Abstract
We performed a systematic review of the current literature addressing the safety and efficacy of photobiomodulation therapy (PBMT) in cancer patients. In this systematic review, the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) guidelines were used. In vitro, in vivo, and clinical studies, which investigated the effect of PBMT on cell proliferation/differentiation, tumor growth, recurrence rate, and/or overall survival were included. The Medline/PubMed, EMBASE, and Scopus databases were searched through April 2020. A total of 67 studies met the inclusion criteria with 43 in vitro, 15 in vivo, and 9 clinical studies identified. In vitro studies investigating the effect of PBMT on a diverse range of cancer cell lines demonstrated conflicting results. This could be due to the differences in used parameters and the frequency of PBM applications. In vivo studies and clinical trials with a follow‐up period demonstrated that PBMT is safe with regards to tumor growth and patient advantage in the prevention and treatment of specific cancer therapy‐related complications. Current human studies, supported by most animal studies, show safety with PBMT using currently recommended clinical parameters, including in Head & Neck cancer (HNC) in the area of PBMT exposure. A significant and growing literature indicates that PBMT is safe and effective, and may even offer a benefit in patient overall survival. Nevertheless, continuing research is indicated to improve understanding and provide further elucidation of remaining questions regarding PBM use in oncology.
Keywords: cancer, photobiomodulation, safety, supportive care, systematic review
We performed a systematic review of the current literature addressing the safety and efficacy of photobiomodulation therapy (PBMT) in cancer patients. A significant and growing literature indicates that PBMT is safe and effective, and may even offer a benefit in patient overall survival. Nevertheless, continuing research is indicated to improve understanding and provide further elucidation of remaining questions regarding PBM use in oncology.
1. INTRODUCTION
In 1967, Dr. Endre Mester was the first scientist to discover that a low power laser had a stimulating effect on hair regrowth in mice. 1 Since then, low‐level laser (LLL) has been applied for a variety of conditions and to boost physiological function in both humans and animals. In the past decade or so, the term photobiomodulation (PBM) replaced the former low‐level laser (LLL), and PBM was introduced as MESH word in PUBMED in 2015. Subsequently, the North American Association for Light Therapy (NAALT) 2 and the World Association for Laser Therapy (WALT) defined photobiomodulation therapy (PBMT) as a form of light therapy that utilizes non‐ionizing forms of light sources, including laser diodes (LD), light‐emitting diodes (LEDs), and broadband light, in the visible and infrared spectrum. PBM provokes a nonthermal process whereby endogenous chromophores elicit photophysical and photochemical events at diverse biological levels. This process results in positive therapeutic outcomes including the stimulation of tissue regeneration and wound healing, the reduction of inflammation and pain, and immunomodulation. 3
Since 1967, the number of clinical applications of light therapy has increased steadily in multiple medical fields, and in recent years PBM has been widely used for supportive care of cancer patients. 4 , 5 The best‐studied cancer therapy‐related complication, for which PBM is recommended, is oral mucositis (OM). The Mucositis Study Group of the Multinational Association of Supportive Care in Cancer/International Society for Oral Oncology (MASCC/ISOO) recommends the use of PBMT in the prevention of OM in patients with head and neck cancer undergoing chemoradiotherapy (CRT) and in stem cell transplant patients treated with high‐dose cytoreductive medications. 6 PBM also has beneficial effects in the management of soft tissue necrosis in patients with head and neck cancer (HNC), and therapy‐induced bone necrosis. 7 , 8 , 9 Also the potential application of PBM for management of xerostomia, dysgeusia, radiodermatitis, post‐RT fibrosis, chronic oral graft‐versus‐host disease (GVHD), and breast cancer‐related lymphedema has been reported. 10 , 11 , 12 , 13 , 14 , 15 , 16 , 17
The basic principle of supportive care in cancer is to provide effective management of complications of cancer treatment without compromising or inducing negative effects on oncology outcomes; also bearing in mind unwanted and dire consequences such as tumor persistence, new secondary tumors, or recurrence of the primary disease. Various in vitro studies have suggested that PBM may induce accelerated growth in some malignant cell lines and/or development of malignancy in dysplastic cells. Due to its increasing utilization in oncology care, and the better understanding of the biologic mechanisms and clinical outcomes, it is important to document the safety of PBM use in oncology settings. The aim of the present systematic review is to evaluate the available literature describing the safety and intervention outcomes in cancer patients receiving PBMT.
2. MATERIALS AND METHODS
2.1. Protocol
For this systematic review, the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) statement was used. 18
2.2. Eligibility criteria
We considered for inclusion all in vivo and human clinical trials articles dealing with treatment and/or prevention of cancer therapy‐related complications, as well as in vitro studies on the safety of PBMT on cell lines. Case reports, cohort studies, case‐control studies, systematic and literature reviews, letters to the editor, theses, studies published in a language other than English, monographs, commentaries, conference abstracts, and unpublished data were excluded.
2.2.1. Interventions
Studies that investigated the safety of PBM in an oncological setting, the potential tumor effects of PBM in cancer therapy, the mechanisms of PBM activity, molecular pathways, and differential effects upon tumor were reviewed. Robust safety data were desirable, considering the potential utility of PBM in supportive cancer care.
2.2.2. Outcome measures
Mitotic rate, Tumor growth, Overall survival, Recurrence rate.
2.3. Information sources
We queried three databases (Medline/PubMed, EMBASE, and Scopus). To detect other potentially eligible reports that could meet the inclusion criteria, the reference list from all selected studies were checked by the reviewers. In addition, the included studies were screened to identify key authors. This allowed us for extra database searches based on author name. The last search was performed in April 2020.
2.4. Search strategy
Electronic searches were conducted in Medline/PubMed, EMBASE and Scopus using the following keywords alone or together: ("Laser Therapy"[Mesh] OR "Low‐Level light Therapy"[Mesh] OR "Laser Phototherapy"[Mesh] OR "Photobiomodulation Therapy"[Mesh] OR "Low‐intensity laser therapy"[Mesh] OR "Low‐Level Laser Irradiation"[Mesh]) AND ("cancer cell"[Mesh] OR "oncology"[Mesh] OR "tumor"[Mesh] OR "carcinoma"[Mesh] OR "neoplasm"[Mesh] OR "cancer"[Mesh] OR "radiotherapy"[Mesh] OR "chemotherapy"[Mesh] OR "oral mucositis"[Mesh] OR "radiodermatitis"[Mesh] OR "lymphedema"[Mesh] OR "dysgeusia"[Mesh] OR "xerostomia"[Mesh]) OR "hyposalivation"[Mesh] OR "trismus"[Mesh] OR "peripheral neuropathy"[Mesh] OR "osteoradionecrosis"[Mesh] AND "Overall survival"[Mesh] OR "Disease‐free survival"[Mesh] OR "Progression‐free survival"[Mesh] OR "proliferation"[Mesh] OR "apoptosis"[Mesh] OR "cell differentiation"[Mesh] OR "cell biology"[Mesh] OR "cell survival"[Mesh] OR "radiation effects"[Mesh]).
2.5. Study selection
All papers were systematically ordered in a Microsoft Office Excel 2016 document (Microsoft Corporation). The titles were checked and the duplicates excluded. Afterward, titles and abstracts were read for inclusion in the systematic review. Studies were classified into different categories: in vitro studies, in vivo studies, clinical studies, duplicates, no follow‐up/safety information, and language other than English. Two independent reviewers (RJB, JR) reviewed the studies assessed for eligibility in full‐text version. The studies lacking relevant methodological information were excluded.
2.6. Data extraction
Data from the included studies were extracted according to the following: (a) Author and publication year; (b) Study type (clinical trials and in vivo/in vitro);(c) PBM properties and treatment protocol; (d) type of animal models; (e) types of cells; (f) patient population; (g) duration of follow‐up; (h) outcome measures.
2.7. Data analysis
For this systematic review, a meta‐analysis was not feasible due to the great variation in PBM protocols used in the included studies. This systematic review presents a comprehensive qualitative synthesis of the results from the incorporated studies.
3. RESULTS
3.1. Study selection
The flow diagram (Figure 1) gives on overview of the selection process of the included studies. In total, 870 studies were collected via the searches on the databases and 5 additional studies via the manual search. After a first review process, 585 duplicate studies were removed. One hundred and nine studies were excluded after reviewing the abstracts, and 114 studies were excluded as they did not meet the inclusion criteria. In total, 67 studies were included in the present systematic review: 43 in vitro, 15 in vivo, and 9 human clinical studies were analyzed.
FIGURE 1.
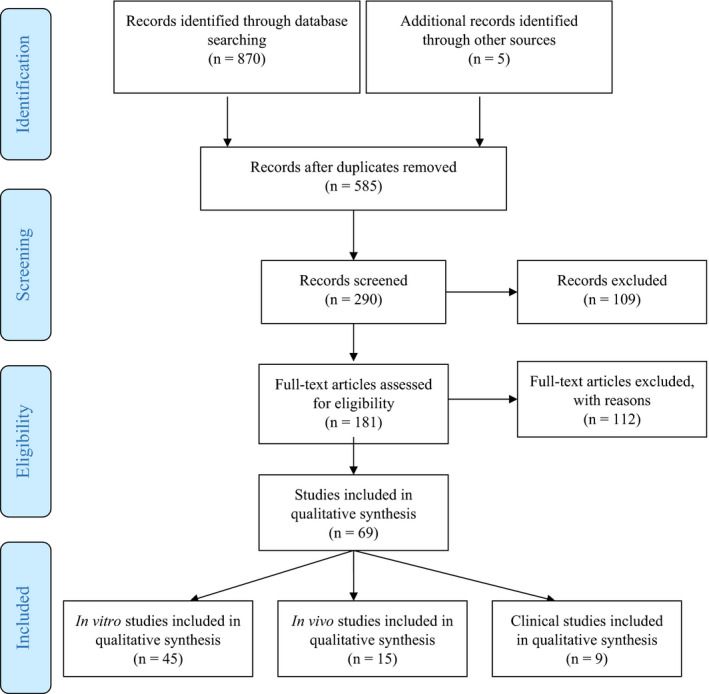
PRISMA flow diagram mapping out the number of records identified, included, and excluded
3.2. Study characteristics
The study characteristics of the in vitro, in vivo, and human clinical studies are presented in Tables 1, 2, and 3, respectively.
TABLE 1.
