ABSTRACT
Alzheimer disease (AD) is a global health concern with the majority of pharmacotherapy choices consisting of symptomatic treatment. Recently, ketogenic therapies have been tested in randomized controlled trials (RCTs), focusing on delaying disease progression and ameliorating cognitive function. The present systematic review aimed to aggregate the results of trials examining the effects of ketogenic therapy on patients with AD/mild cognitive impairment (MCI). A systematic search was conducted on PubMed, CENTRAL, clinicaltrials.gov, and gray literature for RCTs performed on adults, published in English until 1 April, 2019, assessing the effects of ketogenic therapy on MCI and/or AD compared against placebo, usual diet, or meals lacking ketogenic agents. Two researchers independently extracted data and assessed risk of bias with the Cochrane tool. A total of 10 RCTs were identified, fulfilling the inclusion criteria. Interventions were heterogeneous, acute or long term (45–180 d), including adherence to a ketogenic diet, intake of ready-to-consume drinks, medium-chain triglyceride (MCT) powder for drinks preparation, yoghurt enriched with MCTs, MCT capsules, and ketogenic formulas/meals. The use of ketoneurotherapeutics proved effective in improving general cognition using the Alzheimer's Disease Assessment Scale-Cognitive, in interventions of either duration. In addition, long-term ketogenic therapy improved episodic and secondary memory. Psychological health, executive ability, and attention were not improved. Increases in blood ketone concentrations were unanimous and correlated to the neurocognitive battery based on various tests. Cerebral ketone uptake and utilization were improved, as indicated by the global brain cerebral metabolic rate for ketones and [11C] acetoacetate. Ketone concentrations and cognitive performance differed between APOE ε4(+) and APOE ε4(−) participants, indicating a delayed response among the former and an improved response among the latter. Although research on the subject is still in the early stages and highly heterogeneous in terms of study design, interventions, and outcome measures, ketogenic therapy appears promising in improving both acute and long-term cognition among patients with AD/MCI.
This systematic review was registered at www.crd.york.ac.uk/prospero as CRD42019128311.
Keywords: ketosis, MCT, neurologic disease, dementia, cognitive impairment, ketoneurotherapeutics, cognitive decline, brain metabolism, amyloid, APOE
Introduction
Alzheimer disease (AD) is the most prevalent form of progressive dementia (1), and is expected to increase in prevalence as a result of the aging boom generation (2). As a result, AD is considered a “growing global health concern” (3), with ∼70% of the cases being attributed to a genetic predisposition, and the remaining effectors stemming from the environment (3). AD is characterized by brain accumulation of pathological amyloid β (Aβ), predating the initiation of cognitive impairment by ∼1 decade (4–6).
On the primary prevention level, diet has been shown to either accelerate, or delay AD onset, with many cohort studies supporting the existence of this relationship (7). Fish intake (8–10) and greater adherence to traditional dietary patterns rich in antioxidants, PUFAs, and MUFAs like the Japanese, Argentinean, and Mediterranean diets (11) have been shown to postpone AD onset. In parallel, other healthy diet regimes, like the Dietary Approaches to Stop Hypertension (DASH), and the Mediterranean-DASH Intervention for Neurodegenerative Delay diets (12–14), have also been shown to protect against the development of AD. A recent meta-analysis revealed that adherence to an AD protective dietary pattern induced a significantly lower deposition of Aβ, although this difference was not clinically important (15).
During AD, a progressive synaptic dysfunction is taking place, resulting in neuronal death (16, 17), developing a deficit in brain glucose utilization as a result of the reduced neuron count (18). In parallel, nutrient deprivation reduces mitochondrial ATP synthesis. In turn, the observed mitochondrial dysfunction compromises brain regulation of the glucose transporter GLUT1, further aggravating brain glucose uptake and glycolysis (19, 20). The loss of neurons and the impaired brain substrate use exert a synergistic effect in tamping down cognitive function. Adherence to a high-glycemic diet is associated with an increased cerebral amyloid burden among older adults (21). To correct the induced substrate deficit, the use of ketogenic therapy has been proposed and assessed in both animal (22–24) and human studies (25–32). Popular ketogenic remedies include 1) the adherence to ketogenic diet (KD) (high fat content at the expense of carbohydrates) and 2) the intake of medium-chain triglycerides (MCTs) with 6–12 carbon atoms, which are metabolized in the liver (33–35). Owing to the surplus of fatty acid availability, their breakdown synthetizes ketones, which in turn produce acetyl-CoA (36). Accumulating experimental data suggest that ketoneurotherapeutics entail neuroprotective benefits for the ageing brain (35, 37, 38), by replicating the effects of caloric restriction, boosting the production of ketone bodies (39–41), and increasing energy availability.
The aim of the present study was to systematically review all randomized controlled trials (RCTs) assessing the efficacy of ketogenic therapy among patients with AD or mild cognitive impairment (MCI).
Methods
Study design and protocol
The present systematic review was based on a predefined protocol registered in PROSPERO (CRD42019128311), and adhered to the reporting guidelines of the Preferred Reporting Items for Systematic reviews and Meta-Analyses statement (42).
Research question and search strategy
A systematic search was conducted on PubMed, CochranE coNTrolled Register of triALs (CENTRAL), ClinicalTrials.gov, and gray literature, for trials published in the English language assessing the effect of ketogenic therapy on AD indicators, published until 1 April, 2019.
The research question was addressed in the form of population, intervention, control, and outcomes (PICO) as follows: in patients with an MCI and/or AD diagnosis (P), what is the efficacy of ketogenic therapy via adherence to a KD, or consumption of ketogenic agents/supplements/meals (I), compared with placebo/usual diet or non–ketogenic agent administration (C) on disease-related indicators such as cognition or biomarkers associated with AD (O)?
Keywords used in the searches included (ketogenic), (ketosis), (ketone), (MCT), (medium-chain triglycerides), (beta-hydroxybutyrate), (caprylic), (acetoacetate), (Alzheimer), (Alzheimer's disease), and (mild cognitive impairment). Supplemental Figure 1 presents the detailed search string for PubMed.
Eligibility criteria
Two reviewers independently performed the search and selected eligible trials. In the case of disagreement concerning the eligibility of a study, a third reviewer weighed the evidence to provide a solution.
Inclusion criteria involved all 1) clinical trials; 2) performed on adult humans; 3) assessing the effect of nutritional ketosis on AD indicators; 4) compared against placebo, usual diet, or meals lacking ketogenic agents; and, 5) published in the English language. No restrictions were applied concerning the duration of the studies, ketogenic therapy mode (diet/supplementation), ketone dose, cognitive outcomes, participant's sex, age, or sample size.
Reasons for trial exclusion involved 1) clinical trials without a comparator group; 2) results published in languages other than English;3) involving nutritional or pharmacological interventions not inducing nutritional ketosis; 4) trials performed on animals; 5) including healthy participants or patients without cognitive impairment, AD, or dementia; 6) trials performed on children (<18 y old); or, 7) examining the effects of diabetic ketoacidosis.
Data extraction
Data were extracted by 2 independent reviewers in standardized forms. In cases of disagreement, a third reviewer resolved the issue. Extracted data included study details (first author, year of publication, masking of interventions, multicenter design and countries involved, design, having informed consent/approval of protocol, source of funding, duration), AD diagnostic criteria, detailed intervention and comparator group characteristics (age, n, female ratio, condition, etc.), intervention and comparator characteristics (duration of intervention and washout), compliance assessment, primary and secondary outcomes, results, and other trial characteristics (funding, country of implementation, trial registry and number, dropouts, adverse events, evaluation time points, etc.).
Risk of bias
Eligible studies were assessed for bias with the Cochrane Risk of Bias (RoB) tool (43). Two authors examined the studies for sources of bias and concluded if there were low, high, or moderate concerns of bias regarding the randomization process, deviations from the intended interventions, measurement of the outcomes, missing outcome data, selection of the reported results, and overall bias. Disagreements were resolved by discussion with a more experienced investigator.
Quality assessment of the studies
The Oxford quality score (44) was applied to each RCT by 2 independent reviewers. This score assesses and assigns a positive value of 1 based on the randomization criteria, masking, and adequacy in the reporting of withdrawals and dropouts. In addition, 1 point is deducted when the randomization scheme or the masking is not deemed appropriate.
Results
Search results and characteristics of the retrieved trials
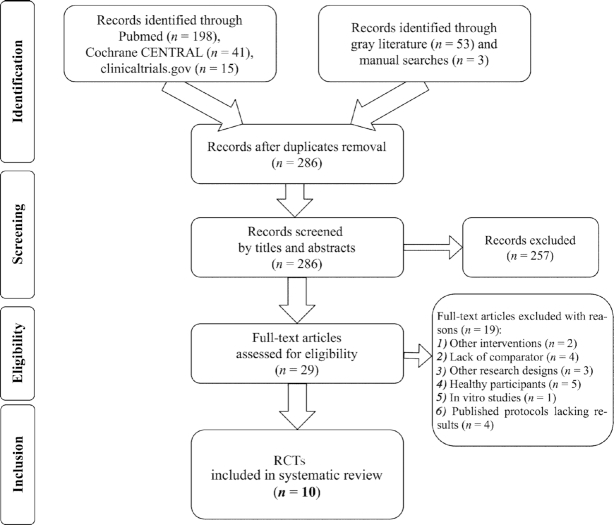
Out of 310 entries in total, 10 RCTs (45–54) fulfilled the protocol's criteria (Figure 1). Table 1 details characteristics concerning the design and protocol of the included trials. Three RCTs (46, 51, 53) were crossover, and the remainder (48–50, 52, 54) were of parallel design. The majority of trials had a published protocol (45–50, 52), with 2 sharing the same protocol (48, 49). Except for the Ota et al. (51) and Fortier et al. (47) trials that originated from Japan and Canada, respectively, the remaining RCTs were conducted in the United States (45, 46, 48–50, 52–54). By default, masking of diet RCTs was not double-blind, and was either single-blind (45, 46) or open-label (50). Table 2 stresses the participant characteristics of the included trials. The number of participants in each trial ranged between 6 (52) and 152 (48), all ketogenic treatment–naïve, with MCI or AD diagnosis. Based on the per protocol (PP) analyses, the pooled sample of patients included in the present systematic review involved 456 adults in total.
FIGURE 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (42) flowchart of the study selection process for the identification of RCTs investigating the effects of ketogenic therapy on patients with Alzheimer disease or mild cognitive impairment. RCT, randomized controlled trial.
TABLE 1.
