Abstract
In the last ten years, there has been increasing recognition that cells can acquire genetic variants during cortical development that can give rise to brain malformations as well as nonlesional focal epilepsy. These often brain tissue-specific, de novo variants can result in highly variable phenotypes based on the burden of a variant in specific tissues and cells. By discovering these variants, shared pathophysiological mechanisms are being revealed between clinically distinct disorders. Beyond informing disease mechanisms, mosaic variants also offer a powerful research tool to trace cellular lineages, to study the roles of specific cell types in disease presentation, and to establish the cell-type specific genomic consequences of a variant.
Keywords: somatic variants, epilepsy, neurogenetics, brain
Introduction
Genome-wide genotyping and sequencing technologies facilitated the discovery of more than 100 specific epilepsy genes and revealed many novel aspects of the underlying genetic architecture. This work has revealed a significant role of de novo variants in the genetic risk of epilepsy. In fact, the vast majority of epilepsy genes identified to date were discovered either by sequencing trios to directly isolate de novo variants or by sequencing individual probands to ascertain the ultra-rare variants with dramatic effects on proteins critical to brain development that presumably arose de novo [1–5]. Most de novo variants associated with epilepsy were discovered in DNA derived from blood, and therefore likely to have arisen largely in parental gametes and transmitted to the germline of their offspring.
De novo variants can also arise post-zygotically and result in a mosaic organism if the cells continue to divide after the mutation occurs. The distribution and extent of mosaicism depends on the timing of the mutation. Mutations that happen post-zygotically within the first few cell divisions will result in the variant being present in the majority of the cells comprising the organism, whereas variants arising much later in embryogenesis after gastrulation may be found in only a small fraction of cells and localized to specific tissue or cell types. Studies have estimated that neural progenitors accumulate mutations at a rate of at least 1.6 per cell division [6], suggesting genetic risk for epilepsy may be in part conferred by brain tissue specific genetic variants that would be overlooked in traditional studies of leukocyte-derived DNA. This possibility is particularly plausible for focal epilepsy types where localization of the variant may in part explain the focal disease presentation. Refractory focal epilepsy, where surgical resections are relatively common, offers a unique opportunity to much more comprehensively study the role of de novo variants in epilepsy, including the analysis of post-zygotically acquired de novo variants localized to epileptogenic neural tissue. These collective observations and opportunities motivated extensive research in recent years towards the identification of the role of somatic variants in epilepsy disorders [7–11]. This review focuses on the recent discoveries of somatic variants in epilepsy, highlights novel insights gleaned from work in somatic genetics, and discusses future avenues to continue to advance the field.
Somatic mosaicism in epilepsy
Very early evidence for somatic mosaicism in epilepsy was identified in monozygotic twins who were discordant for Dravet syndrome, a severe sporadic disorder that is typically caused by de novo variants in SCN1A [12]. A pathogenic SCN1A variant was detected in all tissue types analyzed in the affected twin that was undetectable in the healthy twin, providing clear evidence for a very early post-zygotically acquired variant. Since this finding a number of early postzyotically-acquired variants have been identified in leukocyte-derived DNA in a range of epilepsy disorders. Somatic variants arising after the formation of the germ layers and enriched in the neural tissue have also been shown in a range of epilepsy disorders, and in particular brain malformations associated with seizures (Table 1) [2,3,13–16]. In some cases, the somatic variant is a second hit, in which a post-zygotically acquired variant arises in combination with an inherited variant, whereas in other instances only a single somatic variant is sufficient to give rise to the phenotype (Table 1).
Table 1.
Brain tissue localized/enriched somatic variants in epilepsy
| Epilepsy-associated disorder | Gene(s) | References |
|---|---|---|
| brain arteriovenous malformation | BRAF, KRAS, MAP2K1, NRAS | [17–19] |
| cerebral cavernous malformation | CCM2*, KRIT*, PDCD10* | [20–22] |
| focal cortical dysplasia type Ia/mild malformation of cortical development | SLC35A2 | [23–25] |
| focal cortical dysplasia type IIb/hemimegalencephaly | ATK1, AKT3, DEPDC5*, MTOR, PIK3CA, RHEB, TSC1*, TSC2* | [24,26–32] |
| hypothalamic hamartoma | DYNC2H1*, GLI3, OFD1, PRKACA, PTPN11 | [33–36] |
| non-lesional epilepsy (radiographically non-lesional) | SLC35A2 | [23–25] |
| Sturge-Weber syndrome/leptomeningeal angiomatosis | GNAQ | [37–39] |
two hit model (somatic and germline variants may be required for phenotype)
Focal cortical dysplasia Type II (FCD II)
Brain malformations with FCD II pathology, including hemimegalencephaly, represent the best documented examples of lesional epilepsy caused by somatic variants. The pathogenic somatic variants identified in brain malformations with FCD II pathology affect components of the PI3K-AKT-mTOR signaling pathway (Table 1) [24,26–32]. Prior to identification of the somatic variants in these disorders, a shared histopathological phenotype had been observed across these brain malformations, including the presence of cytomegalic neurons and cortical dyslamination [40]. The identification of somatic variants across PI3K-AKT-mTOR signaling pathway genes, many of which were proven to activate mTOR signaling in independent model system, validated the suggested shared pathophysiology. The majority of the somatic variants identified in the PI3K-AKT-mTOR signaling pathway are exclusively present or are greatly enriched in the malformed brain regions compared to blood [10].
