Abstract
3D Bioprinting directly into injured sites in a surgical setting, intraoperative bioprinting (IOB), is an effective process, in which the defect information can be rapidly acquired and then repaired via bioprinting on a live subject. In patients needing tissue resection, debridement, traumatic reconstruction, or fracture repair, the ability to scan and bioprint immediately following surgical preparation of the defect site has great potential to improve the precision and efficiency of these procedures. In this Opinion, we provided the reader with current major limitations of IOB from engineering and clinical points of view as well as possibilities of future translation of bioprinting technologies from bench to bedside, and expounded our perspectives in the context of IOB of composite and vascularized tissues.
Keywords: intraoperative bioprinting, clinical translation, bioprinting technologies, surgery
Advances from in vitro bioprinting to intraoperative bioprinting
In the last decade, bioprinting has considerably advanced and a large number of studies have shown attractive outcomes in different applications, such as engineering of various organs for regenerative medicine (i.e., skin [1], cartilage [2], and bone [3]), tissue vascularization [4], drug discovery [5] and disease modeling [6]. However, the majority of bioprinting endeavors have been conducted in vitro, and in some cases, further validated in vivo [7]. Although bioprinting of solid organs directly into human body remains elusive, some attempts have been practiced in the realm of intraoperative bioprinting (IOB), also known as in-situ or in-vivo bioprinting [8–10], which refers to the bioprinting process performed on a live subject in a surgical setting, in which defect imaging, data processing, process planning, and bioprinting are performed consecutively in a single process. IOB of tissue substitutes directly into injury sites is greatly beneficial as it can facilitate the complex tissue heterogeneity in an anatomically accurate manner. Although IOB has not been performed in clinics yet, the field is steadily moving forward owing to the rapid developments in bioprinting technologies with contributions from interdisciplinary teams of researchers from different domains spanning from engineering and materials to medical sciences and surgery.
The realization of IOB will bring about several benefits. First, bioprinted tissue substitutes, especially hydrogel- or cell aggregate-based constructs, usually have weak initial mechanical strengths due to the fluid-rich nature, which makes them difficult to handle with surgical tools, and further increases the likelihood of construct disintegration while being transferred to the surgical site and suturing. In contrast, with the assistance of a reverse engineering methodology enabling the real-time design of the graft, IOB is an effective process, where the defect can be repaired with minimum risk of contamination, graft disintegration, and manual interventions such as stacking multiple layers, in vitro culturing bioprinted scaffolds, transportation during surgery, or modifying prefabricated scaffolds conforming the defect shape [11]. More importantly, IOB is able to tackle natural defects with irregular topographies, which is common in clinical cases due to trauma and surgical excision. Comparatively, in vitro bioprinting usually assumes a flat working surface, which is inconsistent with clinical scenarios. Moreover, since intraoperatively repaired defects are surrounded by native tissue, endogenous cells could be directed by proper biochemical and biophysical cues to migrate into the bioprinted constructs and differentiate into target tissue-specific lineages [11, 12]. In addition, compared to manual injection of biomaterials into defect sites, IOB enables the precise deposition of cells, genes, or cytokines with localized control and anatomical biomimicry. For tissues that are heterocellular and formed of zonally-stratified arrangement of extracellular matrix (ECM, see Glossary), IOB is a powerful technology to precisely reconstitute multiple layers that is quite challenging using manual approaches. Lastly, shape distortion of hydrogels (e.g., high degree of shrinkage of collagen during skin regeneration [13]) can be an issue during in-vitro maturation of bioprinted constructs. In addition, during in-vitro culture, cells remodel the matrix and eventually change the shape of the bioprinted constructs, which may pose a significant disruption of pre-designed shapes. Therefore, IOB is also advantageous due to the concurrence of in-vivo integration, where the regenerated tissue may occupy the void space due to shrinkage and regulate the remodeling of matrix.
Current developments in IOB
Although the concept of IOB was first proposed in 2007 [14], very few studies have been reported on this topic, as bioprinting on a live subject is not trivial. Indeed, IOB is more demanding with respect to bioink preparation, bioprinter setup, sterilization, and surgical operation as compared to implanting prefabricated constructs. Thus far, some attempts targeting the repair of cartilage, bone and skin defects have been reported by different groups. For example, handheld devices have been developed for cartilage repair in a sheep model [15, 16] (Figure 1A) and skin repair in murine and porcine models [17]. Although the deposition was performed manually, it has come up with a well-developed strategy to miniaturize the number of external equipment for crosslinking of a cell-laden hydrogel. In terms of bone repair, Keriquel and coworkers [18] has performed IOB in the mouse calvaria defect model using an in-house developed biological laser-based bioprinting (LBB) system (Figure 1B). In their follow-up studies, IOB gave rise to organized microvascular networks and bone regeneration in calvaria defects [19, 20]. Currently, the most successful cases on IOB are mostly associated with skin repair due to its ease of access and regenerative potential [21, 22]. Most recently, Albanna and colleagues presented a mobile skin bioprinting system with integrated imaging technology for on-site management of murine and porcine full-thickness skin wound models [23] (Figure 1C). Table 1 gives a summary of the past works performed in IOB and their significant outcomes. A few excellent review articles provided more detailed information on the current status of in situ bioprinting [8–10]. Beyond the prior reviews, we here focus on the current major limitations of IOB from engineering and clinical points of view, highlighted the potential of future translation of IOB technologies, and also expounded the possibilities of using such a technology in reconstruction of composite and vascularized tissues.
Figure 1.
