Abstract
Efficacy of conventional chemoimmunotherapy is limited in patients with Richter syndrome (RS) with anticipated median overall survival (OS) of less than 10 months. Allogeneic hematopoietic cell transplantation (allo-HCT) is commonly offered as a consolidative treatment option in RS. To our knowledge, there are no randomized controlled studies that have compared allo-HCT against other therapies in RS; available allo-HCT data are limited to small case series from single-institution or registry studies. We performed a systematic review and meta-analysis to assess the totality of evidence regarding the efficacy (or lack thereof) of allo-HCT for RS. We extracted data on post-allograft outcomes related to benefits (overall response rate [ORR], complete remission [CR], OS, and progression-free survival [PFS]). For harms, data were extracted on non-relapse mortality (NRM) and relapse post-allografting. Our search strategy identified 240 studies, but only four studies (n = 72 patients) met our inclusion criteria. Pooled ORR, CR, OS, and PFS rates were 79%, 33%, 49%, and 30%, respectively. Pooled NRM and relapse rates were 24% and 28%, respectively. Results of this systematic review and meta-analysis indicate that allo-HCT yields encouraging OS in RS, thus remaining a reasonable treatment option in fit patients whose disease demonstrates a chemosensitive response to pre-transplant salvage therapies. Novel strategies are certainly needed to reduce the risk of relapse to further improve outcomes in these patients.
Keywords: Allogeneic hematopoietic cell transplantation, Overall survival, Richter syndrome
Introduction
Initially described in 1928 [1], Richter syndrome (RS) entails transformation of chronic lymphocytic leukemia (CLL) into an aggressive form of B-cell lymphoma, most commonly a diffuse large B-cell lymphoma (DLBCL), and rarely a classical Hodgkin lymphoma variant [2–4]. It is estimated that 10% of CLL patients would develop RS which is generally associated with limited survival ranging from 5 to 9 months [5–7]. The median time for RS transformation is generally reported to occur within 2–4 years [8]. Notably, presence of bulky extensive adenopathy, Del17p, Del11q, unmutated IGHV, mutated NOTCH1, and stereotyped B-cell receptor has been associated with an increased risk of RS occurrence [3,9–11]. From the treatment standpoint, several multi-agent chemoimmunotherapy regimens, mostly those used to treat de novo DLBCL, have been used in RS [12,13] with an overall response rate (ORR) of approximately 40% and median overall survival (OS) of less than 10 months [6,14–18]. The durability of responses after chemoimmunotherapy is generally short-lived [13].
Ibrutinib is a Bruton’s tyrosine kinase inhibitor approved for various stages of CLL. Studies have demonstrated that patients with CLL treated with ibrutinib, who develop RS, have a dismal median OS of 3.5 (range, 0.3–6) months from the time of documented transformation [19]. Allogeneic hematopoietic cell transplantation (allo-HCT) is commonly offered as a consolidative treatment option, particularly in clonally-related RS which is known to have a worse prognosis than the clonally-unrelated counterpart [3]. A recently published analysis from the German CLL Study Group showed an encouraging median OS of 17.9 months in three patients allografted for RS [7]. Recently published practice guidelines by the American Society of Transplantation and Cellular Therapies (formerly known as the American Society for Blood and Marrow Transplantation) recommend allo-HCT as a front-line consolidation in patients with RS [20]. Guidelines from the National Comprehensive Cancer Network recommend allo-HCT only in clonally-related or unknown clonal status RS with chemosensitive disease response [21].
To our knowledge, there are no randomized controlled studies that have compared allo-HCT with other therapies in patients with RS. Available allo-HCT data are limited to small case series from single-institution or registry studies. Therefore, we conducted a systematic review and meta-analysis of the existing medical literature to assess the totality of evidence regarding the clinical efficacy (or lack thereof) of allo-HCT for RS.
Methods
Search and selection of eligible studies
According to predefined protocol, a comprehensive search of the published medical literature was undertaken using three major databases, namely PubMed/Medline, Embase, and Cochrane on February 13, 2020 (Appendix 1). Additionally, we performed a manual search of cited references listed on relevant nonsystematic or narrative review articles aimed at identifying additional eligible studies that may have been missed by our search strategy. We did not apply any search limits based on language, country of origin, date of study conduct, or type of study (prospective, retrospective from a single center or multiple centers, or registry data), but we excluded studies that were only reported in abstract form.