Hypothalamic hamartomas
Brain tissue localized variants have also been identified in hypothalamic hamartomas (HH), noncancerous tumors of the hypothalamus associated with intractable gelastic seizures. The first somatic variants to be discovered in HH were found in GLI3, a gene encoding a key protein in the Sonic Hedgehog signaling pathway [34,36]. Somatic variants in GLI3 in HH tissue were confirmed in subsequent studies along with additional somatic variants in genes implicated the Hedgehog signaling pathway, including PRKACA and OFD1 [33,35]. In one study, thirty-eight cases were found to have a somatic variant that disrupted a Hedgehog signaling gene, although it should be noted that many of the variants were large multi-genic deletions that also included many genes not known to be involved in the Hedgehog signaling pathway that could contribute to the phenotype [35]. More recently, HH cases with two-hit (germline and somatic) variants were identified in DYNC2H1, encoding a key protein in the dynein-2 complex critical for the formation of cilia [41]. This study also identified somatic variants in PTPN11, encoding a protein involved in the RAS/mitogen-activated protein kinase (MAPK) pathway that also contributes to signaling in the primary cilium [41]. This suggests that disruption of Hedgehog signaling in HH may be specifically related to its role in proper cilia development functioning, and that the mechanisms underlying HH may be attributed more broadly to ciliary defects through multiple different pathways.
Interestingly, one group showed that cortical dyslamination caused by somatic MTOR variants in FCD II patients may be caused in part by defective neuronal ciliogenesis resulting from impaired autophagy [42]. Furthermore, a recurrent early post-zygotically-acquired somatic mutation in SMO that leads to constitutive activation of Hedgehog signaling has been identified in Curry Jones Syndrome, associated with multiple congenital malformations that sometimes includes hemimegalencephaly [43]. Collectively, this suggests that abnormal cilia formation and activity may be critical across a range of brain malformations associated with epilepsy, and not just limited to HH.
Brain arteriovenous malformations
Cerebral vascular malformations are another group of epilepsy associated lesions attributed to mosaic pathogenic variants. Somatic variants have been identified in the MAPK signaling pathway specifically as the cause of arteriovenous malformations [17–19]. Cerebral cavernous malformations are thought to arise from a combined somatic and inherited variant, two-hit model, involving CCM2, KRIT, and PDCD10 [20–22]. Sturge Weber, a sporadic neurocutaneous syndrome associated with leptomeningeal angiomatosis, port-wine stain, and vascular glaucoma, has been associated with a brain tissue enriched recurrent variant in GNAQ [37–38]. This recurrent variant was also later identified in isolated leptomeningeal angiomatosis [39].
One of the most recent somatic variant discoveries in epilepsy was found in SLC35A2 in individuals with radiographically non-lesional focal epilepsy sometimes with FCD Ia pathology, also referred to as a “mild malformation of cortical development” [23–25]. Across the three independent studies reporting mosaic SLC35A2 variants in intractable neocortical epilepsy, more than 15% of individuals undergoing surgical resection for either non-lesional focal epilepsy or a mild malformation of cortical development had a pathogenic somatic variant in this gene. SLC35A2, located on the X chromosome, encodes a UDP-Gal transporter that moves cytosolic UDP-Gal into the Golgi apparatus to serve as a substrate in glycan formation for the post-translational modification of cellular proteins and lipids. De novo germline or very early post-zygotically acquired SLC35A2 variants had been implicated in congenital disorders of glycosylation and in a rare form of X-linked developmental and epileptic encephalopathy [44,45]. This discovery suggests that glycosylation defects may explain a much larger fraction of intractable neocortical epilepsy that previously thought. As there was no evidence of activated mTOR-signaling in any of the individuals with a mosaic SLC35A2 variant, the mechanism is likely novel and independent of that implicated in FCD II associated phenotypes [23–25].