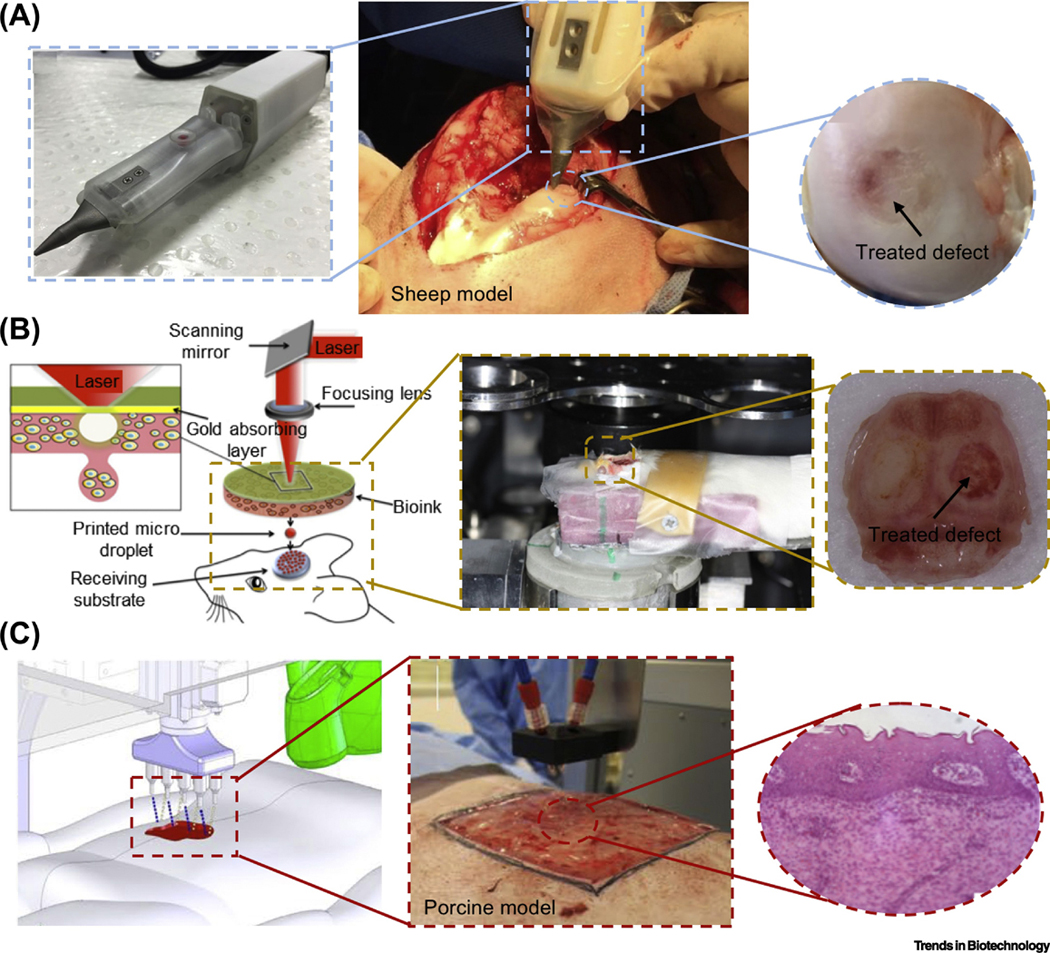
(A) IOB using the biopen for treatment of a full-thickness chondral defect in a sheep; adapted, with permission, from [16]. (B) Laser-based IOB for skull repair in a mouse calvarial defect; adapted, with permission, from [18–20]. (C) Droplet-based IOB for skin repair in a porcine model; adapted, with permission, from [23].
Table 1.
Summary of studies performed in the context of IOB
| Bioprinting modality | Materials | Cell Source | Animal Type | Defect Model | Target Tissue | 3D Scanning | Results | Reference |
|---|---|---|---|---|---|---|---|---|
| Extrusion-based bioprinting | Gelatin methacrylamide (GelMA)/Hyaluronic acid methacrylate (HAMA) | Mesenchymal stem cells (MSCs) | Sheep | Chondral defect | Cartil age | No | • Better overall macroscopic appearance in the handheld printed group compared to control groups; • Handheld printed constructs showed a higher amount of newly regenerated cartilage and the absence of subchondral bone deformation or collapse; • Handheld printed constructs showed positive Safranin O and collagen type II staining. |
[16] |
| Alginate/Fibrin/Collagen/Hyaluronic acid (HA) | Fibroblasts / Keratinocytes | Mice/Pig | Skin defect on dorsa | Skin | No |
Murine Model: • In situ deposition of an architected sheet was performed in the form of a fiber array onto a small and compliant wound surface; Porcine Model: • The porcine model demonstrated successful in situ bioprinting to cover full thickness wounds with a hemostatic barrier where it did not impede normal reepithelialization or wound contraction; • Microscopic analysis of healed wounds on Day 20 revealed that both treated and control wounds formed complete granulation tissue and exhibited comparable levels of collagen deposition and cellularity. |
[17] | |
| Laser-based bioprinting | Nano-hydroxy apatite | --- | Mice | Calvaria defect | Bone | No | • Material was present in close contact with dura mater on the test sites after 1 week; • After 1 month, newly formed mature and immature bones and n-HA aggregates inside macrophages were observed; • Three months after printing, mature bone tissue was observed; • From 1 week to 3 months, the amount of n-HA particles observed in situ decreased. |
[18] |
| Collagen/Nano-hydroxy apatite | MSCs | Mice | Calvaria defect | Bone | No | • Both nHA-collagen and nHA-collagen+cells, printed in a ring geometry, show only a marginal tissue reconstruction at 2 month post printing, particularly from the periphery of the defect; • The nHA-collagen+cells, printed in disk geometry, show a substantial new bone formation, well distributed throughout the defect, even in the center of the defect. |
[19] | |
| Collagen/Vascular endothelial growth factor (VEGF) | Stem cells from the apical papil la (SCAPs)/Human umbilical veinendo thelial cells (HUVECs) | Mice | Calvaria defect | Bone | No | • Randomly seeded cells were poorly organized within the region of interest in the defect at 2 months; • For the ‘ring’ condition, the geometry of vascularized area was consistent with the initial printed pattern. For the ‘disc’ and ‘crossed circle’ patterns, the initial pattern was distorted; • Vascularization rate and bone regeneration rate significantly increased in the ‘disc’ or ‘crossed circle’ patterns of HUVECs; • HUVECs are not the only cells involved in the network formation, and host murine cells seem to have a role in this process. |
[20] | |
| Droplet-based bioprinting | Fibrin/Collagen | Amniotic fluid-derived stem cells (AFSCs)/MSCs/HUVECs | Mice | Skin wound on dorsa | Skin | No | • AFS cell- and MSC-driven wound closure and reepithelialization were significantly greater than wounds treated by fibrin-collagen only; • Microvessel density and capillary diameters in AFS cell-treated wounds increased compared with MSC-treated wounds, whereas the skin treated only with gel showed the least microvessels; • AFS cells secreted growth factors at higher concentrations than MSCs, resulting in increased wound closure rates and angiogenesis. |
[21] |
| HA/Heparin-conjugated hyaluronic acid (HA-HP) | AFSCs | Mice | Skin wound on dorsa | Skin | No | • Deposition of the HA-HP+AFS cells accelerated closure of wounds faster than HA-only hydrogels and treatmentfree controls, and induced increased vascularization; • The increased levels of elastin, GAGs and proteoglycans, and decreased relative expression of collagen type I suggested the regenerated skin using HAHP+AFS cells is more pliable and elastic compared to control groups. |
[22] | |
| Fibrin/Collagen | Fibroblasts / Keratinocytes | Mice/pig | Skin wound on dorsa | Ski | Yes |
Murine Model: • Printed skin cells closed the wound by 3 weeks postsurgery compared to 5 weeks for untreated and matrixonly groups; • Epithelialization of bioprinted wounds was observed, forming an immature epidermal barrier. Porcine Model: • Bioprinting of autologous cells resulted in ~3-week acceleration in wound closure compared to other treatments (i.e., untreated, matrix and allogenic cell-treated); • Bioprinting autologous cells resulted in ~50% reduction in wound contraction compared to other treatments; • Bioprinting autologous cells resulted in a 4-5 week acceleration in wound re-epithelialization compared to other treatments; • Collagen fibers present in the autologous cell-treated wounds appeared larger and more organized than other groups. Comparison with cell spraying: • Bioprinted wounds showed accelerated formation of epidermis and more mature dermis tissue; • Staining of collagen fibers were more prominent in the bioprinted wounds at week 4 compared to wounds treated with the sprayed cells. |
[23] | |
Considerations and future outlook for IOB
Limitations of current bioprinting modalities