Eligibility for inclusion in this systematic review/meta-analysis required that studies must have enrolled ≥ 10 patients who ended up receiving an allo-HCT for the sole purpose of treating RS. Selection of included studies was undertaken by two authors (S.A. and M.A.K-D). Possible disagreements were resolved by consensus majority in consultation with two separate co-authors (T.R. and A.K).
Data collection
Clinical outcome data from eligible studies were extracted in relation to benefits and harms by three authors (S.A., F.Y., and M.A.K-D). To assess clinical benefits, we extracted data on ORR, complete remission rate (CR), OS, and progression-free (PFS) survival. For harms, data were extracted on non-relapse mortality (NRM) and relapse after allo-HCT.
The methodologic quality of eligible studies was assessed by two authors (T.R. and A.K.) using the Newcastle–Ottawa scale modified for single-arm cohort studies [22].
Statistical analysis
In this systematic review/meta-analysis, we calculated proportions for each specific outcome of interest. For the meta-analysis, the proportions were transformed into quantities according to the Freeman–Tukey variant of the arcsine square root-transformed proportion [23,24]. The pooled proportion was calculated as the back-transformation of the weighted mean of the transformed proportions using the random-effects model proposed by DerSimonian and Laird [23], which was used to pool data from studies with similar definitions pertaining to study design, study subjects, and allo-HCT outcomes. All results are reported as rates with their corresponding 95% confidence intervals (CIs).
Analysis of heterogeneity
We assessed heterogeneity among the studies included in this systematic review/meta-analysis using the I2 test as described by Higgins et al. [25]. Moderate heterogeneity was defined as I2 > 30%, and high heterogeneity was defined as I2 > 60%. All analyses reported in this systematic review were performed by Stata version 16 software (StataCorp LP, College Station, TX, USA) [26] and the MetapropOne software package [27]. The review is reported in accordance with PRISMA guidelines [28].
Results
Search results
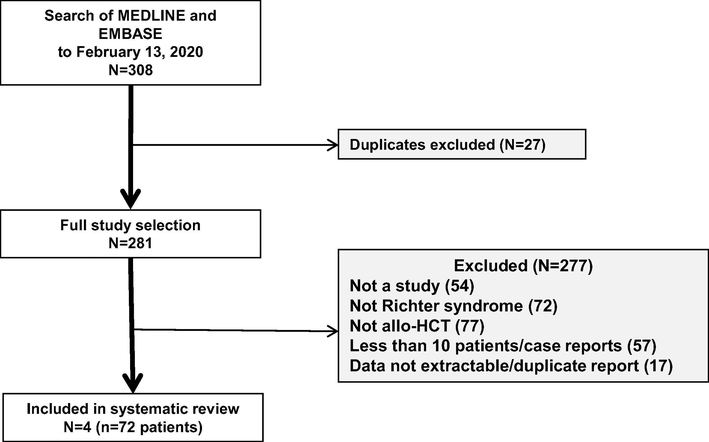
Our implemented search strategy (Appendix 1) identified a total of 240 studies. Only four studies (n = 72 patients) met our inclusion criteria [14,29–30]. The three most common reasons for studies to be excluded were: (a) not allo-HCT, (b) not specific to RS, and (c) included < 10 patients or represented case reports (Fig. 1). Three of the studies represented single-institution data [14,29,30], with two of those published from the same center [14,30]. One study represented registry data from the European Society for Blood and Marrow Transplantation (EBMT) [31].
Fig. 1.
Study selection flow diagram.
Characteristics of included studies
The median age of patients in included studies ranged from 57 to 63 years and reduced intensity conditioning was the preferred preparative regimen in three of four studies. Three studies reported a median follow-up time of 29–46 months. These and other findings are described in Table 1.
Table 1.
