ABSTRACT
Accelerated telomere shortening has been associated with several age-related diseases and/or decreased lifespan in humans. The Mediterranean diet (MedDiet) is considered to be 1 of the most recognized diets for disease prevention and healthy aging, partially due to its demonstrated anti-inflammatory and antioxidative properties which may impact on telomere length (TL). The aim of this meta-analysis was to determine the associations between MedDiet adherence and TL maintenance. MEDLINE-PubMed and Cochrane databases were searched up to December 2018 for studies evaluating the association between MedDiet adherence and TL in blood cells. Two reviewers, working independently, screened all titles and abstracts to identify studies that met the inclusion criteria [cross-sectional, case-control, and prospective cohort studies and randomized clinical trials (RCTs) published in English and excluded nonoriginal articles]. Data were pooled by the generic inverse variance method using the random effects model and expressed as standardized mean difference (SMD). Heterogeneity was identified using the Cochran Q test and quantified by the I2 statistic. A total of 8 original cross-sectional studies were included for the quantitative meta-analysis, comprising a total of 13,733 participants from 5 countries. A positive association between adherence to the MedDiet and TL was observed in all meta-analyses, with the exception of those conducted only in men: SMD (95% CI) of 0.130 (0.029; 0.231) for all subjects, 0.078 (0.005; 0.152) for women, and 0.095 (–0.005; 0.195) for men. Only 1 prospective cohort study and 1 RCT were identified, therefore, we could not undertake a meta-analysis for these study designs. The present meta-analysis of cross-sectional studies demonstrates that higher MedDiet adherence is associated with longer TL. At the same time, larger and high-quality prospective studies and clinical trials are warranted to confirm this association.
Keywords: telomere length, accelerated telomere shortening, Mediterranean diet, dietary pattern, healthy aging, age-associated diseases
Introduction
The Mediterranean diet (MedDiet) is a collection of eating habits, determined by sociability, knowledge, intergenerational transmission, and intercultural dialogue going from the landscape to the table (1). The MedDiet is 1 of the most consistent dietary patterns analyzed in relation to the prevention of cardiovascular disease (CVD) and other health outcomes (2), including reduction of overall mortality (3–5) and increased likelihood of healthy aging (6). The traditional MedDiet is characterized by a high intake of vegetables, fruits, nuts, legumes, and grains (mainly unrefined); a high consumption of olive oil but a low intake of saturated fat; a moderately high intake of fish; a low intake of dairy products, meat, and processed meat; and a regular but moderate intake of alcohol (specifically wine with meals) (7). Therefore, it has been proposed that different nutrient synergistic interactions, albeit difficult to identify, may have a beneficial role into the MedDiet-disease axis (8).
Telomeres (TLs)—the protective ends of linear chromosomes—shorten throughout an individual's lifetime (9, 10). Accumulation of critically short telomeres is proposed to be a primary molecular cause of aging and age-associated diseases such as CVD, type 2 diabetes, neurodegenerative diseases, and decreased life expectancy (9, 11–14). TL is considered to be a biomarker of aging. In this regard, positive relations were established between different pathological conditions modulated by oxidative stress and inflammation and the accelerated shortening of telomeres (15). Indeed, nutrition, oxidative damage, telomere shortening, and cell senescence represent a sequence of processes, which may play an important role in in vivo aging and longevity (16,17), with TL being the potential mediator between lifestyle and risk of disease (18, 19). One of the mechanisms in which diet can reduce the risk of disease is with regards to its impact on telomeres.
Accordingly, findings from different studies have shown that lower stress, physical activity, high-quality diets (e.g. including the intake of ω-3 free fatty acids, some antioxidants, and low consumption of processed red meat), and adequate sleep are related to longer telomeres (20, 21). Therefore, the MedDiet could be a good dietary pattern choice to preserve TL throughout lifespan (reviewed in Davinelli et al.) (22). However, even though the previous meta-analysis found contradictory results in relation to TL and overall diet (23), there have been no meta-analyses focused on the MedDiet.
In an attempt to provide a wide-ranging vision of the field and extend the conclusions, the aim of the present analysis was to systematically review and meta-analyze, for the first time, all the published studies investigating the relation between MedDiet adherence and TL in blood cells.
Methods
Protocol and registration
The present study and the corresponding search protocol have been registered at PROSPEROregistry (http://www.crd.york.ac.uk/PROSPERO) as CRD42020150809.
Literature search strategy
We performed a systematic, comprehensive search of the literature for human studies published in English, Spanish, or French, in 2 electronic bibliographic databases, MEDLINE-PubMed database (http://www.ncbi.nlm.nih.gov/pubmed) and the Cochrane Library/Database of Abstracts of Reviews of Effects, from the earliest available online indexing year up to December 2018. Supplemental Table 1 depicts the complete search strategy. Moreover, we performed hand-searching of the reference list of the retrieved articles.
The following inclusion filters were applied in the search: Classical Article, Clinical Conference, Clinical Study, Clinical Trial, Clinical Trial-Phase I, Clinical Trial-Phase II, Clinical Trial-Phase III, Clinical Trial-Phase IV, Controlled Clinical Trial, English Abstract, Journal Article, Letter, Meta-analysis, Multicenter Study, Pragmatic Clinical Trial, Evaluation Studies, Case Reports, Congresses, Dataset, Introductory Journal Article.
Eligibility criteria and study selection
The titles and abstracts of all the preselected articles were screened for eligibility by 2 independent researchers (SC and SG), who are specialists in TL and human nutrition, respectively. Any discrepancies were re-evaluated together with a third author (JS-S). After primary screening (to evaluate the scope of the study), the full texts of the selected articles were obtained. We included cross-sectional, case-control, and prospective cohort studies, and randomized clinical trials (RCTs) and review articles. Only studies defining MedDiet adherence as the exposure (assessed by a priori index scores in the case of observational studies) and TL in blood cells (peripheral blood leukocytes, peripheral blood mononuclear cells, and whole blood; as the outcome were included in the meta-analysis.
