Abstract
Background
The purpose of this study is to evaluate the use of the tourniquet and its effect on post-operative pain in the paediatric population following lower leg procedures.
Methods
A retrospective study of paediatric patients (under the age of 18) undergoing inpatient orthopaedic procedure below the knee performed at a single academic institution between 1st December 2013 and 31st January 2019 was conducted. Primary outcome measures of total opioid consumption during hospital stay and pre-operative nerve block utilization were retrieved from the electronic medical record (EMR). Secondary outcome measures of blood loss, tourniquet time, procedure time and length of hospital stay were also retrieved. Student’s t-tests were used to assess statistical significance between two sample means.
Results
The final analysis included 204 paediatric procedures, 118 of which used a tourniquet and 86 of which did not. Paediatric patients with a tourniquet had significantly more opioid consumption post-operatively in the form of weight-based morphine equivalents/length of stay (p = 0.01) compared to those who had no tourniquet. This held true for males (p = 0.049) and females (p = 0.04) respectively. We did not see an increase in wound complications or return trips to the operating room in the tourniquet cohort. All procedures included an osseous component except one procedure in the non-tourniquet group.
Conclusion
Minimizing opioid consumption may be achieved by avoiding tourniquet use in paediatric patients with lower leg procedures. In non-anaemic paediatric patients, it is reasonably risk-free to perform these surgeries without the use of tourniquet to decrease opioid dependence in the post-operative period.
Level of evidence
III
Keywords: tourniquet, pain, lower limb, opioid
Introduction
The use of tourniquets in orthopaedic surgery is common practice. Their use in total knee arthroplasty (TKA),1-4 hand surgery,5-8 and adult foot and ankle surgery9-12 has been studied in terms of blood loss, post-operative pain and wound complications. While tourniquet use has been studied in other fields within orthopaedics, there is a paucity of data regarding their use in the paediatric population, particularly as it pertains to paediatric foot and ankle surgery. The purpose of this study was to evaluate the use of tourniquets in the paediatric orthopaedic population undergoing foot and ankle procedures. Our aim was to evaluate the impact of tourniquet use on operative time, blood loss and pain in the immediate post-operative period, as well as to identify any potential complications.
Materials and methods
Study sample
A retrospective study of 204 paediatric patients (under the age of 18 years) undergoing inpatient orthopaedic procedures below the knee performed at a single academic institution between 1st December 2013 and 31st January 2019 was conducted with institutional review board approval. Inclusion criteria included unilateral tibia, ankle, midfoot, hindfoot and forefoot procedures. The procedures included open reduction internal fixation, intramedullary nailing, arthrodesis, osteotomy and tendon transfers. Exclusion criteria included adult patients (over the age of 18 years), bilateral procedures, tumour cases and outpatient procedures.
Measurements
Patient age in years, sex, height, weight, body mass index (BMI), comorbidities including Spinal Muscular Atrophy, adolescent idiopathic scoliosis, spina bifida, Down syndrome and cerebral palsy were recorded. Other variables including procedure type, attending surgeon, pre-operative nerve block use (yes/no and type), length of hospital stay in days, narcotic use, procedure time in minutes, tourniquet use (yes/no), tourniquet time in minutes, post-operative blood loss determined by total drainage in mL, need for blood transfusion (yes/no and amount transfused in mL), most recent follow-up date and need for re-surgery (yes/no, reason and date) were retrieved form the electronic medical record (EMR). Complications were defined as post-operative bleeding, wound complications, infection, non-union, use of wrong implant or incorrect implant positioning.
Outcome measures included blood loss, tourniquet time, procedure time, peri-operative nerve block utilization, length of hospital stay (LOS) and total opioid consumption during hospital stay. Opioid consumption was defined as opioids administered beginning from the patient’s operative day throughout their hospital stay. For those with a LOS of zero, a denominator of one was used, for those with a stay of one night was divided by two (for two days in the hospital). Procedure length was calculated using incision time and incision closing time. Tourniquet time was calculated using inflation and deflation time. Narcotics consumed were recorded and converted into weight-based morphine milligram equivalents (WBME) using previously published methodology.13 Most recent follow-up was defined as the date of last follow-up clinic visit with an orthopaedic provider in regard to the recorded procedure. Procedure descriptions were recorded and then categorized as osseous versus non, trauma versus non and location was documented.