Study design and protocol characteristics of the included RCTs investigating the effects of ketogenic therapy on patients with mild cognitive impairment/Alzheimer disease1
| First author | Brandt (45) | Craft (46) | Fortier (47) | Henderson (48) | Henderson (49) | Krikorian (50) | Ota (51)2 | Rebello (52) | Reger (53) | Torosyan (54) |
|---|---|---|---|---|---|---|---|---|---|---|
| Study name | — | BEAM | BENEFIC | — | — | — | — | — | — | — |
| Study duration | October 2015 to April 2018 | February 2015 to April 2017 | 2015 until now | October 2004 to June 2006 | October 2004 to June 2006 | NR | October 2012 to August 2013 | NR | NR | |
| Publication year | 2019 | 2016 | 2019 | 2009 | 2011 | 2012 | 2019 | 2015 | 2004 | 2018 |
| Publication type | Full-text | Abstract | Full-text | Full-text | Full-text | Full-text | Full-text | Commentary | Brief communication | Full-text |
| Journal | J Alzheimers Dis | Alzheimers Dement | Alzheimers Dement | Nutr Metab | BMC Med Genet | Neurobiol Aging | Neurosci Lett | BBA Clin | Neurobiol Aging | Exp Gerontol |
| Origin | USA | USA | Canada | USA | USA | USA | Japan | USA | USA | USA |
| Registry | NCT02521818 | NCT02984540 | NCT02551419 | NCT00142805 | NCT00142805 | NCT00777010 | NR | NCT01669200 | NR | NR |
| Funding | 1) William & Ella Owens Medical Research Fndn2) Bright Focus Fndn3) NCATS4) Hass Avocado Board | 1) Wake Forest Baptist Medical Center2) NIA3) Hartman Family Fndn4) Dept of Defense5) NCATS | 1) Part-the-Cloud, AA, USA2) Mitacs3) Université de Sher-brooke | Accera, Inc. | Accera, Inc. | 1) Robert C & Veronica Atkins Fndn2) NIA3) Indiana AD Center | 1) Food Science Institute Fndn, Japan2) Meiji Co. Ltd. | NR | NR | 1) John Douglas French AD Fndn 2) Accera, Inc. |
| Ethical permission | Johns Hopkins University, USA | Wake Forest School of Medicine, USA | CIUSSS de l'Estrie–CHUS, Sherbrooke, Quebec, Canada | Essex Institutional Review Board, Lebanon, NJ, USA | Essex Institutional Review Board, Lebanon, NJ, USA | University of Cincinnati, USA | National Center of Neurology & Psychiatry, Japan | Pennington Biomedical Research Center, USA | NR | NR |
| RCT design | Parallel | Crossover | Parallel | Parallel | Parallel | Parallel | Crossover | Parallel | Crossover | Parallel |
| Randomization | Random number table | NR | Randomization sequence with 6 consecutive blocks of 10 participants (1:1 ratio) | Permutated block randomization code with a block size of 4 (1:1 ratio) | NR | NR | Randomized sequence NOD | Random number table NOD | NR | By the pharmacy, NOD |
| Masking | Single-blind | Single-blind (outcomes assessor) | Quadruple-blind | Double-blind | Double-blind | Open label | Double-blind | Double-blind | Double-blind | Double-blind |
| Multicenter | No | No | No | ✓ (23 centers) | ✓ (23 centers) | No | No | No | No | No |
| Concerns | No flowchart, discrepancies between PP analysis and reported dropouts | More outcomes were reported in the protocol registry, lack of flowchart, no tables | Lack of flowchart, most data presented in figures | Patients at each stage were not reported, no flowchart, lack of RCT protocol registry | Very low sample power, lack of statistical analyses and flowchart | Lack of flowchart, data presented in figures | Uneven sample allocation, no flowchart, low sample power in controls | |||
| Oxford quality score (44) | 2 | −1 | 2 | 3 | 3 | −1 | 0 | 1 | 0 | 2 |
AA, Alzheimer's Association; AD, Alzheimer Disease; BEAM, Brain Energy and Memory; BENEFIC, Brain ENErgy Fitness, Imaging and Cognition; CIUSSS de l'Estrie–CHUS: Centre Intégré Universitaire de Santé et de Services Sociaux de l'Estrie–Centre Hospitalier Universitaire de Sherbrooke; Dept, Department; Fndn, Foundation; NCATS, National Center for Advancing Translational Sciences; NIA, National Institute of Aging; NOD, not other defined; NR, not reported; PP, per protocol; RCT, randomized controlled trial.
The study had a double design: an RCT (presented in the table) and a long-term clinical trial without a comparator (omitted for not fulfilling the inclusion criteria).
TABLE 2.
Participant characteristics of the included randomized controlled trials assessing the effects of ketogenic therapy on patients with MCI and/or AD1
| First author | Brandt (45) | Craft (46) | Fortier (47) | Henderson (48) | Henderson (49) | Krikorian (50) | Ota (51) | Rebello (52) | Reger (53) | Torosyan (54) |
|---|---|---|---|---|---|---|---|---|---|---|
| Patients, n | n = 14 (CDR ≤ 1, MoCA: 18–25 2) | n = 10 with MCI and prediabetes | n = 52 with MCI ( 69) | n = 152 with mild to moderate AD (MMSE: 14–24 2) | n = 131 with mild to moderate AD (MMSE: 14–24 2) | n = 23 with acquired MCI based on the CDR | n = 20 AD with MCI (MMSE: 20 ± 4.3 3) | n = 6 AD with MCI (MMSE: 25–28 2) | n = 20 with AD/amnestic MCI | n = 16 with mild to moderate AD (MMSE: 10–28 2) |
| Women, n | n = 7 | NR | NR | n = 85 | NR | n = 13 | n = 9 | NR | NR | NR |
| AD diagnostic criteria | NIA-AA | NR | — | NINCDS-ADRDA and DSM-IV | NINCDS-ADRDA and DSM-IV | NR | NINCDS-ADRDA | NIA-AA | NINCDS-ADRDA | NINCDS-ADRDA |
| Age, y | Intervention2: 74 ± 63Control: 69 ± 53 | 63.64 | ≥55 | 51–932 | NR | 70 ± 63 | 73 ± 63 | 58–782 | 75 ± 73 | 50–902 |
| Intervention arm, n | n = 15 (ITT)n = 9 (PP) | n = 10 | n = 26 (ITT)n = 19 (PP) | n = 86 (ITT) (29 recruited later);5 n = 77 (PP) | n = 86 (ITT)n = 65 (PP)6 | n = 12 | n = 20 | n = 2 | n = 20 | n = 14 |
| Control arm, n | n = 12 (ITT)n = 5 (PP) | n = 10 | n = 26 (ITT)n = 20 (PP) | n = 66 (ITT) (11 recruited later);5 n = 63 (PP) | n = 66 (ITT)n = 55 (PP)6 | n = 11 | n = 20 | n = 2 | n = 20 | n = 2 |
| Inclusion criteria | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | NR | ✓ | ✓ | ✓ |
| Exclusion criteria | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | NR | ✓ | NR | NR |
| Dropouts, n | Intervention: 37Control: 67 unwilling to change their diet and/or considering participation as difficult | None | Intervention: 6 were therapy intolerant, 2 discontinued for other reasons Control: 2 were therapy intolerant, 3 stopped for other reasons | Intervention: 35 stopped, 3 lost to follow-up, 20 had adverse events, 8 withdrew consent, 2 perceived lack of efficacy, 1 protocol violator, 1 moved, 3 for other reasons. Of these 38, 9 were not analyzed (missing data) | Intervention: 8 had incomplete data Control: 3 had incomplete data | NR | NR | 1 GI issues, 1 non-compliant | NR | 6 had incomplete data |
| Control: 12 stopped, 2 lost to follow-up, 4 adverse events, 5 withdrew consent, 2 lack of efficacy, 1 protocol violator. Of these 14, 3 were not analyzed (missing data) |
AD, Alzheimer disease; CDR, Clinical Dementia Rating (70); DSM-IV, Diagnostic and Statistical Manual of Mental Disorders IV; GI, gastrointestinal; ITT, intention to treat; MCI, mild cognitive impairment; MMSE, Mini-Mental State Exam (59); MoCA, Montreal Cognitive Assessment (60); NIA-AA, National Institute of Aging–Alzheimer's Association (71); NINCDS-ADRDA, National Institute of Neurological and Communicative Diseases and Stroke/Alzheimer's Disease and Related Disorders Association (72); NR, not reported; PP, per protocol; SD, standard deviation.
Range.
Mean ± SD.
Mean.
More participants were recruited during the study to attain an adequate sample and increase the response rate; however, the flowchart of participant selection and allocation is not clear.
Not all participants consented to additional genotyping.
7 n of reported dropouts does not coincide with the n calculated when abstracting participants from the PP analysis from those in the ITT.
RoB and quality of the included RCTs
Figure 2 summarizes the RoB of the included RCTs. In most RoB domains, the included studies demonstrated unclear bias. The Fortier et al. trial (47) exhibited less bias than the rest, whereas the RCT by Krikorian et al. (50) was of unclear bias throughout the RoB categories. Regarding quality, most studies received a low Oxford score, with the 2 trials conducted by Henderson et al. (48, 49) demonstrating the highest quality among all included RCTs.
FIGURE 2.
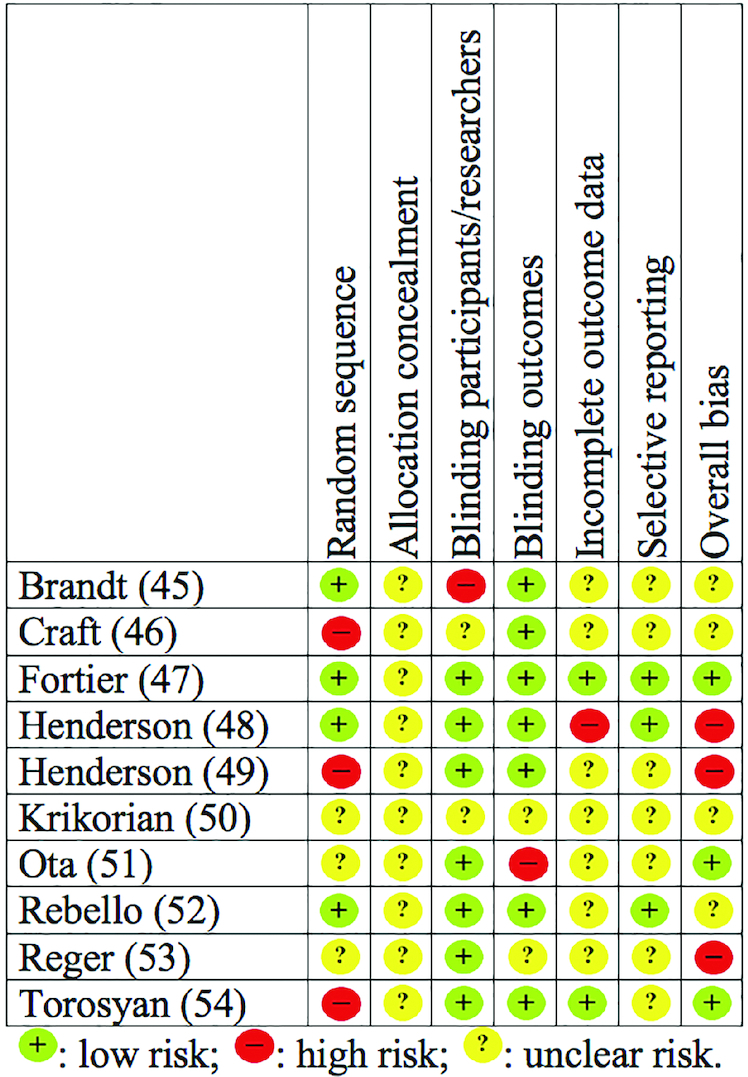
Included randomized controlled trials investigating the effects of ketogenic therapy on patients with Alzheimer disease or mild cognitive impairment, rated against the Cochrane risk of bias tool (43).
Concerning the BEAM (Brain Energy and Memory) trial (46), although 1 full-text publication and another abstract were released during the year 2019 after the end of the present systematic search (55, 56), they both included healthy participants in their sample, without restricting recruitment to persons with AD or MCI only.
Intervention characteristics
Table 3 details the intervention characteristics of the included RCTs, their outcomes, and their findings. A great variety of interventions were identified, including adherence to a KD regime (45, 46, 50), ready-to-consume ketogenic drinks (47, 53), MCT powder for the preparation of ketogenic drinks (48, 49), yoghurt enriched with MCTs (52), MCT capsules (54), and a ketogenic meal (51). In parallel, intervention duration varied considerably, ranging from 45 (54) to 180 d (47), whereas 4 RCTs evaluated the acute effects of consuming a ketogenic meal (51–53) or MCT capsules (54). Intervention compliance was evaluated via plasma (46) or urinary ketone concentrations (45, 50), diet records (50, 52), adherence to the Healthy Eating Index (57) for controls, or consumption count (47, 48), or was not reported at all.
TABLE 3.
