Abstract
Aims:
To test the efficacy of a brief intervention to reduce alcohol or drug use and to promote use of addiction services among patients seeking mental health treatment.
Design and setting:
A multi-centre, longitudinal, two-group randomised controlled trial with randomisation within each of two mental health treatment systems located in Ventura County and Los Angeles County in California, USA.
Participants:
A total of 718 patients (49.2% female) aged 18 and older with a mental health diagnosis and either a heavy drinking day or any use of cannabis or stimulants in the past 90 days.
Intervention and comparator:
A motivation-based brief intervention with personalized feedback (Screening, Brief Intervention and Referral to Treatment (SBIRT) condition) (n=354) or a health education session (control condition) (n=364).
Measurements:
Primary outcomes included frequency of heavy drinking days, days of cannabis use and days of stimulant use at the primary endpoint 3 months post baseline. Secondary outcomes included frequency and abstinence from substance use out to a 12-month follow up and the use of addiction treatment services.
Findings:
Participants in the SBIRT condition had fewer heavy drinking days (odds ratio = 0.53; 95% CrI 0.48 – 0.6) and fewer days of stimulant use (odds ratio = 0.58; 95% CrI 0.50 – 0.66) at the 3-month follow-up compared with participants in the health education condition. Participants in the SBIRT condition did not comparatively reduce days of cannabis use at the 3-month follow-up (odds ratio = 0.93; 95% CrI 0.85 – 1.01). Secondary outcomes indicated sustained effects of SBIRT on reducing the frequency of heavy drinking days and days of stimulant use. No effects were observed on abstinence rates or use of addiction treatment services.
Conclusions:
Screening and brief intervention for unhealthy alcohol and drug use in mental health treatment settings were effective at reducing the frequency of heavy drinking and stimulant use.
Keywords: screening, brief intervention, referral to treatment, alcohol, drug, mental health, psychiatry
Introduction
Screening, Brief Intervention and Referral to Treatment (SBIRT) interventions are designed to reduce risky substance use, mitigate progression to substance use disorder (SUD) and link people who currently have an SUD to specialty treatment. In the United States, of the estimated 47.6 million adults with mental illness in 2018, 9.2 million had both a mental and substance use disorder.1 Moreover, an estimated 31.3 percent of adults with mental illness were binge drinkers in the past year, relative to 25.3 percent of those with no mental illness. Likewise, 36.7 percent of those with mental illness reported drug use in the past year, as compared to those without mental illness (15.7 percent). Among those with co-occurring mental illness and an SUD, only half (51.4 percent) received either mental health care or specialty substance use treatment, and 48.6% received neither type of care.1
SBIRT interventions have been extensively evaluated in medical settings including primary care and emergency departments. Overall, evidence from clinical trials of SBIRT interventions indicate some efficacy for reducing unhealthy alcohol use in emergency department settings, particularly among those with low or moderate levels of use. In contrast, there is less evidence of efficacy of this approach for reducing drug use and for those with an alcohol or other substance use disorder.2 Few studies have evaluated referrals to alcohol and other SUD treatment in medical settings; however, current evidence suggests that extant referral to treatment protocols are ineffective in increasing access to specialty treatment.3 Nevertheless, the current literature in this area is hampered by small sample sizes, potential implementation failure given the lack of reporting fidelity, lack of comparability across studies due to the measurement of outcomes, and self-reported outcomes without biological confirmation.4,5
While general medical settings are ideal for population-based approaches such as SBIRT, given the disproportionately high rates of heavy alcohol and drug use and SUD among those with mental illness,1,6–8 primary mental health treatment settings might be particularly well suited for SBIRT intervention strategies. Moreover, mental health clinics may be limited by time and workforce constraints that preclude the use of intensive assessment and treatment methods to address problematic substance use and SUDs. As such, the SBIRT approach has the potential to meet the need for brief assessment to reduce unaddressed alcohol and substance use in these settings, coupled with brief intervention to raise awareness of the relationship between substance use and mental health conditions among patients with psychiatric illness. Although few randomised-controlled studies of SBIRT-related interventions have been conducted in mental health settings,9,10 at least one investigation in which a brief motivational intervention was delivered in a psychiatric inpatient setting demonstrated evidence of potential efficacy in facilitating engagement in SUD treatment among individuals with schizophrenia or bipolar disorder.11
Given the disparate findings on the efficacy of SBIRT, both generally and specially for patients in mental health settings, this article reports findings from a randomised controlled trial of SBIRT for alcohol and non-prescription substance use targeting adults receiving care in mental health treatment settings. Our primary hypotheses were that SBIRT participants would have (1) fewer days of heavy drinking, (2) fewer days of stimulant use and (3) fewer days of cannabis use at the 3-month follow up compared to participants in an attention-matched health-education control condition. Our secondary hypotheses were that over the 6- and 12-month time points SBIRT participants would have less frequent heavy drinking, stimulant and cannabis use and they would be more likely to abstain from these substances. We also hypothesized that SBIRT participants would demonstrate a higher level of attendance at specialty addiction treatment relative to participants in the control condition.
Method
Setting and Recruitment
The study was conducted in 2 mental health treatment systems: A community-based public system (Ventura County Behavioral Health, n=313) and a university-based health care system (UCLA Psychiatry Clinics, n=405). Across these treatment systems a total of 6 outpatient clinics and 1 inpatient clinic participated. Participants were recruited between June, 2013 and May, 2016 until target sample was reached. Recruitment strategies included flyers, clinician referrals, and word of mouth. Flyers indicated that the study intervention addressed substance use among adults receiving mental health treatment. Follow-ups continued until mid-2017.
To be eligible for the study, participants were required to: (1) be ≥ 18 years old; (2) have a diagnosis of affective disorder (i.e., Major Depressive Disorder, Dysthymia, Bipolar Disorder) or psychotic disorder (i.e., Schizophrenia, Schizoaffective Disorder, or Psychotic Disorder Not Otherwise Specified) assessed using the Mini-International Neuropsychiatric Interview12; and (3) report any use of cannabis or stimulants or one or more heavy drinking days (≥5 drinks for men, ≥4 for women) in the past 90 days. Opioid users were eligible to participate if they also reported any heavy drinking, cannabis or stimulant use. Individuals were excluded if they: (1) had an unstable living situation (i.e., homeless in the past 2 years); (2) were under the influence of alcohol or drugs at the time of enrollment (verified using breathalyzer and clinical judgment); or had (3) received behavioral and/or pharmacological treatment for a SUD in the previous 90 days. The study was approved by both the Ventura County Behavioral Health IRB and the UCLA IRB.
