Abstract
Objective
We aimed to investigate the prognostic value of neutrophil-to-lymphocyte ratio (NLR) and myeloid-derived suppressor cells (MDSCs) in gastric cancer patients treated with second-line ramucirumab plus paclitaxel.
Methods
A total of 116 patients with advanced or metastatic gastric cancer who receive ramucirumab plus paclitaxel were prospectively enrolled. Fresh blood samples were collected before and after treatment, and flow cytometry was performed to assess the proportions of monocytic (mMDSCs) and granulocytic MDSCs (gMDSCs).
Results
Median age was 58 years and 71 (61.2%) patients were male. A baseline NLR≥2.94 was associated with significantly poorer progression-free survival (PFS) and overall survival (OS) vs. an NLR<2.94 (P=0.011 and P=0.002, respectively). In multivariate analysis, an NLR≥2.94 was independently associated with poorer PFS [hazard ratio (HR)=1.58; 95% confidence interval (95% CI): 1.01−2.49, P=0.046] and OS (HR=1.77; 95% CI: 1.04−3.04, P=0.036). While mMDSC counts did not significantly change following two cycles of therapy (P=0.530), gMDSC counts decreased significantly after two treatment cycles (P=0.025) but tended to increase in patients with progressive disease after two treatment cycles (P=0.098). A progressive increase in gMDSC counts (≥44%) was associated with a significantly shorter PFS and OSvs. a gMDSC count increase <44% (P=0.001 and P=0.003, respectively).
Conclusions
The baseline NLR may help guide clinical decisions during ramucirumab plus paclitaxel therapy for gastric cancer. Our gMDSC kinetics data warrant further clinical validation and mechanistic investigation.
Keywords: Gastric cancer, ramucirumab plus paclitaxel, neutrophil-to-lymphocyte ratio, myeloid-derived suppressor cells
Introduction
Gastric cancer is the fifth most common cancer and the third leading cause of cancer-related death worldwide (1). Patients with advanced or metastatic gastric cancer have a poor prognosis and pose significant socioeconomic burdens, especially in Asian countries. Pivotal phase 3 trials have established the role of second-line systemic chemotherapy for patients with disease progression despite platinum and fluoropyrimidine-based first-line systemic therapies, and these studies have demonstrated the superior efficacy of systemic therapy over best supportive care (2-4). Subsequently, the phase 3 Rainbow trial demonstrated the superior efficacy of the combination of anti-angiogenic agent ramucirumab plus paclitaxel vs. paclitaxel monotherapy (5), which led to the designation of this combination as the current standard of second-line care. Although a recent exploratory analysis of the Rainbow trial has suggested the prognostic value of some circulating factors (6), the mechanism underlying the therapeutic response or resistance to ramucirumab plus paclitaxel remains poorly understood, and no predictive biomarker has been identified.
Investigations of systemic inflammatory response markers related to the clinical outcomes of gastric cancer patients are based on the concept that systemic inflammation may contribute to cancer progression through various mechanisms such as induction of cancer proliferation/metastasis, promotion of angiogenesis and attenuation of anti-tumor immune responses (7). Among these potential markers, neutrophil-to-lymphocyte ratio (NLR) was shown to be correlated well with the clinical outcomes of patients with gastric cancer who underwent surgical resection (8-10) or received palliative first-line (11-14) and second-line (15,16) systemic chemotherapy. A previous study suggested NLR as a prognostic factor in patients with gastric cancer who were treated with ramucirumab monotherapy or ramucirumab plus paclitaxel (17); however, inhomogeneous treatment regimen and lack of mechanistic explanation in that study warrant further validation of its clinical value and an investigation of biological features linked to NLR.
Myeloid-derived suppressor cells (MDSCs) are a population of immunosuppressive cells that play a critical role in immune evasion of cancers (18). Reports suggest that the proportion of MDSCs is correlated with NLR (19,20) and cancer stage (21) in patients with gastric cancer. Particularly, an inverse correlation between proportion of MDSCs and function of effector T cells has been observed in gastric cancer patients (22), and this highlights the role of MDSCs in attenuating anti-tumor immune response. Interestingly, both ramucirumab and paclitaxel can potentially modulate MDSCs. Vascular endothelial growth factor (VEGF) can induce the accumulation of aberrant myeloid cells via VEGF receptor 2 (VEGFR2) (23), and a positive correlation between VEGF score and MDSCs has been reported (20,24). Therefore, ramucirumab, a VEGFR2 antagonist, can theoretically modulate MDSCs by interrupting the VEGF/VEGFR2 pathway (25). Paclitaxel has also been shown to reduce MDSC counts (26) and promote differentiation of MDSCs into dendritic cells (27) in preclinical models. However, mechanism by which ramucirumab plus paclitaxel combination therapy affects MDSCs and clinical implications in patients with gastric cancer have not yet been investigated.
Given the potential modulatory effects of ramucirumab and paclitaxel on MDSC count, we hypothesized that the kinetic changes of MDSCs and NLR might be associated with survival outcomes in gastric cancer patients receiving ramucirumab plus paclitaxel treatment. Therefore, we aimed to investigate the prognostic value of NLR and MDSC counts and their kinetics in a prospective cohort of gastric cancer patients treated with second-line ramucirumab plus paclitaxel.
