Abstract
Objective
The transanal approach to specimen collection, combined with the prolapsing technique, is a well-established and minimally invasive surgery for treating rectal cancer. However, reports on outcomes for this approach are sparse. We compared short- and long-term outcomes of conventional laparoscopic surgery (CLS) vs. transanal natural orifice specimen extraction (NOSE) using the prolapsing technique for patients with middle- to low-rectal cancer.
Methods
From January 2013 to December 2017, we enrolled consecutive patients with middle- to low-rectal cancer undergoing laparoscopic anterior resection. Totally, 50 patients who underwent transanal NOSE using the prolapsing technique were matched with 50 patients who received CLS. Clinical parameters and survival outcomes between the two groups were compared.
Results
Estimated blood loss (29.70±29.28 vs. 52.80±45.09 mL, P=0.003), time to first flatus (2.50±0.79 vs. 2.86±0.76, P=0.022), time to liquid diet (3.62±0.64 vs. 4.20±0.76 d, P<0.001), and the need for analgesics (22%vs. 48%, P=0.006) were significantly lower for the NOSE group compared to the CLS group. The incidences of overall complications and fecal incontinence were comparable in both groups. After a median follow-up of 44.52 months, the overall local recurrence rate (6% vs. 5%, P=0.670), 3-year disease-free survival (86.7% vs. 88.0%, P=0.945) and 3-year overall survival (95.6% vs. 96.0%, P=0.708), were not significantly different.
Conclusions
For total laparoscopic rectal resection, transanal NOSE using the prolapsing technique is effective and safe, and associated with less trauma and pain, a faster recovery, and similar survival outcomes compared to CLS.
Keywords: Natural orifice specimen extraction, transanal specimen extraction, rectal cancer, prolapsing technique, survival
Introduction
Laparoscopic surgery is increasingly employed during colorectal cancer treatment. Several randomized controlled trials demonstrated that laparoscopic colorectal cancer resection resulted in reduced blood loss, improved bowel function recovery, shorter hospitalization, and similar long-term survival outcomes without increased perioperative morbidity and mortality when compared with open surgery (1-4). However, current laparoscopic techniques still require a mini-laparotomy to extract specimen and construct bowel anastomosis. This mini-laparotomy may cause postoperative pain, cosmetic problems, and wound-related complications (5,6). The use of laparoscopic low-anterior resection for the treatment of rectal cancer also poses other challenges. These challenges include determining appropriate distal transection line and constructing intracorporeal anastomosis, which may result in positive surgical margins and a high rate of abdominal perineal resection (7-9).
To resolve the problems mentioned above, a novel laparoscopic technique was developed. This technique combines a total intracorporeal anastomosis with natural orifice specimen extraction (NOSE) through the anus or vagina (10,11). This approach has been widely practiced in recent years because it eliminates the abdominal incision, resulting in less pain, better cosmesis, and decreased rate of wound-related complications, when compared with conventional laparoscopic surgery (CLS) (12-14). Moreover, several studies reported that total laparoscopic surgery with NOSE resulted in comparable long-term outcomes to CLS (15-17). NOSE, combined with the prolapsing technique, resolves issues related to selecting an appropriate distal transection line (18-20). This technique can accurately identify distal margin, which may contribute to organ preservation for patients with low-rectal cancer. However, reports on outcomes for this approach are sparse, especially compared with CLS. Therefore, we designed a case-matched study to evaluate short- and long-term outcomes of CLS vs. transanal NOSE using the prolapsing technique for patients with middle- to low-rectal cancer.
Materials and methods
Patient cohort
Data were retrospectively collected from consecutive patients with middle- to low-rectal cancer who underwent laparoscopic anterior resection from January 2013 to December 2017. Patients with the following characteristics were included: clinically staged as T1−3NxM0; a distance of 4−7 cm between the tumor and anal verge; tumor size ≤5 cm; and a body mass index (BMI) ≤30 kg/m2. Patients with distant metastases, multiple primary tumors, those who underwent emergency surgery due to obstruction, bleeding, or perforation, and those who underwent abdominal perineal resection were excluded. Totally, 50 patients who underwent transanal NOSE with the prolapsing technique were identified and assigned to the NOSE group. These 50 cases were then matched with 50 patients who underwent CLS based on age, sex, BMI, surgery date, preoperative chemoradiotherapy, and pathological stage (Figure 1 ). Before surgery, all patients underwent the same assessments, including physical examinations, blood tests, colonoscopy to confirm the pathology, and rectal magnetic resonance imaging (MRI) to determine the local staging. Patients were excluded if pulmonary and abdominopelvic contrast-enhanced computed tomography (CT) scans showed distant metastases. The experienced colorectal surgical team completed curative R0 resections, adhering to the total mesorectal excision (TME) principle. Tumor stage was evaluated according to the American Joint Committee on Cancer (AJCC, seventh edition) staging system. Neoadjuvant chemoradiotherapy was given before curative surgery in patients with clinical stage II and III tumors. Patients diagnosed with pathological stages II and III received adjuvant chemoradiotherapy if they did not receive preoperative chemoradiotherapy. All enrolled patients gave written or oral consent to participate in the study. The Institutional Review Board Committee of the Cancer Hospital at the Chinese Academy of Medical Sciences approved this study (Approval No. 18-015/1617).