Data points were extracted from the EMR without alteration and with blinding using study numbers. Patients were assigned study numbers after data extraction for blinding of statistical analysis.
Statistical analysis
Student’s t-tests were used to assess statistical significance between two sample means. Levene’s tests were conducted to determine if the two groups had equal variance. Welch’s t-tests with unequal variances were used to analyse two sample means with unequal variance. Lastly, Chi-square (χ 2) test with Fisher exact tests were used to compare categorical variables. Results were considered significant when p values were less than 0.05.
Results
Demographics and operative results
In total, 204 patients were screened and included in the study, 118 in the tourniquet group and 86 in non (Fig. 1). Both groups are well matched in regard to age, BMI and procedure type (Fig. 2) and location (Fig. 3). In all, 97% and 90% of procedures were osseous in nature in tourniquet and non-tourniquet group, respectively. Mean age at time of procedure was 13 years, 14 years in the tourniquet group and 12.9 years in non-tourniquet (p = 0.59). Similarly, median BMI in kg/m2 was not statistically different (22.8 in tourniquet group versus 22.4 in non, p = 0.66) (Tables 1 to 3). Procedure time was significantly higher in no tourniquet cases in both males (p = 0.002) and females (p = 0.005). Mean blood loss was significantly higher in non-tourniquet groups in males and females (p < 0.0007), although in both groups the mean difference in blood loss was less than 110 mL. Mean length of stay (LOS) was not statistically significant between tourniquet and no tourniquet groups (1.43 days versus 1.95 days, p = 0.1). There were two patients who required blood transfusions with a tourniquet (2%) and six without the use of tourniquet (7%) (Table 4).
Fig. 1.
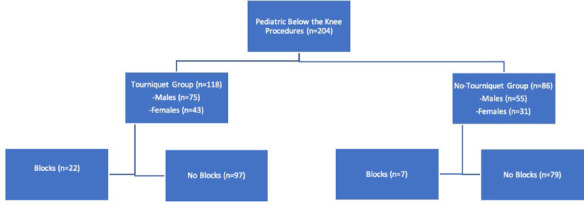
Flowchart of study subject.
Fig. 2.
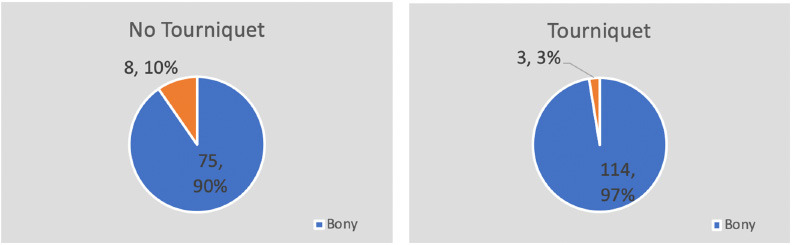
Procedure type in tourniquet and non-tourniquet groups. Procedures were categorized as osseous if there was any osseous component.
Fig. 3.
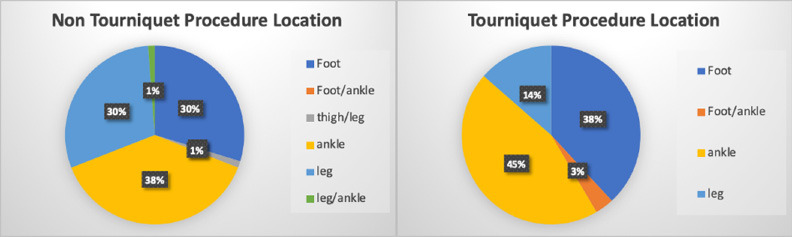
Procedure location in tourniquet and no tourniquet groups.
Table 1.
