Abstract
Background
To best employ radium‐223 dichloride (Ra‐223) for patients with castration‐resistant prostate cancer (CRPC) and bone metastasis, we investigated the bone‐predominant status in patients treated with Ra‐223.
Methods
We retrospectively evaluated 127 CRPC patients who underwent treatment with Ra‐223. The patients were divided into three groups based on the types of dynamic changes of bone metastasis between diagnosis and just before Ra‐223: (a) only known lesions; (b) de novo lesions; (c) new progressive lesions. We developed the risk assessment using predictive factors based on progression‐free survival (PFS).
Results
During the median follow‐up period of 10.4 months, the median PFS in the only known lesions group was 11.3 months compared to 8.1 months in the de novo lesions group and 5.1 months in the new progressive lesions group (P < .001). In multivariate analysis, the type of the new progressive lesions in bone metastasis (HR 1.45, 95% CI 1.13‐1.66, P = .003), performance status of >1 (HR 1.74, 95% CI 1.04‐2.89, P = .034), PSA value of >100 ng/mL (HR 1.59, 95% CI 1.02‐2.50, P = .043), and PSA doubling time (PSADT) of <3 months (HR 1.53, 95% CI 1.11‐2.03, P = .007) were independent unfavorable predictive factors for PFS. The risk assessment for PFS was highlighted when the type of dynamic changes of bone metastasis was combined with PSADT just before Ra‐223 treatment. This was associated with non‐bone metastasis progression, especially visceral metastasis, and overall survival.
Conclusions
Risk assessment in combination with dynamic changes of bone metastasis and PSADT determines the bone‐predominant metastasis type to benefit from Ra‐223.
Keywords: bone‐predominant, castration‐resistant prostate cancer, prognosis, PSA doubling time, radium‐223 dichloride, risk assessment
We developed the risk assessment based on the types of dynamic changes of bone metastasis before the use of radium‐223 dichloride (Ra‐223). This risk assessment can be used to maximize the clinical benefits of Ra‐223 treatment for bone‐predominant metastasis.
![]()
1. INTRODUCTION
Metastatic castration‐resistant prostate cancer (mCRPC) metastasizes to bone in up to 90% of patients with this disease. 1 Patients with bone metastasis are vulnerable to substantial morbidity including bone pain and skeletal‐related events (SREs), which leads to worsening quality‐of‐life (QOL) and survival. 2 This underlines the importance of management for bone metastasis in those patients. However, palliative irradiation and bone‐modifying agents, including zoledronic acid and denosumab, have not been shown to improve survival, and the benefits from these treatments are primarily limited to pain relief and delay of SREs. 3 , 4
Radium‐223 dichloride (Ra‐223) is a targeted alpha emitter that selectively binds to the site of high bone turnover caused by bone metastasis. 5 Alpha particles with a very short range (<100 μm) induce predominantly double‐stranded DNA breaks that result in highly localized cytotoxic effects and less damage to the surrounding tissues. The efficacy and safety of Ra‐223 in mCRPC patients with symptomatic bone metastasis were demonstrated in the ALSYMPCA trial. 6 With six cycles of Ra‐223 plus best standard of care (BSoC) vs placebo plus BSoC, Ra‐223 showed an overall survival benefit of 3.6 months (median 14.9 vs 11.3 months; hazard ratio [HR]), 0.70; 95% confidence interval [CI], 0.58‐0.83). The time to the first symptomatic skeletal event and QOL was also improved by Ra‐223, with a low incidence of grade 3 or 4 adverse events. 7
Although Ra‐223 can play a major role in the management of mCRPC and symptomatic bone metastasis, it remains unclear how to best employ this treatment in clinical practice. 8 , 9 Some patients have disease progression of non‐bone metastasis with eventually elevated PSA levels during Ra‐223 treatment, which can occasionally be too late for the patient to receive subsequent therapies. Thus, the diagnostic certainty of bone‐predominant mCRPC needs to be determined. We hypothesized that the types of dynamic changes of bone metastasis would reflect the differential bone‐predominant status in CRPC. The aim of this study was to clarify which dynamic changes of bone metastasis before the use of Ra‐223 maximized its clinical benefits.
2. MATERIALS AND METHODS
This study was retrospectively conducted using the clinical data of 127 consecutive CRPC patients treated with Ra‐223 at multiple centers from May 2012 to August 2019. Approval of each study center's institutional review board was obtained for this study. All patients were diagnosed with prostate cancer by pathological examination. CRPC was defined by disease progression despite castrate levels of serum testosterone (<50 ng/dL). Bone single‐photon emission computed tomography/computed tomography (SPECT/CT) after injection of 99mTc‐methylene diphosphonate or 99mTc‐hydroxymethylene diphosphonate (bone scintigraphy), and computed tomography (CT), were conducted to identify bone metastases and soft tissue, respectively. All patients had two or more bone metastases (symptomatic or asymptomatic), no lymph node metastasis of more than 3 cm, and no visceral metastasis.
Patients received Ra‐223, 55 kBq/kg, every 4 weeks for up to six cycles and were allowed to receive concomitant BSoC, including external beam radiotherapy, corticosteroids, and first‐generation anti‐androgens such as bicalutamide and flutamide. During the Ra‐223 treatment period, chemotherapy such as docetaxel and cabazitaxel, estrogen agents, and androgen receptor‐targeted agents such as abiraterone and enzalutamide were not used.
