ABSTRACT
Cardiovascular disease (CVD) is the leading cause of death globally and the presence of ≥1 cardiovascular risk factors elevates total risk. Lycopene, a carotenoid with high antioxidant capacity, may be protective. The aim of this systematic review and meta-analyses is to determine the efficacy of consuming dietary and/or supplemental lycopene on cardiovascular risk factors. Using the PRISMA guidelines, 4 databases were systematically searched from inception: Medline, Cinahl, Proquest, and Scopus. Intervention trials assessing dietary or supplemental lycopene on CVD outcomes were included. The Cochrane Risk-of-Bias tool was used to assess the quality of the included papers. Pooled analysis was conducted using outcomes with available data. Forty-three studies were included. Lycopene interventions were highly variable (supplement with or without food, based as tomato juice/paste/raw product, or combined with olive oil), the dose ranged from 1.44 to 75 mg lycopene/d and was not reported in 11 of 43 included studies. Studies reported conflicting findings for the effect of lycopene on cardiovascular risk factors, This was supported by meta-analyses where there were no significant differences between lycopene intervention and control groups for blood pressure and lipids (total cholesterol, LDL cholesterol, HDL cholesterol, and triglycerides). This was observed for overall groups and in subgroup analyses for individuals with elevated risk factor concentrations at baseline. Lycopene interventions for cardiovascular risk factors were highly variable across studies in both the dosage provided and the mode of delivery (supplement or food based). As such, there are conflicting findings regarding the efficacy of lycopene to improve cardiovascular risk factors. This systematic review was registered with PROSPERO as CRD42018112174.
Keywords: lycopene, tomato, cardiovascular disease, blood pressure, carotenoid, Mediterranean diet
Introduction
Cardiovascular disease (CVD) is the leading cause of morbidity and mortality worldwide (1), with rates of CVD mortality projected to increase from 17 million in 2008 to 25 million by 2020 (2).
The risk of CVD increases with the presence of ≥1 CVD risk factors. Well-recognized risk factors for CVD include a range of pathological biomarkers and associated metabolic conditions, such as elevated total cholesterol (TC), elevated LDL cholesterol, low HDL cholesterol, elevated serum triglycerides (TGs), insulin resistance, hypertension (HT), and type 2 diabetes (T2D) (3, 4). The prevention and treatment of CVD risk factors are of paramount importance due to the significant health and economic burden it imposes (5).
Diet is an important modifiable risk factor for CVD. Improved diet quality, characterized by increased intakes of MUFAs and PUFAs and dietary fiber, are linked to improved management and reduction of CVD (3, 6, 7). Conversely, a poor diet with excess intake of SFAs, sugar, and salts, often from highly processed foods, has been shown to increase the occurrence of risk factors and ultimately the prevalence of CVD (3, 6, 7). Investigators from the Lyon Heart Study found that those following the Mediterranean diet had between 50% and 70% lower risk of CVD risk factors (8). This was also reported in the findings from the seminal Prevention with Mediterranean Diet (PREDIMED) trial where a Mediterranean diet reduced the overall risk of CVD and cardiovascular complications (9). In a meta-analysis conducted by Sofi et al. (10) in >4 million participants, an increased adherence to the Mediterranean diet yielded a 10% reduced risk of CVD. Among the numerous beneficial aspects of the Mediterranean dietary pattern is a greater consumption of vegetables, and within this recommendation there is a specific focus on the consumption of tomatoes (11). Tomatoes are a rich and concentrated source of lycopene that has been shown in many epidemiological studies to be protective of CVD risk factors (3). Lycopene is a naturally occurring carotenoid found predominantly in red-pigmented produce (9). Lycopene's antioxidant activity provides a proposed mechanism for potential health benefits in addition to its free-radical scavenging activity, which protects the body against oxidative stress (OS) (12) and subsequently the development and progression of CVD risk factors (13). As well as its antioxidant capacity it is thought that the cardiovascular benefits of lycopene can be attributed to its anti-inflammatory, antiatherogenic, antiplatelet effects and improved endothelial function (EF; blood flow and NO bioavailability) (14). Lycopene can attach to LDL cholesterol in plasma and through this mechanism provides protection against atherosclerosis via lipid peroxidation (14). With known mechanisms showing potential benefits, a systematic literature review and meta-analyses to assess the effects of lycopene on cardiovascular risk factors in a cumulative manner is warranted.
Thus, the aim of this systematic literature review is to determine the effect of dietary and supplemental lycopene in randomized controlled trials (RCTs) on CVD risk factors, including lipids, inflammatory markers, HT, and markers of OS in adults.
Methods
The PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines were followed as a methodological template for this review (15). This systematic review was registered with PROSPERO (CRD42018112174).
Literature search
A comprehensive search of the literature was conducted using 4 databases: MEDLINE, Cinahl, Proquest, and Scopus. Relevant keywords relating to lycopene in combination as MeSH (Medical Subject Headings) terms and text words (lycopene or psi-psi carotene or tomato or carotenoid pigment or watermelon or guava or papaya and their variants) were used in combination with words relating to CVD risk factors (cardiovascular risk or hypertension or atherosclerosis or overweight or obesity or coronary heart disease or myocardial infarct or blood pressure or cholesterol or cardiovascular disease or CVD OR inflammatory or insulin resistance or insulin sensitivity or coronary artery disease or acute coronary event or cardiovascular event or ischemic heart disease or oxidative stress or lipid or endothelial and their variants). The search was limited to human studies in English language and searched from inception to November 2018. The search strategy is shown in Supplemental Table 1.
Eligibility criteria
Titles and abstracts were screened independently by 2 researchers (CER, LMB) according to inclusion criteria. Inclusion criteria were restricted to RCTs that directly involved either a dietary and/or supplementary lycopene intervention and also included ≥1 cardiovascular risk factor as an outcome, which included blood pressure (BP), blood lipids, inflammatory markers, and OS markers. The quality was assessed using the National Health and Medical Research Council (NHMRC) evidence level as well as a risk-of-bias tool described below. Duplicates were removed as were abstracts and papers in languages other than English. Hand-searching of reference lists from included manuscripts was also carried out and eligible papers were included. All eligible articles were then full-text screened and the quality assessed independently by 2 reviewers (CER, LMB). Conflicts were resolved by consultation (ESG, ACT) and based on consensus.
Data extraction
Data were extracted from the included studies using the following considerations: NHMRC level of evidence, study design, study sample, study duration, inclusion criteria, intervention, and effect of intervention on relevant outcomes. Where data were not available, authors were contacted to request this information. If the required data were not available then they were excluded. Full data extraction can be viewed in Table 1.
TABLE 1.
Effects of lycopene (from food, food extracts, or supplements) on BP, lipids, oxidative stress measures, immunoglobulins, and insulin growth factor in healthy populations or populations with CVD or CVD risk factors: data-extraction table1
| Study (reference); country | NHMRC level of evidence/quality Ax | Study design | Study duration | Population | Intervention | Results of outcome of interest |
|---|---|---|---|---|---|---|
| Blood pressure | ||||||
| Paran et al., 2009 (16); Beer Sheba, Israel | III-1/+ | Double-blinded, placebo-controlled, cross-over trial | 12 wk; no wash-out | n = 54; Moderate HPT and on HPT medication; Nonsmoker; Age: 61.4 ± 8.9 y (46–66 y); 26 Males; SBP 144.0 ± 10.0 mmHg; DBP 82.2 ± 7.8mmHg [mean ± SEM] | Dose: 1 capsule standardized tomato extract (15 mg lycopene/d) or Placebo capsule | SBP (mmHg):Baseline: 144.0 ± 10.0Postintervention: 130.4 ± 9.695% CI of difference: 12.1; – 6.3P-value: <0.001DBP (mmHg):Baseline: 82.2 ± 7.8Postintervention: 76.0 ± 7.895% CI of difference: 5.7; – 1.9P-value: <0.001[Mean ± SD] |
