care Model
The Patient-centered HIV Care Model integrated community-based pharmacists with primary medical providers for patient-centered HIV care. The PCHCM was implemented between August 2013 and September 2016 at ten project sites within the United States (Albany, GA; Chicago, IL; Fort Lauderdale, FL; Kansas City, MO; Miami, FL; New York, NY; Palm Springs, CA; Philadelphia, PA; St. Louis, MO; and Washington, D.C.). Each project site was comprised of ≥1 community-based HIV specialty retail pharmacy partnered with a medical clinic. A convenience sample of 765 participants was enrolled in the project; each participant received between 12 and 24 months of model services.
The PCHCM and the main outcomes for the entire participant cohort are described in detail elsewhere. (23, 24) In brief, the model built upon the existing Medication Therapy Management (MTM) model and included additional services. Medication Therapy Management broadly includes a range of pharmacist-led services meant to optimize therapeutic outcomes. (25) These services may include: comprehensive or targeted review of medications for indication, effectiveness, safety and adherence; adherence counseling or other support; and patient education. The PCHCM built upon MTM by requiring: 1) clinic medical providers to share patient clinical information with pharmacists and 2) collaborative action planning to address identified therapy-related problems. The pharmacists’ review of patient clinical information (e.g., HIV viral load, CD4 and drug resistance test results, medical problem lists, and failed medication regimens) enabled the pharmacists to most effectively conduct MTM. In addition, the pharmacists proactively monitored patients’ prescription refill patterns and laboratory test results and provided individualized adherence support. The pharmacists then worked closely with the clinic medical providers (e.g., HIV physicians, physician assistants, nurse practitioners) and/or patients to develop plans to address therapy-related problems identified through the pharmacists’ activities. Patients were scheduled for quarterly follow-up with the pharmacists. At each patient visit with the pharmacist, the patients’ ART was reviewed; other comorbid conditions were reviewed when deemed clinically necessary by the pharmacist. No formalized practice agreements were established between pharmacists and prescribers. The Office of Research Compliance, on behalf of the Institutional Review Board of the University of North Texas Health Science Center, determined the project met criteria for exempt status (exempt category: demonstration projects designed to study public benefit or service programs).
Mental health cohort
A person was considered to have a mental health condition if they had a mental health diagnosis within the pre-implementation clinic record (up to 24 months prior to project enrollment) or had filled ≥1 prescription for a medication within the Medi-Span® Generic Product Identifier (GPI) major class of “antidepressant,” “antianxiety” or “antipsychotic,” in the pre-implementation period. Non-psychotic mental health conditions were defined as a diagnosis of adjustment disorder, anxiety disorder, bipolar disorder, depression, dysthymia, panic disorder or post-traumatic stress disorder or ≥1 prescription fill for an antidepressant or antianxiety medication, in the pre-implementation period. Psychotic mental health conditions were defined as a diagnosis of schizoaffective disorder, schizophrenia, schizophreniform disorder or psychotic disorder (not otherwise specified) or ≥1 prescription fill for an antipsychotic medication, in the pre-implementation period. Substance use was defined by documentation of substance use, abuse or dependence in the pre-implementation clinic record and included illicit (including marijuana) and controlled substances and alcohol.
Retention in care
The proportion of persons with HIV and a mental health condition who were retained in care was calculated, pre- and post-model implementation. Retention in care was defined as ≥1 medical visit with a physician, nurse practitioner, or physician assistant, in each 6-month period of a 12-month measurement period with a minimum of 60 days between medical visits. (26) Retention was measured from 12 months prior to (and included) the enrollment date (pre-implementation) and from one day after the enrollment date to 12 months forward (post-implementation). Persons were included in the analysis if they had a documented HIV diagnosis or evidence of HIV diagnosis (e.g., HIV viral load test result) ≥12 months prior to the enrollment date. A person was excluded if there were no recorded date(s) for clinic visits in the pre-implementation period. (23)
Viral suppression
The proportion of persons with HIV and a mental health condition who were virally suppressed was calculated, pre- and post-model implementation. Viral suppression was defined as an HIV viral load of <200 HIV RNA copies/mL at the last test in the 12–month measurement period. (26) In the pre- and post-implementation periods, the last viral load test was the viral load test result closest to and furthest from the enrollment date, respectively. Measurement periods were the same as those used for the retention analysis. (24) Persons were included in the viral suppression analysis if they had ≥1 viral load test result in the measurement period.
Adherence to psychiatric medications and therapy-related issues
Adherence to psychiatric medications was based on the Proportion of Days Covered (PDC). The PDC is a pharmacy claims-based metric that determines the proportion of days for which a person has medication available during a measurement period. The PDC is calculated by dividing the number of days a person has medication during the measurement period by the length of the measurement period; adjustments are made for fill days’ supply and for days with overlapping medication supply. (27)
Adherence was defined as a PDC ≥0.80 and calculated pre- and post-model implementation. (28) A PDC was calculated, for each medication, from the date of the index fill to the date of the last fill in the 12 months pre- and post-model implementation. For each medication, persons were included in the analysis if they filled prescriptions that covered at least 90 days within the 12 months pre- and post-implementation. Prescriptions for benzodiazepines, hydroxyzine and mental health medications with <30-day supplies, per prescription, were excluded from the analysis; these medications were excluded to limit PDC calculations to medications intended for long-term rather than short-term use.