Tissue and cellular burden contribute to phenotypic variability in mosaic epilepsy
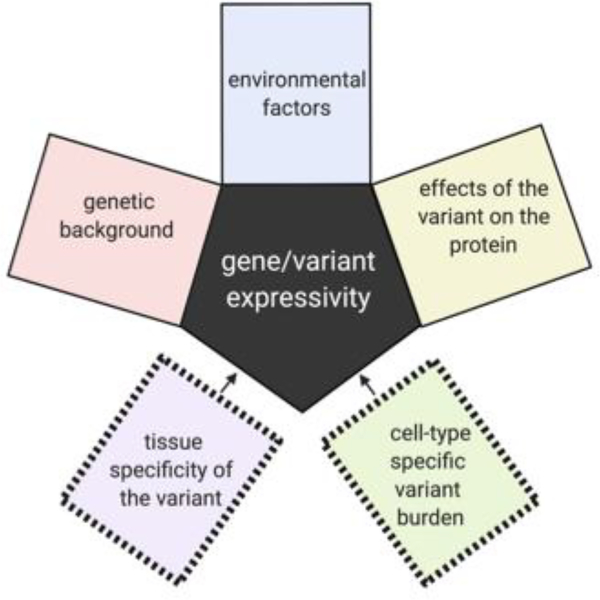
There is extensive discussion in the genetics community about the reasons for the highly variable phenotypic spectrum associated with nearly all genes implicated in neurodevelopmental disorders. These reasons include the potential contributions of modifier loci, differential effects of the variants on the encoded protein, and influential environmental factors. While all possible factors contributing to variable expressivity for germline variants also apply in somatic genetics, there is additional complexity in the case of post-zygotically acquired variants in that tissue and cell-type specific burden also likely contribute to the variability in phenotype (Figure 1).
Figure 1.
Additional complexities affecting the variable expressivity of genes harboring pathogenic post-zygotically acquired genetic variants.
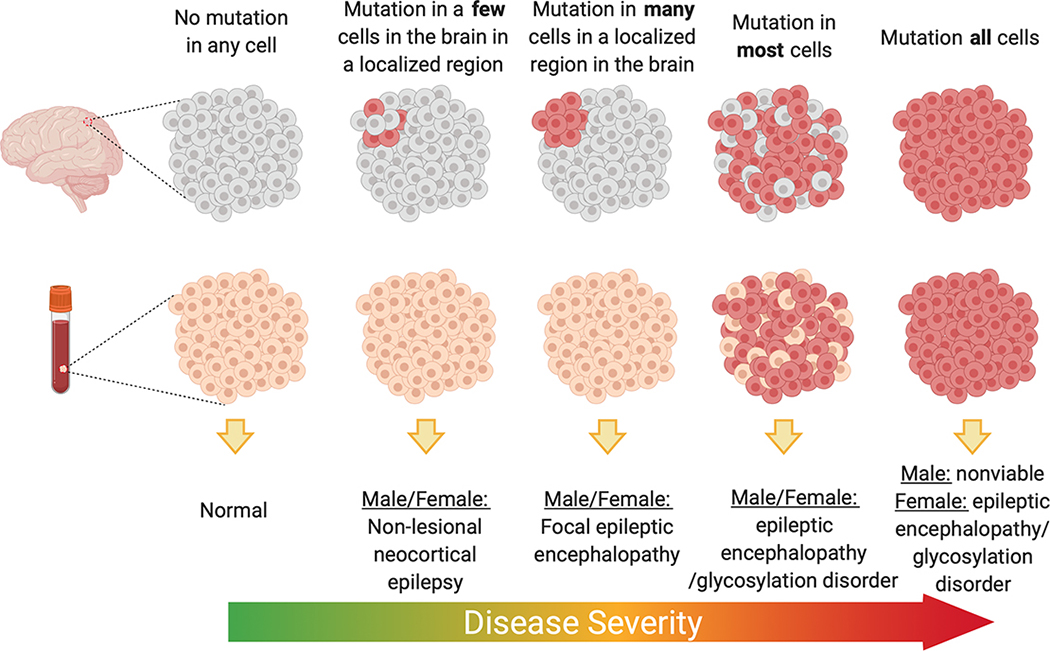
Somatic genetics has revealed multiple instances of how tissue distribution of post-zygotically acquired variants can lead to different disease presentations. GLI3 variants in the germline give rise to Pallister Hall, a syndrome that includes multiple congenital anomalies including HH [46], whereas variants restricted to (or enriched in) the hypothalamus can result in isolated HH. PIK3CA variants can lead to megalencephaly-polymicrogyria-polydactyly-hydrocephalus (MPPH) or congenital lipomatous overgrowth, vascular malformations, and epidermal nevi (CLOVE) syndromes when the mutations arise in the germline or early post-zygotic development, whereas variants in these genes that are acquired later result in hemimegalencephaly [32,47]. Beyond the more obvious tissue localization effects of the variant on phenotype, overall burden within the region with the variant has also been shown to contribute to severity or extent of the clinical presentation for some genes. For hemimegalencephaly, the mutation burden of PI3K-AKT-mTOR pathway mutations tended to be higher compared to that found in more isolated FCD II presentations [27]. A similar pattern is observed for somatic SLC35A2 associated epilepsy where radiographically non-lesional epilepsy patients had on average 6% variant allele burden compared to 22% on average for mild malformations of cortical development [23–25]. In one study that evaluated five individuals with brain-tissue specific SLC35A2 variants, samples with the lowest variant allele burden also showed no evidence of cognitive impairment and later ages of onset compared to that observed in either cases with higher variant burden or cases with germline de novo variants in SLC35A2 (Figure 2). It should be noted, however, that analyses of the landscape of mutation burden across the epileptogenic or dysplastic region are extremely challenging in human tissue given than only small sections are available for research. Much more comprehensive studies in in vitro or ex-vivo analyses of resected tissue would be required to make definitive conclusions about the relationships of variant burden and localization to the clinical phenotype.