All patients were divided into three groups by the types of radiographical dynamic changes of bone metastasis between the diagnosis of prostate cancer and just before treatment with Ra‐223: (a) only known lesions; (b) de novo lesions; (c) new progressive lesions. Representative cases are shown in Figure 1. Only known lesions were defined as bone metastasis just before Ra‐223 treatment observed in only the same lesions that progressed, improved or were not changed compared to the lesions at the diagnosis of prostate cancer. De novo lesions were defined as bone metastasis just before treatment with Ra‐223 newly diagnosed at CRPC. New progressive lesions observed in the newly developed lesions in addition to the lesions at the diagnosis of prostate cancer were defined as bone metastases just before Ra‐223 treatment. Bone scans were evaluated by qualitative and quantitative analyses using a computer‐aided diagnostic system (BONENAVI®, FUJIFILM Toyama Chemical Co. Ltd.). The degree of the bone tumor burden was measured by the bone scan index (BSI). 10 In the case of bone superscans, progression was evaluated by CT findings, which were supported by calculating the mean uptake obtained as the standardized uptake value (SUV) in the trunk using bone SPECT/CT (GIBONE®, Nihon Medi‐Physics Co. Ltd.; Figure S1). The area above SUV = 7.0 was considered to be the active bone metastatic burden. Then the average SUVpeak was obtained as the average of the peaks of the regional SUV. 11 When osteosclerotic lesions on bone superscans extended on CT and SUVpeak increased on bone SPECT/CT, the dynamic changes of bone metastases were classified as the new progressive lesions group. When osteosclerotic lesions on bone superscans extended without a SUVpeak increase, or osteosclerotic lesions reduced regardless of SUVpeak changes, the dynamic changes of bone metastases were classified into the only known lesions group.
Figure 1.
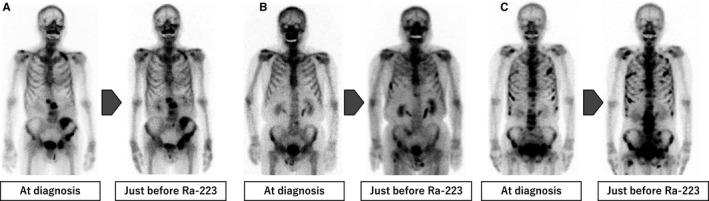
The types of dynamic changes of bone metastasis between the diagnosis of prostate cancer and just before radium‐223 dichloride (Ra‐223). Representative cases are shown. A, Only known lesions with progression, B, De novo lesions, C, New progressive lesions with the known lesions
PSA doubling time was calculated by PSA elevation from 3 months before Ra‐223 treatment. PSA progression was defined as per the criteria of the Prostate Cancer Working Group 3; that is, a 25% increase from the nadir (consisting of a starting value of ≥1.0 ng/mL) with a minimum rise of 2 ng/mL. 12 A PSA response was defined as a PSA decrease of ≥50% at any time. All patients were followed‐up by PSA measurement every 1 month. CT and bone scintigraphy were conducted after Ra‐223 treatment or at need.
Fisher's exact test, the χ 2 test, and the Kruskal‐Wallis test were used as appropriate to evaluate group differences. Progression‐free survival (PFS) was defined as the time from the start of Ra‐223 treatment to the time of PSA progression during the first subsequent therapy, clinical/radiographical progression or death from any cause, whichever occurred first. The cumulative incidence of bone metastasis progression events (bPEs) was defined as the probability that the first evidence of bone metastatic progression such as new lesions, extension of known lesions or the last tumor evaluation occurred after the initiation of Ra‐223 treatment. The cumulative incidence of non‐bone metastasis progression events (nbPEs) was defined as the probability of the first evidence of visceral metastasis, nodal metastatic progression or primary local progression, whichever occurred first after the initiation of Ra‐223 treatment. The Fine–Gray regression model was used to calculate the probability of bPEs or nbPEs. Cumulative incidence curves were used in a competing risk setting, with death without bPEs or nbPEs for the probability of bPEs or nbPEs as a competing event. These were estimated until 3 years because of the number of patients at risk. PFS and overall survival (OS) were evaluated using the Kaplan–Meier method, with the log‐rank test used to evaluate differences between the groups. Multivariate Cox proportional hazards analysis was used to identify factors to predict PFS of Ra‐223 and included all factors that were significantly associated with a univariate analysis model. Based on the HR for PFS, we developed a risk model with three categories: favorable, intermediate, and poor. A P‐value of <.05 was considered statistically significant for all analyses. All statistical analyses were performed using EZR (Jichi Medical University, Saitama, Japan).
3. RESULTS
The characteristics of the patients with the various types of dynamic changes of bone metastasis were compared (Table 1). A higher proportion of patients in the type with de novo lesions had a long time to CRPC compared with other types because of a less advanced T stage at diagnosis and undergoing prior local therapy. Patients with new progressive lesions had a higher PSA value and higher ALP value just before Ra‐223 treatment than those with the other types. There were no significant differences in bone pain or the extent of disease among the types of bone metastasis.
Table 1.