Study characteristics of the in vitro studies investigating the effect of PBMT on cancer cell lines
| Author (Ref.) | Year | Cell type | PBM device | Wavelength | Fluence | Exposition time (sec) | Application protocol | Cell viability /proliferation |
|---|---|---|---|---|---|---|---|---|
| Marchesini 61 | 1989 | Colon carcinoma (HT29), Breast carcinoma (MCF7), Malignant melanoma (M14 and JR1) | Argon LD | N.S | 4.2 and 150 kJ/m2 | N.S. | Single application | Increases tumor cell culture growth |
| Tsai 50 | 1991 | Glioma cell (C6) |
four different types: ‐ CO2 ‐ Argon ‐ HeNe ‐ GaAs |
‐ 488–512 nm ‐ 632.8 nm ‐ 904 nm |
‐ 0.4–22 J/cm2 ‐ 1.1–11 J/cm2 ‐ 2.7–326 mJ/cm2 ‐ 9–380 mJ/cm2 |
‐ 0.1 to 20 s ‐ 0.5 to 5 s ‐ 1 to 120 s ‐ 9 to 350 s |
Single application |
‐ He‐Ne laser induced a dose‐related biostimulatory effect ‐No dose related biostimulatory effect was noted after GaAS laser irradiation |
| Schaffer 49 | 1997 | Human squamous carcinoma cell lines of the gingival mucosa (ZMK) | LD | 805 nm | 2–20 J/cm2 | N.S. | Single application | ZMK cells showed a decreased of mitotic index at 4 and 20 J/cm2 |
| Sroka 51 | 1999 | Skeletal myotubes (C2), normal urothelial cells (HCV29), human squamous carcinoma cells of the gingival mucosa (ZMK1), urothelial carcinoma cells (J82), glioblastoma cells (U373MG), and breast adenocarcinoma cells (MCF7) |
‐Kr+‐laser ‐ Ar+‐laser ‐ Ar+‐pumped tunable dye ‐GaAlAs‐LD ‐Nd:YAG laser |
‐ 410 nm ‐ 630 nm ‐ 635 nm ‐ 640 nm ‐ 805 nm ‐ 1064 nm |
0–20 J/cm2 | N.S. | Single application |
Increased mitotic rate for J82, HCV29 with 410, 635 and 805 nm; C2 with 635 nm Max mitotic rate: J82, HCV29, C2 with 4 and 8 J/cm2 Min mitotic rate: J82, HCV29, C2 with 20 J/cm2 Min mitotic rate for MCF7, U373MG, and ZMK1 with increasing J/cm2; All cell lines with 20 J/cm2 |
| Coombe 59 | 2001 | Human osteosarcoma cell line, (SAOS−2) | GaAlAs LD | 830 nm | 1.7 to 25.1 J/cm2 | N.S. | A single or daily irradiation for a period of 1–10 days | Cellular proliferation or activation was significantly influenced by any of the PBM parameters applied |
| Pinheiro 48 | 2002 | H.Ep.2 cells (SCC type 2) | LD | 635‐ or 670‐nm | 0.04, 0.06, 0.08, 1.2, 2.4, and 4.8 J/cm2 | N.S. | 7 consecutive days at the same daytime | PBM (670 nm) at a dose between 0.04 and 4.8 J/cm2 significantly increased proliferation of H.Ep.2 cells |
| Kreisler 52 | 2003 | Epithelial tumor cells from laryngeal carcinoma | GaAlAs‐LD | 809 nm | 1.96, 3.92, and 7.84 J/cm2 | 75, 150, 300 s | Single application | The irradiated cells demonstrated a higher proliferation rate up to 3 days post‐PBM |
| Liu 61 | 2004 | Human hepatoma cell line (HepG2 and J−5 cells) | GaAlAs‐LD | 808 nm | 5.85 and 7.8 J/cm2 | 90, 120 s | Single application | PBM inhibited the proliferation of HepG2 and J−5 cells |
| Mognato 53 | 2004 | Human epithelial adenocarcinoma (HeLA) and lymphoblast cell line (TK6) | In‐Ga‐As LD | 808–905 nm | 1, 4, 15, 30, and 60 J/cm2 | N.S. | Single application | PBM did not affect HeLa cells at 808 nm but stimulated proliferation at 905 nm and combined wavelengths. TK6 cells were not affected. |
| Werneck 55 | 2005 | H.Ep.2 cells (human SCC larynx) | LD |
685 nm 830 nm |
4 J/cm2 | N.S. | Single application | PBM improved cellular proliferation in cells at 685 nm or 830 nm wavelengths. Top proliferation was detected at 12 h (685 nm) and at 6 h and 48 h (830 nm). |
| De Castro 54 | 2005 | Human oral carcinoma cells 101 | LD |
685 nm 830 nm |
4 J/cm2 | N.S. | One or two applications | PBM (830 nm) increased proliferation at 12 h. The increase was noticeable up to 48 h. No response in cells treated with PBM at 685 nm. |
| Liu 56 | 2006 | Human hepatoma cell line (HepG2 and J−5 cells) | GaAlAs‐LD | 808 nm | 0, 1.95, 3.9, 5.85, 7.8, 9.75, and 11.7 J/cm2 | 0, 30, 60, 90, 120, 150, and 180 s | Single application | PBM at 5.85 and 7.8 J/cm2 inhibited the survival of human HepG2 cells |
| Renno 57 | 2007 | Human osteosarcoma cell line (MG63) | LD |
830 nm 780 nm 670 nm |
0.5, 1, 5, 10 J/cm2 | N.S. | Single application | PBM at 670 nm increased osteosarcoma cell proliferation significantly (at 5 J/cm2). The same was true at 780 nm laser (at 1, 5, and 10 J/cm2), but not after 830 nm PBM |
| Powell 58 | 2010 | Human breast cancer cell line (MCF−7 – adenocarcinoma) and a human melanoma cell line (MDA‐MB−435S/M14) | GaAlAs‐LD |
780 nm 830 nm 904 nm |
0.5, 1, 2, 3, 4, 10, 12, and 15 J/cm2 |
1–3 applications with 24 h in between |
Minimal changes were detected in the growth rates of MDA‐MB−435S (melanoma) cells after a single PBM treatment, regardless of the PBM parameters applied. Increased proliferation of MCF−7 with 1, 2, 4, 10, and 12 J/cm2 at 780 nm and at 0.5, 1, 3, 4 and 15 at 904 nm |
|
| Huang 47 | 2011 | ASTC‐a−1 cells, HeLa cells, human hepatocellular liver carcinoma (HepG2) cells, and African green monkey SV−40‐transformed kidney fibroblast (COS−7) | HeNe LD | 632.8 nm | 20, 40, 80, 120, and 160 J/ cm2 |
1.66, 3.33, 6.66, 10, 13.33 min |
Single application |
PBM increased apoptosis via inactivation of the Akt/GSK3b signaling pathway through ROS production. |
| Al‐Watban 46 | 2012 |
Murine fibrosarcoma (RIF−1) Mouse mammary adenocarcinoma (EMT−6) |
HeNe LD | 632.8 nm | 60, 120, 180, 240, 300, 360, 420, 480, 540, and 600 mJ/cm2 | 16, 32, 48, 64, 80, 96, 112, 128, 144, and 160 s | Three consecutive days | A trend of stimulation, zero‐bioactivation, and inhibition in all cell lines. The ideal biostimulatory dose was 180 mJ/cm2 and bio‐inhibitory doses were from 420–600 mJ/cm2 increasing doses. |
| Schartinger 45 | 2012 | Human oral SCC cell line (SCC−25) | GaAlAs‐LD | 660 nm | N.S. | 15 min | Three consecutive days | PBM led to an increase in the percentage of S‐ phase cells and a decrease in the percentage of G1‐phase cells. PBM induced a pro‐apoptotic effect and no tumor promoting effect. |
| Magrini 44 | 2012 | Human malignant breast cells (MCF−7) | HeNe LD | 633 nm | 5, 28.8, and 1000 mJ/cm2 | 1–16.5 min | Single application | PBM influenced cell metabolism and viability, depending on the fluence, for at least 6.5 days. PBM at 5 mJ∕cm2, had a bio‐inhibitory effect, which led to a decrease in cell metabolism. At 28.8 mJ∕cm2, no proliferation was detected, but there was an increase of the cell metabolism. At 1 J∕cm2, PBM led to an increase of cell metabolism. |
| Murayama 43 | 2012 | Human A−172 glioblastoma cell line | LD | 808 nm | 8, 36, and 54 J/cm2 | 20, 40, and 60 min | Single application | Suppressed proliferation in a fluence‐dependent manner |
| Sperandio 42 | 2013 |
Human dysplastic oral keratinocytes (DOK cell line) Human oral squamous cell carcinoma cell lines (SCC9 and SCC25) |
LD |
660 nm 780 nm |
0, 2.05, 3.07, and 6.15 J/cm2 | N.S. | Single application | PBM changed growth of both cell lines by modulating the Akt/mTOR/CyclinD1 signaling pathway, both up regulating and down regulating depending on the used PBM parameters. |
| Basso 41 | 2014 | Osteosarcoma (Saos2) | InGaAsP LD | 780 nm | 0.5, 1.5, 3, 5, and 7 J/cm2 | 40, 120, 240, 400, and 560 s | Single application | PBM at 0.5 J/cm2 increased cell viability |
| Gomes Henriques 40 | 2014 | Human oral squamous cell carcinoma cell lines (SCC25) | InGaAsP LD | 660 nm | 0, 0.5, 1 J/cm2 | 16 and 33 s | Two applications, 48 hours in between | PBM significantly increased proliferation of SCC25 cells at 1.0 J/cm2. |
| Matsumoto 39 | 2014 | Human Colon cancer cell lines (HT29 and HCT116) | LED |
465 nm 525 nm 635 nm |
N.S. | 10 min | Every 24 h for 5 days | PBM at 465 nm reduced viability of HT29 and HCT116 cells. However, PBM did not change viability of HT29 cells at 525 nm or 635 nm. |
| Tsai 68 | 2015 | Human osteosarcoma cell line (MG−63) | LD | 810 nm | 1.5 J/cm2 | 80 s | Single application before PDT | PBM increases the effect NPe6‐mediated photodynamic therapy via increased ATP synthesis. |
| Obayashi 38 | 2015 | Pancreatic carcinoma cell line (KP4, PK−9, MIA‐PaCa2) | GaAlAs‐LD | 915 nm | N.S. | 3, 5, or 7 min | Single application | Upregulated apoptosis with increasing power and duration of irradiation |
| Cialdai 37 | 2015 | Human breast carcinoma cell lines (MCF−7 and MDA‐MB361) | LD |
808 nm 905 nm |
9 J/cm2 | 10 min | Three consecutive days with | PBMT did not significantly impact the behavior of human breast adenocarcinoma cells, including their clonogenic efficiency |
| Dastanpour 36 | 2015 | Acute myeloid leukemia (AML) cell line (KG−1a) | LD | 810 nm | 5, 10, and 20 J/cm2 | N.S | One to three applications with 48 h in between | PBM significantly increase cell proliferation after two PBM exposures at an energy density of 20 J/cm2. Other PBM parameters did not affect cell proliferation. |
| Crous & Abrahamse 70 | 2016 | Lung cancer stem cells (CSC) isolated from lung cancer cells (A549) | LD | 636 nm | 5, 10, and 20 J/cm2 |
8 min 54 s 17 min 48 s 35 min 36 s |
Single application | PBM increased the cell density due to stimulation of cell proliferation |
| Ramos Silva 34 | 2016 | Human breast cancer cell line (MDA‐MB−231 cells) | GaAlAs LD | 660 nm | 30, 90, 150 J/cm2 | 30, 90, 150 s | Single application | PBM did not influence cell viability. PBM enhanced cell populations in S and G2/M cell cycle phases. PBM led to a decrease in proliferation and increase in senescence. |
| Barasch 33 | 2016 | Normal human lymphoblasts (TK6) Human leukemia cells (HL60) | HeNe LD | 632.8 nm |
0.1, 1, 2, 4, 8,12 J/cm2 |
3, 29, 57, 114, 229, 343 s | Single application | Pre‐radiation exposure to PBM (4.0 J/cm2) followed by 1‐h incubation hindered growth regression in TK6 but not in HL60 cells. PBM made the HL60 cells more susceptible to the killing effects of RT in a dose‐dependent way. Furthermore, exposure of HL60 to PBM alone led to cell death in a dose‐dependent way. |
| Schalch 32 | 2016 | Human lingual squamous cell carcinoma (SCC9) | LD |
660 nm 780 nm |
2.71, 5.43, 8.14 J/cm2 | 12.7, 25.3, 38 s | Single application | PBM of SCC9 cells (4 J/cm2) decreased the pro‐osteoclastogenic potential. |