Bioprinting modalities can be categorized as extrusion-, droplet- and laser-based, which have been comprehensively reviewed elsewhere [24–26], and also summarized in Box 1. For IOB, more challenges appear when adapting knowledge gained through in vitro systems. Extrusion-based bioprinting (EBB) can be considered an appropriate modality for IOB since manual injection (extrusion) of biomaterials has already been clinically applied for decades [27, 28] and current surgical robots, used in some clinical applications such as urology and gynecology [29], can be easily reconfigured to hold EBB tips. However, the print tip might interfere with defect periphery since it is a contact-based technology. In terms of droplet-based bioprinting (DBB), although its resolution is superior than that of EBB, bioink deposition through a small orifice results in higher risk of nozzle clogging, which may increase the duration of surgery and may bring other complications. Limited by poor structural and mechanical integrity of bioprinted constructs, DBB is more suitable for bioprinting thin tissues such as skin. For LBB, it shares similar advantages (e.g., high resolution) and disadvantages (e.g., low integrity of bioprinted constructs) with respect to DBB. The miniaturization of LBB is a major challenge due to its complex setup (such as a laser source and optical components), which constrains its accessibility to internal tissues. Other challenges, such as the immobilization of light sources and the necessity of precise focusing of light, may limit its clinical translation.
Box 1. Comparison of Bioprinting Modalities Regarding Intraoperative Bioprinting.
Extrusion-based bioprinting: Bioprinting of a bioink solution using an extrusion mechanism, resulting in deposition of cells laden in the bioink in the form of cylindrical filaments of customized 3D structures.
Strengths
Compatible with a large variety of bioink material
High mechanical strength with structural integrity, suitable for reconstruction of hard tissues
Appropriate modality for intraoperative bioprinting due to its similarty to manual injection, which has been clinically applied
Enables bioprinting of scaffold-free bioinks such as tissue spheroids
Commercially available with moderate cost.
Limitaitons
Print tip might interfere with defect periphery due to its contact-based mechanism
Cell damage due to high shear stress
Low resolution preventing bioprinting of thin layers of tissues, such as the skin, with a stratified arrangement of multiple layers.
Droplet-based bioprinting: Bioprinting of a bioink solution with a droplet deposition mechanism medicated by etectrical, thermal, or acoustic energy.
Strength
Capable of bioprinting multipie types of cell
High resolution with high-throughput capability
Affordable, versatile, And crommercially available
Non-contact bioprinting (the nozzle does not interfere with the defect).
Limitations
Compatibilty with low viscosity biolinks (in the range of 3.5–12 mPa s)
Poor structural and mechanical integrity of bioprinted constructs
Small orifice results in higher risk of nozzle cloging, which may increase the duration of surgery and associated risks.
Laser-based bioprinting: Bioprinting of bioink with the laser energy as the major deposition mechanism, atowing high-precision patterning of biologics or fabrication of tissue constructs.
Strength
Compatiblity with viscosities in the range of 1–300 mPa s
High resolution with the capability of single cell printing
Nozzle-free bioprinting resulting in negligible cell damage
Non-contact bioprinting.
Limitations
Poor structural and mechanical integrity of bioprinted constructs
Complicated to operate and difficult to miniaturize for a surgical setting
Labor intensive and time consuming leading to increased surgery duration
Higher cost and no commercial availability.
Compatibility of bioinks to surgical settings
Since the deposition is performed directly into a defect in physiological conditions, an ideal bioink should not only meet the general bioink requirements [30] but also possess some features specific to IOB. In particular, such bioinks are expected to be (1) compatible with and intraoperatively bioprintable by the targeted modality to shorten the surgery duration, (2) rapidly crosslinkable in situ to retain the integrity and resolution of bioprinted constructs in physiological temperature and moist environment, and (3) commercially available and affordable to minimize the surgery cost. Therefore, most of the popular biomaterials (e.g., polycaprolactone, polylactic acid, etc.), which rely on volatile organic solvent and high melting temperature, become inappropriate. Among hydrogels, although collagen transitions to gel state at 37 ºC, its slow gelation hinders its use in IOB; however, nano-hydroxyapatite-reinforced collagen has shown promising results in bone regeneration [19]. Fibrinogen is also popular due to its rapid crosslinking when it is interchangeably bioprinted with thrombin into a defect. Photo-crosslinkable bioinks, such as gelatin methacrylamide (GelMA), hyaluronic acid methacrylate (HAMA) and poly(ethylene glycol), have been commonly used in bioprinting [30]. Since exposing UV light directly to a live subject can be dangerous [31], one practical example is to expose UV light towards the side of a transparent nozzle during bioink extrusion [32]. Recently, visible light photo-initiating systems have become popular, which avoided the use of UV light favoring cell viability in photo-crosslinkable bioinks [33–35]. In addition to existing bioinks, more alternatives are expected to be developed to broaden the options for IOB. Newer materials may facilitate retention of the bioprinted constructs at the desired site without the utilization of a support dressing/scaffold, such as a vacuum assisted closure (VAC) device. The manufacture of rapidly integrable bioinks would expand the feasibility of bioprinting into enclosed cavities (e.g. abdomen, thorax), which are not conducive to graft immobilization techniques when compared to more superficial sites, such as the skin.
