Highlights
-
•
The incidence of other respiratory viruses fell dramatically during the first peak.
-
•
SARS-CoV-2 was rarely co-detected with other respiratory viruses.
-
•
SARS-CoV-2 was infrequently associated with exacerbations of COPD or asthma.
-
•
Mortality rate was much higher in patients with COVID-19 compared to other viruses.
Keywords: COVID-19, SARS-CoV-2, Influenza, Respiratory viruses, Pneumonia, Molecular diagnostics, Point-of-care testing
Abstract
Objectives
The effect of SARS-CoV-2 on existing respiratory viruses in circulation and the overall burden of viral respiratory disease remains uncertain. Traditionally, severe viral respiratory disease disproportionally affects those with underlying chronic lung diseases. This study aimed to assess the impact of SARS-CoV-2 on the prevalence and clinical characteristics of respiratory virus disease in hospitalised adults.
Methods
Data for this cohort study were from hospitalised adults who had multiplex PCR testing for respiratory viruses over several seasons in Hampshire, UK. Respiratory virus detection during the first epidemic peak of SARS-CoV-2 was compared to detection during the same time period across previous years.
Results
856 patients had multiplex PCR for respiratory viruses between March and May over 5 years. Before 2020, a non-SARS-CoV-2 virus was detected in 54% patients (202/371) compared to 4.1% (20/485) in 2020 (p < 0.0001). SARS-CoV-2 was associated with asthma or COPD exacerbations in a smaller proportion of infected patients compared to other viruses (1.0% vs 37%, p < 0.0001).
Conclusions
The emergence of SARS-CoV-2 was associated with substantial reductions in the circulation of seasonal respiratory viruses and large differences in the characteristics of viral-associated disease, including illness in a greater proportion of patients without underlying lung disease.
Introduction
A novel coronavirus, SARS-CoV-2, emerged in Hubei Province, China in December 2019.1 In the 10 months since it has spread around the world causing a global pandemic leading to catastrophic loss of life and severe economic consequences that are predicted to endure for many years to come.2 , 3 Policy makers around the world have enforced strict lockdowns to reduce the spread of the virus with unprecedented restrictions on personal freedoms. These appear to be effective but the effect they have on transmission of other respiratory viruses is largely unknown. Our understanding of risk factors, including chronic lung disease, and clinical features of COVID-19 continue to develop but how it compares to disease caused by other respiratory viruses has not been widely considered.
In this study we describe the distribution of respiratory viruses in hospitalised adults in Southampton, UK, during the peak of the coronavirus pandemic from March to May 2020. We compare this to those identified in the same period from preceding years. We compare demographic, clinical and radiographic features in patients testing positive for SARS-CoV-2 with those testing positive for other respiratory viruses.
Methods
Study design and participants
We carried out a large cohort study of patients recruited from hospitals in Hampshire, UK. Data were collected from three large trials carried out between 2015 and 2020: the ResPOC trial4 (ISRCTN90211642), the FluPOC trial (ISRCTN17197293, article in press) and the COV-19POC trial5 , 6 (ISRCTN14966673). Patients were recruited in the Emergency Department (ED) or Acute Medical Unit (AMU) of Southampton General Hospital or the Royal Hampshire County Hospital, Winchester. The former is a large teaching hospital and tertiary referral centre in the South of the UK, and the latter a large District General Hospital. A small number of patients from the CoV-19POC trial (n = 14) were not included in the circulation of respiratory virus data as they were recruited directly from intensive care rather than from the ED or AMU.
All participants were 18 or over, presenting with an acute respiratory illness or suspected viral respiratory tract infection, and recruited within 24 h of presentation to hospital. In each study we attempted to recruit all eligible patients. The inclusion criteria for these trials are very similar and are summarised in Table 1 . A combined nose and throat swab was taken from all participants at recruitment and tested using a multiplex molecular respiratory virus panel for a range of targets which included influenza A, influenza B, human coronaviruses (HKU1, NL63, 229E and OC43), human rhinovirus/enterovirus (hRV), adenovirus, human metapneumovirus (hMPV), parainfluenza virus (1,2,3, and 4), and respiratory syncytical virus (RSV). Patients recruited in 2020 had the same targets tested with the addition of SARS-CoV-2. Testing was performed with the QIAstat-Dx Respiratory SARS-CoV-2 panel (COV-19POC trial) or the BioFire FilmArray Respiratory Panel (ResPOC trial) or the BioFire FilmArray Respiratory Panel 2 (FluPOC trial).
Table 1.
