Abstract
Aims
Bone marrow-derived mononuclear cell (BM-MNC) therapy may improve myocardial recovery in patients following acute myocardial infarction (AMI), though existing trial results are inconsistent.
Methods and results
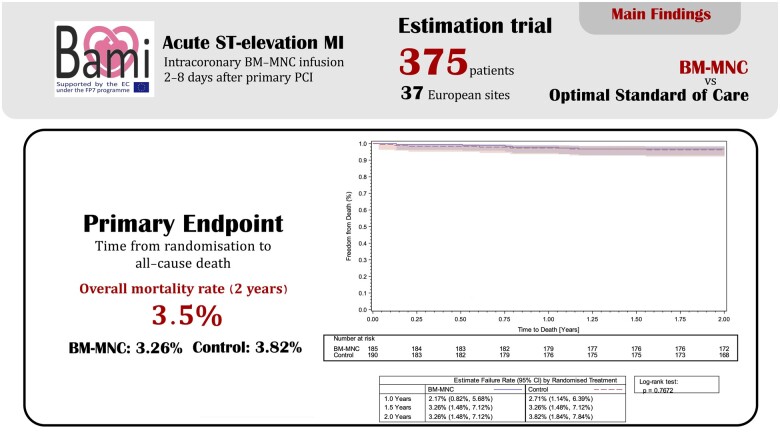
Originally an open-label, multicentre Phase III trial, BAMI was designed to demonstrate the safety and efficacy of intracoronary infusion of BM-MNCs in reducing the time to all-cause mortality in patients with reduced left ventricular ejection fraction (LVEF, ≤45%) after primary angioplasty (PPCI) for ST-elevation AMI. Unexpectedly low recruitment means the trial no longer qualifies as a hypothesis-testing trial, but is instead an observational study with no definitive conclusions possible from statistical analysis. In total, 375 patients were recruited: 185 patients were randomized to the treatment arm (intracoronary infusion of BM-MNCs 2–8 days after PPCI) and 190 patients to the control arm (optimal medical therapy). All-cause mortality at 2 years was 3.26% [6 deaths; 95% confidence interval (CI): 1.48–7.12%] in the BM-MNC group and 3.82% (7 deaths; 95% CI: 1.84–7.84%) in the control group. Five patients (2.7%, 95% CI: 1.0–5.9%) in the BM-MNC group and 15 patients (8.1%, CI : 4.7–12.5%) in the control group were hospitalized for heart failure during 2 years of follow-up. Neither adverse events nor serious adverse events differed between the two groups. There were no patients hospitalized for stroke in the control group and 4 (2.2%) patients hospitalized for stroke in the BM-MNC group.
Conclusions
Although BAMI is the largest trial of autologous cell-based therapy in the treatment of AMI, unexpectedly low recruitment and event rates preclude any meaningful group comparisons and interpretation of the observed results.
Keywords: ST-elevation myocardial infarction, Cell- and tissue-based therapy, Bone marrow cells
Graphical Abstract
Graphical Abstract.
See page 3711 for the editorial comment on this article (doi: 10.1093/eurheartj/ehaa802)
Introduction
Despite more than two decades of pre-clinical and clinical research testing the role of autologous cell-based therapies in the treatment of cardiovascular disease, very few Phase III clinical trials have been conducted. Although attempts were made to unify the experimental approaches used1 , 2 (i.e. the type of cell, method, and timing of delivery), a multitude of Phase II trials have been published using differing methodologies. The European Society of Cardiology’s Task Force for cell-based therapies in cardiovascular disease created a Consortium (listed in Supplementary material online, Appendix S1) to consolidate this considerable pre-clinical research and design a definitive Phase III clinical trial. The Consortium gained funding from the European Commission to design and deliver BAMI: A Phase III clinical trial of autologous cell-based therapies in the treatment of acute myocardial infarction (AMI).
Methods
The full final protocol can be found in Supplementary material online, Appendix S2.
Study design
The autologous bone marrow cell therapy in acute myocardial infarction trial (BAMI) was a multicentre, pan-European, investigator led, randomized, open-label trial co-ordinated by Queen Mary University of London.3 The trial was performed in accordance with the principles of the Declaration of Helsinki. Approvals were obtained from local ethics committees and the pan-European Voluntary Harmonisation Procedure. Funding was provided by the European Union FP7 programme following a competitive application process. After a change in the regulatory processes, all sites contributed additional funding to cover the resultant shortfalls. The trial was designed and conducted by the BAMI Consortium and was co-ordinated from the BAMI Trial Office in London.
Study population
Patients 18 years of age or older with an acute ST-elevation myocardial infarction (as defined by the universal definition of AMI) undergoing acute revascularization (i.e. either acute percutaneous coronary intervention (PCI) within 24 h of symptom onset or thrombolysis within 12 h, followed by acute PCI within 24 h after thrombolysis) were screened at investigational sites.
Potentially eligible patients were consented following their primary PCI. The final eligibility criterion of ≤45% left ventricular ejection fraction (LVEF), based on previous studies suggesting a beneficial effect of cell therapies in patients with impaired cardiac function,4 was confirmed 2–6 days after the primary PCI by the central echocardiography core lab. Patients with cardiogenic shock on admission, and those that developed signs of heart failure following the primary PCI procedure, were excluded. The full list of the inclusion and exclusion criteria can be found in Supplementary material online, Appendix S2.