Characteristics of patients according to the types of dynamic changes of bone metastasis
|
Overall n = 127 |
Dynamic changes of bone metastasis | P value | |||
|---|---|---|---|---|---|
|
Only known n = 66 |
De novo n = 26 |
New progressive n = 35 |
|||
| Age, y, median (IQR) | 75 (69‐79) | 73 (66‐78) | 76 (72‐82) | 76 (70‐80) | .102 |
| ECOG PS, n (%) | .207 | ||||
| 0 | 45 (35) | 26 (39) | 8 (31) | 11 (31) | |
| 1 | 58 (46) | 32 (49) | 13 (50) | 13 (37) | |
| ≥2 | 24 (19) | 8 (12) | 5 (19) | 11 (31) | |
| Gleason score, n (%) | .36 | ||||
| ≤7 | 9 (7) | 2 (3) | 4 (15) | 3 (9) | |
| 8 | 45 (36) | 24 (38) | 9 (35) | 12 (35) | |
| ≥9 | 70 (57) | 38 (59) | 13 (50) | 19 (56) | |
| Unclear | 3 | 2 | 1 | ||
| T stage at diagnosis, n (%) | .004 | ||||
| ≤T2 | 27 (21) | 8 (12) | 13 (50) | 6 (17) | |
| T3 | 73 (58) | 42 (64) | 11 (42) | 20 (57) | |
| T4 | 27 (21) | 16 (24) | 2 (8) | 9 (26) | |
| Time to CRPC, mo, median (IQR) | 14.1 (6.3‐32.2) | 11.4 (5.7‐18.4) | 34.5 (15.7‐67.3) | 15.4 (7.6‐34.8) | <.001 |
| Bone pain at metastasis, n (%) | 61 (48) | 26 (39) | 16 (62) | 19 (54) | .095 |
| PSA, ng/mL, median (IQR) | 45.8 (10.2‐243) | 23.2 (6.8‐95.3) | 45.9 (9.9‐197) | 130 (45.0‐437) | .002 |
| ALP, U/L, median (IQR) | 324 (225‐534) | 273 (197‐459) | 325 (259‐414) | 438 (303‐971) | .045 |
| PSA doubling time, mo, median (IQR) | 2.4 (1.3‐3.8) | 2.6 (1.4‐5.3) | 2.5 (1.2‐3.2) | 2.4 (1.4‐3.0) | .214 |
| Lymph node metastasis, n (%) | 22 (17) | 12 (18) | 3 (12) | 7 (20) | .707 |
| Extent of disease, n (%) | .067 | ||||
| ≤5 | 38 (30) | 23 (35) | 8 (31) | 7 (20) | |
| 6‐20 | 39 (31) | 19 (29) | 12 (46) | 8 (23) | |
| ≥21 | 38 (30) | 19 (29) | 6 (23) | 13 (37) | |
| Superscans | 12 (9) | 5 (7) | 0 | 7 (20) | |
| Concurrent use of zoledronate or denosumab, n (%) | 86 (68) | 47 (71) | 14 (54) | 25 (71) | .237 |
| Prior local treatment, n (%) | 21 (16) | 5 (7) | 14 (54) | 2 (6) | <.001 |
| Prior treatment in CRPC, n (%) | .83 | ||||
| Docetaxel | 58 (45) | 25 (38) | 12 (46) | 21 (60) | |
| Abiraterone | 59 (46) | 26 (39) | 12 (46) | 21 (60) | |
| Enzalutamide | 48 (38) | 18 (27) | 14 (54) | 16 (46) | |
| Numbers of prior treatments, n (%) | .055 | ||||
| ≤2 | 69 (55) | 43 (65) | 14 (54) | 12 (34) | |
| 3 | 22 (17) | 9 (14) | 5 (19) | 8 (23) | |
| ≥4 | 36 (28) | 14 (21) | 7 (27) | 15 (43) | |
Variables are expressed as the median (interquartile range: IQR) or n (%).
Abbreviations: ALP, alkaline phosphatase; CRPC, castration‐resistant prostate cancer; ECOG PS, Eastern Cooperative Oncology Group performance status; PSA, prostate‐specific antigen.
PSA responses were observed in 11 patients (9%) and ALP declines of >30% in 52 (41%). During the median follow‐up period of 10.4 months, 58 patients (46%) died of prostate cancer and three (2%) died from other causes. The median PFS, bPEs, nbPEs, and OS were 8.1, 14.4, 5.7, and 17.7 months, respectively. Patients in the only known lesions group were more likely to have Ra‐223 of ≥5 cycles and six cycles compared to those with de novo lesions and new progressive lesions (83%, 65% vs 66%; P = .070, 77%, 57% vs 43%; P = .002, respectively; Table S1). There were significant differences in PFS and OS among the types of dynamic changes of bone metastasis (Figure 2A,B). The median PFS and OS in the only known lesions group were 11.3 and 21.5 months, respectively, compared to 8.1 and 17.7 months in the de novo lesions group and 5.1 and 11.9 months, respectively, in the new progressive lesions group (P < .001). Univariate analysis revealed that the types of dynamic changes of bone metastasis, Eastern Cooperative Oncology Group performance status (PS), bone pain, PSA value, ALP value, PSADT, and prior use of docetaxel were associated with PFS (Table 2). The number of prior treatments was excluded from the variables because it was a confounder to prior use of docetaxel (P = .001). In multivariate analysis, new progressive lesions in bone metastasis (HR 1.45, 95% CI 1.13‐1.66, P = .003), PS of >1 (HR 1.74, 95% CI 1.04‐2.89, P = .034), a PSA value of >100 ng/mL (HR 1.59, 95% CI 1.02‐2.50, P = .043), and PSADT of <3 months (HR 1.53, 95% CI 1.11‐2.03, P = .007) were independent unfavorable predictive factors for PFS.