Inclusion criteria for contributing studies.
| ResPOC | FluPOC | CoV-19 POC | |
|---|---|---|---|
| Adults ≥18 years | Adults ≥18 years | Adults ≥18 years | |
| ED and AMU | ED and AMU | ED and AMU | |
| Patient consent required | Yes | Yes – provision for consultee assent if lacking capacity | Yes – provision for consultee assent if lacking capacity |
| Maximum duration of symptoms prior to hospitalisation | 7 days | 10 days | No limit |
| Time from presentation patient eligible for recruitment | 24 h | 16 h | 24 h |
Data were prospectively collected for clinical, laboratory and radiographic features of illness. The observations recorded were the first taken on presentation to hospital. Outcomes were collected retrospectively from case notes including electronic records with all cause death (in hospital and all) recorded at 30 days. Readmission to hospital was captured from electronic records up to 30 days after discharge.
A clinically trained investigator summarised each clinical illness into a final diagnosis depending on disease characteristics. Pneumonia was defined as any new pulmonary infiltrate on chest x-ray or CT scan occurring with new respiratory symptoms. If the illness was not defined as pneumonia, then admission symptoms and comorbidities were used to adjudicate the diagnosis.
Each trial was prospectively approved by UK regional ethics committees, and patients had to provide written consent to participate or have a consultee provide assent on their behalf. Full trial protocols are publicly available for each trial.7, 8, 9
Statistical analysis
Data analysis was performed using GraphPad Prism 8.4 (GraphPad Software, LLC). The proportion of positive tests between 1st March and 1st May were calculated for each of the five years within the study (2015, 2016, 2018, 2019, and 2020). Baseline characteristics of all positive viral detections were summarised using appropriate descriptive statistics. Continuous data are presented as medians and interquartile ranges, and categorical data as numbers and percentages. Absolute differences between proportions are presented with 95% confidence intervals. Differences and 95% confidence intervals between medians were calculated using the Hodges-Lehmann estimator. Comparative statistical tests were performed between SARS-CoV-2 and non-SARS-CoV-2 viral positive cases. The Mann-Whitney U test was used for analysis of continuous variables, and the X2 test or Fisher's exact test was used for categorical variables, as appropriate. Missing data were <4% in all analyses unless reported otherwise. This study is reported according to the STROBE guideline.
Results
Respiratory virus circulation
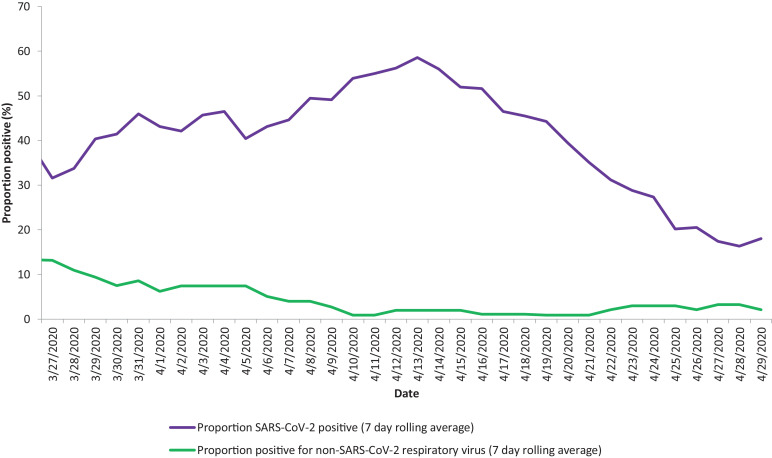
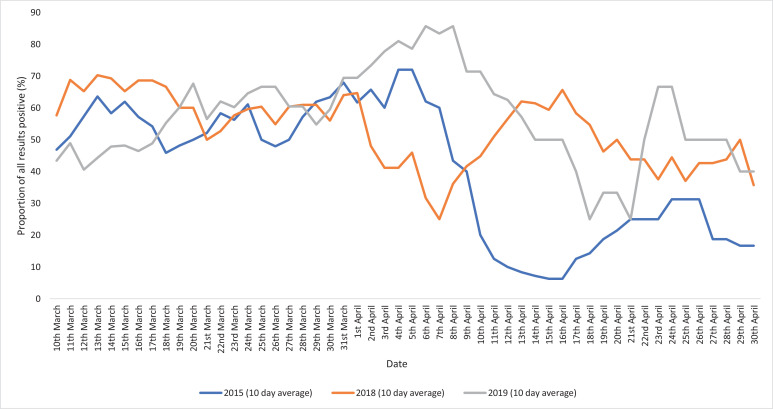
856 patients presented to hospital with acute respiratory illness and were tested for respiratory viruses between March and May since 2015. During the first wave of the 2020 pandemic 38% (185/485) of patients were positive for SARS-CoV-2 and 4.1% (20/485) were positive for other respiratory viruses. By comparison in the 5 years prior to the pandemic 54% (202/371) were positive for a non-COVID-19 respiratory virus in the same 2-month period (difference of 50%, 95%CI 44% to 56%; p < 0.0001). Fig. 1 shows the 7-day rolling average positivity rate for SARS-CoV-2 and other respiratory viruses during the first wave. Fig. 2 shows the positivity rate for non-SARS-CoV-2 respiratory viruses in the preceding 5 years, by year.