1.
Flowchart of patients enrolled in this study. BMI, body mass index; NOSE, transanal natural orifice specimen extraction; CLS, conventional laparoscopic surgery.
Surgery
With the patient in a modified lithotomy position, five trocars (2 mm × 12 mm and 3 mm × 0.5 mm) were placed; one trocar was positioned supraumbilical for the camera; while two trocars were positioned on the right and left quadrants, respectively. A pneumoperitoneum was maintained at 12−15 mmHg. After abdominal exploration, the patient was moved to the Trendelenburg position for full exposure of the pelvic cavity.
Standard techniques were performed laparoscopically in both groups, including high ligation of the inferior mesenteric vessel, medial-to-lateral bowel mobilization, lymph node dissection, sharp pelvic dissection with nerve protection, and division of the distal rectum. Subsequently, the NOSE and CLS groups were subjected to different procedures. In the NOSE group, after adequate mobilization and division of the sigmoid colon and distal rectum, the main challenge associated with intracorporeal anastomosis was how to place the anvil head into the proximal bowel lumen. Here, we introduced the NOSE approach for digestive tract reconstruction. Briefly, after gentle dilation of the anus (three to four finger widths), a sterilized plastic sleeve was introduced into the rectum. The sleeve was usually taken to the distal sigmoid colon. An anvil head was inserted into the proximal sigmoid colon via the sleeve (Figure 2A ), and transection of the sigmoid colon was achieved with a 60 mm linear stapler (Figure 2B ) while the anvil head was in the sigmoid colon. The remaining stump was sterilized by povidone gauze. Then, a grasping forceps, inserted through the anus, was used to grab the rectal stump and gently everted the distal rectum extracorporeally (Figure 2C ). The reverse rectal specimen was washed with 1−2 liters of saline mixed with povidone-iodine. Then, the distal rectum was transected using a curved cutter stapler (Figure 2D ), and the specimen was extracted. Rectal and pelvic irrigation was performed again using the cytotoxic solution. After removing the anvil head from the proximal sigmoid colon (Figure 2E ), a circular stapler was utilized to construct the end-to-end anastomosis (Figure 2F ).
2.
Surgical procedure. (A) Anvil was introduced into bowel lumen through anus; (B) Sigmoid colon was transected using endoscopic linear cutter staplers; (C) Distal rectum was extracted from anus; (D) Distal rectum was transected using curved cutter stapler; (E) Anvil head was extracted from sigmoid colon; (F) An end-to-end anastomosis was performed.
In the CLS group, a 4−7 cm long mini-laparotomy was created to transect the proximal sigmoid colon and the distal rectum for the end-to-end colorectal anastomosis. Generally, protective stomas are made for patients with diabetes, or for those who received neoadjuvant therapies, or those with anastomoses within 2 cm of the dentate line.
Follow-up
Patients’ follow-up was conducted quarterly for 2 years, bi-annually for the next 3 years, then annually for the remainder of the study, as described previously (21). A clinical history, physical exam, blood test (including carcinoembryonic antigen), chest and abdomen CT scans, and a pelvis MRI were completed at each follow-up. A colonoscopy was performed in the first year after surgery. If no advanced adenoma was found, colonoscopies were performed at 3 years, then every 5 years. The deadline for the follow-up was March 1, 2020. At the last follow-up, the Wexner score was collected, which has become a widely used for assessment of severity of fecal incontinence (22).
Statistical analysis
Consecutive patients from the NOSE and CLS groups were matched in a 1:1 ratio using 20% of the standard deviation of the propensity score. Matching conditions were mentioned above. Continuous variables were described as
 , and categorical variables were presented as number (frequency) and compared by Chi-square or Fisher’s exact tests. T-tests and Mann-Whitney U tests for normally and non-normally distributed values, respectively, were utilized to compare continuous data. Disease-free survival (DFS) was defined as the time between the date of surgery and the first tumor recurrence (local or distant metastases), and overall survival (OS) was defined as the time between the date of surgery and the time of death or last follow-up. Local recurrence (LR) rate, DFS and OS rates were calculated using Kaplan-Meier plots. Log-rank tests were utilized to compare LR, DFS, and OS rates. IBM SPSS software (Version 25.0; IBM Corp., New York, USA) was used for all analyses. P<0.05 were deemed statistically significant.