Exclusion criteria were as follows: nonoriginal articles (reviews, commentaries, editorials, or letters). Ecologic assessments, nonpeer-reviewed articles, languages other than English, Spanish, or French, mechanistic studies, studies conducted in pregnant women, and supplements to the main manuscript (e.g. Author's Reply sections). Pooled analyses and meta-analysis were used as support for interpretation, but the data were not included in the database.
Data extraction
Using a standardized proforma, 2 independent reviewers (SC and SG) extracted the following information from each study: authors, year of publication, journal, title, location of the study, age, population studied, sample size, study design, type of exposure and assessment method, outcome and assessment method, and main conclusions.
For the present study, since TL can be measured as absolute TL (bp) or relative TL [telomere (T) to single-copy gene (S) sequence (T/S ratio)], TL was considered as the main outcome regardless of the unit of measure used to assess it. Because the included studies do not have a consensus analysis in the statistical data, we contacted the corresponding authors of each study to ask them to complete a table (Supplemental Table 2) in order to standardize the results and provide data for us to analyze. In all cases, we asked the corresponding authors to transform TL (natural logarithm), regardless of the unit used to measure it, prior to the analyses to address the issue of positive skewness in the measurements of this outcome variable. We requested the age- and sex-adjusted and fully adjusted means of TL and SE for each category of MedDiet adherence. It is important to adjust effect sizes for age and sex because studies observed that women tend to have longer telomeres than men (24) and that younger individuals have longer telomeres than older individuals (25). We asked for this information for the total population and for men and women separately. The corresponding author of each study was invited to participate in the meta-analysis.
Study quality assessment
Two independent reviewers evaluated the quality of the cross-sectional studies included in the meta-analysis using the National Heart, Lung, and Blood Institute Quality Assessment Tool for Observational Cohort and Cross-Sectional Studies. This tool guided the authors to rate the overall quality of the studies as good, fair, or poor based on 14 different criteria (https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools; accessed on 30 September, 2019).
Statistical analysis
The meta-analysis was conducted using Review Manager (RevMan) software v.5.3 (http://community.cochrane.org/tools/reviewproduction-tools/revman-5) and R software v.3.5.1 (26) including the R packages “meta” (27) and “dmetar” (28). Results were reported following the meta-analysis of observational studies in epidemiology (MOOSE) guidelines. The generic inverse-variance method with random effects models was used to combine the results across studies comparing extreme categories of MedDiet adherence. Since studies used different methods to assess TL (absolute TL in bp and relative TL based on T/S ratio), we standardized the data to a uniform scale in order to be able to pool them. We used the means, SDs, and sample size to estimate the standardized mean difference (SMD) (adjusted Hedge's g) for each study. The Hedge's g is the difference between the mean TL between the highest and lowest category of MedDiet adherence divided by the pooled SD of the categories multiplied by a correction term (29). Pooled SD was estimated using the following formula:  with “ne” and SDe denoting the number of observations and SD for the highest category of MedDiet adherence, respectively, and “nc” and SDc denoting the number of participants and SD for the lowest category of Med Diet, respectively.
with “ne” and SDe denoting the number of observations and SD for the highest category of MedDiet adherence, respectively, and “nc” and SDc denoting the number of participants and SD for the lowest category of Med Diet, respectively.
We ran meta-analyses on results from both age- and sex-adjusted models and fully adjusted models and for the total population and both men and women separately.
Interstudy heterogeneity was assessed by the Cochran Q statistics (P < 0.10 was considered significant) and quantified by the I² statistic (≥50% indicated substantial heterogeneity). Sensitivity analysis was performed by removing 1 study at a time (i.e. leave-1 out approach) from the meta-analysis and recalculating the pooled effect size. If >10 study comparisons were available in each analysis, publication bias via funnel plot asymmetry, and Begg's and Egger's test, as well as potential sources of heterogeneity were assessed.
We performed subgroup analyses using a random effects model to explore the association between MedDiet adherence and TL. Data included in the meta-analysis was split into subgroups according to the type of MedDiet score used and based on the qPCR methodology used for TL measurements.
Results
Study characteristics
A total of 26 articles were identified after a primary search of MEDLINE-PubMed and the Cochrane Library. The whole process is detailed in Figure 1. After analyzing all abstracts, 11 articles were excluded because they were beyond the scope of the present study (did not assess the effect of MedDiet on TL). A total of 15 articles were collected as full texts and were assessed for inclusion based on the inclusion/exclusion criteria. After applying all the eligibility criteria, 8 articles were included for qualitative analyses. Only 1 RCT and 1 prospective cohort study were found and, therefore, we could not perform a meta-analysis for these types of study designs.
FIGURE 1.
Study selection flowchart.
The present analysis included a total of 13,733 subjects from 5 countries: Australia, Italy, Finland, Spain, and the USA. The characteristics of the 8 studies (13, 30– 36) are summarized in Table 1. Most of the studies are equally distributed by sex except Crous-Bou et al. (31) (women only) and Gu et al. (32) (higher proportion of women: 68%). The age of the participants ranged from 20 to >65y. Across all studies, 4 different MedDiet scores were used [i.e. Trichopoulou 2003 (3) or 2005 (37), Mediterranean Diet Adherence Screener (MEDAS) (38) and Panagiotakos 2007 (39) scores], and TL was analyzed by qPCR [following Cawthon 2002 (40) or 2009 (41)] in all the included studies (Table 1).
TABLE 1.