Materials and methods
Study patients and ramucirumab plus paclitaxel therapy
This study was based on a prospective cohort of 116 patients with advanced or metastatic gastric cancer who received ramucirumab plus paclitaxel as a second-line systemic therapy between April 2018 and October 2019. Inclusion criteria were as follows: 1) age of 18 years or older; 2) receipt of ramucirumab plus paclitaxel therapy as second-line systemic therapy; 3) Eastern Cooperative Oncology Group (ECOG) performance status ≤2; and 4) adequate blood sample quality for flow cytometry analysis. The Institutional Review Board approved the study protocol, and all patients provided written informed consent. All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1964 and later versions.
Ramucirumab (8 mg/kg IV on d 1 and 15) and paclitaxel (80 mg/m2 IV on d 1, 8 and 15) were administered every 4 weeks until disease progression or unacceptable adverse events occurred. Study patients were followed up and examined every day of ramucirumab plus paclitaxel administration (d 1, 8, and 15 of each cycle). Complete blood cell counts and routine chemistry labs were measured on the follow-up day. Computed tomography (CT) scans were done every 8−12 weeks for disease evaluation. When disease progression was clinically suspected during follow-up, additional CT scans were performed. The study patients were additionally monitored by a study coordinator. The Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1 were used to grade the tumor responses.
Calculation of NLR and immunophenotyping by flow cytometry
The NLR value was obtained by dividing absolute neutrophil count by absolute lymphocyte count. Both cell counts were based on complete blood analyses conducted routinely in clinical practice.
For immunophenotyping, blood samples were collected at baseline and after two cycles of ramucirumab plus paclitaxel therapy. Baseline blood samples were collected from all study patients (N=116), while follow-up samples were collected from 66 patients after two cycles of chemotherapy. Peripheral blood mononuclear cells were isolated from whole blood using LymphoprepTM (Stem Cell Technologies) according to the manufacturer’s instructions. No patients received granulocyte colony-stimulating factor within 2 weeks prior to NLR and MDSC evaluation.
Flow cytometry was performed using an S1000Exi (Stratedigm) or FACSLyric instrument (BD Biosciences, San Jose, CA, USA). Flow cytometry data were analyzed using FlowJo software (Treestar, San Carlos, USA). The following antibodies were used in the flow cytometry analyses: anti-CD33 (P67.6; BD Biosciences) and anti-HLA-DR (L243), anti-CD11b (M1/70), and anti-CD66b (G10F5) (all Biolegend, San Diego, CA, USA). Monocytic MDSCs (mMDSCs) were defined as HLA-DR−CD11b+CD33+CD66b− cells, while granulocytic MDSCs (gMDSCs) were defined as HLA-DR−CD11b+CD33+CD66b− cells. The MDSC counts were estimated from white blood cell counts and proportions of MDSC subsets in leukocyte counts. The representative flow cytometry gating strategy is presented in Supplementary Figure S1 .
S1.
Gating strategy of flowcytometry analysis of MDSCs. MDSC: myeloid-derived suppressor cell; gMDSC, granulocyte MDSC; mMDSC, monocytic MDSC.
Statistical analysis
Progression-free survival (PFS) was defined as the interval from the initiation of ramucirumab plus paclitaxel (index date) to the date of disease progression (determined using RECIST v1.1) or death. Overall survival (OS) was defined as the interval between the index date and the date of death from any cause. The Kaplan-Meier method was used to estimate survival outcomes, and the log-rank test was used to compare these survival outcomes among the subgroups. The Chi-square test or Fisher’s exact test was used to compare categorical variables among the subgroups. Cox proportional hazard modeling was used to assess the associations between examined factors and PFS and OS. In addition to demographic factors and NLR, several prognostic factors shown to be associated with survival outcomes in the second-line setting, such as the time-to-progression (TTP) with first-line systemic therapy, ECOG performance status, lactate dehydrogenase (LDH) concentration, and hemoglobin concentration, were included in the multivariate analysis (15,28,29). The maximal Chi-square method was used to determine the optimal cut-off value of NLR and the percent change in the gMDSC count that best segregated PFS outcomes. P<0.05 was considered statistically significant. Statistical analyses were performed using R software (Version 3.6.2; R Foundation for Statistical Computing, Vienna, Austria). The following R packages were used in this study: dplyr (Version 1.0.2), survival (Version 3.2.3), survminer (Version 0.4.8), maxstat (Version 0.7.25) and ggpubr (Version 0.4.0).
Results
Patient characteristics
Baseline characteristics of 116 patients included in this study are summarized in Table 1 . The median age was 58 years, and 71 (61.2%) patients were male. Additionally, 108 (93.1%) and eight (6.9%) patients had ECOG performance scores of 0−1 or 2, respectively. The median TTP with first-line systemic therapy was 6.5 [interquartile range (IQR): 3.9−12.1] months. The median NLR and hemoglobin were 2.0 g/dL and 11.4 g/dL, respectively. The baseline mMDSC and gMDSC counts were 154.8/µL and 1,953.1/µL, respectively.