Figure 2.
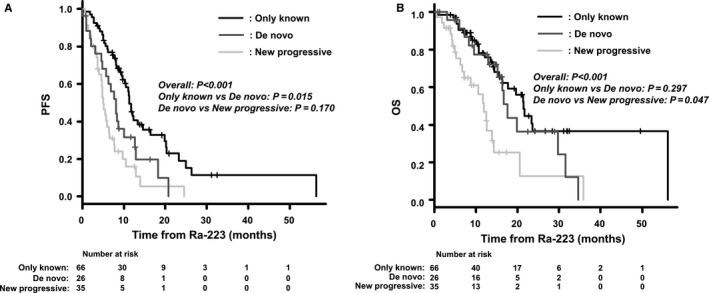
Kaplan–Meier probability curves according to the type of dynamic changes of bone metastasis before treatment with radium‐223 dichloride (Ra‐223). A, progression‐free survival (PFS), B, overall survival (OS)
Table 2.
Factors predicting disease progression during subsequent therapy in patients treated with Ra‐223
| Variable | Category | Univariate | Multivariate | ||
|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | ||
| Dynamic changes of bone metastasis | New progressive (Ref: Only known, De novo) | 1.47 (1.15‐1.86) | <.001 | 1.45 (1.13‐1.66) | .003 |
| Age, y | ≥80 (Ref: <80) | 1.32 (0.81‐2.15) | .269 | ||
| ECOG PS | ≥2 (Ref: <2) | 2.00 (1.22‐3.27) | .006 | 1.74 (1.04‐2.89) | .034 |
| Gleason score | ≥9 (Ref: ≤8) | 1.12 (0.80‐1.56) | .503 | ||
| T stage at diagnosis | ≥T3b (Ref: ≤T3a) | 1.12 (0.74‐1.72) | .586 | ||
| Time to CRPC, mo | <12 (Ref: ≥12) | 1.25 (0.83‐1.89) | .289 | ||
| Bone pain at metastasis | Yes (Ref: No) | 1.26 (1.15‐2.16) | .032 | 1.13 (0.69‐1.71) | .092 |
| PSA, ng/mL | ≥100 (Ref: <100) | 2.00 (1.31‐3.06) | .001 | 1.59 (1.02‐2.50) | .043 |
| ALP, U/L | >ULN (Ref: ≤ULN) | 1.67 (1.11‐2.54) | .015 | 1.22 (0.87‐1.89) | .087 |
| PSA doubling time, mo | <3 (Ref: ≥3) | 1.57 (1.17‐2.10) | .002 | 1.53 (1.11‐2.03) | .007 |
| Lymph node metastasis | Yes (Ref: No) | 0.54 (0.24‐1.49) | .251 | ||
| Extent of disease | Superscans (Ref: <superscans) | 1.13 (0.56‐2.25) | .736 | ||
| Concurrent use of zoledronate or denosumab | No (Ref: Yes) | 1.10 (0.84‐1.37) | .583 | ||
| Prior docetaxel | Yes (Ref: No) | 1.68 (1.11‐2.54) | .015 | 1.34 (0.85‐2.10) | .205 |
Abbreviations: ALP, alkaline phosphatase; CRPC, castration‐resistant prostate cancer; ECOG PS, Eastern Cooperative Oncology Group performance status; HR, hazard ratio; PSA, prostate‐specific antigen; Ra‐223, radium‐223 dichloride; Ref, referent; ULN, upper limit of normal.
To clarify the characteristics of bone metastasis maximizing the benefit of Ra‐223 in patients with bone metastasis, we developed a risk model based on PFS in combination with dynamic changes of bone metastasis by type and PSADT just before treatment with Ra‐223 (Figure 3). Favorable risks were defined as only known lesions and any PSADT, de novo lesions and PSADT of ≥3 months, and any new progressive lesions and PSADT of ≥6 months, whereas intermediate risks were de novo lesions and PSADT of <3 months, or any new progressive lesions and PSADT of 3 to <6 months, and poor risk any new progressive lesions and PSADT of <3 months. The HR for PFS in those with intermediate risk and poor risk was high compared to that for the favorable risk group (HR 1.75, 95% CI 1.02‐2.99; P = .041, and HR 4.16, 95% CI 2.47‐6.99; P < .001, respectively).
Figure 3.