Characteristics of the included studies and the exposures analyzed based on retrieved data
| Reference of the original publication | Study design | Population | Method used for TL | Exposure/Med Score | Variables included/considered in the MVA | QA |
|---|---|---|---|---|---|---|
| Boccardi et al., 2013 (30) | Cross-sectional | 217 (47% women) elderly Italian subjects, aged 71–87 y | PBL/qPCR Cawthon 2002 (42) | Trichopoulou 2003 (3) | Age, sex, inflammation, smoking habit | Good |
| Crous-Bou et al., 2014 (31) | Cross-sectional | 4676 healthy American women within the Nurses’ Health Study (NHS), aged 30–55 y | PBL/qPCR Cawthon 2002 (42) | Trichopoulou 2003 (3) | Age, BMI, pack-years of smoking, physical activity, total caloric intake | Good |
| Gu et al., 2015 (32) | Cross-sectional | 1743 (68% women) American multiethnic community, Washington Heights-Inwood Community Aging Project (WHICAP) study, aged >65 y | PBL/qPCR Cawthon 2009 (41) | Trichopoulou 2003 (3) | Age, sex, total energy intake, BMI, physical activity, smoking status, diabetes status, hypertensive status, dyslipidemia status, group of intervention (EVOO, nuts, Control) | Good |
| García-Calzón et al., 2016 (13) | Cross-sectional1 | 520 (54.8% women) Spanish individuals at high cardiovascular disease risk, PREDIMED-Navarra study, aged 55–80 y | PBL/qPCR Cawthon 2002 (42) | MEDAS score (38) | Age, sex, education, race/ethnicity (white, black, Hispanics), total caloric intake | Good |
| Leung et al., 2018 (33) | Cross-sectional | 4758 (53.6% women) American healthy nonelderly adults from the NHANES study, aged 20–65 y | Whole blood/qPCR Cawthon 2002 (42) | Panagiotakos 2007 (39) | Age, sex, race/ethnicity, educational attainment, marital status, poverty income ratio, alcohol consumption, smoking status, pack years of smoking, physical activity, change in activity over the past year, and total caloric intake | Good |
| Milte et al., 2018 (34) | Cross-sectional | 679 (52.6% women) Australian individuals from the Wellbeing, Eating and Exercise for a Long Life (WELL) study, aged 57–68 y | Whole blood /qPCR Cawthon 2002 (42) | Trichopoulou 2005 (37) | Age, sex, education, smoking, physical activity, BMI | Good |
| Meinilä et al., 2019 (35) | Cross-sectional2 | 1046 (56.4% women) Finnish individuals from the Helsinki Birth Cohort (HBC) study, aged 56–70 y | Whole blood /qPCR Cawthon 2009 (41) | Trichopoulou 2005 (37) | Age, sex, smoking, total caloric intake, BMI, educational attainment, and leisure-time physical activity | Good |
| Ventura et al., 2019 (36) | Cross-sectional | 94 (57.4% women) healthy adults, West Virginia, (USA), aged 45–64 y | PBMC/qPCR Cawthon 2002 (42) | Trichopoulou 2003 (3) | Age, sex, Life's Simple 7 (a tool estimating the cardiovascular health, continuous scale), smoking history, yearly income | Good |
Also included results from a randomized clinical trial.
Also included results from a prospective study. EVOO, extra-virgin olive oil; MEDAS, Mediterranean Diet Adherence Screener; MVA, multivariable adjusted model; PBL, peripheral blood leukocytes; PMBC, peripheral blood mononuclear cell; PREDIMED, Prevention with Mediterranean Diet; QA, quality assessment; TL, telomere length.
Meta-analyses of cross-sectional studies
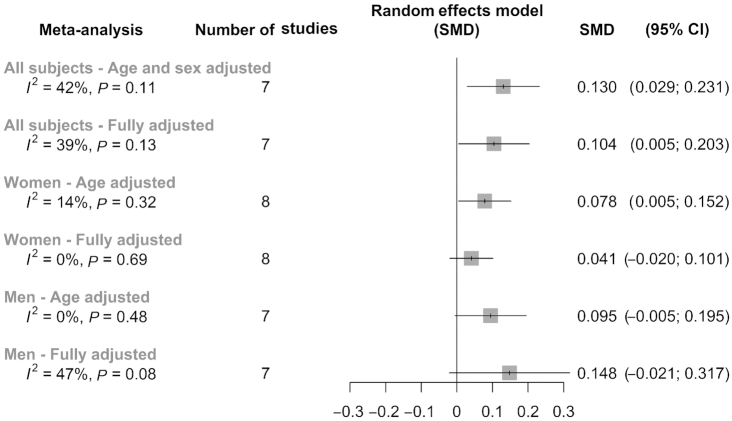
Analyses were performed using unpublished data provided by the authors of each included study. A positive association between MedDiet and TL (measured as the SMD) was found in all meta-analyses with the exception of those conducted only in men, as summarized in the super plot shown in Figure 2. A total of 6 meta-analyses using a random effects model were performed based on type of adjustments (age and/or sex, and fully adjusted) and the population strata [all subjects (Figure 3), specific to women (Figure 4), and specific to men (Figure 5)]. In the case of the analyses specific to women, it included results from Crous-Bou et al. (31) in a cohort of women from the Nurses´ Health Study (NHS). The remaining 7 studies were common to all the analyses (13, 30, 32 – 36). The results showed that for all subjects, age- and sex- adjusted model SMD (95% CI) was 0.130 (0.029; 0.231) (Figure 3). The fully adjusted SMD (95% CI) was 0.104 (0.005; 0.203) (Figure 3). For women, the age-adjusted model SMD was 0.078 (0.005; 0.152) (Figure 4) and for men 0.095 (−0.005; 0.195) (Figure 5). Of note, for women the age-adjusted model was significant, SMD: [0.078 (0.005; 0.152)], whereas this association disappeared in the fully adjusted model, SMD: 0.041 (−0.020; 0.101) (Figure 4). Although the significance was lost, results were still in the same direction as the age-adjusted model, suggesting a putative influence of the variables included in these fully adjusted models that differed between studies. In men, we found no significant associations in any model [age-adjusted model SMD: 0.095 (−0.005; 0.195) and fully adjusted model SMD: 0.148 (−0.021; 0.317)] (Figure 5).
FIGURE 2.
Super plot of the different meta-analyses for the association between telomere length and Mediterranean diet adherence (cross-sectional studies). SMD, standardized mean difference.
FIGURE 3.
