Abstract
Aims
Drug-eluting devices (DED) represent a well-established therapy being widely used for endovascular revascularization (EVR) of peripheral vessels. Recent data indicate a two-fold increased long-term mortality in patients treated with paclitaxel-based DED. The subsequent safety concerns affected international regulatory authorities to enunciate several alerts for further application of DED.
Methods and results
In 9.2 million insurants of the German BARMER Health Insurance, data on the application of paclitaxel-based drug-eluting stents (DES) and drug-coated balloons (DCB) were retrieved from their introduction on the market in 2007 until present. All patients with first EVR between 2007 and 2015 were indexed and followed until 31 December 2017. Each subsequently applied DES, DCB, bare-metal stent, and uncoated balloon was included in further analyses. Multivariable Cox regression analysis considered potential non-linear time-dependent hazard ratios (HRs) of DES and DCB over 11 years. We identified 64 771 patients who underwent 107 112 EVR procedures using 23 137 DED. Multivariable Cox regression analysis showed paclitaxel-based DES not to be associated with increased long-term mortality for over 11 years past application (all P > 0.057). DCB was associated with decreased long-term mortality for the first year past application (HR 0.92; P < 0.001), and indifferent correlation in the years thereafter (all P > 0.202).
Conclusion
Our real-world analysis showed no evidence for increased mortality associated with paclitaxel-based DED for over 11 years.
Keywords: Drug-eluting stent, Drug-coated balloon, Paclitaxel device, Endovascular revascularization, Lower extremity artery disease, Patient safety
See page 3740 for the editorial comment on this article (doi: 10.1093/eurheartj/ehz881)
Introduction
In the last decade, the technology of drug-eluting devices (DED) for endovascular therapy of patients with lower extremity artery disease (LEAD) rapidly developed. Since its introduction on the US market in 2012,1–3 the anti-proliferative drug paclitaxel became widely accepted as a coating substance of drug-eluting stents (DES) and drug-coated balloons (DCB).1,4–7 Randomized clinical trials (RCTs) showed promising results for DES on small-sized selected cohorts to improve late-lumen loss and restenosis rates compared to conventional angioplasty (plain old balloon angioplasty, POBA) or nitinol stents (bare-metal stent, BMS).1,6,8,9 Unlike DES that release their drug coating over a period of 2–4 weeks to the intimal endothelial layer, DCB-mediated paclitaxel application is a one-off procedure leaving nothing behind. Despite critical voices mainly in the face of weak clinical efficacy and cost-effectiveness,10,11 DED of various designs expand on the international market.7,12 Today, DED have become a recommended and commonly used tool for peripheral endovascular revascularization (EVR)13 exceeding annually 55 000 implanted DCB and over 6600 DES (thereof 97% paclitaxel-eluting) alone in Germany (Federal Statistical Offices DESTATIS, 2016).14
Lately, a serious debate on the sensitive issue of patient safety in the use of DED was brought up by unexpected results of a meta-analysis on 28 RCTs (n = 4663 patients, thereof 2552 treated with DED).15 The authors stated an increased risk of all-cause death at two [odds ratio (OR) 1.68; 95% confidence interval (CI) 1.15–2.47] and 4–5 years follow-up (FU; OR 1.93; 95% CI 1.27–2.93) by use of paclitaxel-coated compared to uncoated devices in femoro-popliteal LEAD.
As a consequence, two ongoing major RCTs investigating DED technology (BASIL-3 ISRCTN14469736; SWEDEPAD NCT02051088) halted recruitment.16 Companies of the vascular device sector endeavour to provide preliminary patient-level data in order to address safety concerns of their products.17 From official site, the US Food and Drug Administration (FDA) released a recommendation appealing for critical indication and informed patient consent in the use of DED,18 to which international regulatory authorities were further referring.19 In a recent update, the FDA again tightened its recommendations as a result of preliminary long-term analyses of the three critical RCTs, showing a ‘potentially concerning signal of increased long-term mortality in study subjects treated with paclitaxel-coated products’.20 According to the revalidation of the original trial data, the 5-year mortality risk of DED was 20.1% vs. 13.4% in non-DED treated patients (n = 975). However, the FDA stated persistent doubts in the interpretation of these results due to relevant limitations, most notably the small number of long-term cohorts.
These precautions in the use of state-of-the-art technology reflect the current high uncertainty in the face of the pre-eminent importance of patient safety. The ongoing debate points out the need for continuing critical surveillance of new technologies beyond its establishment in clinical routine. Administrative data related to national and health insurance claims may provide an effective approach to answer these demands.
Herewith, we present a real-world safety analysis on 64 771 patients that covers the entire period from the market introduction of DED until today. Our analysis evaluates if the use of paclitaxel-based DES and DCB represent a potential hazard for the actual non-idealised patients in which these devices were applied during the past decade. Exemplified on DED, our work shows the prospects of health services research to assess patient safety without undue delay.
Methods
The German reimbursement system governs the remuneration of healthcare services subject to encoded diagnoses (International Statistical Classification of Diseases, German Modification; ICD-10 GM) and procedures (German procedure classification; OPS) by means of the ‘German Diagnosis Related Groups’ taxonomy (G-DRG). This obligatory documentation and accounting system is specified and regulated in detail by mandatory coding instructions. Big data derived from national and health insurance claims such as the BARMER are characterized by high trans-sectoral integrity and validity.21,22
Patient selection
We were provided access to the anonymized insurance claims of ∼9.2 million patients of the German BARMER Health Insurance. All patients who were encoded in-hospital balloon- or stent-assisted EVR of the lower limbs (OPS 8-836.0*, 8-836.f,g,t,s,h,j*, 8-840.0-5*, 8-841.0-5*, 8-848.0-5*; *codes 9,b,c,q,s) between 1 January 2007 and 31 December 2015 were indexed for further analyses. Patients aged <18 years at index (n = 32), with incomplete basic information (n = 15), pre-index period <12 months (n = 456), implausible exit of database (n = 11), or encoded DES of non-paclitaxel or unspecified drug-coating (n = 519) were omitted.