Patient- and Transplant-related Characteristics and Post-transplant Outcomes.
| Study | Study type and study period | n (Male sex) | Median (range) age, years | Regimen intensity | Median F/U, months | CR | OS | Relapse | NRM |
|---|---|---|---|---|---|---|---|---|---|
| Tsimberidou et al. 2006 [14] | Single institution (1975–2005) | 17 (Male = 11) | 60 (35–72) | MAC = 2 RIC = 15 |
NE | 6% | 75% In CR/CRu/PR (3-y) | 21% | 12% |
| Cwynarski et al. 2012 [31] | EBMT registry (1997–2007) | 25 (Male = 15) | 57 (31–70) | MAC = 7 RIC = 18 |
29% | NE | 36% (3-y) | 47% (3-y) | 26% (3-y) |
| In CR/Cru/PR 41% (3-y) |
MAC = 43% RIC = 19% (3-y) |
||||||||
| In refractory disease 17% (3-y) |
|||||||||
| Rozovski et al. 2015 [30] | Single institution (1998–2011) | 20 (NE) | 58 (32–72) | MAC = 6 RIC = 14 |
45 | 35% | 36% (2-y) 0 (5-y) |
NE | NE |
| Kharfan-Dabaja et al. 2017 [29] | Single institution (2008–2016) | 10 (Male = 5) | 63 (50–74) | MAC = 7 RIC = 3 |
46 | 70% | 50% (4-y) | 10% (4-y) | 40% (4-y) |
Note. F/U= follow up; CR = complete remission; Cru = complete remission undetermined; EBMT = European Society for Blood and Marrow Transplantation; MAC = myeloablative conditioning; mo = month; n = number of patients; NE = no extractable/not available data; NRM = non-relapse mortality; OS = overall survival; PR = partial response; RIC = reduced intensity conditioning; y = year.
Assessment of methodologic quality of selected studies
In Table 2, we summarize the risk of bias for the studies included in this systematic review/meta-analysis. Three studies [14,29,30] represented single-institution observational data and one study was an EBMT registry study [31]. All included studies [14,29,30] enrolled were at low risk for the representativeness of the cohort, except the study by Cwynarski et al. [31]. All other parameters for methodological quality assessment were judged to be at low risk for all included studies.
Table 2.
Risk of Bias in Included Studies.
| Study | Representativeness of the cohort | Ascertainment of exposure | Demonstration that outcome of interest was not present at start of study | Assessment of outcome | Was follow-up long enough for outcomes to occur? | Adequacy of follow up of cohorts |
|---|---|---|---|---|---|---|
| Tsimberidou et al. 2006 [14] | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk |
| Cwynarski et al. 2012 [31] | Unclear/High risk | Low risk | Low risk | Low risk | Low risk | Low risk |
| Rozovski et al. 2015 [30] | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk |
| Kharfan-Dabaja et al. 2017 [29] | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk |
Outcomes
ORR
ORR was reported in three studies (n = 47 patients) [14,29,30]. The pooled ORR rate was 79% (95% CI = 50–98). Heterogeneity among these studies was high (I2 = 74.6%, p = .02).
CR
CR was reported in three studies (n = 47 patients) [14,29,30]. The pooled CR rate was 33% (95% CI = 4–71). Heterogeneity among these studies was high (I2 = 84.2%, p = .002).
OS
OS was reported in four studies (n = 72 patients) [14,29–31]. The pooled OS rate was 49% (95% CI = 29–69). Heterogeneity among these studies was high (I2 = 63.7%, p = .04; Fig. 2A).
Fig. 2.
(a) Pooled overall survival (OS); (b) pooled progression-free survival (PFS). Note. CI = confidence interval. ES = effect size.
PFS
PFS was reported in three studies (n = 52 patients) [14,29,31]. The pooled PFS rate was 30% (95% CI = 18–44). There was no heterogeneity among the studies (I2 = 0, p = .37; Fig. 2B).
NRM
NRM was reported in three studies (n = 52 patients) [14,29,31]. The pooled NRM rate was 24% (95% CI = 11–41). Heterogeneity among these studies was low (I2 = 29.4%, p = .24).
Relapse
Relapse was reported in three studies (n = 52 patients) [14,29,31]. The pooled relapse rate was 28% (95% CI = 9–52). Heterogeneity among these studies was high (I2 = 62.7%, p = .07).