Forest plot for the association between telomere length and adherence to the Mediterranean diet including all subjects (cross-sectional studies). Figure shows independent meta-analysis for age- and sex-adjusted (A) and fully adjusted (B) of Hedges’ g parameter. SMD, standardized mean difference.
FIGURE 4.
Forest plot for the association between telomere length and adherence to the Mediterranean diet including women only (cross-sectional studies). Figure shows independent meta-analysis for age-adjusted (A) and fully adjusted (B) of Hedges’ g parameter. SMD, standardized mean difference.
FIGURE 5.
Forest plot for the association between telomere length and adherence to the Mediterranean diet including men only (cross-sectional studies). Figure shows independent meta-analysis for age-adjusted (A) and fully adjusted (B) of Hedges’ g parameter. SMD, standardized mean difference.
Results of prospective cohort studies
The study conducted by Meinilä et al. (35), was the only prospective cohort study evaluating the association between MedDiet adherence, measured using the Trichopoulou 2005 score, and TL. This study showed that, after 10 y of follow-up in 456 men, MedDiet adherence was not associated with TL change. However, MedDiet adherence was associated with faster TL shortening in 590 women after 10 y of follow-up. Because only 1 prospective cohort study of MedDiet adherence and TL was found, this study could not be included in the present meta-analysis.
Results of RCTs
To date, only 1 RCT (13) has evaluated the effect of the MedDiet on telomere shortening after 5 y of follow-up. The study was a multicenter parallel group clinical trial conducted in 520 Spanish adults at high risk of CVD. The results showed no effect of the MedDiet supplemented with extra virgin olive oil on telomere change compared with the control group following a low-fat diet. Nonetheless, the MedDiet supplemented with nuts seemed to have a detrimental effect on telomere shortening [P-ANCOVA = 0.003 (MedDiet-Nuts compared with Control)]. When authors analyzed the risk of telomere shortening (Δ age-adjusted z-score TL ≤20th percentile) compared with the control group, individuals following the MedDiet supplemented with nuts had a higher risk of telomere shortening (OR: 3.18; 95% CI: 1.73–5.90), whereas no effect of the MedDiet supplemented with extra virgin olive oil was reported (OR: 1.28; 95% CI: 0.67–2.44). Since only 1 RCT was available, we did not conduct a meta-analysis for this type of study design.
Heterogeneity and publication bias
Significant evidence of heterogeneity (I2) was only reported in the fully adjusted meta-analysis for men (I2 = 47%, P = 0.08). Due to the reduced number of studies included in this meta-analysis we were not able to assess the sources of heterogeneity. For the same reason, publication bias via funnel plots could not be performed (i.e. <10).
Sensitivity analysis
Leave 1 out approach
The individual impact of a single study on overall heterogeneity and its influence on the pooled estimates was analyzed (Supplemental Figures 1–3).
In the fully adjusted meta-analysis using all subjects, the removal of Boccardi et al. (30) or García-Calzón et al. (13) changed the SMD to nonsignificant (Supplemental Figure 1C and D). Of note, the exclusion of Boccardi et al. reduced the I2 from 42% to 3% (age- and sex-adjusted) and from 39% to 0% (fully adjusted) (Supplemental Figure 1B and D).
The SMD became nonsignificant after the exclusion of Leung et al. (33) or Gu et al. (32) from the age-adjusted meta-analysis using only women (Supplemental Figure 2A and B). However, there was no evidence that the null association between MedDiet and TL was attributed to a single study in the fully adjusted meta-analysis using only women (Supplemental Figure 2C and D).
In the meta-analysis conducted only in men, the removal of Leung et al. or Milte et al. modified the SMD which became significant in the age-adjusted model (Supplemental Figure 3B). However, no study omission changed the SMD in the fully adjusted model (Supplemental Figure 3D). Moreover, the removal of Leung et al. explained the observed heterogeneity in the fully adjusted meta-analysis (I2 changed from 47% to 6%) (Supplemental Figure 3D).
Subgroup analyses
We conducted a stratified analysis according to the type of MedDiet score used (Supplemental Figure 4–6). The MEDAS and Panagiotakos scores were used only in 1 study, Garcia et al., and Leung et al., respectively. We observed similar associations in the studies using the MEDAS and Trichopoulou scores in all subjects’ age- and sex-adjusted meta-analysis, whereas in the study using the Panagiotakos score the SMD was not significant (Supplemental Figure 4A). However, in the fully adjusted meta-analysis nonsignificant associations were reported regardless of the MedDiet score (Supplemental Figure 4B). In the case of the meta-analyses conducted only in women, a positive association between MedDiet adherence and TL was observed for the study using the MEDAS score in the age-adjusted model, whereas we found nonsignificant associations in the other scores (Supplemental Figure 5A). However, in the fully adjusted meta-analysis nonsignificant SMD were reported irrespective of the MedDiet score (Supplemental Figure 5B). Finally, for the meta-analyses including men only, any score deviated from the null association reported in both the age-adjusted and fully adjusted meta-analyses (Supplemental Figure 6).
We additionally conducted a subgroup analysis based on the qPCR methodology for TL (i.e. Cawthon 2002 or 2009). Results in all subjects based on the age- and sex-adjusted model showed a significant positive association between MedDiet and TL in those studies using the Cawthon 2002 approach but not in the 2 using Cawthon 2009. However, the heterogeneity was high and significant in the first method and null and nonsignificant in the second (Supplemental Figure 7A). In the fully adjusted model using all subjects, a nonsignificant association was observed regardless of the method used, although the heterogeneity remained significant in Cawthon 2002 (Supplemental Figure 7B). In the case of women, the subgroup analysis using the qPCR TL method was nonsignificant considering age and fully adjusted meta-analyses (9). This same trend was exhibited in the age-adjusted meta-analysis only in men, whereas in the fully adjusted meta-analysis a high heterogeneity was observed for Cawthon 2002 (Supplemental Figure 9).
Discussion
To the best of our knowledge, the present study is the first meta-analysis conducted to date showing a beneficial association between MedDiet adherence and TL in blood cells. However, since all the studies are cross-sectional, observed associations do not imply a causal relation.