Sample Size Determination
Sample size was determined a priori for planned tests of mean differences in alcohol use across the SBIRT and control condition at the 3-month time point. A meta-analysis of motivational interventions included 9 studies with an outcome of quantity of alcohol use spanning 6–26 weeks post baseline.13 The average of those effect sizes was a small-to-medium effect of d = .29 and that effect size was used for sample size planning. Power analysis yielded a sample of n=352 (n=176 for each study condition) based on a 2-tailed test of a mean difference with alpha=.05, power = .80 and 20% attrition. The study was powered to detect an intervention effect within subgroups of high risk and moderate risk users hence the total target enrollment was n=704 (i.e., n=352 high risk users and n=352 moderate risk users).
Study Design and Procedures
A parallel design was used in which participants were randomised within each of the two mental healthcare systems 1:1 to either a single Screening, Brief Intervention, & Referral to Treatment (SBIRT) session (n=156 at Ventura County, n=198 at UCLA) or to a Health Education (HE) session (n=157 at Ventura County, n=207 at UCLA). In-person assessments were conducted at baseline and at 3-, 6-, and 12-months post baseline.
A trained research assistant screened all potential participants for eligibility either in person or by phone using a brief script. Eligible participants were scheduled for a baseline or a baseline was conducted on the spot if time allowed. Participants went through the consent process and completed the baseline assessments in one visit. Immediately after the baseline assessment participants were escorted to another office to receive either the SBIRT or HE session. This approach resulted in almost all enrolled participants (98.5%) receiving the SBIRT or HE session. Participants were not compensated for the intervention session but they received $40 in gift cards as compensation for each of the 4 research assessment visits.
Randomisation
Computer-generated block randomisation was used to balance the two study conditions on (1) primary psychiatric disorder (affective versus bipolar or psychotic disorder), (2) primary substance (alcohol versus other drug) and (3) gender. In total there were 8 stratifying groups and within each strata participants were randomised into condition using a block length of 4 (i.e., for every 4 randomisations within a strata 2 participants were assigned to SBIRT and 2 participants were assigned to health education). This balancing was conducted to minimize between-group differences on variables that could impact participant prognosis. Study staff utilized an online randomisation program created specifically for the project. Blinding to randomisation was not possible for the delivery of the intervention. For the follow-ups there was not perfect blinding, although study staff were instructed not to try to ascertain at the follow-ups which intervention a participant received.
Interventions
Screening, Brief Intervention, and Referral to Treatment (SBIRT).
SBIRT was delivered in a single, face-to-face session by Master’s-level providers (information on providers is given below). The SBIRT condition included the World Health Organization’s Alcohol, Smoking, and Substance Involvement Screening Test (ASSIST) and its accompanying brief intervention.14 The brief intervention included motivational interviewing techniques to provide feedback, emphasize personal responsibility, give advice, provide a menu of options, convey empathy, and promote self-efficacy. The duration of the SBIRT sessions was 33.9 (12.6) minutes (mean(sd)). The duration was based on the length of the audio recordings and it included all aspects of screening, manual scoring, feedback, brief intervention and the referral-to-treatment discussion (when indicated). The time excluded subsequent appointment reminder phone calls. The screening and referral-to-treatment components each lasted approximately 5–10 minutes.
All participants randomised to the SBIRT condition received the ASSIST screening component of the intervention. Participants who scored in the moderate or high-risk range for alcohol, cannabis or stimulants subsequently received the brief intervention. Participants who scored in the low-risk range were given feedback that their alcohol or drug use was low-risk and were encouraged not to increase their level of use. Participants who scored in the high-risk range on the ASSIST (specific substance score of 27 or higher) received the brief intervention and also were encouraged to accept a referral to specialty addiction treatment. Approximately two-thirds of the sample used multiple substances. Clinicians gave feedback about the risk level for all substances used and then focused the brief intervention on the highest-risk substance. In the event that multiple substances were at the same risk level clinicians addressed up to two substances during the brief intervention. If more than two substances were at the same risk level clinicians asked participants which substances were the most important to talk about.
The referral-to-treatment component of the SBIRT condition included the following components: (1) Facilitate a “warm hand-off” with staff at each treatment site. (2) Provide each participant a menu of options (e.g., detoxification, outpatient and inpatient) and discuss his/her preferences. Resistance was addressed in a non-judgmental manner and participants were encouraged to try an initial and noncommittal visit to specialty treatment. (3) If the participant accepted a referral, the counselor either called a point of contact at the designated treatment facility for the next open appointment while the participant was present or delivered a written recommendation for specialty addiction treatment to the patient’s intake clinician. (4) Participants were given the counselor’s telephone number and were encouraged to call the counselor with any questions about the upcoming appointment at specialty care. (5) Discuss and problem solve any potential difficulties related to transportation. Transportation was facilitated by providing participants with bus vouchers (paid for by the study), or with driving directions, bus routes and contact information for the specialty treatment site. (6) Provide a reminder call to participants the day before a scheduled specialty appointment. (7) Provide a follow-up call to participants the day after the appointment.
Health Education.
HE participants received a manualized health psychoeducation session that was intended to be an attention control. The duration of the health education sessions was 20.3 (6.8) minutes (mean(sd)). The intervention focused on the 6 dimensions of health and wellness (e.g., intellectual, social, emotional, physical, environmental, and spiritual). Content was adapted from a wellness manual used in a study of exercise for smoking cessation.15 Of all recordings reviewed, substance use was raised by the participant as a relevant theme in less than 10% of sessions. As such, the effect of these relatively low frequency incidences was unlikely to have impacted treatment outcomes. In those cases in which substance use was brought up, the clinician acknowledged the theme in a similar manner to other health themes that participants spontaneously mentioned in relation to each of the pillars of health discussed.
Training & Fidelity
Six Master’s-level clinicians (5 female and 1 male) were trained by the study P.I.s (MK and SG). Three of the clinicians were advanced graduate students in psychology and three of the clinicians had advanced degrees in social work or public health. The clinicians were employed by the research team and conducted sessions onsite at each of the mental health clinics. For the SBIRT intervention the clinicians participated in a series of training seminars led by a study PI (MK) who is a licensed clinical psychologist with expertise in psychosocial interventions targeting substance use, including SBIRT. The seminars included didactic material, role plays, and supervised practice sessions delivering the intervention. All sessions during the study were audio recorded to promote ongoing training and to assess fidelity. Weekly supervision was provided for the study therapists during all phases of the trial.