, and categorical variables were presented as number (frequency) and compared by Chi-square or Fisher’s exact tests. T-tests and Mann-Whitney U tests for normally and non-normally distributed values, respectively, were utilized to compare continuous data. Disease-free survival (DFS) was defined as the time between the date of surgery and the first tumor recurrence (local or distant metastases), and overall survival (OS) was defined as the time between the date of surgery and the time of death or last follow-up. Local recurrence (LR) rate, DFS and OS rates were calculated using Kaplan-Meier plots. Log-rank tests were utilized to compare LR, DFS, and OS rates. IBM SPSS software (Version 25.0; IBM Corp., New York, USA) was used for all analyses. P<0.05 were deemed statistically significant.
Results
Baseline characteristics
Consecutive patients with middle- to low-rectal cancer undergoing laparoscopic anterior resection were enrolled between January 2013 and December 2017. A total of 100 patients were identified in this study, and 50 patients from each group were matched. The baseline clinicopathological characteristics of the two groups including age, sex, BMI, American Association of Anesthesiologists (ASA) classification, neoadjuvant therapy, history of abdominal surgery, adjuvant therapy, and pathological tumor stage were not significantly different; however, the tumor to anal verge distance in the NOSE group was significantly shorter compared that in the CLS group, as summarized in Table 1 .
1. Patient characteristics.
| Variables | n (%) | P | |
| NOSE (N=50) | CLS (N=50) | ||
| BMI, body mass index; ASA, American Society of Anesthesiologists; NOSE, natural orifice specimen extraction; CLS, conventional laparoscopic surgery. | |||
Age (year) (
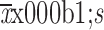 )
)
|
56.94±10.70 | 54.10±9.80 | 0.169 |
| Sex | 0.391 | ||
| Male | 32 (64) | 36 (72) | |
| Female | 18 (36) | 14 (28) | |
BMI (kg/m2) (
 )
)
|
23.90±3.20 | 24.66±2.59 | 0.197 |
| ASA classification | 0.182 | ||
| I | 3 (6) | 7 (14) | |
| II | 47 (94) | 43 (86) | |
| Neoadjuvant therapy | 0.218 | ||
| No | 46 (92) | 42 (84) | |
| Yes | 4 (8) | 8 (16) | |
| History of abdominal surgery | 0.790 | ||
| No | 42 (84) | 41 (82) | |
| Yes | 8 (16) | 9 (18) | |
| Adjuvant therapy | 0.315 | ||
| No | 30 (60) | 25 (50) | |
| Yes | 20 (40) | 25 (50) | |
| Pathological TNM stage | 0.528 | ||
| I | 25 (50) | 24 (48) | |
| II | 6 (12) | 10 (20) | |
| III | 19 (38) | 16 (32) | |
Distance between tumor and the anal verge (cm) (
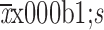 )
)
|
5.26±0.99 | 5.66±0.87 | 0.034 |
Postoperative outcomes
As shown in Table 2 , mean operative times were similar in the two groups (NOSE 162.24±43.26 vs. CLS 145.86±49.97 min, P=0.083). The mean estimated blood loss of the NOSE group was significantly reduced compared with the CLS group (29.70±29.28 vs. 52.80±45.09 mL, P=0.003). All study patients underwent the planned surgery, with no transition to open surgery. Neither group experienced intraoperative complications. Time to first flatus, defecation, and liquid diet, and the postoperative hospitalization period were shorter in the NOSE group compared to the CLS group. However, differences were not statistically significant, except for time to first flatus and liquid diet. Although the visual analog scale score was not prospectively recorded, patients in NOSE group required less additional postoperative analgesia (22% vs. 48%, P=0.006) in comparison to those in CLS group. Furthermore, the NOSE group required fewer analgesia administrations.