Patients were assigned to one of the four sub-groups in hierarchical order according to their first EVR procedure within the index period:
Drug-eluting stents: if ≥1 DES procedure code (OPS 8-836.h*, 8-836.j*, 8-841.0-5*, 8-848.0-5*) combined with paclitaxel material code (OPS 8-83b.03-06) was used.
Drug-coated balloon: if among the remaining patients a balloon angioplasty code (OPS 8-836.0*) combined with ≥1 DCB material code (OPS 8-83b.b2-5, 8-83b.ba-d) was used.
Bare-metal stent: if among the remaining patients ≥1 stent procedure code (OPS 8-840.0-5*, 8-836.f,g,t,s*) was used.
Plain old balloon angioplasty: if among the remaining patients a balloon angioplasty code (OPS 8-836.0*) without any DES, DCB, or BMS codes used.
The selection process including applied ICD-10-GM and OPS codes is presented in detail in the Supplementary material online, Appendix Figure S1 and Table S1.
Cohort characterization
Baseline characteristics were determined for each subgroup according to primary and secondary diagnoses, and procedures during index-hospitalization and within the previous 24 months (Supplementary Appendix Table S2). Diagnoses include LEAD, chronic ischaemic heart disease, previous acute myocardial infarction, chronic heart failure, cerebrovascular disease, and ischaemic stroke, chronic kidney disease, hypertension, diabetes, dyslipidaemia, obesity, smoking, and cancer (Supplementary material online, Appendix Table S2). These were complemented by previous peripheral and coronary vascular procedures and previous amputation of the lower limbs. Notably, all patients were naive related to paclitaxel-coated peripheral devices, since these were not yet available previous to the index-period (before 2007).
Short-term outcome in the four treatment groups at index (DES, DCB, BMS, and POBA) was assessed based on 30-day mortality, amputations, and other complications (for detailed definitions by coding see Supplementary material online, Appendix Table S2).
Follow-up
All patients were continuously followed until death or end of follow-up (FU). All subsequent EVR procedures (inpatient and ambulatory) of individual patients were precisely recorded. For each EVR, the number of applied devices was determined by evaluation of the OPS matrix: the use of up to six BMS (OPS 8-840.0-5), up to six DES (OPS 8-841.0-5, OPS 8-848.0-5), and up to four DCB (OPS 8-83b.ba-bd) per EVR were separately encoded as described in Supplementary material online, Appendix Table S1. The cumulative number of applied DES and DCB served as estimate for patients’ paclitaxel exposure. Data ascertainment reached until 31 December 2017, providing a median FU of 92 months (2760 days). FU time was 98.8% complete.
Statistical methods
Statistical methods are described in detail in the Supplementary material online, Appendix. In brief, logistic regression analyses of 30-day all-cause mortality tested each type of index EVR in hierarchical order (DES, DCB, BMS, and POBA) to evaluate the association between DED and short-term mortality. The model adjusted for the possible confounders age, sex, pre-existing cardiovascular risk factors, and comorbidities as described above.
To evaluate the association between DED and long-term mortality for up to 11 years FU, a multivariable time-dependent Cox regression analysis was performed that adjusted for patients' risk profile at index and during FU. Per definition, the outcome was the time from index EVR to all-cause death if not censored previously for reaching the end of FU, previous exit of the database (n = 1020), exceeding >10 EVR during FU (n = 345), or for being treated with a device coated with a drug other than paclitaxel (n = 456).
The multivariable Cox regression analysis included all devices that were applied during FU. For each individual device, the analysis accounted for its specific type (DES, DCB, BMS, and POBA) and application date. Particularly, the model allowed for a hazard of DED that is non-constant over time and may alter its effect on long-term mortality past device application. Thereby, also a potentially detrimental effect of DED in the later course of time would become verifiable despite a potentially beneficial effect in the early years or potential aggregation of subsequently applied devices. The hazard ratios (HRs) of individual devices of the same type showed no relevant differences in the time course so that in the final model devices of the same type were cumulated in yearly time intervals to serve as estimates of the patients’ paclitaxel exposure. Elementary mortality HRs for each type of device is presented in annual intervals. Combined HRs for any scenario including multiple devices that were applied various years ago can be determined as the product of elementary HRs. Further details of the established Cox model are given in the statistical analysis plan (Supplementary material online, Appendix). All analyses were explorative and P-values are regarded as noticeable if P ≤ 0.05. All statistical analyses were performed using SAS (version 9.4 for Windows, SAS Institute Inc., Cary, NC, USA).