Figure 2.
Suggested model for SLC35A2 epilepsy whereby variant burden and localization dictate phenotypic severity. Figure made with Biorender.com
In addition to overall burden of mutation, there are several examples where the effects of cell-type-specific variant burden has been explored. For a recurrent pathogenic KRAS variant in arteriovenous malformations, the variants are highly enriched in brain endothelial cells and virtually absent from neurons [19]. For hemimegalencephaly and FCD, the somatic variants in the PI3K-AKT-mTOR pathway are always found in neurons and in some cases also present in glia, indicating that neuronal involvement is critical to pathogenesis [30]. Notably, both of these studies demonstrate how somatic variants can also be used more generally a research tool to study the role of various cell types in diseases.
While the effects of variant burden and cellular distribution have only been explored to a very limited extent, the expectation is that variant enrichment in specific brain cell types in conjunction with cell-specific burden will be major contributors to the individual’s phenotype and the phenotypic spectrum associated with a gene harboring somatic variants (Figure 1).
Challenges and explaining the unexplained
Despite remarkable progress in somatic genetics in epilepsy, many cases of presumed somatic epilepsies remain genetically unexplained. For hemimegalencephaly where there is a high likelihood of a pathogenic somatic variant, the percent explained across all published studies approaches only 20% [10]. For FCD II, it is less than 15% [10]. The high fraction of genetically unexplained are likely due to three major contributing factors. The first is the lack of data on the genetic landscape of somatic variants in the normal brain limiting the ability to distinguish benign and pathogenic somatic variation in abnormal brains. It is likely that a significant number of pathogenic variants have been identified that are uninterpretable without more information about how exceptional this type of variant is in normal human brain tissue.
Similar to germline genetic variant detection, the field of somatic genetics is also limited by the technological constraints of short read exome sequencing, specifically the inability to comprehensively detect structural variants, non-coding variants, and short tandem repeat variation. Short tandem repeats are highly mutable DNA sequences that have been associated with many neurological disorders. Given their high potential for mutability, short tandem repeats will likely be important de novo contributors to the genetics of epilepsy both in terms of somatic and germline risk. While in the short term there are budgetary constraints to large scale deployment, long-read sequencing will likely ultimately advance somatic gene discovery in epilepsy by allowing more comprehensive detection of genetic variants (e.g., short tandem repeats, structural variants) and long-range haplotypes that have been largely overlooked by short-read next-generation sequencing technology, the mainstay of studies performed to date.
The third aspect that is likely contributing to the large unexplained fraction in genetic epilepsies due to somatic variation is the inability to detect very low burden variants in bulk tissue and the challenges of determining which cells to pursue for single cell analyses. Single cell DNA and RNA sequencing has experienced tremendous technical advancement in recent years making genome-wide analyses much more affordable. These advancements likely will allow this limitation to be overcome in the near future.
Conclusions
Revealing pathogenic somatic variants concealed in human brain tissue has proven to be a impactful approach to identifying new pathways involved in epilepsy pathogenesis and to linking known genes to novel phenotypes. Beyond this obvious advancement, somatic variation serves as endogenous barcodes and offers an extremely valuable tool for retrospective tracing of cellular origins and fate during brain and tumor development [48–51]. Somatic mutations also enable a powerful means to study the downstream effects of a variant in cells from the same individual, correcting for potential confounding factors that exists when comparing across individuals. Coupling single cell genetic and genomic analyses will likely provide high resolution assessments of the impact of genetic variants in individual cell types in the future. While there remains much work to be done to continue to further somatic gene discovery in the epilepsies and learn about the somatic mutation landscape in the brain, the field is technologically poised to make significant advancements in the near future.