| Massa et al., 2016 (17); Paraiba, Brazil | II/ɸ | Randomized, double-blinded, experimental and placebo-controlled study | 6 wk | n = 40; 21 men, 19 women; BMI: 25–35 kg/m2; HPT stage I or pre-HPT; consistent medication, exercise and dietWatermelon group: Age: 48.7 ± 1.9 y; BMI: 29.6 ± 1.1 k g/m2; WC: 98.1 ± 2.4 cmPlacebo group: Age: 47.4 ± 1.2 y; BMI: 28.2 ± 0.7 kg/m2; WC: 94.6 ± 2.5 cm [mean ± SE] | Dose: 6 g of watermelon extract (1.44 mg lycopene/d) | SBP decreased by 11.8 mmHg for the watermelon group while the placebo group showed no changes (P < 0.0001)DBP decreased by 6.9 mmHg for the watermelon group while the placebo group showed no changes (P < 0.001) |
| Thies et al., 2012 (18); Aberdeen, United Kingdom | II/+ | Single-blinded randomized controlled trial | 16 wk (4-wk wash-out, 12-wk INT) | n = 225; 93 men, 132 women Sedentary or moderately active with signs of MS or moderate HPC; SBP <160 mmHg, DBP <99 mmHg; Age: 51.1 ± 0.8 y (40–65 y); BMI: 26.6 ± 0.5 kg/m2 (18.5–35 kg/m2); WC: 88.4 ± 1.3 cm; SBP: 130.0 ± 1.9 mmHg; DBP: 79.1 ± 1.3 mmHg [mean ± SEM] | Control diet low in tomato based foodsLycopene supplement group: 1 capsule/d containing 10 mg lycopene (70 mg lycopene/wk)High lycopene group: Diet high in tomato-based foods (70 mg lycopene/wk) | SBP (mmHg):Baseline: Lycopene: 127.9 ± 2.0, control 127.4 ± 1.9, P2-value: 0.516Postintervention (PI): Lycopene: 124.7 ± 3.0, control 127.1 ± 1.9, P3-value: 0.286DBP (mmHg):Baseline: Lycopene: 78.0 ± 1.2, control 77.0 ± 1.1, P2-value: 0.933PI: Lycopene: 77.0 ± 1.2, control 76.3± 1.1,P3-value: 0.227[Mean ± SEM]P2- Differences between groups at baselineP3- Changes in concentration from baseline Results are for lycopene and control group |
| Ried et al., 2009 (19); Adelaide, Australia | II/+ | Randomized, placebo-controlled, 3-group-parallel trial | 6 mo [8-wk intervention (phase 1) with 4-wk wash-out, then 8-wk cross-over intervention (phase 2)] | n = 36Pre-HPT; Healthy adults; Not on antihypertensive medication; Age: 22–73 yTomato extract group: Age: 51.2 y (12.1); BMI: 26.2 kg/m2 (3.1); SBP: 128.2 mmHg (11.4); DBP: 79.1 mmHg (7.5) [median (IQR)]Groups were comparable at baseline | Group 1: 50 g dark chocolate/d (750 mg polyphenols)Group 2: 1 tomato extract capsule per day (15 mg lycopene)Group 3: Placebo | SBP (mmHg):Tomato, phase 1:Baseline, 128.2 ± 3.0 (11.4); Week 12, 129.3 ± 3.0 (11.8), P-value: 0.82Phase 2:Week 16, 131.0 ± 4.1 (13.6); Week 24, 127.6 ± 3.9 (8.1), P-value: 0.57DBP (mmHg):Tomato, phase 1:Baseline, 79.1 ± 1.9 (7.5); Week 12, 79.2 ± 2.2 (8.6), P-value: 0.98Phase 2:Week 16, 82.8 ± 2.8 (9.3); Week 24, 79.7 ± 3.7 (12.3), P- value: 0.91[Median (SE)] |
| Gajendragadkar et al., 2014 (20); United Kingdom | III-1/+ | Prospective, randomized, double-blinded, placebo-controlled, parallel-group study | 8 wk | n = 72CVD group: n = 36 Stable CVD with statin therapy; 33 MalesLycopene group: Age: 67 ± 6 y; BMI: 28.6 ± 3.3 kg/m2Placebo group: Age: 68 ± 5 y; BMI: 28.4 ± 4.0 kg/m2HV group: n = 36Lycopene group: Age: 61 ± 13 y; BMI: 25.2 ± 2.8 kg/m2Placebo group: Age: 68 ± 5 y; BMI: 26.7 ± 3.6 kg/m225 Males [mean ± SD] | Dose: 7 mg of lycopene as capsule, once per day (Ateronon) (L)Placebo capsule (P) | Central SBP (mmHg):Day 1:Placebo (P): 129 (4), Lycopene (L):130 (3)Day 56:P: 127 (4), L: 126 (3)P-value: 0.6Central DBP (mmHg):Day 1:P: 77 (2), L: 82 (2)Day 56:P: 78 (3), L: 79 (2)P-value: 0.2P-value: for overall comparison in delta (day 56–day 1) values across placebo and lycopene-treated groups Results only reported for CVD group[Mean (SE)] |
| Engelhard et al., 2006 (21); Beer Sheva, Israel | III-1/ɸ | Single-blinded, placebo-controlled trial | 16 wk (4-wk placebo; 8-wk intervention; 4-wk placebo) | n = 31Grade-1 HTN but otherwise healthy; Nonsmoker; Age: 30–70 y; BMI: 29.4 ± 0.8 kg/m2 [mean ± SE] | Dose: 1 capsule of Lyc-O-Mato per day (15 mg lycopene) | SBP (mmHg):Baseline: 145.0 ± 1.3, Week 4: 144.0 ± 1.1, Week 12: 134.0 ± 2*, Week 16: 144.0 ± 2.1DBP (mmHg):Baseline: 88.9 ± 1.4, Week 4: 87.4 ± 1.2, Week 12: 83.4 ± 1.2*, Week 16: 85.2 ± 2.5*Significant difference from placebo (P < 0.05)[Mean ± SE] |
| Valderas-Martinez et al., 2016 (22); Valencia, Spain | III-1/ɸ | Open, prospective, randomized, cross-over, controlled-feeding trial | 14 wk (3-d wash-out, 1-d intervention, 1-mo wash- out, repeat for each intervention) | n = 4019 Males; No CVD, no medication, nonsmoker; Age: 28 ± 11 y; BMI: 23.30 ± 3.86 kg/m2 [mean ± SD] | Raw Tomato (RT): 7.0 g of raw tomato/kg of body weight (BW)Tomato Sauce (TS): 3.5g of tomato sauce/kg of BW Tomato Sauce with Olive Oil (TSOO): 3.5 g of tomato sauce with refined olive oil/kg of BWControl: 0.25 g of sugar dissolved in water/kg of BW | SBP (mmHg):Before InterventionRT: 120 ± 13, TS: 117 ± 13, TSOO: 120 ± 12, Control: 118 ± 9P-value: 0.135After intervention (6 h post)RT: 115 ± 11, TS: 115 ± 10, TSOO: 115 ± 12, Control: 115 ± 9 DBP (mmHg):Before interventionRT: 72.0 ± 9.5, TS: 73.0 ± 7.8, TSOO: 73.0 ± 8.1, Control: 71.0 ± 6.8P-value: 0.689After interventionRT: 70.0 ± 9.3, TS: 72.0 ± 9.6, TSOO: 70.0 ± 9.0, Control: 69.0 ± 8.0 significant between time in the interventionP-value of the ANOVA for repeated measures from the differences between interventions[Mean ± SD] |
| Abete et al., 2013 (23); Pamplona, Spain | III-1/+ | Randomized, double-blind cross-over study | 10 wk (4-wk intervention, 2-wk wash-out, 4-wk cross-over intervention) | n = 32; Healthy subjects; 18 males; Age: 18–50 y; BMI: 18.5–29.9 kg/m2 | 160 g/d of either high-lycopene (27.2 mg lycopene/d) or low lycopene/commercial tomato sauce (12.3 mg lycopene/d) | SBP (mmHg):High-lycopene:Baseline: 107.4 ± 9.9; Endpoint:108.9 ± 10.1Commercial sauce:Baseline: 107.9 ± 12.5; Endpoint: 111.3 ± 12.1P-value: 0.429DBP (mmHg):High-lycopene:Baseline: 68.9 ± 6.8; Endpoint: 70.6 ± 7.8Commercial sauce:Baseline: 71.0 ± 9.4; Endpoint: 72.4 ± 9.3P-value: 0.826[Mean ± SD] |
| Kim et al., 2011 (24); Seoul, Republic of Korea | II/+ | Randomized, double-blind, placebo-controlled intervention trial | 8 wk | n = 126; Healthy men; Age: 22–57 y; Frequently smoke cigarettes or consume alcohol; <3 servings vegetables and fruit per week; No history of chronic disease | Low dose: 6 mg lycopene/d via capsuleHigh dose: 15 mg lycopene/d via capsulePlacebo capsule | SBP (mmHg):PlaceboPre: 125 ± 1.96, Post: 124.4 ± 1.81Low dosePre: 123.5 ± 1.59, Post: 122.4 ± 1.69 |
| High dose Pre: 126.0 ± 2.16, Post: 122.8 ± 1.78*[Mean ± SE]*P < 0.05 compared with baseline values in each group tested by paired t test | ||||||
| Arranz et al., 2015 (25); Barcelona, Spain | III-1/- | Open, controlled, randomized, cross-over feeding trial | 6 d (3-d wash-out, 1-d intervention, 1-d wash-out, 1-d intervention) | n = 11; 6 Males; Age: 28 ± 3 y; BMI: 23 ± 2 kg/m2; Nonsmokers; No history of heart disease [mean ± SD] | Intervention 1: 750 g tomato juice (TJ) with 10% refined olive oilIntervention 2: 750 g TJ without refined olive oilSingle ingestion for both | SBP (mmHg):TJ with oilBaseline: 120.9 ± 17.5; 6 h: 116.0 ± 9.5Change (%): −1.9 ± 6.3TJ without oilBaseline: 117.5 ± 15.9; 6 h: 117.2 ± 15.3Change (%): −1.0 ± 10.3DBP (mmHg):TJ with oilBaseline: 69.3 ± 7.7; 6 h: 67.0 ± 7.1Change (%): −3.8 ± 12.4TJ without oilBaseline: 70.2 ± 10.5; 6 h: 69.5 ± 11.1Change (%): −0.4 ± 7.8[Mean ± SD] |
| García-Alonso et al., 2012 (26); Murcia, Spain | II/ɸ | Randomized single-blind intervention trial | 2 wk | n = 22; Healthy women; Nonsmokers; no medication; Age: 35–55 y; BMI: 21–30 kg/m2 | Reference group: 500 mL tomato juice/d, ∼50 mg lycopene/dTest group: 500 mL tomato juice enriched with n–3 PUFAs/d | SBP (mmHg):Reference Juice:Day 0: 97.14 ± 3.06; Day 15: 105.86 ± 3.39Test juice:Day 0: 105.18 ± 2.63; Day 15: 106.36 ± 2.79DBP (mmHg):Reference juice:Day 0: 63.57 ± 2.37; Day 15: 65.71 ± 3.85Test juice:Day 0: 65.45 ± 2.82; Day 15: 64.09 ± 3.36[Mean ± SEM] |
| Blood lipids | ||||||