Automation of IOB processes
Imaging of defects during IOB should be performed in a minute timescale immediately after a surgical excision since the prolonged exposure of the defect can increase surgical complications [36]. To match this requirement, scanners based on 3D photogrammetry provide a supreme solution to obtain raw data of the defect topography during IOB. Several models of portable photogrammetric scanners (e.g., Artec Space Spider and CREAFORM HandySCAN) are able to reach a resolution upto ~30 μm with an extremely short scan time (< 5 min), and the portability offers the possibility to get the defect scanned easily. To create a model of tissue constructs, image processing is necessary including image pre-processing, segmentation, feature extraction, and data mining [37]. During IOB, all these processes should be completed in a few minutes after obtaining the scanned data. Hence, segmentation software is vital for convenient extraction of region of interest from 3D images [38, 39]. Since defects have unique textural features case by case, improper post-processing of images might generate pointless 3D models, which needs to be carefully evaluated. Artificial intelligence (AI) and robotics can be incorporated to reduce the process time and variation caused by operators. So far, researchers have strived to optimize path planning targeted at IOB [40, 41], including printing on a free-moving hand anatomy using motion tracking [42, 43]. In the future, machine learning can be used to automatically generate optimal bioprinting strategies [44–47]. The integration of 3D scanner with a fixed relative coordinate to the robotic arm is also required to eliminate the need of pre-bioprinting calibration to minimize total processing time.
Due to shape distortions caused by the sol-gel transition of hydrogels and movement of the live subject during surgery (such as breathing), automation of IOB with tight control on high resolution is also challenging. This issue might be addressed by real-time monitoring technologies to observe construct deformation and provide feedback signal for subsequent deposition [43]. Commercially-available bioprinters with 3-axis coordinates are usually not sufficient for IOB for irregular-shaped defects that necessitates the use of bioprinters with higher degrees of freedom (DOF), where robotic arms (such as surgical robots) can be the ideal solution. Although BioAssemblyBot with a 6-axis robotic arm from Advanced Solutions Inc. (Figure 2A, Key Figure) is currently the only commercially-available bioprinter with higher DOF capability, there are many companies specialized in robotic surgeries (e.g., Intuitive Surgical, MAKO Surgical Corp, etc.) with potential of integrating bioprinting capabilities into surgical robots in the future.
Figure 2, Key Figure.
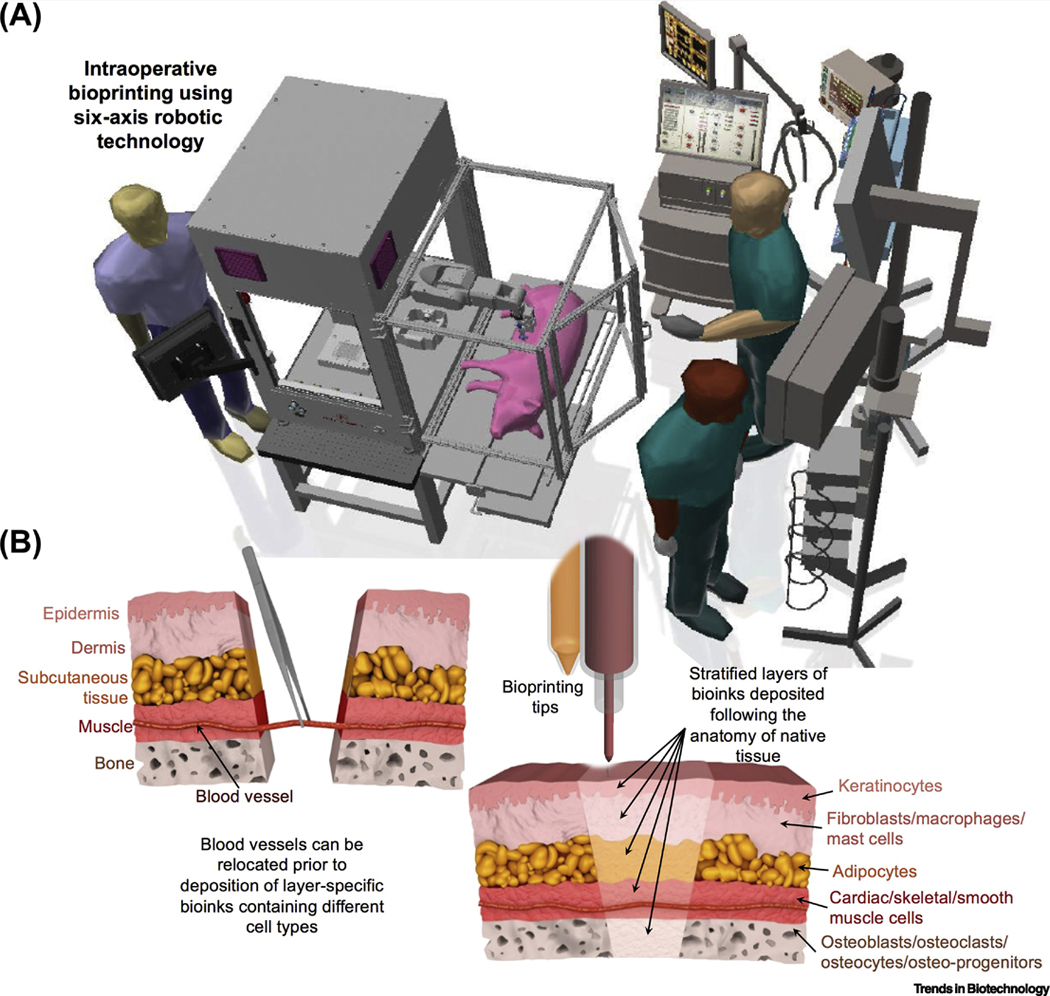
IOB in a surgical setting and its application for repair of composite tissues. Conceptual schematic diagram of (A) IOB in a surgical setting, and (B) the regeneration of composite tissues in a stratified manner. In the case of blood vessels are retained in host tissue; they can be relocated (left) prior to the deposition of layer-specified bioinks containing different cell types (right).