Fig. 1.
Proportion of positive respiratory viral swabs in March to May 2020.
Fig. 2.
Proportion of positive non-SARS-CoV-2 respiratory viral swabs by year.
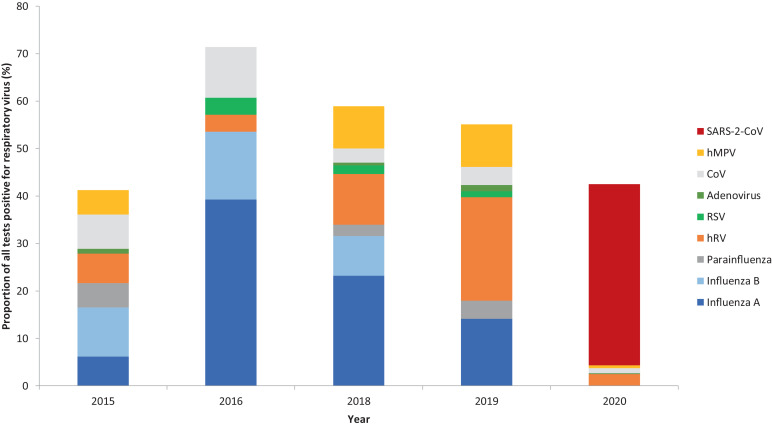
The most frequently detected non-COVID-19 virus in March-May during the 5 years prior to 2020 was influenza, which was detected in 26% of patients tested (95/371) followed by rhinovirus in 11% (42/371) although the proportions varied year on year. The most common non-SARS-CoV-2 virus detected during the pandemic was rhinovirus in 2.5% (12/485) of patients which all occurred in the absence of another virus. Fig. 3 shows the positivity rate of individual virus types by year, before and during the pandemic.
Fig. 3.
Proportion of positive respiratory viral swabs by year, divided by viral type.
768 viruses were detected in 727 patients across all time points from the 3 studies. 194 patients were positive for SARS-CoV-2 and 533 patients had other respiratory viruses. Of these non-COVID-19 viruses, the most frequently detected were influenza A (188), influenza B (83) and rhinovirus (134). Other commonly detected viruses were human metapneumovirus (64), seasonal coronaviruses (47), RSV (29) and parainfluenza viruses (19).
SARS-CoV-2 was co-detected with another respiratory virus in 0.5% of cases (1/194), whereas non-COVID-19 respiratory viruses were detected in combination in 7.7% (41/533) (difference of 7.2%, 95%CI 3.3% to 10.4%; p = 0.0002).
Demographics and comorbidities
There were some clear differences in the baseline characteristics of those with COVID-19 when compared to those with other respiratory viruses. Patients with SARS-CoV-2 were significantly older (median of 65 years, [IQR 50–80] vs 59 years [IQR 39–73], difference of 8 (95%CI 4 to 11; p < 0.0001), were less likely to be of white British ethnicity (72% vs 93%, difference of −21%, 95%CI −16% to −27%; p < 0.0001) and more likely to be of Asian (18% vs 2.1%, difference of 16%, 95%CI 12% to 20%; p < 0.0001) or Black ethnicity (5.9% vs 1.5%, difference of 4.4%, 95%CI 1.7% to 7.1%; p = 0.003). Patients with SARS-CoV-2 infection were also much more likely to work in healthcare, with 21% (39/185) of cases occurring in healthcare workers compared to only 6.0% (23/383) of other respiratory viruses (difference of 15%, 95% CI 9.6% to 20%; p < 0.0001).
5.0% (8/160) of patients with SARS-CoV-2 reported being a current smoker, compared with 24% (127/530) of those infected with other respiratory viruses (difference −19%, 95%CI −26% to −12%; p < 0.0001). This was reflected in the rates of pre-existing respiratory disease, with 31% (58/188) of SARS-CoV-2 cases occurring in patients with an established chronic respiratory diagnosis compared to 59% (313/532) of cases in non-COVID-19 respiratory viruses (difference −30%, 95%CI −36% to −20%; p < 0.0001). Conversely there were greater rates of hypertension (41% vs 25%, difference 16%, 95% CI7.8% to 24%; p = 0.0001), cardiovascular disease (31% vs 22%, difference 8.8%, 95%CI 1.7% to 16%; p = 0.016), chronic renal disease (9.1% vs 4.1%, difference 5.0%, 95%CI 1.2% to 8.8%; p = 0.014) and diabetes (25% vs 13%, difference 12%, 95%CI 5.5% to 18%; p = 0.0002) in those with SARS-CoV-2 infection.