2. Operative and postoperative outcomes.
| Variables |

|
P | |
| NOSE (N=50) | CLS (N=50) | ||
| *, 35 patients are available for analysis; NOSE, transanal natural orifice specimen extraction; CLS, conventional laparoscopic surgery. | |||
| Operation time (min) | 162.24±43.26 | 145.86±49.97 | 0.083 |
| Estimated blood loss (mL) | 29.70±29.28 | 52.80±45.09 | 0.003 |
| Conversion to open surgery | 0 | 0 | − |
| Time to first flatus (d) | 2.50±0.79 | 2.86±0.76 | 0.022 |
| Time to first defecation (d) | 3.28±0.78 | 3.56±0.81 | 0.082 |
| Time to liquid diet (d) | 3.62±0.64 | 4.20±0.76 | <0.001 |
| Postoperative hospitalization (d) | 9.14±4.48 | 9.52±4.32 | 0.667 |
| Analgesia requirement [n (%)] | 0.006 | ||
| No | 39 (78) | 26 (52) | |
| Yes | 11 (22) | 24 (48) | |
| Times of analgesia requirement* | 1.73±0.91 | 4.58±1.44 | <0.001 |
| Protective stoma [n (%)] | 0.836 | ||
| No | 32 (64) | 31 (62) | |
| Yes | 18 (36) | 19 (38) | |
| Postoperative complications [n (%)] | 4 (8) | 7 (14) | 0.324 |
| Anastomotic leakage | 3 | 3 | |
| Anastomotic stenosis | 0 | 0 | |
| Anastomotic bleeding | 0 | 0 | |
| Ileus | 1 | 1 | |
| Rectovaginal fistula | 0 | 1 | |
| Pelvic abscess | 0 | 1 | |
| Urinary retention | 0 | 1 | |
| Wound infection | 0 | 0 | |
Postoperative complications were encountered in four patients in NOSE group and seven patients in CLS group. Three patients in NOSE group developed anastomotic leakage; two were treated with a palliative stoma and one with conservative therapy. One patient in the NOSE group had an intestinal obstruction that required gastrointestinal decompression along with cessation of oral intake. No patients developed complications related to the prolapsing technique, such as anal avulsion or bleeding. In the CLS group, three patients developed anastomotic leakage, and one developed a rectovaginal fistula; all were treated using a palliative stoma. Additional postoperative complications in the CLS group included a pelvic abscess, urinary retention, and ileus; all had a healthy recovery without surgery. No surgery-related deaths occurred.
Pathological outcomes are summarized in Table 3 . No significant differences were detected in tumor size, pathological type, differentiation, pathological T and N category, or perineural and vascular invasion. Although the resected bowel length in the NOSE group was shorter than that in the CLS group (P=0.011), the harvested lymph node quantity was similar in both groups. The distal resection margin (DRM) in the NOSE group was smaller than that in the CLS group (0.94±0.56 vs. 1.28±0.83 cm, P=0.019). Fast frozen pathology was performed in cases with DRM <1 cm. No patients in the NOSE group had a positive distal margin. This indicated that the distal margin could be accurately identified using the prolapsing technique.
3. Pathological outcomes.
| Variables | n (%) | P | |
| NOSE (N=50) | CLS (N=50) | ||
| NOSE, transanal natural orifice specimen extraction; CLS, conventional laparoscopic surgery. | |||
Tumor size (cm) (
 )
)
|
3.29±1.47 | 3.75±1.05 | 0.074 |
Length of bowel resection (cm) (
 )
)
|
13.32±3.94 | 15.49±4.38 | 0.011 |
Distal resection margin (cm) (
 )
)
|
0.94±0.56 | 1.28±0.83 | 0.019 |
| Pathological type | 0.646 | ||
| Adenocarcinoma | 47 (94) | 48 (96) | |
| Mucinous adenocarcinoma | 3 (6) | 2 (4) | |
| Tumor differentiation | 0.461 | ||
| Well | 3 (6) | 5 (10) | |
| Moderate and poor | 47 (94) | 45 (90) | |
| Pathological T stage | 0.151 | ||
| T1−2 | 34 (68) | 27 (54) | |
| T3−4 | 16 (32) | 23 (46) | |
| Pathological N stage | 0.529 | ||
| N0 | 31 (62) | 34 (68) | |
| N1−2 | 19 (38) | 16 (32) | |
Number of lymph node harvested (
 )
)
|
20.54±8.28 | 20.02±6.84 | 0.733 |
| Perineural invasion | 0.134 | ||
| No | 43 (86) | 37 (74) | |
| Yes | 7 (14) | 13 (26) | |
| Vascular invasion | 0.220 | ||
| No | 42 (84) | 37 (74) | |
| Yes | 8 (16) | 13 (26) | |
Long-term outcomes
The median follow-up length was 44.52 months. During the follow-up period, eight patients in the NOSE group developed recurrences, including three lung metastases, three intraluminal local recurrences, one liver metastasis, and one local lymph node metastasis. Seventy-five percent (6/8) of recurrences occurred within 36 months after the initial surgery. Two patients in the NOSE group died because of tumor recurrence, and one for another reason. In the CLS group, eight patients developed recurrences, including three lung metastases, two liver metastases, one intraluminal local recurrence, one local lymph node metastasis, and one concomitant local recurrence and liver metastasis. Seventy-five percent (6/8) of recurrences occurred within 36 months after the initial surgery. Three patients in the CLS group died due to tumor recurrences, and one for another reason. No patients developed recurrences related to the incision site or extraction site in either group. The details of patients with tumor recurrences are summarized in Table 4 . The LR rate was comparable in both groups (6% vs. 5%, P=0.670; Figure 3A ). Three-year DFS and OS rates were similar for the NOSE and CLS groups (DFS: 86.7% vs. 88.0%, P=0.945; Figure 3B ; OS: 95.6% vs. 96.0%, P=0.708; Figure 3C ).
