Abstract
Objective
This study aimed at evaluating the efficacy of the questionnaire-based prediction model in an independent prospective cohort.
Methods
A cluster-randomized controlled trial was conducted in Changsha, Harbin, Luoshan, and Sheyang in eastern China in 2015−2017. A total of 182 villages/communities were regarded as clusters, and allocated to screening arm or control arm randomly. Face-to-face interview through a questionnaire interview, including of relevant risk factors of gastric cancer, was administered for each subject. Participants were further classified into high-risk or low-risk groups based on their exposure to risk factors. All participants were followed up until December 31, 2019. Cumulative incidence rates from gastric cancer between high-risk and low-risk groups were calculated and compared using the log-rank test. Cox proportional hazard regression models were applied to estimate hazard ratio (HR) and 95% confidence interval (95% CI).
Results
Totally, 89,914 residents were recruited with a mean follow-up of 3.47 years. And 42,015 (46.73%) individuals were classified into high-risk group and 47,899 (53.27%) subjects were categorized into low-risk group. Gastric cancer was diagnosed in 131 participants, of which 91 were in high-risk group. Compared with the low-risk participants, high-risk individuals were more likely to develop gastric cancer (adjusted HR=2.15, 95% CI, 1.23−3.76). The sensitivity of the questionnaire-based model was estimated at 61.82% (95% CI, 47.71−74.28) in a general population.
Conclusions
Our questionnaire-based model is effective at identifying high-risk individuals for gastric cancer.
Keywords: Gastric cancer, risk assessment, risk factors, China
Introduction
Gastric cancer caused about 1.0 million new cases and 0.8 million deaths in 2018 worldwide, making it the fifth most common and the third most fatal cancer (1). The 5-year survival rate of gastric cancer was 90.0% for early-stage patients, and only 10.0% for those in advanced stages (2). In Asia, only Korea and Japan have population-based national screening program for gastric cancer (3). Because of early detection and treatment, the 5-year survival rate in Korea (73.1%) (4) and Japan (62.1%) (5), respectively, was much higher than that in China (35.1%) (6). In addition, the age-standardized disability-adjusted life years per 100,000 populations for gastric cancer was highest in China when compared with Japan and South Korea (7).
Reducing gastric cancer mortality through screening on the general population level, rather than focusing on high-risk individuals, is not cost-effective and unpractical, due to high cost and the invasive procedure of endoscopy or gastroscopy (8). Additionally, the efficacy to detect gastric cancer is mainly dependent on gastric cancer risk. As a result, some guidelines or consensus (9,10) considered that screening for gastric cancer should be carried out among high-risk individuals. In recent decades, several screening programs for gastric cancer have been conducted in certain areas in China (2,11,12), which were limited at high-risk population. But there is no consensus on the definition of “high-risk”.
A series of epidemiological studies have been conducted to identify potential risk factors for gastric cancer, such as unhealthy dietary habits and obesity (13-16). Notably, individuals with different risk factors present different level risk of developing gastric cancer. Thus, it is sensible to apply a risk assessment tool to stratify individuals. Several scoring systems were developed globally to identify high-risk individuals for gastric cancer. ABC method, which combined the assay of Helicobacter pylori(H. pylori) antibody and serum pepsinogen (PG) to category participants into 4 level risk (16,17). However, the ability to discriminate false negative of this strategy was weak (18,19), and the optimal PG cut-off value was unclear in Chinese population. In addition, the procedure of this method to identify individuals at high-risk is complex and it is difficult to apply in a large population.
In May 2015, we initiated a multicenter cluster-randomized controlled trial. A simple and easy-to-use questionnaire-based prediction model was used to identify individuals at high-risk. Here, the aim of this study was to estimate the effectiveness of this risk assessment model.
Materials and methods
Study design and participants
This study was based on a multicenter population-based cluster-randomized controlled trial for upper gastrointestinal cancer from 2015 to 2017. The study protocol has been previously published (20). The seven study sites, Cixian, Linzhou, Wuwei, Changsha, Harbin, Luoshan and Sheyang, were chosen according to the quality of cancer registration data and death surveillance data, population stability, and good working basis of screening projects.