Summary of results
| Total | ||||
|---|---|---|---|---|
| Tourniquet | No tourniquet | Statistic | p value* | |
| N | 118 | 86 | ||
| Mean age (range) | 13.14 (0.13–17.5) | 12.90 (5–17) | t = 0.54 | 0.59 |
| Mean BMI (range) | 22.8 (12–39) | 22.4 (14–39) | t = 0.45 | 0.66 |
| Mean length of stay (range, days) | 1.43 (0–19) | 1.95 (0–12) | t = 1.68 | 0.1 |
| Mean morphine equivalents (range) | 105.53 (0–1233.9) | 56.52 (0–1148.5) | t = 1.03 | 0.3 |
| Mean weight-based morphine (range) | 1.45 (0–11.40) | 0.95 (0–9.94) | t = 1.37 | 0.17 |
| Mean WBME/LOS (range) | 0.44 (0–1.61) | 0.24 (0–0.98) | t = 2.6 | 0.01 |
| Mean procedure time (range, min.) | 124.8 (23–318) | 210.5 (19–565) | t = 4.14 | 0.00002 |
| Mean tourniquet time (range, mL) | 75.91 (7–195) | N/A | N/A | N/A |
| Mean EBL (range, mL) | 24.10 (0–350) | 117.85(0–800) | t = 5.27 | 0.00000008 |
| Blood transfusions | 2/118 (0.02) | 6/86 (0.07) | X 2 = 3.68 | 0.054 |
Students T test
BMI, body mass index; EBL, estimated blood loss; LOS, length of stay; WBME, weight-based morphine milligram equivalent
Table 3.
Female specific results
| Female | ||||
|---|---|---|---|---|
| Tourniquet | No tourniquet | Statistic | p value* | |
| N | 44 | 31 | ||
| Mean age (range, years) | 13.0 (0.13–17) | 11.82 (4–16) | t = 1.57 | 0.12 |
| Mean BMI (range) | 23.5 (15–39) | 20.1 (14–34) | t = 2.34 | 0.02 |
| Mean length of stay (range, days) | 1.5 (0–19) | 1.81 (0–12) | t = 1.73 | 0.09 |
| Mean morphine equivalents (range) | 169.04 (0–1130.8) | 27.16 (0–137.5) | t = 1.75 | 0.09 |
| Mean weight-based morphine (range) | 2.20 (0–11.40) | 0.70 (0–4.81) | t= =.67 | 0.1 |
| Mean WBME/LOS (range) | 0.59 (0.33–0.71) | 0.21 (0–0.98) | t = 2.1 | 0.04 |
| Mean procedure time (range, min.) | 122.8 (23–518) | 218.2 (19–537) | t = 2.29 | 0.005 |
| Mean tourniquet time (range, min.) | 82.83 (22–180) | N/A | N/A | N/A |
| Mean EBL (range, mL) | 14.83 (0–200) | 123.10 (0–600) | t =.74 | 0.0007 |
Students T test
BMI, body mass index; EBL, estimated blood loss ; LOS, length of stay; WBME, weight-based morphine milligram equivalent
Table 4.
Surgical complications
| Complications | ||
|---|---|---|
| Tourniquet | No tourniquet | |
| n = 118 | n = 86 | |
| Infection/wound complications | 2% (2/118) | 1% (1/86) |
| Bleeding | 1% (1/118) | 0% (0/86) |
| Total | 3% (3/118) | 1% (1/86) |
There were more males in both the tourniquet (64%) and non-tourniquet groups (64%). Subsequently, males and females were analysed separately and together (Tables 2 and 3).
Table 2.