Bone marrow cell infusion
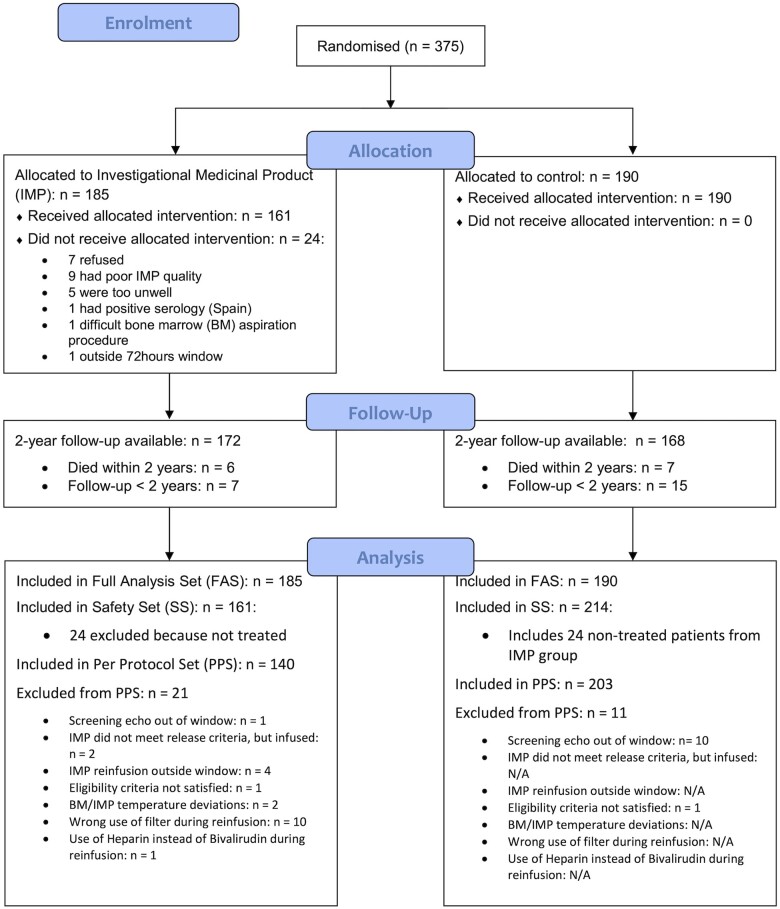
Eligible patients were randomized to the control arm or treatment arm in a 1:1 ratio, which was stratified by country (summarized in Figure 1). Patients in both arms underwent full medical optimization in alignment with existing local protocols.
Figure 1.
CONSORT diagram for patient flow in the BAMI trial.
For patients randomized to the treatment arm, a bone marrow aspirate was performed under local anaesthesia within 2–8 days of the primary PCI. Fifty millilitres of bone marrow was drawn into 5 10 ml heparinized syringes. The aspirates were immediately transported to a dedicated cell processing facility where the bone marrow-derived mononuclear cells (BM-MNCs) were isolated using a Ficoll density gradient as per the t2cure method (Supplementary material online, Appendix S3). Following separation, the BM-MNCs were re-suspended in X-Vivo 10 medium and returned to the source site in a Cryocyte® bag. The BM-MNCs (range 25–500 × 106 as per protocol) were infused into the culprit coronary artery, that was treated with primary PCI at the index admission, using an over-the-wire balloon that matched the vessel dimensions. Infusion used the stop-flow technique: 3 min of balloon inflation with cells delivered through the central lumen of the catheter, followed by 3 min of flow with the balloon down.5 This cycle was repeated three times to deliver the full 10 mls of cells—3 stop-flow cycles of 3.3 ml each. Patients randomized to the treatment arm were infused with BM-MNCs 2–8 days following the primary PCI. Procedural anticoagulation was performed with bivalirudin not heparin due to the possible interaction between the latter and the BM-MNCs.6
Study outcomes
The initial study plan was to demonstrate a 25% reduction in all-cause mortality at 2 years in patients treated with BM-MNCs. When the study was conceived in 2011, contemporary data suggested an event (death) rate of 12% in patients with reduced LVEF7 , 8 , 9 and a sample size of 3000 patients was calculated.3 The primary endpoint of the BAMI trial was the time from randomization to all-cause mortality. The secondary endpoints included time from randomization to cardiac mortality, the composite of cardiovascular death or heart failure re-hospitalization, and the composite of re-hospitalization for repeat myocardial infarction, revascularization, heart failure, implantable cardioverter-defibrillator (ICD), or stroke. Safety endpoints included: adverse events (collected up to 6 months) and serious adverse events, syncope, arrhythmia, neoplastic disease, and bleeds at 2 years.
However, due to a combination of factors including a markedly reduced number of patients with LVEF ≤45%, a reduction in the mortality in patients presenting with AMI (even with impaired left ventricular function), and a limitation on the duration of funding, the steering committee re-issued the statistical analysis plan to present the data as observational results (an ‘estimation’ trial10). This decision was scrutinized and approved by the sponsor and regulatory authorities with a view that the BAMI analysis would produce valuable indicators of effect size for future studies.
All study patients returned for an onsite visit 30 days after hospital discharge. Patients then underwent follow-up by telephone every 3 months from randomization until the end of the trial hospital visit—each patient was followed up for a minimum of 2 years. Endpoints were reported throughout the follow-up period and were adjudicated by an independent Clinical Event Committee (CEC, Supplementary material online, Appendix S6) blinded to the patient treatment allocation.
Statistical analysis
The original trial design was event-driven using existing registry data that suggested a 12% mortality at 2 years in the control group,3 which would be reduced to 9% (25% relative reduction, 1.355 hazard ratio) in the cell-treated group. All patients recruited would complete a 2-year follow-up, even if the trial was stopped prematurely. Using an alpha-spending function according to Lan-DeMets,11 450 events would have been required to have 90% statistical power to detect treatment effect, requiring the recruitment of 1500 patients to each arm. However, the aforementioned markedly reduced recruitment and lower than expected event rates meant that the trial’s statistical analysis would only be sufficient to report effect size based on the reduced number of patients.