| Thies et al., 2012 (18); Aberdeen, United Kingdom | II/+ | Single-blinded randomized controlled trial | 16 wk (4-wk wash-out, 12-wk INT) | n = 225; 93 men, 132 women; Sedentary or moderately active with signs of MS or moderate HPC; SBP <160 mmHg, DBP <99 mmHg; Age: 51.1 ± 0.8 y (40-65 y); BMI: 26.6 ± 0.5 kg/m2 (18.5-35kg/m2); WC: 88.4 ± 1.3 cm; SBP: 130.0 ± 1.9 mmHg; DBP: 79.1 ± 1.3 mmHg [mean ± SEM] | Control diet low in tomato-based foodsLycopene supplement group: 1 capsule/d containing 10 mg lycopene (70 mg lycopene/wk)High lycopene group: Diet high in tomato-based foods (70 mg lycopene/wk) | Cholesterol (mg/dL):Baseline: L 215.39 ± 5.03, control 215.01 ± 4.25P2-value: 0.981, PI: L 212.69 ± 5.03; control 217.71 ± 6.57HDL-C (mg/dL):Baseline: L 64.68 ± 1.93, control 64.97 ± 1.95, P2 0.679, PI: L 64.97 ± 2.32; control 65.74 ± 1.55LDL-C (mg/dL):Baseline: L 131.09 ± 4.25, control 131.48 ± 3.87, P 2 0.782, PI: L 128.38 ± 4.25; control 132.64 ± 3.87 |
| P 2 = Differences between groups at baseline[Mean ± SEM] | ||||||
| Gajendragadkar et al., 2014 (27); United Kingdom | III-1/+ | Prospective, randomized, double-blinded, placebo-controlled, parallel-group study | 8 wk | n = 72CVD group: n = 36; Stable CVD with statin therapy; 33 MalesLycopene group: Age: 67 ± 6 y; BMI: 28.6 ± 3.3 kg/m2Placebo group: Age: 68 ± 5 y; BMI: 28.4 ± 4.0 kg/m2HV group: n = 36Lycopene group: Age: 61 ± 13 y; BMI: 25.2 ± 2.8 kg/m2Placebo group: Age: 68 ± 5 y; BMI: 26.7 ± 3.6 kg/m225 Males [mean ± SD] | Dose: 7 mg of lycopene as capsule, once per day (Ateronon) (L)Placebo capsule (P) | HDL-C (mg/dL):Day 1:Placebo (P): 57.23 (5.41), Lycopene (L): 46.40 (1.93)Day 56:P: 56.84 (6.19), L: 45.24 (2.32)P-value: 0.7LDL-C (mg/dL):Day 1: P: 93.19 (5.80), L: 93.19 (5.41)Day 56: P: 83.53 (5.41), L: 93.19 (4.64)P-value: 0.1P-value: for overall comparison in delta (day 56–day 1) values across placebo and lycopene treated groupsResults only reported for CVD group[Mean (SE)] |
| Engelhard et al., 2006 (21); Beer Sheva, Israel | III-1/ɸ | Single-blinded, placebo-controlled trial | 16 wk (4-wk placebo; 8-wk intervention; 4-week placebo) | n = 31; Grade-1 HTN but otherwise healthy; Nonsmoker; Age: 30–70 y; BMI: 29.4 ± 0.8 kg/m2 [mean ± SE] | Dose: 1 capsule of Lyc-O-Mato per day (15 mg lycopene) | TGs (mg/dL):Baseline: 201.8 ± 15.8, Week 4: 177.7 ± 17.3, Week 12: 182.5 ± 18.0Total cholesterol (mg/dL):Baseline: 213.0 ± 6.4, Week 4: 199.2 ± 6.5, Week 12: 207.4 ± 6.5HDL-C (mg/dL):Baseline: 43.7 ± 1.6, Week 4: 41.3 ± 1.80, Week 12: 43.6 ± 1.8LDL-C (mg%):Baseline: 126.2 ± 6.0, Week 4: 121.9 ± 6.2, Week 12: 128.0 ± 6.0[Mean ± SE] |
| Abete et al., 2013 (28); Pamplona, Spain | III-1/+ | Randomized, double-blind cross-over study | 10 wk (4-wk intervention, 2-wk wash-out, 4-wk cross-over intervention) | n = 32; Healthy subjects; 18 males; Age: 18–50 y; BMI: 18.5–29.9 kg/m2 | 160 g/d of either high-lycopene (27.2 mg lycopene/d) or low lycopene/commercial tomato sauce (12.3 mg lycopene/d) | LDL-C (mg/dL)High-lycopene:Baseline: 118.6 ± 35.1; Endpoint: 114.4 ± 35.8Commercial sauce:Baseline: 118.1 ± 36.2; Endpoint: 115.6 ± 35.5P value: 0.736HDL-C (mg/dL)High-lycopene:Baseline: 60.5 ± 13.7; Endpoint: 65.0 ± 14.1 |
| Commercial sauce:Baseline: 64.7 ± 14.1; Endpoint: 64.2 ± 15.2P-value: 0.486TGs (mg/dL)High-lycopene:Baseline: 79.6 ± 33.1Endpoint: 84.3 ± 37.9Commercial sauce:Baseline: 82.2 ± 32.9Endpoint: 89.0 ± 41.4P- value: 0.839[Mean ± SD] | ||||||
| Valderas-Martinez et al., 2016 (14); Valencia, Spain | III-1/ɸ | Open, prospective, randomized, cross-over, controlled-feeding trial | 14 wk (3-d wash-out, 1-d intervention, 1-mo wash-out, repeat for each intervention) | n = 40; 19 Males; No CVD, no medication, nonsmoker; Age: 28 ± 11 y; BMI: 23.30 ± 3.86 kg/m2 [mean ± SD] | Raw Tomato (RT): 7.0 g of raw tomato/kg of body weight (BW)Tomato Sauce (TS): 3.5 g of tomato sauce/kg of BWTomato Sauce with Olive Oil (TSOO): 3.5 g of tomato sauce with refined olive oil/kg of BWControl: 0.25 g of sugar dissolved in water/kg of BW | Total-C (mg/dL):Before intervention: RT: 167 ± 28, TS: 170 ± 29, TSOO: 167 ± 23, Control: 168 ± 16P 3 value: 0.005After intervention (6 h post): RT: 160 ± 28, TS: 162 ± 26, TSOO: 158 ± 22, Control: 167 ± 13HDL-C (mg/dL):Before intervention: RT: 52.0 ± 11.2, TS: 52.0 ± 11.8, TSOO: 52.0 ± 11.3 P 3 value: 0.404Control: 50.0 ± 12.7After intervention: RT: 53.0 ± 11.1, TS: 53.0 ± 12.4, TSOO: 55.0 ± 13.3*, Control: 50.0 ± 11.9LDL-C (mg/dL):Preintervention: RT: 95.0 ± 19.6, TS: 96.0 ± 22.6, TSOO: 95.0 ± 20.5, Control: 95.0 ± 13.9P 3 value: 0.184Postintervention: RT: 92.0 ± 19.0, TS: 92.0 ± 18.2*, TSOO: 93.0 ± 18.9, Control: 95.0 ± 11.8TGs (mg/dL):Preintervention: RT: 84.0 ± 54.7, TS: 86.0 ± 42.0, TSOO: 83.0 ± 36.5, Control: 86.0 ± 23.8P 3 value: 0.002Postintervention: RT: 62.0 ± 34.8, TS: 57.0 ± 22.7, TSOO: 68.0 ± 31.7, Control: 83.0 ± 31.6 |
| *Significant differences significant between time in the interventionP 3 = value of the ANOVA for repeated measures from the differences between interventions[Mean ± SD] | ||||||
| Arranz et al., 2015 (29); Barcelona, Spain | III-1/- | Open, controlled, randomized, cross-over feeding trial | 6 d (3-d wash-out, 1-d intervention, 1-d wash-out, 1-d intervention) | n = 11; 6 Males; Age: 28 ± 3 y; BMI: 23 ± 2 kg/m2; Nonsmokers; No history of heart disease [mean ± SD] | Intervention 1: 750 g tomato juice (TJ) with refined olive oilIntervention 2: 750 g TJ without refined olive oilSingle ingestion | TGs (mg/dL):TJ with oilBaseline: 81.3 ± 17.9; 6 h: 83.7 ± 37.2Change (%): 3.7 ± 43.9TJ without oilBaseline: 87.5 ± 28.7; 6 h: 69.7 ± 18.6*Change (%): −21.3 ± 18.4Total cholesterol (mg/dL)TJ with oilBaseline: 160 ± 27.6; 6 h: 150 ± 22.4*Change (%): −6.2 ± 5.1TJ without oilBaseline: 160 ± 24.8; 6 h: 153 ± 23.2*Change (%): −5.8 ± 4.4LDL-C (mg/dL):TJ with oilBaseline: 102 ± 17.9; 6 h: 96.4 ± 16.3*Change (%): −5.5 ± 7.2TJ without oilBaseline: 100 ± 16.0; 6 h: 99.3 ± 16.3Change (%): −1.0 ± 7.4HDL-C (mg/dL)TJ with oilBaseline: 41.6 ± 10.9; 6 h: 38.9 ± 10.9*Change (%): −2.7 ± 2.8TJ without oilBaseline: 42.5 ± 11.1; 6 h: 40.5 ± 11.1*Change (%): −2.0 ± 2.9*Statistically different from baseline[Mean ± SD] |
| García-Alonso et al., 2012 (26); Murcia, Spain | II/ɸ | Randomized single-blind intervention trial | 2 wk | n = 22 Healthy women; Nonsmokers; no medications; Age: 35–55 y; BMI: 21–30 kg/m2 | Reference group: 500 mL tomato juice/d, ∼50 mg lycopene/dTest group: 500 mL tomato juice enriched with n–3 PUFAs/d | Total cholesterol (mg/dL):Reference juice:Day 0: 201.86 ± 12.45Day 15: 202.29 ± 8.98Test juice:Day 0: 192.64 ± 10.10Day 15: 202.27 ± 10.28HDL-C (mg/dL):Reference juice:Day 0:76.00 ± 5.36Day 15: 79.43 ± 5.51TEST JUICEDay 0: 68.91 ± 4.61Day 15: 73.73 ± 4.99LDL-C (mg/dL):Reference juice:Day 0: 115.21 ± 11.39Day 15:112.29 ± 10.36Test juice:Day 0:110.55 ± 8.60Day 15: 115.36 ± 5.45TGs (mg/dL):Reference juice:Day 0: 53.00 ± 4.68Day 15: 52.86 ± 3.88Test juice:Day 0: 65.91 ± 8.51Day 15: 65.45 ± 7.93Day 15: 0.27 ± 0.001*[Mean ± SEM]*Significantly different from day 0 within treatment group |
| McEneny et al., 2013 (30); Aberdeen, United Kingdom | II/+ | Single-blinded, randomized controlled trial | 16 wk (4-wk wash-out, 12-wk intervention) | n = 234; Signs of MS or moderate HPC; No CVD; No medication; Age: 40–65 y; BMI: 18.5–35 kg/m2 | Lycopene-rich diet group: 224–350 mg lycopene/wk from a diet high in tomato-based foodsLycopene supplement group: 70 mg lycopene/wkControl: <10 mg lycopene/wk | HDL2:Baseline: control: 0.26 (0.03), supplement: 0.70 (0.18)P-value: 0.023Postintervention:control: 0.20 (0.04), supplement: 1.54 (0.27)P < 0.001HDL3:Baseline: control: 0.03 (0.01), supplement: 0.05 (0.01)P-value: 0.092Postintervention: control: 0.04 (0.01), 0.13 (0.02)P < 0.001[Mean (SEM)] |
| Jacob et al., 2008 (31); Jena, Germany | II/- | Randomized intervention study | 4 wk (2-wk wash-out, 2-wk intervention) | n = 24 Healthy volunteers; Nonsmokers; Age: 23 ± 2 y; BMI: 21.5 ± 2.8 kg/m2 [mean ± SD] | Group L: 250 mL tomato juice twice daily (20.9 mg lycopene/d)Group LC: Same tomato juice enriched with vitamin C | Cholesterol (mg/dL):Lycopene group (L)T-2: 157.1 ± 27.6; T0: 157.6 ± 40.8; T±2: 153.2 ± 30.8P-value: 0.008*Lycopene/Vitamin C Group (LC)T-2: 156.6 ± 28.3; T0: 153.4 ± 29.3; T±2: 147.4 ± 31.9P -value: 0.002*P- values for statistical difference between the groups in T+2 (ANOVA)T-2 = Baseline, T0 = Post-wash-out, T+2 = Postintervention[Mean ± SD] |
| Silaste et al., 2007 (32); Oulu, Finland | III-1/- | Randomized cross-over controlled trial | 8 wk (2-wk baseline period, 3-wk low tomato diet, 3-wk high lycopene diet) | n = 21; 5 Males; Healthy; nonsmoker; Age: 20–49 y (mean: 30 y); BMI: 23.5 ± 2.3 kg/m2; 7 women used oral contraceptives [mean ± SD] | High tomato: 400 mL tomato juice (5.9 mg lycopene) AND 30g tomato ketchup (12.4 mg lycopene)Low tomato: No tomato products allowed | Total cholesterol (mmol/L):Baseline: 4.43 (0.64)P-value: 0.005Low-Tomato: 4.50 (0.63)P-value: 0.002High-Tomato: 4.19 (0.78)LDL-C (mmol/L):Baseline: 2.44 (0.51)P-value: 0.002Low-Tomato: 2.56 (0.56)P-value: 0.0002High-Tomato: 2.18 (0.62)P-value not specified[Mean (SD)] |