Male specific results
| Male | ||||
|---|---|---|---|---|
| Tourniquet | No tourniquet | Statistic | p value* | |
| N | 74 | 55 | ||
| Mean age (range, years) | 13.22 (1–18) | 13.49 (6–17) | t = 0.53 | 0.6 |
| Mean BMI (range) | 22.4 (12–37) | 23.7 (14–39) | t = 1.14 | 0.26 |
| Mean length of stay (range, days) | 1.4 (0–14) | 2.04 (0–9) | t = 1.54 | 0.13 |
| Mean morphine equivalents (range) | 77.4 (0.24–1233.9) | 73.68 (0–1148.5) | t = 0.14 | 0.89 |
| Mean weight-based morphine (range) | 1.14 (0.01-9.36) | 1.05 (0–9.94) | t = 0.29 | 0.78 |
| Mean WBME/LOS (range) | 0.38 (0.002–1.61) | 0.24 (0–0.83) | t = 2.0 | 0.049 |
| Mean procedure time (range, min.) | 126.1 (35–392) | 205.88 (125–565) | t = 3.29 | 0.002 |
| Mean tourniquet time (range, min.) | 71.96 (7–195) | N/A | N/A | N/A |
| Mean EBL (range, mL) | 29.36 (0–350) | 114.85 (0–800) | t = 3.77 | 0.0004 |
Students T test
BMI, body mass index; EBL, estimated blood loss; LOS, length of stay; WBME, weight-based morphine milligram equivalent
Post-operative pain and length of stay
Administered post-operative morphine milligram equivalents was calculated using a previously published method.14 Morphine milligram equivalents was then divided by weight (kg) to derive WBME (mg/kg). Lastly, this value was divided by LOS to calculate WBME/LOS. This value was statistically significant in males alone (p = 0.049), females alone (p = 0.04) and in total (p = 0.01) (Fig. 4). Additionally, there were 22 (19%) pre-operative regional pain blocks in the tourniquet group and seven (8%) in the non-tourniquet group (Fig. 5). Of the 29 blocks, 22 were popliteal blocks, one caudal, two saphenous and two femoral. At the most recent follow-up (315 days in tourniquet, 316 days in no tourniquet), complications were reported in three of the tourniquet patients (3%) and one in the non-tourniquet (1%) (Table 4). Two patients in the tourniquet group returned to the operating room due to infection (surface osteomyelitis and pin tract infection). One returned for post-operative bleeding. In the non-tourniquet group, one patient returned for drainage of an abscess. In trauma cases, patients in the tourniquet group had significantly more WBME/LOS (p = 0.032) (Table 5). This held true for foot and ankle osseous procedures analysed alone (p = 0.046) (Table 6), and in patients aged between 12 and 18 years (p = 0.005) (Table 7). WBME/LOS was significantly greater in tourniquet patients with LOS of zero, one to two, and more than two (Table 8).
Fig. 4.
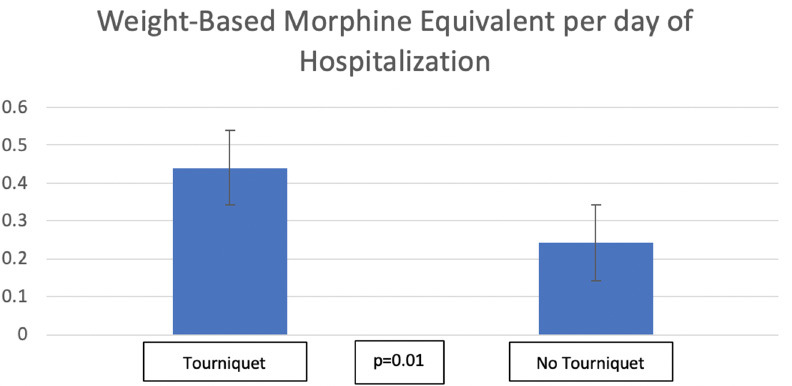
Weight-based morphine equivalents/length of stay.
Fig. 5.
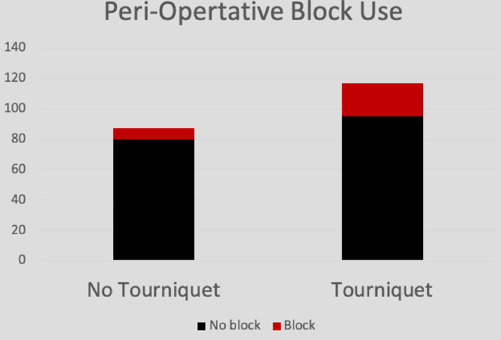
Peri-operative block usage in tourniquet and no tourniquet groups.
Table 5.