| Kara 31 | 2017 |
Saos−2 osteoblast‐like cells (ATCC85‐HTB) Human lung carcinoma cells (A549) |
Nd:YAG laser | 1064 nm | N.S. | 0.5 min | Single application | PBM increased cancer cell proliferation, depending on the applied PBM parameters. |
| Djavid 30 | 2017 | Human cervix adenocarcinoma cell line (HeLa) | LD | 685 nm | 0, 5, 10, 20 J/cm2 | N.S. | Single application | PBM at different energy densities (5–20 J/cm2) was not cytotoxic. However, HeLa cells pre‐exposed to 20 J/cm2 showed improved inhibition of colony formation following RT. Enhanced radiosensitivity was related to more DNA damage, and oxidative stress, and radiation‐induced apoptosis and autophagy, |
| Bamps 29 | 2018 | Head and neck cancer (HNSCC) cell lines (SCC154, SQD9, and SCC61) | AsGaAl LD | 830 nm | 1–2 J/cm2 | N.S. | Single application | PBM increased cell proliferation of HNSCC cell lines at 1 J/cm2, while no significant increase was seen after PBM at 2 J/cm2. |
| Schalch 28 | 2018 | Head and neck cancer (HNSCC) cell line (SCC9) | LD |
660 nm 780 nm |
1–6 J/cm2 | 8.4, 16.9, 12.7, 25.3, 38 s | Single application | PBM reduced mitochondrial activity in the SCC9 cells using 11 diverse PBM parameters. PBM at 780 nm (4 J/cm2) was the safest and led to a reduction in cell viability, the induction of apoptosis, and a reduction in the migration capacity of the cancer cells. |
| Diniz 27 | 2019 |
Oral keratinocytes (HaCat) Tongue squamous cell carcinoma cells (SCC25) Upper aerodigestive tract carcinoma cells (HN12) |
GaAlAs LD | 660 nm | 11.7 J/cm2 | 6 s | Single application | PBM led to an increase in sensitivity to cisplatin. PBM could potentiate the effects of cisplatin, leading to increased drug cytotoxicity and enhanced apoptosis. |
| Chen 26 | 2019 | Melanoma cells (B16F10 melanoma cells) | LED |
418 nm 457 nm 630 nm |
0.04,0.07,0.15, 0.22, 0.30, 0.37, 0.45, 0.56, 1.12 | 0, 450, 900, 1800 s | Single application | PBM at 418–457 nm inhibited the growth of the B16F10 melanoma cells and a high energy density had better results. |
| Takemoto 25 | 2019 | Human OSCC cell line (CAL27) | LED | 660 nm | 3, 6 J/cm2, 9, 12, 24, and 36 J/cm2 | N.S. | Three applications | PBM at high doses hindered the progression and number of OSCC colonies without affecting the surrounding stromal fibroblasts. |
| Levchenko 24 | 2019 | HeLa cells | LD | 808 nm | 0.3, 3, 10, and 30 J/cm2 | 6, 60, 200, and 600 s | Single application | PBM (0.3, 3, and 30 J/cm2) induced apoptosis along a gradual increase over time, in contrast to non‐irradiated cells and cells irradiated at 10 J/cm2 |
| Matsuo 23 | 2019 | Squamous cell carcinoma cell line (HSC−3) | LED | 630 nm | N.S. | N.S | Single application | PBM increased the migration ability of HSC−3 cells |
| Kianmehr 22 | 2019 |
HDF cell line Human melanoma cancer cell lines (A375 and SK‐MEL−37) |
LD | 660 nm | 3 J/cm2 | 90 s | Single application | PBM alone is not able to destroy human normal fibroblast and human melanoma cancer cells. PBM in combination with p‐Coumaric acid did not alter the cell viability in human fibroblasts but reduced the cell viability in melanoma cells probably via the apoptosis pathway. |
| Abuelmakarem 21 | 2019 | Colon cancer cell line (Caco−2 cell line) | LD | 660 nm | N.S. | 5 min | Single application | PBM decreased the cell viability. |
| Kiro 64 | 2019 | Isolated CSCs adenocarcinoma MCF7 | LD |
636 nm 825 nm 1060 nm |
5, 10, 20, 40 J/cm2 |
10 min 48 s 20 min 9 s 40 min 21 s 1 h 20 min 30 s |
Single application | PBM increased the cell proliferation and viability of BCCs and BCSCs after being exposed to 5–40 J/cm2 using wavelengths of 636, 825 and 1060 nm. PBM decreased cytotoxicity in both BCCs and BCSCs after treatment with low energy densities. |
| Khorsandi 20 | 2020 |
Breast cancer cell lines (MDA‐MB−231) Melanoma cancer cell line (A375) Human dermal fibroblast cell line (HDF) |
LD | 660 nm | 3 J/cm2 | 90 s | Single application | PBM alone cannot induce cell death in human normal and cancerous cells. PBM in combination with gallic acid (GA) treatment did not alter the cell viability in human normal cells but significantly reduced the survival of cancer cells more than GA alone. |
| Shakibaie 19 | 2020 | Breast cancer cell lines (MCF−7) | LED | 435 and 629 nm | 7.9 and 17.5 J/cm2 | N.S. | Single application | PBM (435 nm) decreased the proliferation and metabolic activity of MCF−7 cells. PBM (626 nm) increased the metabolic activity and proliferation of MCF−7 cells. |
Abbreviations: BCC, breast cancer cell; CSC, cancer stem cell; HNC, head and neck; LD, laser diode; LED, light emitting diode; PBMT, photobiomodulation therapy; ROS, reactive oxygen species; SCC, squamous cancer cell.
TABLE 2.
Study characteristics of the in vivo studies investigating the effect of PBMT on cancer animal models
| Author (Ref.) | Year | Animal type | Tumor type | PBM device | Wavelength | Fluence | Exposition time (sec) | Application protocol | Tumor growth rate/Tumorigenicity |
|---|---|---|---|---|---|---|---|---|---|
| Mikhailov 82 | 1993 | Rat |
Walker's carcinosarcoma Cancer of the mammary gland (RMK−1) |
LD | 890 nm | 0.46, 1.53 J/cm2 | 15 s | Five applications on consecutive days directly on the tumor |
PBM at 0.46 J/cm2 led to retardation in tumor growth and life span was prolonged versus control animals. PBM increased dystrophic and necrotic changes in the tumor. Tumor weight increased at 1.53 J/cm2. |
| Abe 81 | 1993 | Mouse | Glioma | GaAlAs LD | 830 nm | N.S. | 15 s | Two applications/day, one day post implantation and Two applications/day, 14 days post implantation directly on the skin over the tumor site or indirect on the abdominal skin |
PBM applied on the first day after glioma implantation, both in a direct and indirect manner, inhibited the tumor growth. At 14 days postimplantation indirect PBM enhanced tumor growth. |
| Ulrich 71 | 1996 | Rat | Rhabdomyosarcomas (R1H) | LD | 830 nm | 1 and 100 J/cm2 | N.S. | 15 fractions over 3 weeks |
Single doses PBM do not inhibit nor stimulate tumor growth. Fractionated PBM does not alter growth kinetics of the tumors. Increase in tumor necrosis after 15 fractions of 100 J/cm2 |
| Frigo 62 | 2009 | Mouse | Melanoma cell line (B16F10) | LD | 660 nm |
150 J/cm2 1050 J/cm2 |
60 s 420 s |
Once a day for three consecutive days | PBM at 150 J/cm2 was safe with only negligible effects on cell proliferation in vitro and no significant effect on tumor growth in vivo. PBM at a high irradiance (2.5 W/cm2) combined with high dose of 1050 J/cm2, could stimulate melanoma tumor growth. |
| Zhang 79 | 2009 | Mouse | Human cervical carcinoma cell line (HeLa) | LED | 650 nm | N.S | N.S. | Single application | PBM diminished the tumor growth of tumors on day 50 and weakened the elevation of vascular endothelial growth factor (VEGF). PBM could induce HeLa cell apoptosis and have antitumor properties. |
| Monteiro 76 | 2011 | Hamster | Squamous cell carcinoma (SCC) | LD | 660 nm | 56.4 J/cm2 | 133 s | Every other day for 4 weeks | PBM led to a significant progression of the severity of SCC |
| Myakishev‐Rempel 77 | 2012 | Mouse | UV‐induced skin cancer | GaAlAs LED | 760 nm | 2.5 J/cm2 | 312 s | Twice daily for 37 days | PBM did not have an effect on the growth of the UV‐induced skin cancer. |
| Monteiro 78 | 2013 | Hamster | Squamous cell carcinoma (SCC) | LD | 660 nm | 95 J/cm2 | 133 s | Every other day for 4 weeks | PBM did not influence tumor behavior, four weeks after tumor induction. |
| Wikramanayake 83 | 2013 | Rat | Chemotherapy induced alopecia | LD | 655 nm | N.S. | 60 s | Daily for 10 days | PBM did not affect the efficacy of chemotherapy |
| Ottaviani 63 | 2016 | Mouse | Melanoma cell line (B16F10) | InGaAlAsP LD |
660 nm 800 nm 970 nm |
3 or 6 J/cm2 | 30–60 s | Once a day for 4 consecutive days | PBM hindered tumor progression, provoked tumor vessel normalization and stimulated the immune system to produce type I interferons. |
| Rhee 75 | 2016 | Mouse | Human anaplastic thyroid cell line (FRO) | LD | 650 nm | 15, 30 J/cm2 | 150 and 300 s | Single application |
PBM decreased TGF‐β1 and increased p‐Akt/HIF−1α which resulted in proliferation and angiogenesis of anaplastic thyroid carcinoma (ATC) |
| Khori 74 | 2016 | Mouse | Mouse mammary carcinoma (4T1, ATCC CRL−2539) | LD | 405, 532, and 632 nm | N.S | N.S | 10 treatments three times a week with a weekend break | PBM (405–532 nm) significantly reduced the tumor size. |
| Petrellis 73 | 2017 | Rat | Walker's carcinosarcoma | LD | 660 nm | 35.7, 107.14, 214.28 J/cm2 |
10 s 30 s 60 s |
Three times on alternate days |
PBM increased inflammatory markers IL−1β, COX−2, iNOS. PBM decreased inflammatory markers IL−6, IL−10, and TNF‐α. PBM at 1 J−35,7 J/cm2 produced cytotoxic effects by ROS generation. |
| Frigo 62 | 2018 | Mouse | Melanoma cell line (B16F10) | InGaAlP LD | 660 nm | 150, 450, 1050 J/cm2 |
60 s 180 s 420 s |
Each 24 h for three consecutive days | High PBM doses (≥ 9 J) showed a dose‐dependent tumor growth, different collagen fibers characteristics, and eventually blood vessel growth. A PBM dose of 3 J did not affect the melanoma cell activity. |
| Barasch 72 | 2019 | Mouse | Human squamous cell carcinoma of the oral tongue (Cal−33) | LD |
660 nm 850 nm |
18.4 J/cm2 3.4 J/cm2 |
75 s |
(1) PBM at 660 nm, 18.4 J/cm2, and 5 RT ×4 Gy doses delivered daily; (2) PBM at 660 nm, 18.4 J/cm2, and 1 × 15 Gy RT; (3) PBM at 660 nm +850 nm, 45 mW/cm2, 3.4 J/cm2, and 1 × 15 Gy RT |
RT‐treated animals survived significantly longer and had significantly smaller tumor volume when matched with the control and PBM treatment groups. No significant differences were discovered between the RT alone and PBM +RT groups in any of the experiments. |
Abbreviations: LD, laser diode; LED, light emitting diode; PBMT, photobiomodulation therapy; ROS, reactive oxygen species; SCC, squamous cancer cell.