Presenting features and investigations
Patients with SARS-CoV-2 infection had a median duration of symptoms of 7 days [IQR 2–10], compared to 4 days [IQR 3–6] for other respiratory viruses (difference of 3 days, 95%CI 1 to 2; p = 0.0002). At presentation to hospital, they were more likely to treated with supplementary oxygen therapy than those with another respiratory virus infection (43% vs 21%, difference 22.4%, 95%CI 15% to 29%; p<0.0001) and had a marginally higher respiratory rate (25 breaths/ minute [21–30] vs 24 [20–28], difference 2, 95% CI 1 to 3; p < 0.0001) although there was no significant difference in oxygen saturations between the two groups. There was no difference in the proportion of patients presenting with a fever of ≥38 °C between the two groups (29% SARS-CoV-2 vs 27% other respiratory virus, difference 2.2%, 95% CI −5.2% to 9.6%; p = 0.57).
Median [IQR] C-reactive protein (CRP) of patients with SARS-CoV-2 was higher, 89 [44–145] compared to 49 [16–115] in non-COVID-19 respiratory viral infection (difference of 40, 95%CI 16 to 41; p < 0.0001). Conversely, total white cell count (7.1 [5.5–10.3] vs 9.6 [7.2–13.3], difference of −2.5, 95%CI −1.6 to −2.8; p < 0.0001) and neutrophil count (5.5 [4–8.2] vs 7.6 [5.3–10.6], difference of −2.1, 95% CI −1.2 to −2.4; p < 0.0001) were substantially lower. Median lymphocyte count was 0.9 [0.7–1.3] for SARS-2-CoV and 1.0 [0.7–1.6] for other respiratory viruses (difference −0.1, 95%CI −0.2 to 0; p = 0.062). Pulmonary infiltrates were present on 77% (149/193) of chest radiographs performed on patients with SARS-CoV-2 and only 22% (117/523) of those performed on patients with other respiratory viruses (difference 55%, 95%CI 47% to 63%; p < 0.0001). Baseline characteristics and investigations are summarised in Table 2 .
Table 2.
Patient characteristics at hospital presentation, by respiratory virus.
| SARS-CoV-2 | Non-SARS-CoV-2 respiratory virus | Absolute difference (95% CI) | p value | |
|---|---|---|---|---|
| n = 194a | n = 533 | |||
| Single detections | 193 | 492 | ||
| Co-detections | 1 (1%) | 41 (8%) | −7.2% (−3.3% to −10.4%) | 0.0002 |
| Demographics | ||||
| Age, years | 65 (50–80) | 59 (39–73) | 8 (4 to 11) | <0.0001 |
| >65 | 95 (49%) | 216 (41%) | 8.0% (1.2% to 14.8%) | 0.042 |
| Male | 106 (55%) | 244 (45%) | 8.7% (0.7% to 17.1%) | 0.03 |
| Female | 88 (45%) | 289 (54%) | ||
| Healthcare worker | 39b (21%) | 23b (6%) | 15.1% (9.6% to 20.5%) | <0.0001 |
| Current smoker | 8c (5%) | 127 (24%) | −19.0% (−26.0% to −12.0%) | <0.0001 |
| Symptom duration at presentation | ||||
| Duration, days | 7 (2–10) | 4 (3–6) | 3 (1 to 2) | 0.0002 |
| Ethnicity | ||||
| White British | 134 (72%) | 496 (93%) | −21.2% (−15.7% to −26.7%) | <0.0001 |
| White other | 7 (4%) | 10 (2%) | 1.9% (−0.7% to 4.4%) | 0.163 |
| Black | 11 (6%) | 8 (2%) | 4.4% (1.7% to 7.1%) | 0.0027 |
| Asian | 34 (18%) | 11 (2%) | 16.2% (12.2% to 20.3%) | <0.0001 |
| Other | 0 | 7 (1%) | −1.3% (−3.0% to 0.3%) | 0.2 |
| Comorbidity | ||||
| Hypertension | 76d (41%) | 97d (25%) | 15.8% (7.8% to 23.9%) | 0.0001 |
| Cardiovascular disease | 58 (31%) | 118 (22%) | 8.8% (1.7% to 16%) | 0.016 |
| Respiratory disease | 58 (31%) | 313 (59%) | −30.0% (−36.3% to −19.7%) | <0.0001 |
| Renal disease | 17 (9%) | 22 (4%) | 5.0% (1.2% to 8.8%) | 0.014 |
| Liver disease | 9 (5%) | 11 (2%) | 2.8% (0% to 5.5%) | 0.067 |
| Diabetes mellitus | 47 (25%) | 71 (13%) | 11.7% (5.5% to 17.8%) | 0.0002 |
| Cancer | 10 (5%) | 27 (5%) | 0.3% (−3.4% to 4%) | 0.849 |
| Immunosuppression | 10 (5%) | 25 (5%) | 0.7% (−2.9% to 4.3%) | 0.694 |
| Observations at admission | ||||
| Temperature, °C | 37.1 (36.6–38.1) | 37.2 (36.6–38) | −0.15 (−0.2 to 0.2) | 0.984 |