Discussion
Findings of this systematic review/meta-analysis indicate that allo-HCT yields encouraging pooled ORR of 79% and OS rates of 49% in patients with RS. Unfortunately, a pooled relapse rate of 28% was observed, suggesting that novel strategies are certainly warranted to reduce the risk of relapse to further improve outcomes in these patients. Pertaining to the pooled NRM rate of 24% (95% CI = 11–41), this is consistent with reported NRM for allo-HCT in various lymphoid malignancies [32].
Our analysis has several limitations, namely the small number of identified studies and a small number of patients per study. Additionally, in the absence of individual patient data, it is difficult to ascertain patients’ performance status and presence of specific comorbidities to shed light into their fitness and ultimate impact on post-transplant outcomes. Moreover, studies included in this systematic review/meta-analysis did not specify whether a clonal relation to CLL was confirmed in all patients who received an allo-HCT, or whether the intensity of the conditioning regimen impacts post-transplant outcomes. Besides, clinical outcomes of two studies from the same institution were published in 2006 [14] and 2015 [30], raising concerns of possible overlap of cases. Furthermore, we did not identify any randomized controlled trials (RCTs) comparing allo-HCT with any other treatment modality in patients with RS. Another major limitation is the absence of data assessing the role of novel therapies, such as BCL-2 inhibitors like venetoclax when prescribed alone or in combination with other new agents in the studies included herein, consistent with contemporary practice. In fact, in one single-institution study, only one of 10 patients received ibrutinib and later idelalisib for treatment of CLL prior to transformation into RS [29]. Two of the remaining studies enrolled patients before ibrutinib or venetoclax became approved for commercial use [14,31]. Also, in one single-institution study, the authors reported a 2-year OS of 36% and no survivors reported after 5 years [30]. Methodologically, we assessed OS including the 2-year OS of 36% reported in that study [30] (Fig. 2A) yielding a pooled OS rate of 49%. When the pooled OS rate was assessed including the 5-year OS data (5-year OS = 0) [30], the resulting pooled OS rate was 35% (95% CI = 4–76). Heterogeneity was lower when the 2-year OS was included instead of the 5-year OS (I2 = 63.7% vs. 91.5%).
While novel therapeutics, such as BTK, PI3K, and BCL-2 inhibitors, have drastically shifted the treatment paradigm in CLL, the impact of these targeted therapies on RS remains less encouraging. A phase 2 study has demonstrated promising efficacy of pembrolizumab in CLL patients with RS [33]. Evaluating the role of allo-HCT in patients with RS in the setting of prior treatment with novel targeted therapies remains an area of continuous investigation. The recent emergence of a new effective T-cell engineered therapy, namely chimeric antigen receptor T-cell (CAR-T) therapy, has already revolutionized the treatment algorithm of DLBCL (de novo or transformed from follicular lymphoma) [34,35]; clinical studies are showing encouraging activity in high-risk CLL including cases of RS [36]. The rare incidence of RS makes it difficult to conduct large RCT comparing novel therapies such as CAR-T against allo-HCT unless performed in the context of research consortiums or clinical trial networks.
Conclusion
Notwithstanding all aforementioned limitations, allo-HCT remains a reasonable treatment option for patients with RS who are physically fit and have adequate organ function, have a suitable HLA-compatible donor, and demonstrate chemosensitive disease response prior to the procedure. These cases should be referred to transplant centers as soon as possible to confirm eligibility for allo-HCT in a timely manner, considering the short-lived duration of responses with current chemoimmunotherapy regimes. Clinical trials focusing on incorporating novel therapies as a maintenance/consolidative strategy to help reduce the risk of disease relapse after allo-HCT are definitely needed. Finally, patients with RS who remain refractory to salvage treatments would be better served by being considered for enrollment in clinical trials.
Acknowledgments
This work was presented (poster presentation, number 585) in part at the annual meeting of the American Society for Transplantation and Cellular Therapies (ASTCT) in Houston, TX, USA on February 23, 2019. At the time of the poster presentation, the literature search had been conducted on September 28, 2018. The search results described in this manuscript were updated on February 13, 2020. The number of eligible studies (n=4) remained unchanged.