1. Baseline characteristics of study patients.
| Characteristics | Full cohort (n=116) | NLR<2.94 (n=85) | NLR≥2.94 (n=31) | P* |
| IQR, interquartile range; ECOG PS, Eastern Cooperative Oncology Group performance status; TTP, time-to-progression; NLR, neutrophil-to-lymphocyte ratio; LDH, lactate dehydrogenase; mMDSC, monocytic myeloid-derived suppressor cells; gMDSC, granulocytic myeloid-derived suppressor cells; *, P-values represent comparison between NLR<2.94 and NLR≥2.94 groups. **, Baseline LDH levels were available in 107 patients: 77 and 30 patients in NLR<2.94 and NLR≥2.94 groups, respectively. | ||||
| Age (year) [median (IQR)] | 58 (47−63) | 57 (47−63) | 58 (49−63) | 0.689 |
| Male gender [n (%)] | 71 (61.2) | 51 (60.0) | 20 (64.5) | 0.821 |
| ECOG PS [n (%)] | 0.764 | |||
| 0−1 | 108 (93.1) | 80 (94.1) | 28 (90.3) | |
| 2 | 8 (6.9) | 5 (5.9) | 3 (9.7) | |
| First-line TTP (month) [median (IQR)] | 6.5 (3.9−12.1) | 7.9 (4.7−13.2) | 4.2 (1.6−6.2) | <0.001 |
| NLR [median (IQR)] | 2.0 (1.4−3.0) | 1.7 (1.2−2.1) | 4.4 (3.6−5.4) | <0.001 |
Hemoglobin (g/dL) (
 )
)
|
11.3±1.7 | 11.6±1.7 | 10.6±1.6 | 0.008 |
| LDH elevation [n (%)]** | 36 (33.6) | 24 (31.2) | 12 (40.0) | 0.522 |
| mMDSC (count/µL) [median (IQR)] | 154.8 (29.8−306.7) | 123.9 (22.2−238.7) | 255.2 (100.1−81.2) | 0.001 |
| gMDSC (count/µL) [median (IQR)] | 1,953.1 (937.0−2,948.0) | 1,480.0 (98.5−2,561.6) | 2,508.0 (1,770.9−5,523.4) | 0.001 |
The median PFS and OS of study patients were 3.35 [95% confidence interval (95% CI): 3.29−4.47] months and 7.76 (95% CI: 6.51−11.54) months, respectively. The objective response rate was 36.3%, and the disease control rate was 80.3%, with no confirmed complete responses among 66 patients with measurable disease.
Clinical outcomes according to baseline NLR
To examine the prognostic value of NLR in the context of ramucirumab plus paclitaxel therapy, we subdivided the study patients according to a cut-off NLR of 2.94, which best segregated PFS outcomes into a high NLR group (NLR≥2.94, n=31) and a low NLR group (NLR<2.94, n=85). The high NLR group had a significantly shorter TTP with first-line systemic therapy than the low NLR group (median: 4.2vs. 7.9 months, respectively, P<0.001). The high NLR group also had a significantly lower mean hemoglobin concentration as well as lower median mMDSC and gMDSC count than the low NLR group; in contrast, the baseline LDH levels were comparable between the two groups (Table 1 ).
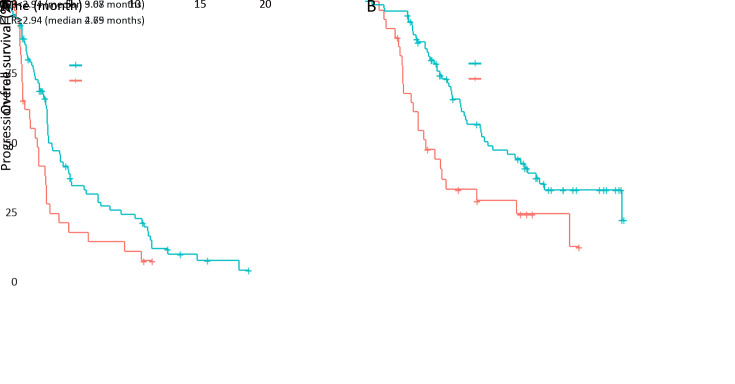
During a median follow-up duration of 12.1 months for surviving patients, the high NLR group had a significantly poorer PFS [median, 2.79 (95% CI: 1.71−3.52) months] than the low NLR group [median, 3.68 (95% CI: 3.55−5.29) months] (P=0.011;Figure 1A ). The high NLR group also had a significantly poorer OS [median, 4.64 (95% CI: 3.48−11.54) months] than the low NLR group [median, 9.07 (95% CI: 7.56−13.32) months] (P=0.002;Figure 1B ).
1.
Survival outcomes according to baseline neutrophil-to-lymphocyte ratio (NLR). (A) Progression-free survival (P=0.011); (B) overall survival (P=0.002).
We next performed univariate and multivariate analyses of PFS and OS using the prognostic factors previously identified in the second-line setting (Table 2 ). In the univariate analysis, an NLR≥2.94, TTP<6 months with first-line systemic therapy, and a hemoglobin concentration <10 g/dL were associated with poorer PFS [hazard ratio (HR)=1.77, 95% CI: 1.13−2.77, P=0.012; HR=2.11, 95% CI: 1.39−3.20, P<0.001 and HR=1.67, 95% CI: 1.05−2.67, P=0.031, respectively], while an NLR≥2.94, TTP <6 months with first-line therapy and a hemoglobin concentration <10 g/dL were associated with poorer OS (HR=2.18, 95% CI: 1.32−3.60, P=0.002; HR=2.21, 95% CI: 1.38−3.54, P=0.001 and HR=2.05, 95% CI: 1.19−3.53, P=0.010 respectively). Multivariate analyses revealed that both an NLR≥2.94 and TTP<6 months were independently associated with shorter PFS (HR=1.58, 95% CI: 1.01−2.49, P=0.046 and HR=1.99, 95% CI: 1.31−3.05, P=0.001, respectively) and OS (HR=1.77, 95% CI: 1.04−3.04, P=0.036 and HR=1.99, 95% CI: 1.20−3.29, P=0.008, respectively). Additionally, an ECOG performance status of 2 was independently associated with shorter OS (HR=1.56, 95% CI: 1.04−2.34, P=0.034). Notably, neither the mMDSC count nor the gMDSC count was associated with PFS and OS in either the univariate or multivariate analysis.