Results
We identified 64 771 patients with an index procedure, defined as first EVR of the iliac and lower limb arteries between 2007 until 2015. DED applied in 5.1% of index EVRs with 2648 DCB (4.1%) and 676 DES (1.0%) procedures (Table 1, Supplementary material online, Figure S1). Of those who had no DED at index (n = 61 447), 5184 (8.4%) received at least one DED during FU, and 2064 patients had repeated DED exposure. Baseline characteristics of the four index cohorts are shown in Table 1. These illustrate relatively homogeneous subgroups in terms of age (average 71.5 years), sex (45.3% female), and cardiovascular comorbidity. LEAD was encoded in 94.9% of patients as the underlying reason for EVR, thereof 42.7% at critical stages of disease. Every second patient had coronary heart disease at baseline, one-third had cerebrovascular disease. Common comorbidities comprised arterial hypertension (90.0%), dyslipidaemia (72.2%), diabetes (47.3%), chronic heart failure (30.9%), and chronic kidney disease (28.1%). Within two years afore index, amputation of the lower limbs was performed in 2.8% of patients, and vascular surgery was performed in 7.1%. EVR previous to the index-procedure applied in 3.1% of patients, notably none of these with DED.
Table 1.
Patient characteristics at baseline according to index procedure
| Characteristics | DES procedure (N = 676) | DCB procedure (N = 2648) | BMS procedure (N = 28 290) | POBA procedure (N = 33 157) | All (N = 64 771) | P |
|---|---|---|---|---|---|---|
| Mean age (year) | 73 | 73 | 70 | 74 | 72 | <0.001 |
| Female sex, n (%) | 293 (43.34) | 1249 (47.17) | 12 438 (43.97) | 15 385 (46.40) | 29 365 (45.34) | <0.001 |
| Lower extremity artery disease, n (%) | ||||||
| Lower extremity artery disease (any) | 622 (92.01) | 2603 (98.30) | 26 830 (94.84) | 31 416 (94.75) | 61 471 (94.91) | <0.001 |
| Lower extremity artery disease (Rutherford Stage 1–3) | 364 (53.85) | 1517 (57.29) | 17 952 (63.46) | 15 391 (46.42) | 35 224 (54.38) | |
| Lower extremity artery disease (Rutherford Stage 4) | 83 (12.28) | 268 (10.12) | 3161 (11.17) | 3527 (10.64) | 7039 (10.87) | |
| Lower extremity artery disease (Rutherford Stage 5) | 88 (13.02) | 409 (15.45) | 2627 (9.29) | 5688 (17.15) | 8812 (13.60) | |
| Lower extremity artery disease (Rutherford Stage 6) | 86 (12.72) | 407 (15.37) | 3059 (10.81) | 6763 (20.40) | 10 315 (15.93) | |
| Previous procedures of lower limb arteries, n (%) | ||||||
| Endovascular revascularization | 16 (2.37) | 76 (2.87) | 666 (2.35) | 1256 (3.79) | 2014 (3.11) | <0.001 |
| Vascular surgery | 42 (6.21) | 187 (7.06) | 1740 (6.15) | 2643 (7.97) | 4612 (7.12) | <0.001 |
| Amputation | 19 (2.81) | 71 (2.68) | 467 (1.65) | 1278 (3.85) | 1835 (2.83) | <0.001 |
| Arteriosclerotic co-diagnoses, n (%) | ||||||
| Coronary heart disease | 346 (51.18) | 1250 (47.21) | 13 235 (46.78) | 17 222 (51.94) | 32 035 (49.49) | <0.001 |
| Previous myocardial infarction | 90 (13.31) | 318 (12.01) | 3560 (12.58) | 4405 (13.29) | 8373 (12.93) | 0.032 |
| Previous coronary revascularization | 65 (9.62) | 218 (8.23) | 2157 (7.62) | 2562 (7.73) | 5002 (7.72) | 0.191 |
| Cerebrovascular disease | 239 (35.36) | 906 (34.21) | 9147 (32.33) | 11 332 (34.18) | 21 624 (33.39) | <0.001 |
| Previous stroke | 109 (16.12) | 398 (15.03) | 3684 (13.02) | 5479 (16.52) | 9670 (14.93) | <0.001 |
| Cardiovascular risk factors, n (%) | ||||||
| Atrial fibrillation or flutter | 160 (23.67) | 588 (22.21) | 4548 (16.08) | 8217 (24.78) | 13 513 (20.86) | <0.001 |
| Chronic kidney disease | 207 (30.62) | 801 (30.25) | 6587 (23.28) | 10 604 (31.98) | 18 199 (28.10) | <0.001 |
| Chronic heart failure | 221 (32.69) | 842 (31.80) | 7295 (25.79) | 11 656 (35.15) | 20 014 (30.90) | <0.001 |
| Diabetes mellitus | 329 (48.67) | 1306 (49.32) | 11 589 (40.97) | 17 409 (52.50) | 30 633 (47.29) | <0.001 |
| Diabetes mellitus (on insulin) | 149 (22.04) | 595 (22.74) | 4337 (15.33) | 8257 (24.90) | 13 338 (20.59) | <0.001 |
| Dyslipidaemia | 512 (75.74) | 1975 (74.58) | 20 567 (72.70) | 23 706 (71.50) | 46 760 (72.19) | <0.001 |
| Hypertension | 614 (90.83) | 2439 (92.11) | 24 850 (87.84) | 30 393 (91.66) | 58 296 (90.00) | <0.001 |
| Nicotine abuse | 190 (28.11) | 807 (30.48) | 10 844 (38.33) | 8062 (24.31) | 19 903 (30.73) | <0.001 |
| Obesity | 158 (23.37) | 646 (24.40) | 6370 (22.52) | 8619 (25.99) | 15 793 (24.38) | <0.001 |
| Cancer, n (%) | 168 (24.85) | 607 (22.92) | 6197 (21.91) | 7734 (23.33) | 14 706 (22.70) | <0.001 |
BMS, bare-metal stent; DCB, drug-coated balloon; DES, drug-eluting stent; POBA, plain old balloon angioplasty.