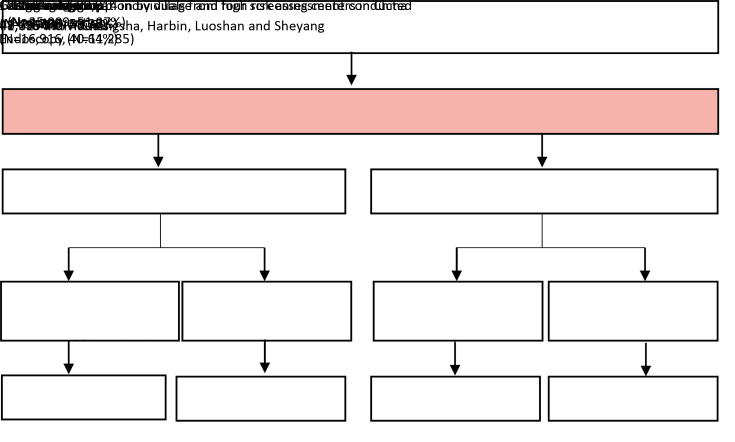
The inclusion criteria of participants were as follows: 1) local registered residents in a target village; 2) aged 40−69 years; 3) no history of cancer and/or mentally and physically competent; 4) no history of endoscopic examination in the recent three years; and 5) voluntary participation. Each participant had signed an informed consent voluntarily before recruitment. The original design of trial included 230,583 subjects, of which 152,172 completed the baseline survey completely. Subjects who had cancer before entry (n=1,057), had a history of endoscopic screening in the latest 3 years (n=521), had duplicates/erroneous baseline data (n=55) and those outside the target age range (n=583) were excluded. A total of 149,956 individuals recruited from 345 villages/communities between May 2015 and July 2017 formed the final cohort. The risk assessment was only conducted at Changsha, Harbin, Luoshan and Sheyang, due to the low incidence for gastric cancer in these four areas. Therefore, subjects from these four regions with 182 villages were included in this study. The flow diagram of the study cohort is shown inFigure 1 .
1.
Flow diagram of study cohort and risk assessment of individuals.
Ethical considerations
Approval of the study was obtained from the independent Ethics Committee of National Cancer Center/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College. The trial has been registered with the Protocol Registration System in Chinese Clinical Trial Registry (identifier: ChiCTR-EOR-16008577). All participants provided written informed consent prior to participation.
Randomization
The randomization procedure was described in detail in previous protocol (20). A stratified cluster sampling design was conducted using computer-generated sequence in a 1:1 ratio to ensure balance within different clusters. Villages/communities regarded as clusters were allocated into either intervention group or control group randomly.
Assessment of exposure
The screening strategy for gastric cancer in the present study was executed as follows: at the first stage, after explaining the study and obtaining written informed consent, basic information about participants’ exposure was acquired from trained interviewers, using a computer-aided standardized questionnaire, which included demographic information, data of dietary habit and lifestyles and comorbidities. This questionnaire-based prediction model was constructed by several risk factors of upper gastrointestinal cancer based on the existing evidence, which was developed by the expert group led by the National Cancer Center of China. The detailed items are presented in Supplementary Table S1 . The cut-off value was two for identifying high-risk individuals, considering that this questionnaire prediction model was a qualitative tool. The risk level was estimated for each subject and participants were further classified into high-risk and low-risk groups based on their risk level. Participants at high-risk in the screening arm were invited to receive endoscopy and participants in the control arm received no screening and were followed up.
S1. Definition of high-risk individuals.
| Item | Risk level |
| *, Hot food means that the temperature of food is over 70 ℃, and hard food generally refers to food that is difficult to digest. **, Including gastric ulcer, superficial gastritis, atrophic gastritis. | |
| Smoking at least 20 cigarettes per day and last for 10 years or more | 1 |
| Drinking at least 28 g ethanol per day and last for 10 years or more | 1 |
| Eating salt-preserved food at least once per week | 1 |
| Eating habit of very hot and hard food* | 1 |
| Family history of upper gastrointestinal cancer | 2 |
| Current symptom of chest pain, pressure or burning | 2 |
| Dysphagia | 2 |
| Chronic heartburn or indigestion | 2 |
| Vomiting or hemoptysis | 2 |
| Progressive weight loss | 2 |
| Esophageal reflux | 2 |
| History of gastric disease** | 2 |
Ascertainment of outcomes and follow-up
The primary outcome was gastric cancer development. Gastric cancer was diagnosed at baseline based on standard upper endoscopy and biopsy. During the follow-up period, passive follow-up and active follow-up annually were used to ascertain gastric cancer. The total population of selected areas in this study was covered by population-based cancer registry and death surveillance system which would facilitate follow-up. Firstly, gastric cancer and causes of death were identified by linkage to the cancer registration database and death cause database. Information from medical records or local health care centers was also referred. Then, we track individual vital status through telephone follow-up or home visit. The International Classification of Diseases for Oncology, 3rd edition (ICD-O-3) and the International Statistical Classification of Diseases and Related Health and Problems 10th Revision (ICD-10) are both applied for coding. The enrolment period extended from 2015 and follow-up here was documented to December 31, 2019. Individuals unavailable for follow-up or without the incident date were excluded.