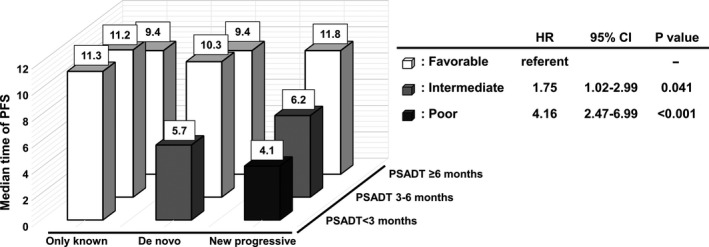
The risk assessment of progression‐free survival (PFS) in combination with the dynamic changes of bone metastasis by type and PSA doubling time (PSADT) just before radium‐223 dichloride (Ra‐223) treatment (white cuboid, favorable; gray cuboid, intermediate; black cuboid, poor). A, median time of PFS, B, hazard ratio (HR) according to the risk category
The PSA response, ALP value, and completion of Ra‐223 were assessed for the risk groups (Table 3). A PSA response was observed in 11 patients (13%) in the favorable risk group, but in none in the intermediate risk and poor risk groups (P = .046). There was no significant difference in the decline in ALP levels among the risk groups. Completion of Ra‐223 treatment was achieved in 64 patients (77%) in the favorable risk group, 10 (48%) in the intermediate risk group, and 7 (30%) in the poor risk group (P < .001). Patients with a favorable risk or intermediate risk were more likely to have a PSADT of ≥6 months during Ra‐223 treatment even if they had a PSADT of <3 months just before it (Figure S2). Patients in the poor risk group were more likely to have the best supportive care as sequential therapy.
Table 3.
Response during Ra‐223 treatment and the kinds of subsequent therapy according to the risk category
| Risk category |
Favorable n = 83 |
Intermediate n = 21 |
Poor n = 23 |
P value |
|---|---|---|---|---|
| PSA decline, n (%) | ||||
| Any | 17 (20) | 2 (10) | 0 | .024 |
| ≥30% | 13 (16) | 1 (5) | 0 | .062 |
| ≥50% | 11 (13) | 0 | 0 | .046 |
| ALP decline, n (%) | ||||
| ≥30% | 33 (40) | 9 (43) | 9 (39) | .965 |
| ≥50% | 27 (32) | 4 (19) | 6 (26) | .487 |
| Ra‐223 cycles, n (%) | ||||
| ≥5 | 69 (83) | 14 (67) | 12 (52) | .006 |
| 6 | 64 (77) | 10 (48) | 7 (30) | <.001 |
| Subsequent therapy, n (%) | <.001 | |||
| Docetaxel | 20 (24) | 4 (19) | 2 (9) | |
| Abiraterone | 17 (20) | 2 (9) | 2 (9) | |
| Enzalutamide | 13 (16) | 7 (33) | 0 | |
| Cabazitaxel | 2 (2) | 1 (5) | 0 | |
| BSC | 18 (22) | 6 (29) | 17 (73) | |
| Others | 13 (16) | 1 (5) | 2 (9) | |
Variables are expressed as n (%).
Abbreviations: ALP, alkaline phosphatase; BSC, best supportive care; PSA, prostate‐specific antigen; Ra‐223, radium‐223 dichloride.
We assessed PFS, bPEs, nbPEs, and OS according to the risk group. PFS was significantly longer in the favorable risk group than in the intermediate and poor risk groups (median, 11.3 vs 5.9 vs 4.6 months, P < .001; Figure 4A). In the poor risk group, the 1‐year bPEs rate was 45% compared to 32% in the favorable risk group and 41% in the intermediate risk group (P = .115), while the 1‐year nbPEs rate was 81% in the poor risk group compared to 18% in the favorable risk group and 40% in the intermediate risk group (P < .001; Figure 4B,C). Fifteen (94%) of the 16 patients in the poor risk group who had non‐bone metastasis progression developed visceral metastasis. The median OS in the favorable risk group was 21.5 months compared to 13.8 months in the intermediate risk group and 7.2 months in the poor risk group (P < .001; Figure 4D).
Figure 4.
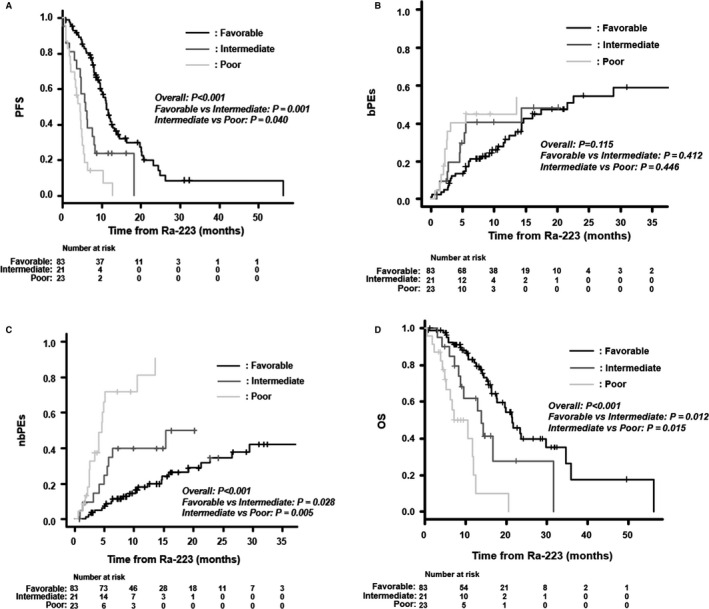
Kaplan‐Meier probability curves (A, D) and Fine–Gray incidence curves (B, C) according to the risk category: A, progression‐free survival (PFS), B, bone metastasis progression events (bPEs), C, non‐bone metastasis progression events (nbPEs), D, overall survival (OS)
4. DISCUSSION
We developed a risk assessment model to determine the bone‐predominant metastasis type in CRPC patients that will benefit from Ra‐223 treatment. The dynamic changes of bone metastasis, PSADT, PSA just before Ra‐223 treatment, and PS were significantly associated with PFS in patients treated with Ra‐223. In particular, the dynamic changes of bone metastasis combined with PSADT highlighted the optimal decision for the use of Ra‐223. The favorable risks, defined as only known lesions and any PSADT, de novo lesions, and PSADT of ≥3 months, or any new progressive lesions and PSADT of ≥6 months, were associated with better nbPEs and OS as well. In contrast, the poor risk, defined as any new progressive lesions and PSADT of <3 months, was associated with poor nbPEs and OS. Those with the poor risk were presumably more likely to have the risk of progression of visceral metastasis following Ra‐223 treatment. This risk assessment may help to determine the ideal sequencing of other life‐prolonging therapies following Ra‐223 treatment.