Adherence was calculated for persons on medications within the following three drug groupings: antidepressant and/or antianxiety medication(s) only; antipsychotic medication(s) only; both antidepressant and/or antianxiety medication(s) and antipsychotic medication(s). If, during the measurement period, more than one medication was filled within any of the drug groupings, a PDC was calculated for each medication and adherence was based on the average PDC. The overall mean PDC, PDC range and the proportion adherent was calculated for each category of psychiatric medication, pre- and post-model implementation. In addition, pharmacist identified psychiatric therapy-related problems and suggested resolutions were determined.
Retention in care and viral suppression by psychiatric medication adherence level
The proportion of persons retained in care and the proportion virally suppressed were determined by psychiatric medication adherence (PDC ≥0.80 and <0.80) within each drug grouping. Persons with a diagnosis of depression, dysthymia or anxiety in the pre-implementation clinic record but who never filled a prescription for an antidepressant/antianxiety medication were included in a “not on therapy” level in the antidepressant/antianxiety drug grouping. A “not on therapy” level was not included in the antipsychotic or both antidepressant/antianxiety and antipsychotic drug groupings because of the small number of persons diagnosed with a psychotic disorder who were not on therapy.
Censoring
Persons were censored from the analyses at the first date that one of the following occurred: person died, too ill (e.g., moved into hospice), moved out of area, transferred care to non-project participating clinic or provider, incarcerated, voluntarily withdrew from project, or transferred prescriptions to a non-project (or non-project network) pharmacy. If one of the aforementioned conditions occurred but no date was recorded for the event, then the person was censored one day after their last clinic visit. Two project sites did not collect censoring data. For these two sites, persons were censored one day after the date of the last clinic visit if a person had no clinic visit, HIV viral load, or CD4 test drawn for >6 months, but continued to fill prescriptions at the project pharmacies in the last six months of the project implementation period.
Statistical analysis
The proportion of persons with mental health conditions who were retained in care and the proportion virally suppressed were modeled using log-binomial regression. Generalized estimating equations (GEE) with an exchangeable working correlation structure accounted for repeated measures. Overall, pre- and post-implementation retention and viral suppression was compared by including the implementation period (pre- or post-) as the sole main effect in the model. Similar comparisons were made within each level of the demographic and other characteristics (e.g., category of mental health diagnosis and baseline substance use diagnosis) by including the main effect terms for the implementation period and the demographic or other characteristic, and an implementation period by demographic or other characteristic interaction term. The relative percentage change in the proportion of persons retained and virally suppressed was also calculated, pre- to post-model implementation. Pre- and post-implementation mean PDC and the proportion adherent were compared using GEE with normal and binomial distributions, respectively.
Lastly, multivariable log-binomial regression was used to calculate the relative risk (RR) and 95% confidence intervals of being retained in care and the RR of being virally suppressed by psychiatric medication adherence category within each drug grouping, post-implementation. The relative risks of retention and the risks of viral suppression in the non-adherent and “not on therapy” groups were estimated relative to the risk in the adherent group. The RR estimates for retention in care were adjusted for pre-implementation retention in care. In cases where data were too sparse to generate stable estimates using log binomial regression, we performed stratified contingency table analyses and computed the adjusted logit estimates of the RR with exact unconditional 95% confidence limits. The RR estimates for viral suppression were initially adjusted for both pre-implementation viral suppression and adherence to ART (defined as three antiretroviral medications [excluding boosters], as outlined by treatment guidelines, or one of the following nonstandard regimens: lamivudine used in combination with two other antiretroviral drugs or the combined use of darunavir/dolutegravir/ritonavir (29, 30); an ART PDC of ≥0.90 was considered adherent (26)). However, because approximately 90% of study participants were virally suppressed post-implementation, the RR estimates were unstable due to sparse data. The RR estimates for viral suppression were, therefore, stratified by drug grouping and left unadjusted.
Results
Of the 765 persons enrolled in the project, 453 (59%) had a mental health condition in the pre-implementation period and were included in this analysis. (Table I) Among these individuals, the largest proportions were non-Hispanic black (36%), male (71%) and Medicaid-insured (32%). The median age was 49 years (interquartile range: 40 – 56). The majority (88%) had a diagnosis for a non-psychotic mental health condition and 15% had a diagnosis for substance (alcohol or drug) use in the pre-implementation period. (Table I) The analytic cohorts are shown in Figure 1.