2. Factors associated with PFS and OS.
| Variables | PFS | OS | |||||||
| Univariate | Multivariate | Univariate | Multivariate | ||||||
| HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | ||
| PFS, progression-free survival; OS, overall survival; NLR, neutrophil-to-lymphocyte ratio; ECOG PS, Eastern Cooperative Oncology Group performance status; TTP, time-to-progression; gMDSC, granulocytic myeloid-derived suppressor cell; mMDSC, monocytic myeloid-derived suppressor cell; 95% CI, 95% confidence interval; HR, hazard ratio. | |||||||||
| NLR≥2.94 | 1.77 (1.13−2.77) | 0.012 | 1.58 (1.01−2.49) | 0.046 | 2.18 (1.32−3.60) | 0.002 | 1.77 (1.04−3.04) | 0.036 | |
| Age | 1.00 (0.98−1.02) | 0.903 | − | − | 0.99 (0.97−1.02) | 0.610 | 0.98 (0.96−1.01) | 0.136 | |
| Male gender | 0.93 (0.62−1.41) | 0.735 | − | − | 0.77 (0.48−1.23) | 0.278 | − | − | |
| ECOG PS 2 | 1.12 (0.76−1.65) | 0.558 | − | − | 1.46 (0.98−2.18) | 0.062 | 1.56 (1.04−2.34) | 0.034 | |
| First-line TTP<6 months | 2.11 (1.39−3.20) | <0.001 | 1.99 (1.31−3.05) | 0.001 | 2.21 (1.38−3.54) | 0.001 | 1.99 (1.20−3.29) | 0.008 | |
| Hemoglobin <10 g/dL | 1.67 (1.05−2.67) | 0.031 | − | − | 2.05 (1.19−3.53) | 0.010 | 1.74 (0.98−3.08) | 0.057 | |
| gMDSC count | 1.00 (0.99−1.00) | 0.705 | − | − | 1.00 (1.00−1.00) | 0.128 | − | − | |
| mMDSC count | 1.00 (0.99−1.00) | 0.367 | − | − | 1.00 (1.00−1.00) | 0.044 | − | − | |
Kinetics of MDSC counts and NLR after ramucirumab plus paclitaxel therapy
Uncertainty regarding the clinical value of the baseline mMDSC and gMDSC counts led us to further examine whether kinetics of MDSC counts following ramucirumab plus paclitaxel therapy would be associated with clinical outcomes. Although the mMDSC count did not change significantly (Figure 2A , left column), the gMDSC counts were significantly reduced after two cycles of ramucirumab plus paclitaxel therapy (Figure 2B , left column). These kinetic changes were further analyzed according to the occurrence of disease progression after two cycles of therapy. Again, the mMDSC count kinetics did not change significantly, regardless of disease progression (Figure 2B , right columns). In contrast, although the gMDSC count decreased significantly in patients who did not experience disease progression after two cycles of therapy, the gMDSC count tended to increase in patients with progressive disease after the second cycle (Figure 2B , right columns). NLR value also decreased significantly when taking the entire cohort into account (Figure 2C , left column); it also decreased among patients whose disease did not progress after two cycles of therapy (Figure 2C , right columns). However, NLR did not decrease among patients with early disease progression (Figure 2C , right columns). The similar patterns of change in gMDSC and NLR were supported by a positive correlation between gMDSC count and NLR at baseline and after two cycles of ramucirumab plus paclitaxel therapy (Supplementary Figure S2A ). Sensitivity analysis showed that correlation between NLR and gMDSC counts was still significant even after exclusion of some influence points (Supplementary Figure S2B ).
2.
Kinetics of myeloid-derived suppressor cell (MDSC) counts and neutrophil-to-lymphocyte ratio (NLR) after ramucirumab plus paclitaxel therapy. (A) Kinetics of monocytic MDSC (mMDSC) counts; (B) granulocyte MDSC (gMDSC) counts; (C) NLR. The left column indicates overall changes, while the right columns indicate changes in subgroups with and without disease progression after two cycles of chemotherapy (Post_C2, post cycle 2).
S2.
Correlation between NLR and gMDSC counts at baseline and after two cycles of ramucirumab plus paclitaxel therapy. (A) Full cohort; (B) After exclusion of some influence points. NLR, neutrophil-to-lymphocyte ratio; gMDSC, granulocyte myeloid-derived suppressor cell.