Intervention particularities, outcomes, and findings of the included randomized controlled trials assessing the effects of ketogenic therapy on patients with mild cognitive impairment and/or AD1
| First author | Brandt (45) | Craft (46) | Fortier (47) | Henderson (48) | Henderson (49) | Krikorian (50) | Ota (51)2 | Rebello (52) | Reger (53) | Torosyan (54) |
|---|---|---|---|---|---|---|---|---|---|---|
| Intervention | MAD with ≤20 g net CHO (total CHO minus fiber), large amounts of fat to satiety, moderate protein, ample hydration and unrestricted energy intake. | MMKD (<15 g CHO/d) | kMCT drink: emulsion with 12% Captex 355 [60% caprylic acid (8:0), 40% capric acid; Abitec Corp.] in lactose-free skim milk. The drink provided 30 g kMCT in 250 mL (Nalgene). | Powder sachets with 10 g AC1202 (MCT composed of glycerin and caprylic acid, known as tricaprylin/ trioctanoin), mixed with a meal replacement drink (Ensure, Abbott Lab Inc.), taken at breakfast for 7 d, and a double dose thereafter (2 × 10 g AC1202) | 2 × powder sachets per day with 10 g AC1202 each (MCT, >95% of the fatty acids being caprylic acid). Product luted in water or other liquids, forming a drink. | Dietary education and counseling for adherence to a KD (5%–10% of calories from protein, <20 g CHO/d), with fruit restriction and a CHO intake based on small portions of vegetables. MUFA was the preferred fat source. | Meal: 50 g ketogenic formula (Ketonformula) containing 20 g of MCTs. | 56 g MCTs (MCT oil, Nestlé) added to 6 oz Yoplait 99% fat-free fruit yogurt. | MCTs (40 mL) (Neo-Bee 895, Stepan Inc.), blended with 152 mL heavy whipping cream to form a 690-kcal drink. | 40 g caprylidene (MCT) Axona® in capsules (Accera, Inc.). |
| Comparator | NIA diet: high intake of fruit, whole-gains, fat-free/low-fat dairy, seafood, lean meat, eggs, poultry, oils, beans, peas, vegetable, nuts/seeds. Low intake of sodium, solid fats, refined gains, sugar. | Equicaloric AHAD (<10 g fat/d). | Placebo drink: refined, bleached, winterized, deodorized, high-oleic-acid sunflower oil, as the non-ketogenic lipid. | Powder sachets with 10 g placebo, mixed with a meal replacement drink (Ensure, Abbott Lab, Inc.), taken at breakfast for 7 d, and a double dose thereafter (2 × 10 g placebo). | 2 × powder sachets per day, each with 10 g placebo (isocaloric to the active powder, consisting of 51% gum acacia, 37% dextrose, 10% safflower oil, and 2% syloid). | Healthy diet with ≥50% of calories from CHO, high fruit and vegetable intake. MUFA as the preferred fat source. | Meal: isocaloric placebo formula without MCTs. | Placebo (canola oil, color matched) added to 177.4 mL Yoplait 99% fat-free fruit yogurt. | Placebo drink with heavy whipping cream alone (232 mL, 690 kcal). | Placebo capsule. |
| Intervention duration | 12 wk | 6 wk | 6 mo | 90 d | 90 d | 6 wk | 1 meal (single intake), consumed 120 min before tests | 24 wk | 1 meal consumed 90 min before tests | 45 d |
| Acute intervention | No | No | No | NR3 | NR3 | No | ✓ | ✓ | ✓ | ✓ |
| Long-term intervention | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | No2 | ✓ | No | ✓ |
| Washout duration | N/A | 6 wk | N/A | 2 wk post-intervention | 2 wk post-intervention | N/A | 1 visit (NOD) | N/A | 1 visit | N/A |
| Compliance assessment | Urinary ketone levels (intervention) and HEI ≥85 (controls) | Plasma ketone levels | Bottle count, blind blood analyses | Consumption log >80% of intended dose | NR | Diet records evaluated by a dietitian and urinary ketones | N/A (acute intervention only) | By a registered dietitian via daily diet diaries | N/A (acute intervention only) | NR |
| APOE ε4 status | ✓ | No | ✓ | ✓ | ✓ | No | No | ✓ | ✓ | ✓ |
| Adverse events | Minor GI issues and 1 vasovagal episode | NR | Nausea, therapy intolerance, headache, GI issues (diarrhea, reflux, bloating, constipation) | GI adverse events | NR | NR | Diarrhea | GI issues | NR | NR |
| Primary outcomes | MCS | Δ in CSF levels of Aβ42 and t-tau (INNO-BIA-Alzbio3) | Δ in CMRAcAc and CMRKetones by PET imaging (Patlak method) | Improvements in cognition (Δ in the ADAS-Cog and ADCS-CGIC) | ADAS-Cog | TMT, V-PAL, GDS | Acute WAIS-III, WMS-R, Stroop, TMT, all at 120 min post-meal | ADAS-Cog, TMT, and Digit Symbol tests | ADAS-Cog, MMSE, Stroop | rCBF (15O-water PET scans) analyzed with sVOI and voxel-based spm |
| Secondary outcomes | MMSE, MDS-HC, POMS-Bi | Δ in memory composite score, insulin sensitivity, [11C] AcAc PET uptake | CMRGlu, plasma medium-chain fatty acidsCognition: RL/RI-16, MMSE, MoCA, VF, BVMT-R, BNT, TMT, Stroop | MMSE, serum BHB | Serum BHB | Urinary ketone levels, fasting plasma insulin, anthropometry, FPG | Plasma ketone bodies (AcAc and BHB) concentrations | Plasma Glu, insulin, and pre-/post-prandial BHB | BHB | rCBF by sVOI and voxel-based spm |
| Time points | Baseline, at 3, 6, 9, and 12 wk | Baseline, at 6, 12, and 18 wk | Baseline, end (last wk of mo 6) | Baseline, days 45 and 90 | Baseline, days 45, 90, and 104 | Baseline and end of intervention (post-6 wk) | Baseline, at 120 min post–meal ingestion | Baseline, weeks 4, 8, 12, 16, and 20 | Baseline, 90 min | Baseline (before, after first pill) and on day 45 (before, after final pill) |
| Results | MCS of the MAD group increased slightly over the trial, whereas in the NIA group it declined slightly (not significantly). MCS and MMSE scores of compliant patients did not differ from baseline. Mood of MAD subjects improved at wk 6. | Global [11C] AcAc uptake increased after the KD intervention. CSF t-tau increased with the KD, with a similar trend noted for Aβ42. Global [11C] AcAc uptake decreased after the low-fat diet. No changes were observed after adoption of the low-fat diet regime. | Brain ketone metabolism increased (230%) in the kMCT group, whereas brain Glu uptake remained unchanged. Episodic memory, language, executive function, and processing speed improved on the kMCT vs. baseline. Increased brain ketone uptake was related to several cognitive scales. | AC-1202 elevated serum ketone bodies and improved ADAS-Cog scores compared with the placebo. Effects were most notable in APOE ε4(−) subjects. A significant pharmacologic response was observed between serum BHB levels and change in the ADAS-Cog scores in APOE ε4(−) subjects, at day 90. | Among APOE genotypes, homozygous carriers of the ε3 on AC-1202 had improved cognition relative to placebo. Improved cognition was noted among APOE ε4(−) subjects on AC-1202 relative to placebo. Specific genotype combinations of IDE and APOE ε4(−), and IL1B and APOE ε4(−) produced more improvements. | The intervention improved secondary memory performance and reduced energy intake and anthropometric parameters. No effect was recorded on the depressive symptoms and the TMT. Ketone levels were positively correlated with memory performance. | The ketogenic formula increased plasma ketone bodies. No differences were noted between scores after taking the ketogenic or the placebo formula, in any cognitive test after correction for multiple testing. | MCT oil intake increased serum ketone bodies and improved memory in the APOE ε4(−) participant, whereas intake of placebo did not improve any cognitive measure. In the APOE ε4(+) patient an increase in post-prandial serum BHB was noted. | MCT treatment improved ADAS-Cog performance for APOE ε4(−) subjects, but not for the (+) ones. Higher ketone levels were associated with greater improvement in paragraph recall among those on MCT treatment. | APOE ε4(−) patients had elevated rCBF in the left superior lateral temporal cortex after 45 d of following adopting a caprylidene diet. APOE ε4(+) patients did not display changes in rCBF. |
1AcAc, acetoacetate; AD, Alzheimer disease; ADAS-Cog, Alzheimer's Disease Assessment Scale-Cognitive subscale (58); ADCS-CGIC, AD Cooperative Study—Clinical Global Impression of Change (76); AHAD, American Heart Association diet; APOE (ε4), apoε4 allele; Aβ42, amyloid β 42; BHB, serum β-hydroxybutyrate; BNT, Boston Naming Test (65); BVMT-R, Brief Visuospatial Memory Test-Revised (62); CHO, carbohydrates; CMR, cerebral metabolic rate; CSF, cerebrospinal fluid; FPG, fasting plasma glucose; GDS, Geriatric Depression Scale (73); GI, gastrointestinal; Glu, glucose; HEI, Healthy Eating Index (57); IDE, insulin-degrading enzyme; IL1B, interleukin 1β; KD, ketogenic diet; kMCT, commercially available drink; MAD, modified Atkins diet; MCS, Memory Composite Score (Hopkins Verbal Learning Test-Revised and BVMT-R); MCT, medium-chain triglyceride; MDS-HC, Minimum Data Set for Home Care (75); MMKD, Modified Mediterranean Ketogenic diet; MMSE, Mini-Mental State Exam (59); MoCA, Montreal Cognitive Assessment (60, 64); N/A, not applicable; NIA, National Institute of Aging; NOD, not other defined; NR, not reported; PET, positron emission tomography; POMS-Bi, Profile of Mood States Bipolar Scale (74); rCBF, regional cerebral blood flow; RL/RI-16, Rappel Libre/Rappel Indicé (64); spm, statistical parametric mapping; Stroop, Stroop Color Word Interference (66); sVOI, standardized volumes of interest; TMT, Trail Making Test (68); t-tau, total tau; VF, Verbal Fluency (67); V-PAL, Verbal Paired Associate Learning Test (63); WAIS-III, Wechsler Adult Intelligence Scale-3rd (77); WMS-R, Wechsler Memory Scale-Revised (78).
2The study had a double design: a RCT (presented herein) and a longitudinal clinical trial without a comparator (omitted for not fulfilling the inclusion criteria).
3Some outcomes were also measured after the first intervention.
Outcomes of interest
As Table 3 displays, a variety of outcomes were assessed in the included trials, involving subjective neurocognitive tests and combinations of tests (batteries), psychological indexes, brain imaging, genetic predisposition, and a variety of biomarkers (blood and biopsy-derived). The Alzheimer's Disease Assessment Scale-Cognitive (ADAS-Cog) (58) and the Mini-Mental State Exam (MMSE) (59) were the most used tests for assessing general cognition. Other cognitive tests applied included the Montreal Cognitive Assessment (MoCA) (60), the Memory Composite Score—comprised of the Hopkins Verbal Learning Test-Revised (61) and the Brief Visuospatial Memory Test-Revised (BVMT-R) (56), the Wechsler Adult Intelligence Scale-3rd (57), and the Wechsler Memory Scale-Revised (WMS-R) (62). Secondary memory was assessed with the Verbal Paired Associate Learning Test (V-PAL) (63), whereas changes in episodic memory were evaluated with the Rappel Libre/Rappel Indicé (RL/RI-16) (64), the BVMT-R (62), or the paragraph recall test. Language ability was assessed with the Boston Naming Test (BNT) (65). For executive ability and attention, the Stroop Color Word Interference (66), Verbal Fluency (VF) (67), Digit Symbol Substitution Test, and Trail Making Test (TMT) (68) were applied.
Outcomes of psychological interest included the Geriatric Depression Scale (73) and the Profile of Mood States Bipolar Scale (74), applied by Krikorian et al. (50) and Brandt et al. (45), respectively. The latter study also evaluated efficacy in everyday behavior via the Minimum Data Set for Home Care (75).
Most studies assessed urinary (50) or blood ketone bodies concentrations (47–49, 51–53) in the form of acetoacetate (AcAc) or serum β-hydroxybutyrate (BHB). In the BENEFIC (Brain ENErgy Fitness, Imaging and Cognition) trial (47), positron emission tomography (PET) imaging was applied to assess the cerebral metabolic rate (CMR) of AcAc (CMRAcAc) and ketones (CMRKetones). In the BEAM trial (46), a lumbar puncture was performed pre- and postintervention for the collection of cerebrospinal fluid (CSF) and the assay of Aβ42 and total tau (t-tau) concentrations via INNO-BIA-Alzbio3 fluorimetric immunoassay. In addition, [11C] AcAc brain uptake was evaluated via PET. Finally, Torosyan et al. (54) assessed the regional cerebral blood flow (rCBF) using 15O-water PET scans, by quantifying 47 standardized volumes of interest (sVOI).
The majority of RCTs (45, 47–49, 52–54) assessed the apolipoprotein ε4 allele (APOE ε4) status of participants from blood samples, by categorizing subjects with ≥1 copy of the APOE ε4 gene as APOE ε4(+) (45, 54). In the Fortier et al. (47) trial, APOE genotyping was performed by real-time PCR (79), while Henderson et al. (48, 49) used allele-specific extension (80). Apart from the APOE ε4, Henderson et al. (49) additionally performed genotyping on APOE ε2, 3 and single nucleotide polymorphisms (SNPs) in the interleukin1β (IL1β and insulin-degrading enzyme (IDE) gene.