Kaplan–Meier curves have summarized the overall mortality by treatment group and have not censored patients at 2 years, but have included their entire follow-up (i.e. up to their time of death, study completion or study termination). Event rates at 2 years are presented together with their associated 95% confidence interval, obtained using a log(-log)-transformation. The treatment effect is estimated by means of a hazard ratio and its associated 95% confidence interval.
Cumulative incidence functions (CIFs) and their 95% CIs at 2 years were calculated for the secondary endpoints and presented for each randomized arm. The treatment effect is estimated by means of a hazard ratio for the sub-distribution hazard of the event of interest using a Fine–Gray regression model. The primary and secondary endpoints are analysed for Full Analysis Set (FAS i.e. intention to treat) and Per Protocol Set (PPS).
The safety endpoints were analysed using the methods described for the secondary efficacy endpoints. For the analysis of adverse events, CIFs have been calculated up to 6 months as only serious events were reported thereafter. Additional analyses were performed for the occurrence of any serious events within the Safety Set. For clarity, only the FAS and Safety Set analyses are presented in the Results section.
The FAS included all patients who were randomized according to their randomized allocation. The Safety Set included all patients according to their actual treatment, i.e. untreated patients randomized to BM-MNC were included in the control group.
All significance testing was two-sided and done at a significance level of 5%. However, due to the nature of the trial, with the aim of the trial being the estimation of event rates and treatment effects, all significance testing is of a purely exploratory nature. No adjustment was made to the significance levels to account for multiple testing.
All analyses were performed using SAS version 9.4 and SAS/STAT version 15.1 for Windows.
Results
Patient and procedural characteristics
From the trial’s start date (10 September 2013) to the last recruited patient (25 October 2017), 518 patients with local LV function thought to be ≤45% were screened, from which 375 patients were enrolled into the BAMI trial after core lab analysis confirmed an echocardiographic LVEF ≤45%. One hundred eighty-five patients were randomized to the BM-MNC group and 190 patients to the control arm. Thirty-seven sites were initiated in the study, out of which 28 contributed to recruitment and 23 delivered BM-MNC therapy (see Supplementary material online, Appendix S4 for the list of sites). In the BM-MNC group, 161 patients received cell therapy [median BM-MNC infused 140 × 106, interquartile range (IQR) 138–340 × 106] with 140 receiving cell therapy per protocol (see Supplementary material online, Appendix S5 for the breakdown in discrepancy between randomized and treated patients).
The baseline characteristics of all trial participants are presented in Table 1. Both groups were well matched for age, ethnicity, and vital signs. Nominal differences in pre-morbid state were observed; the treatment arm contained more patients with insulin-dependent diabetes (8.1% in the BM-MNC arm and 2.6% in the control arm), prior history of myocardial infarction (11.4% and 5.3%), and previous percutaneous revascularization (10.3% and 3.2%).
Table 1.
Baseline characteristics for patients enrolled into BAMI
| Baseline characteristic | Statistic | BM-MNC | Control | Total | P-value |
|---|---|---|---|---|---|
| Total population | N | 185 | 190 | 375 | |
| Age [years] | [n] Mean (SD) | [185] 59 (11) | [190] 60 (11) | [375] 59 (11) | 0.29 |
| Female (%) | n/N (%) | 30/185 (16.22) | 43/190 (22.63) | 73/375 (19.47) | 0.12 |
| Race | |||||
| White | n/N (%) | 165/185 (89.19) | 179/190 (94.21) | 344/375 (91.73) | 0.15 |
| Black | n/N (%) | 3/185 (1.62) | 4/190 (2.11) | 7/375 (1.87) | |