During the study, in total, 107 112 inpatient and ambulatory EVR procedures were identified, thereof 9401 DCB and 1395 DES procedures (10 796 DED procedures, 10.1%). These correspond to the use of 1973 DES and 21 164 DCB devices (in total ≥ 23 137 DED), accounting for 11.5% of all 200 681 devices being applied (Supplementary material online, Appendix Figure S2).
Acute outcomes
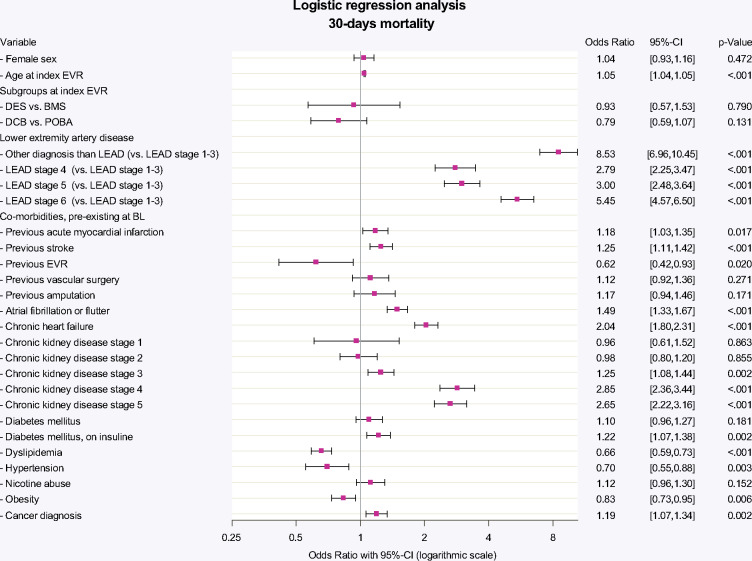
Observed 30-day mortality was 1.6% in DED vs. 2.0% in non-DED procedures (2.1% DES, 1.5% DCB, 1.6% BMS, 2.4% POBA; P < 0.001; Table 2). Multivariable logistic regression was established and detailed model performance is shown in the Supplementary material online, Appendix Figure S3). The logistic regression analysis allowing for co-prevalent risk factors showed adjusted 30-day mortality to be independent from the use of DED, both for DES (vs. BMS: OR 0.93, P = 0.790) and DCB (vs. POBA: OR 0.79, P = 0.131) (Figure 1).
Table 2.
In-hospital outcome according to index procedure
| In-hospital outcome | DES procedure (N = 676) | DCB procedure (N = 2648) | BMS procedure (N = 28 290) | POBA procedure (N = 33 157) | All (N = 64 771) | P |
|---|---|---|---|---|---|---|
| Cardiovascular events, n (%) | ||||||
| Acute myocardial infarction | 6 (0.89) | 16 (0.60) | 294 (1.04) | 375 (1.13) | 691 (1.07) | 0.070 |
| Acute stroke | <5 (<0.70) | 8 (0.30) | 140 (0.49) | 228 (0.69) | 378 (0.58) | <0.001 |
| Lower limb complications, n (%) | ||||||
| Amputation, any | 46 (6.80) | 183 (6.91) | 1199 (4.24) | 3282 (9.90) | 4710 (7.27) | <0.001 |
| Minor amputation | 36 (5.33) | 159 (6.00) | 917 (3.24) | 2605 (7.86) | 3717 (5.74) | <0.001 |
| Major amputation | 10 (1.48) | 24 (0.91) | 282 (1.00) | 677 (2.04) | 993 (1.53) | <0.001 |
| Other complications, n (%) | ||||||
| Acute renal failure | 18 (2.66) | 40 (1.51) | 394 (1.39) | 591 (1.78) | 1043 (1.61) | <0.001 |
| Bleeding event | 73 (10.80) | 196 (7.40) | 2257 (7.98) | 3643 (10.99) | 6169 (9.52) | <0.001 |
| Infection including sepsis | 15 (2.22) | 32 (1.21) | 288 (1.02) | 595 (1.79) | 930 (1.44) | <0.001 |
| Death from any cause, n (%) | 14 (2.07) | 39 (1.47) | 440 (1.56) | 787 (2.37) | 1280 (1.98) | <0.001 |
BMS, bare-metal stent; DCB, drug-coated balloon; DES, drug-eluting stent; POBA, plain old balloon angioplasty.
Figure 1.
Thirty-day mortality adjusted for baseline risk. Thirty-day mortality after multivariable adjustment for baseline characteristics as assessed within 24 months previous to index EVR. Logistic regression model included co-prevalent cardiovascular risk factors, previous vascular procedures, as well as in-hospital complications and adverse events. Death (from any cause) at 30 days did not differ significantly between stent nor balloon angioplasty with vs. without paclitaxel (DES vs. BMS: OR 0.93, 95% confidence interval 0.57–1.53; P = 0.790; drug-coated balloon vs. POBA: OR 0.79, 95% confidence interval 0.59–1.07; P = 0.131). BL, baseline; BMS, bare-metal stent; CI, confidence interval; DCB, drug-coated balloon; DES, drug-eluting stent; EVR, endovascular revascularization; LEAD, lower extremity artery disease; OR, odds ratio; POBA, plain balloon angioplasty.