Intervention results on cognition
Table 4 summarizes the findings of each intervention. The majority of trials (45, 47–53) assessed the effect of nutritional ketosis on a variety of cognition outcomes. The ketogenic therapy proved successful in improving general cognition using the ADAS-Cog, in both acute (53) and long-term (48, 49, 52) interventions. The MMSE appeared less sensitive to ketogenic treatment, with the Fortier et al. (47) and Brandt et al. (45) RCTs indicating lack of significant improvement, and only 1 trial (48) suggesting ameliorated results posttreatment.
TABLE 4.
Overview of the findings associated with ketogenic therapy in patients with AD or mild cognitive impairment1
| Effects | First author | Cognitive battery | Mood/depression | Efficacy in everyday behavior | Blood ketone bodies concentrations | AD protein and peptide concentrations in CSF | Brain ketone uptake | rCBF |
|---|---|---|---|---|---|---|---|---|
| Long-term | Brandt (45) | ∼ MMSE and MCS | ↑ POMS-Bi (at wk 6) | ∼ MDS-HC | ||||
| Craft (46) | ↑ t-tau, ∼ Aβ42 | ↑ [11C] AcAc | ||||||
| Fortier (47) | ∼ MMSE and MoCA,↑ Stroop,↑ RL/RI-16,↑ BVMT-R | ↑ BHB | ↑ Global brain CMRAcAc and CMRKetones∼ CMRGlu | |||||
| Henderson (48) | ↑ε4− ADAS-Cog,↑ε4− ADCS-CGIC (day 45),↑ε4+ MMSE (day 104) | ↑ BHB | ||||||
| Henderson (49) | ↑2 ADAS-Cog↑ε4− ADAS-Cog | |||||||
| Krikorian (50) | ∼ TMT, ↑ V-PAL | ∼ GDS | ||||||
| Rebello (52) | ↑ε4−ADAS-Cog,3∼ TMT and Digit Symbol | |||||||
| Torosyan (54) | ↑ε4− rCBF in the left superior lateral temporal cortex | |||||||
| Acute | Ota (51) | ∼ WAIS-III, WMS-R, Stroop, TMT | ↑ AcAc and BHB | |||||
| Rebello (52) | ↑ε4− BHB3 | |||||||
| Reger (53) | ↑ε4− ADAS-Cog, ∼ Stroop | ↑ BHB | ||||||
| Torosyan (54) | ↑ε4− rCBF in dorsolateral prefrontal and temporal cortices |
AcAc, acetoacetate; AD, Alzheimer disease; ADAS-Cog, Alzheimer's Disease Assessment Scale-Cognitive subscale (58); ADCS-CGIC, AD Cooperative Study–Clinical Global Impression of Change (76); APOE, apoE; Aβ42, amyloid β 42; BHB, serum β-hydroxybutyrate; BVMT-R, Brief Visuospatial Memory Test-Revised (62); CMR, cerebral metabolic rate; CSF, cerebrospinal fluid; GDS, Geriatric Depression Scale (73); Glu, glucose; MCS, Memory Composite Score; MDS-HC, Minimum Data Set for Home Care (75); MMSE, Mini-Mental State Exam (59); MoCA, Montreal Cognitive Assessment (60); POMS-Bi, Profile of Mood States Bipolar Scale (74); rCBF, regional cerebral blood flow; RL/RI-16, Rappel Libre/Rappel Indicé (64); Stroop, Stroop Color Word Interference (66); TMT, Trail Making Test (68); t-tau, total tau; V-PAL, Verbal Paired Associate Learning Test (63); WAIS-III, Wechsler Adult Intelligence Scale-3rd (77); WMS-R, Wechsler Memory Scale-Revised (78); ε4−, APOE ε4(−); ε4+, APOE ε4(+); ↑, improved; ∼, no change.
Homozygous carriers of the ε3 allele.
n = 1 participant only.
Long-term ketogenic therapy appeared to improve episodic memory as demonstrated by improved scores in the RL/RI-16 and BVMT-R tests (47), as well as secondary memory, assessed via V-PAL (50). However, consumption of a ketogenic formula (51) failed to induce acute improvements in either the WAIS-III, or the WMS-R tests.
As far as executive ability and attention are concerned, none of the included trials revealed either acute (51) or long-term (50, 52) improvements based on the TMT or the Digit Symbol Substitution Test (52). On the other hand, results concerning the Stroop Color Word Interference test appear to be time-dependent, with improved results noted in patients with MCI treated with ketogenic agents for 6 months (47), and nonsignificant changes over a short-term treatment duration (51, 53).
Adherence to a KD failed to improve mood among patients with MCI (45, 50). In parallel, when efficacy in everyday behavior was assessed postintervention, no changes were noted (45). According to the trial performed by Krikorian et al. (50), adherence to a very low carbohydrate diet decreased body weight and waist circumference and ameliorated fasting plasma glucose and insulin concentrations.
Intervention results on ketone bodies production and utilization
Increases in blood ketone bodies concentrations were unanimous in all RCTs, after either acute (51–53) or long-term ketogenic therapy (47, 48). In addition, brain ketone uptake and utilization were also improved, as indicated by global brain CMRAcAc, CMRKetones (47), and [11C] AcAc (46). In parallel, brain uptake and utilization of glucose appeared to remain unchanged (47). According to Torosyan et al. (54), adherence to ketogenic therapy for 45 days increased rCBF in the left superior lateral temporal cortex.
Effects of intervention according to APOE status
In most studies (48, 53), APOE ε4(+) participants failed to increase blood ketone concentrations to the same extent as the APOE ε4(−) ones. Rebello et al. (52) reported an increase in BHB concentrations among the APOE ε4(−) group at baseline; however, at 24 wk the increase was higher among the APOE ε4(+) group, indicating an adaptation to the intervention among APOE ε4(−) participants. In addition, the BENEFIC (47) and Brandt et al. trials (45) failed to demonstrate differences in the ketosis and cognitive outcomes according to APOE status. APOE ε4(−) patients exhibited elevated rCBF in the left superior lateral temporal cortex (54) and improved cognition as evaluated with the ADAS-Cog (48, 49, 53).
Overall, ketone bodies concentrations were directly correlated to the neurocognitive batteries including paragraph recall (53), secondary memory performance (V-PAL) (50), TMT (47), BNT, VF (47), and general cognition using the ADAS-Cog (48, 52).
Discussion
The present systematic review indicated that, based on the available RCTs, adherence to a ketogenic therapy appears to induce many cognition-related benefits among adults with MCI and/or AD. In further detail, both the direct administration of ketogenic agents and the use of high-fat, low-carbohydrate ketogenic meals/diets increased serum and brain ketone availability and induced beneficial effects on a variety of neurocognitive outcomes. Among APOE ε4(+) and (−) carriers, the effect appears to be higher in the latter than in the former.
Adherence to ketogenic therapy and neurocognition
The present results collectively indicate that adherence to ketogenic therapy among adults with MCI and/or AD improves both acute and long-term cognition. The findings reveal that the effects of ketogenic therapy appear similar, either when delivered acutely (through 1 meal) or over a longer period (months). Ketone availability appears to provide an alternative energy source for the aging brain cells (81–85), both directly, and indirectly via effects on the synaptic transmission and the intrinsic neuronal excitability. The identified pathways explaining the efficacy of the ketogenic therapy include modulation of oxidative stress and inflammatory responses, inhibition of the α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor, attenuation of Aβ-induced mitochondrial alterations and associated toxicity, and activation of the peroxisome proliferator–activated γ receptor (81, 83, 86–88). According to Croteau et al. (89), when MCTs are provided as treatment, all types of MCTs induce similar brain ketone uptake improvements.
When considering the ketogenic therapy narrative, however, one should also take into account that the improved cognition is only apparent when patients with MCI are concerned, because RCTs performed on cognitive-healthy participants failed to manifest further cognitive function improvements, as expected (90).
Glucose metabolism and cerebral substrate use post-intervention
Long-term adherence to ketogenic therapy does not appear to affect glucose metabolism in a negative manner (47, 50, 52). The BENEFIC trial (47) showed that cerebral glucose uptake remained unchanged after 6 mo of ketogenic therapy, whereas Rebello et al. (52) reported normal blood glucose and insulin concentrations after 24 wk consuming yoghurt with MCT oil every day. Thus, the availability of glucose and the insulin response appear unaffected. In parallel, Krikorian et al. (50) reported ameliorated fasting glucose and insulin concentrations 6 wk postintervention, as a possible epiphenomenon of the observed reduction in body weight and waist circumference of participants. After all, low-carbohydrate diets appear promising for both diabetes treatment and weight loss (91–93). On the primary prevention level, the risk of developing AD is increased when a trend toward insulin resistance is observed among older adults (94, 95). Based on the RCTs included in the present systematic review, and individual case studies (96), the use of ketoneurotherapeutics can improve the glycemic profile, which in turn, by inference, might delay progression to AD.
Although the low glucose uptake of the aging brain has long been identified in AD (97, 98) and has been associated with cognitive decline (99, 100), very few AD-related therapies have focused on addressing the issue of the induced energy availability (101, 102). Based on the CMR and [11C] AcAc PET uptake, ketogenic interventions increased brain ketone metabolism (46, 47) and this produced subsequent increments in the total brain metabolism as indicated by the sVOI and voxel-based spm (54). These findings suggest that the metabolism gap observed during AD progression as a result of reduced brain glucose availability (103) is compensated for by the increased utilization of ketones. Ketones serve as alternative substrate sources for the brain, a “super fuel,” preventing energy starvation (91, 103–105). Thus, ketones seem to be an obligatory brain substrate among persons with MCI, owing to the low net cerebral glucose uptake (103).
Ketogenic therapy according to APOE status
With the suggested etiology of AD being a complex interplay of genetic and environmental causes, genome-wide association studies have revealed many genes associated with increased risk of AD development (106–114). Among those, the APOE gene is a major genetic risk determinant for the development of sporadic late-onset AD (115, 116). Moreover, the APOE ε4 variant has been suggested to affect the clearance capacity and degradation of β-amyloid from the brain, CMR, cholesterol transport, glucose metabolism, Αβ deposition, as well as tau pathology and degeneration (28, 117–120).
In general, ketone concentrations were directly correlated to the neurocognitive battery (47, 48, 50, 53), indicating a dose–response association (48). This effect was significant among the APOE ε4(−) subjects and the total sample; however, it was missing from the APOE ε4(+) participants (48). Owing to the aforementioned dose–response association, an important prerequisite for this effect was that participants were intervention-compliant (48). In addition, a time frame was suggested by Henderson et al. (48) for the results to become efficient. In greater detail, the significant pharmacologic response of the ketogenic intervention among APOE ε4(−) participants was initiated 90 d post-administration (48). Torosyan et al. (54) showed that adherence to the ketogenic therapy increased rCBF in the left superior lateral temporal cortex, as indicated by the sVOI, corroborated by the spm. Based on the spm, the long-term increase in rCBF was noted in the anterior cerebellum, left inferior temporal cortex, and hypothalamus. When more genes and loci were analyzed (49), improvements in cognition outcomes were more significant among the ε3/ε3 genotype group, and specific genotype combinations of IDE (heterozygous C/T for IDE rs2251101SNP) and ε4(−) produced similar positive outcomes. In addition, specific genotype combinations of IL1B (homozygous for T/T for IL1B rs1143627 and homozygous for the C/C allele of rs16944) and ε4(−) resulted in additive cognition improvements post-intervention.
However, it should be noted that APOE ε4(+) participants did not fail to respond to the treatment. The effect of ketogenic therapy on APOE ε4(+) participants appears delayed, with 1 trial showing improvement in the MMSE score on day 104 compared with the controls (48). On the other hand, Torosyan et al. (54) failed to indicate regions of increased rCBF among APOE ε4(+) participants; however, the trial lasted for a total of 45 days, a duration much shorter than the cutoff suggested by Henderson et al. (48) as the time frame needed for induction of a significant response among APOE ε4(+) participants. In parallel, Torosyan et al. (54) failed to assess the degree of compliance to the therapy among participants; thus, a high prevalence of noncompliance might have diminished the expected response further.