Statistical analysis
The baseline characteristics of subjects were described using frequency and percentage for categorical variables and
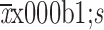 for continuous variables. Chi-squared test or Fisher’s exact test was applied to test the difference for categorical variables, and continuous variable was compared using the Student’s ttest. Comparisons of the cumulative incidence between high-risk group and low-risk group were modelled by the log-rank test. Cox proportion hazard models were applied to estimate hazard ratios (HRs) and 95% confidence intervals (95% CIs). All statistical analyses were conducted using SAS software (Version 9.4; SAS Institute, Cary, NC, USA). Two-tailed P values less than 0.05 was considered statistically significant.
for continuous variables. Chi-squared test or Fisher’s exact test was applied to test the difference for categorical variables, and continuous variable was compared using the Student’s ttest. Comparisons of the cumulative incidence between high-risk group and low-risk group were modelled by the log-rank test. Cox proportion hazard models were applied to estimate hazard ratios (HRs) and 95% confidence intervals (95% CIs). All statistical analyses were conducted using SAS software (Version 9.4; SAS Institute, Cary, NC, USA). Two-tailed P values less than 0.05 was considered statistically significant.
Results
Baseline characteristics of study population
A total of 89,914 (48,295 in the screening arm and 41,619 in the control arm) eligible residents aged 40−69 years were included with a mean follow-up of 3.47 years. Thirty-eight participants unavailable for follow-up were excluded during this period. The baseline demographical characteristics of the eligible population are shown inTable 1 . The mean age of the study subjects in low-risk and high-risk group was 53.29±8.14 years and 54.44±7.87 years, respectively. And 54.73% of participants were females. Based on our risk assessment model, 42,015 (46.73%) individuals were classified into high-risk group and 47,899 (53.27%) subjects were categorized as low-risk group. There were significant differences between high-risk and low-risk group in terms of smoking and alcohol consumption (P<0.001). Overall, 131 (0.15%) gastric cancer cases were observed, most of them were adenocarcinoma, including 91 patients in high-risk group and 40 patients in low-risk group (P<0.001).
1. Baseline characteristics of cohort.
| Variables | n (%) | P | ||
| Overall | Low risk | High risk | ||
| *, Data are not available for one participant. | ||||
| Participants | 89,914 (100.00) | 47,899 (53.27) | 42,015 (46.73) | |
Age (
 ) (year)
) (year)
|
53.83 (8.04) | 53.29 (8.14) | 54.44 (7.87) | <0.001 |
| Sex | 0.110 | |||
| Male | 40,703 (45.27) | 21,564 (45.02) | 19,139 (45.55) | |
| Female | 49,211 (54.73) | 26,335 (54.98) | 22,876 (54.45) | |
| Smoking* | <0.001 | |||
| Never | 73,131 (81.34) | 42,069 (87.83) | 31,062 (73.93) | |
| Ever | 16,782 (18.66) | 5,830 (12.17) | 10,952 (26.07) | |
| Drinking | <0.001 | |||
| No | 77,931 (86.67) | 44,431 (92.76) | 33,500 (79.73) | |
| Yes | 11,983 (13.33) | 3,468 (7.24) | 8,515 (20.27) | |
| Family history of upper gastrointestinal cancer | 8,211 (100.00) | 0 (0) | 8,211 (100.00) | |
| Gastric cancer cases | <0.001 | |||
| No | 89,783 (99.85) | 47,859 (99.92) | 41,924 (99.78) | |
| Yes | 131 (0.15) | 40 (0.08) | 91 (0.22) | |
Risk comparisons
Table 2 presents the risk level distribution of participants and the risk of gastric cancer by cohort. In a total cohort, there were more gastric cancer cases in high-risk group and incidence from gastric cancer between high-risk group and low-risk group showed a significant statistical difference (P<0.001). The number needed to be screened to detect one case in low-risk and high-risk groups were 1,197 and 462, respectively. Compared to participants in low-risk group, those in high-risk group had higher risk of gastric cancer [adjusted hazard ratio (aHR)=2.37, 95% CI, 1.63−3.45].