Acknowledgements
The author would like to thank Dulcie Lai, Tristan Sands, and Jinfeng Lu for the helpful comments and discussions. ELH is supported by the NINDS (R01-NS094596), and previously by NINDS (R01-NS089552) and by the Irving Institute for Clinical and Translational Research at Columbia University.
Footnotes
Competing Interests
The author declares no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Epi4K Consortium, Epilepsy Phenome/Genome Project: Ultra-rare genetic variation in common epilepsies: a case-control sequencing study. Lancet Neurology 2017, 16:135–143. [DOI] [PubMed] [Google Scholar]
- 2.EuroEPINOMICS-RES Consortium, Epilepsy Phenome/Genome Project, Epi4K Consortium: De novo mutations in synaptic transmission genes including DNM1 cause epileptic encephalopathies. Am J Hum Genet 2014, 95:360–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Epi4K Consortium, Epilepsy Phenome/Genome Project: De novo mutations in epileptic encephalopathies. Nature 2013, 501:217–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Epi25 Collaborative: Ultra-Rare Genetic Variation in the Epilepsies: A Whole-Exome Sequencing Study of 17,606 Individuals. Am J Hum Genet 2019, 105:267–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.EuroEPINOMICS-RES Consortium, Heyne HO, Singh T, Stamberger H, Jamra RA, Caglayan H, Craiu D,Jonghe PD,Guerrini R, Helbig KL, et al. : De novo variants in neurodevelopmental disorders with epilepsy. Nat Genet 2018, 50:1048–1053.* The Epi25 Collaborative reports the results of the largest exome sequencing study performed to date oncomparing 9,170 individuals with epilepsy, including individuals with developmental and epileptic encephalopathies, genetic generalized epilepsy, and nonacquired focal epilepsy, to ~8,500 controls. In all types of epilepsy there was an enrichment of ultra-rare deleterious variants in genes intolerant to functional variation suggesting that ultra-rare variants contribute to epilepsy risk. This study confirms the findings from an earlier study showing overlapping genetic contributors in both rare and common epilepsies.
- 6.Bae T, Tomasini L, Mariani J, Zhou B, Roychowdhury T, Franjic D, Pletikos M, Pattni R, Chen B-J,Venturini E, et al. : Different mutational rates and mechanisms in human cells at pregastrulation and neurogenesis. Science 2017, 359:550–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Koh HY, Lee JH: Brain Somatic Mutations in Epileptic Disorders. Mol Cells 2018, 41:881–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Poduri A, Evrony GD, Cai X, Walsh CA: Somatic mutation, genomic variation, and neurological disease. Sci New York N Y 2013, 341:1237758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.D’Gama AM, Walsh CA: Somatic mosaicism and neurodevelopmental disease. Nat Neurosci 2018, 21:1504–1514. [DOI] [PubMed] [Google Scholar]
- 10.Ye Z, McQuillan L, Poduri A, Green TE, Matsumoto N, Mefford HC, Scheffer IE, Berkovic SF, HildebrandMS: Somatic Mutation: The Hidden Genetics of Brain Malformations and Focal Epilepsies. Epilepsy Res 2019, 155:106161. [DOI] [PubMed] [Google Scholar]
- 11.Jamuar SS, Lam A-TN, Kircher M, D’Gama AM, Wang J, Barry BJ, Zhang X, Hill RS, Partlow JN, Rozzo A, et al. : Somatic Mutations in Cerebral Cortical Malformations. New Engl J Med 2014, 371:733–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vadlamudi L, Dibbens LM, Lawrence KM, Iona X, McMahon JM, Murrell W, Mackay-Sim A, Scheffer IE,Berkovic SF: Timing of De Novo Mutagenesis — A Twin Study of Sodium-Channel Mutations. New Engl J Med 2010, 363:1335–1340. [DOI] [PubMed] [Google Scholar]