Conflict of interest
J.P-I reports honoraria from Abbvie, Janssen, Takeda, AstraZeneca, Pharmacyclics, Sanofi; consultancy for Abbvie, Janssen, AstraZeneca, Novartis, TG therapeutics, Takeda; member in the speaker’s bureau for Abbvie, Janssen, Astrazeneca, Takeda; and research funding from MEI, Sunesis. M.A.K-D reports consultancy for Pharmacyclics and Daiichi Sankyo. Other authors S.A., T.R., F.Y., E.A., J.C.C., A.C-K, and A.K. report no relevant conflicts of interest.
Appendix 1. Electronic search strategies
| Search terms for MEDLINE via PubMed | |
| #1 | Richter syndrome[MeSH Terms] |
| #2 | (Richter* Syndrome*[Title/Abstract])) OR (Richter* Transformation*) |
| #3 | #1 OR #2 |
| #4 | “Hematopoietic Stem Cell Transplantation”[Mesh] |
| #5 | (Hematopoietic Stem Cell Transplantation) |
| #6 | (allogeneic[Title/Abstract] AND transplant*[Title/Abstract]) |
| #7 | “Bone Marrow Transplantation”[Mesh] |
| #8 | (Bone Marrow Transplantation[Title/Abstract]) |
| #9 | #4 OR #5 OR #6 OR #7 OR #8 |
| #10 | #3 AND #9 |
| Search terms for EMBASE | |
| #1 | ’richter syndrome’/exp OR ’richter syndrome’ |
| #2 | ’richter transformation’/exp OR ’richter transformation’ |
| #3 | richter:ti,ab |
| #4 | #1 OR #2 OR #3 |
| #5 | ’allogeneic hematopoietic cell transplantation’/exp OR ’allogeneic hematopoietic cell transplantation’ |
| #6 | transplant*:ab,ti |
| #7 | #5 OR #6 |
| #8 | #4 AND #7 |
| Search terms for Cochrane | |
| #1 | Richter syndrome |
| #2 | Richter Transformation |
| #3 | #1 OR #2 |
| #4 | MeSH descriptor: [Hematopoietic Stem Cell Transplantation] explode all trees |
| #5 | MeSH descriptor: [Bone Marrow Transplantation] explode all trees |
| #6 | Transplantation |
| #7 | #4 OR #5 OR #6 |
| #8 | #3 AND #7 |
References
- [1].Richter MN. Generalized reticular cell sarcoma of lymph nodes associated with lymphatic leukemia. Am J Pathol 1928;4:285. [PMC free article] [PubMed] [Google Scholar]
- [2].Maddocks-Christianson K, Slager SL, Zent CS, Reinalda M, Call TG, Habermann TM, et al. Risk factors for development of a second lymphoid malignancy in patients with chronic lymphocytic leukaemia. Br J Haematol 2007;139:398–404 [DOI] [PubMed] [Google Scholar]
- [3].Rossi D, Spina V, Deambrogi C, Rasi S, Laurenti L, Stamatopoulos K, et al. The genetics of Richter syndrome reveals disease heterogeneity and predicts survival after transformation. Blood 2011;117:3391–401. [DOI] [PubMed] [Google Scholar]
- [4].Lortholary P, Boiron M, Ripault P, Levy JP, Manus A, Bernard J. Chronic lymphoid leukemia secondarily associated with a malignant reticulopathy: Richter’s syndrome. Nouv Rev Fr Hematol 1964;4:621–44. [PubMed] [Google Scholar]
- [5].Thornton PD, Bellas C, Santon A, Shah G, Pocock C, Wotherspoon AC, et al. Richter’s transformation of chronic lymphocytic leukemia. The possible role of fludarabine and the Epstein-Barr virus in its pathogenesis. Leuk Res 2005;29:389–95. [DOI] [PubMed] [Google Scholar]
- [6].Tsimberidou AM, Keating MJ. Richter syndrome: biology, incidence, and therapeutic strategies. Cancer 2005;103:216–28. [DOI] [PubMed] [Google Scholar]
- [7].Al-Sawaf O, Robrecht S, Bahlo J, Fink AM, Cramer P, J VT, et al. Richter transformation in chronic lymphocytic leukemia (CLL)-a pooled analysis of German CLL Study Group (GCLLSG) front line treatment trials. Leukemia 2020. doi: 10.1038/s41375-020-0797-x. [DOI] [PubMed] [Google Scholar]
- [8].Robertson LE, Pugh W, O’Brien S, Kantarjian H, Hirsch-Ginsberg C, Cork A, et al. Richter’s syndrome: a report on 39 patients. J Clin Oncol 1993;11:1985–9. [DOI] [PubMed] [Google Scholar]