2. Distribution of risk level among target participants and risk of GC by risk group.
| Cohort | Risk group | Risk level | N | GC | NNS* | P | Sensitivity [% (95% CI)] | aHR (95% CI) |
| Adjusted for age group (40−49, 50−59, 60−69 years old), sex, ethnicity and marital status (never married, married, divorced and widow); *, The number of participants who should undergo endoscopy screening to identify one incident case; GC, gastric cancer; NNS, number needed to screen; 95% CI, 95% confidence interval; aHR, adjusted hazard ratio. | ||||||||
| All | Low | 0 | 33,934 | 19 | 1,197 | <0.001 | 69.47 (60.72−77.05) | Reference |
| 1 | 13,965 | 21 | ||||||
| High | ≥2 | 42,015 | 91 | 462 | 2.37 (1.63−3.45) | |||
| Screening group | Low | 0 | 17,150 | 11 | 1,221 | <0.001 | 75.00 (63.52−83.92) | Reference |
| 1 | 6,046 | 8 | ||||||
| High | ≥2 | 25,099 | 57 | 440 | 2.58 (1.53−4.34) | |||
| Control group | Low | 0 | 16,784 | 8 | 1,176 | 0.001 | 61.82 (47.71−74.28) | Reference |
| 1 | 7,919 | 13 | ||||||
| High | ≥2 | 16,916 | 34 | 498 | 2.15 (1.23−3.76) | |||
In the screening arm, 23,196 (48.03%) participants were identified as low risk, of whom 19 had gastric cancer. Whereas there were 57 cases in high-risk group, accounting for 0.23%. The number needed to be screened to detect one case in low-risk and high-risk groups were 1,221 and 440, respectively. High-risk individuals were more likely to develop gastric cancer, and the aHR was estimated at 2.58 (95% CI, 1.53−4.34). In the control arm, 40.64% of participants were estimated at high risk, among them 34 developed gastric cancer. The number needed to be screened to identify one case in low-risk and high-risk groups were 1,176 and 498, respectively. Compared to individuals in low-risk group, high-risk participants also had a higher risk of gastric cancer (aHR=2.15, 95% CI, 1.23−3.76). The sensitivities to identify cases in the screening and control arm were 75.00% (95% CI, 63.52−83.92) and 61.82% (95% CI, 47.71−74.28), respectively (Table 2 ).
Cumulative incidence of gastric cancer among target population
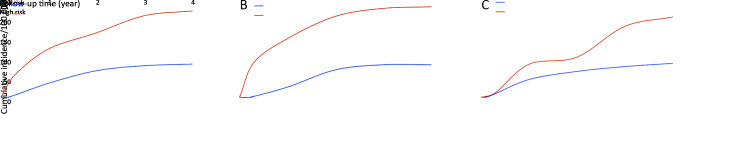
Figure 2A shows cumulative incidence from gastric cancer in total cohort. Over the whole observation period, the cumulative incidence from gastric cancer in high-risk group was much higher, compared with individuals at low risk (log-rank test, P<0.001). Similar trends were also observed in the screening arm and control arm (Figure 2B ,C ).
2.
Cumulative incidence from gastric cancer. (A) All participants (Log-rank test χ2=26.58, P<0.001); (B) Participants in screening arm (Log-rank test χ2=15.95, P<0.001); (C) Participants in control arm (Log-rank test χ2=9.89, P<0.001).
Sensitivity analysis
We excluded individuals in the screening arm who had undergone endoscopy at baseline to avoid the effect of endoscopy (Table 3 ). A total of 78,629 participants were selected as the target population. The sensitivity to identify case was 54.02% (95% CI, 43.04−64.64). Compared to low-risk participants, high-risk individuals were associated with a higher risk of gastric cancer (aHR=1.67, 95% CI, 1.10−2.56). We also afraid that some esophageal symptoms may exert influence on the risk assessment of gastric cancer, we further changed the rules to re-estimate the risk of developing gastric cancer, excluding esophageal cancer symptoms, and population were re-stratified (Table 4 ). A higher risk of gastric cancer was also observed among high-risk population (aHR=1.90, 95% CI, 1.24−2.91). More results about sensitivity analysis could be found inSupplementary Table S2 .
3. Distribution of risk level among all participants, and risk of GC by risk group after excluding participants received endoscopy.
| Risk group | Risk level | N | GC | NNS* | P | Sensitivity [% (95% CI)] | aHR (95% CI) |
| Adjusted for age group (40−49, 50−59, 60−69 years old), sex, ethnicity and marital status (never married, married, divorced and widow); *, The number of participants who should undergo endoscopy screening to identify one incident case; GC, gastric cancer; NNS, number needed to screen; 95% CI, 95% confidence interval; aHR, adjusted hazard ratio. | |||||||
| Low | 0 | 33,934 | 19 | 1,197 | 0.004 | 54.02% (43.04−64.64) | Reference |
| 1 | 13,965 | 21 | |||||
| High | ≥2 | 30,730 | 47 | 654 | 1.67 (1.10−2.56) | ||
4. Distribution of re-assessment risk level among all population after excluding participants received endoscopy (excluding esophageal cancer symptoms in questionnaire-based risk prediction model)*.