TABLE 3.
Study characteristics of the clinical trials investigating the safety of PBMT in patients with cancer
| Author (Ref.) | Year | Type of study | Patient type | PBM device | Wavelength | Fluence | Exposition time (sec) | Application protocol | Disease free survival/overall survival |
|---|---|---|---|---|---|---|---|---|---|
| Mikhailov 96 | 2000 | Prospective, interventional cohort | Breast cancer patients | N.S | N.S | N.S | N.S | Before surgery and in postoperative during 2 years. | PBM decreased postoperative complications by 15.3% and the duration of lymphedema. 86.9% of patients with 2nd stage breast cancer and 83.3% of the patients with 3rd stage breast cancer survived 10 years after PBM. 82.6% of patients with 2nd stage and 77.7% with 3rd stage breast cancer treated by PBM had no recurrences in 10 years period. |
| Santana‐Blank 95 | 2002 | Prospective, interventional cohort | Different cancer types (colon, breast, non‐Hodgkin lymphoma, lung, oral, brain, bone, gallbladder) | LD | 904 nm | 4.5 × 105 J/m2 | N.S. | Daily application up to 39 months | PBM dose‐limiting toxicity was not observed. PBM is safe for clinical use and may have potential effects on Karnofsky Performance Status, antitumor activity, and quality of life, in patients with advanced cancer. |
| Samoilova 94 | 2015 | Prospective, randomized controlled trial | Breast cancer patients | LD | 480–3400 nm | 24 J/cm2 | N.S. | Daily for 1 week on the sacral area | PBM may stimulate growth of human skin cells and concurrently downregulate the proliferation of tumor cells, including breast cancer cells via a systemic manner. |
| Antunes 92 | 2017 | Prospective, randomized, double‐blind, placebo controlled | Patients with squamous cell carcinoma of the head and neck | InGaAlP LD | 660 nm | 4 J/cm2 |
10 s/point Total 12 min |
Daily from Monday to Friday, every week, immediately before RT | PBM upregulated genes related to differentiation of human epidermal keratinocytes while PBM downregulated genes associated with cytotoxicity and immune response. |
| Antunes 93 | 2017 | Prospective, randomized, double‐blind, placebo controlled | Patients with squamous cell carcinoma of the head and neck | InGaAlP LD | 660 nm | 4 J/cm2 |
10 s/point Total 12 min |
Daily from Monday to Friday, every week, immediately before RT | Patients who underwent PBM had a statistically significant better complete response to treatment than patients in the placebo group (LG = 89.1%; PG = 67.4%; p = 0.013) after a median follow‐up of 41.3 months (range 0.7–101.9). Patients in the PBM group showed an increase in progression‐free survival in comparison with patients in the placebo group (61.7% vs. 40.4%; p = 0.030; HR: 1:93; CI 95%: 1.07–3.5) and had a tendency for better overall survival (57.4% vs. 40.4%; p = 0.90; HR: 1.64; CI 95%: 0.92–2.91). |
| Marin‐Condé 91 | 2018 | Prospective, randomized controlled trial | Patients diagnosed with oral or oropharyngeal SCC | LD | 940 nm | 83.3 J/cm2 | 360 s | N.S | There was no statistically significant difference between the PBM and control group with regard to the development of side effects |
| Brandão 90 | 2018 | Retrospective | Patients diagnosed with oral SCC | LD | 660 nm | 10 J/cm2 | 10 s/point 16 points in total | Daily applications for five consecutive days (Monday to Friday) throughout RT, immediately before each RT session. | The overall survival and disease‐free survival rates were 46.7 and 51.8%, respectively, after a mean follow‐up of 40.84 (± 11.71) months. 29.6% patients developed local‐regional recurrence, 6.57% patients developed distant relapse, and 12.5% patients developed new (second) primary tumors. Prophylactic PBM therapy did not alter treatment outcomes of the primary cancer, recurrence or new primary tumors, or survival. |
| Genot‐Klastersky 89 | 2019 | Retrospective | Patients diagnosed with head and neck SCC | LD | 630 nm | 2–3 J/cm2 | 33 s/point 6 min in total | Three times weekly up to 1 month | There was no significant difference in overall survival, time to local recurrence, and progression‐free survival, between the PBM and control patients. |
| Morais 88 | 2020 | Prospective, interventional cohort | Head and neck cancer patients | InGaAIP LD | 660 nm | 6.2 J/cm2 | 620 s/ session | Daily sessions (5 days per week) | The overall survival rate was 77% and disease‐free survival was 73.8%. PBM seemed to be safe in HNC patients. |
Abbreviations: LD, laser diode; LED, light emitting diode; PBMT, photobiomodulation therapy.
3.3. In vitro studies
A total of 43 in vitro studies examining the effect of PBMT on diverse cancer cells types with varying PBM parameters were included (Table 1). 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 , 55 , 56 , 57 , 58 , 59 , 60 , 61 , 62 , 63 , 64
The potential for PBM to negatively influence tumor growth and/or reaction to cytotoxic treatment has had a limited evaluation to date and is currently unresolved. Conflicting data refute or support the potential for PBM to impact tumor activity and responsiveness to treatment. As noted above, given the lack of uniformity, which characterizes tumor biology, it seems probable that different tumors vary in reaction to the range of biomodulatory activities associated with PBM exposure. PBM may affect various pathways linked to negative tumor behavior, including cell proliferation and anti‐apoptosis effects. Different malignant cell lines have been used in in vitro studies to investigate the effects of PBM on cell proliferation and differentiation. They showed conflicting data by exploiting a wide diversity of PBM parameters and tumor cell lines. 48 , 49 , 52 , 54 , 61 , 65
Concerning the effect of PBM on malignant transformation in non‐cancer cells, an in vitro study applied PBM (660 nm, 350 mW, 15 min) during three consecutive days to epithelial cells and/or fibroblasts and no change in cell behavior was shown. 45 Besides, in an in vitro study with normal breast epithelial cells, no malignant transformation was detected when different PBM doses and wavelengths were applied during numerous exposures. 58
When PBM parameters were used outside the range recommended in oncology (GaAIAs laser, 809 nm, 1.96–7.84 J/cm2), a clear proliferation was seen in laryngeal carcinoma cells. 52 Another study applying PBM at different wavelengths (685 and 830 nm) to Hep2 carcinoma cells, clearly showed an increase in proliferation. 55 The differential effect of PBM on normal and cancer cells was tested in a study with osteoblasts and osteosarcoma cells. The study demonstrated that only a laser diode (830 nm) at 10 J/cm2 was able to increase osteoblast proliferation. On the contrary, a laser diode (780 nm) decreased osteoblast proliferation at energy densities of 1, 5, and 10 J/cm2. PBM did not affect osteosarcoma cells by using an 830 nm laser, while a minor proliferative effect was detected at 670 nm. 57 In another study, human breast carcinoma and melanoma cell lines were used to investigate the effects of diverse doses of PBM at different wavelengths on cancer cells 58 : the proliferation of breast carcinoma cells increased at specific PBM doses, while numerous exposures had either no effect or reduced cell proliferation. An in vitro study on oral cancer cell lines with 1 J/cm2 PBM (660 nm) showed a nonsignificant increase in the invasive potential of these cell lines. 40 Another in vitro study in oral dysplastic and oral cancer cells suggested that PBM (660 nm or 780 nm, 40 mW, 2.05, 3.07, or 6.15 J/cm2) could modulate the Akt/mTOR/CyclinD1 signaling pathway linked to more aggressive cell behavior. 42 Another report of PBM exposure of three head and neck cancer (HNC) cell lines was reported to increase the proliferation of cells in each tumor line, but not in normal tissue control. 29
While the limits of basing broad‐reaching conclusions on in vitro assays have been noted, collectively the reports suggest it would be irresponsible to ignore the possibility that PBM could negatively impact tumor behavior. Therefore, understanding how PBM may modify tumor biology, both positively and negatively, is a research priority. 66
Direct investigation of the radio‐modulatory effects of PBM as it affects tumor response is limited, but as with other types of cytotoxic cancer therapy, PBM may affect tumor response to radiation by the dose, fractions, and timing of PBM or radiotherapy (RT). While the data are sparse and limited to in vitro studies, the evidence suggests that PBM may act as a radiosensitizer. 30 Another in vitro study with three HNC cell lines suggests that PBMT can enhance the sensitivity of cancer cells to chemotherapy (CT). 27 Conversely, PBM’s reported induction of cell survival suggests a potential pathway for tumor self‐preservation. 67 An in vitro study with oral squamous cell carcinoma (OSCC) demonstrated that PBM induced apoptosis in the absence of radiation. Moreover, PBM did not induce anti‐apoptotic effects that might stimulate tumor cell resistance to cancer therapy. 45 When PBM (810 nm, continuous wave, 20 mW/cm2, 1.5 J/cm2) was applied to human osteosarcoma cells before NPe6‐mediated photodynamic therapy, increased apoptosis was detected as a result of a higher uptake of the photosensitizer and an increased cellular ATP. 68 The potential enhancement of ionizing RT and CT in OSCC, was seen in PBM when applied shortly before RT and suggested that increased loco‐regional blood flow may have contributed to local tissue oxygenation, which may translate into enhanced tumor effect. 69
Several in vitro studies demonstrated that PBM could also inhibit the proliferation of malignant cells. A study using PBM (805 nm, 4 J/cm2 or 20 J/cm2) in gingival SCC demonstrated a decrease in mitotic rate. 49 In an in vitro study with osteosarcoma cells, PBM (830 nm) did not influence cell proliferation or protein expression. 59 A decreased proliferation of human hepatoma cells was detected after PBM (808 nm; 5.85 and 7.8 J/cm2). 60 A study with glioblastoma and astrocytoma cells showed a minor reduction in mitotic rate after PBM (805 nm and 5–20 J/cm2). 51 Comparably, glioblastoma cell proliferation was inhibited by PBM (808 nm, 5 J/cm2). 43 PBM at rather high cumulative doses resulted in growth inhibition of various malignant cell lines. 46 This suggested the hypothesis that PBM may have favorable effects in the treatment of lung cancer. 70 An in vitro study in B16F10 melanoma cells showed that high‐dose PBM (50 J/cm2) seemed safe, with only insignificant effects on proliferation. Furthermore, no noteworthy effect on tumor growth in a melanoma mouse model was shown. PBM at a high power density (2.5 W/cm2) with a very high dose of 1050 J/cm2 induced tumor growth in the melanoma mouse model. 62