| Temperature ≥38 °C | 55 (29%) | 141 (27%) | 2.2% (−5.2% to 9.6%) | 0.57 |
| Pulse rate, bpm | 93 (82–109) | 100 (88–116) | −7 (−4 to −10) | <0.0001 |
| Respiratory rate, bpm | 25 (21–30) | 24 (20–28) | 1.5 (1 to 3) | <0.0001 |
| Oxygen saturations,% | 96 (92–97) | 95 (93–97) | 1 (0 to −1) | 0.661 |
| Supplementary O2 | 84 (43%) | 111 (21%) | 22.4% (15.2% to 29.7%) | <0.0001 |
| Systolic blood pressure, mmHg | 130 (120–145) | 134 (119–148) | −4 (−6 to 2) | 0.3 |
| NEWS2 score | 6 (3–7) | 4 (3–6) | 2 (0 to 1) | 0.002 |
| Laboratory and radiology at admission | ||||
| CRP, mg/L | 89 (44–145) | 49 (16–115) | 40 (16 to 41) | <0.0001 |
| WCC, x109/L | 7.1 (5.5–10.3) | 9.6 (7.2–13.3) | −2.5 (−1.6 to −2.8) | <0.0001 |
| neutrophils, x109 | 5.5 (4–8.2) | 7.6 (5.3–10.6) | −2.1 (−1.2 to −2.4) | <0.0001 |
| lymphocytes x109 | 0.9 (0.7–1.3) | 1 (0.7–1.6) | −0.1 (−0.2 to 0) | 0.062 |
| CXR performed | 193 (99%) | 523 (98%) | ||
| Infiltrates/consolidation | 149 (77%) | 117 (22%) | 54.8% (46.9% to 62.8%) | <0.0001 |
Data are n (%) or median (IQR).
Includes 185 patients recruited in AMU/ED and 9 recruited directly on ICU within 24 h of admission.
n=185 and 383 respectively.
n=160 (some patients unable to communicate this result).
n=185 and n = 384 respectively (not collected as part of ResPOC trial). NEWS2, National Early Warning Score 2; CXR, Chest X-ray.
Outcomes and diagnoses
Differences in clinical outcomes were marked: the 30-day mortality was 26% (47/184) in SARS-CoV-2 infection, compared to 1.7% (9/532) with other respiratory virus infection (difference 24%, 95% CI 19% to 28%; p < 0.0001). 19% (26/194) of SARS-CoV-2 positive patients required ICU level care compared with only 1.5% (8/532) of those with another respiratory virus (difference 17%, 95%CI 13% to 21%; p < 0.0001).
81% (156/193) of patients with SARS-CoV-2 infection had a radiological diagnosis of pneumonia, compared to 24% (125/531) of other respiratory viral infections (difference 57%, 95%CI 49% to 65%; p < 0.0001). SARS-CoV-2 was associated with fewer exacerbations of chronic obstructive pulmonary disease (COPD) (0.5% vs 17%, difference −17%, 95%CI −22% to −11%; p < 0.0001) and asthma (0.5% vs 20%, difference −19%, 95%CI −25% to −14%; p < 0.0001) compared with other respiratory viruses.
91% of patients with SARS-CoV-2 infection were treated with antibiotics, compared to 89% of those with other respiratory viruses (difference 2.1%, 95%CI −2.9% to 7.1%; p = 0.317). Outcome measures and diagnoses are summarised in Table 3 .
Table 3.
Therapy, outcomes and diagnosis by virus.
| SARS-CoV-2 | Non-SARS-CoV-2 virus | Absolute difference (95% CI) | p value | |
|---|---|---|---|---|
| n = 194 | n = 533 | |||
|
Therapy Antibiotics used |
176 (91%) | 475 (89%) | 2.1% (−2.9% to 7.1%) | 0.317 |
| Outcome | ||||
| ICU admission | 36 (19%) | 9 (2%) | 16.9% (12.9% to 20.8%) | <0.0001 |
| In hospital mortality to 30 days | 41 (21%) | 8 (2%) | 19.7% (15.6% to 23.9%) | <0.0001 |
| 30-day mortality | 47a (26%) | 9 (2%) | 23.9% (19.4% to 28.4%) | <0.0001 |
| Readmission | 15b (12%) | 35 (7%) | 5.0% (−0.1% to 10.2%) | 0.065 |
| Investigator final diagnosis | ||||
| Pneumonia | 156 (81%) | 125 (24%) | 57.3% (49.3% to 65.3%) | <0.0001 |
| Influenza like illness | 18 (9%) | 114 (21%) | −12.1% (−18.5% to −5.8%) | 0.0002 |
| Non-pneumonic lower respiratory tract infection | 15 (8%) | 67 (13%) | −4.8% (−10.1% to 0.4%) | 0.069 |
| Exacerbation asthma | 1 (1%) | 106 (20%) | −19.4% (−25.3% to −13.6%) | <0.0001 |
| Exacerbation COPD | 1 (1%) | 91 (17%) | −16.6% (−22.1% to −11.1%) | <0.0001 |
| Exacerbation other underlying respiratory disease | 1 (1%) | 17 (3%) | −2.7% (−5.3% to 0%) | 0.055 |
| Other | 1 (1%) | 11 (2%) | −1.6% (−3.7% to 0.6%) | 0.198 |
Data are n (%).
n=145.
n=128.