The same pool of clinicians delivered the HE sessions. Training in the HE intervention included didactics, role plays, and supervised intervention delivery, provided by one of the study P.I.’s (SG), a licensed clinical psychologist who had used and supervised delivery of the intervention in a prior investigation. Weekly supervision, based upon feedback concerning audiotaped intervention sessions, was provided to the study therapists throughout the trial, to ensure adherence to the HE protocol and discriminability of the intervention from the SBIRT condition.
Fidelity ratings were conducted using a session content checklist16 on about half (48.4%) of all SBIRT sessions. Fidelity ratings were completed on a rolling weekly basis such that each study clinician received information during supervision about the ratings from 1 session during the prior week. In the event that a clinician conducted multiple sessions during a given week the coded session was selected in a non-systematic manner from a list of audio files but formal random selection was not used. These ratings evaluated the entirety of each session and showed a high degree of completion of all required elements (mean(sd) percentage of completed elements per session = 96.1% (0.05)). The completion rates for required elements ranged from 100% to a low of 77.4%.
Measures
Baseline Measures
Diagnoses.
A trained research assistant administered the Mini-International Neuropsychiatric Interview (MINI), a brief structured diagnostic interview for assessing DSM-IV psychiatric disorders.12 Diagnoses of affective, bipolar and psychotic disorders were made based upon the MINI.
Drug Abuse Screening Test (DAST-10).
The DAST-10 is a 10-item questionnaire designed to identify drug-use related problems in the past year.18 It was used to assess overall severity of drug use at baseline.
Alcohol Use Disorders Identification Test (AUDIT).
The AUDIT is a 10-item self-report measure designed to identify individuals with unhealthy alcohol use.19 It was used to assess severity of alcohol use at baseline.
Kessler Psychological Distress Scale (Kessler-6).
The Kessler-6 is a well-validated, highly useful clinical measure of symptoms of psychological distress.20,21
Primary Outcome Measures
Timeline Follow Back (TLFB).
The TLFB was administered at the 3-month follow-up to provide data on the quantity of alcohol consumed on each drinking day for the calculation of the number of heavy drinking days (≥5 drinks for men, ≥4 drinks for women) and the number of days of stimulant and cannabis use in the past 90 days.17 These outcomes were assessed for all participants irrespective of which substances were discussed during the intervention or control session.
Secondary Outcome Measures
The TLFB was used to assess the number of heavy drinking days, days of stimulant use and days of cannabis use over the 90 days preceding the 6- and 12-month follow-ups. The TLFB was also used to assess abstinence from alcohol, stimulants and cannabis at each follow-up timepoint.
Attendance at addiction treatment was evaluated by study staff based on a review of electronic medical records, provider reports of session attendance and participant self-report during the TLFB interview. Data from medical providers and electronic health records were counted first. If participants reported any additional services beyond those in official records then those additional services were added to the count. Participants were coded as having any addiction treatment if they received at least one specialty service (i.e., outpatient group or individual therapy, medication visit, inpatient, partial hospitalization, intensive outpatient) within 30 days of entry into the study. A count of addiction services received during the 3 months between the baseline and the 3-month follow up yielded the total services received. Units of service comprised an outpatient therapy visit or a day in inpatient or intensive outpatient treatment.
Statistical Analysis
For the primary outcomes estimated group effects were evaluated at the 3-month follow-up. All outcomes were modeled using binomial hurdle distributions because there was a fixed number of trials (days) in which a substance was used and a disproportionate number of zeros (non-users) for each outcome. These distributions can be separated into two parts: a logistic model to estimate whether or not any use occurred (zero model) and a zero-truncated binomial model to estimate the days of use given that some amount of use occurred (count model). The count models yield odds ratios that refer to the likelihood of engaging in a target outcome among users on each day in the 90-day assessment period. Higher odds ratios are indicative of more frequent substance use days. The count models were used to examine the primary substance use outcomes. The zero models yield odds ratios that indicate the likelihood of participants being users versus non-users (i.e., abstainers). The zero models were used to test secondary substance use outcomes related to abstinence.
Both the zero and count models reflected the longitudinal study design by including subject level random intercepts to account for the autocorrelation of each individual’s outcome data over time. Models were adjusted for the covariates of study site, race, age, employment status, psychological distress, and severity of alcohol or drug use (models predicting alcohol use were adjusted for baseline drug use severity and vice versa). The analyses were not pre-registered and the results should be considered exploratory.
All models were fit using Bayesian Hamiltonian Monte Carlo algorithms, after attempts at standard frequentist attempts failed to produce viable estimates.22 Group and covariate effects were quantified using the posterior means of odds ratios and associated 95% Bayesian credible intervals (CrI). All analyses were done using R 3.5.1. Bayesian estimates were produced using the RStan package23 and Stan software.24 Missing outcome data were assumed to be Missing at Random and this assumption was supported by Hosmer-Lemeshow goodness of fit tests in multivariate logistic regressions that regressed missingness on a set of observed baseline variables (all p-values > .2). All of those variables were included as covariates in the analyses presented herein. Also, missingness at each time point was independent of study condition (all p-values > .2). Missingness was deemed to be ignorable and no missingness mechanism was modeled. Missing data were not imputed. There were very few missing covariates (<.004%) among cases that had reported substance use during the follow-up period. Sensitivity analyses were conducted such that missing data were replaced with baseline values. Those sensitivity analyses yielded results consistent with the primary analyses that did not impute missing data.
At each follow-up participants provided a urine sample that was analyzed for the presence of cannabis and stimulant metabolites using enzyme immunoassay test procedures. In preliminary work (not shown) we conducted sensitivity analyses to assess if discordance between the urinalysis and self-report data (i.e., positive urinalysis and no self-report of recent use of a given substance) influenced the study findings. We created 2 modified datasets in which discordant cases were either deleted or were re-coded as ‘positive’ for any cannabis and/or methamphetamine use. Analyses predicting abstinence at each follow up using those modified datasets did not provide substantively different results compared to analyses of abstinence based solely on self-report data. Given the lack of substantive differences this paper reports on analyses from the self-report TLFB data.