- [9].Parikh SA, Rabe KG, Call TG, Zent CS, Habermann TM, Ding W, et al. Diffuse large B-cell lymphoma (Richter syndrome) in patients with chronic lymphocytic leukaemia (CLL): a cohort study of newly diagnosed patients. Br J Haematol 2013;162:774–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Rossi D, Cerri M, Capello D, Deambrogi C, Rossi FM, Zucchetto A, et al. Biological and clinical risk factors of chronic lymphocytic leukaemia transformation to Richter syndrome. Br J Haematol 2008;142:202–15. [DOI] [PubMed] [Google Scholar]
- [11].Rossi D, Spina V, Cerri M, Rasi S, Deambrogi C, De Paoli L, et al. Stereotyped B-cell receptor is an independent risk factor of chronic lymphocytic leukemia transformation to Richter syndrome. Clin Cancer Res 2009;15:4415–22. [DOI] [PubMed] [Google Scholar]
- [12].Coiffier B, Lepage E, Briere J, Herbrecht R, Tilly H, Bouabdallah R, et al. CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma. N Engl J Med 2002;346:235–42. [DOI] [PubMed] [Google Scholar]
- [13].Langerbeins P, Busch R, Anheier N, Durig J, Bergmann M, Goebeler ME, et al. Poor efficacy and tolerability of R-CHOP in relapsed/refractory chronic lymphocytic leukemia and Richter transformation. Am J Hematol 2014;89:E239–43. [DOI] [PubMed] [Google Scholar]
- [14].Tsimberidou AM, O’Brien S, Khouri I, Giles FJ, Kantarjian HM, Champlin R, et al. Clinical outcomes and prognostic factors in patients with Richter’s syndrome treated with chemotherapy or chemoimmunotherapy with or without stem-cell transplantation. J Clin Oncol 2006;24:2343–51. [DOI] [PubMed] [Google Scholar]
- [15].Dabaja BS, O’Brien SM, Kantarjian HM, Cortes JE, Thomas DA, Albitar M, et al. Fractionated cyclophosphamide, vincristine, liposomal daunorubicin (daunoXome), and dexamethasone (hyperCVXD) regimen in Richter’s syndrome. Leuk Lymphoma 2001;42:329–37. [DOI] [PubMed] [Google Scholar]
- [16].Tsimberidou AM, Kantarjian HM, Cortes J, Thomas DA, Faderl S, Garcia-Manero G, et al. Fractionated cyclophosphamide, vincristine, liposomal daunorubicin, and dexamethasone plus rituximab and granulocyte-macrophage-colony stimulating factor (GM-CSF) alternating with methotrexate and cytarabine plus rituximab and GM-CSF in patients with Richter syndrome or fludarabine-refractory chronic lymphocytic leukemia. Cancer 2003;97:1711–20. [DOI] [PubMed] [Google Scholar]
- [17].Tsimberidou AM, Murray JL, O’Brien S, Wierda WG, Keating MJ. Yttrium-90 ibritumomab tiuxetan radioimmunotherapy in Richter syndrome. Cancer 2004;100:2195–200. [DOI] [PubMed] [Google Scholar]
- [18].Rogers KA, Huang Y, Ruppert AS, Salem G, Stephens DM, Heerema NA, et al. A single-institution retrospective cohort study of first-line R-EPOCH chemoimmunotherapy for Richter syndrome demonstrating complex chronic lymphocytic leukaemia karyotype as an adverse prognostic factor. Br J Haematol 2018;180:259–66. [DOI] [PubMed] [Google Scholar]
- [19].Maddocks KJ, Ruppert AS, Lozanski G, Heerema NA, Zhao W, Abruzzo L, et al. Etiology of ibrutinib therapy discontinuation and outcomes in patients with chronic lymphocytic leukemia. JAMA Oncol 2015;1:80–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Kharfan-Dabaja MA, Kumar A, Hamadani M, Stilgenbauer S, Ghia P, Anasetti C, et al. Clinical practice recommendations for use of allogeneic hematopoietic cell transplantation in chronic lymphocytic leukemia on behalf of the Guidelines Committee of the American Society for Blood and Marrow Transplantation. Biol Blood Marrow Transplant 2016;22:2117–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Wierda WG, Byrd JC, Abramson JS, Bilgrami SF, Bociek G, Brander D, et al. NCCN guidelines insights: chronic lymphocytic leukemia/small lymphocytic lymphoma, version 2.2019. J Natl Compr Canc Netw 2019;17:12–20. [DOI] [PubMed] [Google Scholar]
- [22].Wells G, Shea B, O’Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Available from: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed 6 May 2020).