COPD, Chronic obstructive pulmonary disease.
Discussion
This large study of adults demonstrates the impact of the emergence of SARS-CoV-2 on the frequency of detection of other respiratory viruses in those presenting to hospital with acute respiratory illness, and on the patterns of associated illness.
We report a dramatic drop in the detection of other viruses in hospitalised adults during the March-May peak of the COVID-19 pandemic in the UK when compared to previous years. Typically, before COVID-19, almost half of the patients presenting to our institution with acute respiratory illness would have a non-SARS respiratory virus detected. Remarkably these viruses were only detected in 4% of a comparable patient cohort in 2020. The explanation for this is likely to be multifactorial. Seasonal respiratory viruses typically have a shorter incubation period10, 11, 12 than SARS-CoV-2, therefore transmission of these viruses may have been impacted earlier by the effects of social distancing measures and the nationwide lockdown that was introduced on 23rd March 2020. Indeed, it seems highly likely that the measures introduced to reduce transmission of SARS-CoV-2 are the main reason for the significant reduction in spread of other respiratory viruses. Other transmissible diseases, such as measles,13 , 14 have reported a similar decline.
It has been theorised that the spread of previous epidemic influenza viruses have been slowed by interaction with existing viral infections,15 and there is a growing body of epidemiological evidence to support this phenomenon in other respiratory viruses.16 The mechanisms for this are poorly understood. It was highly unusual for SARS-CoV-2 to be co-detected with other viruses, occurring in only 1% compared to 8% of other seasonal respiratory virus infections. This finding raises the possibility that viral interference may have played a role in the reduced prevalence of other respiratory viruses.
SARS-CoV-2 had a disproportionate effect on those without existing respiratory disease when compared to other respiratory viruses. Pre-existing lung disease was present in almost twice as many patients with non-SARS-CoV-2 respiratory virus infection. This is reflected in the pattern of clinical illness, where exacerbations of asthma and COPD associated with SARS-CoV-2 were rare. It is well established that respiratory viruses are a frequent cause of exacerbation in these diseases,17 , 18 and yet these were the main clinical diagnosis in only 1% of SARS-CoV-2 infections.
The rate of smoking (5%) in those testing positive for SARS-CoV-2 is consistent with other large reported UK datasets19 and substantially below the 24% of patients with other respiratory virus detection. It is notable that smoking appears to be a risk factor for developing severe disease but is infrequently present in hospitalised patients with COVID-19.20 , 21
The disproportionately high rates of infection and mortality of COVID-19 on BAME (Black, Asian and Minority Ethnic) groups has been widely acknowledged.22 Our study shows that this was also the case in our region where Black and Asian patients accounted for a much higher proportion of SARS-CoV-2 infections compared with seasonal respiratory virus infection. Locally these data may be confounded by the rate of infection observed in cruise ship workers who were from a diverse range of ethnic backgrounds. Southampton is a large, maritime city where a number of cruise ships were berthed during the height of the pandemic. More studies are urgently required to investigate the disproportionate effect of COVID-19 on BAME populations.
Other groups have described the disproportionate risk to healthcare workers of developing COVID-19 in observational studies.23 Our findings also suggest this, with 1 in 5 infections being reported in this group, matching other large reports during the height of the pandemic.24 As a novel finding, we report that this rate of infection requiring hospitalisation was more than five times greater than rates for existing respiratory viruses in previous years. This difference may be explained in part by the impact of annual influenza vaccination of healthcare workers in the UK, which would limit the number of staff developing severe disease. Another contributing factor could be the disproportionately high rates of infection and transmission of SARS-CoV-2 in care homes in the UK.25
Almost all patients with any virus detected were given antibiotic therapy, despite the lack of evidence for their utility use in viral infection. Presumably, this was given for suspected secondary bacterial infection and no microbiological data was collected to this end, however it continues to highlight an unmet need for better diagnostic tests which allow differentiation between viral and bacterial infections and the safe withholding of unnecessary antibiotics.
Lymphopaenia is a reliable feature of COVID-1926 , 27 and a lower count predicts severe disease.28 Our work highlights that it remains a common feature of other viral respiratory tract infections and therefore cannot be used to help differentiate between different viral aetiologies. Our data show that CRP and neutrophil count are potentially more useful to differentiate between SARS-CoV-2 and other viral infections although there remains considerable overlap.