Secondary outcomes for the utilization of addiction treatment services (receipt of any service and total number of services received) were examined with regression analyses. Prior to regression analysis the addiction services data were 90% Winsorized to reduce the influence of outliers. Models were adjusted for the covariates of study site, race, age, employment status, psychological distress and severity of alcohol and drug use.
Results
Participants
A total of 1,965 people were screened for the study and 1,050 people were deemed eligible to participate. Of those eligible, 718 individuals (68% of those who screened eligible) provided consent and were randomised to study condition. Figure 1 depicts the study participant flow. There were no significant differences in follow-up rates between the two study conditions. Studywide follow-up rates for the 3-, 6- and 12-month assessments were 80.9%, 82.5% and 79.2%, respectively.
Figure 1.
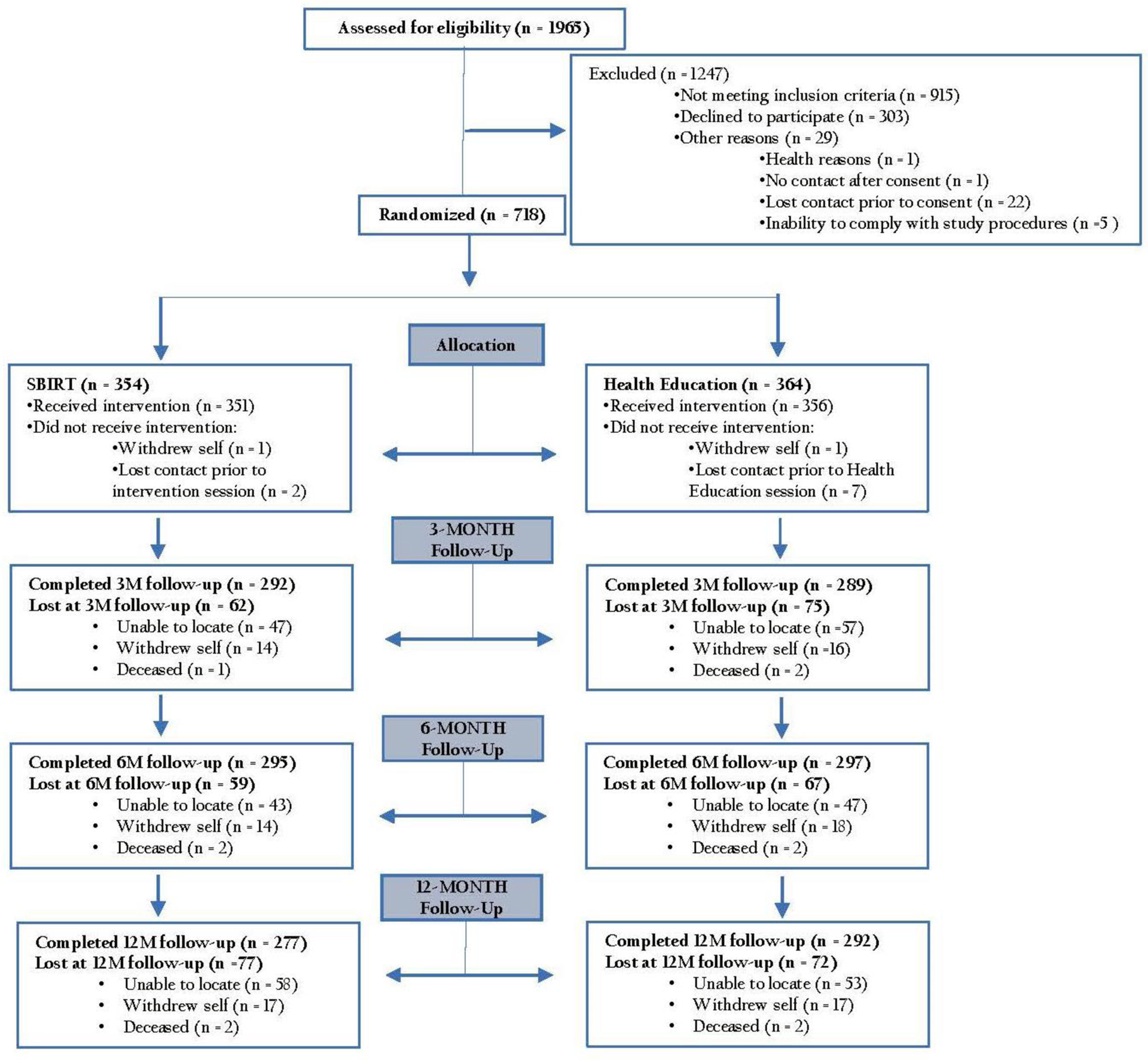
CONSORT flowchart of study enrollment.
Demographics for the 718 participants are shown in Table 1. The sample comprised 49% females and 47% racial and ethnic minorities. Based on mental health chart reviews 49.9% of study participants were diagnosed with an alcohol or drug use disorder. Scores on the Kessler-6 (mean(sd) = 12.4(5.9)) indicated that on average the sample was close to the threshold of severe mental illness (Kessler-6 = 13).20 About one-half of the sample (52.4%) exceeded this threshold.
Table 1.
Baseline characteristics of study participants.