In several studies, Ra‐223 was found to confer a survival benefit even after docetaxel therapy. 13 , 14 However, there may still be room for improvement regarding the timing for the use of Ra‐223 in real clinical practice because some patients have an early cessation of Ra‐223 due to death or disease progression. Recent studies have shown that patients with 1‐4 doses of Ra‐223 are more likely to have shorter OS than those with 5‐6 doses. 15 , 16 , 17 , 18 , 19 The cessation of Ra‐223 was associated with poor PS, severe pain, and a higher PSA level at baseline and a lower hemoglobin level at baseline. Likewise, the present study demonstrated that patients with PSA values of >100 ng/mL at baseline and PS ≥2 were more likely to have worse PFS with Ra‐223. Thus, it appears to be clear that earlier use of Ra‐223, when the patient has a lesser disease burden than indicated by these poor baseline factors, should be considered. These findings are supported by the international early access program (iEAP) findings that mCRPC patients with no symptoms had better OS than those with symptoms. 20
Another concern is the occasional loss of the opportunity to receive subsequent life‐prolonging therapies even if the patient is treated with a full schedule of Ra‐223. We must avoid a treatment period of Ra‐223 that sacrifices the remaining time of patients due to the uncontrolled progression of non‐bone metastasis. In the present study, 41 of the patients (32%) had no choice but best supportive care following Ra‐223 treatment in spite of rates of PSA responses similar to those in other reports. 6 , 21 Of those, 21 had two or more choices for life‐prolonging therapies before the initiation of Ra‐223 treatment. This indicates that we could not have identified bone‐predominant mCRPC in some patients treated with Ra‐223. A recent new imaging study showed the utility of gallium Ga 68‐labeled positron emission tomography tracer targeting of prostate‐specific membrane antigen (68Ga‐PSMA‐11 PET) for planning Ra‐223 treatment. 22 In the study, patients selected by PSMA PET and bone scintigraphy were more likely to have a PSA decline (44%) with Ra‐223 treatment. Therefore, in addition to the completion of the Ra‐223 course, which patients will benefit most from Ra‐223 should be determined for bone‐predominant mCRPC to best address the following treatment with maintenance for control of bone metastasis.
Assessment discriminating progression of bone and/or non‐bone metastasis is needed to clarify bone‐predominant mCRPC. We focused on dynamic changes of bone metastasis having differential biological progression. The risk assessment using dynamic changes of bone metastasis by type was highlighted by the PSADT just before Ra‐223 treatment, which was one of the significant factors associated with PFS, because PSADT just before Ra‐223 well reflected the biological dynamics in the progression of bone and/or non‐bone metastasis. This risk assessment consisting of the dynamic status of bone metastasis by the type and PSADT just before Ra‐223 treatment was significantly associated with the rate of the PSA response and the completion rate of Ra‐223 treatment, which was supported by nbPEs, OS, and the proportion of best supportive care in the first subsequent therapy. Actually, patients with a favorable risk were more likely to have a slow elevation of the PSA level even if the value was increased during Ra‐223 treatment, whereas those with the poor risk were less likely to have one. The decline in the ALP level was similar to those in other reports and was steadily observed regardless of the risk group. 6 , 21 It is likely that Ra‐223 exerted a biological effect on bone metastasis even in those with the poor risk, but its duration was limited based on the results of bPEs in the present study. This risk assessment clearly appeared to predict bone‐predominant mCRPC in that there were significant differences in PFS and nbPEs among the risk groups.
The limitations of our study should be acknowledged. Foremost are the retrospective design of our study, the short follow‐up period, and the small number of patients. Dynamic radiographical changes of bone metastases were evaluated between the diagnosis of prostate cancer, not CRPC, and just before treatment with Ra‐223 because of the various treatment lines of Ra‐223. These might have substantially affected the results. Moreover, the efficacy of Ra‐223 alone was insufficient for patients with the intermediate risk. For such patients, it remains challenging to determine whether combination therapy can be effective and safe. In the ERA 223 trial, a double‐blind, randomized, placebo‐controlled, phase 3 study, concurrent treatment with Ra‐223, and abiraterone acetate plus prednisone or prednisolone did not improve symptomatic skeletal event‐free survival or OS in chemotherapy‐naive mCRPC patients with bone metastasis. 23 In that study, there was a higher incidence of bone fracture in the Ra‐223 and abiraterone arm. The issue of concurrent treatment with Ra‐223 and abiraterone acetate plus prednisone or prednisolone inducing extremely poor bone health has been discussed in terms of several findings such as obvious depletion of testosterone and its metabolite, estrogen, and inhibition of osteoblast activity in the healthy bone microenvironment. 24 Concurrent use of a bone‐mediating agent such as zoledronate or denosumab strongly appeared to be important in Ra‐223 treatment. Another phase 3 trial (PEACE III, NCT02194842) with Ra‐223 and a placebo, each in combination with enzalutamide, is ongoing. Further study is needed to clarify the benefit of Ra‐223 in combination with other agents involved in the setting of each treatment line for CRPC.