It is important to highlight that the present results are not completely in line with the only 2 previous prospective studies conducted so far, a cohort study and an RCT, where a deleterious association between MedDiet adherence and TL was reported (13, 35). In the prospective cohort study, conducted in 1046 subjects aged between 56 and 70 y, MedDiet was associated with a faster TL shortening in women (35), whereas their null association in men mirror our present results. In the RCT, conducted in 520 subjects aged between 55 and 80 y, the MedDiet supplemented with nuts had a detrimental effect in telomere shortening compared with the low-fat control group (13). However, small effect sizes in combination with middle-age participants and short follow-up times, makes it difficult to assess the association between TL and MedDiet. These contradictory results are difficult to explain since it is expected that the MedDiet, due to its intrinsic antioxidant and anti-inflammatory properties, should prevent TL shortening. However, as stated by the authors, in the prospective cohort study the effect estimate was small and clinically nonsignificant (35). Moreover, the clinical trial was conducted in Mediterranean individuals and the authors suggested that the lifelong exposure to the MedDiet could be more important in determining TL than a dietary intervention of only 5 y. In fact, results could also be affected by those participants with a suboptimal compliance of the intervention. In any case, there is an urgent need to conduct prospective cohorts and RCTs to confirm our findings in relation to the beneficial association of MedDiet adherence and TL. In the present meta-analysis, considering sex subgroup meta-analysis, we have reported that the associations between MedDiet adherence and TL, was only significant in the age-adjusted meta-analysis for women. This may suggest a bigger impact of potential confounders regarding the MedDiet-TL axis in women.
Different underlying mechanisms could explain the positive association between MedDiet and TL. Some key foods of the MedDiet, such as vegetables, fruits, olive oil, nuts, and wine, are especially rich in antioxidant and anti-inflammatory components that have been implicated in TL maintenance (43). Their consumption has been broadly associated with an improvement of several inflammatory and oxidant biomarkers [reviewed in (44)]. In fact, the MedDiet may positively influence telomere attrition by reducing inflammation and oxidative stress (45). It is unclear whether the protective effects on TL provided by the MedDiet result from its individual constituents or synergistic combinations of them. Such interactions induce, by multifactorial protective specific aging mechanisms (i.e. inflammation and oxidative stress), a reduction in the risk of disease (46, 47).
Only 1 study [Boccardi et al. (30)] has analyzed the effect of a food pattern on telomerase—the enzyme responsible for telomere elongation—activation showing a positive association between MedDiet adherence and TL or telomerase activity. Importantly, there is a huge need for prospective studies in order to unravel whether MedDiet contributes to telomere elongation and/or to prevent accelerated attrition. In the present study, a low interstudy heterogeneity was observed in all the meta-analyses conducted except for the fully adjusted meta-analysis in men. Even though we cannot explore the sources of heterogeneity, the exclusion of Leung et al.—the only 1 using the Panagiotakos score—resulted in a significant positive association between MedDiet and TL together with the removal of heterogeneity. We may speculate that the interstudy heterogeneity observed in our analyses could be partially due to the different MedDiet scores used in each study and the differential population analyzed (i.e. Australia, Finland, Italy, Spain, and the USA). In regards to the subgroup analysis by the qPCR TL method, the studies using Cawthon 2002 resulted in a higher heterogeneity in the fully adjusted meta-analysis for men, which was not exhibited in those using Cawthon 2009. Notably, there were only 2 studies assessing qPCR TL using Cawthon 2009. However, due to the limited number of studies included in each meta-analyses (<10) we could not assess the contribution of the different scores or qPCR TL methods to the overall heterogeneity.
Strengths and limitations
The present study has several strengths, first, principal investigators of the retrieved articles were contacted, agreed to participate, and provided standardized data in order to be able to pool the study results. Second, 2 different databases were used to identify available published studies regarding this topic using a systematic review. Third, all the studies adjusted their results for age and sex, which are 2 well-known factors associated with TL, as well as for other potential cofounders. Fourth, the overall heterogeneity was low and nonsignificant in our meta-analyses.
This study also has several limitations that need to be discussed. Since only 1 RCT and 1 prospective cohort study were identified, it was not possible to conduct a meta-analysis for these types of study design. Moreover, a relatively small number of cross-sectional studies were identified and included in the meta-analyses. In addition, in the fully adjusted meta-analyses in men, considerable interstudy heterogeneity was observed, but sources contributing to it could not be explored because of the few studies included (<10 in each analysis). However, removal of Leung et al. in those analyses reduced heterogeneity. Furthermore, published studies used different MedDiet scores to measure MedDiet adherence or qPCR TL methods which could influence the observed results, although we could not evaluate to what extent it affected the pooled effect estimates. It is important to highlight that the meta-analyses were based on cross-sectional studies and, although we also asked corresponding authors to report the fully adjusted means, each 1 included different covariables in the models. Therefore, residual confounding could not be excluded from the results.
Conclusions
The present study showed a positive association between MedDiet adherence and TL in blood cells. This systematic review and meta-analysis of cross-sectional studies provides the most wide-ranging analysis to date on the association between MedDiet and TL.
Taking into account that biological aging is caused in part by telomere shortening, a greater adherence to a healthy dietary pattern such as MedDiet may be used to counteract multiple age-related diseases through counteracting telomere shortening. However, large prospective observational studies and clinical trials are warranted to determine with a high level of evidence the beneficial effect of MedDiet on TL or telomerase activity. In addition, since genetic factors are also involved in TL maintenance, nutrigenetic/nutrigenomic approaches are required in order to gain deeper insights on the potential benefits of MedDiet on telomere maintenance.
Supplementary Material
ACKNOWLEDGEMENTS
The authors’ contributions were as follows—SC and JS-S: initiated the idea of this review and participated in the study design; SC and SG: selected the data and assessed the articles; CL, MC-B, IDV, YG, TG, JM, CM, SG-C, AM, VB, and MV-M: prepared data for the analyses and critically reviewed the article for important intellectual content; SC: contributed to collecting the data, performed statistical analyses, and wrote the manuscript; NB-T and PH-A, participated in the study design, statistical analyses, data interpretation, and manuscript drafting: JS-S critically reviewed the article for important intellectual content; NB-T: reviewed the meta-analysis process; and all authors: read and approved the final manuscript.