Most trials demonstrated differences in the blood ketone concentrations, ketone uptake, and cognition outcomes postintervention among participants with APOE ε4(−) or APOE ε4(+) status. However, 4 RCTs failed to reveal such differences (45, 47, 52, 53). The trial by Rebello et al. (52) was extremely underpowered and lacked statistical analyses, because it included only 2 participants in the intervention group, 1 being APOE ε4(−) and the other APOE ε4(+). On the other hand, the BENEFIC (47) and Brandt et al. trials (45) were also underpowered. In parallel, the lack of differences reported in the Rebello et al. (52) and Reger et al. (53) trials can also be attributed to the delayed response suggested to occur among APOE ε4(+) participants. Given that both trials assessed the effects of a short-term intervention, it is possible that the trial duration was inadequate for APOE ε4(+) subjects to respond adequately.
Effects of ketogenic therapy on t-tau and Aβ42 CSF concentrations
Concerning other AD biomarkers like t-tau and Aβ42 concentrations, an increase in CSF t-tau and a trend toward an increase in Aβ42 were reported after a KD (46). However, research appears conflicting regarding the relation of these biomarkers with cognitive decline (121). Some researchers (122, 123) have reported increased CSF Aβ42 concentrations, whereas others have suggested reduced concentrations, among patients with cognitive decline (124–126). According to Lee et al. (121), although the aggregation of tau and Aβ isoforms is typical in AD, research attempting to establish their reliability as biomarkers has produced contradictory results. CSF t-tau and Aβ isoforms, in particular, rely on insufficient analytical standardization and are not AD-specific biomarkers; however, they are often used as proxy biomarkers for detecting changes in AD degeneration (121). Overall, CSF Aβ42 concentrations tended to correlate well with brain Aβ content (127). However, fluctuations, especially concerning t-tau, have been recorded in acute disorders, and the invasive nature of a lumbar puncture might also affect the results via the induced pain, stress, and possible CSF contamination. However, differences in the results associated with increased AD risk might be attributed to the fact that these biomarkers are not so accurate among patients in the prodromal stages of AD (121), as most patients included in the present systematic review were. Subsequently, it is difficult to interpret the findings of the Craft et al. trial (46), especially given the small number of participants and the fact that APOE status was not assessed, although carriers have been shown to carry a higher amyloid burden (128, 129). In parallel, the literature is still too limited to discern whether ketogenic therapy entails antiamyloid effects or just cognition benefits for patients with MCI/AD.
Research in the pipeline
The need for effective MCI remedies has created an upsurge in ketogenic therapy research, with a plethora of additional outcomes being examined (31, 55). During the search process, protocols of ongoing RCTs were identified meeting the review's criteria, without having published any results yet (Table 5). A total of 4 ongoing parallel RCTs were identified in clinicaltrials.gov, comparing adherence to a KD, a modified Mediterranean KD, consumption of ketogenic drinks, or MCT oil against other diet regimes, placebo drinks, or lower intake of MCT oil among patients with MCI/AD. The MCT and brain Metabolism in Alzheimer's disease (MCT-MA) trial (NCT02709356) appears to have completed the intervention and results are expected. In the MINT-01 (Medium-chain triglyceride INTervention for patients with Alzheimer disease) RCT (NCT02912936), the intervention was completed in December 2019. The TDAD (Therapeutic Diets in Alzheimer's Disease) (NCT03860792) and BEAT-AD (Brain Energy for Amyloid Transformation in Alzheimer's Disease) (NCT03472664) trials are expected to publish results after the year 2023. Once more, although important for AD research, the reported primary outcomes are heterogeneous, with only 1 trial reporting the assessment of cognitive ability postintervention.
TABLE 5.
RCTs in the pipeline, investigating the use of ketogenic diet/agents on patients with Alzheimer disease or mild cognitive impairment1
| Clinical trial identifier | Study | Collaborators | Design | Intervention/comparator duration | Intervention | Comparator | Estimated study duration | Primary outcomes | Secondary outcomes |
|---|---|---|---|---|---|---|---|---|---|
| NCT03860792 | TDAD2 | 1) University of Kansas Medical Center2) NIA | Parallel, single-blind (outcomes assessor) RCT | 3 mo | KD (1:1) (70% fat, <10% CHO, and 20% protein) | TLC diet (20%–35% fat, 50%–60% CHO, and ∼15% protein as energy. Fat intake is comprised of <7% SFA, ≤20% MUFA, and ≤10% PUFA as total energy. Consumption of ≤200 cholesterol mg/d, ≥2 servings of fruit and ≥5 servings of vegetables daily). | October 2019 to November 2023 | 1) ADAS-Cog2) MMSE3) Logical memory test (WMS-R)4) Stroop5) CDR | 1) Cerebral NAA concentration2) Blood platelet mitochondrial function3) Self-reported symptoms4) Blood ketone concentrations5) Days positive for urinary ketones6) Dietary intake |
| NCT02912936 | MINT-012 | 1) University of British Columbia2) Université de Sherbrooke | Parallel RCT with quadruple masking | 10 d | Ketogenic MCT drink (lactose-free skim milk drink containing 25 g MCT oil per 250 mL). | Placebo drink (lactose-free skim milk drink containing high-oleic sunflower oil with the equivalent amount of energy as the active arm). | September 2016 to December 2019 | 1) Adverse events2) Plasma ketone concentrations in ascending MCT dose | 1) AUC of MCT2) Δ in cerebral metabolic rate of glucose, blood flow, and MRS after a ketogenic drink3) Physical activity |
| NCT03472664 | BEAT-AD3 | 1) Wake Forest University2) NIA | Parallel, double-blind RCT | 4 mo | Modified Mediterranean KD (low-CHO/high-fat diet) including extra virgin olive oil, fish, lean meats, <20 g CHO/d, and a daily MV tablet. | AHA diet (low fat/high CHO). Diet includes fat intake <40 g/d, fruits, vegetables, CHO, adequate fiber, and a daily MV tablet. | July 2018 to April 2023 | 1) CSF Aβ42 | 1) CSF Aβ42/tau2) PACC3) Cerebral blood flow with ASL |
| NCT02709356 | MCT-MA4 | 1) Université de Sherbrooke2) Fondation Vitae | Parallel,5 single-blind (patients) RCT | 1 mo | Supplementation of caprylic acid MCT oil [60% capric acid (10:0) + 40% caprylic acid] per day. | Supplementation of 30 g MCT oil (100% caprylic acid) per day. | October 2015 to February 2018 | 1) Brain glucose uptake2) Brain AcAc uptake | NR |
AcAc, acetoacetate; ADAS-Cog, Alzheimer's Disease Assessment Scale-Cognitive subscale (58); AHA, American Heart Association; ASL, arterial spin labeling; Aβ42, Amyloid β 42; BEAT-AD, Brain Energy for Amyloid Transformation in Alzheimer's Disease; CDR, Clinical Dementia Rating (70); CHO, carbohydrates; CSF, cerebrospinal fluid; KD, ketogenic diet; MCT, medium-chain triglyceride; MCT-MA, Medium Chain Triglycerides and brain Metabolism in Alzheimer's disease; MINT-01, Medium Chain Triglyceride INTervention for patients with Alzheimer disease; MMSE, Mini-Mental State Exam (59); MRS, magnetic resonance spectroscopy; MV, multivitamin; NAA, N-acetylaspartate; NIA, National Institute on Aging; NR, not reported; PACC, Preclinical Alzheimer Cognitive Composite (Free and Cued Selective Reminding Test, Logical Memory IIa, Digit Symbol Substitution Test, MMSE); RCT, randomized controlled trial; TDAD, Therapeutic Diets in Alzheimer's Disease; TLC, Therapeutic Lifestyle Changes (131); WMS-R, Wechsler Memory Scale-Revised (78).
RCTs not yet recruiting.
RCTs with a “recruiting” status on www.clinicaltrials.gov.
“Completed” RCT status.
Registration indicates parallel design; however, the explanation of the procedures denotes a crossover intervention.
In addition, after the present search was completed, the BEAM trial (46, 55, 56) published another full-text publication (130) during early 2020, based on the same protocol. The results revealed that adherence to a modified Mediterranean KD increased cerebral perfusion and ketone body uptake.
Limitations of the present study
The present systematic review accrued the best available evidence (i.e., from RCTs) on the effects of ketogenic therapy on patients with AD/MCI. Important shortcomings of the included trials involve the relatively small sample sizes used in the majority of RCTs, as well as the lack of compliance assessment. In addition, as Tolar et al. noted (129), patient heterogeneity is often common in MCI RCTs owing to the coexistence of distinct neuropathologies resulting in increased variability in the induced biomarker changes and the cognitive decline of participants, and, in parallel, APOE status is not always considered in AD research. However, treatment responses appear mediated by APOE status. Inarguably, as for every systematic review, the present review also inherits all the limitations of its included trials, namely the increased RoB demonstrated by the RoB assessment and the low methodological quality. On these premises, it was difficult to meta-analyze extracted data, and, for this, future studies with specific designs and outcomes are required.
A holistic view of the available RCTs applying ketogenic therapy to patients with MCI and/or AD also indicates a high degree of heterogeneity in the study design and methodology of the published research. Induction of ketosis was not always measured or achieved (50), despite the study protocols. A variety of agents and methods were applied to increase ketone bodies production, using both short- and long-term interventions, not allowing for the synthesis of the results. According to Margolis and O'Fallon (132), exogenous ketone supplementation, as in the form of MCT or ketogenic agents, consists of an alternative strategy aiming to increase ketone bodies concentrations, while reducing possible adverse events associated with adherence to a KD.
Admittedly, however effective it may be, the KD is not an “easy” diet regime. Compared with the other “healthy” diets, it is restrictive (129), and its initiation is often associated with adverse gastrointestinal events and hypoglycemic episodes (133). According to Taylor et al. (134), the KD can be nutritionally dense, while meeting the RDAs of older adults. However, adherence to the KD requires drastic changes, and, for this, long-term compliance is challenging to maintain (135). For all these reasons, the duration of the majority of interventions did not appear to exceed 6 mo.
Conclusions
Currently, there are no approved drugs to delay or halt the progress of cognitive decline in AD (124). Although faith in the therapeutic effects of the KD was initially attributed to Hippocrates (136), research on ketoneurotherapeutics for AD appears young. The results underline that, collectively, the efficacy of ketogenic therapy in MCI/AD appears promising, indicating that it is more than a symptomatic remedy (137). Nevertheless, research is still scattered and heterogeneous in terms of study design, intervention, participants, and outcomes of interest. Predefining a set of important outcomes for relevant RCTs would add weight to the evidence and aid toward the development of recommendations advocating for the usefulness of ketogenic therapy in AD. Thus, apart from reviewing the available RCTs assessing the efficacy of ketoneurotherapeutics on AD, the present study can also serve as a primer for the design of future clinical trials, to support public health translation and promote the KD as an evidence-based AD prescription remedy.
Supplementary Material
ACKNOWLEDGEMENTS
The authors’ responsibilities were as follows—MGG and DGG: designed the study, performed the data extraction, drafted the manuscript, and are responsible for the final content; MGG, KG, and XT: assessed the risk of bias and quality of the included studies; MGG, DGG, and KG: performed the systematic search; KG, MGG, and DPB: drafted the systematic review protocol; KKG, AE, ED, and DPB: discussed and commented on the manuscript; MGG and KG: created the figures presented in the manuscript; and all authors: read and approved the final manuscript.
Notes
The authors reported no funding received for this study.
Author disclosures: The authors report no conflicts of interest.
Supplemental Figure 1 is available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/advances.
Abbreviations used: AcAc, acetoacetate; AD, Alzheimer disease; ADAD-Cog, Alzheimer's Disease Assessment Scale-Cognitive; APOE ε4, apoε4 allele; Aβ, amyloid β; BEAM, Brain Energy and Memory; BENEFIC, Brain ENErgy Fitness, Imaging and Cognition; BHB, β-hydroxybutyrate; BNT, Boston Naming Test; BVMT-R, Brief Visuospatial Memory Test-Revised; CMR, cerebral metabolic rate; CSF, cerebrospinal fluid; DASH, Dietary Approaches to Stop Hypertension; IDE, insulin-degrading enzyme; IL1B, interleukin 1β; KD, ketogenic diet; MCI, mild cognitive impairment; MCT, medium-chain triglyceride; MMSE, Mini-Mental State Exam; MoCA, Montreal Cognitive Assessment; MUFA, mono-unsaturated fatty acids; PET, positron emission tomography; PUFA, poly-unsaturated fatty acids; rCBF, regional cerebral blood flow; RCT, randomized controlled trial; RL/RI-16, Rappel Libre/Rappel Indicé; RoB, risk of bias; SNP, single nucleotide polymorphism; spm, statistical parametric mapping; sVOI, standardized volumes of interest; TMT, Trail Making Test; t-tau, total tau; VF, verbal fluency; V-PAL, Verbal Paired Associate Learning Test; WMS-R, Wechsler Memory Scale-Revised.