Long-term outcomes
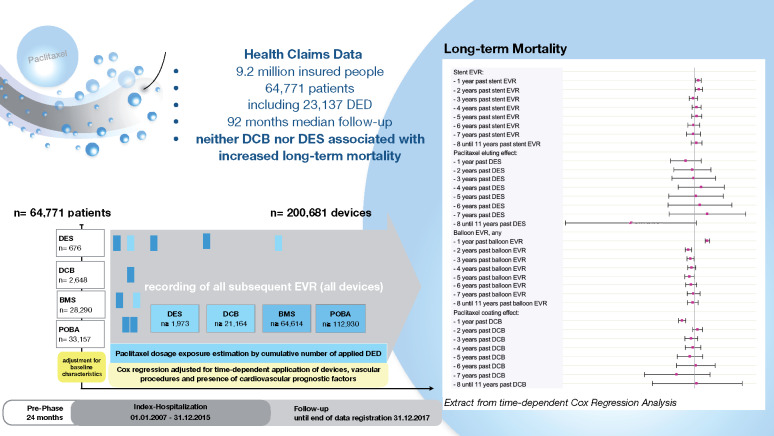
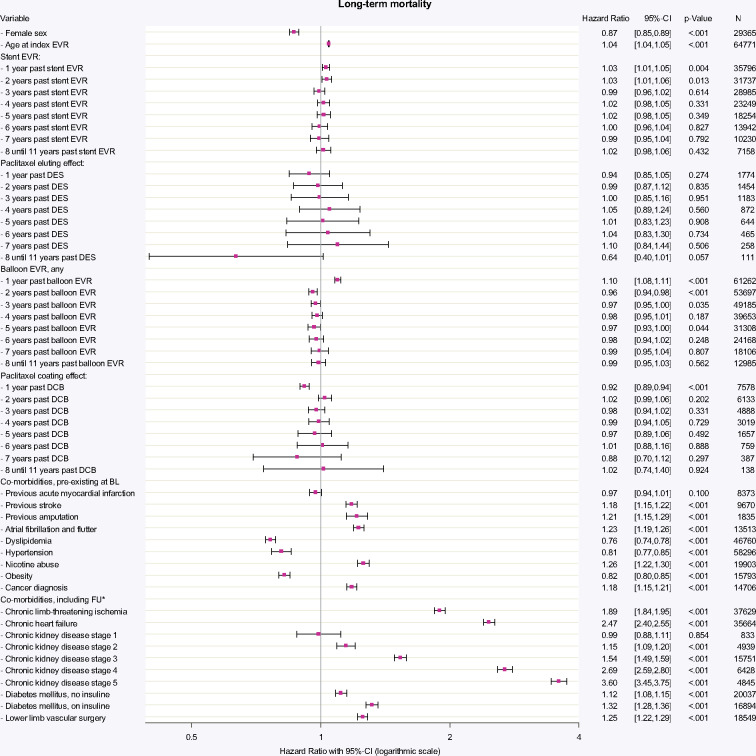
Over the entire study period, 41.9% of patients died. A time-dependent Cox regression analysis was performed that adjusted long-term mortality for DED application and cardiovascular risk indicators at baseline and during FU (Figure 2). The performance of any stent EVR was associated with increased mortality risk within the first two years (first and second year: HR 1.03; P = 0.004 and P = 0.013), which was not verifiable in the following years. For the use of paclitaxel-based DES, a tendency towards increased hazards became apparent beyond the fourth year past application. However, these associations with increased long-term mortality could not be statistically confirmed for up to 11 years after DES application (HR between 0.64 and 1.10; all P > 0.057). Balloon angioplasty was associated with increased mortality for the first year past balloon EVR (HR 1.10; P < 0.001). Paclitaxel coating was not associated with increased long-term mortality for up to 11 years past DCB application. On the contrary, DCB use was associated with decreased mortality for the first year (HR 0.92; P < 0.001), which however became irrelevant in the subsequent years. The mortality hazard for the use of multiple devices, e.g. repeated DED exposure, is the product of elementary hazards as illustrated in the example in Figure 2. Since none of the elementary hazards for DES or DCB reached statistical noticeability, also multiple DED exposure within the same time period will not result in a statistically noticeable increase of long-term mortality.
Figure 2.
Time-dependent Cox-regression allowing for non-linear and time-dependent hazard ratio of the respective devices. All devices applied between 1 January 2007 and 31 December 2017 were included in the model. HRs for each type of device are given per application of one distinct device over annual time intervals. HR for stent EVR (any device) was increased for the first 2 years (both HR 1.03; P = 0.004 and P = 0.013), reflecting the procedural risk and general hazard being involved with the need for stent implantation. The additional effect by use of paclitaxel-based DES was not associated with increased mortality in up to 11 years past application. Likewise, balloon EVR (any device) was associated with evident increased mortality in the first year of application (HR 1.10; P < 0.001). Paclitaxel coating effect in DCB was associated with a protective effect in the first year of DCB application (HR 0.92; P < 0.001), which became irrelevant in the years thereafter. The HR of any combination of applied devices including use of multiple devices in different years and concomitant risk factors can be calculated by multiplying elementary HRs: e.g. a patient with previous stroke, diabetes without insulin and one DES 1 year ago plus two POBA 3 years ago compared to a patient without stroke, without diabetes, only one POBA 3 years ago and identical baseline characteristics (e.g. age and gender) has a HR = [1.18 (previous stroke) * 1.12 (diabetes w/o insulin) * 1.03 (1 stent one year ago) * 0.94 (DES) * 0.972 (two POBAs 3 years ago)]/[0.97 (1 POBA three years ago)] = 1.20/0.97 = 1.24. BL, baseline; CI, confidence interval; DCB, drug-coated balloon; DES, drug-eluting stent; EVR, endovascular revascularization; FU, follow-up period; N, number of patients at end of FU. aTime-dependent adjustment of covariates.
Take home figure.