4. Clinicopathological details of patients with tumor recurrence.
| Case | Group | Gender | Age (year) | Stage | Time of recurrence
(month) |
Site of recurrence | Treatment after recurrence | Outcome |
| NOSE, transanal natural orifice specimen extraction; CLS, conventional laparoscopic surgery. | ||||||||
| 1 | NOSE | Male | 71 | T2N0M0 | 6.47 | Local | Surgery | Alive |
| 2 | NOSE | Male | 58 | T2N0M0 | 23.27 | Lung | Surgery | Alive |
| 3 | NOSE | Male | 40 | T3N2M0 | 23.83 | Lung | Chemotherapy | Dead |
| 4 | NOSE | Male | 42 | T2N0M0 | 24.77 | Local lymph node | Radiotherapy | Alive |
| 5 | NOSE | Male | 65 | T3N1M0 | 34.97 | Liver | Chemotherapy | Alive |
| 6 | NOSE | Female | 52 | T3N1M0 | 35.30 | Lung | Surgery | Alive |
| 7 | NOSE | Male | 71 | T3N1M0 | 44.00 | Local | Radiotherapy | Dead |
| 8 | NOSE | Female | 46 | T2N1M0 | 48.87 | Local | Surgery | Alive |
| 9 | CLS | Male | 61 | T4aN2M0 | 6.83 | Local and liver | Chemotherapy | Dead |
| 10 | CLS | Male | 44 | T3N2M0 | 13.23 | Lung | Chemotherapy | Dead |
| 11 | CLS | Male | 59 | T3N2M0 | 13.87 | Liver | Radiofrequency ablation | Alive |
| 12 | CLS | Female | 34 | T3N2M0 | 15.23 | Lung | Radiofrequency ablation | Alive |
| 13 | CLS | Male | 64 | T3N2M0 | 22.23 | Liver | Chemotherapy | Dead |
| 14 | CLS | Male | 42 | T2N0M0 | 25.30 | Local lymph node | Radiotherapy | Alive |
| 15 | CLS | Female | 54 | T3N2M0 | 42.87 | Lung | Radiofrequency ablation | Alive |
| 16 | CLS | Male | 49 | T3N2M0 | 63.33 | Local | Surgery | Alive |
3.
Survival outcomes. (A) Overall local recurrence rate in NOSE group and CLS group (P=0.670); (B) Three-year DFS rate in NOSE group and CLS group (P=0.945); (C) Three-year OS rate in NOSE group and CLS group (P=0.708). NOSE, transanal natural orifice specimen extraction; CLS, conventional laparoscopic surgery; DFS, disease-free survival; OS, overall survival.
Wexner score was collected at the last follow-up, and data were available for 38 patients in the NOSE group and 36 patients in the CLS group. Patients with a stoma (n=7), or who were dead (n=7), or who refused to answer the questionnaire (n=12) were excluded from the analysis. In both groups, 21 (55.3%) patients had no incontinence (0 point). Each group had 14 (36.8%) patients with minor incontinence (1−9 points). Three (7.9%) patients in the NOSE group and one patient in the CLS group (2.8%) experienced severe incontinence (10−19 points). There were no significant between-group differences in postoperative complications (P=0.633) (Figure 4 ).
4.
Wexner score in NOSE group and CLS group (P=0.633). NOSE, transanal natural orifice specimen extraction; CLS, conventional laparoscopic surgery.
Discussion
We successfully performed total laparoscopic anterior resection using the prolapsing technique for rectal cancer with transanal specimen extraction and demonstrated the effectiveness and safety of this technique. Compared with the CLS group, patients in the NOSE group experienced less estimated blood loss and pain, and a faster recovery. Meanwhile, there was no increase in complications associated with the NOSE procedure, thus demonstrating the superiority of this approach. More importantly, long-term survival outcomes and bowel function were similar for the two groups.