Contributor Information
Maria G Grammatikopoulou, Department of Rheumatology and Clinical Immunology, Faculty of Medicine, School of Health Sciences, University of Thessaly, Larissa, Greece.
Dimitrios G Goulis, Unit of Reproductive Endocrinology, 1st Department of Obstetrics and Gynecology, Medical School, Faculty of Health Sciences, Aristotle University of Thessaloniki, Thessaloniki, Greece.
Konstantinos Gkiouras, Department of Rheumatology and Clinical Immunology, Faculty of Medicine, School of Health Sciences, University of Thessaly, Larissa, Greece.
Xenophon Theodoridis, Department of Rheumatology and Clinical Immunology, Faculty of Medicine, School of Health Sciences, University of Thessaly, Larissa, Greece.
Kalliopi K Gkouskou, Embiodiagnostics Biology Research Company, Crete, Greece.
Athanasios Evangeliou, 4th Department of Pediatrics, Medical School, Faculty of Health Sciences, Aristotle University of Thessaloniki, Papageorgiou General Hospital, Thessaloniki, Greece.
Efthimis Dardiotis, Department of Neurology, Laboratory of Neurogenetics, Faculty of Medicine, School of Health Sciences, University of Thessaly, University Hospital of Larissa, Larissa, Greece.
Dimitrios P Bogdanos, Department of Rheumatology and Clinical Immunology, Faculty of Medicine, School of Health Sciences, University of Thessaly, Larissa, Greece; Division of Transplantation Immunology and Mucosal Biology, MRC Centre for Transplantation, King's College London Medical School, London, United Kingdom.
References
- 1. McDonald TJW, Cervenka MC. The expanding role of ketogenic diets in adult neurological disorders. Brain Sci. 2018;8:148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Alzheimer's Association 2016 Alzheimer's disease facts and figures. Alzheimers Dement. 2016;12:459–509. [DOI] [PubMed] [Google Scholar]
- 3. Lane CA, Hardy J, Schott JM. Alzheimer's disease. Eur J Neurol. 2018;25:59–70. [DOI] [PubMed] [Google Scholar]
- 4. Selkoe DJ, Hardy J. The amyloid hypothesis of Alzheimer's disease at 25 years. EMBO Mol Med. 2016;8:595–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lopez Lopez C, Tariot PN, Caputo A, Langbaum JB, Liu F, Riviere M-E, Langlois C, Rouzade-Dominguez M-L, Zalesak M, Hendrix S et al. The Alzheimer's Prevention Initiative Generation Program: study design of two randomized controlled trials for individuals at risk for clinical onset of Alzheimer's disease. Alzheimers Dement (N Y). 2019;5:216–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Jansen WJ, Ossenkoppele R, Knol DL, Tijms BM, Scheltens P, Verhey FRJ, Visser PJ, Aalten P, Aarsland D, Alcolea D et al. Prevalence of cerebral amyloid pathology in persons without dementia. JAMA. 2015;313:1924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Solfrizzi V, Custodero C, Lozupone M, Imbimbo BP, Valiani V, Agosti P, Schilardi A, D'Introno A, La Montagna M, Calvani M et al. Relationships of dietary patterns, foods, and micro- and macronutrients with Alzheimer's disease and late-life cognitive disorders: a systematic review. J Alzheimers Dis. 2017;59:815–49. [DOI] [PubMed] [Google Scholar]
- 8. Samieri C, Morris M-C, Bennett DA, Berr C, Amouyel P, Dartigues J-F, Tzourio C, Chasman DI, Grodstein F. Fish intake, genetic predisposition to Alzheimer disease, and decline in global cognition and memory in 5 cohorts of older persons. Am J Epidemiol. 2018;187:933–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. van de Rest O, Wang Y, Barnes LL, Tangney C, Bennett DA, Morris MC. APOE ε4 and the associations of seafood and long-chain omega-3 fatty acids with cognitive decline. Neurology. 2016;86:2063–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Morris MC, Brockman J, Schneider JA, Wang Y, Bennett DA, Tangney CC, van de Rest O. Association of seafood consumption, brain mercury level, and APOE ε 4 status with brain neuropathology in older adults. JAMA. 2016;315:489–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dohrmann DD, Putnik P, Bursać Kovačević D, Simal-Gandara J, Lorenzo JM, Barba FJ. Japanese, Mediterranean and Argentinean diets and their potential roles in neurodegenerative diseases. Food Res Int. 2019;120:464–77. [DOI] [PubMed] [Google Scholar]
- 12. van den Brink AC, Brouwer-Brolsma EM, Berendsen AAM, van de Rest O. The Mediterranean, Dietary Approaches to Stop Hypertension (DASH), and Mediterranean-DASH Intervention for Neurodegenerative Delay (MIND) diets are associated with less cognitive decline and a lower risk of Alzheimer's disease—a review. Adv Nutr. 2019;10:1040–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Morris MC, Tangney CC, Wang Y, Sacks FM, Bennett DA, Aggarwal NT. MIND diet associated with reduced incidence of Alzheimer's disease. Alzheimers Dement. 2015;11:1007–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hosking DE, Eramudugolla R, Cherbuin N, Anstey KJ. MIND not Mediterranean diet related to 12-year incidence of cognitive impairment in an Australian longitudinal cohort study. Alzheimers Dement. 2019;15:581–9. [DOI] [PubMed] [Google Scholar]
- 15. Hill E, Goodwill AM, Gorelik A, Szoeke C. Diet and biomarkers of Alzheimer's disease: a systematic review and meta-analysis. Neurobiol Aging. 2019;76:45–52. [DOI] [PubMed] [Google Scholar]
- 16. Terry RD, Masliah E, Salmon DP, Butters N, DeTeresa R, Hill R, Hansen LA, Katzman R. Physical basis of cognitive alterations in alzheimer's disease: synapse loss is the major correlate of cognitive impairment. Ann Neurol. 1991;30:572–80. [DOI] [PubMed] [Google Scholar]
- 17. Antonell A, Tort-Merino A, Ríos J, Balasa M, Borrego-Écija S, Auge JM, Muñoz-García C, Bosch B, Falgàs N, Rami L et al. Synaptic, axonal damage and inflammatory cerebrospinal fluid biomarkers in neurodegenerative dementias. Alzheimers Dement. 2019;16(2):262–72. [DOI] [PubMed] [Google Scholar]
- 18. Cunnane SC, Courchesne-Loyer A, St-Pierre V, Vandenberghe C, Pierotti T, Fortier M, Croteau E, Castellano C-A. Can ketones compensate for deteriorating brain glucose uptake during aging? Implications for the risk and treatment of Alzheimer's disease. Ann N Y Acad Sci. 2016;1367:12–20. [DOI] [PubMed] [Google Scholar]
- 19. Castellano C-A, Nugent S, Paquet N, Tremblay S, Bocti C, Lacombe G, Imbeault H, Turcotte É, Fulop T, Cunnane SC. Lower brain 18f-fluorodeoxyglucose uptake but normal 11c-acetoacetate metabolism in mild Alzheimer's disease dementia. J Alzheimers Dis. 2014;43:1343–53. [DOI] [PubMed] [Google Scholar]
- 20. Koppel SJ, Swerdlow RH. Neuroketotherapeutics: a modern review of a century-old therapy. Neurochem Int. 2018;117:114–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Taylor MK, Sullivan DK, Swerdlow RH, Vidoni ED, Morris JK, Mahnken JD, Burns JM. A high-glycemic diet is associated with cerebral amyloid burden in cognitively normal older adults. Am J Clin Nutr. 2017;106:1463–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Murray AJ, Knight NS, Cole MA, Cochlin LE, Carter E, Tchabanenko K, Pichulik T, Gulston MK, Atherton HJ, Schroeder MA et al. Novel ketone diet enhances physical and cognitive performance. FASEB J. 2016;30:4021–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kashiwaya Y, Pawlosky R, Markis W, King MT, Bergman C, Srivastava S, Murray A, Clarke K, Veech RL. A ketone ester diet increases brain malonyl-CoA and uncoupling proteins 4 and 5 while decreasing food intake in the normal Wistar rat. J Biol Chem. 2010;285:25950–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hernandez AR, Hernandez CM, Campos K, Truckenbrod L, Federico Q, Moon B, McQuail JA, Maurer AP, Bizon JL, Burke SN. A ketogenic diet improves cognition and has biochemical effects in prefrontal cortex that are dissociable from hippocampus. Front Aging Neurosci. 2018;10:391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Castellano C-A, Croteau E, Bocti C, Fulop T, Cunnane S. Strategies to increase brain energy supply in mild Alzheimer's disease. Alzheimers Dement. 2017;13:P128. [Google Scholar]
- 26. Swerdlow RH. The KU Alzheimer's disease ketogenic diet feasibility and retention trial: results from a pilot study. Alzheimers Dement. 2017;13:P883. [Google Scholar]
- 27. Nygaard HB. Pharmacokinetics and dynamics of a ketogenic intervention in Alzheimer's disease and frontotemporal dementia. Alzheimers Dement. 2017;13:P883. [Google Scholar]
- 28. Stoykovich S, Gibas K. APOE ε4, the door to insulin-resistant dyslipidemia and brain fog? A case study. Alzheimers Dement (Amst). 2019;11:264–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Morrill SJ, Gibas KJ. Ketogenic diet rescues cognition in ApoE4+ patient with mild Alzheimer's disease: a case study. Diabetes Metab Syndr Clin Res Rev. 2019;13:1187–91. [DOI] [PubMed] [Google Scholar]
- 30. Davis JJ, Fournakis N, Ellison J. Ketogenic diet for the treatment and prevention of dementia: a review. J Geriatr Psychiatry Neurol. 2020;891988720901785. [DOI] [PubMed] [Google Scholar]
- 31. Morrison SA, Fazeli PL, Gower B, Willig AL, Younger J, Sneed NM, Vance DE. Cognitive effects of a ketogenic diet on neurocognitive impairment in adults aging with HIV: a pilot study. J Assoc Nurses AIDS Care. 2020;31:312–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kim E-J, Park J-S, Choi W-S, Park YK. Effects of low-carbohydrate and high-fat diet supplemented with ketogenic drink on cognitive function and physical performance in the elderly at high risk for dementia. Korean J Community Nutr. 2019;24:525–34. [Google Scholar]
- 33. Puchalska P, Crawford PA. Multi-dimensional roles of ketone bodies in fuel metabolism, signaling, and therapeutics. Cell Metab. 2017;25:262–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Dhillon KK, Gupta S. Biochemistry, ketogenesis. In: StatPearls. Treasure Island, FL: StatPearls Publishing; 2020. Available from: https://www.ncbi.nim.nih.gov/books/NBK493179. [PubMed] [Google Scholar]
- 35. Rusek M, Pluta R, Ułamek-Kozioł M, Czuczwar SJ. Ketogenic diet in Alzheimer's disease. Int J Mol Sci. 2019;20(16):3892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Broom GM, Shaw IC, Rucklidge JJ. The ketogenic diet as a potential treatment and prevention strategy for Alzheimer's disease. Nutrition. 2019;60:118–21. [DOI] [PubMed] [Google Scholar]
- 37. Newport MT, VanItallie TB, Kashiwaya Y, King MT, Veech RL. A new way to produce hyperketonemia: use of ketone ester in a case of Alzheimer's. Alzheimers Dement. 2015;11:99–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Steindler DA, Reynolds BA. Perspective: neuroregenerative nutrition. Adv Nutr. 2017;8:546–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Boison D. New insights into the mechanisms of the ketogenic diet. Curr Opin Neurol. 2017;30:187–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Freeman JM, Kossoff EH. Ketosis and the ketogenic diet, 2010: advances in treating epilepsy and other disorders. Adv Pediatr. 2010;57:315–29. [DOI] [PubMed] [Google Scholar]