Safety analysis of paclitaxel based drug eluting devices for application in peripheral arteries.
Discussion
Based on our analysis, from introduction to the market until present, the use of DED was not associated with exceeding death rates compared to non-DED. Therefore, our study debilitates current safety concerns resulting from previous findings.15
Our analysis on 64 771 patients and 107 112 peripheral interventions over a median time period of 92 months (7.6 years) implies high data validity and informational value of the presented results. Compared to the meta-analysis by Katsanos et al.15 that resulted from small-sized selected cohorts of the underlying RCTs, our data reflect the unselected real-world patient collective to which the devices actually apply to. Our cohort is representative compared to other large epidemiological studies in terms of age, sex, cardiovascular risk burden, and LEAD severity.23,24
Long-term mortality
Our database included a high percentage of patients with chronic limb-threatening ischaemia (CLTI 42.7%) which corresponds well with the 43.5% reported for German nationwide inpatient LEAD cases.25 Since CLTI is associated with dramatically increased death rates ranging between 40% and 50% within 5 years,21,24 this explains the observed 41.9% overall mortality during 7.6 years FU in our real-world cohort.
In contrast, Katsanos et al.15 reported markedly lower mortality rates with 14.7% in DED and to 8.1% in non-DED at 4–5 years. The three underlying RCTs, ZILVER-PTX,1 IN.PACT SFA,3 and THUNDER6 included in total n = 268 DCB, n = 214 DES, and n = 403 unspecified non-DED procedures. All procedures were performed on femoro-popliteal lesions in patients at moderate LEAD Rutherford stages (ZILVER-PTX: 9% CLTI; IN.PACT SFA: 5.4% CLTI only Rutherford stage, RF 4; THUNDER: mean RF at index 3.1–3.4, no RF 6). Study protocols further limited risk and complexity of the vascular status, as for example, patients with low life expectancy, poor inflow or absence of at least one patent crural artery were excluded upfront from the trials. Importantly, analyses did not include potential subsequent EVR procedures involving paclitaxel exposure outside the frame of the studies.
The hereupon applied meta-analysis resulted in an almost two-fold increased mortality risk for DED (RR 1.93, 95% CI 1.27–2.93) and indicated an increasing risk per paclitaxel dosage. However, reasonable doubts on these results involve methodical issues such as lack of information on the original patient data and a relevant loss of FU in the RCTs. Moreover, a missing discrimination between stent and balloon EVR as well as statistical simplification of bail-out changeovers between treatment arms in ZILVER-PTX were discussed.26
Value of health claims data for safety concerns
Large-sized administrative data related to national reimbursement and/or health insurance claims may provide an advantageous approach for surveillance after regulatory approval to address patient safety in the long-term. Based on 9.2 million insurants, respectively over 10% of the German population, our data represent the current practice in endovascular treatment of LEAD.
As expected from other trials,27,28 acute complications and deaths during the first 30 days occurred at relatively low levels irrespective of the devices applied. In the long-term, the categorical risk being involved with medical indication for a repeated EVR was reflected in the slightly increased HR for stent and balloon application in the first year. However, thereafter the EVR the procedure itself had no negative impact on overall mortality anymore. Adjusting for an additional effect by the use of paclitaxel-based DED did not result in increased mortality risks.
DES analysis, based on 1973 encoded devices, indicated slightly decreased mortality in the first year past application. Thereafter, DES indeed trended towards hazardous risks although all of these missed statistical conspicuities. Given the relatively high share of DES (44% of DED cases) in the designated low-risk cohorts, the previous meta-analysis15 fits in the greater picture drawn by our analysis. However, the reported hazardous effects were likely overweighted due to the afore-mentioned limitations.26
DCB, based on 21 164 encoded devices, were associated with an 8% decreased mortality hazard per applied DCB within the first year. Up to 8 years past application, a protective or hazardous association was not verifiable. Beyond the eighth year past DED application, the model faces the limitations of decreasing sample sizes for DES and DCB due to the timeliness of the index period.
Strengths and limitations
Strengths of our analysis are its large and comprehensive database of unselected real-world patients, covering the entire period of DED usage from its introduction on the market until today. Pursuant to the structure of the German health care system and legal framework the study contains no missing values. Since exact coding of each device of DES, BMS, and DCB trigger a markedly increase in reimbursement in the G-DRG-System, completeness of the applicable codes could be expected to be very high. The methodical approach of our analysis overcomes changeover between EVR treatment strategies during FU and further considered any aggregation of subsequently applied devices. To detect any possible detrimental effect of DED in the later course of time against a potential early benefit, the chosen model was also able to consider a non-linear time-dependent effect of DED changing its mortality HR over the time course.
Our study is limited by the general constraints in the use of secondary health care data as previously described in detail.21 Specifically, real-world administrative data do not provide information on the underlying reason for DED treatment, representing a potential selection bias. Further, the restricted level of detail in some of the codes affected the present study: paclitaxel exposure was estimated by means of the cumulative number of each device. Up to six DES and a maximum of four DCB can be encoded per procedure. However, the collected numbers for DED as estimates for paclitaxel exposure are correct in all probability since the use of more than four DCB or six DES within the same procedure is certainly a rare exception. Further, the OPS coding system does not register the exact product and manufacturer; individual paclitaxel load or length of device are missing.
Our time-dependent Cox regression analysis cumulated devices of each type within annual time intervals. Preliminary working models showed that this methodological simplification was justified as the temporal course of the HR of each device proved to be independent from the chronological order of its application. Finally, our explorative model needs to be validated externally. We will provide this confirmation in a timely manner in a subsequent publication.