| Misra et al., 2006 (33); New Delhi, India | II/ɸ | Randomized controlled trial | 6 mo | n = 41; Postmenopausal women aged <60 y, cessation of menses >1 y ago, or >6 mo; FSH level >40 u/L; NonsmokerHRT group: n = 21; Age: 46.2 y; BMI: 25.3 kg/m2LycoRed group: n = 22; Age: 46.4 y; BMI: 25.8 kg/m2No statistically significant differences between groups | Group 1: Oral HRT dailyGroup 2: 2 x LycoRed softules (containing 2000 μg lycopene each) daily | Total cholesterol (mg/dL):Baseline: 215.1 ± 31.3; 3 Mo: 189.2 ± 27.2; 6 Mo: 161.5 ± 34.3P-value: 0.0001HDL-C (mg/dL):Baseline: 47.6 ± 1.9; 3 Mo: 53.7 ± 7.8; 6 Mo: 59.1 ± 7.4P-value: 0.001LDL-C (mg/dL)Baseline: 132.0 ± 32.8; 3 Mo: 116.7 ± 24.7; 6 Mo: 107.8 ± 19.6P-value: 0.001VLDL-C (mg/dL)Baseline: 25.6 ± 1.1; 3 Mo: 26 ± 3.5; 6 Mo: 25.7 ± 4.2P-value: 0.92TG (mg/dL)Baseline: 123.0 ± 28.3; 3 Mo: 127.8 ± 21.6; 6 Mo: 134.3 ± 16.9P-value: 0.001Significant difference in mean levels between the 2 groups at 0 and 6 moResults reported are for LycoRed group.[Mean (SD)] |
| Collins et al., 2004 (14); Beltsville, MD, USA | III-2/- | Diet-controlled, repeated-measures, cross-over controlled trial | 19 wk (2-wk washout, 3-wk intervention, 4-wk wash-out, repeated for each intervention) | n = 10; 5 Males; Healthy, nonsmokers; Mean age: 50 y; Mean BMI: 27.7 kg/m2 | Watermelon juice: 20.1 mg lycopene/d from watermelon juiceTomato juice: 18.4 mg lycopene/d from tomato juiceControl: no added lycopene | TGs (mg/dL):Depletion: 185.9 ± 16.9Postintervention (PI): Control: 181.7 ± 16.9; Watermelon: 198.9 ± 18.3; Tomato: 174.7 ± 15.6HDL-C (mg/dL):Depletion: 56.9 ± 5.15PI: Control: 58.65 ± 4.31; Watermelon: 58.00 ± 5.96; Tomato: 59.38 ± 4.55Total cholesterol (mg/dL):Depletion: 220.9 ± 9.5PI: Control: 223.4 ± 7.9; Watermelon: 224.6 ± 8.2; Tomato: 233.6 ± 6.2Results reported on group 1 [Mean ± SE] |
| Cuevas-Ramos et al., 2013 (3); Tlalpan, Mexico | II/+ | Randomized, single-blinded, controlled clinical trial | 6 wk (2-wk run-in, 4-wk intervention) | n = 52; 11 Males; Age: 18–65 y; Low HDL-C, normal TGTomato group: n = 26; Age: 43.4 ± 15.5 y; BMI: 27.1 ± 5.0 kg/m2Control group: n = 26 (24?); Age: 40.3 ± 16.0 y; BMI: 27.1 ± 4.0 kg/m2 [mean ± SD] | Dose: 300 g Roma tomatoes (∼2)/dControl: 300 g raw cucumber/d | TGs (mg/dL):Tomato, Baseline: 113.4 ± 46.4; Final: 122.7 ± 21.8P-value: 0.18Control, Baseline: 107.5 ± 36.3; Final: 106.9 ± 41.5P-value: 0.89Cholesterol (mg/dL):Tomato, Baseline: 165.9 ± 44.7; Final: 169.7 ± 39.7P-value: 0.62Control, Baseline: 162.5 ± 31.0; Final: 159.9 ± 33.2P-value: 0.41HDL-C (mg/dL):Tomato, Baseline: 36.5 ± 7.5; Final: 41.6 ± 6.9 *P-value: <0.0001Control, Baseline: 36.8 ±7.2; Final: 35.8 ± 7.3P value: 0.08LDL-C (mg/dL):Tomato, Baseline: 108.1 ± 38.1; Final: 104.5 ± 31.0P-value: 0.63Control, Baseline: 103.2 ± 28.0; Final: 104.3 ± 30.0P value: 0.71*P < 0.05 considered significant[Mean ± SD] |
| Tsitsimpikou et al., 2014 (34); Crete, Greece | III-2/+ | Parallel, controlled trial (randomization unclear) | 8 wk | n = 27; Patients with MS; High TG, Low HDL-C, High BP; High fasting blood glucose or on medicationTomato group: n = 15; 13 Males; Age: 53.5 ± 9.8 yControl group: n = 12; 11 Males; Age: 56.6 ± 10.2 y [mean ± SD] | Regular diet supplemented with tomato juice 4 times/wkDose: TJ with 2.51 mg lycopene per 100 mL | TC baseline (mg/dL): 212 ± 24.9; TCsupplement (mg/dL): 208 ± 33.3P-value: 0.889TG baseline (mg/dL): 284 ± 68.3; TG supplement (mg/dL): 287 ± 77.1P-value: 0.695HDL-C baseline (mg/dL): 41.0 ± 7.81; HDL-C supplement (mg/dL): 44.4 ± 6.72P-value: 0.049*LDL-C baseline (mg/dL): 144 ± 38.9; LDL-C supplement (mg/dL): 123 ± 30.5P-value: <0.001*[Mean ± SD] |
| Devaraj et al., 2008 (13); Sacramento, CA, USA | II/+ | Double-blind, randomized, placebo-controlled trial | 10 wk (2-wk wash-out, 8-wk intervention) | n = 82; Healthy subjects; 19 Males; Mildly elevated cholesterol; nonsmokers, <30 mL alcohol/d; >40 y of age | 6.5 mg, 15 mg, or 30 mg/d via lycopene capsulesPlacebo capsule | Serum total cholesterol (mg/dL):Placebo: Visit A: 208.2 ± 71.9; Visit B: 205.7 ± 68.3; Visit C: 204.7 ± 30.2Lycopene 6.5 mg: Visit A: 203.8 ± 25.6; Visit B: 205.1 ± 27.1; Visit C: 203.3 ± 27.2Lycopene 15 mg: Visit A: 197.6 ± 20.2; Visit B: 207.3 ± 22.4; Visit C: 210.4 ± 19.9Lycopene 30 mg: Visit A: 194.6 ± 20.4; Visit B: 199.2 ± 22.5; Visit C: 201.5 ± 23.0LDL-C (mg/dL):Placebo: Visit A: 124.8 ± 30.9; Visit B: 124.7 ± 24.6; Visit C: 123.9 ± 27.0Lycopene 6.5 mg: Visit A: 127.9 ± 22.8; Visit B: 129.4 ± 19.4; Visit C: 125.1 ± 21.3Lycopene 15 mg: Visit A: 120.0 ± 21.6; Visit B: 131.4 ± 19.3; Visit C: 125.4 ± 21.4Lycopene 30 mg: Visit A: 120.9 ± 19.5; Visit B: 121.4 ± 24.7; Visit C: 123.4 ± 23.0HDL-C (mg/dL):Placebo: Visit A: 56.8 ± 9.2; Visit B: 56.7 ± 9.2; Visit C: 56.9 ± 10.2Lycopene 6.5 mg: Visit A: 54.4 ± 13.9; Visit B: 55.5 ± 11.2; Visit C: 56.2 ± 10.9Lycopene 15 mg: Visit A: 64.8 ± 1.3; Visit B: 62.6 ± 13.2; Visit C: 66.7 ± 15.7Lycopene 30 mg:Visit A: 56.5 ± 19.8; Visit B: 56.5 ± 17.8; Visit C: 55.8 ± 16.8Serum triacylglycerol (mg/dL)Placebo: Visit A: 94.8 ± 27.4; Visit B: 99.6 ± 40.8; Visit C: 103.5 ± 44.5Lycopene 6.5 mg: Visit A: 91.1 ± 36.6; Visit B: 87.7 ± 36.9; Visit C: 95.9 ± 36.3Lycopene 15 mg: Visit A: 88.1 ± 24.3; Visit B: 87.2 ± 23.8; Visit C: 90.1 ± 39.4Lycopene 30 mg: Visit A: 107.5 ± 45.9; Visit B: 100.7 ± 45.8; Visit C: 100.9 ± 45.6No significant effects of time or treatment[Mean ± SD] |
| Massa et al., 2016 (23); Paraíba, Brazil | II/+ | Randomized, double-blind, placebo-controlled trial | 6 wk | n = 43; Dyslipidemic; Age: 47.22 ± 6.5 y, between 20–60 y; No lipid-lowering medicationExperimental Group: n = 22; Age: 47.1 ± 7.5 y; 11 MalesControl Group: n = 21; Age: 47.33 ± 5.5 y; 11 Males [mean ± SD] | Dose: 6 g of watermelon extract (1.44 mg/lycopene), once per day | Sufficient outcome data not reportedSignificant reduction in TC and LDL-CTGs: –38.05 mg/dL |
| Burton-Freeman et al., 2012 (35); Bedford Park, IL, USA | II/+ | Single-center, randomized, cross-over, 2-arm, 2-sequence, placebo-controlled, 360-min postprandial trial | 360 min | n = 25; Healthy; nonsmokers; hs-CRP <1.0 mL/L; 13 Males; Age: 27 ± 8 y; BMI: 22, >19 and <24 kg/m2 [mean ± SD] | Tomato-containing mealDose: ∼85 g tomato paste | TGs (mg/dL): Tomato: 122.6 ± 11.3; Control: 111.7 ± 11.3P -value: 0.14[LSM ± SEM] |
| Samaras et al., 2014 (25); Larissa, Greece | III-2/+ | Parallel controlled trial (randomization unclear) | 8 wk | n = 27; Ultra-marathon runners; nonsmokers; no medical history of HT; Tomato juice: 13 Males, 2 Females; Age: 44.9 ± 8.53 y; BMI: 24.1 ± 2.46 kg/m2; Control: 12 Males; Age: 46.6 ± 15.3 y; BMI: 24.3 ± 1.56 kg/m2 [mean ± SD] | Tomato juice dailyDose: Amount required to match subjects with usual carbohydrate supplementation (unspecified amount) | TCbaseline (mg/dL): 207 ± 55.2; TCSupplement (mg/dL): 181 ± 23.1P-value: 0.048*TGBaseline(mg/dL): 84.9 ± 41.3; TGSupplement(mg/dL): 87.1 ± 29.4P-value: 0.823HDL-CBasline(mg/dL): 80.7 ± 18.70; HDL-CSupplement(mg/dL): 80.5 ± 9.81P-value: 0.954LDL-CBaseline(mg/dL): 110.0 ± 41.20; LDL-CBaseline(mg/dL): 82.8 ± 22.0P-value: 0.011*[Mean ± SD] All values reported on tomato supplementation group |
| Blum et al., 2006 (36); Haifa, Israel | III-2/- | Nested case-controlled study (no randomization) | 4 wk | n = 98; Healthy >18 y; Age: 45.5 ± 14.1 y; Tomato group: 16 Males; Control group: 16 Males | Regular diet supplemented with tomatoDose: 300 g tomato products (including TS, TJ, FT, tomato soup) per day | Total cholesterol (mg/dL):Before: 207.5 ± 44.3; Postintervention: 204.1 ± 45.1P-value: 0.68TGs (mg/dL):Before: 170.8 ± 85.4; Postintervention: 167.4 ± 99.4P-value: 0.98HDL-C (mg/dL):Before: 46.1 ± 10.6; Postintervention: 53.4 ± 13.3P-value: 0.03*LDL-C (mg/dL):Before: 127.7 ± 41.8; Postintervention: 119.1 ± 41.7P-value: 0.57VLDL-C (mg/dL):Before: 34.2 ± 17.1; Postintervention: 32.9 ± 19.4P-value: 0.78[Mean ± SD]*Results reported on intervention group |
| Oxidative stress | ||||||
| Abete et al., 2013 (28); Pamplona, Spain | III-1/+ | Randomized, double-blind cross-over study | 10 wk (4-wk intervention, 2-wk wash-out, 4-wk cross-over intervention) | n = 32; Healthy subjects; 18 males; Age: 18–50 y; BMI: 18.5-29.9 kg/m2 | 160 g/d of either high-lycopene (27.2 mg lycopene/d) or low lycopene/commercial tomato sauce (12.3 mg lycopene/d) | TAC (mM Trolox):High-lycopene: Baseline:1.3 ± 0.8, Endpoint: 1.5 ± 0.8Commercial sauce: Baseline:1.4 ± 0.6, Endpoint: 1.3 ± 0.6P-value:0.058Oxidized LDL-C (U/L):High-lycopene: Baseline:45.7 ± 18.7, Endpoint:40.4 ± 15.4Commercial sauce: Baseline: 43.9 ± 18.0, Endpoint: 44.2 ± 17.4P-value: 0.080GPx activity (nmol/min/mL):High-lycopene: Baseline: 158.5 ± 90.5, Endpoint: 156.8 ± 85.1Commercial sauce: Baseline: 152.1 ± 69.5, Endpoint: 150.9 ± 68.0P-value: 0.615MDA (equivalents):High-lycopene: Baseline: 0.6 ± 0.3, Endpoint: 0.6 ± 0.3Commercial sauce: Baseline: 0.6 ± 0.2, Endpoint: 0.5 ± 0.3P-value: 0.899[Mean ± SD] |