The wide variety of PBM parameters utilized in these studies constitutes an obstacle to arriving at meaningful conclusions, especially when the parameters are outside the scope of the MASCC/ISOO guideline that recommended PBM therapy in cancer care. Additionally, even studies using similar parameters can have differing or contradictory results. To wit, some in vitro studies suggest that PBM may favor tumor progression of oral SCC cells by activation of Akt/mTOR pathway, 42 cellular proliferation, 29 , 40 and cellular migration, 28 while other studies report a reduction in tumor growth. 25 , 28 , 63 It is also important to comment that the results suggesting PBM tumor enhancement were not replicated in other studies. Generally, in vitro studies have limited applicability when compared with in vivo studies where various physiologically active cells and systems interact in the targeted tissue. There is a concomitant effect of light on endothelial, epithelial, mesenchymal, and immune cells, which must be studied together to identify real‐time effects.
3.4. In vivo studies
A total of 15 in vivo studies investigating the safety of PBMT in different animal cancer models were identified (Table 2). 62 , 83 In a study with a chemically induced OSCC hamster cheek pouch model, PBM (660 nm, 30 mW, 424 mW/cm2, 56.4 J/cm2, and 133 s, 4 J) led to tumor progression. 76 In contrast, a study with a mouse model of multiple UV‐induced skin tumors, full‐body PBM (670 nm, twice a day, 5 J/cm2 for 37 days) did not enhance tumor growth in comparison with sham‐treated animals. Moreover, the tumor area slightly decreased after PBM, possibly associated with PBM‐induced antitumor immune activity or a local photodynamic effect. 77 Similar results were seen in a rat study demonstrating that PBM was able to reduce and let even completely disappear small tumors. 82 This led to the hypothesis that the upregulation of ATP signaling by PBM stimulated differentiation of tumor cells and apoptosis, leading to an inhibition of tumor proliferation. 84 , 85 A normal cell produces ATP via the process of oxidative phosphorylation. This gives a yield of around 32–38 ATPs per glucose molecule. Cancer cells naturally change from “cellular respiration” to the very ineffective glycolysis for their ATP needs (i.e. Warburg effect). Cancer cells perform anaerobic glycolysis, which implies that they produce most of their energy from glycolysis. This produces only two ATPs per glucose molecule. 86 The potential of PBM to promote anti‐inflammatory and repair of normal tissue while not enhancing tumor cell proliferation may be related to this differential effect.
PBM was tested to stimulate hair regrowth in an animal model with leukemia, which developed chemotherapy‐induced alopecia (CIA). PBM did not alter the efficacy of CT, as 22% of the PBM‐treated rats and 20% of the control rats remained leukemia‐free. 83 An in vivo study showed that PBM reduced the tumor growth and invasiveness in xenograft OSCC and melanoma mouse models. The authors suggested that PBM may have impacted tumor proliferation by stimulating antitumor immunity and normalizing tumor vessels. 63 A recent study in an orthotopic animal model of head and neck squamous cell carcinoma (HNSCC) suggests that the use of PBMT does not safeguard tumor cells against the cytotoxic effect of RT. 72
The described results indicate that diverse malignant cells may react differently to specific PBM parameters and doses. A possible explanation for this lies in the dissimilarities in the cellular microenvironment between different tumor models, as PBM has a clear effect on the cellular signal transduction pathways. The microenvironment of cancer cells differs between in vitro studies. Moreover, it is not possible to compare the microenvironment of cell culture studies with that in animal models. To improve the understanding of the dissimilarities in tumor response to PBM and how pretreatment molecular and genomic characterization of tumors can be used to establish the most appropriate PBM parameters, more in vitro and in vivo studies are needed. 87
3.5. Clinical human studies
We identified nine clinical trials studying the safety of PBMT in patients with cancer, reporting disease‐free survival, overall survival, and recurrence rates (Table 3). 88 , 89 , 90 , 91 , 92 , 93 , 94 , 95 , 96 In a prospective, randomized‐controlled trial (RCT) with HNC patients (SCC of the nasopharynx, oropharynx, and hypopharynx) undergoing CRT, PBM was used to prevent OM. The average follow‐up time was 18 months (range 10–48 months). Results showed that PBM treated patients had improved loco‐regional disease control, and a better progression‐free and overall survival. 93 In a retrospective study with 152 advanced OSCC patients PBM (660 nm, 40 mW, 10 J/cm2) applied for the prevention of OM. In overall, PBM did not affect the treatment result of the primary tumor, relapse rate, development of new primary tumors, and overall survival. 90 Similarly, a retrospective study of 222 patients with HNC who were treated with RT with or without cisplatin‐based CT investigated the safety of PBM in the management of OM. PBM did not affect the time to local recurrences, the disease‐free survival, and the overall survival. 89
4. DISCUSSION
Evidence from the clinical follow‐up, and animal models and in vitro data indicate that the possible negative effect of PBM on tumor biology is not clinically relevant at the doses applied in the management of cancer therapy‐related complications. It is key to understand the effect of different PBM parameters (wavelength, fluence, energy, and time) and experimental models before applying and evaluating PBM correctly. As there is a clear contrast between in vivo and human studies versus in vitro cell culture studies, related to the tumor microenvironment. 66 , 97 , 98
In vitro studies strengthen the meaning of dosimetry and exact description and control of PBM parameters for clinical, oncologic use. The benefits of the use of PBM and levels of theoretical risk should be considered for the best patient care. In our opinion, current evidence supports the use of PBM in accepted indications. The Mucositis Study Group of the MASCC/ISOO has completed a systematic review and recommended the use of PBM for management of OM in stem cell transplant and HNC patients receiving stomatotoxic cancer therapies. 6 NICE guidelines in the UK recommend PBM also in those indications. 99
For more than 20 years, PBM has been used in the management of OM in HNC patient and no significant adverse effects have been documented. Clinical studies have assessed tumor outcomes in patients treated with PBM. 88 , 89 , 90 , 91 , 92 , 93 While these clinical trial outcomes support safety in the clinical application of PBM for oral/oropharyngeal complications of cancer therapy, continuing research is warranted due to the potential diverse biological impact of PBM, and due to tumor heterogeneity and evaluating tumor behavior and overall response to therapy. Indeed tumor heterogeneity and the tumor microenvironment may have been reflected in contradictions observed in some in vitro studies. For example, 35% expressing dysregulation in the PI3K pathway, a putative site of action of PBM in OSCC. 100 Animal and clinical trials allow assessment of the effect of PBM on the tumor and the tumor microenvironment (e.g. immune function, the surface microenvironment, and epithelial and connective tissue interactions). While additional clinical trials and follow‐up are desirable, the current evidence supports the safety of PBM in the established protocols through consensus guidelines in the management of cancer therapy‐related side effects.
4.1. Limitations
Due to heterogeneity of the used PBM parameters and outcome measures, a meta‐analysis of the reported data was not possible. Moreover, in this systematic review, in vitro, in vivo, and clinical trials were considered and therefore it was impractical to find a rigorous model to determine the risk of bias. This would be implemented in case the review only included clinical trials.
5. CONCLUSION
PBM (in the red or NIR spectrum by definition) appears safe and successful in the management of cancer therapy‐related side effects. Therefore, PBM should be considered as part of the standard of care for specific oncology purposes. 10 Clinicians should be able to prescribe PBM by guideline recommendations using appropriate approved parameters of PBM in a clinical setting. 101 The safety of PBM concerning the effect on the tumor response and most importantly the benefit of PBM to patients in the management of cancer treatment‐related toxicities have been shown in in vivo studies and clinical trials. Vigilance remains warranted for applications that have not been adequately documented or without guidelines due to a lack of evidence. Therefore, studies concerning the effect of PBM on malignant cell protection or enhancement of tumor growth are still needed.
The increasing number of published data in OM prevention in HNC and stem cell transplantation patients suggest that PBM does not influence tumor or treatment outcomes and overall survival. In recognition of the complexities, which govern tumor responsiveness, 102 it remains obligatory upon the clinician to notify patients of the potential benefits and risks related to PBM. Based on the demonstrated value of PBM in supportive cancer care, continuing research may also be directed to elucidate its effect on respondents and nonrespondents to PBM, like any other treatment or mitigation modality in modern precision oncology.