Notes
The Physiopathology of Obesity and Nutrition Networking Biomedical Research Center (CIBEROBN) is an initiative of the Carlos III Health Institute (ISCIII) of Spain, which is financed by the European Regional Development Fund (ERDF) (CB07/03/2004). SC is supported by a RYC-2013–12598 grant. PH-A and NB-T are supported by a postdoctoral fellowship (Juan de la Cierva-Formación), FJCI-2017–32205 and FJC2018–036016-I, respectively, funded by the Ministry of Science and Innovation. SG is a doctoral fellow from AGAUR No. 2018FI_B_00444, Generalitat de Catalunya. MC-B holds a Miguel Servet fellowship (Grant CP19/00035) funded by Acción Estratégica de Salud - Carlos III Health Institute (ISCIII), Spain. This study was funded by the NIH, the Spanish Ministry of Health and the Ministry of Economy and Business-European Regional Development Fund. This work is partially supported by ICREA under the ICREA Academia programme.
Author disclosures: The authors report no conflicts of interest.
Supplemental Tables 1 and 2 and Supplemental Figures 1–9 are available from “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/advances.
*SC and NB-T contributed equally to this review.
Abbreviations used: CVD, cardiovascular disease; MEDAS, Mediterranean Diet Adherence Screener; MedDiet, Mediterranean diet; RCT, randomized clinical trial; SMD, standardized mean difference; TL, telomere length.
Contributor Information
Silvia Canudas, Departament of Biochemistry and Biotechnology, Human Nutrition Unit, Universitat Rovira i Virgili. Sant Joan de Reus University Hospital, Reus, Spain; Pere Virgili Institut of Health (IISPV), Reus, Spain; Physiopathology of Obesity and Nutrition Networking Biomedical Research Center (CIBEROBN), Carlos III Health Institute, Madrid, Spain.
Nerea Becerra-Tomás, Departament of Biochemistry and Biotechnology, Human Nutrition Unit, Universitat Rovira i Virgili. Sant Joan de Reus University Hospital, Reus, Spain; Pere Virgili Institut of Health (IISPV), Reus, Spain; Physiopathology of Obesity and Nutrition Networking Biomedical Research Center (CIBEROBN), Carlos III Health Institute, Madrid, Spain; Department of Preventive Medicine and Public Health, School of Medicine, University of Valencia, Valencia, Spain.
Pablo Hernández-Alonso, Departament of Biochemistry and Biotechnology, Human Nutrition Unit, Universitat Rovira i Virgili. Sant Joan de Reus University Hospital, Reus, Spain; Pere Virgili Institut of Health (IISPV), Reus, Spain; Physiopathology of Obesity and Nutrition Networking Biomedical Research Center (CIBEROBN), Carlos III Health Institute, Madrid, Spain; Virgen de la Victoria University Hospital, Institute of Biomedical Research of Malaga (IBIMA)Málaga, Spain.
Serena Galié, Pere Virgili Institut of Health (IISPV), Reus, Spain; Physiopathology of Obesity and Nutrition Networking Biomedical Research Center (CIBEROBN), Carlos III Health Institute, Madrid, Spain.
Cindy Leung, Department of Nutritional Sciences, School of Public Health, University of Michigan, Ann Arbor, MI, USA.
Marta Crous-Bou, Barcelona Beta Brain Research Center (BBRC), Pasqual Maragall Foundation, Barcelona, Spain; IMIM (Hospital del Mar Medical Research Institute), Barcelona, Spain; Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA, USA; Frailty and Healthy Ageing Networking Biomedical Researcher Center (CIBERFES), Carlos III Health Institute, Madrid, Spain.
Immaculata De Vivo, Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA, USA; Channing Division of Network Medicine, Brigham and Women's Hospital - Harvard Medical School, Boston, MA, USA; Radcliffe Institute for Advanced Study, Harvard University, Cambridge, MA, USA.
Yawen Gao, Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA, USA.
Yian Gu, The Taub Institute for Research in Alzheimer's Disease and the Aging Brain, Columbia University Medical Center, New York, NY, USA.
Jelena Meinilä, Public Health Research Program, Folkhälsan Research Center, Helsinki, Finland.
Catherine Milte, Institute for Physical Activity and Nutrition (IPAN), School of Exercise and Nutrition Sciences, Deakin University, Geelong, Australia.
Sonia García-Calzón, Epigenetics and Diabetes Unit, Department of Clinical Sciences CRC, Lund University Diabetes Centre, Scania University Hospital, Malmö, Sweden.
Amelia Marti, Physiopathology of Obesity and Nutrition Networking Biomedical Research Center (CIBEROBN), Carlos III Health Institute, Madrid, Spain; Department of Nutrition, Food Science and Physiology, University of Navarra, Pamplona, Spain.
Virginia Boccardi, Department of Internal Medicine, Surgical, Neurological Metabolic Disease and Geriatric Medicine, Second University of Naples, Naples, Italy.
Melissa Ventura-Marra, Division of Animal and Nutritional Sciences, West Virginia University, Morgantown, WV, USA.
Jordi Salas-Salvadó, Departament of Biochemistry and Biotechnology, Human Nutrition Unit, Universitat Rovira i Virgili. Sant Joan de Reus University Hospital, Reus, Spain; Pere Virgili Institut of Health (IISPV), Reus, Spain; Physiopathology of Obesity and Nutrition Networking Biomedical Research Center (CIBEROBN), Carlos III Health Institute, Madrid, Spain.