| Silaste et al., 2007 (37); Oulu, Finland | III-1/- | Randomized cross-over controlled trial | 8 wk (2-wk baseline period, 3-wk low tomato diet, 3-wk high lycopene diet) | n = 21; 5 Males; Healthy; nonsmoker; Age: 20–49 y (mean: 30 y); BMI: 23.5 ± 2.3 kg/m2; 7 women used oral contraceptives [mean ± SD] | High tomato: 400 mL tomato juice (5.9 mg lycopene) AND 30 g tomato ketchup (12.4mg lycopene) dailyLow tomato: No tomato products allowed | LDL-C oxidation– EO6 binding (RLU/ms)Baseline: 107978 ± 38478; Low-Tomato: Value not reported; High-Tomato: 93220 ± 40732P -value: 0.02[Mean ± SD] |
| Kim et al., 2011 (38); Seoul, Republic of Korea | II/+ | Randomized, double-blind, placebo-controlled intervention trial | 8 wk | n = 126; Healthy men; Age: 22–57 y; Frequently smoke cigarettes or consume alcohol; <3 servings vegetables and fruit per week; No history of chronic disease | Low dose: 6 mg lycopene/d via capsuleHigh dose: 15 mg lycopene/d via capsulePlacebo | Tail DNA (%):Placebo: Pretreatment: 10.7 ± 0.33; Post-treatment: 9.87 ± 0.37Low-dose: Pretreatment: 10.8 ± 0.55; Post-treatment: 9.39 ± 0.38*High-dose: Pretreatment: 11.2 ± 0.52; Post-treatment: 9.30 ± 0.36LDL-C particle size (nm):Placebo: Pretreatment: 23.76 ± 0.10; Post-treatment: 23.79 ± 0.10Low-dose: Pretreatment: 23.63 ± 0.10; Post-treatment: 23.74 ± 0.10High-dose: Pretreatment: 23.80 ± 0.10; Post-treatment: 23.93 ± 0.09 |
| *[Mean ± SE]*P < 0.05 compared with baseline values in each group tested by paired t test = P < 0.001 compared with baseline values in each group tested by paired t test | ||||||
| Stangl et al., 2011 (39); Berlin, Germany | III-1/ɸ | Randomized cross-over, controlled trial | 4 wk (1-wk intervention, 2-wk wash-out, 1-wk cross-over intervention) | n = 19; Healthy postmenopausal women, nonsmoking, no CVD risk factors; Age: 58.9 ± 6.3 y; BMI: 25.0 ± 3.3 kg/m2; SBP: 115 ± 10 mmHg; DBP: 70 ± 6 mmHg [mean ± SD] | Intervention: Buttered roll with tomato puree (70 g, 46.2 mg lycopene) consumed daily for 7 dControl: Buttered roll without tomato puree consumed daily for 7 d | FMD (%):Baseline: 7.8 ± 3.3; 24h: 8.2 ± 2.8; 7d: 8.3 ± 2.7Prior to control: 7.7 ± 3.2; 24h: 8.6 ± 3.5; 7d: 8.2 ± 2.7[Mean ± SEM] |
| Jacob et al., 2008 (31); Jena, Germany | II- | Randomized intervention study | 4 wk (2-wk wash-out, 2-wk intervention) | n = 24 Healthy volunteers; Nonsmokers; Age: 23 ± 2 y; BMI: 21.5 ± 2.8 kg/m2 [mean ± SD] | Group L: 250 mL tomato juice twice daily (20.9 mg lycopene/d)Group LC: Same tomato juice enriched with vitamin C | TGs (mg/dL):L: T-2: 123.3 ± 74.9; T0: 105.7 ± 43.5; T±2: 153.2 ± 30.8; LC: T-2: 82.5 ± 36.3; T0: 91.1 ± 34.6; T±2:81.3 ± 33.5P-value: 0.072FRAP (mmol/L):L: T-2: 0.84 ± 0.18; T0: 0.82 ± 0.16; T±2: 0.85 ± 0.20; LC: T-2: 0.79 ± 0.27; T0: 0.83 ± 0.23; T±2: 0.82 ± 0.25P-value: 0.667TBARS (μmol MDA/L):L: T-2: 0.55 ± 0.10; T0: 0.54 ± 0.10; T±2: 0.53 ± 0.10; LC: T-2: 0.60 ± 0.14; T0: 0.56 ± 0.14; T±2: 0.50 ± 0.09P-value: 0.271TNF-ɑ (ng/L):L: T-2: 6.97 ± 4.69; T0: 6.01 ± 5.27; T±2: 3.45 ± 1.32; LC: T-2: 2.93 ± 1.49; T0: 3.35 ± 2.23; T±2:3.28 ± 0.97P-value: 0.609P-values for statistical difference between the groups in T+2 (ANOVA)[Mean ± SD] |
| García-Alonso et al., 2012 (26); Murcia, Spain | II/ɸ | Randomized single-blind intervention trial | 2 wk | n = 22; Healthy women; Nonsmokers; no medication; Age: 35–55 y; BMI: 21-30 kg/m2 | Reference group: 500 mL tomato juice/d, ∼50 mg lycopene/dTest group: 500 mL tomato juice enriched with n–3 PUFAs/d | TEAC (nM Trolox):Reference juice: Day 0: 2.17 ± 0.27; Day 15: 2.90 ± 0.23Test juice: Day 0: 2.50 ± 0.19; Day 15: 2.41 ± 0.25FRAP (mM Trolox):Reference juice: Day 0: 0.22 ± 0.02; Day 15: 0.24 ± 0.10Test juice: Day 0: 0.22 ± 0.01; Day 15: 0.27 ± 0.001*MDA (μM MDA):Reference juice: Day 0: 0.62 ± 0.06; Day 15: 0.54 ± 0.06Test juice: Day 0: 0.75 ± 0.11; Day 15: 0.61 ± 0.03*Significantly different from day 0 within treatment group [Mean ± SEM] |
| Burton-Freeman et al., 2012 (35); Bedford Park, IL, USA | II/+ | Single-center, randomized, cross-over, 2-arm, 2-sequence, placebo-controlled, 360-min postprandial trial | 360 min | n = 25 Healthy; nonsmokers; hs-CRP <1.0 mL/L; 13 Males; Age: 27 ± 8 y; BMI: 22, >19, and <24 kg/m2 [mean ± SD] | Tomato-containing mealDose: ∼85 g tomato paste | OxLDL-C (U/L):Tomato: 72.0 ± 4.3, Control: 77.8 ± 4.4P-value: 0.02IL-6 (pg/mL):Tomato: 2.2 ± 0.3, Control: 2.6 ± 0.3P-value: 0.19[LSM ± SEM] |
| Tsitsimpikou et al., 2014 (34); Crete, Greece | III-2/+ | Parallel, controlled trial (randomization unclear) | 8 wk | n = 27; Patients with MS; High TG, Low HDL-C, High BP; High fasting blood glucose or on medicationTomato group: n = 15, 13 Males; Age: 53.5 ± 9.8 yControl group: n = 12, 11 Males; Age: 56.6 ± 10.2 y [mean ± SD] | Regular diet supplemented with tomato juice 4 times/wk; unspecified amountDose: TJ with 2.51 mg lycopene per 100 mL | IL-6baseline (pg/mL): 38.1± 22.2; IL-6supplement (pg/mL): 35.19 ± 18.7P -value: 0.126TNF-ɑbaseline (pg/mL): 35.5 ± 21.9; TNF -ɑ supplement (pg/mL): 28.0 ± 15.6P -value: 0.021[Mean ± SD] |
| Devaraj et al., 2008 (13); Sacramento, CA, USA | II/+ | Double-blind, randomized, placebo-controlled trial | 10 wk (2-wk wash-out, 8-wk intervention) | n = 82 Healthy subjects; 19 Males; Mildly elevated cholesterol; nonsmokers, <30 mL alcohol/d; >40 y of age | 6.5 mg, 15 mg, or 30,mg/d via lycopene capsulesPlacebo capsule | LDL-C oxidation rate (nmol/min):Placebo: Visit A: 3.83 ± 1.8; Visit B: 4.03 ± 1.7; Visit C: 3.68 ± 1.6Lycopene 6.5 mg: Visit A: 4.59 ± 2.04; Visit B: 4.35 ± 1.81; Visit C: 4.17 ± 1.76Lycopene 15 mg: Visit A: 4.42 ± 2.15; Visit B: 4.67 ± 1.79; Visit C: 4.04 ± 1.89Lycopene 30 mg: Visit A: 3.74 ± 1.64; Visit B: 4.05 ± 1.66; Visit C: 3.80 ± 1.70LDL-C lag time (min):Placebo: Visit A: 67.8 ± 11.3; Visit B: 67.8 ± 11.6; Visit C: 69.0 ± 15.6Lycopene 6.5 mg: Visit A: 64.0 ± 9.80; Visit B: 66.1 ± 12.7; Visit C: 67.6 ± 13.6Lycopene 15 mg: Visit A: 65.6 ± 9.40; Visit B: 65.4 ± 9.9; Visit C: 63.7 ± 12.8Lycopene 30 mg: Visit A: 70.6 ± 9.1; Visit B: 72.5 ± 9.9; Visit C: 68.6 ± 18.0No significant effects of time or treatment[Mean ± SD] |
| Rao and Shen, 2002 (40); Toronto, Canada | III-1- | Randomized, cross-over design | 24 wk (2-wk wash-out, 2-wk treatment period, repeated for each of the 6 treatments) | n = 12; 6 Males; Nonsmokers; Age: 31 ± 2.7 y; Weight: 64.6 ± 3.7 kg; BMI: 22.6 ± 1.2 kg/m2 [mean ± SEM] | Tomato sauce: 5, 10, 20 mg lycopene as tomato ketchup dailyTomato supplement: 5, 10, 20 mg lycopene as Lyc-O-Mato capsule daily | Serum MDA (μM):The mean reduction of serum MDA was 10% for all interventionsSerum thiols (μM):Mean 23.6% decrease in thiols for all treatment groupsSufficient outcome data not reported |
| Misra et al., 2006 (33); New Delhi, India | II/ɸ | Randomized controlled trial | 6 mo | n = 41 Postmenopausal women; Aged <60 y, cessation of menses >1 y ago, or >6 mo; FSH level >40 u/L; NonsmokerHRT group: n = 21; Age: 46.2 y; BMI: 25.3 kg/m2LycoRed group: n = 22; Age: 46.4 y; BMI: 25.8 kg/m2No statistically significant differences between groups | Group 1: Oral HRT dailyGroup 2: 2 x LycoRed softules (containing 2000 μg lycopene each) daily | MDA (μmol/L):Baseline: 38.3 ± 6.3; 3 Mo: 34.7 ± 4.0; 6 Mo: 34.0 ± 3.7P-value: 0.001*GSH (μmol/L):Baseline: 7.5 ± 1.2; 3 Mo: 9.0 ± 1.3; 6 Mo: 9.8 ± 1.9P-value: 0.001*Significant difference in mean levels between the 2 groups at 0 and 6 moResults reported are for LycoRed group.[Mean (SD)] |
| Collins et al., 2004 (14); Beltsville, MD, USA | III-2/- | Diet-controlled, repeated measures, cross-over controlled trial | 19 wk (2-wk wash-out, 3-wk intervention, 4-wk wash-out, repeated for each intervention) | n = 10; 5 Males; Healthy, nonsmokers; Mean age: 50 y; Mean BMI: 27.7 kg/m2 | Watermelon juice: 20.1 mg lycopene/d from watermelon juiceTomato juice: 18.4 mg lycopene per day from tomato juiceControl: no added lycopene | MDA (μmol/L):Depletion: 1.21 ± 0.11Postintervention: Control: 1.12 ± 0.11; Watermelon: 1.15 ± 0.12; Tomato: 1.37 ± 0.11FRAP (μmol/L):Depletion: 831.6 ± 24.9Postintervention: Control: 871.7 ± 26.7; Watermelon: 900.9 ± 25.2; Tomato: 861.6 ± 23.4GPX (μmol/L):Depletion: 2728 ± 219Postintervention: Control: 2728 ± 222; Watermelon: 2263 ± 169; Tomato: 2574 ± 187 Results reported on group 1[Mean ± SE] |