| Asian | n/N (%) | 12/185 (6.49) | 6/190 (3.16) | 18/375 (4.80) | |
| Other | n/N (%) | 5/185 (2.70) | 1/190 (0.53) | 6/375 (1.60) | |
| Vital signs | |||||
| Systolic BP [mmHg] | [n] Mean (SD) | [184] 114 (15) | [190] 113 (18) | [374] 114 (17) | 0.63 |
| Diastolic BP [mmHg] | [n] Mean (SD) | [183] 69 (10) | [190] 69 (12) | [373] 69 (11) | 0.79 |
| Heart rate [b.p.m.] | [n] Mean (SD) | [184] 77 (13) | [190] 78 (12) | [374] 77 (13) | 0.58 |
| Killip class | |||||
| 1 | n/N (%) | 140/176 (79.55) | 136/179 (75.98) | 276/355 (77.75) | 0.28 |
| 2 | n/N (%) | 32/176 (18.18) | 38/179 (21.23) | 70/355 (19.72) | |
| 3 | n/N (%) | 2/176 (1.14) | 5/179 (2.79) | 7/355 (1.97) | |
| 4 | n/N (%) | 2/176 (1.14) | 0/179 (0.00) | 2/355 (0.56) | |
| Hypertension | n/N (%) | 83/185 (44.86) | 94/190 (49.47) | 177/375 (47.20) | 0.41 |
| Hypercholesterolaemia | n/N (%) | 69/185 (37.30) | 72/190 (37.89) | 141/375 (37.60) | 0.92 |
| Insulin-dependent diabetes | n/N (%) | 15/185 (8.11) | 5/190 (2.63) | 20/375 (5.33) | 0.02 |
| Non-insulin-dependent diabetes | n/N (%) | 23/185 (12.43) | 17/190 (8.95) | 40/375 (10.67) | 0.32 |
| Current smoker | n/N (%) | 79/185 (42.70) | 76/190 (40.00) | 155/375 (41.33) | 0.60 |
| Prior smoker | n/N (%) | 33/185 (17.84) | 41/190 (21.58) | 74/375 (19.73) | 0.39 |
| Prior MI | n/N (%) | 21/185 (11.35) | 10/189 (5.29) | 31/374 (8.29) | 0.04 |
| Prior percutaneous revascularization | n/N (%) | 19/185 (10.27) | 6/189 (3.17) | 25/374 (6.68) | 0.01 |
| CABG | n/N (%) | 0/185 (0.00) | 2/189 (1.06) | 2/374 (0.53) | 0.50 |
| Stroke | n/N (%) | 5/185 (2.70) | 3/189 (1.59) | 8/374 (2.14) | 0.50 |
| Renal insufficiency | n/N (%) | 8/185 (4.32) | 5/189 (2.65) | 13/374 (3.48) | 0.41 |
| Malignancy | n/N (%) | 5/185 (2.70) | 13/189 (6.88) | 18/374 (4.81) | 0.09 |
| Time intervals | |||||
| Time from onset to PCI [h] | [n] Median (Q1; Q3) | [176] 3.6 (2.2; 7.2) | [187] 3.8 (2.3; 7.6) | [363] 3.7 (2.3; 7.5) | 0.64 |
| Time from onset to randomization [h] | [n] Median (Q1; Q3) | [179] 81 (66; 106) | [187] 80 (61; 103) | [366] 81 (63; 103) | 0.32 |
| Time from PCI to randomization [h] | [n] Median (Q1; Q3) | [181] 74 (61; 97) | [190] 73 (57; 97) | [371] 74 (57; 97) | 0.28 |
| Location of culprit lesion | |||||
| LM | n/N (%) | 2/185 (1.08) | 4/190 (2.11) | 6/375 (1.60) | 0.69 |
| LAD | n/N (%) | 160/185 (86.49) | 162/190 (85.26) | 322/375 (85.87) | 0.77 |
| CX | n/N (%) | 6/185 (3.24) | 9/190 (4.74) | 15/375 (4.00) | 0.60 |
| RCA | n/N (%) | 17/185 (9.19) | 15/190 (7.89) | 32/375 (8.53) | 0.71 |
| Unknown | n/N (%) | 0/185 (0.00) | 0/190 (0.00) | 0/375 (0.00) | |
| Core Lab LVEF [%] | [n] Mean (SD) | [185] 39 (5) | [190] 39 (5) | [375] 39 (5) | 0.24 |
Primary PCI was performed in a timely manner from the onset of chest pain in both groups [3.6 h (IQR 2.2–7.2) BM-MNC and 3.8 h (IQR 2.3–7.6) control]. Likewise, the time from PCI to randomization into the trial was similar for both groups [74 h (IQR 61–97) BM-MNC and 73 h (IQR 57–97) control].
In both groups, the culprit lesion was most frequently located in the left anterior descending coronary artery (86.5% in the BM-MNC and 85.3% control group).
Echocardiographic ejection fraction as determined by the core lab assessment prior to inclusion was 39% ± 5% in the BM-MNC and 39% ± 5% in the control group.
Endpoint analyses
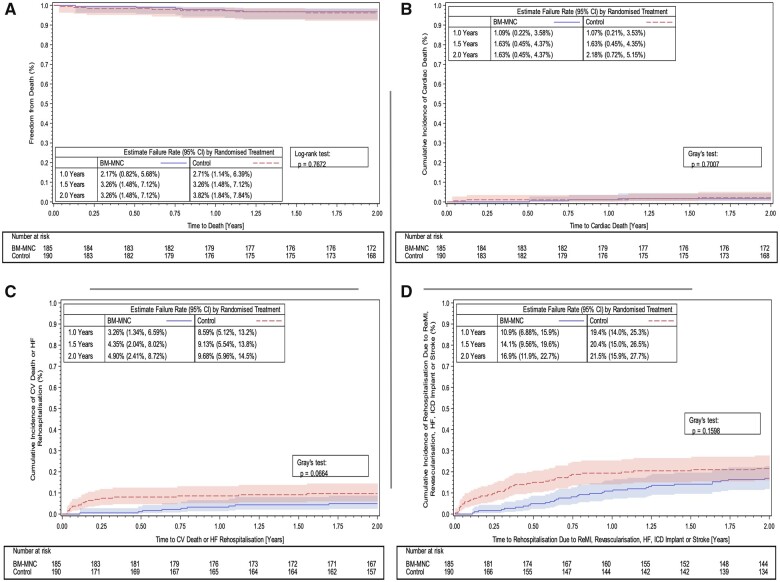
The results are summarized in Table 2. There were six deaths in the BM-MNC group [n = 185, estimated event rate 3.26%, 95% confidence interval (CI): 1.48–7.12%] and seven deaths in the control group (n = 190, 3.82%, 95% CI: 1.84–7.84% control) (Figure 2A). There were three cardiac deaths in the BM-MNC group (1.63%, 95% CI: 0.45–4.37%) and four cardiac deaths in the control group (2.18%, 95% CI: 0.72–5.15%). Kaplan–Meier curves illustrate the temporal occurrence of the composite secondary endpoints in Figure 2C and D.
Table 2.