Trauma versus non-trauma procedures
| Trauma: | ||||
|---|---|---|---|---|
| LOS | Morphine equiv. | WBME/LOS | Blocks | |
| Tourniquet (n = 57) |
1.5 | 109.06 | 0.51 | 17.5% |
| No tourniquet (n = 33) |
1.8 | 79.35 | 0.25 | 0% |
| p value* | 0.684 | 0.309 | 0.032** | |
| Non-trauma: | ||||
| LOS | Morphine equiv. | WBME/LOS | Blocks | |
| Tourniquet (n = 61) |
1.4 | 91.71 | 0.31 | 19.7% |
| No tourniquet (n = 53) |
2.4 | 48.02 | 0.25 | 13.2% |
| p value* | 0.037** | 0.230 | 0.140 | |
Students T test
denotes statistical significance
LOS, length of stay; WBME, weight-based morphine milligram equivalent
Table 6.
Foot and ankle boney procedures
| LOS | Morphine equiv. | ME/LOS | Blocks | |
|---|---|---|---|---|
| Tourniquet | 0.8 | 40.92 | 0.35 | |
| (n = 84) | 23.8% | |||
| No tourniquet | 1.1 | 32.60 | 0.21 | |
| (n = 33) | 12.1% | |||
| p value* | 0.251 | 0.208 | 0.046** |
Students T test
denotes statistical significance
LOS, length of stay; ME, morphine equivalents
Table 7.
Summary of results by age
| Age 0 to 1 | n | LOS | ME | WBME | WBME/LOS |
|---|---|---|---|---|---|
| Tourniquet | 3 | 0.33 | 0.90 | 0.06 | 0.04 |
| No tourniquet | 0 | NA | NA | NA | NA |
| p value* | NA | NA | NA | NA | |
| 1 to 5 | |||||
| Tourniquet | 2 | 0.50 | 7.98 | 0.53 | 0.26 |
| No tourniquet | 1 | 2.00 | 8.98 | 0.73 | 0.24 |
| p value* | NA | NA | NA | NA | |
| 5 to 12 | |||||
| Tourniquet | 28 | 9.92 | 0.79 | 14.38 | 0.62 |
| No tourniquet | 23 | 1.97 | 31.20 | 0.81 | 0.21 |
| p value* | 0.01** | 0.06 | 0.26 | 0.20 | |
| 12 to 18 | |||||
| Tourniquet | 85 | 1.94 | 172.96 | 2.13 | 0.56 |
| No tourniquet | 60 | 2.11 | 67.33 | 0.99 | 0.26 |
| p value* | 0.39 | 0.03** | 0.03** | 0.005**x |
Students T test
denotes statistical significance
LOS, length of stay, ME,morphine equivalents; WBME, weight-based morphine milligram equivalent
Table 8.
Summary of results by length of hospital stay
| Tourniquet | ME | WBME | WBME/LOS |
|---|---|---|---|
| LOS of 0 | 23.22 | 0.38 | 0.38 |
| LOS of 1–2 | 41.22 | 0.89 | 0.39 |
| LOS of > 2 | 440.88 | 5.24 | 0.81 |
| No tourniquet | ME | WBME | WBME/LOS |
| LOS of 0 | 5.37 | 0.08 | 0.08 |
| LOS of 1–2 | 33.01 | 0.62 | 0.26 |
| LOS of > 2 | 144.29 | 2.34 | 0.36 |
LOS, length of stay, ME, morphine equivalents; WBME, weight-based morphine milligram equivalent
Discussion
The findings in this study demonstrate that lower extremity tourniquet use in the paediatric population is associated with lower blood loss and lower operative time, but requires a higher daily opioid requirement during the inpatient stay. This is despite a higher percentage of patients with tourniquet also receiving peri-operative anaesthetic blocks. These findings agree with previous studies in the adult arthroplasty literature, where less pain and a better early range of motion was found in cases performed without the use of a tourniquet.1-3 Our results also agree with similar findings in the adult foot and ankle literature as well where post-operative pain was more difficult to control following ankle fracture fixation in those patients whom underwent surgery with the use of a tourniquet .11 A sub-group analysis of the females in our cohort demonstrated a three-fold increase in the mean WBME consumed in the tourniquet group, agreeing with a recent study that demonstrated females with a tourniquet post-TKA reported significantly more pain and required more opioids within the first 24 hours after surgery than females without tourniquet use.14 However, this finding only trended towards significance in our study. Tourniquet-related pain is a common early post-operative complaint likely due to a combination of neuromuscular injury, ischemic effects in the nerve or muscle, disturbances in nerve conduction and slowly resolving axonal compression syndrome as a result of tourniquet use.3,16-20
As expected, our results demonstrated differences in blood loss and operative time with tourniquet use, falling in line with several other studies.2,4,9 However they differ from findings in the context of ankle arthroscopy, where no difference was found in length of procedure, maximum intraoperative fluid pressure or visibility when comparing those undergoing the procedure with or without a tourniquet.10 Unlike previous studies in the adult literature where tourniquet use has been correlated with increased wound complications as well,12 we did not see an increase in wound problems or return trips to the operating room in the tourniquet cohort.