| Ghavipour et al., 2015 (12); Tehran, Iran | II/ɸ | Randomized controlled clinical trial | 20 d | n = 64; Overweight or obese female students; Aged between 20 and 30 y; BMI >25 kg/m2; Nonsmoker; no inflammatory diseasesIntervention group: n = 32; Age: 25.2 ± 0.6 y; BMI: 29.4 ±0.23 kg/m2Control group: n = 32; Age: 25.4 ± 0.7 y; BMI: 29.1 ± 0.30 kg/m2 [mean ± SEM] | Intervention group: 330 mL (37.0 mg lycopene) tomato juice dailyControl: Water | TAC (mg/dL):Baseline: Intervention: 0.4 ± 0.4, Control: −0.2 ± 0.4P-value: 0.03SOD (U/gHb):Baseline: Intervention: 27.4 ± 10.2, Control: 1.9 ± 1.1P-value: 0.01*GPx (U/gHb):Baseline: Intervention: 28.4 ± 2.5, Control: 2.0 ± 0.0P-value: 0.01*CAT(U/gHb):Baseline: Intervention: 4.9 ± 0.0, Control: 0.92 ± 0.77P-value: 0.02*MDA (μmol/mL):Baseline: Intervention: -0.4 ± 0.8, Control: 0.3 ± 0.6P value: 0.01 ANCOVA, adjusted for age, BMI, and daily energy intake[Mean ± SEM] |
| Colmán Martínez et al., 2017 (41); Valencia, Spain | II/ɸ | Open, prospective, randomized, cross-over and | 18 wk (4-wk intervention, 3-wk wash-out) | n = 28; High cardiovascular risk; Mean age: 69.7 ± 3.1 y; Mean BMI: 31.5 ± 3.6 kg/m2 | Low dose: 200 mL Tomato juice dailyHigh dose: 400 mL Tomato | ICAM-1 (ng/mL):Control: 3624 ± 773; Postintervention: 156 ± 688 |
| controlled clinical trial | juice dailyControl: Water | P ≤ 0.001VCAM-1 (ng/mL):Control: 3945 ± 561; Postintervention: 213 ± 540P ≤ 0.001IL-8 (pg/mL):Control: 40 ± 90; Postintervention: 24 ± 79P-value: 0.135IFN-ɣ (pg/mL):Control: 450 ± 307; Postintervention: 399 ± 265P- value: 0.791[Mean ± SD] Results are for high-dose intervention | ||||
| Carroll et al., 2000 (42); Cork, Ireland | II+ | Randomized, placebo-controlled double-blind study | 12 wk | n = 51; Healthy elderly people over 65 y; nonsmoking; 22 males, 25 femalesLycopene group: n = 16; Age: 70 ± 5 y; BMI: 26.0 ± 3 kg/m2; Total-C: 5.7 ± 0.8 mmol/L; HDL-C: 1.2 ± 0.3 mmol/L; TGs: 1.2 ± 0.5 mmol/L [mean ± SD] | Lycopene: One Lyc-O-Pen capsule per day (13.3 mg lycopene/d)Carotene: One carotene capsule per day (8.2 mg B-carotene)Placebo capsule | Maximal oxidation rate (nmol/mg) LDL-C protein/min):Placebo: Baseline: 8.61 ± 2.94; 12 wk: 8.73 ± 3.03Lycopene: Baseline: 8.69 ± 2.51; 12 wk: 8.47 ± 1.56Lag Phase (min):Placebo: Baseline: 57 ± 21; 12 wk: 56 ± 11Lycopene: Baseline: 54 ± 20; 12 wk: 62 ± 22Maximum diene concentration (nmol/mg LDL-C protein):Placebo: Baseline: 705 ± 153; 12 wk: 701 ± 169Lycopene: Baseline: 696 ± 142; 12 wk: 679 ± 99[Mean ± SD] |
| Sarkar et al., 2012 (43); Indore, India | III-2/ɸ | Case-control intervention study | 60 d | n = 60; Aged 40–60 y, nonsmokersStudy subjects: n = 30; Oxidative stress; No history of chronic illnessControl subjects: n = 30; Healthy and without illness or oxidative stress | 180 g tomato (e.g., soups, paste, ketchup) containing 12 mg lycopene/d | MDA (nmol/mL):Baseline: 6.5 ± 0.7; Postintervention: 3.73 ± 0.7*SOD (units/mL)Baseline: 3.2 ± 0.7; Postintervention: 5.9 ± 0.7*GPX *(unit/gHb):Baseline: 48.3 ± 5.4; Postintervention: 73.4 ± 7.6*GSH (unit/gHb):Baseline: 8.1 ± 1.1; Postintervention: 9.3 ± 0.1 Statistically significant, P-value < 0.05[Mean ± SD] |
| Xaplanteris et al., 2012 (44); Athens, Greece | III-1/+ | Randomized, single-blinded, cross-over trial | 8 wk | n = 19; Healthy volunteers; 8 males | Supplementation: 70 g tomato paste/d (33.3 mg lycopene/d)Control: No tomato products | FMD (%):Tomato supplement: Baseline: 4.2 ± 5.1; Day 1: 5.6 ± 3.6; Day 15: 7.5 ± 3.5 Control: Baseline: 5.0 ± 3.5; Day 1: 4.7 ± 3.5; Day 15: 4.5 ± 3.5P-value: 0.047NMD (%)LTomato supplement: Baseline: 15.5 ± 6.1; Day 1: 16.2 ± 6.1; Day 15: 14.6 ± 6.1Control: Baseline: 13.6 ± 10.0; Day 1: 15.2 ± 10.5; Day 15: 12.6 ± 8.3P -value: 0.358Baseline diameter (mm):Tomato supplement: Baseline: 2.94 ± 0.61; Day 1: 2.95 ± 0.61; Day 15: 2.93 ± 0.83Control: Baseline: 2.99 ± 0.57; Day 1: 2.93 ± 0.57; Day 15: 2.99 ± 0.52P-value: 0.592[Mean ± SD] P < 0.05 vs. first day for paired samples t test comparison between 2 time points |
| Steinberg and Chait, 1998 (45); Seattle, WA, USA | II/ɸ | Randomized placebo-controlled trial | 8 wk (4-wk wash-out, 4-wk intervention) | n = 39; No marker of hyperlipidemiaControl group: n = 19 (12 women, 7 men); Age: 26.8 ± 2.2 y; BMI: 23.4 ± 0.8 kg/m2Test group: n = 20 (12 women, 8 men); Age: 29.8 ± 2.6 y; BMI: 26.5 ± 1.1 kg/m2 [mean±SD] | Intervention: 237mL tomato juice, supplemented with 600 mg ascorbic acid, 30 mg B-carotene, 400 mg all-rac-a-tocopherol/dPlacebo: 237 mL Nonsupplemented tomato juice per day | LDL-C oxidation:Sufficient outcome data not reported |
| Hininger et al., 2001 (46); Tronche, France | II/- | Randomized controlled trial | 12 wk | n = 175; Healthy adult males with a stable lifestyle; nonsmokers; Serum retinol >1 μmol/L; BMI <28 kg/m2; Age: 25–45 y | Four groups receiving 15 mg/d vi. capsule of either:- Lycopene- β-carotene- Lutein- Placebo | GSH (μmol/L):Lycopene group: Week 0: 1022 ± 29; Week 12: 877 ± 32Placebo group: Week 0: 955 ± 29; Week 12: 857 ± 29GSSG (μmol/L):Lycopene group: Week 0: 29.1 ± 1.7; Week 12: 28.5 ± 1.9Placebo group: Week 0: 28.9 ± 2.1; Week 12: 27.7 ± 2.6SH groups (μmol/g proteins):Lycopene group: Week 0: 6.54 ± 0.10; Week 12: 6.06 ± 0.05Placebo group: Week 0: 6.32 ± 0.16; Week 12: 6.03 ± 0.07[Mean ± SEM] |
| Riso et al., 2006 (42); Milan, Italy | III-2/ɸ | Double-blind, placebo-controlled, cross-over trial | 78 d (∼11 wk) | n = 26; Healthy; no history of chronic illnessGroup 1: n = 13; age: 25.7 ± 2.1 y; BMI: 21.2 ± 2.2 kg/m2Study sequence: Placebo/wash-out/Lyc-O-MatoGroup 2: n = 13; Age: 25.9 ± 3.4 y; BMI: 20.9 ± 1.9 kg/m2 [mean ± SD]Study sequence: Lyc-o-Mato/wash-out/placebo | Lyc-O-Mato: 1 bottle (250 mL) of Lyc-O-Mato (5.7 mg lycopene/d)Placebo: 1 bottle (250 mL) of placebo drink/d | IFN-y (ng/mL):Placebo: Pre-placebo: 9.3 (6.2); Post-placebo: 21.3 (15.7)Supplement: 34.4% reduction from before intervention to after8-iso-PGF2a (ng/mL):Placebo: Pre: 2.4 (0.2), Post: 2.1 (0.2)Supplement: Pre: 2.3(0.2), Post: 2.4(0.2)Endogenous lymphocyte DNA damage—DNA in tail:Placebo: Pre: 2.6 ± 1.3, Post: 2.6 ± 1.1Supplement: Pre: 3.3 ± 1.9, Post: 2.8 ± 1.2[Mean ± SD] or [median (range)] |
| Upritchard et al., 2000 (46); Dunedin, New Zealand | II/ɸ | Randomized placebo-controlled parallel trial | 8 wk (4-wk placebo, 4-wk intervention) | n = 57; T2D; <75 y; nonsmoker; HbA1c level <10%; Fasting plasma glucose level <11 mmol/L;Tomato juice: n = 15; Age: 63±8 y; M/F: 10/5; Duration of diabetes: 4.9 ± 5.5 y; BMI: 30.9±7.0 kg/m2Vitamin E: n = 12; Age: 56 ± 9 y; M/F: 6/6; Duration of diabetes: 5.8 ± 7.6 y; BMI: 31.5 ± 7.4 kg/m2Vitamin C: n = 12; Age: 56 ± 9 y; M/F: 6/6; Duration of diabetes: 1.9 ± 1.3 y; BMI: 30.7 ± 6.3 kg/m2Placebo: n = 13; Age: 60 ± 6 y; M/F: 10/3; Duration of diabetes: 3.2 ± 2.4 y; BMI: 31.8 ± 2.4 kg/m2 [mean ± SD] | 800 IU/d, vitamin E500 mg/d, vitamin C250 mL tomato juice twice a dayPlacebo capsule | Lag time (min):Tomato juice: Baseline: 69 ± 18; End of run-in: 71 ± 24; Postintervention: 101 ± 27*Placebo:Baseline: 81 ± 21: End of run-in: 86 ± 23; Postintervention: 80 ± 23[Mean ± SD] or [median (range)]*Statistically significant from baseline, P < 0.05 |
| Briviba et al., 2004 (47); Karlsruhe, Germany | III-1/- | Randomized, cross-over trial | 10 wk (2 × 2-wk intervention, 3 × 2-wk wash-out period) | n = 22 Healthy men; Nonsmokers | 330 mL/d tomato juice (37 mg/lycopene)330 mL/d carrot juice (27 mg/β-carotene) | LDL-C oxidation lag time (min):Increased by 4.5% for both groups after 2-wk interventionP-value: 0.08Plasma MDA (μmol/L):Tomato juice: Before: 0.46 ± 0.17; After: 0.43 ± 0.16; 2 wk after: 0.42 ± 0.15Carrot juice: Before: 0.43 ± 0.17; After: 0.43 ± 0.20; 2 wk after: 0.42 ± 0.17[Mean ± SD]*P-value considered significant from baseline at <0.05 |
| MacKinnon et al., 2011 (45); Toronto, Canada | II/+ | Randomized, placebo-controlled intervention study | 5 mo (1-mo wash-out, 4-mo intervention) | n = 60 Females; >1-y postmenopausal; nonsmokers; aged 50–60 y | Twice daily of either: 1) 15 mg lycopene from tomato juice; 2) 35 mg lycopene from lycopene-rich tomato juice; 3) 15 mg lycopene from Lyc-O-Mato capsules; 4) Placebo (0 mg lycopene/d) | Protein thiols (μM):15.56 ± 3.75% increase from baseline in lycopene supplemented group; P < 0.0015.09 ± 3.19% decrease from baseline in placebo group; P < 0.005TBARS (nmol/mL):Lycopene supplement group: Baseline: 7.91 ± 0.46; Postintervention: 6.80 ± 0.35P < 0.00111.93 ± 2.16% significant decrease |