| Control (N=364) | SBIRT (N=354) | All (N=718) | |
|---|---|---|---|
| Categorical Variables | n (%) | n (%) | n (%) |
| Site | |||
| Ventura County | 157 (43.1%) | 156 (44.1%) | 313 (43.6%) |
| UCLA | 207 (56.9%) | 198 (55.9%) | 405 (56.1%) |
| Gender | |||
| Male | 187 (51.4%) | 178 (50.3%) | 365 (50.8%) |
| Female | 177 (48.6%) | 176 (49.7%) | 353 (49.2%) |
| Ethnicity/Race | |||
| Latino (White, non-White) | 106 (29.1%) | 94 (26.6%) | 200 (27.9%) |
| non-Latino White | 192 (52.8%) | 189 (53.4%) | 381 (53.1%) |
| non-Latino non-White | 66 (18.1%) | 71 (20.1%) | 137 (19.1%) |
| Employment (Last 30 days)a | |||
| Employed | 98 (26.9%) | 103 (29.1%) | 201 (28%) |
| Unemployed | 266 (73.1%) | 251 (70.9%) | 517 (72%) |
| AUDIT Score | |||
| 0–7 (Low risk) | 162 (44.5%) | 143 (40.4%) | 305 (42.5%) |
| 8–15 (Moderate risk) | 104 (28.6%) | 103 (29.1%) | 207 (28.8%) |
| 16+ (High risk) | 98 (26.9%) | 108 (30.5%) | 206 (28.7%) |
| DAST-10 Score | |||
| 0–2 (Low risk) | 138 (37.9%) | 136 (38.4%) | 274 (38.2%) |
| 3–5 (Moderate risk) | 105 (28.8%) | 100 (28.2%) | 205 (28.6%) |
| 6+ (High risk) | 121 (33.2%) | 118 (33.3%) | 239 (33.3%) |
| Continuous Variables | Mean (SD) | Mean (SD) | Mean (SD) |
| Age | 34.78 (12.85) | 32.74 (12.28) | 33.78 (12.6) |
| Education (years) | 13.63 (2.68) | 13.71 (2.49) | 13.67 (2.59) |
| Kessler-6 Score | 12.35 (5.8) | 12.38 (5.94) | 12.36 (5.86) |
| Tobacco use (days in past 90) | 32.32 (39.44) | 31.25 (40.26) | 31.79 (39.82) |
| Alcohol use (days in past 90) | 18.84 (23.33) | 21.46 (25.7) | 20.14 (24.55) |
| Primary outcome variables | |||
| Heavy drinking (days in past 90) | 8.91 (16.08) | 12.18 (21.62) | 10.52 (19.07) |
| Cannabis use (days in past 90) | 25.77 (34.46) | 28.82 (35.72) | 27.27 (35.09) |
| Stimulant use (days in past 90) | 7.29 (17.57) | 6.84 (16.61) | 7.07 (17.09) |
| Secondary outcome variables | n (%) | n (%) | n (%) |
| Alcohol abstinence (past 90 days) | 69 (19.0%) | 45 (12.7%) | 114 (15.9%) |
| Cannabis abstinence (past 90 days) | 137 (37.6%) | 124 (35.0%) | 261 (36.4%) |
| Stimulant abstinence (past 90 days) | 238 (65.4%) | 234 (66.1%) | 472 (65.7%) |
‘Employed’ category includes full-time, part-time or military service; ‘Unemployed’ category includes student, retired or homemaker.
Effect of intervention on primary outcomes
Frequency of heavy drinking days
Results from the count model showed that those in the SBIRT condition were likely to have fewer heavy drinking days at the 3-month follow-up compared to participants in the HE control condition (OR=0.53, 95% CrI 0.47–0.59). Adjusted mean for participants in the SBIRT condition were 3.6 heavy drinking days at the 3-month follow-up compared to 7.5 heavy drinking days for participants in the control group. Results are shown in Table 2 and Figure 2.
Table 2.
Odds Ratio (OR) posterior estimates and 95% credible intervals (CrI) from Multivariable Binomial Hurdle models predicting primary substance use outcomes at 3-month follow up.
| Count Modelsa | |||
|---|---|---|---|
| Variable | Heavy Drinking Days (n=505)b OR (95% CrI) | Days of cannabis use (n=523)b OR (95% CrI) | Days of stimulant use (n=299)b OR (95% CrI) |
| Site | .86 (.62, 1.21) | .35 (.19, .6)* | .42 (.26, .67)* |
| Female | 1.1 (.83, 1.46) | .77 (.44, 1.31) | .89 (.59, 1.35) |
| Latinoc | .78 (.55, 1.11) | .68 (.35, 1.32) | .95 (.59, 1.52) |
| non-Latino non-Whitec | .71 (.48, 1.05) | .83 (.4, 1.6) | .78 (.44, 1.38) |
| Employed | .84 (.61, 1.16) | 1.51 (.81, 2.76) | .84 (.52, 1.34) |
| Age | 1.05 (.89, 1.23) | .8 (.61, 1.05) | 1.02 (1, 1.04) |
| DAST | 1.14 (.98, 1.33) | .77 (.6, .99)* | .98 (.96, 1) |
| Kessler | 1.22 (1.17, 1.27)* | 1.3 (1.26, 1.33)* | 1.04 (1.04, 1.05)* |
| 3 Monthd | .87 (.81, .94)* | .56 (.53, .6)* | 1.13 (1.02, 1.24)* |
| 3 Month × Groupe | .53 (.48, .6)* | .93 (.85, 1.01) | .58 (.5, .66)* |
Count models predicted likelihood of an outcome on any one day among participants with any use during the follow-up period.
Analytic sample of participants with at least 1 day of use during the follow-up period.
Compared to non-Latino Whites.
Compared to baseline.
SBIRT compared to Health Education.
95% CrI does not include zero.
Figure 2.