Weaknesses of the current study include its retrospective nature, varied heterogeneity of the surgical procedures and reliance on opioid use alone rather than validated pain scores to assess post-operative pain. Unfortunately, these scores were inconsistently recorded over the study period. As opposed to the high volume and often standard approach used in primary TKA, high volumes of identical procedures are rarely performed in most paediatric centres. Often the paediatric surgical practice is diverse, making single centre direct comparisons of identical procedures difficult. While this confounding factor cannot be completely overlooked, the fact that opioid use was still greater in those receiving regional anaesthesia suggests that the tourniquet and not potential differences in the procedures is the cause for the differences in morphine equivalents found. In the tourniquet group three children had cognitive delay (two autism, one cerebral palsy) and 13 in the non-tourniquet group (four autism, nine cerebral palsy). With such a low number of children with neurological delay (16 in total, only one trauma procedure in a child with autism), we did not think it would be beneficial to analyse them separately. In these children, nursing staff assessed pain using the Faces pain scale and recorded pain scores every four hours. Parental input was utilized in these children for pain management. The authors feel that this study provides the justification for a more rigorous multicentre prospective comparison, which would allow for procedures to be matched, regional anaesthesia to be standardized and validated pain scores to be recorded in a standardized fashion.
Given the recent opioid epidemic, decreasing opioid use in orthopaedic patients is paramount. In 2015, the American Academy of Orthopaedic Surgeons (AAOS) released a statement detailing strategies for safer and more effective pain management and treatment: ‘The AAOS believes that a comprehensive opioid program is necessary to decrease opioid use, misuse, and abuse in the United States. New, effective education programs for physicians, caregivers, and patients; improvements in physician monitoring of opioid prescription use; increased research funding for effective alternative pain management and coping strategies; and support for more effective opioid abuse treatment programs are needed.’ While the surgeon must balance many factors when performing a surgery, these data suggest that patients may have a lower opioid requirement if the use of a tourniquet can be safely avoided. However, as our data shows, this may increase the operative time, blood loss and risk of transfusion, thus it is up to each surgeon to assess the risks and benefits of tourniquet use in each of their patients.
Open access
This article is distributed under the terms of the Creative Commons Attribution-Non Commercial 4.0 International (CC BY-NC 4.0) licence (https://creativecommons.org/licenses/by-nc/4.0/) which permits non-commercial use, reproduction and distribution of the work without further permission provided the original work is attributed.
Compliance with Ethical Standards
Funding Statement
No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
Ethical Statement
Ethical approval: This article does not contain any studies with human and/or animal subjects performed by the any of the authors. Institutional Review Board/Ethics committee approval IRB#2018-0059.
Informed consent: Informed consent was not obtained because it was waived by the IRB for this study.
ICMJE Conflict of Interest Statement
None declared.
Author Contributions
RBH: Performed measurements and statistical analysis, Manuscript preparation, Design, data acquisition and analysis, Interpretation of data.
MN: Study design, Manuscript preparation, Design, Analysis and interpretation of data.
PJL: Conceived and designed analysis, Manuscript preparation, Design, Interpretation of data.
MH: Conceived study and study design, Manuscript preparation, Design, data acquisition and analysis, Interpretation of data.