| Sarkar et al., 2012 (48); Madhya, Pradesh | II/- | Randomized controlled trial | 12 wk (2-wk lycopene-restricted diet, 10-wk intervention) | n = 75Patient group: n = 45; Had oxidative stress; Age: 52.4 ± 4.8 y, 25/20 (M/F)Control group: n = 30; Controls with sedentary lifestyle doing yoga regularly; Age: 50.4 ± 5.7 y; 16/14 (M/F) [mean ± SD] | Group 1: 1 capsule (15 mg lycopene)Group 2: 200 mg of tomato products (soup, paste, ketchup) with 15 mg lycopeneGroup 3: Placebo capsule | GPX (unit/gHb)MDA (nmol/mL)SOD (units/mL)Sufficient outcome data not reported |
| Agarwal and Rao, 1998 (49); Toronto, Canada | III-1/- | Randomized, cross-over trial | 8 wk (1-wk intervention, 1-wk wash-out, repeated for each intervention) | n = 19; 10 Males; Nonsmoker; Aged 25–40 y (mean 29 y); Mean weight: 67.6 ± 11.6 kg; Mean BMI: 24.0 ± 2.8 kg/m2 [mean ± SD] | Group 1: 126 g spaghetti sauce (39.2 mg lycopene)Group 2: 540 mL tomato juice (50.4 mg lycopene)Group 3: 1.243 g of 6% lycopene oleoresin from tomatoes (75.0 mg lycopene)PlaceboDaily | LDL-C oxidation (mmol/mol LDL-C): All dietary lycopene treatments significantly lowered serum LDL oxidation over the placeboLDL-TBARS (mmol/mol LDL): Mean decrease over placebo was 25%LDL-CD (mmol/mol LDL): Mean decrease over placebo was 13% |
| Porrini et al., 2002 (40); Milan, Italy | III-1/- | Controlled cross-over intervention Study | 8 wk (3-wk intervention 1, 2-w wash-out, 3-wk intervention 2) | n = 9 Healthy females; Nonsmoking; Age: 25.2 ± 2.2 y; BMI: 20.2 ± 1.6 kg/m2 | Intervention 1: 150 g spinach + 10g olive oil daily Intervention 2: 150 g spinach + 25g tomato puree (7 mg lycopene/d) + 10g olive oil | Lymphocyte oxidative damage: % DNA in tailSufficient outcome data not reported |
| Samaras et al., 2014 (47); Larissa, Greece | III-2/+ | Parallel-controlled trial (randomization unclear) | 8 wk | n = 27 Ultra-marathon runners; nonsmokers; no medical history of HTTomato juice: 13 Males, 2 Females; Age: 44.9 ± 8.53 y; BMI: 24.1 ± 2.46 kg/m2Control: 12 Males; Age: 46.6 ± 15.3 y; BMI: 24.3 ± 1.56 kg/m2 [mean ± SD] | Tomato juiceDose: Amount required to match subjects with usual carbohydrate supplementation (unspecified amount) | FMDBaseline: 20.2 ± 9.9; FMDSupplement: 25.7 ± 10.2P- value: 0.028*GSHBaseline (μmol/L): 35.4 ± 15.8; GSHSupplement (μmol/L): 34.9 ± 6.08P-value: 0.902 TBARSBaseline (μmol/L): 7.11 ± 1.44; TBARSSupplement (μmol/L): 4.94 ± 12.6P-value: 0.001*TACBaseline (mmol DPPH/L): 0.961 ± 0.126; TACSupplement (mmol DPPH/L): 1.020 ± 0.092P-value: 0.065 All values reported on intervention group[Mean ± SD] |
| Blum et al., 2007 (44); Haifa, Israel | III-2/ɸ | Parallel-controlled trial | 4 wk | n = 103; Healthy overweightIntervention group: n = 50; 33 Women; Age: 45.5 ± 14 y; BMI: 28.1 ± 3.155 kg/m2 Control group: n = 53; 35 Women; Age: 45.1 ± 13.5 y; BMI: 30.5 ± 1.51 kg/m2 [mean ± SD] | Regular diet supplemented with tomatoDose: 300 g tomato products (including TS, TJ, FT, tomato soup) per day | ICAM-1: Baseline: 286.47 ± 80.80; Postintervention: 307.44 ± 89.80P-value: 0.07E-selectin: Baseline: 55.37 ± 34.35; Postintervention: 61.20 ± 40.20P-value: 0.14 Results are for tomato-rich diet group[Mean ± SD] |
| Inflammatory biomarkers | ||||||
| Biddle et al., 2015 (50); Kentucky, USA | II/+ | 2-Group, randomized controlled intervention pilot study | 30 d | n = 40; Patients with HF; hospitalized for HF within the last 6 mo; >21 y; Males = 30.7 kg/m2, Females = 31.9 kg/m2Intervention group: n = 22; 8 Males, 10 females; Age: 65 ± 11 yControl group: n = 18; 15 Males, 7 females; Age: 65 ± 9 y | Regular diet supplemented with tomato juice Dose: 11.5 ounces of V8 tomato juice per day (29.4 mg lycopene/d) | CRP (mg/L)Interventionbaseline: 3.4 ± 3.1; InterventionPostintervention: 3.1 ± 2.8Controlbaseline: 4.8 ± 3.4; ControlPostintervention: 4.5 ± 3.8 P < 0.5 for time and group effect[Mean ± SD] |
| Blum et al., 2007 (44); Haifa, Israel | III-2/ɸ | Parallel-controlled trial | 4 wk | n = 103 Healthy overweightIntervention group: n = 50; 33 Women; Age: 45.5 ± 14 y; BMI: 28.1 ± 3.155 kg/m2Control group: n = 53; 35 Women; Age: 45.1 ± 13.5 y; BMI: 30.5 ± 1.51 kg/m2 [mean ± SD] | Regular diet supplemented with tomatoDose: 300 g tomato products (including TS, TJ, FT, tomato soup) per day | hsCRP: Baseline: 0.43 ± 0.59; Postintervention: 0.41 ± 0.40P-value: 0.70 |
| Upritchard et al., 2000 (46); Dunedin, New Zealand | II/ɸ | Randomized placebo-controlled parallel trial | 8 wk (4-wk placebo, 4-wk intervention) | n = 57; T2D; <75 y; nonsmoker; HbA1c level <10%; Fasting plasma glucose level <11 mmol/LTomato juice: n = 15; Age: 63 ± 8 y; M/F: 10/5; Duration of diabetes: 4.9 ± 5.5 y; BMI: 30.9±7.0 kg/m2Vitamin E: n = 12; Age: 56 ± 9 y; M/F: 6/6; Duration of diabetes: 5.8 ± 7.6 y; BMI: 31.5 ± 7.4 kg/m2Vitamin C: n = 12; Age: 56 ± 9 y; M/F: 6/6; Duration of diabetes: 1.9 ± 1.3 y; BMI: 30.7±6.3kg/m2Placebo: n = 13; Age: 60 ± 6 y; M/F: 10/3; Duration of diabetes: 3.2 ± 2.4 y; BMI: 31.8 ± 2.4 kg/m2 [mean ± SD] | 800 IU/d, vitamin E500 mg/d, vitamin C250 mL tomato juice twice a dayPlacebo capsule | CRP (mg/L):Tomato juice: Baseline:3.8 (0.5–17.4); End of run-in:3.5 (0.5–16.2); Postintervention:4.1 (1.2-14.6)Placebo: Baseline: 3.1 (0.5–19.5); End of run-in: 2.9 (1.0–10.6); Postintervention: 3.1 (0.6–12.3)[Mean ± SD] or [median (range)]Statistically significant from baseline, P < 0.05 |
| Kim et al., 2011 (24); Seoul, Republic of Korea | II/+ | Randomized, double-blind, placebo-controlled intervention trial | 8 wk | n = 126; Healthy men; Age: 22–57 y; Frequently smoke cigarettes or consume alcohol; <3 servings vegetables and fruit per week; No history of chronic disease | Low dose: 6 mg lycopene/d via capsuleHigh dose: 15 mg lycopene/d via capsulePlacebo | hs-CRP (mg/dL):Placebo: Pretreatment: 1.14 ± 0.07; Post-treatment: 1.10 ± 0.27Low dose: Pretreatment: 1.39 ± 0.33; Post-treatment: 1.40 ± 0.37High dose: Pretreatment: 1.25 ± 0.44; Post-treatment: 0.54 ± 0.10*[Mean ± SE]*P < 0.05 compared with baseline values in each group tested by paired t test = P < 0.001 compared with baseline values in each group tested by paired t test |
| Colmán Martínez et al., 2017 (51); Valencia, Spain | II/ɸ | Open, prospective, randomized, cross-over, and controlled clinical trial | 18 wk (4-wk intervention, 3-wk wash-out) | n = 28; High cardiovascular risk; Mean age: 69.7 ± 3.1 y; Mean BMI: 31.5 ± 3.6 kg/m2 | Low dose: 200 mL Tomato juice dailyHigh dose: 400 mL Tomato juice dailyControl: Water | CRP (ng/mL):Control: 546 ± 243; Postintervention: 530 ± 228P-value: 0.228[Mean ± SD] Results are for high-dose intervention |
| Jacob et al., 2008 (52); Jena, Germany | II/- | Randomized intervention study | 4-wk (2-wk wash-out, 2-wk intervention) | n = 24 Healthy volunteers; Nonsmokers; Age: 23 ± 2 y; BMI: 21.5 ± 2.8 kg/m2 [mean ± SD] | Group L: 250 mL tomato juice twice daily (20.9 mg lycopene/d)Group LC: Same tomato juice enriched with vitamin C | CRP (μg/L):L: T-2: 336.2 ± 267.3; T0: 315.6 ± 257.7; T±2: 262.3 ± 215.4; LC: T–2: 349.5 ± 279.4; T0: 319.2 ± 212.5; T±2: 247.1 ± 179.3P- value: 0.792[Mean ± SD] |
| Other measures | ||||||
| Neyestani et al., 2007 (33); Tehran, Iran | III-1/ɸ | Placebo-controlled clinical trial | 10 wk (2-wk wash-out, 8-wk intervention) | n = 35 T2D patients; nonsmokers; Aged 35–70 y; 19 women, 16 men | Lycopene supplement (10 mg lycopene/d)Placebo capsule | IgM (mg/dL):Lycopene: Initial: 207.7 ± 35.7; Final: 227.2 ± 42.6Placebo: Initial: 219.4 ± 71.2; Final: 215.4 ± 57.7IgG (mg/dL):Initial: 1471.3 ± 572.8; Final: 1462.8 ± 572.9Placebo: Initial: 1422.3 ± 318.6; Final: 1251.8 ± 261.7slgA (mg/dL):Lycopene: Initial: 9.3 ± 3.8; Final: 10.0 ± 3.4Placebo: Initial: 9.4 ± 2.6; Final: 17.4 ± 32.9[Mean±SD]significant difference between initial and final |
| Graydon et al., 2007 (31); Belfast, United Kingdom | II/ɸ | Randomized, double-blinded, placebo-controlled trial | 4 wk | n = 20 Healthy male volunteers; Aged 18–60 yearsLycopene group: n = 10; Age: 32 y (26, 41 y); 10% smokersPlacebo: n = 10; Age: 39 y (28, 48 y); 10% smokers [median (IQR)] | Dose: 15 mg lycopene capsule, once per dayPlacebo capsule | IGF-I (ng/mL):Lycopene: 0.29 (0.09, 0.46); Placebo: 0.03 (−0.11, 0.08)P-value: 0.52IGFBP-3 (ng/mL):Lycopene: 245 (−109, 484); Placebo: 101 (−34, 234)P value: 0.55[Median (IQR)] |