Conclusions
In summary, our analysis found the use of DED to be safe for endovascular therapy of the lower limbs. Particularly with regard to long-term mortality, neither DCB nor DES was associated with increased risk compared to non-DED. Furthermore, our analysis exemplarily demonstrates the significance of health claims data for assessing urgent safety concerns without undue delay.
Supplementary Material
Acknowledgements
The authors thank the personnel of the BARMER health insurance for their technical support.
Conflict of interest: Dr Freisinger reports grants from BAYER and Pfizer outside the submitted work; Dr Gerß received personal honoraria for consultancy, participation in advisory boards and invited lectures from the companies Dr August Wolff GmbH & Co. KG Arzneimittel, QUIRIS Healthcare GmbH & Co. KG, Roche Pharma AG, TESARO Bio Germany GmbH, TESARO Bio GmbH, and Ecker + Ecker GmbH, all outside the submitted work. Dr Malyar reports personal fees from BAYER, other grants from BARD, Cordis, and Medtronic, and personal fees from Daiichi-Sankyo all outside the submitted work. Prof. Dr Reinecke reports personal fees from MedUpdate, personal fees from DiaPlan, personal fees from Pfizer, personal fees from NeoVasc, other from Bristol Myers Squibb, personal fees from Pluristem, personal fees from Daiichi Sankyo, other from Bard, other from Biotronik, and personal fees from NovoNordisk, all outside the submitted work.
References
- 1. Dake MD, Ansel GM, Jaff MR, Ohki T, Saxon RR, Smouse HB, Zeller T, Roubin GS, Burket MW, Khatib Y, Snyder SA, Ragheb AO, White JK, Machan LS; Zilver PTX Investigators. Paclitaxel-eluting stents show superiority to balloon angioplasty and bare metal stents in femoropopliteal disease: twelve-month Zilver PTX randomized study results. Circ Cardiovasc Interv 2011;4:495–504. [DOI] [PubMed] [Google Scholar]
- 2. Liistro F, Grotti S, Porto I, Angioli P, Ricci L, Ducci K, Falsini G, Ventoruzzo G, Turini F, Bellandi G, Bolognese L. Drug-eluting balloon in peripheral intervention for the superficial femoral artery: the DEBATE-SFA randomized trial (drug eluting balloon in peripheral intervention for the superficial femoral artery). JACC Cardiovasc Interv 2013;6:1295–1302. [DOI] [PubMed] [Google Scholar]
- 3. Rosenfield K, Jaff MR, White CJ, Rocha-Singh K, Mena-Hurtado C, Metzger DC, Brodmann M, Pilger E, Zeller T, Krishnan P, Gammon R, Müller-Hülsbeck S, Nehler MR, Benenati JF, Scheinert D; LEVANT 2 Investigators. Trial of a paclitaxel-coated balloon for femoropopliteal artery disease. N Engl J Med 2015;373:145–153. [DOI] [PubMed] [Google Scholar]
- 4. Mehili J, Kastrati A, Wessely R, Dibra A, Hausleiter J, Jaschke B, Dirschinger J, Schömig A; Intracoronary Stenting and Angiographic Restenosis–Test Equivalence Between 2 Drug-Eluting Stents (ISAR-TEST) Trial Investigators. Randomized trial of a nonpolymer-based rapamycin-eluting stent versus a polymer-based paclitaxel-eluting stent for the reduction of late lumen loss. Circulation 2006;113:273–279. [DOI] [PubMed] [Google Scholar]
- 5. Lammer J, Bosiers M, Zeller T, Schillinger M, Boone E, Zaugg MJ, Verta P, Peng L, Gao X, Schwartz LB. First clinical trial of nitinol self-expanding everolimus-eluting stent implantation for peripheral arterial occlusive disease. J Vasc Surg 2011;54:394–401. [DOI] [PubMed] [Google Scholar]
- 6. Tepe G, Zeller T, Albrecht T, Heller S, Schwarzwälder U, Beregi JP, Claussen CD, Oldenburg A, Scheller B, Speck U. Local delivery of paclitaxel to inhibit restenosis during angioplasty of the leg. N Engl J Med 2008;358:689–699. [DOI] [PubMed] [Google Scholar]
- 7. Scheinert D, Duda S, Zeller T, Krankenberg H, Ricke J, Bosiers M, Tepe G, Naisbitt S, Rosenfield K. The LEVANT I (Lutonix paclitaxel-coated balloon for the prevention of femoropopliteal restenosis) trial for femoropopliteal revascularization: first-in-human randomized trial of low-dose drug-coated balloon versus uncoated balloon angioplasty. JACC Cardiovasc Interv 2014;7:10–19. [DOI] [PubMed] [Google Scholar]
- 8. Werk M, Albrecht T, Meyer DR, Ahmed MN, Behne A, Dietz U, Eschenbach G, Hartmann H, Lange C, Schnorr B, Stiepani H, Zoccai GB, Hänninen EL. Paclitaxel-coated balloons reduce restenosis after femoro-popliteal angioplasty: evidence from the randomized PACIFIER trial. Circ Cardiovasc Interv 2012;5:831–840. [DOI] [PubMed] [Google Scholar]
- 9. Laird JR, Schneider PA, Tepe G, Brodmann M, Zeller T, Metzger C, Krishnan P, Scheinert D, Micari A, Cohen DJ, Wang H, Hasenbank MS, Jaff MR; IN.PACT SFA Trial Investigators. Durability of treatment effect using a drug-coated balloon for femoropopliteal lesions: 24-month results of IN.PACT SFA. J Am Coll Cardiol 2015;66:2329–2338. [DOI] [PubMed] [Google Scholar]