Vascularization in intraoperatively bioprinted tissues
Vascularization is crucial for maturation of bioprinted constructs, especially in segmental defects. Although bioprinted constructs with an embedded microvasculature have been described [4], it is still not feasible to directly anastomose them to the recipient vasculature. Therefore, engineered grafts still rely on inosculation from the recipient bed for perfusion. In order to drive micro-vascularization within intraoperatively bioprinted tissues, there are multiple ways, such as bioprinting of endothelial progenitor cells, hypoxia-inducible factor (HIF) or vascular endothelial growth factor (VEGF). Since it usually takes more than 10 days for angiogenesis to take place [48], temporary strategies can be followed to extend the oxygen supply prior to inosculative angiogenesis. For example, oxygen (O2)-filled microparticles or oxygen-generating biomaterials (OGBs) can be bioprinted within the bioink, which are expected to feed the cells until capillaries can actively transfer the blood [49, 50]. Oxygenation rate of OGBs (e.g., peroxides) is the key parameters for viable outcome of organs, which can be controlled by factors such as pH, temperature and solubility of peroxides [51]. Involvement of porous internal structures is an advantage of bioprinting and another option to facilitate infiltration of blood from host tissue, which offers the possibility for IOB of porous constructs. Introduction of macro-pores in IOB can be realized by bioprinting filaments in cross-hatched patterns similar to what has been achieved in conventional in-vitro bioprinting [52].
However, the ultimate goal is to create a bioprinted construct that includes an embedded microvasculature with a continuous anastomosable artery and vein. With the development of super-microsurgical techniques, it is now feasible to perform a direct anastomosis on vessels with an internal diameter < 150 μm [53]. This advancement may provide a mechanism for the rapid establishment of graft blood flow without requisite exposure to main vascular tree. This is significant as the vascular pedicle of the graft can be kept quite short, simplifying the bioprinting process. To facilitate graft anastomosis and vascularization, the implanting surgeon can also utilize a variety of autologous conduits, including the creation of arteriovenous loops.
IOB of composite tissues and their translational potential
Currently, seamless reconstruction of tissues with multiple components such as craniomaxillofacial (CMF) defects (skin, bone and muscle), osteochondral defects (cartilage and bone), and musculoskeletal defects (bone, muscle, tendon and skin) possesses several limitations. Different tissue types exhibit local variations in terms of physiological, anatomical, and histological aspects, and precise layer-by-layer stacking of multiple tissue compartments is not trivial. Such compartmentalization necessitates the precision and effective use of stem cells and differentiation factors, since differentiating stem cells into multiple lineages is crucial in order to recapitulate the native tissue anatomy. Taking a major musculoskeletal defect as an example, it comprises layers of bone, blood vessel, muscle, subcutaneous fat, and skin tissues from inside out, which contains more than 10 cell types (Figure 2B), and has traditionally required an autologous composite fibula free-flap for correction.
IOB of composite tissue should allow rapid acquirement of the defect information with minimum manual interventions, enabling personalized reconstructions in an anatomically accurate and cosmetically appealing manner immediately after characterization of the defect. Although bioprinted constructs are usually designed based on the compartmentalization of native tissues, the maturation of bioprinted tissues may alter its anatomy and phenotype, leading to differences from the native tissue. Since composite tissues are usually thick, vascularization is particularly vital to enhance the proper tissue regeneration, such as bone formation in CMF defects, as necrotic scar tissue would take over if vascularization is not sufficient [54]. Currently, free-flap surgery is the standard of care for the repair of composite segmental defects. A free-flap is an autologous tissue transplant where expendable donor tissue along with its feeding vascular pedicle (artery and vein) is transferred to a remote recipient site [55]. This approach has revolutionized the treatment of large traumatic and oncologic defects. However, free-flap surgery can be restricted by limited donor site supply and donor site morbidity. Additionally, although the goal is to replace like tissue with like tissue, this is often impossible. For example, a fibula free-flap can be used for intra-oral bone and soft tissue replacement in mandible reconstruction but the amount of bone stock is reduced, sensation is lost, and skin is not equivalent to native mucosa. Therefore, it may be better to combine the principles of reconstructive microsurgery and IOB. The surgeon could configure the recipient vasculature to perfuse an adjacent bioprinted construct either via direct anastomosis or angiogenic induction. This would eliminate the concerns of donor supply and morbidity while providing an exact match of the desired replacement tissue. In the future, it is anticipated that bioprinted or tissue engineered grafts will be available instead of harvesting autologous flaps.
Another concern about IOB of composite tissues is the integration of bioprinted tissues to native tissue, especially for avascular tissues, such as cartilage due to its low metabolism and anti-adhesive ECM [56]. Vertical integration of cartilage to underlying bone occurs due to the innate repair capability of bone [56]. In order to enhance the healing capability, intraoperatively bioprinted composite tissues with a histologically similar osteochondral interface will be a promising solution [2], which can be integrated into full-thickness bone-cartilage defects. However, lateral integration of the bioprinted cartilage to the adjacent cartilage is a major roadblock to achieve permanent cartilage replacement [56]. Strategies to enhance lateral integration may include bioink functionalization to enable direct bonding to the adjacent cartilage [57].
So far, animal models for IOB are almost non-load-bearing, such as skin and calvarial defects (Table 1). IOB for repairing load-bearing defect models, such as segmental bone defects in long bones and joint defects, will gain more attention in the future. Mechanical stiffness of intraoperatively bioprinted constructs depends on their inherent properties determined by the bioink, which can be resolved by developing new materials such as tough hydrogels [58] or integrating mechanically-strong thermoplastics. In this regard, bioprinting of soft bioinks can be coupled with thermoplastics (e.g., PCL or PLGA), where one can envision a technology that can facilitate rapid cooling of deposited thermoplastics into a defect in a safe manner (such as laser-based cooling systems [59]). In addition, postoperative care and rehabilitation are still necessary (as in conventional surgeries) until intraoperatively bioprinted tissue restores sufficient mechanical strength.