- 41. Hernández-Saavedra D, Moody L, Xu GB, Chen H, Pan Y-X. Epigenetic regulation of metabolism and inflammation by calorie restriction. Adv Nutr. 2019;10:520–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group . Preferred Reporting Items for Systematic Reviews and Meta-Analyses: the PRISMA Statement. PLoS Med. 2009;6:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Higgins JPT, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JAC et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, McQuay HJ. Assessing the quality of reports of randomized clinical trials: is blinding necessary?. Control Clin Trials. 1996;17:1–12. [DOI] [PubMed] [Google Scholar]
- 45. Brandt J, Buchholz A, Henry-Barron B, Vizthum D, Avramopoulos D, Cervenka MC. Preliminary report on the feasibility and efficacy of the modified Atkins diet for treatment of mild cognitive impairment and early Alzheimer's disease. J Alzheimers Dis. 2019;68:969–81. [DOI] [PubMed] [Google Scholar]
- 46. Craft S, Neth BJ, Mintz A, Sai K, Shively N, Dahl D, Baker LD, Cunnane S, Register TC, Gage HD. O4‐05‐03: ketogenic diet effects on brain ketone metabolism and Alzheimer's disease Csf biomarkers. Alzheimers Dement. 2016;12(7S):P342–3. [Google Scholar]
- 47. Fortier M, Castellano C-A, Croteau E, Langlois F, Bocti C, St-Pierre V, Vandenberghe C, Bernier M, Roy M, Descoteaux M et al. A ketogenic drink improves brain energy and some measures of cognition in mild cognitive impairment. Alzheimers Dement. 2019;15(5):625–34. [DOI] [PubMed] [Google Scholar]
- 48. Henderson ST, Vogel JL, Barr LJ, Garvin F, Jones JJ, Costantini LC. Study of the ketogenic agent AC-1202 in mild to moderate Alzheimer's disease: a randomized, double-blind, placebo-controlled, multicenter trial. Nutr Metab (Lond). 2009;6:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Henderson ST, Poirier J. Pharmacogenetic analysis of the effects of polymorphisms in APOE, IDE and IL1B on a ketone body based therapeutic on cognition in mild to moderate Alzheimer's disease; a randomized, double-blind, placebo-controlled study. BMC Med Genet. 2011;12:137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Krikorian R, Shidler MD, Dangelo K, Couch SC, Benoit SC, Clegg DJ. Dietary ketosis enhances memory in mild cognitive impairment. Neurobiol Aging. 2012;33:425.e19–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Ota M, Matsuo J, Ishida I, Takano H, Yokoi Y, Hori H, Yoshida S, Ashida K, Nakamura K, Takahashi T et al. Effects of a medium-chain triglyceride-based ketogenic formula on cognitive function in patients with mild-to-moderate Alzheimer's disease. Neurosci Lett. 2019;690:232–6. [DOI] [PubMed] [Google Scholar]
- 52. Rebello CJ, Keller JN, Liu AG, Johnson WD, Greenway FL. Pilot feasibility and safety study examining the effect of medium chain triglyceride supplementation in subjects with mild cognitive impairment: a randomized controlled trial. BBA Clin. 2015;3:123–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Reger MA, Henderson ST, Hale C, Cholerton B, Baker LD, Watson GS, Hyde K, Chapman D, Craft S. Effects of β-hydroxybutyrate on cognition in memory-impaired adults. Neurobiol Aging. 2004;25:311–14. [DOI] [PubMed] [Google Scholar]
- 54. Torosyan N, Sethanandha C, Grill JD, Dilley ML, Lee J, Cummings JL, Ossinalde C, Silverman DH. Changes in regional cerebral blood flow associated with a 45 day course of the ketogenic agent, caprylidene, in patients with mild to moderate Alzheimer's disease: results of a randomized, double-blinded, pilot study. Exp Gerontol. 2018;111:118–21. [DOI] [PubMed] [Google Scholar]
- 55. Nagpal R, Neth BJ, Wang S, Craft S, Yadav H. Modified Mediterranean-ketogenic diet modulates gut microbiome and short-chain fatty acids in association with Alzheimer's disease markers in subjects with mild cognitive impairment. EBioMedicine. 2019;47:529–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Brinkley TE, Register TC, Zetterberg H, Dahl D, Neth BJ, Craft S. Changes in adiposity and CSF biomarkers following a modified Mediterranean diet. Alzheimers Dement. 2019;15:P581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Reedy J, Lerman JL, Krebs-Smith SM, Kirkpatrick SI, Pannucci TE, Wilson MM, Subar AF, Kahle LL, Tooze JA. Evaluation of the Healthy Eating Index-2015. J Acad Nutr Diet. 2018;118:1622–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Rosen WG, Mohs RC, Davis KL. A new rating scale for Alzheimer's disease. Am J Psychiatry. 1984;141:1356–64. [DOI] [PubMed] [Google Scholar]
- 59. Folstein MF, Folstein SE, McHugh PR. “Mini-mental state.” A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–98. [DOI] [PubMed] [Google Scholar]
- 60. Nasreddine ZS, Phillips NA, Bédirian V, Charbonneau S, Whitehead V, Collin I, Cummings JL, Chertkow H. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53:695–9. [DOI] [PubMed] [Google Scholar]
- 61. Brandt J, Benedict RHB. Hopkins Verbal Learning Test – Revised. Administration manual. Lutz, FL: Psychological Assessment Resources; 2001. [Google Scholar]
- 62. Benedict R. Brief Visuospatial Memory Test-revised professional manual 1997. Odessa, FL: Psychological Assessment Resources; 1997. [Google Scholar]
- 63. Krikorian R. Independence of verbal and spatial paired associate learning. Brain Cogn. 1996;32:219–23. [Google Scholar]
- 64. Van der Linden M, Coyette F, Jgremem P. L’épreuve de Rappel Libre/Rappel Indicé à 16 items (RL/RI-16). In: Van der Linden M, Agniel AEeditors. L’évaluation des troubles de la mémoire: présentation de quatre tests de mémoire épisodique (avec leur étalonnage). Marseille, France: Solal; 2004. p. 25–47. [Google Scholar]
- 65. Kaplan E, Goodglass H, Weintraub S. The Boston Naming Test 1983. Philadelphia, PA: Lea & Febiger; 1983. [Google Scholar]
- 66. Stroop JR. Studies of interference in serial verbal reactions. J Exp Psychol. 1935;18:643–62. [Google Scholar]
- 67. Delis D, Kaplan E, Kramer J. Delis-Kaplan Executive Function System® (D-KEFS®): examiner's manual: flexibility of thinking, concept formation, problem solving, planning, creativity, impulse control, inhibition. The Psychological Corporation, editor. San Antonio, TX: Pearson; 2001. [Google Scholar]
- 68. Reitan RM. Validity of the Trail Making Test as an indicator of organic brain damage. Percept Mot Skills. 1958;8:271–6. [Google Scholar]
- 69. Petersen RC. Mild cognitive impairment as a diagnostic entity. J Intern Med. 2004;256:183–94. [DOI] [PubMed] [Google Scholar]
- 70. Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 1993;43:2412–14. [DOI] [PubMed] [Google Scholar]
- 71. Albert MS, DeKosky ST, Dickson D, Dubois B, Feldman HH, Fox NC, Gamst A, Holtzman DM, Jagust WJ, Petersen RC et al. The diagnosis of mild cognitive impairment due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7:270–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984;34:939–44. [DOI] [PubMed] [Google Scholar]
- 73. Yesavage JA, Brink TL, Rose TL, Lum O, Huang V, Adey M, Leirer VO. Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res. 1982;17:37–49. [DOI] [PubMed] [Google Scholar]
- 74. Lorr M, McNair DM, Heuchert JW. Profile of Mood States: Bi-polar Form. North Tonawanda, NY: Multi-Health Systems (MHS); 2003. Available from: https://www.ncbi.nlm.nih.gov/books/NBK493179/. [Google Scholar]
- 75. Landi F, Tua E, Onder G, Carrara B, Sgadari A, Rinaldi C, Gambassi G, Lattanzio F, Bernabei R, SILVERNET-HC Study Group of Bergamo . Minimum data set for home care: a valid instrument to assess frail older people living in the community. Med Care. 2000;38:1184–90. [DOI] [PubMed] [Google Scholar]
- 76. Schneider LS, Clark CM, Doody R, Ferris SH, Morris JC, Raman R, Reisberg B, Schmitt FA. ADCS Prevention Instrument Project: ADCS-Clinicians’ Global Impression of Change Scales (ADCS-CGIC), Self-rated and Study Partner-rated Versions. Alzheimer Dis Assoc Disord. 2006;20:S124–38. [DOI] [PubMed] [Google Scholar]
- 77. Wechsler D. Wechsler Adult Intelligence Scale. 3rd ed San Antonio, TX: Psychological Corporation; 1997. [Google Scholar]
- 78. Wechsler D. Wechsler Memory Scale-Revised. San Antonio, TX: Psychological Corporation; 1987. [Google Scholar]
- 79. Koch W, Ehrenhaft A, Griesser K, Pfeufer A, Müller J, Schömig A, Kastrati A. Taqman systems for genotyping of disease-related polymorphisms present in the gene encoding apolipoprotein E. Clin Chem Lab Med. 2002;40:1123–31. [DOI] [PubMed] [Google Scholar]
- 80. Nalbantoglu J, Gilfix BM, Bertrand P, Robitaille Y, Gauthier S, Rosenblatt DS, Poirier J. Predictive value of apolipoprotein E genotyping in Alzheimer's disease: results of an autopsy series and an analysis of several combined studies. Ann Neurol. 1994;36:889–95. [DOI] [PubMed] [Google Scholar]
- 81. Augustin K, Khabbush A, Williams S, Eaton S, Orford M, Cross JH, Heales SJR, Walker MC, Williams RSB. Mechanisms of action for the medium-chain triglyceride ketogenic diet in neurological and metabolic disorders. Lancet Neurol. 2018;17:84–93. [DOI] [PubMed] [Google Scholar]
- 82. Fernando WM, Martins IJ, Goozee KG, Brennan CS, Jayasena V, Martins RN. The role of dietary coconut for the prevention and treatment of Alzheimer's disease: potential mechanisms of action. Br J Nutr. 2015;114:1–14. [DOI] [PubMed] [Google Scholar]
- 83. Yang H, Shan W, Zhu F, Wu J, Wang Q. Ketone bodies in neurological diseases: focus on neuroprotection and underlying mechanisms. Front Neurol. 2019;10:585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. deCampo DM, Kossoff EH. Ketogenic dietary therapies for epilepsy and beyond. Curr Opin Clin Nutr Metab Care. 2019;22:264–8. [DOI] [PubMed] [Google Scholar]
- 85. Omar SH. Mediterranean and MIND diets containing olive biophenols reduces the prevalence of Alzheimer's disease. Int J Mol Sci. 2019;20:2797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Nafar F, Mearow KM. Coconut oil attenuates the effects of amyloid-β on cortical neurons in vitro. J Alzheimers Dis. 2014;39:233–7. [DOI] [PubMed] [Google Scholar]
- 87. Kanabus M, Fassone E, Hughes SD, Bilooei SF, Rutherford T, Donnell MO, Heales SJR, Rahman S. The pleiotropic effects of decanoic acid treatment on mitochondrial function in fibroblasts from patients with complex I deficient Leigh syndrome. J Inherit Metab Dis. 2016;39:415–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Nafar F, Clarke JP, Mearow KM. Coconut oil protects cortical neurons from amyloid beta toxicity by enhancing signaling of cell survival pathways. Neurochem Int. 2017;105:64–79. [DOI] [PubMed] [Google Scholar]
- 89. Croteau E, Castellano C-A, Richard MA, Fortier M, Nugent S, Lepage M, Duchesne S, Whittingstall K, Turcotte ÉE, Bocti C et al. Ketogenic medium chain triglycerides increase brain energy metabolism in Alzheimer's disease. J Alzheimers Dis. 2018;64:551–61. [DOI] [PubMed] [Google Scholar]
- 90. Iacovides S, Goble D, Paterson B, Meiring RM. Three consecutive weeks of nutritional ketosis has no effect on cognitive function, sleep, and mood compared with a high-carbohydrate, low-fat diet in healthy individuals: a randomized, crossover, controlled trial. Am J Clin Nutr. 2019;110:349–57. [DOI] [PubMed] [Google Scholar]