DISCLAIMER
This systematic review has been performed by an international multidisciplinary panel of clinicians and researchers, with expertise in the area of PBM. This article is informational in nature and should be used with the clear understanding that continued research and practice could result in new insights and recommendations. In no event shall the authors be liable for any decision made or action taken in reliance on the protocols included in this review paper.
CONFLICTS OF INTEREST
The authors declare that they have no conflict of interest.
AUTHOR CONTRIBUTION
RJB, JBE, RGN, AB, JRD, and JR conceived of the idea and the design of this systematic literature review. JR took the lead in conducting the literature search, analyzing articles, and in creating the tables and figures. RJB, JR, JBE, RGN, and JRD played a leading role in writing the manuscript. All authors discussed the results and contributed to the final manuscript. Each author has contributed in the same manner to this manuscript.
Bensadoun RJ, Epstein JB, Nair RG, et al. Safety and efficacy of photobiomodulation therapy in oncology: A systematic review. Cancer Med. 2020;9:8279–8300. 10.1002/cam4.3582
REFERENCES
- 1. Mester A, Mester A. The history of photobiomodulation: Endre Mester (1903–1984). Photomed Laser Surg. 2017;35(8):393–394. 10.1089/pho.2017.4332 [DOI] [PubMed] [Google Scholar]
- 2. WALT/NAALT (2014) Photobiomodulation: mainstream medicine and beyond. WALT Biennial Congress and NAALT Annual Conference, Arlington, VI. (September 2014). [Google Scholar]
- 3. Anders JJ, Lanzafame RJ, Arany PR. Low‐level light/laser therapy versus photobiomodulation therapy. Photomed Laser Surg. 2015;33(4):183–184. 10.1089/pho.2015.9848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hamblin M, Ferraresi C, Huang YY, de Freitas LF, Carroll J. Low‐level light therapy: Photobiomodulation, vol TT115. Tutorial Texts in Optical Engineering. Bellingham, WA: SPIE Press; 2018. [Google Scholar]
- 5. Zecha JAEM, Raber‐Durlacher JE, Nair RG, et al. Low‐level laser therapy/photobiomodulation in the management of side effects of chemoradiation therapy in head and neck cancer: part 1: mechanisms of action, dosimetric, and safety considerations. Support Care Cancer. 2016;24(6):2781–2792. 10.1007/s00520-016-3152-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zadik Y, Arany PR, Fregnani ER, et al. Systematic review of photobiomodulation for the management of oral mucositis in cancer patients and clinical practice guidelines. Supportive Care Cancer. 2019;27(10):3969–3983. 10.1007/s00520-019-04890-2 [DOI] [PubMed] [Google Scholar]
- 7. Scoletta M, Arduino PG, Reggio L, Dalmasso P, Mozzati M. Effect of low‐level laser irradiation on bisphosphonate‐induced osteonecrosis of the jaws: preliminary results of a prospective study. Photomed Laser Surg. 2010;28(2):179–184. 10.1089/pho.2009.2501 [DOI] [PubMed] [Google Scholar]
- 8. Epstein JB, Song PY, Ho AS, Larian B, Asher A, Bensadoun RJ. Photobiomodulation therapy: management of mucosal necrosis of the oropharynx in previously treated head and neck cancer patients. Supportive Care Cancer. 2017;25(4):1031–1034. 10.1007/s00520-016-3525-3 [DOI] [PubMed] [Google Scholar]
- 9. Epstein JB, Song P, Ho A, Larian B, Asher A, Bensadoun RJ. Management of radiation‐induced mucosal necrosis with photobiomodulation therapy. Supportive Care Cancer. 2018;26(8):2493 10.1007/s00520-018-4228-8 [DOI] [PubMed] [Google Scholar]
- 10. Zecha JA, Raber‐Durlacher JE, Nair RG, et al. Low‐level laser therapy/photobiomodulation in the management of side effects of chemoradiation therapy in head and neck cancer: part 2: proposed applications and treatment protocols. Support Care Cancer. 2016;24(6):2793–2805. 10.1007/s00520-016-3153-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Robijns J, Lodewijckx J, Mebis J. Photobiomodulation therapy for acute radiodermatitis. Curr Opin Oncol. 2019;31(4):291–298. 10.1097/CCO.0000000000000511 [DOI] [PubMed] [Google Scholar]
- 12. Epstein JB, Raber‐Durlacher JE, Lill M, et al. Photobiomodulation therapy in the management of chronic oral graft‐versus‐host disease. Supportive Care Cancer. 2017;25(2):357–364. 10.1007/s00520-016-3401-1 [DOI] [PubMed] [Google Scholar]
- 13. Epstein JB, Raber‐Durlacher JE, Huysmans MC, et al. Photobiomodulation therapy alleviates tissue fibroses associated with chronic graft‐versus‐host disease: two case reports and putative anti‐fibrotic roles of TGF‐beta. Photomed Laser Surg. 2018;36(2):92–99. 10.1089/pho.2017.4297 [DOI] [PubMed] [Google Scholar]
- 14. Heiskanen V, Zadik Y, Elad S. Photobiomodulation therapy for cancer treatment‐related salivary gland dysfunction: a systematic review. Photobiomodul Photomed Laser Surg. 2020;38(6):340–347. 10.1089/photob.2019.4767 [DOI] [PubMed] [Google Scholar]
- 15. El Mobadder M, Farhat F, El Mobadder W, Nammour S. Photobiomodulation therapy in the treatment of oral mucositis, dysphagia, oral dryness, taste alteration, and burning mouth sensation due to cancer therapy: a case series. Int J Environ Res Public Health. 2019;16(22):4505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chen HY, Tsai HH, Tam KW, Huang TW. Effects of photobiomodualtion therapy on breast cancer‐related lymphoedema: a systematic review and meta‐analysis of randomised controlled trials. Complement Ther Med. 2019;47:102200 10.1016/j.ctim.2019.102200 [DOI] [PubMed] [Google Scholar]
- 17. Bensadoun RJ. Photobiomodulation or low‐level laser therapy in the management of cancer therapy‐induced mucositis, dermatitis and lymphedema. Curr Opin Oncol. 2018;30(4):226–232. 10.1097/CCO.0000000000000452 [DOI] [PubMed] [Google Scholar]
- 18. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. PLoS Medicine. 2009;6(7):e1000097 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Shakibaie M, Vaezjalali M, Rafii‐Tabar H, Sasanpour P. Phototherapy alters the oncogenic metabolic activity of the breast cancer cells. Photodiagnosis Photodyn Ther. 2020;30:101695 10.1016/j.pdpdt.2020.101695 [DOI] [PubMed] [Google Scholar]
- 20. Khorsandi K, Kianmehr Z, Hosseinmardi Z, Hosseinzadeh R. Anti‐cancer effect of gallic acid in presence of low level laser irradiation: ROS production and induction of apoptosis and ferroptosis. Cancer Cell Int. 2020;20:18 10.1186/s12935-020-1100-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Abuelmakarem HS, Sliem MA, El‐Azab J, Farghaly MMA, Ahmed WA. Toward highly efficient cancer imaging and therapy using the environment‐friendly chitosan nanoparticles and NIR laser. Biosensors (Basel). 2019;9(1):28 10.3390/bios9010028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kianmehr Z, Khorsandi K, Mohammadi M, Hosseinzadeh R. Low‐level laser irradiation potentiates anticancer activity of p‐coumaric acid against human malignant melanoma cells. Melanoma Res. 2020;30(2):136–146. 10.1097/CMR.0000000000000603 [DOI] [PubMed] [Google Scholar]
- 23. Matsuo K, Suzuki H, Yatagai N, et al. Red LED light is influenced by IL‐6 to promote the migration ability of oral squamous cell carcinoma cell line. Kobe J Med Sci. 2019;64(6):E210–E216. [PMC free article] [PubMed] [Google Scholar]
- 24. Levchenko SM, Kuzmin AN, Pliss A, Ohulchanskyy TY, Prasad PN, Qu J. Cellular transformations in near‐infrared light‐induced apoptosis in cancer cells revealed by label‐free CARS imaging. J Biophotonics. 2019;12(12):e201900179 10.1002/jbio.201900179 [DOI] [PubMed] [Google Scholar]
- 25. Takemoto MM, Garcez AS, Sperandio M. High energy density LED‐based photobiomodulation inhibits squamous cell carcinoma progression in co‐cultures in vitro. J Photochem Photobiol B. 2019;199:111592 10.1016/j.jphotobiol.2019.111592 [DOI] [PubMed] [Google Scholar]
- 26. Chen Z, Li W, Hu X, Liu M. Irradiance plays a significant role in photobiomodulation of B16F10 melanoma cells by increasing reactive oxygen species and inhibiting mitochondrial function. Biomed Opt Express. 2020;11(1):27–39. 10.1364/BOE.11.000027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Diniz IMA, Souto GR, Freitas IDP, et al. Photobiomodulation enhances cisplatin cytotoxicity in a culture model with oral cell lineages. Photochem Photobiol. 2020;96(1):182–190. 10.1111/php.13152 [DOI] [PubMed] [Google Scholar]
- 28. Schalch TD, Fernandes MH, Destro Rodrigues MFS, et al. Photobiomodulation is associated with a decrease in cell viability and migration in oral squamous cell carcinoma. Lasers Med Sci. 2019;34(3):629–636. 10.1007/s10103-018-2640-4 [DOI] [PubMed] [Google Scholar]
- 29. Bamps M, Dok R, Nuyts S. Low‐level laser therapy stimulates proliferation in head and neck squamous cell carcinoma cells. Front Oncol. 2018;8:343 10.3389/fonc.2018.00343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Djavid GE, Bigdeli B, Goliaei B, Nikoofar A, Hamblin MR. Photobiomodulation leads to enhanced radiosensitivity through induction of apoptosis and autophagy in human cervical cancer cells. J Biophotonics. 2017;10(12):1732–1742. 10.1002/jbio.201700004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kara C, Selamet H, Gokmenoglu C, Kara N. Low level laser therapy induces increased viability and proliferation in isolated cancer cells. Cell Prolif. 2018;51(2):e12417 10.1111/cpr.12417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Dias Schalch T, Porta Santos Fernandes K, Costa‐Rodrigues J, et al. Photomodulation of the osteoclastogenic potential of oral squamous carcinoma cells. J Biophotonics. 2016;9(11–12):1136–1147. 10.1002/jbio.201500292 [DOI] [PubMed] [Google Scholar]