| Risk group | Risk level | N | GC | NNS** | P | Sensitivity [% (95% CI)] | aHR (95% CI) |
| Adjusted for age group (40−49, 50−59, 60−69 years old), sex, ethnicity and marital status (never married, married, divorced and widow); *, Items, including dysphagia, esophageal reflux, hemoptysis, chronic heartburn, and current symptom of chest pain, were excluded. All population after excluding participants received endoscopy were re-stratified; **, The number of participants who should undergo endoscopy screening to identify one incident case; GC, gastric cancer; NNS, number needed to screen; 95% CI, 95% confidence interval; aHR, adjusted hazard ratio. | |||||||
| Low | 0 | 35,616 | 19 | 1,269 | <0.001 | 54.02% (43.04−64.64) | Reference |
| 1 | 15,139 | 21 | |||||
| High | ≥2 | 27,874 | 47 | 593 | 1.90 (1.24−2.91) | ||
S2. Distribution of re-assessment risk level among participants in the control arm (excluding esophageal cancer symptoms in questionnaire-based risk prediction model).
| Risk group | Risk level | N | GC | NNS* | P | Sensitivity [% (95% CI)] | aHR (95% CI) |
| Adjusted for age group (40−49, 50−59, 60−69 years old), sex, ethnicity and marital status (never married, married, divorced and widow); *, The number of participants who should undergo endoscopy screening to identify one incident case; GC, gastric cancer; NNS, number needed to screen; 95% CI, 95% confidence interval; aHR, adjusted hazard ratio. | |||||||
| Low | 0 | 17,481 | 8 | 1,240 | <0.001 | 61.82 (47.71−74.28) | Reference |
| 1 | 8,552 | 13 | |||||
| High | ≥2 | 15,586 | 34 | 458 | 2.43 (1.39−4.25) | ||
Discussion
Our results showed that this questionnaire-based prediction model was useful in identifying individuals at high risk through a multicenter cluster-randomized controlled cohort study. The sensitivity was 61.82% for detecting high-risk individual for gastric cancer in a general population.
Endoscopy offers a great opportunity to find possible gastric cancer. Ideally, endoscopy could be administered for every individual to prevent gastric cancer. However, the incidence for gastric cancer in a general population is low (33/100,000) (21,22) and only 1%−3% of the population is predicted to have gastric cancer (8). So unnecessary endoscopy would be performed if endoscopy were offered to the whole population. Thus, an imperative strategy to find potential individuals who were at high risk of gastric cancer is required. This questionnaire-based prediction model was developed by several epidemiologists according to their experience and it is not validated yet in another population. The significant difference of incidence from gastric cancer between high-risk and low-risk groups suggested that this questionnaire-based prediction model to identify high-risk population is effective. We noted that individuals in the screening arm had a higher risk of gastric cancer, because more gastric cancer could be found through endoscopy at baseline. Thus, we excluded individuals who had undergone endoscopy, to avoid the influence of intervention. In addition, individuals with esophageal symptom were also excluded. High-risk individuals still had a higher risk of gastric cancer. We also compared gastric cancer incidence between screening arm and control arm in low-risk group, presenting no statistical difference (P=0.323). These results suggest that the ability of this questionnaire prediction model to identify high-risk population of gastric cancer is stable.
The questionnaire-based prediction model was a simple model to estimate risk level of gastric cancer, comprising history of smoking, alcohol consumption, dietary habits, family history of upper gastrointestinal cancers and gastric diseases history. The association between cigarette smoking and gastric cancer was evident, which was supported by previous studies (23,24). Such association may increase with smoking intensity and duration (25). Alcohol use was associated with the risk of gastric cancer. A recent meta-analysis of case-control studies had suggested that alcohol consumption could elevate the risk of gastric cancer with an odds ratio (OR) of 1.39 (26). A prospective cohort study conducted in Japan during a mean follow-up of 13.4 year also reported that alcohol use was associated with the increased risk of gastric cancer in men, with an OR of 1.82 (27). Concerning dietary habits such as hard food, the positive association had been confirmed by other studies (28,29). All of them can cause chronic injury to the upper digestive tract and making it more vulnerable when exposed to detrimental carcinogenesis (13). In addition, salted food may increase the risk of H. pylori infection, which can also promote the development of gastric cancer (30).