- 13.Carvill GL, Heavin SB, Yendle SC, McMahon JM, O’Roak BJ, Cook J, Khan A, Dorschner MO, Weaver M, Calvert S, et al. : Targeted resequencing in epileptic encephalopathies identifies de novo mutations in CHD2 and SYNGAP1. Nat Genet 2013, 45:825–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xu X, Yang X, Wu Q, Liu A, Yang X, Ye AY, Huang AY, Li J, Wang M, Yu Z, et al. : Amplicon Resequencing Identified Parental Mosaicism for Approximately 10% of “ de novo ” SCN1A Mutations in Children with Dravet Syndrome. Hum Mutat 2015, 36:861–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stosser MB, Lindy AS, Butler E, Retterer K, Piccirillo-Stosser CM, Richard G, McKnight DA: High frequency of mosaic pathogenic variants in genes causing epilepsy-related neurodevelopmental disorders. Genetics Medicine Official J Am Coll Medical Genetics 2017, 20:403–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Uddin M, Woodbury-Smith M, Chan A, Brunga L, Lamoureux S, Pellecchia G, Yuen RKC, Faheem M,Stavropoulos DJ, Drake J, et al. : Germline and somatic mutations in STXBP1 with diverse neurodevelopmental phenotypes. Neurology Genetics 2017, 3:e199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hong T, Yan Y, Li J, Radovanovic I, Ma X, Shao YW, Yu J, Ma Y, Zhang P, Ling F, et al. : High prevalence of KRAS/BRAF somatic mutations in brain and spinal cord arteriovenous malformations. Brain J Neurology 2019, 142:23–34. [DOI] [PubMed] [Google Scholar]
- 18.Al-Olabi L, Polubothu S, Dowsett K, Andrews KA, Stadnik P, Joseph AP, Knox R, Pittman A, Clark G,Baird W, et al. : Mosaic RAS/MAPK variants cause sporadic vascular malformations which respond to targeted therapy. J Clin Investigation 2018, 128:1496–1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nikolaev SI, Vetiska S, Bonilla X, Boudreau E, Jauhiainen S, Jahromi BR, Khyzha N, DiStefano PV,Suutarinen S, Kiehl T-R, et al. : Somatic Activating KRAS Mutations in Arteriovenous Malformations of the Brain. New Engl J Med 2018, 378:250–261.* Nikolaev et al. identified somatic activating KRAS variants in >60% of individuals with arteriovenousmalformations studied. These variants were found to be selective enriched in endothelial cells populations suggesting that MAPK-ERK pathway activation in endothelial cells leads to arteriovenous malformations.
- 20.Pagenstecher A, Stahl S, Sure U, Felbor U: A two-hit mechanism causes cerebral cavernous malformations: complete inactivation of CCM1, CCM2 or CCM3 in affected endothelial cells. Hum Mol Genet 2008, 18:911–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Craig HD, Gunel M, Cepeda O, Johnson EW, Ptacek L, Steinberg GK, Ogilvy CS, Berg MJ, Crawford SC,Scott RM, et al. : Multilocus linkage identifies two new loci for a mendelian form of stroke, cerebral cavernous malformation, at 7p15–13 and 3q25.2–27. Hum Mol Genet 1998, 7:1851–1858. [DOI] [PubMed] [Google Scholar]
- 22.Cavé-Riant F, Denier C, Labauge P, Cécillon M, Maciazek J, Joutel A, Couteulx SL, Tournier-Lasserve E: Spectrum and expression analysis of KRIT1 mutations in 121 consecutive and unrelated patients with Cerebral Cavernous Malformations. Eur J Hum Genet 2002, 10:733–740. [DOI] [PubMed] [Google Scholar]
- 23.Sim NS, Seo Y, Lim JS, Kim WK, Son H, Kim HD, Kim S, An HJ, Kang H-C, Kim SH, et al. : Brain somatic mutations in SLC35A2 cause intractable epilepsy with aberrant N-glycosylation. Neurology Genetics 2018, 4:e294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baldassari S, Ribierre T, Marsan E, Adle-Biassette H, Ferrand-Sorbets S, Bulteau C, Dorison N, Fohlen M,Polivka M, Weckhuysen S, et al. : Dissecting the genetic basis of focal cortical dysplasia: a large cohort study. Acta Neuropathol 2019, 138:885–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Winawer MR, Griffin NG, Samanamud J, Baugh EH, Rathakrishnan D, Ramalingam S, Zagzag D, Schevon CA, Dugan P, Hegde M, et al. : Somatic SLC35A2 variants in the brain are associated with intractable neocortical epilepsy. Ann Neurol 2018, 83:1133–1146.** This study reported the discovery of somatic SLC35A2 variants in both nonlesional focal epilepsy and individuals with a mild malformation of cortical development, implicating glycosylation pathway as a cause for intractable focal epilepsy. Seventeen percent of cases studied with nonlesional focal epilepsy were found to have a somatic variant in SLC35A2.