Concluding remarks
IOB has already shown promise for regeneration of tissues, including cartilage, skin and bone, in animals. However, regeneration of composite tissues, which are composed of hard and soft tissues, and the interface layers in between, have hardly been explored, which require the development of new bioinks with rapid and stable crosslinking, and the integration of advanced bioprinting modalities.
In addition, seeking a practical way to facilitate and enhance vascularization is vital for long-term functionality of intraoperatively bioprinted tissues. IOB is especially appealing to combine with vascular anastomosis to repair composite segmental defects, which is currently treated by free-flap surgeries. In long term, automation of IOB not only relies on the integration of sequential processes, but also requires a large amount of clinical cases to optimize the bioprinting strategies (see Outstanding Questions). Beyond scientific considerations, clinical studies will be impeded by ethical and regulatory issues, and sharing of patient-specific information for database development will be related to the protection of private information and intellectual properties. Although significant efforts will be required to address all these issues, we do not doubt that IOB will be a game changer in regenerative surgical care.
Outstanding Questions
What are some efficient methods to facilitate vascularization and micro-capillarization in an intraoperatively bioprinted construct?
How can bioprinting modalities be integrated and miniaturized for intraoperative bioprinting of composite tissues?
How can image processing, robotics and artificial intelligence contribute to the automation of intraoperative bioprinting technologies?
How can current surgical methods be combined with intraoperative bioprinting to promote its clinical translation, especially in the context of repairing of composite tissues?
Beyond the scientific barriers, how can ethical, regulatory, and intellectual property issues be addressed for successful clinical translation of intraoperative bioprinting technology?
Highlights
Recent progress in bioprinting has advanced beyond in vitro applications toward intraoperative bioprinting.
Different intraoperative bioprinting modalities possess pros and cons in specific biomedical applications.
The advantages of intraoperative bioprinting bring forth opportunities to clinically translate bioprinting technologies from bench to bedside.
Intraoperative bioprinting of composite tissues has drawn particular interest since the repair of natural defects usually entails the reconstruction of multiple different tissue types.
Acknowledgement
This research was funded by Osteology Foundation (15–042), International Team for Implantology (1275_2017), and National Science Foundation (1600118) and National Institutes of Health (1R01DE028614-01A1, RDE024790A). The authors are also thankful to Advanced Solutions Inc. and Ms. Talley Fisher (from Penn State) for providing Figure 2A and 2B, respectively.
Glossary
- 3D photogrammetry
The process of getting shape measurements and extracting 3D information from photographs. Digital photogrammetry operates on images of an object those captured from different locations and angles using digital cameras, and has the computer detected overlapping patterns to 3D reconstruct the photographed model.
- Arteriovenous loop
AL is an anastomosis between an artery and a vein, where arterial blood is pumped into the vein.
- Artificial intelligence
AI is a field of science and engineering concerned with the computational understanding of intelligent behavior and with the creation of artifacts that exhibit such behavior.
- Craniomaxillofacial defects
CMF defects are major facial defects where hard and soft tissues are rendered abnormal, dysfunctional or deformed due to the trauma, resection of a tumor, or the result of a disease process.
- Extracellular matrix
ECM is the non-cellular portion of a tissue, which is produced and secreted by cells. The main function of ECM is to provide structural and biochemical support to surrounding cells.
- Inosculation
In plastic surgery, inosculation means blood vessels from the recipient site connects with those of the graft in order to facilitate subsequent blood perfusion and restore vascularity.
- Intraoperative bioprinting (IOB)
A bioprinting process that is performed on a live subject during the course of a surgical operation, in which defect imaging, data processing, process planning, and bioprinting are performed consecutively in a single process.
- Miniaturization
To manufacture ever smaller mechanical, optical and electronic devices.
- Oxygen-generating biomaterials
OGBs are designed to provide a gradual and prolonged oxygen supply to cells as rapid or high concentration of oxygen potentially causes cell death due to the formation of free radicals.
- Path planning
Generation of a path for a print head to deposit bioink to fill the defect with designated pattern based on the scanned 3D model.
- Stem cells
One of the human body’s master cells which are unspecialized (undifferentiated) and retain the ability to divide throughout life and give rise to cells that can become highly specialized and take the place of cells that die or are lost. Popular stem cells in bioprinting including mesenchymal stem cells (MSCs), induced pluripotent stem cells (iPSCs), adipose-derived stem cells (ADSCs), etc.
- Vacuum assisted closure
VAC is a type of therapy to help wounds heal. During the treatment, a device decreases air pressure on the wound, which can help the wound heal more quickly.