Our questionnaire-based prediction model is just a qualitative tool to identify population at high risk, which is significantly suitable to be used in a large-scale population-based screening program or in a limited resource community setting. Because it does not require clinicians to perform complex examination and risk predictors could be obtained without laboratory testing. Thus, the acceptancy was extremely high, with an overall response proportion of 80.40% in these four areas. But the efficacy was inferior, compared to quantitative tools. A new score-based prediction rule was developed by Cai et al., which included age, sex, pepsinogen (PG) I/II ratio, gastrin-17 (G-17) concentration, anti-H. Pylori IgG status, consumption of pickled food and fried food (8). The area under curve (AUC) was 0.76 (95% CI: 0.73−0.79) and sensitivity was 70.8%, showing a better performance. Biochemical parameters including PG I/II ratio and G-17 level are associated with high risk of gastric cancer (31), which could be used in the screening projects and diagnosis of gastric cancer (32). Another new risk scoring-system reported by National Clinical Research Center for Digestive Diseases et al., contained age, sex, anti-H. pylori IgG status, PG I/II ration and G-17 (9), which has affirmed the irreplaceability of PG I/II ration and G-17 for defining high-risk population for gastric cancer. Since the 1990s, serum PG was considered as a marker for chronic atrophic gastritis and has been incorporated into gastric cancer screening programs (33). However, the cut-off value was difficult to define. PG I ≤70 ng/mL and PG I/II ratio ≤3.0 have been frequently applied as the threshold for defining population at risk in Japan (34,35). While the optimal cut-off levels were 59.3 μg/L for PG I (sensitivity, 83.3%; specificity, 78.4%) and 3.6 μg/L for PG I/II ratio (sensitivity, 70.0%; specificity, 78.4%) in a Korean study (36). Thus, if the standard of PG I and PG I/II ratio is unclear or some poor areas have not adequate health resources, our questionnaire-based prediction model is preferred to detect high-risk individuals of gastric cancer.
Two risk prediction models were developed in the Japanese population, who share similar risk factors of gastric cancer with the Chinese population. Charvat et al. (37) showed that their prediction model consists of 5 regular variables (age, gender, smoking status, family history of gastric cancer and consumption of highly salted food) and 2 biological markers (anti-H. pylori IgG and serum PG status) to estimate the 10-year probability of gastric cancer. Another risk assessment tool was modelled and validated to predict future risk of gastric cancer by Iida et al. (38). This risk prediction model incorporated age, sex, H. pylori antibody, PG status, hemoglobin A1c level, and smoking status, which showed that the AUC was 0.79 (95% CI, 0.74−0.83). In these two studies, the main point was predicting gastric cancer risk, rather than stratifying people into low risk or high risk. Besides, the cut-off value indicating that screening is adaptable and practical is unknown. Although simple information was used in our risk assessment tool, the ability to discriminate high-risk individuals of gastric cancer was enough.
This study had several limitations. Firstly, people may exaggerate their unhealthy behaviors to increase the possibility of examination freely, which may increase the likelihood of high risk. Secondly, the attitude to visit doctor was not considered in our study. Medical treatment of gastric diseases, including gastric ulcer, superficial gastritis, atrophic gastritis, might exert influence on the risk of gastric cancer. Thirdly, current questionnaire-based prediction model was only used in a Chinese population, the application of this questionnaire-based prediction model to people with different characteristics should be carefully validated. Lastly, the corresponding scores and cut-off value of the questionnaire-based prediction model to define high-risk population were determined by the expert experience in the field of gastric cancer prevention. In the future, we will develop a new risk prediction model to quantity the risk of developing of gastric cancer based on an ongoing prospective cohort. Despite of these limitations, there are several strengths, including large sample size and multicenter randomized design, which may increase the credibility. This study, to our knowledge, is the first study to prospectively explore the effectiveness of this questionnaire-based prediction model through multicenter cluster-randomized controlled trial in a Chinese population. And we nearly grasp the survival status of all participants through annually passive and active follow-up.
Conclusions
The present study demonstrates that our questionnaire-based prediction model to identify high-risk population for gastric cancer is effective and practicable. Individuals in high-risk group have two-fold risk of gastric cancer than those in low-risk group. This risk assessment model could be used widely in resource-limited settings.
Acknowledgements
This work was supported by the National Key Research and Development Program of China (No. 2018YFC1313100), the CAMS Innovation Fund for Medical Sciences (CIFMS) (No. 2019-I2M-2-004), and Sanming Project of Medicine in Shenzhen (SZSM201911015).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Contributor Information
Ji Peng, Email: pengji126@126.com.
Wanqing Chen, Email: chenwq@cicams.ac.cn.