- 33. Barasch A, Raber‐Durlacher J, Epstein JB, Carroll J. Effects of pre‐radiation exposure to LLLT of normal and malignant cells. Supportive Care Cancer. 2016;24(6):2497–2501. 10.1007/s00520-015-3051-8 [DOI] [PubMed] [Google Scholar]
- 34. Ramos Silva C, Cabral FV, de Camargo CF, et al. Exploring the effects of low‐level laser therapy on fibroblasts and tumor cells following gamma radiation exposure. J Biophotonics. 2016;9(11–12):1157–1166. 10.1002/jbio.201600107 [DOI] [PubMed] [Google Scholar]
- 35. Crous A, Abrahamse H. Low‐intensity laser irradiation at 636 nm induces increased viability and proliferation in isolated lung cancer stem cells. Photomedicine Laser Surg. 2016;34(11):525–532. 10.1089/pho.2015.3979 [DOI] [PubMed] [Google Scholar]
- 36. Dastanpour S, Momen Beitollahi J, Saber K. The effect of low‐level laser therapy on human leukemic cells. J Lasers Med Sci. 2015;6(2):74–79. [PMC free article] [PubMed] [Google Scholar]
- 37. Cialdai F, Landini I, Capaccioli S, et al. In vitro study on the safety of near infrared laser therapy in its potential application as postmastectomy lymphedema treatment. J Photochem Photobiol B. 2015;151:285–296. 10.1016/j.jphotobiol.2015.08.003 [DOI] [PubMed] [Google Scholar]
- 38. Obayashi T, Funasaka K, Ohno E, et al. Treatment with near‐infrared radiation promotes apoptosis in pancreatic cancer cells. Oncol Lett. 2015;10(3):1836–1840. 10.3892/ol.2015.3399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Matsumoto N, Yoshikawa K, Shimada M, et al. Effect of light irradiation by light emitting diode on colon cancer cells. Anticancer Res. 2014;34(9):4709–4716. [PubMed] [Google Scholar]
- 40. Gomes Henriques AC, Ginani F, Oliveira RM, et al. Low‐level laser therapy promotes proliferation and invasion of oral squamous cell carcinoma cells. Lasers Med Sci. 2014;29(4):1385–1395. 10.1007/s10103-014-1535-2 [DOI] [PubMed] [Google Scholar]
- 41. Basso FG, Turrioni AP, Soares DG, Bagnato VS, Hebling J, de Souza Costa CA. Low‐level laser therapy for osteonecrotic lesions: effects on osteoblasts treated with zoledronic acid. Supportive Care Cancer. 2014;22(10):2741–2748. 10.1007/s00520-014-2267-3 [DOI] [PubMed] [Google Scholar]
- 42. Sperandio FF, Giudice FS, Correa L, Pinto DS Jr, Hamblin MR, de Sousa SC. Low‐level laser therapy can produce increased aggressiveness of dysplastic and oral cancer cell lines by modulation of Akt/mTOR signaling pathway. J Biophotonics. 2013;6(10):839–847. 10.1002/jbio.201300015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Murayama H, Sadakane K, Yamanoha B, Kogure S. Low‐power 808‐nm laser irradiation inhibits cell proliferation of a human‐derived glioblastoma cell line in vitro. Lasers Med Sci. 2012;27(1):87–93. 10.1007/s10103-011-0924-z [DOI] [PubMed] [Google Scholar]
- 44. Magrini TD, dos Santos NV, Milazzotto MP, Cerchiaro G, da Silva MH. Low‐level laser therapy on MCF‐7 cells: a micro‐Fourier transform infrared spectroscopy study. J Biomed Opt. 2012;17(10):101516 10.1117/1.JBO.17.10.101516 [DOI] [PubMed] [Google Scholar]
- 45. Schartinger VH, Galvan O, Riechelmann H, Dudas J. Differential responses of fibroblasts, non‐neoplastic epithelial cells, and oral carcinoma cells to low‐level laser therapy. Support Care Cancer. 2012;20(3):523–529. 10.1007/s00520-011-1113-0 [DOI] [PubMed] [Google Scholar]
- 46. Al‐Watban FA, Andres BL. Laser biomodulation of normal and neoplastic cells. Lasers Med Sci. 2012;27(5):1039–1043. 10.1007/s10103-011-1040-9 [DOI] [PubMed] [Google Scholar]
- 47. Huang L, Wu S, Xing D. High fluence low‐power laser irradiation induces apoptosis via inactivation of Akt/GSK3beta signaling pathway. J Cell Physiol. 2011;226(3):588–601. 10.1002/jcp.22367 [DOI] [PubMed] [Google Scholar]
- 48. Pinheiro ALB, Nascimento SCD, Vieira ALdB, Rolim AB, Silva PSD, Brugnera A Jr. Does LLLT stimulate laryngeal carcinoma cells? An in vitro study. Braz Dent J. 2002;13(2):109–112. 10.1590/s0103-64402002000200006 [DOI] [PubMed] [Google Scholar]
- 49. Schaffer M, Sroka R, Fuchs C, et al. Biomodulative effects induced by 805 nm laser light irradiation of normal and tumor cells. J Photochem Photobiol B. 1997;40(3):253–257. 10.1016/s1011-1344(97)00065-1 [DOI] [PubMed] [Google Scholar]
- 50. Tsai JC, Kao MC. The biological effects of low power laser irradiation on cultivated rat glial and glioma cells. J Clin Laser Med Surg. 1991;9(1):35–41. 10.1089/clm.1991.9.35 [DOI] [PubMed] [Google Scholar]
- 51. Sroka R, Schaffer M, Fuchs C, et al. Effects on the mitosis of normal and tumor cells induced by light treatment of different wavelengths. Lasers Surg Med. 1999;25(3):263–271. [DOI] [PubMed] [Google Scholar]
- 52. Kreisler M, Christoffers AB, Willershausen B, d'Hoedt B. Low‐level 809 nm GaAlAs laser irradiation increases the proliferation rate of human laryngeal carcinoma cells in vitro. Lasers Med Sci. 2003;18(2):100–103. 10.1007/s10103-003-0265-7 [DOI] [PubMed] [Google Scholar]
- 53. Mognato M, Squizzato F, Facchin F, Zaghetto L, Corti L. Cell growth modulation of human cells irradiated in vitro with low‐level laser therapy. Photomed Laser Surg. 2004;22(6):523–526. 10.1089/pho.2004.22.523 [DOI] [PubMed] [Google Scholar]
- 54. de Castro JL, Pinheiro AL, Werneck CE, Soares CP. The effect of laser therapy on the proliferation of oral KB carcinoma cells: an in vitro study. Photomed Laser Surg. 2005;23(6):586–589. 10.1089/pho.2005.23.586 [DOI] [PubMed] [Google Scholar]
- 55. Werneck CE, Pinheiro AL, Pacheco MT, Soares CP, de Castro JL. Laser light is capable of inducing proliferation of carcinoma cells in culture: a spectroscopic in vitro study. Photomed Laser Surg. 2005;23(3):300–303. 10.1089/pho.2005.23.300 [DOI] [PubMed] [Google Scholar]
- 56. Liu YH, Ho CC, Cheng CC, Hsu YH, Lai YS. Photoradiation could influence the cytoskeleton organization and inhibit the survival of human hepatoma cells in vitro. Lasers Med Sci. 2006;21(1):42–48. 10.1007/s10103-005-0369-3 [DOI] [PubMed] [Google Scholar]
- 57. Renno AC, McDonnell PA, Parizotto NA, Laakso EL. The effects of laser irradiation on osteoblast and osteosarcoma cell proliferation and differentiation in vitro. Photomed Laser Surg. 2007;25(4):275–280. 10.1089/pho.2007.2055 [DOI] [PubMed] [Google Scholar]
- 58. Powell K, Low P, McDonnell PA, Laakso EL, Ralph SJ. The effect of laser irradiation on proliferation of human breast carcinoma, melanoma, and immortalized mammary epithelial cells. Photomed Laser Surg. 2010;28(1):115–123. 10.1089/pho.2008.2445 [DOI] [PubMed] [Google Scholar]
- 59. Coombe AR, Ho CT, Darendeliler MA, et al. The effects of low level laser irradiation on osteoblastic cells. Clin Orthod Res. 2001;4(1):3–14. [DOI] [PubMed] [Google Scholar]
- 60. Liu YH, Cheng CC, Ho CC, et al. Effects of diode 808 nm GaAlAs low‐power laser irradiation on inhibition of the proliferation of human hepatoma cells in vitro and their possible mechanism. Res Commun Mol Pathol Pharmacol. 2004;115–116:185–201. [PubMed] [Google Scholar]
- 61. Marchesini R, Dasdia T, Melloni E, Rocca E. Effect of low‐energy laser irradiation on colony formation capability in different human tumor cells in vitro. Lasers Surg Med. 1989;9(1):59–62. 10.1002/lsm.1900090112 [DOI] [PubMed] [Google Scholar]
- 62. Frigo L, Luppi JS, Favero GM, et al. The effect of low‐level laser irradiation (In‐Ga‐Al‐AsP ‐ 660 nm) on melanoma in vitro and in vivo. BMC Cancer. 2009;9:404 10.1186/1471-2407-9-404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Ottaviani G, Martinelli V, Rupel K, et al. Laser therapy inhibits tumor growth in mice by promoting immune surveillance and vessel normalization. EBioMedicine. 2016;11:165–172. 10.1016/j.ebiom.2016.07.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Kiro NE, Hamblin MR, Abrahamse H. Photobiomodulation of breast and cervical cancer stem cells using low‐intensity laser irradiation. Tumour Biol. 2017;39(6):1010428317706913 10.1177/1010428317706913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Ocana‐Quero JM, Gomez‐Villamandos R, Moreno‐Millan M, Santisteban‐Valenzuela JM. Helium‐neon (he‐ne) laser irradiation increases the incidence of unreduced bovine oocytes during the first meiotic division in vitro. Lasers Med Sci. 1998;13(4):260–264. 10.1007/s101030050005 [DOI] [PubMed] [Google Scholar]
- 66. Silveira FM, Paglioni MP, Marques MM, et al. Examining tumor modulating effects of photobiomodulation therapy on head and neck squamous cell carcinomas. Photochem Photobiol Sci. 2019;18(7):1621–1637. [DOI] [PubMed] [Google Scholar]
- 67. Chu J, Wu S, Xing D. Survivin mediates self‐protection through ROS/cdc25c/CDK1 signaling pathway during tumor cell apoptosis induced by high fluence low‐power laser irradiation. Cancer Lett. 2010;297(2):207–219. 10.1016/j.canlet.2010.05.013 [DOI] [PubMed] [Google Scholar]