Footnotes
Declaration of competing interest
The authors confirm that there are no known conflicts of interest associated with this publication and there has been no significant financial support for this work that could have influenced its outcome.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Vijayavenkataraman S, Lu W and Fuh J 2016. 3D bioprinting of skin: a state-of-the-art review on modelling, materials, and processes Biofabrication 8 032001 [DOI] [PubMed] [Google Scholar]
- [2].Wu Y, Kennedy P, Bonazza N, Yu Y, Dhawan A and Ozbolat I 2018. Three-Dimensional Bioprinting of Articular Cartilage: A Systematic Review Cartilage 1947603518809410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Ashammakhi N, Hasan A, Kaarela O, Byambaa B, Sheikhi A, Gaharwar AK and Khademhosseini A 2019. Advancing frontiers in bone bioprinting Advanced healthcare materials 8 1801048 [DOI] [PubMed] [Google Scholar]
- [4].Datta P, Ayan B and Ozbolat IT 2017. Bioprinting for vascular and vascularized tissue biofabrication Acta Biomater. 51 1–20 [DOI] [PubMed] [Google Scholar]
- [5].Peng W, Datta P, Ayan B, Ozbolat V, Sosnoski D and Ozbolat IT 2017. 3D bioprinting for drug discovery and development in pharmaceutics Acta biomaterialia 57 26–46 [DOI] [PubMed] [Google Scholar]
- [6].Vijayavenkataraman S, Yan W-C, Lu WF, Wang C-H and Fuh JYH 2018. 3D bioprinting of tissues and organs for regenerative medicine Advanced drug delivery reviews 132 296–332 [DOI] [PubMed] [Google Scholar]
- [7].Hong N, Yang GH, Lee J and Kim G 2018. 3D bioprinting and its in vivo applications Journal of Biomedical Materials Research Part B: Applied Biomaterials 106 444–59 [DOI] [PubMed] [Google Scholar]
- [8].Ashammakhi N, Ahadian S, Pountos I, Hu S-K, Tellisi N, Bandaru P, Ostrovidov S, Dokmeci MR and Khademhosseini A 2019. In situ three-dimensional printing for reparative and regenerative therapy Biomedical microdevices 21 42. [DOI] [PubMed] [Google Scholar]
- [9].Singh S, Choudhury D, Yu F, Mironov V and Naing MW 2019. In situ bioprinting–Bioprinting from benchside to bedside? Acta biomaterialia [DOI] [PubMed] [Google Scholar]
- [10].Wang M, He J, Liu Y, Li M, Li D and Jin Z 2015. The trend towards in vivo bioprinting International Journal of Bioprinting 1 15–26 [Google Scholar]
- [11].Ozbolat IT 2015. Bioprinting scale-up tissue and organ constructs for transplantation Trends in biotechnology 33 395–400 [DOI] [PubMed] [Google Scholar]
- [12].Qu F, Guilak F and Mauck RL 2019. Cell migration: implications for repair and regeneration in joint disease Nature Reviews Rheumatology 1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Yan W-C, Davoodi P, Vijayavenkataraman S, Tian Y, Ng WC, Fuh JY, Robinson KS and Wang C-H 2018. 3D bioprinting of skin tissue: From pre-processing to final product evaluation Advanced drug delivery reviews [DOI] [PubMed] [Google Scholar]
- [14].Campbell PG and Weiss LE 2007. Tissue engineering with the aid of inkjet printers Expert opinion on biological therapy 7 1123–7 [DOI] [PubMed] [Google Scholar]
- [15].D O’Connell C, Di Bella C, Thompson F, Augustine C, Beirne S, Cornock R, Richards CJ, Chung J, Gambhir S and Yue Z 2016. Development of the Biopen: a handheld device for surgical printing of adipose stem cells at a chondral wound site Biofabrication 8 015019 [DOI] [PubMed] [Google Scholar]
- [16].Di Bella C, Duchi S, O’Connell CD, Blanchard R, Augustine C, Yue Z, Thompson F, Richards C, Beirne S and Onofrillo C 2018. In situ handheld three‐dimensional bioprinting for cartilage regeneration J Tissue Eng Regen Med 12 611–21 [DOI] [PubMed] [Google Scholar]
- [17].Hakimi N, Cheng R, Leng L, Sotoudehfar M, Ba PQ, Bakhtyar N, Amini-Nik S, Jeschke MG and Günther A 2018. Handheld skin printer: in situ formation of planar biomaterials and tissues Lab on a Chip 18 1440–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Keriquel V, Guillemot F, Arnault I, Guillotin B, Miraux S, Amédée J, Fricain J-C and Catros S 2010. In vivo bioprinting for computer-and robotic-assisted medical intervention: preliminary study in mice Biofabrication 2 014101 [DOI] [PubMed] [Google Scholar]
- [19].Keriquel V, Oliveira H, Rémy M, Ziane S, Delmond S, Rousseau B, Rey S, Catros S, Amédée J and Guillemot F 2017. In situ printing of mesenchymal stromal cells, by laser-assisted bioprinting, for in vivo bone regeneration applications Scientific reports 7 1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Kérourédan O, Hakobyan D, Rémy M, Ziane S, Dusserre N, Fricain J-C, Delmond S, Thébaud NB and Devillard R 2019. In situ prevascularization designed by laser-assisted bioprinting: effect on bone regeneration Biofabrication [DOI] [PubMed] [Google Scholar]
- [21].Skardal A, Mack D, Kapetanovic E, Atala A, Jackson J D, Yoo J and Soker S 2012. Bioprinted amniotic fluid‐derived stem cells accelerate healing of large skin wounds Stem cells translational medicine 1 792–802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Skardal A, Murphy SV, Crowell K, Mack D, Atala A and Soker S 2017. A tunable hydrogel system for long‐term release of cell‐secreted cytokines and bioprinted in situ wound cell delivery Journal of Biomedical Materials Research Part B: Applied Biomaterials 105 1986–2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Albanna M, Binder KW, Murphy SV, Kim J, Qasem SA, Zhao W, Tan J, El-Amin IB, Dice DD and Marco J 2019. In Situ Bioprinting of Autologous Skin Cells Accelerates Wound Healing of Extensive Excisional Full-Thickness Wounds Scientific reports 9 1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Ozbolat IT and Hospodiuk M 2016. Current advances and future perspectives in extrusion-based bioprinting Biomaterials 76 321–43 [DOI] [PubMed] [Google Scholar]
- [25].Gudapati H, Dey M and Ozbolat I 2016. A comprehensive review on droplet-based bioprinting: past, present and future Biomaterials 102 20–42 [DOI] [PubMed] [Google Scholar]
- [26].Mandrycky C, Wang Z, Kim K and Kim D-H 2016. 3D bioprinting for engineering complex tissues Biotechnology advances 34 422–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Clark JM and Cook TA 2002. Immediate reconstruction of extruded alloplastic nasal implants with irradiated homograft costal cartilage The laryngoscope 112 968–74 [DOI] [PubMed] [Google Scholar]
- [28].Besalti O, Ozak A, Pekcan Z, Tong S, Eminaga S and Tacal T 2005. The role of extruded disk material in thoracolumbar intervertebral disk disease: a retrospective study in 40 dogs The Canadian Veterinary Journal 46 814. [PMC free article] [PubMed] [Google Scholar]