Laparoscopic surgery has been extensively used in recent years for treating colorectal cancer. However, CLS still requires an abdominal incision for specimen extraction and anastomosis, and this 4−7 cm incision is associated with some complications. The NOSE technique was developed to eliminate the need for an abdominal incision. This allowed the intraoperative abdominal operation and anastomosis procedures to be performed laparoscopically. The specimen was extracted through a natural orifice such as the vagina or anus. Two prospective randomized clinical trials, which compared the short-term outcomes in NOSEvs. CLS groups, were recently published. These trials demonstrated that the NOSE group experienced less pain, required less analgesia, and experienced lower wound infection rates compared to the CLS group; additionally, perioperative morbidity did not increase (12,14). Also, several retrospective studies suggested that patients who underwent NOSE recovered intestinal function faster and experienced better cosmetic outcomes than patients treated with CLS (13,16). More importantly, recent studies have indicated that the long-term survival outcomes, including LR, DFS and OS, were comparable between the NOSE and CLS groups (15,17).
Previous studies have demonstrated that NOSE was effective and safe for use in patients with colorectal cancer. However, this technique might not be suitable for patients whose lesions are located in the lower rectum, because of the difficulty in determining appropriate distal transection and cutting the distal rectum. To resolve this problem, NOSE combined with the prolapsing technique was developed. Several studies suggested that transanal specimen extraction using the prolapsing technique for total laparoscopic rectal resection was effective and safe in selected patients (18-20). However, these studies had some limitations, such as small cohort sizes and no comparison with CLS, for determining clinical outcomes and long-term survival.
In the short-term, patients in the NOSE group experienced less blood loss, required less analgesia, and experienced quicker intestinal function recovery. Compared to the CLS group, there was no change in the mean operative time or in the incidence of perioperative complications. Potential reasons for the superior short-term outcomes include the absence of an abdominal incision. This might reduce both blood loss and the need for analgesics during the intraoperative period. Meanwhile, earlier ambulation, due to less incision pain, might promote bowel functions. Total laparoscopic surgery also prevents the intraabdominal organs from contacting the external environment; thus, the internal environment is less disturbed, contributing to the faster recovery of intestinal function.
This study compared long-term survival outcomes between the NOSE and CLS groups. The major concern surrounding this prolapsing technique is the fear of tumor cell exfoliation and implantation. Although three patients in the NOSE group developed local recurrences, no patients experienced recurrences related to the extraction site or multifocal pelvic sidewall recurrences (23). During the NOSE procedure, the oncological principle was not changed; meanwhile, irrigation of the pelvic cavity and distal rectum after bowel eversion could further decrease the potential risk of tumor spillage. However, we still found that the pathological stage of the tumor recurrence cases in the NOSE group was lower than that in the CLS group. There were several reasons for this situation. First, the sample size of this study was small, and the retrospective cohort might cause selection bias. Second, the follow-up time was not enough, and the longer survival outcomes remained to be investigated. Third, our first experience to perform NOSE might inevitably cause tumor cell exfoliation or implantation, and these three cases with stage T2N0M0 who experienced tumor recurrences in the NOSE group all occurred in 2013 and 2014, although the oncological principle was not changed during the NOSE procedure. On the whole, the LR rate with 6% in the NOSE group was comparable to the historical LR rates after conventional TME or transanal TME (3,24), and large and prospective randomized clinical trials should be conducted to further confirm the safety of NOSE.
The DRM in the NOSE group was shorter, and the mean DRM was <1 cm, possibly due to the closer distance between the tumor and the anal verge afforded by the NOSE procedure. For patients where the DRM was <1 cm, fast-frozen pathology was performed, and no patients had a positive distal margin. During the follow-up period, five patients developed local recurrences, and DRM <1 cm was detected in two cases; more intriguingly, these two local recurrences occurred three years later after initial excision. Hence, a shorter distal resection negative margin is not likely to elevate the LR rate; on the contrary, the distal margin could be accurately identified during the prolapsing procedure.
Given the fact that the rectum is everted, there may be concerns related to bowel function. In this study, we found that the Wexner score was similar in both groups. Although four patients experienced severe incontinence, receiving radiotherapy (n=3) and having a tumor located in lower rectum (n=1) might explain the observed episodes of fecal incontinence. Careful case selection is an essential prerequisite to performing this technique. Patients with large tumors or obesity were excluded from the NOSE group; therefore, this approach was successfully completed in all 50 patients, without conversion to open surgery.
Another concern is the potential risk of contamination in the process of intracorporeal bowel opening. Human and animal studies have found increased peritoneal contamination during the NOSE procedure; however, this did not translate into increased infectious complications (25,26). The prolapsing procedure could decrease the risk of intracorporeal contamination because the distal rectum was transected extracorporeally. Also, repeated pelvic irritation could contribute to a lower risk of infectious morbidity.