Summary of results of BAMI for the full analysis and safety sets
| Endpoints (Type of analyses performed) | BM-MNC N (estimated events rate%: 95% confidence interval) | Control N (estimated events rate%: 95% confidence interval) | Estimated hazard ratio vs. control (95% confidence interval) | Event collection time |
|---|---|---|---|---|
| Primary endpoint (Survival analyses performed) | ||||
| Full analysis set (N) | 185 | 190 | ||
| All-cause mortality | 6 (3.3%:1.5 -7.1%) | 7 (3.8%:1.8–7.8%) | 0.85 (0.29–2.53) | 2 years |
| Secondary efficacy endpoints (Competing risk analyses performed) | ||||
| Cardiac mortality | 3 (1.6%: | 4 (2.2%: | 0.75 | 2 years |
| 0.5–4.4%) | 0.7–5.2%) | (0.17–3.32) | ||
| Cardiovascular death or Re-hospitalization due to heart failure | 9 (4.9%: | 18 (9.7%: | 0.48 | 2 years |
| 2.4–8.7%) | 6.0–14.5%) | (0.22–1.06) | ||
|
Re-hospitalization due to re-myocardial infarction, revascularization, heart failure, implantable cardioverter- defibrillator (ICD) or stroke Breakdown: |
31 (16.9%: | 40 (21.5%: | 0.72 | 2 years |
| 11.9–22.7%) | 15.9–27.7%) | (0.45–1.14) | ||
|
5 (2.7%: | 7 (3.8%: | 0.701 | 2 years |
| 1.0–5.9%) | 1.7–7.3%) | (0.22–2.19) | ||
|
13 (7.1%: | 14 (7.5%: | 0.902 | 2 years |
| 4.0–11.4%) | 4.3–11.9%) | (0.43–1.91) | ||
|
5 (2.7%: | 15 (8.1%: | 0.332 | 2 years |
| 1.0–5.9%) | 4.7–12.5%) | (0.12–0.88) | ||
|
10 (5.4%: | 16 (8.7%: | 0.614 | 2 years |
| 2.8–9.4%) | 5.2–13.4%) | (0.28–1.35) | ||
|
4 (2.2%: | 0 (0.0%) | Not calculable | 2 years |
| 0.7–5.1%) | ||||
| Secondary safety endpoints (Competing risk analyses performed) | ||||
| Safety set (N) | 161 | 214 | ||
| Any adverse events | 101 (62.8%: | 116 (54.9%: | 1.20 | 6 months |
| 54.8–69.8%) | 47.9–61.4%) | (0.92–1.55) | ||
| Any serious adverse events | 57 (41.5%: | 78 (60.9%: | 0.96 | 5 years |
| 31.8–50.9%) | 40.7–76.1%) | (0.68–1.36) | ||
| Re-hospitalization due to stroke* | 7 (6.5%: | 1 (1.1%: | 9.17 | 5 years |
| 2.4–13.4%) | 0.1–5.5%) | (1.14–73.47) | ||
| Bleeds | 28 (18.1%: | 29 (14.2%: | 1.29 | 5 years |
| 12.4–24.6%) | 9.8 -19.4%) | (0.77- 2.16) | ||
| Neoplastic disease | 7 (6.6%: | 8 (5.4%: | 1.14 | 5 years |
| 2.7–13.0%) | 2.3–10.3%) | (0.41 -3.12) | ||
| Syncope | 8 (6.5%: | 7 (4.5%: | 1.48 | 5 years |
| 2.8–12.3%) | 1.7–9.6%) | (0.54–4.06) | ||
| Arrhythmias (atrial fibrillation and ventricular tachycardia) | 9 (6.3%: | 19 (9.6%: | 0.60 | 5 years |
| 3.0–11.2%) | 6.0–14.4%) | (0.27–1.31) |
*Rehospitalization due to stroke is an efficacy endpoint, but additional safety analysis has been performed.
Figure 2.
Kaplan–Meier curves showing primary and secondary endpoint results in the BAMI trial: (A) Primary endpoint—all-cause mortality up to 2 years (full analysis set). (B) Secondary endpoint—cumulative incidence functions for cardiac death up to 2 years (full analysis set). (C) Secondary endpoint—cumulative incidence functions for cardiovascular death or re-hospitalization due to heart failure up to 2 years (full analysis set). (D) Secondary endpoint—cumulative incidence functions for re-hospitalization due to repeat myocardial infarction, revascularization, heart failure, implantable cardioverter-defibrillator, or stroke up to 2 years (full analysis set).
Analysis of the secondary endpoints (FAS) demonstrated that five patients in the BM-MNC group were re-hospitalized due to heart failure (2.7%, 95% CI: 1.0–5.9%) and 15 patients (8.1%, CI: 4.7–12.5%) in the control group (Table 2). Four patients in the BM-MNC group (2.2%, 95% CI: 0.7–5.1%) and 0 patients in the control group were hospitalized due to stroke (Table 2). Site-based assessment of each individual stroke hospitalization (which occurred 1–19 months post-cell infusion) did not suggest that these events were related to BM-MNC therapy.