- [23].DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177–88. [DOI] [PubMed] [Google Scholar]
- [24].Kendall MG, Stuart A, Ord JK. Kendall’s advanced theory of statistics. Oxford: Oxford University Press, Inc.; 1987. [Google Scholar]
- [25].Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003;327:557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].StataCorp. tata Statistical Software: Release 16. College Station, TX: StataCorp LP; 2016. [Google Scholar]
- [27].Nyaga VN, Arbyn M, Aerts M. Metaprop: a Stata command to perform meta-analysis of binomial data. Arch Public Health 2014;72:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Moher D, Liberati A, Tetzlaff J, Altman DG, The PG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009;6 e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Kharfan-Dabaja MA, Kumar A, Stingo FE, Khimani F, Hussaini M, Ayala E, et al. Allogeneic hematopoietic cell transplantation for Richter syndrome: a single-center experience. Clin Lymphoma Myeloma Leuk 2018;18:e35–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Rozovski U, Benjamini O, Jain P, Thompson PA, Wierda WG, O’Brien S, et al. Outcomes of patients with chronic lymphocytic leukemia and Richter’s transformation after transplantation failure. J Clin Oncol 2015;33:1557–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Cwynarski K, van Biezen A, de Wreede L, Stilgenbauer S, Bunjes D, Metzner B, et al. Autologous and allogeneic stem-cell transplantation for transformed chronic lymphocytic leukemia (Richter’s syndrome): A retrospective analysis from the chronic lymphocytic leukemia subcommittee of the chronic leukemia working party and lymphoma working party of the European Group for Blood and Marrow Transplantation. J Clin Oncol 2012;30:2211–7. [DOI] [PubMed] [Google Scholar]
- [32].Kharfan-Dabaja MA, El-Jurdi N, Ayala E, Kanate AS, Savani BN, Hamadani M. Is myeloablative dose intensity necessary in allogeneic hematopoietic cell transplantation for lymphomas?. Bone Marrow Transplant 2017;52:1487–94. [DOI] [PubMed] [Google Scholar]
- [33].Ding W, LaPlant BR, Call TG, Parikh SA, Leis JF, He R, et al. Pembrolizumab in patients with CLL and Richter transformation or with relapsed CLL. Blood 2017;129:3419–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Neelapu SS, Locke FL, Bartlett NL, Lekakis LJ, Miklos DB, Jacobson CA, et al. Axicabtagene Ciloleucel CAR T-Cell Therapy in Refractory Large B-Cell Lymphoma. N Engl J Med 2017;377:2531–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Schuster SJ, Bishop MR, Tam CS, Waller EK, Borchmann P, McGuirk JP, et al. Tisagenlecleucel in adult relapsed or refractory diffuse large b-cell lymphoma. N Engl J Med 2019;380:45–56. [DOI] [PubMed] [Google Scholar]
- [36].Turtle CJ, Hay KA, Hanafi LA, Li D, Cherian S, Chen X, et al. Durable molecular remissions in chronic lymphocytic leukemia treated with CD19-specific chimeric antigen receptor-modified t cells after failure of ibrutinib. J Clin Oncol 2017;35:3010–20. [DOI] [PMC free article] [PubMed] [Google Scholar]