- 10. Garg S, Eisenberg MJ. Drug-eluting stents: more dollars than sense? JACC Cardiovasc Interv 2009;2:1188–1189. [DOI] [PubMed] [Google Scholar]
- 11. Reekers JA, de Vries CJ. A decade of drug-eluting technology in peripheral arterial disease: blurred by dissembling evidence. Cardiovasc Interv Radiol 2016;39:1678–1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Müller-Hülsbeck S, Keirse K, Zeller T, Schroë H, Diaz-Cartelle J. Twelve-month results from the MAJESTIC trial of the eluvia paclitaxel-eluting stent for treatment of obstructive femoropopliteal disease. J Endovasc Ther 2016;23:701–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Aboyans V, Ricco JB, Bartelink MEL, Björck M, Brodmann M, Cohnert T, Collet JP, Czerny M, De Carlo M, Debus S, Espinola-Klein C, Kahan T, Kownator S, Mazzolai L, Naylor AR, Roffi M, Röther J, Sprynger M, Tendera M, Tepe G, Venermo M, Vlachopoulos C, Desormais I; ESC Scientific Document Group. 2017 ESC Guidelines on the diagnosis and treatment of peripheral arterial diseases, in collaboration with the European Society for Vascular Surgery (ESVS): document covering atherosclerotic disease of extracranial carotid and vertebral, mesenteric, renal, upper and lower extremity arteries. Endorsed by: the European Stroke Organization (ESO). The Task Force for the Diagnosis and Treatment of Peripheral Arterial Diseases of the European Society of Cardiology (ESC) and of the European Society for Vascular Surgery (ESVS). Eur Heart J 2018;39:763–816. [DOI] [PubMed] [Google Scholar]
- 14.Federal Statistical Offices DESTATIS, Wiesbaden, Germany; data 2016.
- 15. Katsanos K, Spiliopoulos S, Kitrou P, Krokidis M, Karnabatidis D. Risk of death following application of paclitaxel-coated balloons and stents in the femoropopliteal artery of the leg: a systematic review and meta-analysis of randomized controlled trials. J Am Heart Assoc 2018;7:e011245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.ClinicalTrials.gov. https://www.clinicaltrials.gov/ct2/results?term=paclitaxel&cond=Peripheral+Artery+Disease&Search=Apply&recrs=a&age_v=&gndr=&type=&rslt= (20 February 2019).
- 17.LINC programme. https://www.leipzig-interventional-course.com/fileadmin/images/pdf-downloads/programme/LINC2019__programme.pdf (20 February 2019).
- 18.FDA safety alert. https://www.fda.gov/MedicalDevices/Safety/LetterstoHealthCareProviders/ucm629589.htm (20 February 2019).
- 19.AKDAE safety alert. https://www.akdae.de/en/index.html (20 February 2019).
- 20.FDA safety alert. https://www.fda.gov/MedicalDevices/Safety/LetterstoHealthCareProviders/ucm633614.htm (19 March 2019).
- 21. Reinecke H, Unrath M, Freisinger E, Bunzemeier H, Meyborg M, Lüders F, Gebauer K, Roeder N, Berger K, Malyar NM. Peripheral arterial disease and critical limb ischaemia: still poor outcomes and lack of guideline adherence. Eur Heart J 2015;36:932–938. [DOI] [PubMed] [Google Scholar]
- 22. Reinöhl J, Kaier K, Reinecke H, Schmoor C, Frankenstein L, Vach W, Cribier A, Beyersdorf F, Bode C, Zehender M. Effect of availability of transcatheter aortic-valve replacement on clinical practice. N Engl J Med 2015;373:2438–2447. [DOI] [PubMed] [Google Scholar]
- 23. Olinic DM, Spinu M, Olinic M, Homorodean C, Tataru DA, Liew A, Schernthaner GH, Stanek A, Fowkes G, Catalano M. Epidemiology of peripheral artery disease in Europe: VAS educational paper. Int Angiol 2018;37:327–334. [DOI] [PubMed] [Google Scholar]
- 24. Fowkes FG, Aboyans V, Fowkes FJ, McDermott MM, Sampson UK, Criqui MH. Peripheral artery disease: epidemiology and global perspectives. Nat Rev Cardiol 2017;14:156–170. [DOI] [PubMed] [Google Scholar]
- 25. Malyar N, Fürstenberg T, Wellmann J, Meyborg M, Lüders F, Gebauer K, Bunzemeier H, Roeder N, Reinecke H. Recent trends in morbidity and in-hospital outcomes of in-patients with peripheral arterial disease: a nationwide population-based analysis. Eur Heart J 2013;34:2706–2714. [DOI] [PubMed] [Google Scholar]
- 26.FDA Executive Summary. Circulatory System Devices Panel Meeting June 19 and 20, 2019. Paclitaxel-Coated Drug Coated Balloon and Drug-Eluting Stent Late Mortality Panel. https://www.fda.gov/media/127698/download (19 June 2019).
- 27. Liang P, Li C, O'Donnell TFX, Lo RC, Soden PA, Swerdlow NJ, Schermerhorn ML. In-hospital versus postdischarge major adverse events within 30 days following lower extremity revascularization. J Vasc Surg 2019;69:482–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Secemsky EA, Kundi H, Weinberg I, Jaff MR, Krawisz A, Parikh SA, Beckman JA, Mustapha J, Rosenfield K, Yeh RW. Association of survival with femoropopliteal artery revascularization with drug-coated devices. JAMA Cardiol 2019;4:332–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.