This study has several limitations. First, although we used the matching method to compare the clinical outcomes between the two groups, the analysis was limited by its retrospective nature and selection bias. Second, although we provided the Wexner score at the last follow-up, we did not collect the Wexner form at baseline. Also, we only evaluated the fecal continence score, and subjective data such as urgency and objective data such as manometric pressures, and sonographic anal sphincter appearance were not provided. To address this, we designed a prospective randomized clinical trial to evaluate postoperative health-related quality of life in the CLS group vs. the NOSE group (ChiCTR1900026970). Finally, the sample size of this study was small, which may limit the statistical power.
Conclusions
Our results demonstrate the effectiveness and safety of transanal NOSE using the prolapsing technique compared to CLS in patients with middle- to low-rectal cancer. However, large and prospective randomized clinical trials should be conducted to confirm our conclusions.
Acknowledgements
This work was supported by National Key R&D Program of China (No. 2017YFC0908203), Chinese Academy of Medical Sciences Initiative for Innovative Medicine (No. 2017-I2M-2-003 and 2016-I2M-1-001), Science and Technology Project of Chaoyang District, Beijing (No. CYSF-1931), Beijing Science and Technology Program (No. D17110002617004) and Beijing Gold-Bridge Funds (No. ZZ19055).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Contributor Information
Zhaoxu Zheng, Email: zzx_20003@126.com.
Xishan Wang, Email: wxshan1208@126.com.
References
- 1.The Colon Cancer Laparoscopic or Open Resection Study Group Survival after laparoscopic surgery versus open surgery for colon cancer: long-term outcome of a randomised clinical trial. Lancet Oncol. 2009;10:44–52. doi: 10.1016/S1470-2045(08)70310-3. [DOI] [PubMed] [Google Scholar]
- 2.Jeong SY, Park JW, Nam BH, et al Open versus laparoscopic surgery for mid-rectal or low-rectal cancer after neoadjuvant chemoradiotherapy (COREAN trial): survival outcomes of an open-label, non-inferiority, randomised controlled trial. Lancet Oncol. 2014;15:767–74. doi: 10.1016/S1470-2045(14)70205-0. [DOI] [PubMed] [Google Scholar]
- 3.Bonjer HJ, Deijen CL, Abis GA, et al A randomized trial of laparoscopic versus open surgery for rectal cancer. N Engl J Med. 2015;372:1324–32. doi: 10.1056/NEJMoa1414882. [DOI] [PubMed] [Google Scholar]
- 4.Simillis C, Lal N, Thoukididou SN, et al Open versus laparoscopic versus robotic versus transanal mesorectal excision for rectal cancer: A systematic review and network meta-analysis. Ann Surg. 2019;270:59–68. doi: 10.1097/SLA.0000000000003227. [DOI] [PubMed] [Google Scholar]
- 5.Romy S, Eisenring MC, Bettschart V, et al Laparoscope use and surgical site infections in digestive surgery. Ann Surg. 2008;247:627–32. doi: 10.1097/SLA.0b013e3181638609. [DOI] [PubMed] [Google Scholar]
- 6.Benlice C, Stocchi L, Costedio MM, et al Impact of the specific extraction-site location on the risk of incisional hernia after laparoscopic colorectal resection. Dis Colon Rectum. 2016;59:743–50. doi: 10.1097/DCR.0000000000000632. [DOI] [PubMed] [Google Scholar]
- 7.Scheidbach H, Schneider C, Konradt J, et al Laparoscopic abdominoperineal resection and anterior resection with curative intent for carcinoma of the rectum. Surg Endosc. 2002;16:7–13. doi: 10.1007/s00464-001-8314-4. [DOI] [PubMed] [Google Scholar]
- 8.Laurent C, Leblanc F, Gineste C, et al Laparoscopic approach in surgical treatment of rectal cancer. Br J Surg. 2007;94:1555–61. doi: 10.1002/bjs.5884. [DOI] [PubMed] [Google Scholar]
- 9.Ito M, Sugito M, Kobayashi A, et al Relationship between multiple numbers of stapler firings during rectal division and anastomotic leakage after laparoscopic rectal resection. Int J Colorectal Dis. 2008;23:703–7. doi: 10.1007/s00384-008-0470-8. [DOI] [PubMed] [Google Scholar]
- 10.Akamatsu H, Omori T, Oyama T, et al Totally laparoscopic sigmoid colectomy: a simple and safe technique for intracorporeal anastomosis. Surg Endosc. 2009;23:2605–9. doi: 10.1007/s00464-009-0406-6. [DOI] [PubMed] [Google Scholar]