Table I:
Proportion of persons with HIV and mental health conditionsa who were retained in care and virally suppressed pre- and post-model implementation, by characteristic, Patient-centered HIV Care Model, 2014 – 2016, United States
| Retained in careb | HIV RNA <200 copies/mLc | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Characteristic | Total n (%d) | Baseline (n = 421) n (%) | Follow-up (n = 390) n (%) | % Changee | p-valuef | Baseline (n = 387) n (%) | Follow-up (n = 367) n (%) | % Changee | p-valuef | |
| Total | 453 (100) | 252 (60) | 265 (68) | 13 | 0.009 | 306 (79) | 329 (90) | 13 | <0.001 | |
| Age in Years | 18–24 | 9 (2) | 4 (57) | 4 (67) | 17 | 0.701 | 4 (50) | 6 (86) | 71 | 0.096 |
| 25–34 | 68 (15) | 37 (62) | 38 (69) | 12 | 0.353 | 35 (69) | 45 (82) | 19 | 0.106 | |
| 35–49 | 150 (33) | 83 (59) | 88 (68) | 15 | 0.115 | 92 (73) | 103 (87) | 19 | 0.001 | |
| ≥50 | 226 (50) | 128 (60) | 135 (68) | 13 | 0.076 | 175 (87) | 175 (94) | 9 | 0.008 | |
| Race/ethnicity | Black, non-Hispanic | 165 (36) | 94 (62) | 108 (75) | 21 | 0.007 | 91 (64) | 115 (84) | 32 | <0.001 |
| Hispanic | 54 (12) | 35 (69) | 32 (70) | 2 | 0.960 | 41 (85) | 43 (98) | 14 | 0.040 | |
| White, non-Hispanic | 130 (29) | 64 (54) | 60 (56) | 4 | 0.731 | 90 (86) | 94 (95) | 11 | 0.019 | |
| White, ethnicity unknown | 55 (12) | 30 (57) | 35 (67) | 19 | 0.210 | 48 (98) | 45 (92) | −6 | 0.088 | |
| Other/Unknown | 49 (11) | 29 (63) | 30 (73) | 16 | 0.272 | 36 (86) | 32 (84) | −2 | 0.708 | |
| Sex | Male | 323 (71) | 181 (61) | 187 (68) | 12 | 0.066 | 230 (83) | 246 (93) | 11 | <0.001 |
| Female | 120 (27) | 64 (56) | 71 (68) | 21 | 0.036 | 72 (71) | 76 (82) | 16 | 0.032 | |
| Transgender | 10 (2) | 7 (78) | 7 (70) | −10 | 0.768 | 4 (50) | 7 (88) | 75 | 0.087 | |
| Medical Insurance | Medicaid | 144 (32) | 78 (59) | 76 (63) | 6 | 0.500 | 89 (71) | 100 (86) | 20 | 0.005 |
| Medicare | 37 (8) | 22 (65) | 29 (91) | 40 | 0.028 | 24 (75) | 27 (90) | 20 | 0.030 | |
| Ryan White/ADAPg | 59 (13) | 32 (62) | 39 (80) | 29 | 0.023 | 33 (70) | 41 (84) | 19 | 0.093 | |
| Private Insurance | 39 (9) | 22 (60) | 23 (72) | 21 | 0.207 | 24 (86) | 29 (100) | 17 | 0.052 | |
| Multiple | 85 (19) | 38 (49) | 45 (58) | 20 | 0.242 | 65 (88) | 68 (97) | 11 | 0.026 | |
| Uninsured/Unknown | 89 (20) | 60 (68) | 53 (67) | −2 | 0.867 | 71 (88) | 64 (89) | 1 | 0.691 | |
| Category of diagnosis | Non-psychotic Disorderh | 397 (88) | 220 (60) | 227 (67) | 11 | 0.043 | 274 (80) | 294 (91) | 13 | <0.001 |
| Psychotic Disorderi | 56 (12) | 32 (59) | 38 (78) | 31 | 0.056 | 32 (70) | 35 (83) | 20 | 0.070 | |
| Baseline substance use diagnosisj | No | 386 (85) | 221 (62) | 225 (68) | 10 | 0.058 | 266 (82) | 278 (90) | 11 | <0.001 |
| Yes | 67 (15) | 31 (50) | 40 (68) | 36 | 0.036 | 40 (66) | 51 (86) | 32 | 0.001 | |
Persons were considered to have a mental health condition if they had a mental health diagnosis in the baseline clinic record or had filled ≥1 prescription for an antidepressant, antianxiety or antipsychotic medication in the pre-implementation period.
Retention in care was defined as ≥1 medical visit with a physician, nurse practitioner, or physician assistant, in each 6-month period of a 12-month measurement period with a minimum of 60 days between medical visits. Pre-implementation retention was measured during the 12 months leading up to and including the enrollment date and post-implementation retention was measured from one day after the enrollment date to 12 months forward.
Viral suppression was defined as an HIV viral load of <200 HIV RNA copies/mL at the last test in the 12–month measurement period. In the pre- and post-implementation periods, the last viral load test was the viral load result closest to and furthest from the first comprehensive medication review date, respectively.
Column percentage.
Relative percentage change.
P-values were calculated using log binomial regression with an interaction term for time (pre- and post-implementation) by demographic factor level.
ADAP = AIDS Drug Assistance Program.
Includes persons with either a diagnosis for a non-psychotic mental health condition in the baseline clinic record (adjustment disorder, anxiety disorder, bipolar disorder, depression, dysthymia, panic disorder or post-traumatic stress disorder) and persons who filled ≥1 antidepressant/antianxiety prescription in the pre-implementation period. Persons who had a diagnosis for both a psychotic disorder and a non-psychotic disorder or who had filled both an antipsychotic medication and an antidepressant/antianxiety were categorized as having a psychotic disorder.
Includes persons with a diagnosis for schizoaffective disorder, schizophrenia, schizophreniform disorder or psychotic disorder (not otherwise specified) in the baseline clinic record and persons who filled ≥1 antipsychotic medication in the pre-implementation period.
Includes persons with a mental health condition and a diagnosis of substance (alcohol or drug) use, abuse or dependence in the pre-implementation period.
Figure 1: Flow diagram of inclusion in retention in care, HIV viral suppression and adherence analyses among persons with HIV and comorbid mental health conditions, Patient-centered HIV Care Model, 2014 – 2016, United States.