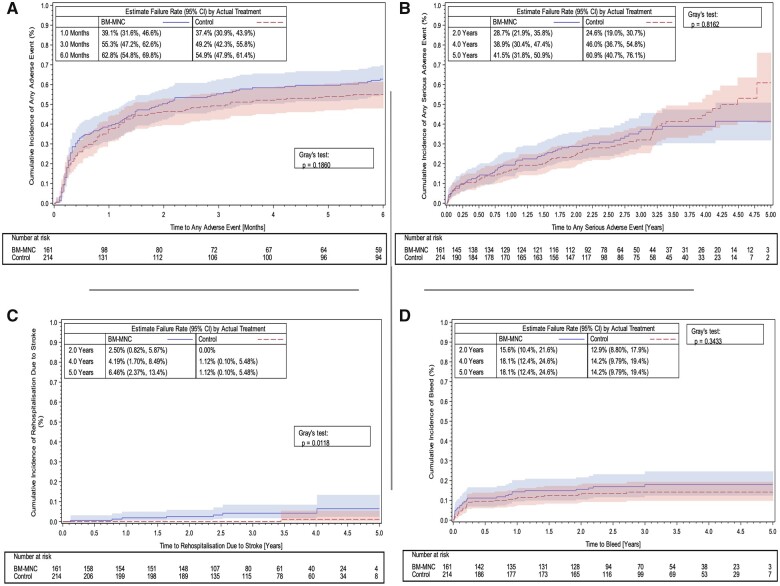
Figure 3 illustrates cumulative event rates in both groups for any adverse event (Figure 3A), any serious adverse event (Figure 3B), re-hospitalization for stroke (Figure 3C), and the occurrence of bleeding (Figure 3D).
Figure 3.
Kaplan–Meier curves showing safety and efficacy endpoints in the BAMI trial. (A) Cumulative incidence functions for any adverse event up to 6 months (safety set). (B) Cumulative incidence functions for serious adverse events up to 5 years (trial end) (safety set). (C) Cumulative incidence functions for re-hospitalization due to stroke up to 5 years (trial end) (safety set). (D) Cumulative incidence functions for bleeding up to 5 years (trial end) (safety set).
Discussion
Given the low recruitment and event rates, BAMI does not qualify for assessing the efficacy of the administration of autologous BM-MNCs on all-cause mortality, nor can it be viewed as a hypothesis-testing trial in patients with successfully re-perfused AMI and depressed LV function 2–6 days post-reperfusion therapy. Consequently, no group comparisons are possible and the data presented are descriptive in nature. However, since BAMI is the largest trial investigating the use of autologous BM-MNC administration in AMI, we believe that the results do provide insights into the future of new therapeutics in the treatment of AMI, and the challenges of designing and conducting trials of autologous BM-MNC-based therapies in AMI.
Firstly, one of the most striking observations is the trial’s very low mortality rate. When the study was conceived in 2011 existing registries suggested that in patients with an LVEF ≤45% post-reperfusion therapy, an all-cause mortality of 12% at 2 years could be expected3 , 7 , 9 reflecting the result of primary angioplasty services that were in evolution during the preceding years. Moreover, the REPAIR-AMI trial, performed in 2004 and 2005, showed that the beneficial effects of BM-MNC administration on clinical outcome (mortality and re-hospitalization for heart failure) was entirely confined to patients with a baseline LVEF ≤45%. Finally, in BAMI, all-cause mortality was chosen as the primary endpoint in order to mitigate any effects of the open-label trial design without a placebo control group, as the intracoronary instrumentation for infusion of a placebo preparation was previously criticized as it may increase event rates in the placebo group and, thereby, skew the results towards the treatment arm. The trial’s very low all-cause mortality (composite of 3.5%) demonstrates the overwhelmingly successful implementation of primary angioplasty across the 28 recruiting sites in 10 European countries, as well as the effectiveness of concomitant medical therapy. Likewise, the difficulty in identifying patients with an ejection fraction ≤45% also points to the success in improved management of myocardial infarction in BAMI sites. Thus, if all-cause mortality is the primary outcome of a trial of new therapeutics for myocardial infarction, the sample size of enrolled patients will need to be much larger (over 10 000 patients, using a mortality rate of 3.5%). This would likely make the trial futile based on cost and logistics. This suggests that future studies will need careful consideration with respect to target population and the use of composite endpoints to meet regulatory approval.
Notwithstanding all these limitations, the results of the present study illustrate that besides the very low mortality rates, re-hospitalization for heart failure occurs very infrequently during 2 years of follow-up, even in patients with a mean LVEF of 39% within 2–6 days of successful acute PCI for AMI.
Thus, for future trials, the logistical and regulatory considerations currently required to administer cell-based products,12 even if efficacy is shown, could only be offset by seeing a dramatic beneficial effect of BM-MNC therapy. Given the low event rate seen in BAMI, the number of patients needed to demonstrate a significant treatment effect, and the logistical costs involved, it is suggested that future trials would be prohibitively expensive. It is important to consider that the largest advance in the treatment of AMI in recent years has been primary angioplasty, which was affected by a change in clinical pathways, not the discovery of a new therapeutic. Whether BM-MNC therapy has a role in other forms of cardiac disease (e.g. heart failure and refractory angina13 , 14) remains to be seen.
The BAMI trial was pivotal due to several important considerations. This was the first attempt at a Phase III study of BM-MNC therapy in the treatment of AMI delivered by an academic Consortium. The Consortium achieved an agreement on a definitive trial design based on existing Phase II trial data collected over the preceding decade (which included patient selection, the processing of cells and the timing of infusion) and took into consideration the various changes in regulatory approvals that occurred between the trial’s planning and completion. The BAMI trial most closely followed the design and methodologies used in the Phase II REPAIR-AMI study, including the delivery of the same order of magnitude of cells. REPAIR-AMI demonstrated improved clinical outcomes including mortality and re-hospitalization for heart failure in patients with reduced LVEF during long-term follow-up after AMI, but was performed in 2004 and 2005.15 Furthermore, meta-analyses demonstrated a signal towards reduced mortality and morbidity following BM-MNC therapy16 , 17; this was in the context of clinical trials of cell-based therapies in patients with AMI which initially did not show dramatic changes in LVEF, but went on to show significant reductions in mortality.18
The trial’s conduct highlighted several important difficulties regarding the regulatory processes surrounding the use of cell-based products in large multicentre, pan-European studies. Although ultimately the logistical issues were solved, the cost in time and money significantly impacted the success of the trial.19
In conclusion, while BAMI cannot provide any definitive answers regarding the effect of BM-MNC therapy on all-cause mortality in AMI patients due to the significant under recruitment, it did demonstrate that autologous cell-based therapy can be delivered following AMI in multiple sites across Europe. The very low 2-year all-cause mortality rate, and the small number of patients developing post-infarction heart failure within 2 years following primary angioplasty for AMI provides useful information regarding the challenges and difficulties involved in future trials of cell-based therapies.