- 68. Tsai SR, Yin R, Huang YY, Sheu BC, Lee SC, Hamblin MR. Low‐level light therapy potentiates NPe6‐mediated photodynamic therapy in a human osteosarcoma cell line via increased ATP. Photodiagnosis Photodyn Ther. 2015;12(1):123–130. 10.1016/j.pdpdt.2014.10.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Schaffer M, Bonel H, Sroka R, et al. Effects of 780 nm diode laser irradiation on blood microcirculation: preliminary findings on time‐dependent T1‐weighted contrast‐enhanced magnetic resonance imaging (MRI). J Photochem Photobiol B. 2000;54(1):55–60. [DOI] [PubMed] [Google Scholar]
- 70. Crous AM, Abrahamse H. Lung cancer stem cells and low‐intensity laser irradiation: a potential future therapy? Stem Cell Res Ther. 2013;4(5):129 10.1186/scrt340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Ulrich M, Jahn R, Zywietz F, Schäfer H, Jungblut KH. (1996) Influence of laser light (830nm) on the growth kinetics of rat rhabdomyosarcomas In: Waidelich WSG, Waidelich R. (eds). Laser in der Medizin / Laser in Medicine. Berlin, Heidelberg: Springer. [Google Scholar]
- 72. Barasch A, Li H, Rajasekhar VK, et al. Photobiomodulation effects on head and neck squamous cell carcinoma (HNSCC) in an orthotopic animal model. Supportive Care Cancer. 2020;28(6):2721–2727. 10.1007/s00520-019-05060-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Petrellis MC, Frigo L, Marcos RL, et al. Laser photobiomodulation of pro‐inflammatory mediators on walker tumor 256 induced rats. J Photochem Photobiol B. 2017;177:69–75. 10.1016/j.jphotobiol.2017.09.011 [DOI] [PubMed] [Google Scholar]
- 74. Khori V, Alizadeh AM, Gheisary Z, et al. The effects of low‐level laser irradiation on breast tumor in mice and the expression of Let‐7a, miR‐155, miR‐21, miR125, and miR376b. Lasers Med Sci. 2016;31(9):1775–1782. 10.1007/s10103-016-2049-x [DOI] [PubMed] [Google Scholar]
- 75. Rhee YH, Moon JH, Choi SH, Ahn JC. Low‐level laser therapy promoted aggressive proliferation and angiogenesis through decreasing of transforming growth factor‐beta1 and increasing of Akt/hypoxia inducible factor‐1alpha in anaplastic thyroid cancer. Photomed Laser Surg. 2016;34(6):229–235. 10.1089/pho.2015.3968 [DOI] [PubMed] [Google Scholar]
- 76. de C Monteiro JS, Pinheiro AN, de Oliveira SC, et al. Influence of laser phototherapy (lambda660 nm) on the outcome of oral chemical carcinogenesis on the hamster cheek pouch model: histological study. Photomed Laser Surg. 2011;29(11):741–745. 10.1089/pho.2010.2896 [DOI] [PubMed] [Google Scholar]
- 77. Myakishev‐Rempel M, Stadler I, Brondon P, et al. A preliminary study of the safety of red light phototherapy of tissues harboring cancer. Photomed Laser Surg. 2012;30(9):551–558. 10.1089/pho.2011.3186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. de C Monteiro JS, de Oliveira SC, Reis Junior JA, et al. Effects of imiquimod and low‐intensity laser (lambda660 nm) in chemically induced oral carcinomas in hamster buccal pouch mucosa. Lasers Med Sci. 2013;28(3):1017–1024. 10.1007/s10103-012-1192-2 [DOI] [PubMed] [Google Scholar]
- 79. Zhang L, Xiong Z, Li Z, Yao B, Zhang D. Effects of red light emitting diode on apoptosis of HeLa cells and suppression of implanted HeLa cells growth in mice. J Radiat Res. 2009;50(2):109–117. 10.1269/jrr.08003 [DOI] [PubMed] [Google Scholar]
- 80. Frigo L, Cordeiro JM, Favero GM, et al. High doses of laser phototherapy can increase proliferation in melanoma stromal connective tissue. Lasers Med Sci. 2018;33(6):1215–1223. 10.1007/s10103-018-2461-5 [DOI] [PubMed] [Google Scholar]
- 81. Abe M, Fujisawa K, Suzuki H, Sugimoto T, Kanno T. Role of 830 nm low reactive level laser on the growth of an implanted glioma in mice. Keio J Med. 1993;42(4):177–179. 10.2302/kjm.42.177 [DOI] [PubMed] [Google Scholar]
- 82. Mikhailov VA, Skobelkin OK, Denisov IN, Frank GA, Voltchenko NN. Investigations on the influence of low level diode laser irradiation on the growth of experimental tumours. Laser Therapy. 1993;5(1):33–38. 10.5978/islsm.93-OR-03 [DOI] [Google Scholar]
- 83. Wikramanayake TC, Villasante AC, Mauro LM, et al. Low‐level laser treatment accelerated hair regrowth in a rat model of chemotherapy‐induced alopecia (CIA). Lasers Med Sci. 2013;28(3):701–706. 10.1007/s10103-012-1139-7 [DOI] [PubMed] [Google Scholar]
- 84. Shabbir M, Ryten M, Thompson C, Mikhailidis D, Burnstock G. Purinergic receptor‐mediated effects of ATP in high‐grade bladder cancer. BJU Int. 2008;101(1):106–112. 10.1111/j.1464-410X.2007.07286.x [DOI] [PubMed] [Google Scholar]
- 85. Karu T. Mitochondrial mechanisms of photobiomodulation in context of new data about multiple roles of ATP. Photomed Laser Surg. 2010;28(2):159–160. 10.1089/pho.2010.2789 [DOI] [PubMed] [Google Scholar]
- 86. Fadaka A, Ajiboye B, Ojo O, Adewale O, Olayide I, Emuowhochere R. Biology of glucose metabolization in cancer cells. J Oncological Sci. 2017;3(2):45–51. 10.1016/j.jons.2017.06.002 [DOI] [Google Scholar]
- 87. Laakso EL. Personalizing photobiomodulation therapy. Photomed Laser Surg. 2017;35(1):1–2. 10.1089/pho.2016.4218 [DOI] [PubMed] [Google Scholar]
- 88. Morais MO, Martins AFL, de Jesus APG, et al. A prospective study on oral adverse effects in head and neck cancer patients submitted to a preventive oral care protocol. Supportive Care Cancer. 2020;28(9):4263–4273. [DOI] [PubMed] [Google Scholar]
- 89. Genot‐Klastersky MT, Paesmans M, Ameye L, et al. Retrospective evaluation of the safety of low‐level laser therapy/photobiomodulation in patients with head/neck cancer. Supportive Care Cancer. 2020;28(7):3015–3022. 10.1007/s00520-019-05041-3 [DOI] [PubMed] [Google Scholar]
- 90. Brandão TB, Morais‐Faria K, Ribeiro ACP, et al. Locally advanced oral squamous cell carcinoma patients treated with photobiomodulation for prevention of oral mucositis: retrospective outcomes and safety analyses. Supportive Care Cancer. 2018;26(7):2417–2423. 10.1007/s00520-018-4046-z [DOI] [PubMed] [Google Scholar]
- 91. Marin‐Conde F, Castellanos‐Cosano L, Pachon‐Ibanez J, Serrera‐Figallo MA, Gutierrez‐Perez JL, Torres‐Lagares D. Photobiomodulation with low‐level laser therapy reduces oral mucositis caused by head and neck radio‐chemotherapy: prospective randomized controlled trial. Int J Oral Maxillofac Surg. 2019;48(7):917–923. 10.1016/j.ijom.2018.12.006 [DOI] [PubMed] [Google Scholar]
- 92. Antunes HS, Wajnberg G, Pinho MB, et al. cDNA microarray analysis of human keratinocytes cells of patients submitted to chemoradiotherapy and oral photobiomodulation therapy: pilot study. Lasers Med Sci. 2018;33(1):11–18. 10.1007/s10103-017-2313-8 [DOI] [PubMed] [Google Scholar]
- 93. Antunes HS, Herchenhorn D, Small IA, et al. Long‐term survival of a randomized phase III trial of head and neck cancer patients receiving concurrent chemoradiation therapy with or without low‐level laser therapy (LLLT) to prevent oral mucositis. Oral Oncol. 2017;71:11–15. 10.1016/j.oraloncology.2017.05.018 [DOI] [PubMed] [Google Scholar]
- 94. Samoilova KA, Zimin AA, Buinyakova AI, Makela AM, Zhevago NA. Regulatory systemic effect of postsurgical polychromatic light (480–3400 nm) irradiation of breast cancer patients on the proliferation of tumor and normal cells in vitro. Photomed Laser Surg. 2015;33(11):555–563. 10.1089/pho.2014.3878 [DOI] [PubMed] [Google Scholar]
- 95. Santana‐Blank LA, Rodriguez‐Santana E, Vargas F, et al. Phase I trial of an infrared pulsed laser device in patients with advanced neoplasias. Clin Cancer Res. 2002;8(10):3082–3091. [PubMed] [Google Scholar]
- 96. Mikhailov V, Denisov I, Frank G, Voltchenko N. Results of treatment of patients with second‐ to third‐stage breast cancer by combination of low‐level laser therapy (LLLT) and surgery: ten‐year experience, vol 4166. Laser Florence ‘99. SPIE; 2000.
- 97. da Silva JL, Silva‐de‐Oliveira AFS, Andraus RAC, Maia LP. Effects of low level laser therapy in cancer cells‐a systematic review of the literature. Lasers Med Sci. 2020;35(3):523–529. 10.1007/s10103-019-02824-2 [DOI] [PubMed] [Google Scholar]
- 98. de Pauli PM, Araujo ALD, Arboleda LPA, et al. Tumor safety and side effects of photobiomodulation therapy used for prevention and management of cancer treatment toxicities. A systematic review. Oral Oncol. 2019;93:21–28. 10.1016/j.oraloncology.2019.04.004 [DOI] [PubMed] [Google Scholar]
- 99. National Institute for Health and Care Excellence . Low‐level laser therapy for preventing or treating oral mucositis caused by radiotherapy or chemotherapy. Accessed on 05/06/2020, https://www.nice.org.uk/guidance/ipg615
- 100. Hedberg ML, Peyser ND, Bauman JE, et al. Use of nonsteroidal anti‐inflammatory drugs predicts improved patient survival for PIK3CA‐altered head and neck cancer. J Exp Med. 2019;216(2):419–427. 10.1084/jem.20181936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Elad S, Arany P, Bensadoun RJ, Epstein JB, Barasch A, Raber‐Durlacher J. Photobiomodulation therapy in the management of oral mucositis: search for the optimal clinical treatment parameters. Support Care Cancer. 2018;26:3319–3321. [DOI] [PubMed] [Google Scholar]
- 102. Sonis S. Could the impact of photobiomodulation on tumor response to radiation be effected by tumor heterogeneity? Support Care Cancer. 2020;28:423–424. [DOI] [PubMed] [Google Scholar]