Large data sets have shown a typical rate of admission to ICU of around 15%19 , 29 of hospitalised patients with COVID-19 which is consistent with our findings (19%). The stark difference in mortality rate between SARS-CoV-2 infection and other respiratory viruses are highlighted by our data, with 30-day mortality around 10 times greater.
The strengths of our study are that large amounts of data were collected prospectively with a high degree of detail and accuracy. There is minimal missing data, and all of the collection procedures were standardised for each study (i.e. the same electronic data and paper sources were accessed in the same way). As a result, we have a very large cohort who had comprehensive multiplex testing for respiratory viral infection who are directly comparable. The findings of this study are highly generalisable to adults presenting with acute respiratory illness at a time of peak prevalence of disease in a developed healthcare setting.
A limitation of the trial is that it was only carried out in two centres. Furthermore, we did not record whether patients were residents of long-term care facilities in these trials. This was an area of widespread transmission that emerged as a potential confounding factor midway through our trial during the UK pandemic.30
Conclusion
We report a significant drop in the circulation of non-SARS-CoV-2 respiratory viruses during first wave of the 2020 pandemic when compared to the same time period in previous years. SARS-CoV-2 infection was associated with major differences in the clinical characteristics and outcome of respiratory virus associated diseases. These include infrequent association with exacerbations of airways disease, a higher rate of severe pneumonia, and a mortality rate around 10 times higher than that seen with seasonal respiratory virus infection.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. FluPOC was funded by a NIHR Fellowship awarded to TWC (PDF 2016-09-061) and ResPOC and CoV-19POC were funded by the University of Southampton and University Hospital Southampton NHS Foundation Trust respectively.
Declaration of Competing Interest
Poole, S. - Declarations of interest: none.
Brendish, N.J. - Declarations of interest: none.
Clark, T.W. - TWC has received equipment and consumables free of charge or at discounted rates from QIAGEN, Biofire LLC and BioMerieux for the purposes of independent research, for the 3 studies listed in this work. TWC has received speaker fees, honoraria, travel reimbursement, and equipment and consumables free of charge for the purposes of research outside of this submitted study, from BioFire diagnostics LLC and BioMerieux. TWC has received consultancy fees from Synairgen research Ltd, Randox laboratories Ltd and Cidara therapeutics. He a member of an advisory board for Roche and a member of two independent data monitoring committees for trials sponsored by Roche. He has acted as the UK chief investigator for an IMP study sponsored by Janssen.
Acknowledgements
We would like to thank all of the participants in the FluPOC, CoV-19POC and ResPOC studies. We would also like to thank the recruiting fellows, research nurses and clinical trials assistants for the conduct of the studies.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.jinf.2020.11.010.
Appendix. Supplementary materials
References
- 1.Zhu N., Zhang D., Wang W. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nicola M., Alsafi Z., Sohrabi C. The socio-economic implications of the coronavirus pandemic (COVID-19): a review. Int J Surg. 2020;78:185–193. doi: 10.1016/j.ijsu.2020.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McKee M., Stuckler D. If the world fails to protect the economy, COVID-19 will damage health not just now but also in the future. Nat Med. 2020;26:640–642. doi: 10.1038/s41591-020-0863-y. [DOI] [PubMed] [Google Scholar]
- 4.Brendish N.J., Malachira A.K., Armstrong L. Routine molecular point-of-care testing for respiratory viruses in adults presenting to hospital with acute respiratory illness (ResPOC): a pragmatic, open-label, randomised controlled trial. Lancet Respir Med. 2017;5:401–411. doi: 10.1016/S2213-2600(17)30120-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brendish N.J., Poole S., Naidu V V. et al. Clinical impact of molecular point-of-care testing for suspected COVID-19 in hospital: a prospective, interventional, non-randomised, controlled study (COV-19POC) Lancet Respir Med. 2020 doi: 10.1016/S2213-2600(20)30454-9. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brendish N.J., Poole S., Naidu V V. et al. Clinical characteristics, symptoms and outcomes of 1054 adults presenting to hospital with suspected COVID-19: a comparison of patients with and without SARS-CoV-2 infection. J Infect. 2020;9:32. doi: 10.1016/j.jinf.2020.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beard K., Brendish N., Malachira A. Pragmatic multicentre randomised controlled trial evaluating the impact of a routine molecular point-of-care ‘test-and-treat’ strategy for influenza in adults hospitalised with acute respiratory illness (FluPOC): trial protocol. BMJ Open. 2019;9 doi: 10.1136/bmjopen-2019-031674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clark T.W. Evaluating the clinical impact of routine molecular point-of-care testing for. https://eprints.soton.ac.uk/439309/2/CoV_19POC_Protocol_v2_0_eprints.pdf (Accessed 6 August 2020).