- 91. Ludwig DS. The ketogenic diet: evidence for optimism but high-quality research needed. J Nutr. 2020;150:1354–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Snorgaard O, Poulsen GM, Andersen HK, Astrup A. Systematic review and meta-analysis of dietary carbohydrate restriction in patients with type 2 diabetes. BMJ Open Diabetes Res Care. 2017;5:e000354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Masood W, Uppaluri KR. Ketogenic diet. In: StatPearls. Treasure Island, FL: StatPearls Publishing; 2019. Available from: https://www.ncbi.nim.nih.gov/books/NBK499830. [PubMed] [Google Scholar]
- 94. Craft S. The role of metabolic disorders in Alzheimer disease and vascular dementia. Arch Neurol. 2009;66:300–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Baker LD, Cross DJ, Minoshima S, Belongia D, Watson GS, Craft S. Insulin resistance and Alzheimer-like reductions in regional cerebral glucose metabolism for cognitively normal adults with prediabetes or early type 2 diabetes. Arch Neurol. 2011;68:51–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Mohorko N, Černelič-Bizjak M, Poklar-Vatovec T, Grom G, Kenig S, Petelin A, Jenko-Pražnikar Z. Weight loss, improved physical performance, cognitive function, eating behavior, and metabolic profile in a 12-week ketogenic diet in obese adults. Nutr Res. 2019;62:64–77. [DOI] [PubMed] [Google Scholar]
- 97. Lying-Tunell U, Lindblad BS, Malmlund HO, Persson B. Cerebral blood flow and metabolic rate of oxygen, glucose, lactate, pyruvate, ketone bodies and amino acids. Acta Neurol Scand. 2009;63:337–50. [DOI] [PubMed] [Google Scholar]
- 98. Mosconi L, Andrews RD, Matthews DC; for the Alzheimer's Disease Neuroimaging Initiative (ADNI) . Comparing brain amyloid deposition, glucose metabolism, and atrophy in mild cognitive impairment with and without a family history of dementia. J Alzheimers Dis. 2013;35:509–24. [DOI] [PubMed] [Google Scholar]
- 99. Anchisi D, Borroni B, Franceschi M, Kerrouche N, Kalbe E, Beuthien-Beumann B, Cappa S, Lenz O, Ludecke S, Marcone A et al. Heterogeneity of brain glucose metabolism in mild cognitive impairment and clinical progression to Alzheimer disease. Arch Neurol. 2005;62:1728–33. [DOI] [PubMed] [Google Scholar]
- 100. Ou Y-N, Xu W, Li J-Q, Guo Y, Cui M, Chen K-L, Huang Y-Y, Dong Q, Tan L, Yu J-T. FDG-PET as an independent biomarker for Alzheimer's biological diagnosis: a longitudinal study. Alzheimers Res Ther. 2019;11:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Gejl M, Brock B, Egefjord L, Vang K, Rungby J, Gjedde A. Blood-brain glucose transfer in Alzheimer's disease: effect of GLP-1 analog treatment. Sci Rep. 2017;7:17490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. de la Monte SM. Insulin resistance and neurodegeneration: progress towards the development of new therapeutics for Alzheimer's disease. Drugs. 2017;77:47–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Cunnane SC, Courchesne-Loyer A, Vandenberghe C, St-Pierre V, Fortier M, Hennebelle M, Croteau E, Bocti C, Fulop T, Castellano C-A. Can ketones help rescue brain fuel supply in later life? Implications for cognitive health during aging and the treatment of Alzheimer's disease. Front Mol Neurosci. 2016;9:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Cahill GF Jr, Veech RL. Ketoacids? Good medicine?. Trans Am Clin Climatol Assoc. 2003;114:149–61.; discussion 162–3. [PMC free article] [PubMed] [Google Scholar]
- 105. Cunnane SC. Ketones, omega-3 fatty acids and the Yin-Yang balance in the brain: insights from infant development and Alzheimer's disease, and implications for human brain evolution. OCL. 2018;25:D409. [Google Scholar]
- 106. Kunkle BW, Grenier-Boley B, Sims R, Bis JC, Damotte V, Naj AC, Boland A, Vronskaya M, van der Lee SJ, Amlie-Wolf A et al. Genetic meta-analysis of diagnosed Alzheimer's disease identifies new risk loci and implicates Aβ, tau, immunity and lipid processing. Nat Genet. 2019;51:414–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Chandler HL, Wise RG, Murphy K, Tansey KE, Linden DEJ, Lancaster TM. Polygenic impact of common genetic risk loci for Alzheimer's disease on cerebral blood flow in young individuals. Sci Rep. 2019;9:467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Glorioso CA, Pfenning AR, Lee SS, Bennett DA, Sibille EL, Kellis M, Guarente LP. Rate of brain aging and APOE ε4 are synergistic risk factors for Alzheimer's disease. Life Sci Alliance. 2019;2:e201900303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Westfall S, Iqbal U, Sebastian M, Pasinetti GM. Gut microbiota mediated allostasis prevents stress-induced neuroinflammatory risk factors of Alzheimer's disease. Prog Mol Biol Transl Sci. 2019;168:147–81. [DOI] [PubMed] [Google Scholar]
- 110. Guerreiro R, Hardy J. Genetics of Alzheimer's disease. Neurotherapeutics. 2014;11:732–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Samadi M, Moradi S, Moradinazar M, Mostafai R, Pasdar Y. Dietary pattern in relation to the risk of Alzheimer's disease: a systematic review. Neurol Sci. 2019;40:2031–43. [DOI] [PubMed] [Google Scholar]
- 112. Huq AJ, Fransquet P, Laws SM, Ryan J, Sebra R, Masters CL, Winship IM, James PA, Lacaze P. Genetic resilience to Alzheimer's disease in APOE ε4 homozygotes: a systematic review. Alzheimers Dement. 2019;15(12):1612–23. [DOI] [PubMed] [Google Scholar]
- 113. Kellar DA, Lockhart SN, Neth BJ, Whitlow CT, Jung Y, Craft S. Diet-related alterations in white matter microstructure in participants at risk for AD. Alzheimers Dement. 2019;15:P1106. [Google Scholar]
- 114. Stamati P, Siokas V, Aloizou A-M, Karampinis E, Arseniou S, Rakitskii VN, Tsatsakis A, Spandidos DA, Gozes I, Mitsias PD et al. Does SCFD1 rs10139154 polymorphism decrease Alzheimer's disease risk?. J Mol Neurosci. 2019;69:343–50. [DOI] [PubMed] [Google Scholar]
- 115. Slot RER, Sikkes SAM, Berkhof J, Brodaty H, Buckley R, Cavedo E, Dardiotis E, Guillo-Benarous F, Hampel H, Kochan NA et al. Subjective cognitive decline and rates of incident Alzheimer's disease and non–Alzheimer's disease dementia. Alzheimers Dement. 2019;15:465–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Raber J, Huang Y, Ashford JW. ApoE genotype accounts for the vast majority of AD risk and AD pathology. Neurobiol Aging. 2004;25:641–50. [DOI] [PubMed] [Google Scholar]
- 117. Lane-Donovan C, Philips GT, Herz J. More than cholesterol transporters: lipoprotein receptors in CNS function and neurodegeneration. Neuron. 2014;83:771–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Zhao N, Liu C-C, Van Ingelgom AJ, Martens YA, Linares C, Knight JA, Painter MM, Sullivan PM, Bu G. Apolipoprotein E4 impairs neuronal insulin signaling by trapping insulin receptor in the endosomes. Neuron. 2017;96:115–129.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Zhong N, Weisgraber KH. Understanding the association of apolipoprotein E4 with Alzheimer disease: clues from its structure. J Biol Chem. 2009;284:6027–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Yamazaki Y, Zhao N, Caulfield TR, Liu C-C, Bu G. Apolipoprotein E and Alzheimer disease: pathobiology and targeting strategies. Nat Rev Neurol. 2019;15:501–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Lee JC, Kim SJ, Hong S, Kim Y. Diagnosis of Alzheimer's disease utilizing amyloid and tau as fluid biomarkers. Exp Mol Med. 2019;51:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Nakamura T, Shoji M, Harigaya Y, Watanabe M, Hosoda K, Cheung TT, Shaffer LM, Golde TE, Younkin LH, Younkin SG et al. Amyloid β protein levels in cerebrospinal fluid are elevated in early-onset Alzheimer's disease. Ann Neurol. 1994;36:903–11. [DOI] [PubMed] [Google Scholar]
- 123. Bowman S. Low economic status is associated with suboptimal intakes of nutritious foods by adults in the National Health and Nutrition Examination Survey 1999-2002. Nutr Res. 2007;27:515–23. [Google Scholar]
- 124. de Leon MJ, DeSanti S, Zinkowski R, Mehta PD, Pratico D, Segal S, Rusinek H, Li J, Tsui W, Saint Louis LA et al. Longitudinal CSF and MRI biomarkers improve the diagnosis of mild cognitive impairment. Neurobiol Aging. 2006;27:394–401. [DOI] [PubMed] [Google Scholar]
- 125. Kanai M, Matsubara E, Isoe K, Urakami K, Nakashima K, Arai H, Sasaki H, Abe K, Iwatsubo T, Kosaka T et al. Longitudinal study of cerebrospinal fluid levels of tau, Aβ1-40, and Aβ1-42(43) in Alzheimer's disease: a study in Japan. Ann Neurol. 1998;44:17–26. [DOI] [PubMed] [Google Scholar]
- 126. Mollenhauer B, Bibl M, Trenkwalder C, Stiens G, Cepek L, Steinacker P, Ciesielczyk B, Neubert K, Wiltfang J, Kretzschmar HA et al. Follow-up investigations in cerebrospinal fluid of patients with dementia with Lewy bodies and Alzheimer's disease. J Neural Transm. 2005;112:933–48. [DOI] [PubMed] [Google Scholar]
- 127. Blennow K, Zetterberg H, Fagan AM. Fluid biomarkers in Alzheimer disease. Cold Spring Harb Perspect Med. 2012;2:a006221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Sperling RA, Jack CR, Black SE, Frosch MP, Greenberg SM, Hyman BT, Scheltens P, Carrillo MC, Thies W, Bednar MM et al. Amyloid-related imaging abnormalities in amyloid-modifying therapeutic trials: recommendations from the Alzheimer's Association Research Roundtable Workgroup. Alzheimers Dement. 2011;7:367–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Tolar M, Abushakra S, Sabbagh M. The path forward in Alzheimer's disease therapeutics: reevaluating the amyloid cascade hypothesis. Alzheimers Dement. 2020. [DOI] [PubMed] [Google Scholar]
- 130. Neth BJ, Mintz A, Whitlow C, Jung Y, Solingapuram Sai K, Register TC, Kellar D, Lockhart SN, Hoscheidt S, Maldjian J et al. Modified ketogenic diet is associated with improved cerebrospinal fluid biomarker profile, cerebral perfusion, and cerebral ketone body uptake in older adults at risk for Alzheimer's disease: a pilot study. Neurobiol Aging. 2020;86:54–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. NIH, National Heart, Lung and Blood Institute Your guide to lowering cholesterol with therapeutic lifestyle changes (TLC). Bethesda, MD: National Heart, Lung and Blood Institute; 2005. [Google Scholar]
- 132. Margolis LM, O'Fallon KS. Utility of ketone supplementation to enhance physical performance: a systematic review. Adv Nutr. 2020;11(2):412–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Lin A, Turner Z, Doerrer SC, Stanfield A, Kossoff EH. Complications during ketogenic diet initiation: prevalence, treatment, and influence on seizure outcomes. Pediatr Neurol. 2017;68:35–9. [DOI] [PubMed] [Google Scholar]
- 134. Taylor MK, Swerdlow RH, Burns JM, Sullivan DK. An experimental ketogenic diet for Alzheimer disease was nutritionally dense and rich in vegetables and avocado. Curr Dev Nutr. 2019;3:nzz003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Włodarek D. Role of ketogenic diets in neurodegenerative diseases (Alzheimer's disease and Parkinson's disease). Nutrients. 2019;11:169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Walshe T. Neurological concepts in Ancient Greek medicine. New York, NY: Oxford University Press; 2016. [Google Scholar]
- 137. Gasior M, Rogawski MA, Hartman AL. Neuroprotective and disease-modifying effects of the ketogenic diet. Behav Pharmacol. 2006;17:431–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.