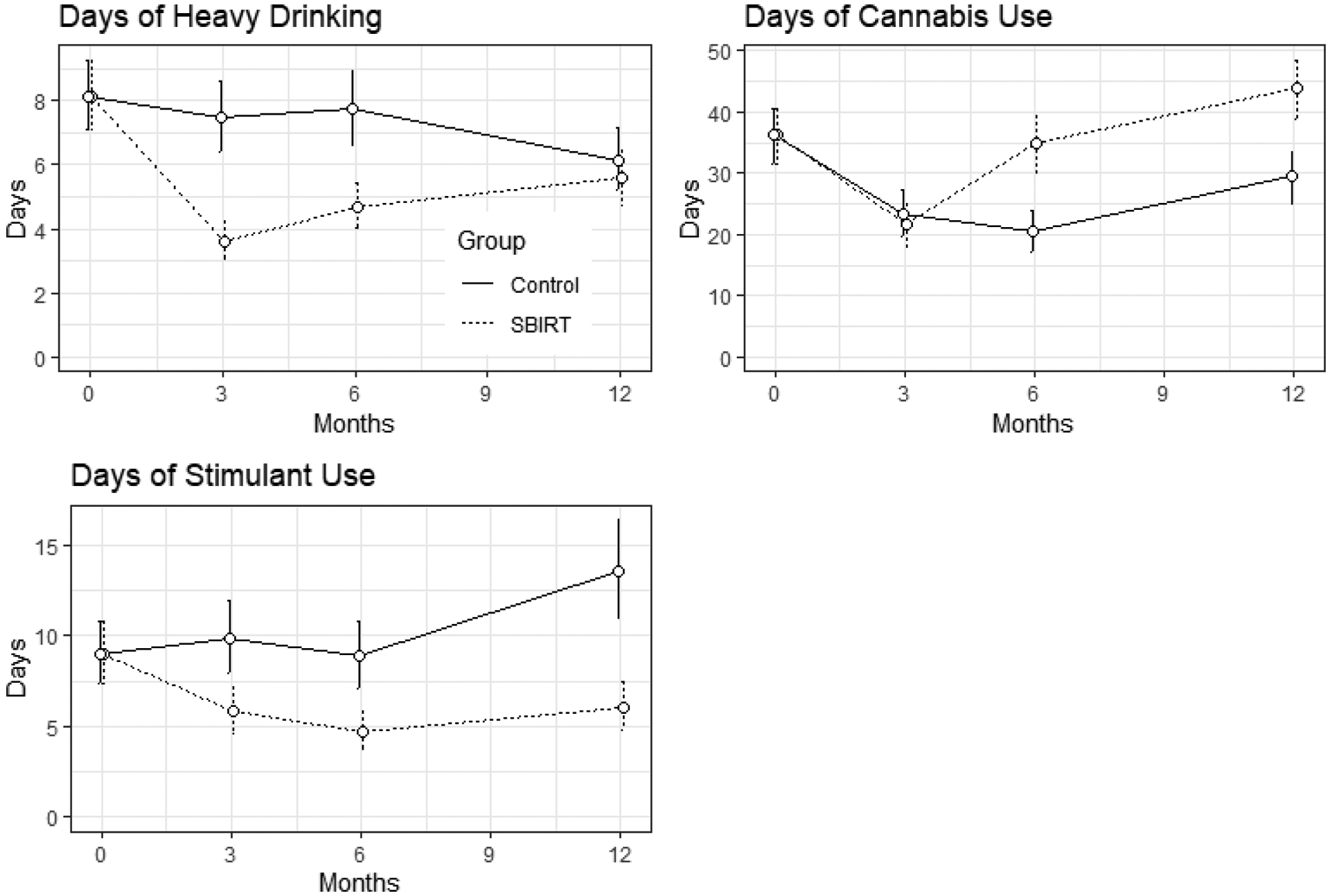
Count model point estimates of primary substance use outcomes in the past 90 days measured at the baseline, 3-, 6- and 12-month follow-ups.
Frequency of cannabis use days
Results from the count model did not support a difference in frequency of cannabis use between conditions at the 3-month follow-up (OR= 0.93, 95% CrI 0.85–1.01). Results are shown in Table 2 and count model estimates of days of cannabis use are displayed in Figure 2.
Frequency of stimulant use days
Results from the count model showed a positive effect of the SBIRT condition on reducing the frequency of stimulant use at the 3-month follow-up (OR= 0.58, 95% CrI 0.5–0.66). Adjusted mean for participants in the SBIRT condition was 5.8 days of stimulant use at the 3-month follow-up compared to 9.8 days for participants in the control group. These results are shown in Table 2 and count model estimates of days of stimulant use are displayed in Figure 2.
Effect of intervention on secondary outcomes
6- and 12-month frequency of heavy drinking, cannabis and stimulant use days
Results from the count model showed that those in the SBIRT condition were likely to have fewer heavy drinking days at the 6-month follow-ups compared to participants in the HE control condition (6-month: OR=0.62, 95% CrI 0.55–0.69). Adjusted mean at the 6-month follow-up for the SBIRT group was 4.7 heavy drinking days and for the control group was 7.7 heavy drinking days. The effect did not persist to the 12-month follow-up (OR=0.99, 95% CrI 0.87–1.13). Contrary to hypothesis, at the 6- and 12-month follow-ups participants in the SBIRT condition used cannabis more frequently than those participants in the control condition. Results from the count model showed a consistent and enduring effect of the SBIRT condition on reducing the frequency of stimulant use at the 6- and 12-month follow-ups (6-month: OR=0.58, 95% CrI 0.5–0.68; 12-month: OR=0.42, 95% CrI 0.36–0.48). At the 6-month follow-up the SBIRT group had an adjusted mean of 4.7 stimulant use days and the control group had 8.9 days. At the 12-month follow-up the SBIRT group had 6.1 stimulant use days and the control group had 13.5 days. These outcomes are shown in Figure 2.
Abstinence from alcohol, stimulants and cannabis
Results from all of the zero models did not support an intervention effect on abstinence. No difference was found between the SBIRT and control condition in the likelihood of being a non-drinker at the 3-month, 6-month or 12-month follow-up. Similarly there was no support for an intervention effect on the likelihood of abstaining from cannabis or stimulants at the 3-, 6- or 12-month follow-up. Detailed results are provided in a supplemental table.
Addiction treatment utilization
Among the entire sample, 22.8% received an addiction-focused treatment service within 30 days after study enrollment. No significant difference was observed between the SBIRT (21.3%) and HE (24.3%) conditions (OR (95% CI): 0.79 (0.55–1.14)). Participants who received an addiction service in the first 30 days (n=164) attended a median of 5 addiction services between baseline and the 3-month follow up (mean (sd) = 10.96 (17.4) services). No significant difference (p = .10) was observed in the number of addiction services between SBIRT (adjusted mean (95% CI) of 11.34 (8.2–14.48) services) and HE (adjusted mean (95% CI) of 8.33 (5.35–11.31) services).
Discussion
This is the first study to implement and systematically evaluate in a large, multi-site, well-controlled RCT, the efficacy of SBIRT in reducing both alcohol and other drug use in mental health treatment settings. Major findings from this investigation suggest that: (1) SBIRT effectively reduced the frequency of heavy alcohol use among individuals receiving mental health treatment; (2) effects of SBIRT on heavy alcohol use were observed out to 6 months post-intervention, but not at a longer-term 12-month follow-up; and (3) SBIRT effectively reduced the frequency of stimulant use relative to an attention-control condition up to 12-months post-intervention. Comparable findings were not observed with respect to changes in cannabis use. To the contrary, participants in the SBIRT condition generally reported more cannabis use during the later part of the follow-up period than the control condition. This finding suggests that caution should be taken in applying the SBIRT approach to reduce cannabis use. In the context of legalized medical cannabis in California during the course of this study, the use of cannabis by mental health patients may be perceived as particularly helpful or desirable. More research is needed to examine if there is indeed an iaotrogenic effect of SBIRT on cannabis use.
SBIRT was not shown to increase the likelihood of abstinence from alcohol, stimulants and cannabis. Abstinence was generally not a goal that participants set for themselves and clinicians did not impose an abstinence goal. Participants typically had a moderation goal and we speculate that goal choice was the reason the intervention did not impact abstinence.