- [29].Hussain A, Malik A, Halim M and Ali A 2014. The use of robotics in surgery: a review International journal of clinical practice 68 1376–82 [DOI] [PubMed] [Google Scholar]
- [30].Jungst T, Smolan W, Schacht K, Scheibel T and Groll J r 2015. Strategies and molecular design criteria for 3D printable hydrogels Chemical reviews 116 1496–539 [DOI] [PubMed] [Google Scholar]
- [31].Duchi S, Onofrillo C, O’Connell CD, Blanchard R, Augustine C, Quigley AF, Kapsa RM, Pivonka P, Wallace G and Di Bella C 2017. Handheld co-axial bioprinting: application to in situ surgical cartilage repair Sci Rep 7 5837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Ouyang L, Highley CB, Sun W and Burdick JA 2017. A generalizable strategy for the 3D bioprinting of hydrogels from nonviscous photo ‐ crosslinkable inks Advanced materials 29 1604983 [DOI] [PubMed] [Google Scholar]
- [33].Wang Z, Abdulla R, Parker B, Samanipour R, Ghosh S and Kim K 2015. A simple and high-resolution stereolithography-based 3D bioprinting system using visible light crosslinkable bioinks Biofabrication 7 045009 [DOI] [PubMed] [Google Scholar]
- [34].Lim KS, Schon BS, Mekhileri NV, Brown GC, Chia CM, Prabakar S, Hooper GJ and Woodfield TB 2016. New visible-light photoinitiating system for improved print fidelity in gelatin-based bioinks ACS Biomaterials Science & Engineering 2 1752–62 [DOI] [PubMed] [Google Scholar]
- [35].Wang Z, Kumar H, Tian Z, Jin X, Holzman JF, Menard F and Kim K 2018. Visible light photoinitiation of cell-adhesive gelatin methacryloyl hydrogels for stereolithography 3D bioprinting ACS applied materials & interfaces 10 26859–69 [DOI] [PubMed] [Google Scholar]
- [36].Cheng H, Chen BP-H, Soleas IM, Ferko NC, Cameron CG and Hinoul P 2017. Prolonged operative duration increases risk of surgical site infections: a systematic review Surgical infections 18 722–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Sun W, Starly B, Darling A and Gomez C 2004. Computer ‐ aided tissue engineering: application to biomimetic modelling and design of tissue scaffolds Biotechnology and applied biochemistry 39 49–58 [DOI] [PubMed] [Google Scholar]
- [38].Ozbolat I and Gudapati H 2016. A review on design for bioprinting Bioprinting 3 1–14 [Google Scholar]
- [39].Xu S, Mundra PA, Li H, Zhu S, Welsch RE, Rajapakse JC 2013. Imaging in Cellular and Tissue Engineering, ed Yu H, Rahim NAA (Boca Raton: CRC Press; ) p 223 [Google Scholar]
- [40].Ding H and Chang RC 2018. Simulating image-guided in situ bioprinting of a skin graft onto a phantom burn wound bed Additive Manufacturing 22 708–19 [Google Scholar]
- [41].Li X, Lian Q, Li D, Xin H and Jia S 2017. Development of a robotic arm based hydrogel additive manufacturing system for in-situ printing Applied Sciences 7 73 [Google Scholar]
- [42].O’Neill JJ, Johnson RA, Dockter RL and Kowalewski TM 2017. 3D bioprinting directly onto moving human anatomy. In: 2017 IEEE/RSJ International Conference on Intelligent Robots and Systems (IROS): IEEE; ) pp 934–40 [Google Scholar]
- [43].Zhu Z, Guo SZ, Hirdler T, Eide C, Fan X, Tolar J and McAlpine MC 2018. 3D printed functional and biological materials on moving freeform surfaces Advanced Materials 30 1707495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Shi J, Song J, Song B and Lu WF 2019. Multi-Objective Optimization Design through Machine Learning for Drop-on-Demand Bioprinting Engineering [Google Scholar]
- [45].Gu GX, Chen C-T, Richmond DJ and Buehler MJ 2018. Bioinspired hierarchical composite design using machine learning: simulation, additive manufacturing, and experiment Materials Horizons 5 939–45 [Google Scholar]
- [46].Menon A, Póczos B, Feinberg AW and Washburn NR 2019. Optimization of Silicone 3D Printing with Hierarchical Machine Learning 3D Printing and Additive Manufacturing [Google Scholar]
- [47].Dernowsek J, Rezende R and da Silva J 2017. BioCAE: a new strategy of complex biological systems for biofabrication of tissues and organs J Tissue Sci Eng 8 2 [Google Scholar]
- [48].Staton CA, Stribbling SM, Tazzyman S, Hughes R, Brown NJ and Lewis CE 2004. Current methods for assaying angiogenesis in vitro and in vivo International journal of experimental pathology 85 233–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Kheir JN, Scharp LA, Borden MA, Swanson EJ, Loxley A, Reese JH, Black KJ, Velazquez LA, Thomson LM and Walsh BK 2012. Oxygen gas–filled microparticles provide intravenous oxygen delivery Science translational medicine 4 140ra88-ra88 [DOI] [PubMed] [Google Scholar]
- [50].Pedraza E, Coronel MM, Fraker CA, Ricordi C and Stabler CL 2012. Preventing hypoxia-induced cell death in beta cells and islets via hydrolytically activated, oxygen-generating biomaterials Proceedings of the National Academy of Sciences 109 4245–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Ward CL, Corona BT, Yoo JJ, Harrison BS and Christ GJ 2013. Oxygen generating biomaterials preserve skeletal muscle homeostasis under hypoxic and ischemic conditions PloS one 8 e72485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Lee JM and Yeong WY 2016. Design and printing strategies in 3D bioprinting of cell‐hydrogels: A review Advanced healthcare materials 5 2856–65 [DOI] [PubMed] [Google Scholar]
- [53].Walløe L 2016. Arterio-venous anastomoses in the human skin and their role in temperature control Temperature 3 92–103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Hankenson KD, Dishowitz M, Gray C and Schenker M 2011. Angiogenesis in bone regeneration Injury 42 556–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Shandalov Y, Egozi D, Koffler J, Dado-Rosenfeld D, Ben-Shimol D, Freiman A, Shor E, Kabala A and Levenberg S 2014. An engineered muscle flap for reconstruction of large soft tissue defects Proceedings of the National Academy of Sciences 111 6010–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Huey DJ, Hu JC and Athanasiou KA 2012. Unlike bone, cartilage regeneration remains elusive Science 338 917–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Wang D-A, Varghese S, Sharma B, Strehin I, Fermanian S, Gorham J, Fairbrother DH, Cascio B and Elisseeff JH 2007. Multifunctional chondroitin sulphate for cartilage tissue–biomaterial integration Nature materials 6 385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Hong S, Sycks D, Chan HF, Lin S, Lopez GP, Guilak F, Leong KW and Zhao X 2015. 3D printing of highly stretchable and tough hydrogels into complex, cellularized structures Advanced materials 27 4035–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Nemova G and Kashyap R 2010. Laser cooling of solids Reports on Progress in Physics 73 086501 [Google Scholar]