Clinical outcomes according to changes in gMDSCs
The opposing trends in gMDSC kinetics according to the occurrence of disease progression after cycle 2 led us to assume that an insufficient reduction or progressive increase in gMDSC count may be associated with the clinical outcomes. The percent changes in gMDSC counts are presented in Supplementary Figure S3 , and a gMDSC percent increase ≥44% was identified as a hotspot cut-off for a conceptual comparison. Clinical characteristics of patient subgroups subdivided according to this criterion were comparable (Supplementary Table S1 ). However, patients with gMDSC percentage increases ≥44% had a significantly shorter median PFS of 2.96 (95% CI: 2.00−4.50) months than those with gMDSC percentage increases <44% [median PFS of 5.16 (95% CI: 3.68−7.66) months] (P=0.001;Figure 3A ). Patients with gMDSC percentage increase ≥44% also had poorer OS of median 6.51 (95% CI: 4.70−9.73) months than those with gMDSC percentage increases <44% [median OS of 13.05 months (95% CI: 7.76−NA) months] (P=0.003;Figure 3B ).
S3.
Percent changes in gMDSC counts from baseline to after two cycles of ramucirumab plus paclitaxel therapy. gMDSC, granulocyte myeloid-derived suppressor cell.
S1. Clinical characteristics according to percent change of gMDSC count.
| Variables | gMDSC increase <44% (n=45) | gMDSC increase ≥44% (n=21) | P |
| gMDSC, granulocytic myeloid-derived suppressor cell; ECOG PS, Eastern Cooperative Oncology Group performance status; TTP, time-to-progression; NLR, neutrophil-to-lymphocyte ratio; LDH, lactate dehydrogenase. | |||
Age (year) (
 )
)
|
54.5±10.9 | 54.2±11.9 | 0.943 |
| Male gender [n (%)] | 30 (66.7) | 11 (52.3) | 0.535 |
| ECOG PS [n (%)] | 0.763 | ||
| 0−1 | 43 (95.6) | 19 (90.5) | |
| 2 | 2 (4.4) | 2 (9.5) | |
| TTP with first-line therapy (month) [median (IQR)] | 6.6 (4.7−10.9) | 5.7 (3.2−12.8) | 0.334 |
| NLR [median (IQR)] | 1.8 (1.3−2.7) | 1.9 (1.2−2.5) | 0.983 |
Hemoglobin (g/dL) (
 )
)
|
11.7±1.4 | 11.4±1.9 | 0.426 |
| LDH (IU/L) [median (IQR)] | 212.0 (182.5−251.5) | 194.0 (187.0−240.0) | 0.776 |
3.
Survival outcomes according to percent increase in granulocyte myeloid-derived suppressor cell (gMDSC) count following ramucirumab plus paclitaxel therapy. (A) Progression-free survival (P=0.001); (B) overall survival (P=0.003).
To exclude the possibility that a higher degree of myelosuppression reflected a reduced gMDSC count after ramucirumab plus paclitaxel therapy, we compared the survival outcomes of subgroups with absolute neutrophil counts (ANCs) ≥1,000/µL and ≥1,500/µL after cycle 2. Among patients with post-treatment ANCs ≥1,000/µL or ≥1,500/µL, those with a gMDSC percentage increase ≥44% also had shorter median PFS and OS durations (PFS, P<0.001 and OS, P=0.020; PFS, P=0.001 and OS, P=0.015;Supplementary Figure S4 , respectively).
S4.
Survival outcomes according to percent increase in gMDSC count following ramucirumab plus paclitaxel therapy. (A) ANC≥1,000/µL (PFS, P<0.001; OS, P=0.020) and (B) ANC≥1,500/µL (PFS, P=0.001; OS, P=0.015) after cycle 2 of ramucirumab plus paclitaxel therapy. gMDSC, granulocyte myeloid-derived suppressor cell; ANC, absolute neutrophil counts; PFS, progress-free survival; OS overall survival.
When the therapeutic response to ramucirumab plus paclitaxel was compared according to the gMDSC kinetics, patients with gMDSC percentage increases ≥44% had a higher proportion of progressive disease (30.7% vs. 0%, P=0.033 for measurable disease and 50% vs. 10%, P=0.009 for non-measurable disease; Table 3 ).
3. Response to ramucirumab plus paclitaxel according to gMDSC kinetics.
| Disease status | n (%) | P | |
| gMDSC
increase <44% |
gMDSC
increase ≥44% |
||
| gMDSC, granulocytic myeloid-derived suppressor cell; PR, partial response; SD, stable disease; PD, progressive disease; CR, complete remission. | |||
| Measurable disease | 0.033 | ||
| PR+SD | 24 (100) | 8 (66.7) | |
| PD | 0 (0) | 4 (33.3) | |
| Non-measurable disease | 0.009 | ||
| Non-CR/non-PD | 18 (90.0) | 4 (50.0) | |
| PD | 2 (10.0) | 4 (50.0) | |
Discussion
In this study, we investigated the clinical value of NLR and kinetics of MDSCs in patients with gastric cancer who received second-line ramucirumab plus paclitaxel treatment. Notably, NLR was independently associated with survival outcomes in our analysis. The overall gMDSC counts decreased after two cycles of ramucirumab plus paclitaxel therapy, but patients with progressive disease after cycle 2 tended to exhibit increasing gMDSC counts. We also found that a progressive increase in MDSC count was correlated with poor clinical outcomes. Our study appears to validate the prognostic value of baseline NLR in patients with gastric cancer who receive ramucirumab plus paclitaxel treatment. In addition, our study is the first to focus on the dynamic changes in MDSC populations and NLR of patients with gastric cancer in a therapeutic setting.