Supplementary Material
Acknowledgements
We would like to acknowledge the financial contribution to the trial made by the Williamson Trust.
Funding
This work was supported by the European Commission under the 7th Framework Programme (Grant Agreement number 278967).
Conflict of interest: J.B. reports grants from European Union FP7 Grant received by Cardiovascular Research Centre Aalst, during the conduct of the study; F.C. reports personal fees from Biotronic, Amgen, Astra Zeneca, Servier, Menarini, and BMS, outside the submitted work; J.H. reports grants from Ministry of Social Affairs and Health (Finland), during the conduct of the study; A.Z. reports personal fees from Sanofi, Boehringer Ingelheim, Amgen, and Novo Nordisk, outside the submitted work; and Co-founder and scientific advisor of t2cure, manufacturer of cellular products. All other authors reported no conflict of interest.
Contributor Information
Anthony Mathur, Centre for Cardiovascular Medicine & Devices, Queen Mary University of London, London EC1M 6BQ, UK.
Francisco Fernández-Avilés, Hospital General Universitario Gregorio Marañón, Instituto de Investigación Sanitaria del Hospital Gregorio Marañón, CIBERCV, Madrid, Spain.
Jozef Bartunek, Cardiovascular Center, OLV Hospital Aalst, Aalst, Belgium.
Ann Belmans, KU, Leuven, Leuven, Belgium.
Filippo Crea, Catholic University of the Sacred Heart, Rome, Italy; Fondazione Policlinico Universitario A. Gemelli IRCCS, Rome, Italy.
Sheik Dowlut, Centre for Cardiovascular Medicine & Devices, Queen Mary University of London, London EC1M 6BQ, UK.
Manuel Galiñanes, Department of Cardiac Surgery, Reparative Therapy of the Heart, Vall d’Hebron Research Institute, University Hospital Vall d’Hebron, Autonomous University of Barcelona, Barcelona, Spain.
Marie-Claire Good, Queen Mary University of London, London, UK.
Juha Hartikainen, Kuopio University Hospital, Kuopio, Finland.
Christine Hauskeller, University of Exeter, Exeter, UK.
Stefan Janssens, University Hospitals (UZ) Leuven, Belgium.
Petr Kala, University Hospital Brno and Medical Faculty of Masaryk University, Brno, Czech Republic.
Jens Kastrup, Rigshospitalet and University of Copenhagen, Denmark.
John Martin, University College London, London, UK.
Philippe Menasché, Department of Cardiovascular Surgery, Hôpital Européen Georges Pompidou and University of Paris, Paris, France.
Ricardo Sanz-Ruiz, Hospital General Universitario Gregorio Marañón, Instituto de Investigación Sanitaria del Hospital Gregorio Marañón, CIBERCV, Madrid, Spain.
Seppo Ylä-Herttuala, University of Eastern Finland, Finland.
Andreas Zeiher, Department of Medicine III, Goethe University of Frankfurt, Frankfurt, Germany.
References
- 1. Bartunek J, Dimmeler S, Drexler H, Fernández-Avilés F, Galinanes M, Janssens S, Martin J, Mathur A, Menasche P, Priori S, Strauer B, Tendera M, Wijns W, Zeiher A. The consensus of the task force of the European Society of Cardiology concerning the clinical investigation of the use of autologous adult stem cells for repair of the heart. Eur Heart J 2006;27:1338–1340. [DOI] [PubMed] [Google Scholar]
- 2. Mathur A, Fernández-Avilés F, Dimmeler S, Hauskeller C, Janssens S, Menasche P, Wojakowski W, Martin J, Zeiher A, the BAMI Investigators. The consensus of the Task Force of the European Society of Cardiology concerning the clinical investigation of the use of autologous adult stem cells for the treatment of acute myocardial infarction and heart failure: update 2016. Eur Heart J 2017;38:2930–2935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mathur A, Arnold R, Assmus B, Bartunek J, Belmans A, Bönig H, Crea F, Dimmeler S, Dowlut S, Fernández-Avilés F, Galiñanes M, Garcia-Dorado D, Hartikainen J, Hill J, Hogardt-Noll A, Homsy C, Janssens S, Kala P, Kastrup J, Martin J, Menasche P, Miklik R, Mozid A, Román J, Sanz-Ruiz R, Tendera M, Wojakowski W, Ylä-Herttuala S, Zeiher A. The effect of intracoronary infusion of bone marrow-derived mononuclear cells on all-cause mortality in acute myocardial infarction: rationale and design of the BAMI trial. Eur J Heart Fail 2017;19:1545–1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Assmus B, Leistner DM, Schächinger V, Erbs S, Elsässer A, Haberbosch W, Hambrecht R, Sedding D, Yu J, Corti R, Mathey DG, Barth C, Mayer-Wehrstein C, Burck I, Sueselbeck T, Dill T, Hamm CW, Tonn T, Dimmeler S, Zeiher AM, Estel S, Braun H, Geweyer I, Palapies L, REPAIR-AMI Study Group. Long-term clinical outcome after intracoronary application of bone marrow-derived mononuclear cells for acute myocardial infarction: migratory capacity of administered cells determines event-free survival. Eur Heart J 2014;35:1275–1283. [DOI] [PubMed] [Google Scholar]