5. CONCLUSIONS
We have shown that the type of dynamic changes of bone metastasis is useful to determine the indication for Ra‐223 treatment in patients with mCRPC. The risk assessment for PFS was highlighted when the type of dynamic changes of bone metastasis was combined with PSADT just before the use of Ra‐223. Patients with the favorable risk had a high proportion of completion of Ra‐223 treatment and a decline or a slow elevation in the PSA level during the treatment with Ra‐223. They had better nbPEs and OS. Our findings suggest that this risk assessment can be used to maximize the clinical benefits of Ra‐223 treatment for bone‐predominant metastasis, which may make possible ideal sequencing of other life‐prolonging therapies following the use of Ra‐223.
CONFLICT OF INTEREST
No potential conflict of interest was reported by the authors.
AUTHOR CONTRIBUTIONS
Kohei Hashimoto: Conceptualization; data curation, investigation, methodology, project administration, formal analysis, and writing. Yasuhide Miyoshi: Investigation, data curation, and project administration. Tetsuya Shindo: Investigation, data curation, and project administration. Masakazu Hori: Project administration. Yasumasa Tsuboi: Data acquisition. Ko Kobayashi: Investigation and data curation. Fumimasa Fukuta: Investigation and data curation. Toshiaki Tanaka: Investigation and data curation. Shintaro Miyamoto: Data acquisition. Takeshi Maehana: Data acquisition. Manabu Okada: Data acquisition. Naotaka Nishiyama: Data acquisition. Masahiro Yanase: Investigation. Ryuichi Kato: Investigation. Hiroshi Hotta: Investigation. Yasuharu Kunishima: Investigation. Atsushi Takahashi: Investigation and project administration. Shiro Hinotsu: Formal analysis and project administration. Koh‐ichi Sakata: Project administration. Hiroshi Kitamura: Investigation, data curation, and project administration. Hiroji Uemura: Investigation and project administration. Naoya Masumori: Conceptualization, methodology, writing, and supervision.
Supporting information
Fig S1
Fig S2
Table S1
ACKNOWLEDGMENTS
This project was made possible with help of the data collected in the Sapporo Medical University Urologic Oncology Consortium (SUOC), and we would like to thank the members of the SUOC: Naoki Itoh, Masanori Matsukawa, Keisuke Taguchi, Hitoshi Tachiki, Toshihiro Maeda, Akio Takayanagi, Koji Ichihara, Shuichi Kato, Shunsuke Sato, Masahiro Matsuki, Hidetoshi Tabata, Kosuke Shibamori, Seisuke Nogfuji, and Kimihito Tachikawa.
Hashimoto K, Miyoshi Y, Shindo T, et al. Dynamic changes of bone metastasis predict bone‐predominant status to benefit from radium‐223 dichloride for patients with castration‐resistant prostate cancer. Cancer Med. 2020;9:8579–8588. 10.1002/cam4.3459
Funding information
No specific funding was disclosed.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author, NM, upon reasonable request.
REFERENCES
- 1. Bubendorf L, Schöpfer A, Wagner U, et al. Metastatic patterns of prostate cancer: an autopsy study of 1,589 patients. Hum Pathol. 2000;31:578‐583. [DOI] [PubMed] [Google Scholar]
- 2. Sathiakumar N, Delzell E, Morrisey MA, et al. Mortality following bone metastasis and skeletal‐related events among men with prostate cancer: a population‐based analysis of US Medicare beneficiaries, 1999–2006. Prostate Cancer Prostatic Dis. 2011;14:177‐183. [DOI] [PubMed] [Google Scholar]
- 3. Saad F, Gleason DM, Murray R, et al. A randomized, placebo‐controlled trial of zoledronic acid in patients with hormone‐refractory metastatic prostate carcinoma. J Natl Cancer Inst. 2002;94:1458‐1468. [DOI] [PubMed] [Google Scholar]
- 4. Smith MR, Egerdie B, Toriz NH, et al. Denosumab in men receiving androgen‐deprivation therapy for prostate cancer. N Engl J Med. 2009;361:745‐755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bruland ØS, Nilsson S, Fisher DR, et al. High‐linear energy transfer irradiation targeted to skeletal metastases by the alpha‐emitter 223Ra: adjuvant or alternative to conventional modalities? Clin Cancer Res. 2006;12:6250s‐6257s. [DOI] [PubMed] [Google Scholar]
- 6. Parker C, Nilsson S, Heinrich D, et al. Alpha emitter radium‐223 and survival in metastatic prostate cancer. N Engl J Med. 2013;369:213‐223. [DOI] [PubMed] [Google Scholar]
- 7. Sartor O, Coleman R, Nilsson S, et al. Effect of radium‐223 dichloride on symptomatic skeletal events in patients with castration‐resistant prostate cancer and bone metastases: results from a phase 3, double‐blind, randomised trial. Lancet Oncol. 2014;15:738‐746. [DOI] [PubMed] [Google Scholar]