Findings from this investigation suggest that introducing an intervention targeting alcohol and stimulant use in mental health treatment settings is feasible and confers therapeutic effects in psychiatrically comorbid populations. Although the reductions in the frequency of substance use were not large in absolute terms, there were substantial percentage reductions in stimulant use and heavy drinking days in the SBIRT group. Notably these positive effects were not accompanied by SBIRT-induced attendance at specialty addiction treatment. Taken together, the positive effects of SBIRT and the lack of addiction treatment utilization suggest that engaging people in specialty addiction treatment may not be an essential component to promote positive behavior change. It remains to be seen if addiction treatment can enhance the effects of brief intervention or be effective for those who do not respond to brief intervention.
Our findings concerning the impact of SBIRT on heavy alcohol use join and extend a growing literature on the efficacy of this approach in primary care settings. Extant studies demonstrate that screening and brief intervention targeting unhealthy alcohol use yields modest reductions in self-reported drinking behaviors.10,25 Yet the efficacy of this approach for individuals with moderate to severe alcohol use disorders remains unclear.26 The present study overcomes several of the methodological limitations of prior studies by including a range of alcohol use severity, employing a psychoeducational attention control condition, and conducting long-term follow-up. Critically, the current study replicates and extends findings on the beneficial effects of screening and brief intervention for heavy alcohol use to adults with co-occurring psychiatric disorders.
The present study’s finding of a sustained positive effect of SBIRT on reducing the frequency of stimulant use in a sample that was predominantly using amphetamine-type stimulants is novel in the SBIRT literature. Other research found a benefit of screening and brief intervention for cocaine users, yet not for amphetamine users.27,28 One difference between these studies is that the current analyses grouped individuals based on any use of a particular drug while Gelberg et al.27 grouped individuals based on which drug was the most problematic. This difference suggests that SBIRT can be helpful for reducing the use of amphetamine-type stimulants when delivered to all users rather than exclusively to those for whom it is the most problematic. Collectively these studies indicate a promising pattern of findings for screening and brief intervention targeted to stimulant users.
Consistent with other research,29 the present study’s efforts to engage high-risk individuals in specialty addiction treatment (i.e., the “referral to treatment” (RT) component of SBIRT) was not successful. Although the relatively high rate of use of any addiction treatment in the current study suggests that mental health settings are important conduits, the challenge remains how to improve RT practices to better promote use of addiction services. A recent meta-analysis underscores that alcohol brief interventions alone are inadequate to help link patients to specialty substance use services.3 Significant barriers remain on this issue that likely also underlie the low rates of addiction treatment received by people with substance use disorders in the general population.30
Limitations of this study include an emphasis on retrospective self-report of alcohol and drug use and the lack of blinding of study condition. The study has a number of strengths. It is the largest trial evaluating SBIRT in mental health treatment settings and follow-up rates were high. Generalizability to other psychiatric and/or primary care settings in which high rates of psychiatric illness are treated was enhanced by including participants with a range of psychiatric and substance use severity, including severe SUDs. Fidelity to the SBIRT intervention was excellent.
Based on our findings, SBIRT targeting individuals with psychiatric illness has beneficial effects on reducing alcohol and stimulant use. Based on anecdotal feedback from study clinicians the intervention was well-received and acceptable to patients in this setting. The utility of SBIRT in targeting cannabis use is doubtful. The identification of alternative approaches to reduce cannabis use and to optimize the referral-to-treatment process remain priority areas. Given the prevalence of substance use by patients receiving mental health treatment and the effect of substance use on mental health conditions and their treatment, substance use in these treatment settings should not be ignored. The present findings indicate that substance use should be identified and that brief behavioral intervention is beneficial. In practice, the feasibility of providing SBIRT for alcohol and drug use in mental health treatment settings is enhanced by the presence of clinical staff already trained in basic counseling skills. We recommend that alcohol and drug screening be incorporated into the routine mental health intake process and that a portion of an early treatment session focus on alcohol and/or drug use and their relationship to mental health. An important task for future work is to calculate the specific costs and resources needed to deliver SBIRT in mental health settings so that decision makers can make an informed decision about whether to implement this approach.
Supplementary Material
Acknowledgements
We gratefully acknowledge the hard work of study staff including Samuel Ballou, Anne Bellows Lee, Helene Chokron Garneau, Blanca Dominguez, Michael Denin, and Alexandra Venegas.
FUNDING: The study was funded by the US National Institutes of Health grant #R01DA032733.
Footnotes
DECLARATIONS OF INTEREST: The authors have no conflicts of interest. Dr. Saitz discloses the following interests: (1) Alkermes provide medication for a study supported by NIAAA which is awarded to Boston University for which Dr Saitz is PI. (2) Dr. Saitz as PI or co-investigator receives research funding to his institution from the NIH and PCORI. (3) The American Medical Association and American Society of Addiction Medicine and Wolters Kluwer support Dr Saitz’ work as a medical editor. (4) Dr Saitz is President of the International Society of Addiction Journal Editors. (5) Dr. Saitz has been compensated for speaking about addiction at universities and healthcare facilities, and has provided expert review and testimony for malpractice cases. Details available on request.