- 5. Schächinger V, Erbs S, Elsässer A, Haberbosch W, Hambrecht R, Hölschermann H, Yu J, Corti R, Mathey D, Hamm C, Süselbeck T, Assmus B, Tonn T, Dimmeler S, Zeiher A; REPAIR-AMI Investigators. Intracoronary bone marrow-derived progenitor cells in acute myocardial infarction. N Engl J Med 2006;355:1210–1221. [DOI] [PubMed] [Google Scholar]
- 6. Seeger FH, Rasper T, Fischer A, Muhly-Reinholz M, Hergenreider E, Leistner D, Sommer K, Manavski Y, Henschler R, Chavakis E, Assmus B, Zeiher A, Dimmeler S. Heparin disrupts the CXCR4/SDF-1 axis and impairs the functional capacity of bone marrow-derived mononuclear cells used for cardiovascular repair. Circ Res 2012;111:854–862. [DOI] [PubMed] [Google Scholar]
- 7. Ng VG, Lansky AJ, Meller S, Witzenbichler B, Guagliumi G, Peruga JZ, Brodie B, Shah R, Mehran R, Stone GW. The prognostic importance of left ventricular function in patients with ST-segment elevation myocardial infarction: the HORIZONS-AMI trial. Eur Heart J Acute Cardiovasc Care 2014;3:67–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sutton NR, Li S, Thomas L, Wang T, Lemos J, Enriquez J, Shah R, Fonarow G. The association of left ventricular ejection fraction with clinical outcomes after myocardial infarction: findings from the Acute Coronary Treatment and Intervention Outcomes Network (ACTION) Registry-Get with the Guidelines (GWTG) Medicare-linked database. Am Heart J 2016;178:65–73. [DOI] [PubMed] [Google Scholar]
- 9. Daneault B, Généreux P, Kirtane AJ, Witzenbichler B, Guagliumi G, Paradis JM, Fahy MP, Mehran R, Stone GW. Comparison of Three-year outcomes after primary percutaneous coronary intervention in patients with left ventricular ejection fraction <40% versus ≥ 40% (from the HORIZONS-AMI trial). Am J Cardiol 2013;111:12–20. [DOI] [PubMed] [Google Scholar]
- 10. Claridge-Chang A, Assam PN. Estimation statistics should replace significance testing. Nat Methods 2016;13:108–109. [DOI] [PubMed] [Google Scholar]
- 11. Lan KKG, DeMets DL. Trust discrete sequential boundaries for clinical trials. Biometrika 1983;70:659–663. [Google Scholar]
- 12. Hauskeller C, Baur N, Harrington J. Standards, harmonization and cultural differences: examining the implementation of a European Stem Cell Clinical Trial. Sci Culture 2019;28:174–199. [Google Scholar]
- 13. Hamshere S, Arnous S, Choudhury T, Choudry F, Mozid A, Yeo C, Barrett C, Saunders N, Gulati A, Knight C, Locca D, Davies C, Cowie MR, Prasad S, Parmar M, Agrawal S, Jones D, Martin J, McKenna W, Mathur A. Randomized trial of combination cytokine and adult autologous bone marrow progenitor cell administration in patients with non-ischaemic dilated cardiomyopathy: the REGENERATE-DCM clinical trial. Eur Heart J 2015;36:3061–3069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Henry TD, Losordo DW, Traverse JH, Schatz RA, Jolicoeur EM, Schaer GL, Clare R, Chiswell K, White CJ, Fortuin FD, Kereiakes DJ, Zeiher AM, Sherman W, Hunt AS, Povsic TJ. Autologous CD34+ cell therapy improves exercise capacity, angina frequency and reduces mortality in no-option refractory angina: a patient-level pooled analysis of randomized double-blinded trials. Eur Heart J 2018;39:2208–2216. [DOI] [PubMed] [Google Scholar]
- 15. Assmus B, Rolf A, Erbs S, ElsäSser A, Haberbosch W, Hambrecht R, Tillmanns H, Yu J, Corti R, Mathey DG, Hamm CW, SüSelbeck T, Tonn T, Dimmeler S, Dill T, Zeiher AM, SchäChinger V, REPAIR-AMI Investigators. Clinical outcome 2 years after intracoronary administration of bone marrow-derived progenitor cells in acute myocardial infarction. Circ Heart Fail 2010;3:89–96. [DOI] [PubMed] [Google Scholar]
- 16. Jeevanantham V, Butler M, Saad A, Abdel-Latif A, Zuba-Surma EK, Dawn B. Heart failure adult bone marrow cell therapy improves survival and induces long-term improvement in cardiac parameters a systematic review and meta-analysis. Circulation 2012;126:551–568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Martin-Rendon E, Brunskill SJ, Hyde CJ, Stanworth SJ, Mathur A, Watt SM. Autologous bone marrow stem cells to treat acute myocardial infarction: a systematic review. Eur Heart J 2008;29:1807–1818. [DOI] [PubMed] [Google Scholar]
- 18. Reffelmann T, Könemann S, Kloner RA. Promise of blood- and bone marrow-derived stem cell transplantation for functional cardiac repair. Putting it in perspective with existing therapy. J Am Coll Cardiol 2009;53:305–308. [DOI] [PubMed] [Google Scholar]
- 19. Hauskeller C. Between the local and the global: evaluating European regulation of stem cell regenerative medicine. Perspect Biol Med 2018;61:42–58. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.