In our cohort, ramucirumab plus paclitaxel treatment yielded numerically shorter median PFS and OS durations (3.35 and 7.76 months, respectively) than those previously reported in the Expanded Access Program (17) (3.8 and 8.6 months, respectively) and the Rainbow trial (5) (4.4 and 9.6 months, respectively). When considered together with the higher percentage of patients with an ECOG performance status 2 in our study (6.9% vs. 3.1%) (17) and 0% (5), these decreases in survival may reflect the application of this regimen to a wider range of patients in routine practice.
The prognostic value of NLR was previously examined in patients with gastric cancer who received second-line paclitaxel (16) and ramucirumab with or without paclitaxel (17). However, the specific clinical value of NLR in patients receiving ramucirumab plus paclitaxel was not previously reported. In our study, we determined that along with a shorter TTP with first-line systemic therapy, a high NLR was independently associated with both PFS and OS in patients with gastric cancer who were treated with ramucirumab plus paclitaxel. Our results suggest that an “easily obtainable” baseline NLR may help guide clinical decisions in this clinical context. Notably, in addition to the prognostic implication of baseline NLR, we newly discovered that kinetic changes in NLR coincide with the patients’ clinical outcomes. The potential association of a high NLR with poor clinical outcomes in cancer patients may be explained by several mechanisms. Neutrophils, the core component of NLR value, can suppress T-cell function by producing arginase-1 and hydrogen peroxide (30,31). Circulating factors implicated in cancer progression, such as granulocyte colony-stimulating factor (32,33) and VEGF (20,24), were also shown to be associated with the NLR. Additionally, cancer patients with high NLRs may also have elevated MDSC counts (19,20,34). However, our data suggest that baseline MDSC count appears to have no additional prognostic value than the NLR, which discourages the use of MDSC count as a prognostic factor. The exact mechanism by which NLR is correlated with the clinical outcomes of patients with gastric cancer requires further elucidation.
Examination of kinetics of MDSC counts before and after ramucirumab plus paclitaxel therapy revealed an overall decrease in gMDSC counts after treatment. This is consistent with preclinical data that demonstrated the potential effects of ramucirumab and paclitaxel on the depletion of MDSCs or differentiation of these cells into dendritic cells (25-27). Notably, opposing trends in gMDSC kinetics were observed according to the occurrence of disease progression after two cycles of therapy, and similar patterns were observed in NLR kinetics. Together with the association between changes in gMDSC counts and clinical outcomes, these results suggest the potential implications of dynamic changes of MDSCs in this clinical setting. The observed differential clinical outcomes according to gMDSC changes are in line with previous studies that highlighted the clinical value of dynamic changes in immune subsets (35-37). However, one critical consideration is the remaining uncertainty regarding whether a progressive increase in gMDSCs directly contributes to tumor progression or is itself a bystander phenomenon driven by other crucial factors. Nevertheless, our data raise an important question regarding the delineation of critical factors that contribute to disease progression in association with the dynamic changes of gMDSC counts. Future studies should aim to validate the clinical relevance and elucidate the mechanism underlying our findings. In particular, the identification of baseline factors associated with differential immunomodulatory effects of ramucirumab plus paclitaxel treatment will be clinically important.
Reductions in the proportions of MDSCs after systemic chemotherapy regimens including bevacizumab or paclitaxel have been reported in other types of cancer. In patients with non-small cell lung cancer, bevacizumab-containing chemotherapy regimens reduced the proportion of gMDSCs, in contrast to non-bevacizumab regimens (38). Significant reductions in the MDSC counts were also reported in patients with breast cancer after treatment with paclitaxel (39). Limagne et al. also reported that a proportional decrease in gMDSC count was associated with favorable survival outcomes in colorectal cancer patients who received bevacizumab-based FOLFOX chemotherapy (40). However, when evaluating a reduction in the MDSC count, one should consider that variations in the degree of myelosuppression can be related to different MDSC counts after systemic chemotherapy, which was not addressed by these previous studies. To avoid this potential bias, we performed subgroup analyses of patients with preserved ANC counts and achieved consistent outcomes. Future studies that address changes in the MDSC counts should consider this aspect.