References
- 1. Trichopoulou A, Lagiou P. Healthy traditional Mediterranean diet: an expression of culture, history, and lifestyle. Nutr Rev. 1997;55(11 Pt 1):383–9. [DOI] [PubMed] [Google Scholar]
- 2. Salas-Salvadó J, Bullo M, Babio N, Martinez-Gonzalez M, Ibarrola-Jurado N, Basora J, Estruch R, Covas M, Corella D, Aros F et al. Reduction in the incidence of type 2 diabetes with the Mediterranean diet. Diabetes Care. 2011;34(1):14–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Trichopoulou A, Costacou T, Bamia C, Trichopoulos D. Adherence to a Mediterranean diet and survival in a Greek population. N Engl J Med. 2003;348:2599–608. [DOI] [PubMed] [Google Scholar]
- 4. Lopez-Garcia E, Rodriguez-Artalejo F, Li TY, Fung TT, Li S, Willett WC, Rimm EB, Hu FB. The Mediterranean-style dietary pattern and mortality among men and women with cardiovascular disease. Am J Clin Nutr. 2014;99:172–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Guasch-Ferré M, Babio N, Martínez-González MA, Corella D, Ros E, Martín-Peláez S, Estruch R, Arós F, Gómez-Gracia E, Fiol M et al. Dietary fat intake and risk of cardiovascular disease and all-cause mortality in a population at high risk of cardiovascular disease. Am J Clin Nutr. 2015;102(6):1563–73. [DOI] [PubMed] [Google Scholar]
- 6. Samieri C, Sun Q, Townsend MK, Chiuve SE, Okereke OI, Willett C, Stampfer M, Grodstein F. The association between dietary patterns at midlife and health in aging: an observational study. Ann Intern Med. 2013;159(9):584–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Willett WC, Sacks F, Trichopoulou A, Drescher G, Ferro-Luzzi A, Helsing E, Trichopoulos D. Mediterranean diet pyramid: a cultural model for healthy eating. Am J Clin Nutr. 1995;61:1402S–06S. [DOI] [PubMed] [Google Scholar]
- 8. Salas-Salvadó J, Becerra-Tomás N, García-Gavilán JF, Bulló M, Barrubés L. Mediterranean diet and cardiovascular disease prevention: what do we know?. Prog Cardiovasc Dis. 2018;61(1):62–67. [DOI] [PubMed] [Google Scholar]
- 9. Cech TR, Lingner J, Telomerase and the chromosome end replication problem. Ciba Found Symp. 1997;211:20–8. [DOI] [PubMed] [Google Scholar]
- 10. Frenck RW, Blackburn EH, Shannon KM, Shannon KM. The rate of telomere sequence loss in human leukocytes varies with age. Proc Natl Acad Sci. 1998;95(10):5607–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Blackburn EH. Structure and function of telomeres. Nature. 1991;350(6319):569–73. [DOI] [PubMed] [Google Scholar]
- 12. Zee RYL, Castonguay AJ, Barton NS, Germer S, Martin M. Mean leukocyte telomere length shortening and type 2 diabetes mellitus: a case-control study. Transl Res. 2010;55:166–9. [DOI] [PubMed] [Google Scholar]
- 13. García-Calzón S, Martínez-González MA, Razquin C, Arós F, Lapetra J, Martínez JA, Zalba G, Marti A. Mediterranean diet and telomere length in high cardiovascular risk subjects from the PREDIMED-NAVARRA study. Clin Nutr. 2016;35:1399. [DOI] [PubMed] [Google Scholar]
- 14. Zhang J, Rane G, Dai X, Shanmugam MK, Arfuso F, Samy RP, Lai MKP, Kappei D, Kumar AP, Sethi G. Ageing and the telomere connection: an intimate relationship with inflammation. Ageing Res Rev. 2016;25:55–69. [DOI] [PubMed] [Google Scholar]
- 15. O'Donovan A, Pantell MS, Puterman E, Dhabhar FS, Blackburn EH, Yaffe K, Cawthon RM, Opresko PL, Hsueh WC, Satterfield S et al. Cumulative inflammatory load is associated with short leukocyte telomere length in the health, aging and body composition study. PLoS One. 2011;6:e19687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Babizhayev MA, Savel'yeva EL, Moskvina SN, Yegorov YE. Telomere length is a biomarker of cumulative oxidative stress, biologic age, and an independent predictor of survival and therapeutic treatment requirement associated with smoking behavior. Am J Ther. 2011;18:e209–26. [DOI] [PubMed] [Google Scholar]
- 17. Von Zglinicki T. Oxidative stress shortens telomeres. Trends Biochem Sci. 2002;27(7):339–44. [DOI] [PubMed] [Google Scholar]
- 18. Cassidy A, De Vivo I, Liu Y, Han J, Prescott J, Hunter DJ, Rimm EB. Associations between diet, lifestyle factors, and telomere length in women. Am J Clin Nutr. 2010;91(5):1273–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Puterman E, Lin J, Blackburn E, O'Donovan A, Adler N, Epel E. The power of exercise: buffering the effect of chronic stress on telomere length. PLoS One. 2010;5(5):e10837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Farzaneh-Far R, Lin J, Epel ES, Harris WS, Blackburn EH, Whooley MA. Association of marine omega-3 fatty acid levels with telomeric aging in patients with coronary heart disease. JAMA. 2010;303(3):250–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ornish D, Lin J, Daubenmier J, Weidner G, Epel E, Kemp C, Magbanua MJM, Marlin R, Yglecias L, Carroll PR et al. Increased telomerase activity and comprehensive lifestyle changes: a pilot study. Lancet Oncol. 2008;9(11):1048–57. [DOI] [PubMed] [Google Scholar]
- 22. Davinelli S, Trichopoulou A, Corbi G, De Vivo I, Scapagnini G. The potential nutrigeroprotective role of Mediterranean diet and its functional components on telomere length dynamics. Ageing Res Rev. 2018;49:1–10. [DOI] [PubMed] [Google Scholar]
- 23. Pérez LM, Amaral MA, Mundstock E, Barbé-Tuana FM, Guma FTCR, Jones MH, Machado DC, Sarria EE, Marques Marques M, Preto LT et al. Effects of diet on telomere length: systematic review and meta-analysis. Public Health Genomics. 2018;20:286–92. [DOI] [PubMed] [Google Scholar]