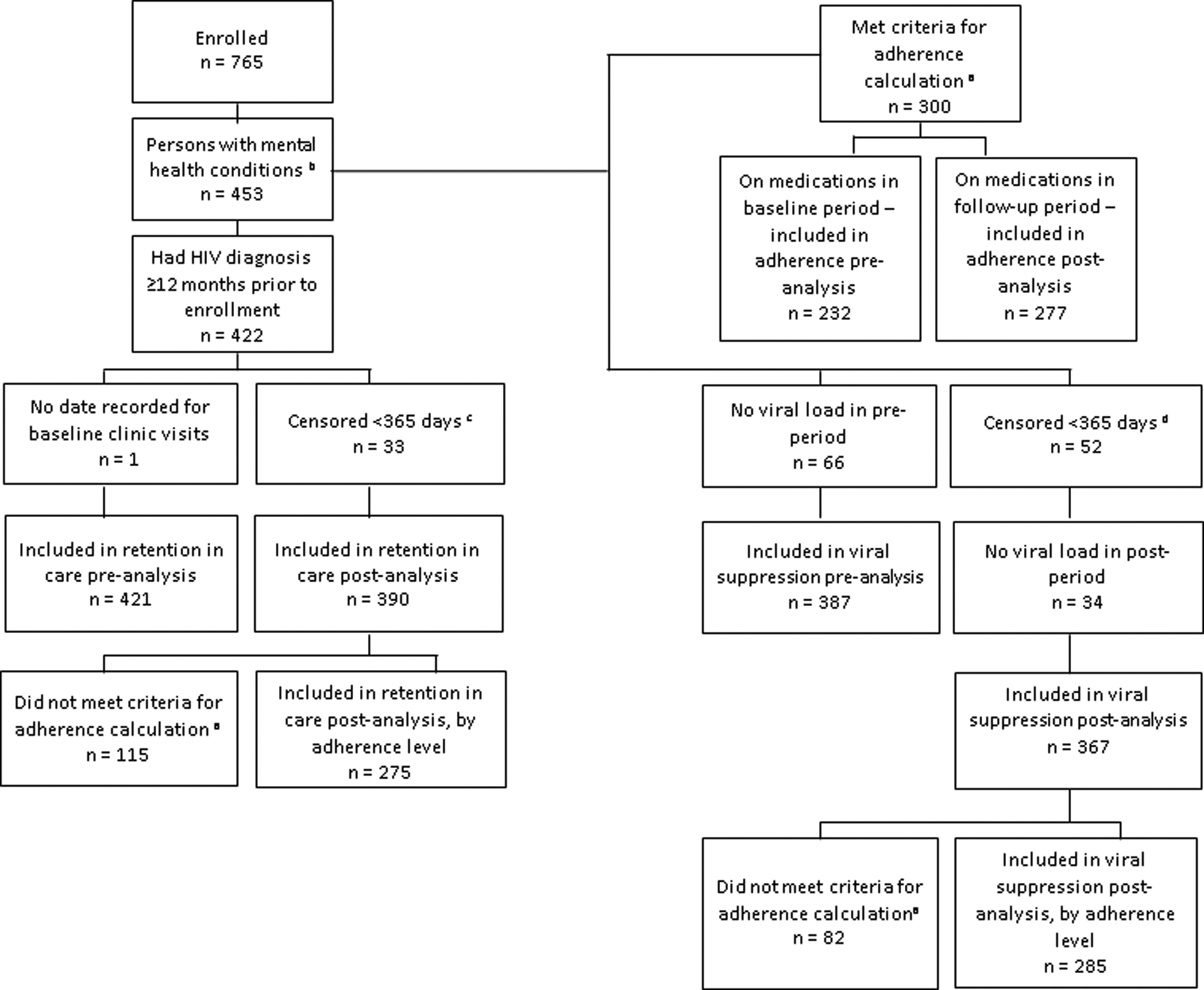
a Adherence was calculated for persons with at least a total of 90 days’ supply (in a 12 month measurement period) of an antidepressant, antianxiety or antipsychotic medication, excluding prescriptions for benzodiazepines, hydroxyzine and for <30-day supplies of medications.
b Persons were considered to have a mental health condition if they had a mental health diagnosis in the pre-implementation clinic record or had filled ≥1 mental health prescription (for an antidepressant, antianxiety, or antipsychotic medication) in the pre-implementation period.
c Persons were censored in the post-implementation period and were excluded from the retention in care analysis. Persons were censored for the following reasons: 1 immediately censored; 4 died; 2 too ill; 7 moved out of the area; 7 transferred care; 5 no longer able to fill at project (or project network) pharmacy for insurance reasons; 2 transferred prescriptions to a non-project (or non-project network) pharmacy. Two project sites did not collect censoring data. For individuals from these two sites, persons were censored one day after the date of the last clinic visit if a person had no clinic visit or HIV laboratory test drawn for >6 months but continued to fill prescriptions in the last six months of the project implementation period; 5 persons were censored for this reason.
d Persons were censored in the post-implementation period and were excluded from the viral suppression analysis. Persons were censored for the following reasons: 7 patients died; 3 too ill; 10 moved out of area; 9 transferred care; 2 incarcerated; 10 no longer able to fill at project (or project network) for insurance reasons; 5 transferred prescriptions to a non-project (or non-project network) pharmacy. Two project sites did not collect censoring data. For individuals from these two sites, persons were censored one day after the date of the last clinic visit if a person had no clinic visit or HIV laboratory test drawn for >6 months but continued to fill prescriptions in the last six months of the project implementation period; 6 persons were censored for this reason.
Psychiatric therapy-related issues and suggested resolutions
The largest proportion of psychiatric therapy-related issues identified by project pharmacists were related to suboptimal therapy (12%), patient adherence (11%), and medical record documentation (10%). Medical record documentation refers to updating the medical record to reflect any changes to patients’ medical history or treatment (e.g., update patient medication list to remove discontinued medications). The largest proportion of pharmacist suggested resolutions to identified problems were medication modification (16%) and medical record update (10%) (data not shown).