Conclusions
Our results suggest that NLR is an independent prognostic factor in patients with gastric cancer who are receiving second-line ramucirumab plus paclitaxel treatment. NLR may help guide clinical decisions in this clinical setting. The association between an increase in the gMDSC count and a poor clinical outcome raises an important issue regarding further investigations of mechanism and validation of clinical relevance of MDSC kinetics in gastric cancer patients.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Ferlay J, Soerjomataram I, Dikshit R, et al Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–86. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 2.Kang JH, Lee SI, Lim DH, et al Salvage chemotherapy for pretreated gastric cancer: a randomized phase III trial comparing chemotherapy plus best supportive care with best supportive care alone. J Clin Oncol. 2012;30:1513–8. doi: 10.1200/JCO.2011.39.4585. [DOI] [PubMed] [Google Scholar]
- 3.Ford HE, Marshall A, Bridgewater JA, et al Docetaxel versus active symptom control for refractory oesophagogastric adenocarcinoma (COUGAR-02): an open-label, phase 3 randomised controlled trial. Lancet Oncol. 2014;15:78–86. doi: 10.1016/S1470-2045(13)70549-7. [DOI] [PubMed] [Google Scholar]
- 4.Fuchs CS, Tomasek J, Yong CJ, et al Ramucirumab monotherapy for previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (REGARD): an international, randomised, multicentre, placebo-controlled, phase 3 trial. Lancet. 2014;383:31–9. doi: 10.1016/S0140-6736(13)61719-5. [DOI] [PubMed] [Google Scholar]
- 5.Wilke H, Muro K, Van Cutsem E, et al Ramucirumab plus paclitaxel versus placebo plus paclitaxel in patients with previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (RAINBOW): a double-blind, randomised phase 3 trial. Lancet Oncol. 2014;15:1224–35. doi: 10.1016/S1470-2045(14)70420-6. [DOI] [PubMed] [Google Scholar]
- 6.Van Cutsem E, Muro K, Cunningham D, et al Biomarker analyses of second-line ramucirumab in patients with advanced gastric cancer from RAINBOW, a global, randomized, double-blind, phase 3 study. Eur J Cancer. 2020;127:150–7. doi: 10.1016/j.ejca.2019.10.026. [DOI] [PubMed] [Google Scholar]
- 7.Grivennikov SI, Greten FR, Karin M Immunity, inflammation, and cancer. Cell. 2010;140:883–99. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miyamoto R, Inagawa S, Sano N, et al The neutrophil-to-lymphocyte ratio (NLR) predicts short-term and long-term outcomes in gastric cancer patients. Eur J Surg Oncol. 2018;44:607–12. doi: 10.1016/j.ejso.2018.02.003. [DOI] [PubMed] [Google Scholar]
- 9.Shimada H, Takiguchi N, Kainuma O, et al High preoperative neutrophil-lymphocyte ratio predicts poor survival in patients with gastric cancer. Gastric Cancer. 2010;13:170–6. doi: 10.1007/s10120-010-0554-3. [DOI] [PubMed] [Google Scholar]
- 10.Jung MR, Park YK, Jeong O, et al Elevated preoperative neutrophil to lymphocyte ratio predicts poor survival following resection in late stage gastric cancer. J Surg Oncol. 2011;104:504–10. doi: 10.1002/jso.21986. [DOI] [PubMed] [Google Scholar]
- 11.Wang F, Liu ZY, Xia YY, et al Changes in neutrophil/lymphocyte and platelet/lymphocyte ratios after chemotherapy correlate with chemotherapy response and prediction of prognosis in patients with unresectable gastric cancer. Oncol Lett. 2015;10:3411–8. doi: 10.3892/ol.2015.3783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee S, Oh SY, Kim SH, et al Prognostic significance of neutrophil lymphocyte ratio and platelet lymphocyte ratio in advanced gastric cancer patients treated with FOLFOX chemotherapy. BMC Cancer. 2013;13:350. doi: 10.1186/1471-2407-13-350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jeong JH, Lim SM, Yun JY, et al Comparison of two inflammation-based prognostic scores in patients with unresectable advanced gastric cancer. Oncology. 2012;83:292–9. doi: 10.1159/000342376. [DOI] [PubMed] [Google Scholar]
- 14.Cho IR, Park JC, Park CH, et al Pre-treatment neutrophil to lymphocyte ratio as a prognostic marker to predict chemotherapeutic response and survival outcomes in metastatic advanced gastric cancer. Gastric Cancer. 2014;17:703–10. doi: 10.1007/s10120-013-0330-2. [DOI] [PubMed] [Google Scholar]
- 15.Fanotto V, Cordio S, Pasquini G, et al Prognostic factors in 868 advanced gastric cancer patients treated with second-line chemotherapy in the real world. Gastric Cancer. 2017;20:825–33. doi: 10.1007/s10120-016-0681-6. [DOI] [PubMed] [Google Scholar]
- 16.Ryu MH, Kim J, Oh S, et al Neutrophil-lymphocyte ratio (NLR) as an important prognostic factor for paclitaxel as a second line chemotherapy in advanced gastric cancer (AGC): Results from phase III DREAM study. Ann Oncol. 2018;29:viii232. [Google Scholar]
- 17.Jung M, Ryu MH, Oh DY, et al Efficacy and tolerability of ramucirumab monotherapy or in combination with paclitaxel in gastric cancer patients from the Expanded Access Program Cohort by the Korean Cancer Study Group (KCSG) Gastric Cancer. 2018;21:819–30. doi: 10.1007/s10120-018-0806-1. [DOI] [PubMed] [Google Scholar]
- 18.Fleming V, Hu X, Weber R, et al Targeting myeloid-derived suppressor cells to bypass tumor-induced immunosuppression. Front Immunol. 2018;9:398. doi: 10.3389/fimmu.2018.00398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ohki S, Shibata M, Gonda K, et al Circulating myeloid-derived suppressor cells are increased and correlate to immune suppression, inflammation and hypoproteinemia in patients with cancer. Oncol Rep. 2012;28:453–8. doi: 10.3892/or.2012.1812. [DOI] [PubMed] [Google Scholar]