References
- 1. Liu D, Graham D, Gillies K, Gillies RM. Effects of tourniquet use on quadriceps function and pain in total knee arthroplasty. Knee Surg Relat Res 2014;26:207-213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ledin H, Aspenberg P, Good L. Tourniquet use in total knee replacement does not improve fixation, but appears to reduce final range of motion. Acta Orthop 2012;83:499-503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ejaz A, Laursen AC, Kappel A, et al. Faster recovery without the use of a tourniquet in total knee arthroplasty. Acta Orthop 2014;85:422-426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Aglietti P, Baldini A, Vena LM, Abbate R, Fedi S, Falciani M. Effect of tourniquet use on activation of coagulation in total knee replacement. Clin Orthop Relat Res 2000:169-177. [DOI] [PubMed]
- 5. Tzarnas CD. Carpal tunnel release without a tourniquet. J Hand Surg Am 1993;18:1041-1043. [DOI] [PubMed] [Google Scholar]
- 6. Nitz AJ, Dobner JJ. Upper extremity tourniquet effects in carpal tunnel release. J Hand Surg Am 1989;14:499-504. [DOI] [PubMed] [Google Scholar]
- 7. Odinsson A, Finsen V. Tourniquet use and its complications in Norway. J Bone Joint Surg [Br] 2006;88:1090-1092. [DOI] [PubMed] [Google Scholar]
- 8. Hutchinson DT, McClinton MA. Upper extremity tourniquet tolerance. J Hand Surg Am 1993;18:206-210. [DOI] [PubMed] [Google Scholar]
- 9. Michelson JD, Perry M. Clinical safety and efficacy of calf tourniquets. Foot Ankle Int 1996;17:573-575. [DOI] [PubMed] [Google Scholar]
- 10. Zaidi R, Hasan K, Sharma A, Cullen N, Singh D, Goldberg A. Ankle arthroscopy: a study of tourniquet versus no tourniquet. Foot Ankle Int 2014;35:478-482. [DOI] [PubMed] [Google Scholar]
- 11. Omeroğlu H, Günel U, Biçimoğlu A, Tabak AY, Uçaner A, Güney O. The relationship between the use of tourniquet and the intensity of postoperative pain in surgically treated malleolar fractures. Foot Ankle Int 1997;18:798-802. [DOI] [PubMed] [Google Scholar]
- 12. Wiewiorski M, Barg A, Hoerterer H, Voellmy T, Henninger HB, Valderrabano V. Risk factors for wound complications in patients after elective orthopedic foot and ankle surgery. Foot Ankle Int 2015;36:479-487. [DOI] [PubMed] [Google Scholar]
- 13. Bagsby DT, Ireland PH, Meneghini RM. Liposomal bupivacaine versus traditional periarticular injection for pain control after total knee arthroplasty. J Arthroplasty 2014;29:1687-1690. [DOI] [PubMed] [Google Scholar]
- 14. Kheir MM, Ziemba-Davis M, Dilley JE, Hood MJ Jr, Meneghini RM. Tourniquetless total knee arthroplasty with modern perioperative protocols decreases pain and opioid consumption in women. J Arthroplasty 2018;33:3455-3459. [DOI] [PubMed] [Google Scholar]
- 15. Kumar N, Yadav C, Singh S, Kumar A, Vaithlingam A, Yadav S. Evaluation of pain in bilateral total knee replacement with and without tourniquet; a prospective randomized control trial. J Clin Orthop Trauma 2015;6:85-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zhou K, Ling T, Wang H, et al. Influence of tourniquet use in primary total knee arthroplasty with drainage: a prospective randomised controlled trial. J Orthop Surg Res 2017;12:172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Vandenbussche E, Duranthon L-D, Couturier M, Pidhorz L, Augereau B. The effect of tourniquet use in total knee arthroplasty. Int Orthop 2002;26:306-309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Abdel-Salam A, Eyres KS. Effects of tourniquet during total knee arthroplasty. A prospective randomised study. J Bone Joint Surg [Br] 1995;77:250-253. [PubMed] [Google Scholar]
- 19. Wakai A, Winter D C, Street J T. et al. Pneumatic tourniquets in extremity surgery. J Am Acad Orthop Surg 2001;9:345–351. [DOI] [PubMed] [Google Scholar]
- 20. Worland RL, Arredondo J, Angles F, Lopez-Jimenez F, Jessup DE. Thigh pain following tourniquet application in simultaneous bilateral total knee replacement arthroplasty. J Arthroplasty 1997;12:848-852. [DOI] [PubMed] [Google Scholar]