Retention in care
Overall, retention in care improved among persons with mental health conditions a relative 13% from 60% to 68% (p=0.009), pre- to post-model implementation. Increases in retention in care were seen among persons who were: non-Hispanic black (+21%; 62% to 75% [p=0.007]); female (+21%; 56% to 68% [p=0.036]); Medicare insured (+40%; 65% to 91% [p=0.028]); covered by the Ryan White/AIDS Drug Assistance Program (ADAP) (+29%; 62% to 80% [p=0.023]); and diagnosed with baseline substance use (+36%; 50% to 68% [p=0.036]). There were also substantial increases among persons with a psychotic disorder (+31%; 59% to 78% [p=0.056] and persons covered by private insurance (+21%; 60% to 72% [p=0.207]) although these increases were not significant at the p<0.05 level. (Table I)
Viral suppression
Overall, viral suppression improved among persons with mental health conditions a relative 13% from 79% to 90% (p<0.001), pre- to post-model implementation. Increases in viral suppression were seen within most demographic groups with notable improvements among non-Hispanic black persons (+32%; from 64% to 84% [p<0.001]) and persons with a baseline diagnosis of substance use (+32%; 66% to 86% [p=0.001]). Viral suppression improved a relative 20% (70% to 83%, [p=0.070]) among persons with a psychotic disorder although this improvement was not significant at the p<0.05 level. (Table I)
Adherence to psychiatric medications
The mean PDC for each drug grouping was ≥0.80 in both the pre- and post-implementation periods. Overall, there were no statistically significant differences in the mean PDC or the proportions of persons adherent to psychiatric medications in any drug grouping, pre- to post-implementation. (Table II)
Table II.
Mean adherence and the proportion of persons adherent (Proportion of Days Covered [PDC] ≥0.80) to mental health medications, pre- and post-model implementation Patient-centered HIV Care Model, 2014 – 2016, United States
| Mean PDC and rangea | Proportion with PDC ≥0.80b | ||||||
|---|---|---|---|---|---|---|---|
| Drug Category | Baseline Mean (range) | Follow-up Mean (range) | p-value | Baseline % | Follow-up % | % Changec | p-value |
| Antidepressant/Antianxietyd | 0.88 (0.28 – 1.00) | 0.90 (0.37 – 1.00) | 0.207 | 79% | 84% | +7 | 0.186 |
| Antipsychotice | 0.80 (0.42 – 1.00) | 0.86 (0.48 – 1.00) | 0.276 | 50% | 65% | +30 | 0.302 |
| Both Antidepressant/Antianxiety & Antipsychoticf | 0.87 (0.31 – 1.00) | 0.88 (0.32 – 1.00) | 0.061 | 78% | 80% | +3 | 0.470 |
Adherence was calculated for persons with at least a total of 90 days’ supply of an antidepressant, antianxiety or antipsychotic medication, excluding prescriptions for benzodiazepines, hydroxyzine and for <30-day supplies of medications. The PDC was calculated from the index fill date to the date of the last fill within a 12-month measurement period.
A PDC ≥0.80 was considered adherent.
Relative percentage change.
The total number of persons who filled antidepressant/antianxiety medications only, in either the pre- or post-implementation period: n = 211; of these individuals 166 and 172 filled this category of medication in the baseline and follow-up periods, respectively.
The total number of persons who filled antipsychotic medications only, in either the pre- or post-implementation period: n = 22; of these individuals 16 and 20 filled this category of medication in the baseline and follow-up periods, respectively.
The total number of persons who filled antidepressant/antianxiety medications and antipsychotic medications, in either the pre- or post-implementation period: n = 67; of these individuals 50 and 85 filled both categories of medications in the baseline and follow-up periods, respectively.
Retention in care and viral suppression by psychiatric medication adherence level
After adjustment for baseline retention in care, there were no statistically significant differences in the proportion of persons retained in care by adherence level in any psychiatric drug grouping (including persons not on therapy), post-implementation. There were no statistically significant differences in the proportion of persons virally suppressed by adherence level in any psychiatric drug category, post-implementation. (Table III)
Table III:
Proportion of persons with HIV and mental health conditions who were retained in care and the proportion virally suppressed post-implementation, by drug category and adherence level, Patient-centered HIV Care Model, 2014 – 2016, United States
| Retained in carea | |||||
|---|---|---|---|---|---|
| Drug category | Adherence levelb | nc | % retained in cared | RRe (95% CI)f | p-value |
| Antidepressant/Antianxiety | |||||
| Adherent | 127 | 68% | ref | --- | |
| Non-adherent | 25 | 64% | 0.943 (0.691, 1.29) | 0.713 | |
| Not on therapy | 29 | 69% | 1.11 (0.890, 1.37) | 0.329 | |
| Antipsychotic | |||||
| Adherent | 12 | 83% | ref | --- | |
| Non-adherent | 7 | 86% | 1.69 (0.588, 4.89) | 0.343 | |
| Both Antidepressant/Antianxiety & Antipsychotic | |||||
| Adherent | 60 | 75% | ref | --- | |
| Non-adherent | 15 | 73% | 1.02 (0.742, 1.39) | 0.919 | |
| Virally suppressed (HIV RNA <200 copies/mL) | |||||
| Drug category | Adherence level b | n c | % virally suppressed d | RRg (95% CI) f | p-value |
| Antidepressant/Antianxiety | |||||
| Adherent | 129 | 92% | ref | --- | |
| Non-adherent | 24 | 83% | 0.905 (0.731, 1.12) | 0.358 | |
| Not on therapy | 38 | 79% | 0.844 (0.685, 1.04) | 0.112 | |
| Antipsychotic | |||||
| Adherent | 13 | 92% | ref | --- | |
| Non-adherent | 6 | 67% | 0.733 (0.404, 1.33) | 0.308 | |
| Both Antidepressant/Antianxiety & Antipsychotic | |||||
| Adherent | 61 | 92% | ref | --- | |
| Non-adherent | 14 | 100% | 0.376 (0.022, 6.43) | 0.607 | |
Retention in care was defined as ≥1 medical visit with a physician, nurse practitioner, or physician assistant, in each 6-month period of a 12-month measurement period with a minimum of 60 days between medical visits. Pre-implementation retention was measured during the 12 months leading up to and including the enrollment date and post-implementation retention was measured from one day after the enrollment date to 12 months forward.