References
- 1.Ferlay J, Ervik M, Lam F, et al. Global Cancer Observatory: Cancer Today. Lyon, France: International Agency for Research on Cancer 2018. Available online: https://gco.iarc.fr/today
- 2.Chen Q, Yu L, Hao CQ, et al Effectiveness of endoscopic gastric cancer screening in a rural area of Linzhou, China: results from a case-control study. Cancer Med. 2016;5:2615–22. doi: 10.1002/cam4.812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Suh YS, Yang HK Screening and early detection of gastric cancer: East versus West. Surg Clin North Am. 2015;95:1053–66. doi: 10.1016/j.suc.2015.05.012. [DOI] [PubMed] [Google Scholar]
- 4.Oh CM, Won YJ, Jung KW, et al Cancer statistics in Korea: Incidence, mortality, survival, and prevalence in 2013. Cancer Res Treat. 2016;48:436–50. doi: 10.4143/crt.2016.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Matsuda T, Ajiki W, Marugame T, et al Population-based survival of cancer patients diagnosed between 1993 and 1999 in Japan: A chronological and international comparative study. Jpn J Clin Oncol. 2011;41:40–51. doi: 10.1093/jjco/hyq167. [DOI] [PubMed] [Google Scholar]
- 6.Zeng H, Chen W, Zheng R, et al Changing cancer survival in China during 2003-15: a pooled analysis of 17 population-based cancer registries. Lancet Glob Health. 2018;6:e555–67. doi: 10.1016/S2214-109X(18)30127-X. [DOI] [PubMed] [Google Scholar]
- 7.Sun D, Cao M, Li H, et al Cancer burden and trends in China: A review and comparison with Japan and South Korea. Chin J Cancer Res. 2020;32:129–39. doi: 10.21147/j.issn.1000-9604.2020.02.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cai Q, Zhu C, Yuan Y, et al Development and validation of a prediction rule for estimating gastric cancer risk in the Chinese high-risk population: a nationwide multicentre study. Gut. 2019;68:1576–87. doi: 10.1136/gutjnl-2018-317556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.National Clinical Research Center for Digestive Disease, Chinese Society of Digestive Endoscopy, Chinese Health Management Association Expert consensus on the early gastric cancer screening procedure in China. Zhonghua Xiao Hua Za Zhi. 2018;38:87–92. doi: 10.3760/cma.j.issn.0254-1432.2018.02.006. [DOI] [Google Scholar]
- 10.Santaballa A, Pinto Á, Balanyà RP, et al SEOM clinical guideline for secondary prevention (2019) Clin Transl Oncol. 2020;22:187–92. doi: 10.1007/s12094-020-02302-0. [DOI] [PubMed] [Google Scholar]
- 11.Zheng X, Mao X, Xu K, et al Massive endoscopic screening for esophageal and gastric cancers in a high-risk area of China. PloS One. 2015;10:e0145097. doi: 10.1371/journal.pone.0145097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen WQ, Li N, Shi JF, et al Preliminary analysis of cancer screening program in urban China from 2013 to 2017. Zhongguo Zhong Liu. 2019;28:23–5. doi: 10.11735/j.issn.1004-0242.2020.01.A001. [DOI] [Google Scholar]
- 13.Chen P, Lin Y, Zheng K, et al Risk factors of gastric cancer in high-risk region of China: A population-based case-control study. Asian Pac J Cancer Prev. 2019;20:775–81. doi: 10.31557/APJCP.2019.20.3.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang XQ, Yan H, Terry PD, et al Interaction between dietary factors and Helicobacter pylori infection in noncardia gastric cancer: a population-based case-control study in China . J Am Coll Nutr. 2012;31:375–84. doi: 10.1080/07315724.2012.10720447. [DOI] [PubMed] [Google Scholar]
- 15.Chen W, Xia C, Zheng R, et al Disparities by province, age, and sex in site-specific cancer burden attributable to 23 potentially modifiable risk factors in China: a comparative risk assessment. Lancet Glob Health. 2019;7:e257–e69. doi: 10.1016/S2214-109X(18)30488-1. [DOI] [PubMed] [Google Scholar]
- 16.Chen XZ, Huang CZ, Hu WX, et al Gastric cancer screening by combined determination of serum helicobacter pylori antibody and pepsinogen concentrations: ABC method for gastric cancer screening. Chin Med J (Engl) 2018;131:1232–9. doi: 10.4103/0366-6999.231512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yamaguchi Y, Nagata Y, Hiratsuka R, et al Gastric cancer screening by combined assay for serum anti-Helicobacter pylori IgG antibody and serum pepsinogen levels--The ABC method . Digestion. 2016;93:13–8. doi: 10.1159/000441742. [DOI] [PubMed] [Google Scholar]
- 18.Kwon H, Lee SY, Kim JH, et al ABC classification is less useful for older Koreans born before 1960. Gut Liver. 2019;13:522–30. doi: 10.5009/gnl18399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kiso M, Yoshihara M, Ito M, et al Characteristics of gastric cancer in negative test of serum anti-Helicobacter pylori antibody and pepsinogen test: a multicenter study . Gastric Cancer. 2017;20:764–71. doi: 10.1007/s10120-016-0682-5. [DOI] [PubMed] [Google Scholar]