- 8. Uemura H, Uemura H, Matsubara N, et al. Safety and efficacy of radium‐223 dichloride in Japanese patients with castration‐resistant prostate cancer and bone metastases. Int J Clin Oncol. 2017;22:954‐963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Uemura H, Uemura H, Nagamori S, et al. Three‐year follow‐up of a phase II study of radium‐223 dichloride in Japanese patients with symptomatic castration‐resistant prostate cancer and bone metastases. Int J Clin Oncol. 2019;24:557‐566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Uemura K, Miyoshi Y, Kawahara T, et al. Prognostic value of a computer‐aided diagnosis system involving bone scans among men treated with docetaxel for metastatic castration‐resistant prostate cancer. BMC Cancer. 2016;16:109 10.1186/s12885-016-2160-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Umeda T, Koizumi M, Fukai S, et al. Evaluation of bone metastatic burden by bone SPECT/CT in metastatic prostate cancer patients: defining threshold value for total bone uptake and assessment in radium‐223 treated patients. Ann Nucl Med. 2018;32(2):105‐113. 10.1007/s12149-017-1224-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Scher HI, Morris MJ, Stadler WM, et al. Trial design and objectives for castration‐resistant prostate cancer: updated recommendations from the prostate cancer clinical trials working group 3. J Clin Oncol. 2016;34:1402‐1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hoskin P, Sartor O, O'Sullivan JM, et al. Efficacy and safety of radium‐223 dichloride in patients with castration‐resistant prostate cancer and symptomatic bone metastases, with or without previous docetaxel use: a prespecified subgroup analysis from the randomised, double‐blind, phase 3 ALSYMPCA trial. Lancet Oncol. 2014;15:1397‐1406. [DOI] [PubMed] [Google Scholar]
- 14. Dizdarevic S, Petersen PM, Essler M, et al. Interim analysis of the REASSURE (Radium‐223 alpha Emitter Agent in non‐intervention Safety Study in mCRPC popUlation for long‐teRm Evaluation) study: patient characteristics and safety according to prior use of chemotherapy in routine clinical practice. Eur J Nucl Med Mol Imaging. 2019;46:1102‐1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Alva A, Nordquist L, Daignault S, et al. Clinical correlates of benefit from radium‐223 therapy in metastatic castration resistant prostate cancer. Prostate. 2017;77:479‐488. [DOI] [PubMed] [Google Scholar]
- 16. Wong WW, Anderson EM, Mohammadi H, et al. Factors associated with survival following radium‐223 treatment for metastatic castration‐resistant prostate cancer. Clin Genitourin Cancer. 2017;15:e969‐e975. [DOI] [PubMed] [Google Scholar]
- 17. McKay RR, Jacobus S, Fiorillo M, et al. Radium‐223 use in clinical practice and variables associated with completion of therapy. Clin Genitourin Cancer. 2017;15:e289‐e298. [DOI] [PubMed] [Google Scholar]
- 18. Parikh S, Murray L, Kenning L, et al. Real‐world outcomes and factors predicting survival and completion of radium 223 in metastatic castrate‐resistant prostate cancer. Clin Oncol (R Coll Radiol). 2018;30:548‐555. [DOI] [PubMed] [Google Scholar]
- 19. Saad F, Gillessen S, Heinrich D, et al. Disease Characteristics and completion of treatment in patients with metastatic castration‐resistant prostate cancer treated with radium‐223 in an International early access program. Clin Genitourin Cancer. 2019; 17(5):348‐355.e5. [DOI] [PubMed] [Google Scholar]
- 20. Heidenreich A, Gillessen S, Heinrich D, et al. Radium‐223 in asymptomatic patients with castration‐resistant prostate cancer and bone metastases treated in an international early access program. BMC Cancer. 2019;19:12 10.1186/s12885-018-5203-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Matsubara N, Nagamori S, Wakumoto Y, et al. Phase II study of radium‐223 dichloride in Japanese patients with symptomatic castration‐resistant prostate cancer. Int J Clin Oncol. 2018;23(1):173‐180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ahmadzadehfar H, Azgomi K, Hauser S, et al. 68Ga‐PSMA‐11 PET as a gatekeeper for the treatment of metastatic prostate cancer with 223Ra: proof of concept. J Nucl Med. 2017;58:438‐444. [DOI] [PubMed] [Google Scholar]
- 23. Smith M, Parker C, Saad F, et al. Addition of radium‐223 to abiraterone acetate and prednisone or prednisolone in patients with castration‐resistant prostate cancer and bone metastases (ERA 223): a randomised, double‐blind, placebo‐controlled, phase 3 trial. Lancet Oncol. 2019;20:408‐419. [DOI] [PubMed] [Google Scholar]
- 24. Mizokami A, Kimura GO, Fujii Y, et al. Considering bone health in the treatment of prostate cancer bone metastasis based on the results of the ERA‐223 trial. Int J Clin Oncol. 2019;24:1629‐1631. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1
Fig S2
Table S1
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, NM, upon reasonable request.