Adherence was calculated for persons with at least a total of 90 days’ supply of an antidepressant, antianxiety or antipsychotic medication, excluding prescriptions for benzodiazepines, hydroxyzine and for <30-day supplies of medications. Adherence was measured as the Proportion of Days Covered (PDC). The PDC was calculated from the index fill date to the date of the last fill within the 12-month measurement period. Adherence was defined as a PDC ≥0.80. A PDC <0.80 was considered non-adherent. The “not on therapy” level included persons with a diagnosis of depression or dysthymia and/or anxiety (without any other co-morbid mental health condition) who never filled a prescription for a mental health medication during the measurement period.
The number of persons in each adherence level.
Proportion of persons retained in care or virally suppressed within each adherence level.
RR = relative risk of retention in care by adherence level (i.e., risk of retention in care among persons non-adherent or “not on therapy” relative to the risk among persons adherent to their psychiatric medication); the RR estimates were adjusted for baseline retention in care.
CI = confidence interval.
RR = relative risk of viral suppression by adherence level (i.e., risk of viral suppression among persons non-adherent or “not on therapy” relative to the risk among persons adherent to their psychiatric medication); the RR estimates are unadjusted.
Discussion
The Patient-centered HIV Care Model increased both retention in care and viral suppression among persons with HIV and co-morbid mental health conditions, a relative 13%. Post-implementation, both the proportion retained in care and virally suppressed were higher than national estimates for the general HIV population which are 57% and 60%, respectively. (31) In addition, viral suppression improved among most demographic groups with the overall proportion suppressed reaching 90%, a level which reached the Joint United Nations Programme on HIV/AIDS (UNAIDS) viral suppression goal of 90% and surpassed the 2020 U.S. National HIV/AIDS Strategy viral suppression goal of 80%. (32, 33) Persons with a baseline substance use diagnosis had notable improvements in both retention in care and viral suppression. Noteworthy improvements were also seen among persons with a baseline diagnosis of a psychotic disorder; although these differences did not reach statistical significance at the p-value threshold of <0.05 (which was likely due to small sample size) the improvement may, nonetheless, be clinically significance. The model did not improve adherence to psychiatric medications. There was no association found between adherence to psychiatric medications and retention in care or viral suppression which might be reflective of high baseline psychiatric medication adherence.
Large improvements were seen in retention in care and viral suppression among persons with co-occurring mental health condition(s) and substance use. These improvements are meaningful because substance use is, by itself, associated with key determinants of health outcomes such as ART non-adherence and immunosuppression (34) and because psychosocial co-morbidities have shown a dose response relationship to poor HIV care continuum outcomes (i.e., the greater number of syndemic factors the greater likeliness of poor care continuum outcomes). (17, 18, 20, 21) Further, persons triply diagnosed with HIV, mental illness and substance use have been shown to have lower viral suppression than either persons with substance use only or persons with neither condition. (35, 36) For example, a cross-sectional study from seven HIV Research Network clinical sites demonstrated that persons with HIV who had both mental illness and illicit drug use (Odds ratio 0.66 [95% CI: 0.58 – 0.75]) or illicit drug use only (Odds ratio 0.77 [0.67 – 0.88]) had lower odds of viral suppression compared with persons with neither condition. (35) Also, HIV outbreaks among persons who inject drugs have resulted in large numbers of new infections and rapid transmission. (37–39) A key goal of HIV outbreak response is to quickly link newly diagnosed persons into care and to achieve viral suppression. Collaborative models of care that improve viral suppression among this population can not only improve the health of individuals with HIV but can help reduce transmission risk.
Although less is known about the syndemic effect of mental illness and substance use on retention in HIV care, studies have shown an inverse relationship between substance use and retention in care. (13, 40, 41) A study from the multisite Center for AIDS Research Network of Integrated Clinical Systems found lower proportions of persons with substance use disorder were retained in care (67%) compared to those without a substance use disorder (76%). (13) Our study found that the PCHCM collaborative model increased retention in HIV care a relative 36%, among persons with substance use and mental health conditions.
The PCHCM did not improve adherence to psychiatric medications. However, baseline adherence to psychiatric medications was high with the mean PDC within each drug category at ≥0.80. After adjusting for baseline retention in care we found no statistically significant difference in post-implementation retention by adherence to any category of psychiatric medication. However, the sample of persons non-adherent (or not on therapy) to their psychiatric medication(s) was small; the viral suppression outcome showed similar results.
The project analyses have limitations. First, we were unable to distinguish between episodic and ongoing mental health conditions. However, we tried to minimize the inclusion of persons with episodic or short-term conditions by excluding persons with prescriptions for psychiatric medications meant for short term use (e.g., benzodiazepines, prescriptions with less than 30-day supply). Utilization of mental health services has been associated with retention in HIV care (5); we, however, were unable to account for this potential confounder. The PDC measure is a proxy for adherence which measures the amount of time a person has medication in hand not whether a person is taking their medication; adherence was, therefore, likely overestimated. Substance use was determined by documentation of a diagnosis of substance use or dependence in the baseline clinic record. The analysis did not account for new substance use diagnoses (i.e., diagnoses during the post-implementation period), the severity of substance use (e.g., use versus dependence), type of substances used and did not distinguish between current and past use. Lastly, the PCHCM was a demonstration project, not a research study; the pretest-posttest evaluation design is not as rigorous as a study with control groups.