CLINICAL TRIAL REGISTRATION: NCT01883791
References
- 1.Substance Abuse and Mental Health Services Administration. Key substance use and mental health indicators in the United States: Results from the 2018 National Survey on Drug Use and Health (HHS Publication No. PEP19–5068, NSDUH Series H-54). 2019; Rockville, MD: Center for Behavioral Health Statistics and Quality, Substance Abuse and Mental Health Services Administration; Retrieved from https://www.samhsa.gov/data/ [Google Scholar]
- 2.Barata IA, Shandro JR, Montgomery M, Polansky R, Sachs CJ, Duber HC, Weaver LM, Heins A, Owen HS, Josephson EB, Macias-Konstantopoulos W Effectiveness of SBIRT for alcohol use disorders in the emergency department: A systematic review. Western J Emerg Medicine 2017; 18:1143–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Glass JE, Hamilton AM, Powell BJ, Perron BE, Brown RT, Ilgen MA Specialty substance use disorder services following brief alcohol intervention: A meta‐analysis of randomized controlled trials. Addiction 2015; 110:1404–1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Saitz R Screening and brief intervention for unhealthy drug use: Little or no efficacy. Frontiers in Psychiatry 2014; 5:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Young MM, Stevens A, Galipuea J, Pirie T, Garritty C, Singh K, et al. Effectiveness of brief interventions as part of the Screening, Brief Intervention and Referral to Treatment (SBIRT) model for reducing the nonmedical use of psychoactive substances: A systematic review. Systematic Reviews 2014; 3:1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clark HW, Power AK, Le Fauve CE, Lopez EI Policy and practice implications of epidemiological surveys on co-occurring mental and substance use disorders. J Subst Abuse Treat 2008; 34:3–13. [DOI] [PubMed] [Google Scholar]
- 7.Grant BF, Stinson FS, Dawson DA, Chou SP, Dufour MC, Compton W, et al. Prevalence and co-occurrence of substance use disorders and independent mood and anxiety disorders: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Arch Gen Psychiatry 2004; 61:807–816. [DOI] [PubMed] [Google Scholar]
- 8.Harris KM, Edlund MJ Use of mental health care and substance abuse treatment among adults with co-occurring disorders. Psychiatr Serv 2005; 56:954–959. [DOI] [PubMed] [Google Scholar]
- 9.Madras BK, Compton WM, Avula D, Stegbauer T, Stein JB, Clark HW Screening, brief interventions, referral to treatment (SBIRT) for illicit drug and alcohol use at multiple healthcare sites: comparison at intake and 6 months later. Drug Alcohol Depend 2009; 99:280–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Babor TF, Del Boca F, Bray JW Screening, brief intervention and referral to treatment: implications of SAMHSA’s SBIRT initiative for substance abuse policy and practice. Addiction 2017; 112 Suppl 2:110–117. [DOI] [PubMed] [Google Scholar]
- 11.Graham HL, Copello A, Griffith E, Freemantle N, McCrone P, Clarke L et al. Pilot randomized trial of a brief intervention for comorbid substance misuse in psychiatric inpatient settings. Acta Psychiatr Scand 2016; 133:298–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry 1998; 59 Suppl(20):22–33. [PubMed] [Google Scholar]
- 13.Burke BL, Arkowitz H, Menchola M The Efficacy of Motivational Interviewing: A Meta-Analysis of Controlled Clinical Trials. J Consult Clin Psychol 2003; 71:843–861. [DOI] [PubMed] [Google Scholar]
- 14.Humeniuk R, Henry-Edwards S, Ali R, Poznyak V, Monteiro MG et al. The ASSIST-linked brief intervention for hazardous and harmful substance use: a manual for use in primary care. 2010; Geneva:World Health Organization. [Google Scholar]
- 15.Kinnunen T, Leeman RF, Korhonen T, Quiles ZN, Terwal DM, Garvey AJ et al. Exercise as an adjunct to nicotine gum in treating tobacco dependence among women. Nicotine Tob Res 2008; 10: 689–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vendetti JA, McRee BG, Del Boca FK Development of the SBIRT checklist for observation in real-time (SCORe). Addiction 2017; 112 Suppl 2:34–42. [DOI] [PubMed] [Google Scholar]
- 17.Sobell LC, Maisto SA, Sobell MB, Cooper AM Reliability of alcohol abusers’ self-reports of drinking behavior. Behav Res Ther 1979; 17:157–160. [DOI] [PubMed] [Google Scholar]
- 18.Skinner HA The Drug Abuse Screening Test. Addict Behav 1982; 7:363–371. [DOI] [PubMed] [Google Scholar]
- 19.Saunders JB, Aasland OG, Babor TF, de la Fuente JR, Grant M Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO Collaborative Project on Early Detection of Persons with Harmful Alcohol Consumption--II. Addiction 1993; 88:791–804. [DOI] [PubMed] [Google Scholar]
- 20.Kessler RC Andrews G, Colpe LJ, Hiripi E, Mroczek DK, Normand SL et al. Short screening scales to monitor population prevalences and trends in non-specific psychological distress. Psychol Med 2002; 32:959–76. [DOI] [PubMed] [Google Scholar]
- 21.Kessler RC, Barker PR, Colpe LJ, Epstein JF, Gfroerer JC, Hiripi E et al. Screening for serious mental illness in the general population. Arch Gen Psychiatry 2003; 60:184–9. [DOI] [PubMed] [Google Scholar]
- 22.Hoffman MD and Gelman A The No-U-Turn sampler: adaptively setting path lengths in Hamiltonian Monte Carlo. Journal of Machine Learning Research 2014; 15: 1593–1623. [Google Scholar]
- 23.Stan Development Team. RStan: the R interface to Stan. R package version 2.17.3 2018. Retrieved from http://mc-stan.org.
- 24.Carpenter B, Gelman A, Hoffman M, Lee D, Goodrich B, Betancourt M, Brubaker M, Guo J, Li P, Riddell A. Stan: A probabilistic programming language. Journal of Statistical Software 2017; 76(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jonas DE, Garbutt JC, Amick HR, Brown JM, Brownley KA, Council CL et al. Behavioral counseling after screening for alcohol misuse in primary care: a systematic review and meta-analysis for the U.S. Preventive Services Task Force. Ann Intern Med 2012; 157:645–654. [DOI] [PubMed] [Google Scholar]
- 26.Saitz R Alcohol screening and brief intervention in primary care: Absence of evidence for efficacy in people with dependence or very heavy drinking. Drug Alcohol Rev 2010; 29:631–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gelberg L, Andersen RM, Afifi AA, Leake BD, Arangua L, Vahidi M et al. Project QUIT (Quit Using Drugs Intervention Trial): a randomized controlled trial of a primary care-based multi-component brief intervention to reduce risky drug use. Addiction 2015; 110:1777–1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bernstein J, Bernstein E, Tassiopoulos K, et al. Brief motivational intervention at a clinic visit reduces cocaine and heroin use. Drug Alcohol Depend 2005; 77:49–59. [DOI] [PubMed] [Google Scholar]
- 29.Kim TW, Bernstein J, Cheng DM, Lloyd-Travaglini C, Samet JH, Palfai TP, Saitz R Receipt of addiction treatment as a consequence of a brief intervention for drug use in primary care: a randomized trial. Addiction 2017; 112:818–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Grella CE, Karno MP, Warda US, Moore AA, Niv N. Perceptions of Need and Help Received for Substance Dependence in a National Probability Survey. Psychiatr Serv 2010; 60: 1068–10754. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