- 9.Brendish N.J., Malachira A.K., Clark T.W. Molecular point-of-care testing for respiratory viruses versus routine clinical care in adults with acute respiratory illness presenting to secondary care: a pragmatic randomised controlled trial protocol (ResPOC) BMC Infect Dis. 2017;17 doi: 10.1186/s12879-017-2219-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Heikkinen T., Järvinen A. Elsevier Limited; 2003. The Common Cold. In: Lancet; pp. 51–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chan J.F.W., Yuan S., Kok K.H. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet. 2020;395:514–523. doi: 10.1016/S0140-6736(20)30154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Backer J.A., Klinkenberg D., Wallinga J. Incubation period of 2019 novel coronavirus (2019- nCoV) infections among travellers from Wuhan, China, 20 28 January 2020. Eurosurveillance. 2020;25 doi: 10.2807/1560-7917.ES.2020.25.5.2000062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen C.-.H., Lai C.-.C., Chao C.-.M., Tang H.-.J. Zero measles after COVID-19 pandemic in Taiwan Running title: measles and COVID-19. J Infect. 2020 doi: 10.1016/j.jinf.2020.10.021. 0. [DOI] [PubMed] [Google Scholar]
- 14.Rana M.S., Usman M., Alam M.M. Impact of COVID-19 pandemic on Measles surveillance in Pakistan. J Infect. 2020 doi: 10.1016/j.jinf.2020.10.008. published online Oct 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Casalegno J.S., Ottmann M., Bouscambert Duchamp M. Rhinoviruses delayed the circulation of the pandemic influenza A (H1N1) 2009 virus in France. Clin Microbiol Infect. 2010;16:326–329. doi: 10.1111/j.1469-0691.2010.03167.x. [DOI] [PubMed] [Google Scholar]
- 16.Nickbakhsh S., Mair C., Matthews L. Virus-virus interactions impact the population dynamics of influenza and the common cold. Proc Natl Acad Sci USA. 2019;116:27142–27150. doi: 10.1073/pnas.1911083116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nicholson K.G., Kent J., Ireland D.C. Respiratory viruses and exacerbations of asthma in adults. Br Med J. 1993;307:982–986. doi: 10.1136/bmj.307.6910.982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Seemungal T., Harper-Owen R., Bhowmik A. Respiratory viruses, symptoms, and inflammatory markers in acute exacerbations and stable chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2001;164:1618–1623. doi: 10.1164/ajrccm.164.9.2105011. [DOI] [PubMed] [Google Scholar]
- 19.Docherty A.B., Harrison E.M., Green C.A. Features of 20 133 UK patients in hospital with covid-19 using the ISARIC WHO clinical characterisation protocol: prospective observational cohort study. BMJ. 2020;369 doi: 10.1136/bmj.m1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rossato M., Russo L., Mazzocut S., Di Vincenzo A., Fioretto P., Vettor R. Early view correspondence current smoking is not associated with COVID-19. DOI:10.1183/13993003.01290-2020. [DOI] [PMC free article] [PubMed]
- 21.Zheng Z., Peng F., Xu B. Risk factors of critical & mortal COVID-19 cases: a systematic literature review and meta-analysis. J Infect. 2020;81:e16–e25. doi: 10.1016/j.jinf.2020.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Patel P., Hiam L., Sowemimo A., Devakumar D., McKee M. Ethnicity and covid-19. BMJ. 2020;369 doi: 10.1136/bmj.m2282. [DOI] [PubMed] [Google Scholar]
- 23.Nguyen L.H., Drew D.A., Graham M.S. Risk of COVID-19 among front-line health-care workers and the general community: a prospective cohort study. Lancet Public Heal. 2020 doi: 10.1016/s2468-2667(20)30164-x. 0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Burrer S.L., de Perio M.A., Hughes M.M. Characteristics of health care personnel with COVID-19 — United States, February 12–April 9, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:477–481. doi: 10.15585/mmwr.mm6915e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vivaldi 1: coronavirus (COVID-19) care homes study report - GOV.UK. https://www.gov.uk/government/statistics/vivaldi-1-coronavirus-covid-19-care-homes-study-report (Accessed 24 August 2020).
- 26.Huang C., Wang Y., Li X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goyal P., Choi J.J., Pinheiro L.C. Clinical Characteristics of Covid-19 in New York City. N Engl J Med. 2020;382:2372–2374. doi: 10.1056/NEJMc2010419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huang I., Pranata R. Lymphopenia in severe coronavirus disease-2019 (COVID-19): systematic review and meta-analysis. J Intensive Care. 2020;8:36. doi: 10.1186/s40560-020-00453-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Richardson S., Hirsch J.S., Narasimhan M. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City Area. JAMA. 2020;323:2052. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.O'Dowd A. Covid-19: deaths in care home deaths in England and Wales rise sharply. BMJ. 2020;369:m1727. doi: 10.1136/bmj.m1727. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.