- 11.McKenzie S, Baek JH, Wakabayashi M, et al Totally laparoscopic right colectomy with transvaginal specimen extraction: the authors’ initial institutional experience. Surg Endosc. 2010;24:2048–52. doi: 10.1007/s00464-009-0870-z. [DOI] [PubMed] [Google Scholar]
- 12.Leung AL, Cheung HY, Fok BK, et al Prospective randomized trial of hybrid NOTES colectomy versus conventional laparoscopic colectomy for left-sided colonic tumors. World J Surg. 2013;37:2678–82. doi: 10.1007/s00268-013-2163-x. [DOI] [PubMed] [Google Scholar]
- 13.Awad ZT, Griffin R Laparoscopic right hemicolectomy: a comparison of natural orifice versus transabdominal specimen extraction. Surg Endosc. 2014;28:2871–6. doi: 10.1007/s00464-014-3540-8. [DOI] [PubMed] [Google Scholar]
- 14.Wolthuis AM, Fieuws S, Van Den Bosch A, et al Randomized clinical trial of laparoscopic colectomy with or without natural-orifice specimen extraction. Br J Surg. 2015;102:630–7. doi: 10.1002/bjs.9757. [DOI] [PubMed] [Google Scholar]
- 15.Park JS, Kang H, Park SY, et al Long-term outcomes after Natural Orifice Specimen Extraction versus conventional laparoscopy-assisted surgery for rectal cancer: a matched case-control study. Ann Surg Treat Res. 2018;94:26–35. doi: 10.4174/astr.2018.94.1.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen C, Chen H, Yang M, et al Laparoscopy-assisted natural orifice specimen extraction to treat tumors of the sigmoid colon and rectum: The short- and long-term outcomes of a retrospective study. J Laparoendosc Adv Surg Tech A. 2019;29:801–8. doi: 10.1089/lap.2018.0601. [DOI] [PubMed] [Google Scholar]
- 17.Zhou S, Wang X, Zhao C, et al Comparison of short-term and survival outcomes for transanal natural orifice specimen extraction with conventional mini-laparotomy after laparoscopic anterior resection for colorectal cancer. Cancer Manag Res. 2019;11:5939–48. doi: 10.2147/CMAR.S209194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Akamatsu H, Omori T, Oyama T, et al Totally laparoscopic low anterior resection for lower rectal cancer: combination of a new technique for intracorporeal anastomosis with prolapsing technique. Dig Surg. 2009;26:446–50. doi: 10.1159/000239761. [DOI] [PubMed] [Google Scholar]
- 19.Endo S, Takehara Y, Tanaka J, et al Complete laparoscopic surgery for early colorectal cancer after endoscopic resection. Asian J Endosc Surg. 2013;6:338–41. doi: 10.1111/ases.12045. [DOI] [PubMed] [Google Scholar]
- 20.Katsuno G, Fukunaga M, Nagakari K, et al Natural orifice specimen extraction using prolapsing technique in single-incision laparoscopic colorectal resections for colorectal cancers. Asian J Endosc Surg. 2014;7:85–8. doi: 10.1111/ases.12063. [DOI] [PubMed] [Google Scholar]
- 21.Lu Z, Cheng P, Yang F, et al Long-term outcomes in patients with ypT0 rectal cancer after neoadjuvant chemoradiotherapy and curative resection. Chin J Cancer Res. 2018;30:272–81. doi: 10.21147/j.issn.1000-9604.2018.02.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jorge JM, Wexner SD Etiology and management of fecal incontinence. Dis Colon Rectum. 1993;36:77–97. doi: 10.1007/BF02050307. [DOI] [PubMed] [Google Scholar]
- 23.Larsen SG, Pfeffer F, Kørner H, on behalf of the Norwegian Colorectal Cancer Group Norwegian moratorium on transanal total mesorectal excision. Br J Surg. 2019;106:1120–1. doi: 10.1002/bjs.11287. [DOI] [PubMed] [Google Scholar]
- 24.Kang L, Chen YG, Zhang H, et al Transanal total mesorectal excision for rectal cancer: a multicentric cohort study. Gastroenterol Rep (Oxf) 2020;8:36–41. doi: 10.1093/gastro/goz049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Costantino FA, Diana M, Wall J, et al Prospective evaluation of peritoneal fluid contamination following transabdominal vs. transanal specimen extraction in laparoscopic left-sided colorectal resections . Surg Endosc. 2012;26:1495–500. doi: 10.1007/s00464-011-2066-6. [DOI] [PubMed] [Google Scholar]
- 26.Senft JD, Dröscher T, Gath P, et al Inflammatory response and peritoneal contamination after transrectal natural orifice specimen extraction (NOSE) versus mini-laparotomy: a porcine in vivo study . Surg Endosc. 2018;32:1336–43. doi: 10.1007/s00464-017-5811-7. [DOI] [PubMed] [Google Scholar]