- 24. Gardner M, Bann D, Wiley L, Cooper R, Hardy R, Nitsch D, Martin-Ruiz C, Shiels P, Sayer AA, Barbieri M et al. Gender and telomere length: systematic review and meta-analysis. Exp Gerontol. 2014;51:15–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Marioni RE, Harris SE, Shah S, McRae AF, von Zglinicki T, Martin-Ruiz C, Wray NR, Visscher PM, Deary IJ. The epigenetic clock and telomere length are independently associated with chronological age and mortality. Int J Epidemiol. 2016;45(2):424–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. core Team R R: a language and environment for statistical computing. R Found Stat Comput Vienna, Austria; 2018. Available from: http://www.R-project.org/. [Google Scholar]
- 27. Schwarzer G. How to perform a meta-analysis with R: a practical tutorial, Evidence-Based Mental Health. R News. 2019;22:(4):153–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Harrer M, Cuijpers P, Furukawa T ED. dmetar: companion R package for the guide “Doing Meta-Analysis in R”. R package version 0.0.9000. 2019, [Internet]. [cited 22 November 2019]. Available from: http://dmetar.protectlab.org. [Google Scholar]
- 29. Decoster J, Hall GP. Meta-analysis notes. Narrative. 2009, Available from:http://www.stat-help.com/Meta%20analysis%202009-07-31.pdf. [Google Scholar]
- 30. Boccardi V, Esposito A, Rizzo MR, Marfella R, Barbieri M, Paolisso G. Mediterranean diet, telomere maintenance and health status among elderly. PLoS One. 2013;8(4):e62781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Crous-Bou M, Fung TT, Prescott J, Julin B, Du M, Sun Q, Rexrode KM, Hu FB, De Vivo I. Mediterranean diet and telomere length in Nurses’ Health Study: population based cohort study. BMJ. 2014;349:g6674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gu Y, Honig LS, Schupf N, Lee JH, Luchsinger JA, Stern Y, Scarmeas N. Mediterranean diet and leukocyte telomere length in a multi-ethnic elderly population. Age (Omaha). 2015;37(2):24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Leung CW, Fung TT, McEvoy CT, Lin J, Epel ES. Diet quality indices and leukocyte telomere length among Healthy US adults: data from the National Health and Nutrition Examination Survey, 1999–2002. Am J Epidemiol. 2018;187(10):2192–2201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Milte CM, Russell AP, Ball K, Crawford D, Salmon J, McNaughton SA. Diet quality and telomere length in older Australian men and women. Eur J Nutr. 2018;57(1):363–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Meinilä J, Perälä MM, Kautiainen H, Männistö S, Kanerva N, Shivappa N, Hébert JR, Iozzo P, Guzzardi MA, Eriksson JG. Healthy diets and telomere length and attrition during a 10-year follow-up. Eur J Clin Nutr. 2019;73(10):1352–60. [DOI] [PubMed] [Google Scholar]
- 36. Marra MV, Drazba MA, Holásková I, Belden WJ. Nutrition risk is associated with leukocyte telomere length in middle-aged men and women with at least one risk factor for cardiovascular disease. Nutrients. 2019;11:508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Trichopoulou A, Orfanos P, Norat T, Bueno-de-Mesquita B, Ocké MC, Peeters PHM, van der Schouw YT, Boeing H, Hoffmann K, Boffetta P et al. Modified Mediterranean diet and survival: EPIC-elderly prospective cohort study. BMJ. 2005;330(7498):991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Martínez-González MA, García-Arellano A, Toledo E, Salas-Salvadó J, Buil-Cosiales P, Corella D, Covas MI, Schröder H, Arós F, Gómez-Gracia E et al. A 14-item Mediterranean diet assessment tool and obesity indexes among high-risk subjects: The PREDIMED trial. PLoS One. 2012;7(8):e43134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Panagiotakos DB, Pitsavos C, Arvaniti F, Stefanadis C. Adherence to the Mediterranean food pattern predicts the prevalence of hypertension, hypercholesterolemia, diabetes and obesity, among healthy adults; the accuracy of the MedDietScore. Prev Med. 2007;44(4):335–40. [DOI] [PubMed] [Google Scholar]
- 40. Cawthon RM. Telomere measurement by quantitative PCR. Nucleic Acids Res. 2002;30:e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Cawthon RM. Telomere length measurement by a novel monochrome multiplex quantitative PCR method. Nucleic Acids Res. 2009;37:e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Cawthon RM. Telomere measurement by quantitative PCR. Nucleic Acids Res. 2002;30(10):e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Garcia-Calzon S, Moleres A, Martinez-Gonzalez MA, Martinez JA, Zalba G, Marti A, GENOI members. Dietary total antioxidant capacity is associated with leukocyte telomere length in a children and adolescent population. Clin Nutr. 2015;34:694–9. [DOI] [PubMed] [Google Scholar]
- 44. Tosti V, Bertozzi B, Fontana L. Health benefits of the Mediterranean diet: metabolic and molecular mechanisms. J Gerontol A Biol Sci Med Sci. 2018;73:318–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Jurk D, Wilson C, Passos JF, Oakley F, Correia-Melo C, Greaves L, Saretzki G, Fox C, Lawless C, Anderson R et al. Chronic inflammation induces telomere dysfunction and accelerates ageing in mice. Nat Commun. 2014;5:4172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kondo T, Hirose M, Kageyama K. Roles of oxidative stress and redox regulation in atherosclerosis. J Atheroscler Thromb. 2009;16(5):532–8. [DOI] [PubMed] [Google Scholar]
- 47. Masi S, Nightingale CM, Day INM, Guthrie P, Rumley A, Lowe GDO, Von Zglinicki T, D'Aiuto F, Taddei S, Klein N et al. Inflammation and not cardiovascular risk factors is associated with short leukocyte telomere length in 13- to 16-year-old adolescents. Arterioscler Thromb Vasc Biol. 2012;32(8):2029–34. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.