- 20.Chen W, Zeng H, Chen R, et al Evaluating efficacy of screening for upper gastrointestinal cancer in China: a study protocol for a randomized controlled trial. Chin J Cancer Res. 2017;29:294–302. doi: 10.21147/j.issn.1000-9604.2017.04.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chinese Society of Digestive Endoscopy Consensus on screening and endoscopic diagnosis and treatment of early gastric cancer in China (changsha, 2014) Zhonghua Xiao Hua Za Zhi. 2014;34:361–77. doi: 10.3760/cma.j.issn.1007-5232.2014.07.001. [DOI] [Google Scholar]
- 22.National Health Commission of the People’s Republic of China Chinese guidelines for diagnosis and treatment of gastric cancer 2018 (English version) Chin J Cancer Res. 2019;31:707–37. doi: 10.21147/j.issn.1000-9604.2019.05.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tan MC, Mallepally N, Liu Y, et al Demographic and lifestyle risk factors for gastric intestinal metaplasia among us veterans. Am J Gastroenterol. 2020;115:381–7. doi: 10.14309/ajg.0000000000000498. [DOI] [PubMed] [Google Scholar]
- 24.Cheng XJ, Lin JC, Tu SP Etiology and prevention of gastric cancer. Gastrointest Tumors. 2016;3:25–36. doi: 10.1159/000443995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Praud D, Rota M, Pelucchi C, et al Cigarette smoking and gastric cancer in the Stomach Cancer Pooling (StoP) Project. Eur J Cancer Prev. 2018;27:124–33. doi: 10.1097/CEJ.0000000000000290. [DOI] [PubMed] [Google Scholar]
- 26.Ma K, Baloch Z, He TT, et al Alcohol consumption and gastric cancer risk: A meta-analysis. Med Sci Monit. 2017;23:238–46. doi: 10.12659/msm.899423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li Y, Eshak ES, Kokoro S, et al Alcohol consumption and risk of gastric cancer: The Japan Collaborative Cohort study. J Epidemiol. 2019 doi: 10.2188/jea.JE20190304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Somi MH, Mousavi SM, Naghashi S, et al Is there any relationship between food habits in the last two decades and gastric cancer in North-Western Iran? Asian Pac J Cancer Prev. 2015;16:283–90. doi: 10.7314/apjcp.2015.16.1.283. [DOI] [PubMed] [Google Scholar]
- 29.Huang L, Chen L, Gui ZX, et al Preventable lifestyle and eating habits associated with gastric adenocarcinoma: A case-control study. J Cancer. 2020;11:1231–39. doi: 10.7150/jca.39023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tsugane S Salt, salted food intake, and risk of gastric cancer: epidemiologic evidence. Cancer Sci. 2005;96:1–6. doi: 10.1111/j.1349-7006.2005.00006.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tu H, Sun L, Dong X, et al A serological biopsy using five stomach-specific circulating biomarkers for gastric cancer risk assessment: A multi-phase study. Am J Gastroenterol. 2017;112:704–15. doi: 10.1038/ajg.2017.55. [DOI] [PubMed] [Google Scholar]
- 32.Afshin Shafaghi, Fariborz Mansour-Ghanaei, Farahnaz Joukar, et al Serum gastrin and the pepsinogen I/II ratio as markers for diagnosis of premalignant gastric lesions. Asian Pac J Cancer Prev. 2013;14:3931–36. doi: 10.7314/apjcp.2013.14.6.3931. [DOI] [PubMed] [Google Scholar]
- 33.Fock KM, Talley N, Moayyedi P, et al Asia-Pacific consensus guidelines on gastric cancer prevention. J Gastroenterol Hepatol. 2008;23:351–65. doi: 10.1111/j.1440-1746.2008.05314.x. [DOI] [PubMed] [Google Scholar]
- 34.Watabe H, Mitsushima T, Yamaji Y, et al Predicting the development of gastric cancer from combining Helicobacter pylori antibodies and serum pepsinogen status: a prospective endoscopic cohort study . Gut. 2005;54:764–68. doi: 10.1136/gut.2004.055400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Watanabe Y, Kurata JH, Mizuno S, et al Helicobacter pylori infection and gastric cancer. A nested case-control study in a rural area of Japan . Dig Dis Sci. 1997;42:1383–7. doi: 10.1023/a:1018833819860. [DOI] [PubMed] [Google Scholar]
- 36.Song HJ, Jang SJ, Yun SC, et al Low levels of pepsinogen I and pepsinogen I/II ratio are valuable serologic markers for predicting extensive gastric corpus atrophy in patients undergoing endoscopic mucosectomy. Gut Liver. 2010;4:475–80. doi: 10.5009/gnl.2010.4.4.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Charvat H, Sasazuki S, Inoue M, et al Prediction of the 10-year probability of gastric cancer occurrence in the Japanese population: the JPHC study cohort II. Int J Cancer. 2016;138:320–31. doi: 10.1002/ijc.29705. [DOI] [PubMed] [Google Scholar]
- 38.Iida M, Ikeda F, Hata J, et al Development and validation of a risk assessment tool for gastric cancer in a general Japanese population. Gastric Cancer. 2018;21:383–90. doi: 10.1007/s10120-017-0768-8. [DOI] [PubMed] [Google Scholar]