- 26.Salinas V, Vega P, Piccirilli MV, Chicco C, Ciraolo C, Christiansen S, Consalvo D, Perez-Maturo J, Medina N, González-Morón D, et al. : Identification of a somatic mutation in the RHEB gene through high depth and ultra-high depth next generation sequencing in a patient with hemimegalencephaly and drug resistant epilepsy. Eur J Med Genet 2018, 62:103571. [DOI] [PubMed] [Google Scholar]
- 27.D’Gama AM, Woodworth MB, Hossain AA, Bizzotto S, Hatem NE, LaCoursiere CM, Najm I, Ying Z,Yang E, Barkovich AJ, et al. : Somatic mutations activating the mTOR pathway in dorsal telencephalic progenitors cause a continuum of cortical dysplasias. Cell Reports 2017, 21:3754–3766.** This study showed using next-generation sequencing that the same mutations are implicated in both FCD II and hemimegalencephaly depending on the timing of the mutational event. All individuals studied were found to have the variants in neurons suggesting that neuronal involvement is critical to the pathology. In a mouse model they were able to show that activation of mTOR in excitatory neurons specifically was associated with the development cortical dysplasia.
- 28.Lim JS, Gopalappa R, Kim SH, Ramakrishna S, Lee M, Kim W-I, Kim J, Park SM, Lee J, Oh J-H, et al. : Somatic Mutations in TSC1 and TSC2 Cause Focal Cortical Dysplasia. Am J Hum Genet 2017, 100:454– 472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lim JS, Kim W, Kang H-C, Kim SH, Park AH, Park EK, Cho Y-W, Kim S, Kim HM, Kim JA, et al. : Brain somatic mutations in MTOR cause focal cortical dysplasia type II leading to intractable epilepsy. Nat Med 2015, 21:395–400. [DOI] [PubMed] [Google Scholar]
- 30.D’Gama AM, Geng Y, Couto JA, Martin B, Boyle EA, LaCoursiere CM, Hossain A, Hatem NE, Barry BJ,Kwiatkowski DJ, et al. : Mammalian target of rapamycin pathway mutations cause hemimegalencephaly and focal cortical dysplasia. Ann Neurol 2015, 77:720–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Poduri A, Evrony GD, Cai X, Elhosary PC, Beroukhim R, Lehtinen MK, Hills LB, Heinzen EL, Hill A, Hill RS, et al. : Somatic Activation of AKT3 Causes Hemispheric Developmental Brain Malformations. Neuron 2012, 74:41–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee JH, Huynh M, Silhavy JL, Kim S, Dixon-Salazar T, Heiberg A, Scott E, Bafna V, Hill KJ, Collazo A, et al. : De novo somatic mutations in components of the PI3K-AKT3-mTOR pathway cause hemimegalencephaly. Nat Genet 2012, 44:941–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Saitsu H, Sonoda M, Higashijima T, Shirozu H, Masuda H, Tohyama J, Kato M, Nakashima M, Tsurusaki Y, Mizuguchi T, et al. : Somatic mutations in GLI3 and OFD1 involved in sonic hedgehog signaling cause hypothalamic hamartoma. Ann Clin Transl Neur 2016, 3:356–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Craig DW, Itty A, Panganiban C, Szelinger S, Kruer MC, Sekar A, Reiman D, Narayanan V, Stephan DA,Kerrigan JF: Identification of somatic chromosomal abnormalities in hypothalamic hamartoma tissue at the GLI3 locus. Am J Hum Genet 2008, 82:366–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hildebrand MS, Griffin NG, Damiano JA, Cops EJ, Burgess R, Ozturk E, Jones NC, Leventer RJ, Freeman JL, Harvey AS, et al. : Mutations of the Sonic Hedgehog Pathway Underlie Hypothalamic Hamartoma with Gelastic Epilepsy. Am J Hum Genetics 2016, 99:423–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wallace RH, Freeman JL, Shouri MR, Izzillo PA, Rosenfeld JV, Mulley JC, Harvey AS, Berkovic SF: Somatic mutations in GLI3 can cause hypothalamic hamartoma and gelastic seizures. Neurology 2007, 70:653–655. [DOI] [PubMed] [Google Scholar]
- 37.Shirley MD, Tang H, Gallione CJ, Baugher JD, Frelin LP, Cohen B, North PE, Marchuk DA, Comi AM,Pevsner J: Sturge-Weber syndrome and port-wine stains caused by somatic mutation in GNAQ. New Engl J Medicine 2013, 368:1971–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nakashima M, Miyajima M, Sugano H, Iimura Y, Kato M, Tsurusaki Y, Miyake N, Saitsu H, Arai H,Matsumoto N: The somatic GNAQ mutation c.548G>A (p.R183Q) is consistently found in Sturge–Weber syndrome. J Hum Genet 2014, 59:691–693. [DOI] [PubMed] [Google Scholar]