Conclusion
The Patient-centered HIV Care Model demonstrated a relative 13% improvement in both retention in care and viral suppression among persons with HIV and mental health conditions. Further, both outcomes improved >30% among persons triply diagnosed with HIV, mental illness and substance use. These improvements are significant because the presence of complex syndemics, such as co-morbid mental illness and substance use, can undercut the benefits of HIV care. Programs, such as the PCHCM, that require collaboration between community-based pharmacists and primary medical providers and extend outside of medical clinics settings can be successfully implemented to improve HIV care continuum interventions among persons with HIV and mental health conditions.
Acknowledgements
This work was supported by the Secretary’s Minority AIDS Initiative Fund and the Centers for Disease Control and Prevention through a co-operative agreement [grant number NU65PS004275] with the University of North Texas Health Science Center System College of Pharmacy. Walgreens Co. provided all pharmacist services in-kind.
Footnotes
The findings and conclusions of this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
References
- 1 ).Tegger MK, Crane HM, Tapia KA, et al. The effect of mental illness, substance use, and treatment for depression on the initiation of highly active antiretroviral therapy among HIV-infected individuals. AIDS Patient Care STDS 2008; 22 (3): 233–243. [DOI] [PubMed] [Google Scholar]
- 2 ).Kessler RC, Demler O, Frank RG, et al. Prevalence and treatment of mental disorders, 1990 to 2003. N Engl J Med 2005; 352 (24): 2515–2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3 ).Bing EG, Burnam MA, Longshore D, et al. Psychiatric disorders and drug use among human immunodeficiency virus-infected adults in the United States. Arch Gen Psychiatry 2001; 58 (8): 721–728. [DOI] [PubMed] [Google Scholar]
- 4 ).Nanni MG, Caruso R, Mitchell AJ, et al. Depression in HIV infected patients: A review. Curr Psychiatry Rep 2015; 17 (1): 530. [DOI] [PubMed] [Google Scholar]
- 5 ).Rooks-Peck CR, Adegbite AH, Wichser ME, et al. Mental health and retention in HIV care: A systematic review and meta-analysis. Health Psychol 2018; 37 (6): 574–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6 ).Gonzalez JS, Batchelder AW, Psaros C, et al. Depression and HIV/AIDS treatment nonadherence: A review and meta-analysis. J Acquir Immune Defic Syndr 2011; 58 (2): 181–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7 ).Springer SA, Dushaj A, Azar MM. The impact of DSM-IV mental disorders on adherence to combination antiretroviral therapy among adult persons living with HIV/AIDS: A systematic review. AIDS Behav 2012; 16 (8): 2119–2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8 ).Pence BW, Miller WC, Gaynes BN, et al. Psychiatric illness and virologic response in patients initiating highly active antiretroviral therapy. J Acquir Immune Defic Syndr 2007; 44 (2): 159–166. [DOI] [PubMed] [Google Scholar]
- 9 ).Yehia BR, Stephens-Shield AJ, Momplaisir F, et al. Health outcomes of HIV-infected people with mental illness. AIDS Behav 2015; 19 (8): 1491–1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10 ).Pence BW, Mills JC, Bengtson AM, et al. Association of increased chronicity of depression with HIV appointment attendance, treatment failure, and mortality among HIV-infected adults in the United States. JAMA Psychiatry 2018; 75 (4): 379–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11 ).Remien RH, Stirratt MJ, Nguyen N, et al. Mental health and HIV/AIDS: The need for an integrated response. AIDS 2019; 33 (9): 1411–1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12 ).Helleberg M, Pedersen MG, Pedersen CB, et al. Associations between HIV and schizophrenia and their effect on HIV treatment outcomes: A nationwide population-based cohort study in Denmark. Lancet HIV 2015; 2 (8): e344–350. [DOI] [PubMed] [Google Scholar]
- 13 ).Hartzler B, Dombrowski JC, Crane HM, et al. Prevalence and predictors of substance use disorders among HIV care enrollees in the United States. AIDS Behav 2017; 21 (4): 1138–1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14 ).Substance Abuse and Mental Health Services Administration, Center for Behavioral Health Statistics and Quality (December 1, 2010). The NSDUH report: HIV/AIDS and substance use. Rockville, MD. [Google Scholar]
- 15 ).Nolan S, Walley AY, Heeren TC, et al. HIV-infected individuals who use alcohol and other drugs, and virologic suppression. AIDS Care 2017; 29 (9): 1129–1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16 ).Hartzler B, Dombrowski JC, Williams JR, et al. Influence of substance use disorders on 2-year HIV care retention in the United States. AIDS Behav 2018; 22 (3): 742–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17 ).Biello KB, Oldenburg CE, Safren SA, et al. Multiple syndemic psychosocial factors are associated with reduced engagement in HIV care among a multinational, online sample of HIV-infected MSM in Latin America. AIDS Care 2016; 28 Suppl 1: 84–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18 ).Blashill AJ, Bedoya CA, Mayer KH, et al. Psychosocial syndemics are additively associated with worse ART adherence in HIV-infected individuals. AIDS Behav 2015; 19 (6): 981–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19 ).Friedman MR, Stall R, Silvestre AJ, et al. Effects of syndemics on HIV viral load and medication adherence in the Multicentre AIDS Cohort Study. AIDS 