- 20.Nakamura I, Shibata M, Gonda K, et al Serum levels of vascular endothelial growth factor are increased and correlate with malnutrition, immunosuppression involving MDSCs and systemic inflammation in patients with cancer of the digestive system. Oncol Lett. 2013;5:1682–6. doi: 10.3892/ol.2013.1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang L, Chang EW, Wong SC, et al Increased myeloid-derived suppressor cells in gastric cancer correlate with cancer stage and plasma S100A8/A9 proinflammatory proteins. J Immunol. 2013;190:794–804. doi: 10.4049/jimmunol.1202088. [DOI] [PubMed] [Google Scholar]
- 22.Mao FY, Zhao YL, Lv YP, et al CD45+CD33lowCD11bdim myeloid-derived suppressor cells suppress CD8+ T cell activity via the IL-6/IL-8-arginase I axis in human gastric cancer . Cell Death Dis. 2018;9:763. doi: 10.1038/s41419-018-0803-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang Y, Chen X, Dikov MM, et al Distinct roles of VEGFR-1 and VEGFR-2 in the aberrant hematopoiesis associated with elevated levels of VEGF. Blood. 2007;110:624–31. doi: 10.1182/blood-2007-01-065714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Horikawa N, Abiko K, Matsumura N, et al Expression of vascular endothelial growth factor in ovarian cancer inhibits tumor immunity through the accumulation of myeloid-derived suppressor cells. Clin Cancer Res. 2017;23:587–99. doi: 10.1158/1078-0432.CCR-16-0387. [DOI] [PubMed] [Google Scholar]
- 25.Yang J, Yan J, Liu B Targeting VEGF/VEGFR to modulate antitumor immunity. Front Immunol. 2018;9:978. doi: 10.3389/fimmu.2018.00978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sevko A, Michels T, Vrohlings M, et al Antitumor effect of paclitaxel is mediated by inhibition of myeloid-derived suppressor cells and chronic inflammation in the spontaneous melanoma model. J Immunol. 2013;190:2464–71. doi: 10.4049/jimmunol.1202781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Michels T, Shurin GV, Naiditch H, et al Paclitaxel promotes differentiation of myeloid-derived suppressor cells into dendritic cells in vitro in a TLR4-independent manner . J Immunotoxicol. 2012;9:292–300. doi: 10.3109/1547691X.2011.642418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Catalano V, Graziano F, Santini D, et al Second-line chemotherapy for patients with advanced gastric cancer: who may benefit? Br J Cancer. 2008;99:1402–7. doi: 10.1038/sj.bjc.6604732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kanagavel D, Pokataev IA, Fedyanin MY, et al A prognostic model in patients treated for metastatic gastric cancer with second-line chemotherapy. Ann Oncol. 2010;21:1779–85. doi: 10.1093/annonc/mdq032. [DOI] [PubMed] [Google Scholar]
- 30.Schmielau J, Finn OJ Activated granulocytes and granulocyte-derived hydrogen peroxide are the underlying mechanism of suppression of t-cell function in advanced cancer patients. Cancer Res. 2001;61:4756–60. [PubMed] [Google Scholar]
- 31.Bronte V, Zanovello P Regulation of immune responses by L-arginine metabolism. Nat Rev Immunol. 2005;5:641–54. doi: 10.1038/nri1668. [DOI] [PubMed] [Google Scholar]
- 32.Mouchemore KA, Anderson RL, Hamilton JA Neutrophils, G-CSF and their contribution to breast cancer metastasis. FEBS J. 2018;285:665–79. doi: 10.1111/febs.14206. [DOI] [PubMed] [Google Scholar]
- 33.Coffelt SB, Kersten K, Doornebal CW, et al IL-17-producing γδ T cells and neutrophils conspire to promote breast cancer metastasis. Nature. 2015;522:345–8. doi: 10.1038/nature14282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Riemann D, Cwikowski M, Turzer S, et al Blood immune cell biomarkers in lung cancer. Clin Exp Immunol. 2019;195:179–89. doi: 10.1111/cei.13219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim KH, Cho J, Ku BM, et al The first-week proliferative response of peripheral blood PD-1+CD8+ T cells predicts the response to anti-PD-1 therapy in solid tumors . Clin Cancer Res. 2019;25:2144–54. doi: 10.1158/1078-0432.CCR-18-1449. [DOI] [PubMed] [Google Scholar]
- 36.Huang AC, Postow MA, Orlowski RJ, et al T-cell invigoration to tumour burden ratio associated with anti-PD-1 response. Nature. 2017;545:60–5. doi: 10.1038/nature22079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zappasodi R, Budhu S, Hellmann MD, et al Non-conventional inhibitory CD4+Foxp3-PD-1hi T cells as a biomarker of immune checkpoint blockade activity . Cancer Cell. 2018;33:1017–32. doi: 10.1016/j.ccell.2018.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Koinis F, Vetsika EK, Aggouraki D, et al Effect of first-line treatment on myeloid-derived suppressor cells’ subpopulations in the peripheral blood of patients with non-small cell lung cancer. J Thorac Oncol. 2016;11:1263–72. doi: 10.1016/j.jtho.2016.04.026. [DOI] [PubMed] [Google Scholar]
- 39.Diaz-Montero CM, Salem ML, Nishimura MI, et al Increased circulating myeloid-derived suppressor cells correlate with clinical cancer stage, metastatic tumor burden, and doxorubicin-cyclophosphamide chemotherapy. Cancer Immunol Immunother. 2009;58:49–59. doi: 10.1007/s00262-008-0523-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Limagne E, Euvrard R, Thibaudin M, et al Accumulation of MDSC and Th17 cells in patients with metastatic colorectal cancer predicts the efficacy of a FOLFOX-bevacizumab drug treatment regimen. Cancer Res. 2016;76:5241–52. doi: 10.1158/0008-5472.CAN-15-3164. [DOI] [PubMed] [Google Scholar]