- 39.Hildebrand MS, Harvey AS, Malone S, Damiano JA, Do H, Ye Z, McQuillan L, Maixner W, Kalnins R,Nolan B, et al. : Somatic GNAQ mutation in the forme fruste of Sturge-Weber syndrome. Neurology Genetics 2018, 4:e236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ljungberg MC, Bhattacharjee MB, Lu Y, Armstrong DL, Yoshor D, Swann JW, Sheldon M, D’Arcangelo G: Activation of mammalian target of rapamycin in cytomegalic neurons of human cortical dysplasia. Ann Neurol 2006, 60:420–429. [DOI] [PubMed] [Google Scholar]
- 41.Fujita A, Higashijima T, Shirozu H, Masuda H, Sonoda M, Tohyama J, Kato M, Nakashima M, Tsurusaki Y, Mitsuhashi S, et al. : Pathogenic variants of DYNC2H1, KIAA0556, and PTPN11 associated with hypothalamic hamartoma. Neurology 2019, 93:e237–e251. [DOI] [PubMed] [Google Scholar]
- 42.Park SM, Lim JS, Ramakrishina S, Kim SH, Kim WK, Lee J, Kang H-C, Reiter JF, Kim DS, Kim HH, et al. : Brain Somatic Mutations in MTOR Disrupt Neuronal Ciliogenesis, Leading to Focal Cortical Dyslamination. Neuron 2018, 99:83–97.e7.* Park et al. showed that human resected brain tissue from individuals with an FCD II associated brainmalformations harboring a somatic MTOR variant showed defective neuronal ciliogenesis. This finding was confirmed in a mouse model and in vitro using a human cell line. The MTOR variant associated altered ciliogenesis was found to be due disruptions in autophagy leading to accumulation of OFD1 at centriolar satellites; and that defective ciliogenesis accounted for the observed cortical dyslamination.
- 43.Twigg SRF, Hufnagel RB, Miller KA, Zhou Y, McGowan SJ, Taylor J, Craft J, Taylor JC, Santoro SL,Huang T, et al. : A Recurrent Mosaic Mutation in SMO, Encoding the Hedgehog Signal Transducer Smoothened, Is the Major Cause of Curry-Jones Syndrome. Am J Hum Genet 2016, 98:1256–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kodera H, Nakamura K, Osaka H, Maegaki Y, Haginoya K, Mizumoto S, Kato M, Okamoto N, Iai M,Kondo Y, et al. : De Novo Mutations in SLC35A2 Encoding a UDP-Galactose Transporter Cause EarlyOnset Epileptic Encephalopathy. Hum Mutat 2013, 34:1708–1714. [DOI] [PubMed] [Google Scholar]
- 45.Yates TM, Suri M, Desurkar A, Lesca G, Wallgren-Pettersson C, Hammer TB, Raghavan A, Poulat A-L,Møller RS, Thuresson A-C, et al. : SLC35A2-related congenital disorder of glycosylation: Defining the phenotype. Eur J Paediatr Neuro 2018, 22:1095–1102. [DOI] [PubMed] [Google Scholar]
- 46.Kang S, Graham JM, Olney AH, Biesecker LG: GLI3 frameshift mutations cause autosomal dominant Pallister-Hall syndrome. Nat Genet 1997, 15:266–268. [DOI] [PubMed] [Google Scholar]
- 47.Mirzaa G, Timms AE, Conti V, Boyle EA, Girisha KM, Martin B, Kircher M, Olds C, Juusola J, Collins S, et al. : PIK3CA-associated developmental disorders exhibit distinct classes of mutations with variable expression and tissue distribution. Jci Insight 2016, 1:e87623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Evrony GD, Lee E, Mehta BK, Benjamini Y, Johnson RM, Cai X, Yang L, Haseley P, Lehmann HS, Park PJ, et al. : Cell lineage analysis in human brain using endogenous retroelements. Neuron 2015, 85:49–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lodato MA, Woodworth MB, Lee S, Evrony GD, Mehta BK, Karger A, Lee S, Chittenden TW, D’Gama AM, Cai X, et al. : Somatic mutation in single human neurons tracks developmental and transcriptional history. Science 2015, 350:94–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Woodworth MB, Girskis KM, Walsh CA: Building a lineage from single cells: genetic techniques for cell lineage tracking. Nat Rev Genet 2017, 18:230–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nik-Zainal S, Loo PV, Wedge DC, Alexandrov LB, Greenman CD, Lau KW, Raine K, Jones D, Marshall J,Ramakrishna M, et al. : The life history of 21 breast cancers. Cell 2012, 149:994–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]