2015; 29 (9): 1087–1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20 ).Glynn TR, Safren SA, Carrico AW, et al. High levels of syndemics and their association with adherence, viral non-suppression, and biobehavioral transmission risk in Miami, a U.S. City with an HIV/AIDS epidemic. AIDS Behav 2019; 23 (11): 2956–2965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21 ).Harkness A, Bainter SA, O’Cleirigh C, et al. Longitudinal effects of syndemics on ART non-adherence among sexual minority men. AIDS Behav 2018; 22 (8): 2564–2574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22 ).Mizuno Y, Purcell DW, Knowlton AR, et al. Syndemic vulnerability, sexual and injection risk behaviors, and HIV continuum of care outcomes in HIV-positive injection drug users. AIDS Behav 2015; 19 (4): 684–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23 ).Byrd KK, Hardnett F, Clay PG, et al. Retention in HIV care among participants in the Patient-centered HIV Care Model: A collaboration between community-based pharmacists and primary medical providers. AIDS Patient Care STDS 2019; 33 (2): 58–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24 ).Byrd KK, Hou JG, Bush T, et al. Adherence and viral suppression among participants of the Patient-centered HIV Care Model project-a collaboration between community-based pharmacists and HIV clinical providers. Clin Infect Dis 2019; 70 (5): 789–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25 ).American Pharmacists Association, National Association of Chain Drug Stores Foundation. Medication Therapy Management in pharmacy practice: Core elements of an MTM service model (version 2.0). J Am Pharm Assoc (2003) 2008; 48: 341–353. [DOI] [PubMed] [Google Scholar]
- 26 ).Department of Health and Human Services. HIV/AIDS Bureau performance measures. Available at: https://hab.hrsa.gov/sites/default/files/hab/clinical-quality-management/coremeasures.pdf. Accessed June 5, 2018.
- 27 ).Nau D Proportion of Days Covered (PDC) as a preferred method of measuring medication adherence. Available at: http://www.pqaalliance.org/images/uploads/files/pqa%20pdc%20vs%20%20mpr.pdf. Accessed June 5, 2018.
- 28 ).PQAalliance. PQA measure overview. Available at: https://www.PQAalliance.org/assets/measures/2019_pqa_measure_overview.pdf. Accessed September 14, 2019.
- 29 ).Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in adults and adolescents living with HIV. Department of Health and Human Services. Available at http://www.aidsinfo.nih.gov/contentfiles/adultandadolescentgl.pdf. Accessed June 5, 2018. [Google Scholar]
- 30 ).Günthard HF, Saag MS, Benson CA, et al. Antiretroviral drugs for treatment and prevention of HIV infection in adults: 2016 recommendations of the International Antiviral Society–USA Panel. JAMA. 2016; 316(2): 191–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31 ).Centers for Disease Control and Prevention. Monitoring selected national HIV prevention and care objectives by using HIV surveillance data—United States and 6 dependent areas, 2016. HIV surveillance supplemental report 2018; 23 (no. 4). http://www.cdc.gov/hiv/library/reports/hiv-surveillance.html. Published June 2018 Accessed September 10, 2019. [Google Scholar]
- 32 ).White House Office of National AIDS Policy. National HIV/AIDS strategy for the United States: Updated to 2020. Available at: https://files.hiv.gov/s3fs-public/nhas-update.pdf.
- 33 ).Joint United Nations Programme on HIV/AIDS (UNAIDS). 90–90-90 an ambitious treatment target to help end the AIDS epidemic. Available at: https://www.unaids.org/sites/default/files/media_asset/90-90-90_en_0.pdf Accessed April 8, 2020.
- 34 ).Durvasula R, Miller TR. Substance abuse treatment in persons with HIV/AIDS: Challenges in managing triple diagnosis. Behav Med 2014; 40 (2): 43–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35 ).Chander G, Himelhoch S, Fleishman JA, et al. HAART receipt and viral suppression among HIV-infected patients with co-occurring mental illness and illicit drug use. AIDS Care 2009; 21 (5): 655–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36 ).Yellin H, Beckwith C, Kurth A, et al. Syndemic effect of mental illness and substance use on viral suppression among recently-incarcerated, HIV-infected individuals in the CARE+ corrections study. AIDS Care 2018; 30 (10): 1252–1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37 ).Cranston K, Alpren C, John B, et al. Notes from the field: HIV diagnoses among persons who inject drugs - northeastern Massachusetts, 2015–2018. MMWR Morb Mortal Wkly Rep 2019; 68 (10): 253–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38 ).Golden MR, Lechtenberg R, Glick SN, et al. Outbreak of human immunodeficiency virus infection among heterosexual persons who are living homeless and inject drugs - Seattle, Washington, 2018. MMWR Morb Mortal Wkly Rep 2019; 68 (15): 344–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39 ).Peters PJ, Pontones P, Hoover KW, et al. HIV infection linked to injection use of oxymorphone in Indiana, 2014–2015. N Engl J Med 2016; 375 (3): 229–239. [DOI] [PubMed] [Google Scholar]
- 40 ).Mugavero MJ, Lin HY, Willig JH, et al. Missed visits and mortality among patients establishing initial outpatient HIV treatment. Clin Infect Dis 2009; 48 (2): 248–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41 ).Hall HI, Gray KM, Tang T, et al. Retention in care of adults and adolescents living with HIV in 13 U.S. Areas. J Acquir Immune Defic Syndr 2012; 60 (1): 77–82. [DOI] [PubMed] [Google Scholar]