1BP, blood pressure; CRP, C-reactive protein; CVD, cardiovascular disease; DBP, diastolic blood pressure; DPPH, 2,2-diphenyl-1-picrylhydrazyl; HbA1c, glycated hemoglobin; HDL-C, HDL cholesterol; HPT, hypertensive; hsCRP, high-sensitivity C-reactive protein; HT, hypertension; Ig, immunoglobulin; IGF-I, insulin-like growth factor I; IGFBP3, IGF binding protein 3; LDL-C, LDL cholesterol; LSM, least-square means; MDA, malondialdehyde; MetS, metabolic syndrome; NHMRC, National Health and Medical Research Council; Quality Ax, Quality Assessment; SBP, systolic blood pressure; TAC, total active cannabinoids; TBARS, thiobarbituric acid reactive substances; TC, total cholesterol; TG, triglyceride; TJ, tomato Juice; TS, tomato sauce; T2D, type 2 diabetes; WC, waist circumference (cm).
Risk of bias
Studies were assessed for quality and risk of bias, independently by 2 authors (CER, LMB), using the Cochrane Collaboration Tool to assess any risk of bias in individual studies; results are shown in Table 1. Studies were deemed of a positive, neutral, or negative quality based on the tool; and those that were determined to be of lower quality and assigned a negative score were excluded from the meta-analysis.
Data analysis
When outcomes of included studies were adequately reported, data were pooled using Review Manager (version 5.3, The Cochrane Collaboration 2014) (CER). To calculate the overall treatment effect, the differences between the intervention and comparison groups’ outcomes at follow-up were considered and analyzed using a random-effects model. Continuous outcome data were calculated using the inverse variance test as mean differences (MDs) for studies that used the same measurement. Where biochemistry variables were reported in different units (e.g., mmol/L vs. mg/dL), the measures were converted to the same unit and an MD was calculated. There were no categorical variables pooled. For outcomes related to lipid profile and hemodynamics, subgroup analyses were undertaken for “healthy” patients versus those with hyperlipidemia or hypertension, respectively. “Healthy” status was defined as participants whose baseline biomarkers were within the recommended reference range. Due to the many OS biomarkers reported and the heterogeneity in the studies included, analysis was not carried out on this dataset.
Results
Results of searches
From the initial database searches a total of 6533 articles were identified. All duplicates, animal or cell studies, studies not in English, and those without full-text versions available were excluded. All remaining abstracts were screened. Sixty-seven full-text articles remained and were assessed for eligibility. Studies were excluded at this stage if the outcome, study design, and/or interventions did not meet the inclusion criteria. An additional 5 studies were identified through hand-searching reference lists and were included. In total, 43 RCTs were identified and included in this review. Figure 1 outlines the inclusion process.
FIGURE 1.
PRISMA flow diagram illustrating the identification and selection of studies. PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses.
Study characteristics
Across the 43 included articles, the interventions of either lycopene supplementation or lycopene-containing foods involved a total of 2306 participants, ranging from 11 to 234 participants. Ten studies focused on a population diagnosed with ≥1 CVD risk factors (12, 16, 17, 21, 34, 46, 49, 50, 33, 26), 30 studies looked at the effects of lycopene intervention on healthy individuals (3, 13, 18, 19, 22–25, 35–37, 40, 42, 45, 47, 44, 51, 52, 31, 28–30, 32, 38, 39, 14, 41, 48, 53, 54), and 3 involved both groups (20, 43, 27). Six studies involved females only (12, 39, 28, 41, 53, 54), whereas 3 focused on only males (24, 31, 32). Eleven of the studies were interventions with a placebo-controlled group (13, 17, 20, 21, 24, 35, 42, 46, 33, 31, 55), and 16 used a cross-over study design (14, 16, 22, 23, 25, 35, 37, 40, 49, 51, 26, 38, 39, 30, 29, 41).
Delivery of lycopene intervention
Studies included in this review were heterogeneous with regard to the delivery of lycopene as an intervention. Fifteen studies used lycopene supplementation (13, 16, 17, 19–21, 24, 42, 33, 31, 26, 38, 32, 29, 53), whereas 22 used a whole-food approach (3, 12, 14, 22, 23, 25, 34, 35, 36, 37, 45, 46, 47, 44, 50, 51, 52, 39, 30, 29, 54, 41, 27), with the remaining 6 studies using both supplements and dietary lycopene delivery (18, 40, 49, 28, 48, 43). Of the studies that used a whole-food approach, 15 used tomato juice (12, 14, 25, 34, 37, 45, 46, 49, 47, 50, 51, 52, 28, 30, 54), 10 used tomato paste (22, 23, 37, 40, 49, 39, 29, 41, 43, 27), 4 used raw tomato (3, 22, 36, 44), and 3 paired their tomato product with extra-virgin olive oil (EVOO) to improve the bioavailability (22, 25, 39). While the dosage of lycopene varied greatly throughout the studies, the range was between 1.44 and 75 mg lycopene/d and the mean dosage was 20.6 mg lycopene/d. Fourteen studies used interventions of >20.6 mg lycopene/d (12–14, 23, 49, 50, 52, 28, 30, 29, 54, 41, 48) and 11 studies did not report lycopene dosage (3, 22, 25, 34, 35, 36, 45, 46, 47, 44, 51). Where possible, we contacted the authors of these studies to obtain the lycopene dosage, 1 no longer had the data available, 2 provided us with the whole-food dose but not lycopene concentration, 1 gave us the lycopene dose, and the remaining authors did not reply. The duration of the lycopene interventions varied widely, ranging from measuring outcomes after a 30-min delivery to post–6-mo intervention.
Effect of lycopene on BP
Eleven of the 43 studies reported BP as an outcome measure (16–25, 54). Five of these papers reported statistically significant decreases in BP when compared with a control or placebo group (16, 17, 21, 22, 24), whereas 3 reported nonsignificant improvements (18, 20, 25) and the remaining 3 saw no effect (19, 23, 54). The 5 studies that achieved a significant improvement in BP were blinded, and 4 used a supplementation intervention. The largest significant effects were observed by Massa et al. (17) and Paran et al. (16); both assessed hypertensive participants, where mean diastolic BP (DBP) decreased by 6.9 mmHg (P < 0.001) and 6.2 mmHg (P < 0.001), respectively. Systolic BP (SBP) decreased by 11.8 mmHg (P < 0.0001) and 13.6 mmHg (P < 0.001), respectively. Massa et al. (17) investigated the effect of 1.44 mg lycopene via 6 g watermelon extract, and Paran et al. (16) investigated the effect of 15 mg lycopene via tomato extract capsules. Based on the variable doses administered within these 2 studies and the magnitude of effects observed, a dose-response cannot be hypothesized.
The 5 studies that showed improvements for BP demonstrated a mean significant reduction in SBP of −7.4 mmHg and DBP of −4.0 mmHg among 291 participants. Despite the significant findings for DBP and SBP in these 5 studies, there were 6 studies where improvements were not significant or there was no change observed. The data extracted from these articles can be seen in Table 1.
Effect of lycopene on blood lipids
The effects of lycopene on TC were reported in 14 studies (3, 13, 14, 18, 21, 22, 25, 34, 36, 37, 47, 52, 53, 54). There were also 14 studies that reported on LDL cholesterol (3, 13, 18, 20–23, 25, 34, 36, 37, 47, 53, 54) and HDL cholesterol (3, 13, 14, 18, 20–23, 25, 34, 36, 47, 53, 54), and 11 studies reported on TGs (3, 14, 21–23, 25, 34, 35, 47, 53, 54). Ten studies found significant improvements for the effect of lycopene on ≥1 measure of blood lipids (3, 22, 25, 36, 37, 47, 52, 53, 54, 48), while the remaining 8 studies showed no significant improvements for any measure of blood lipids (13, 14, 18, 20, 21, 23, 35, 26). Samaras et al. (47) found significant reductions for TC and LDL cholesterol in ultra-marathon runners with daily tomato juice consumption over a 2-mo period. Tsitsimpikou et al. (34) observed a mean increase of 8.2% in HDL cholesterol (P = 0.049) and a significant decrease of ≤14.5% in LDL cholesterol (P = 0.001) with daily supplementation of tomato juice (2.51 mg lycopene/100 mL) for 2 mo in participants with metabolic syndrome (MetS).
Cuevas-Ramos et al. (3) and Arranz et al. (25) (intervening with 300 g Roma tomatoes and 750 g tomato juice with 10% refined olive oil, respectively, although lycopene dosage was not reported) observed significant changes in HDL cholesterol [+5.1 mg/dL (P < 0.0001) and −2.7% (P value not supplied), respectively]. The largest improvement was seen in the Cuevas-Ramos et al. (3) study, which looked at overweight participants with low HDL-cholesterol concentrations at baseline (36.5 ± 7.5 mg/dL). The study by Arranz et al. (25) studied the effect with healthy participants. Arranz et al. (25) also saw significant decreases in LDL cholesterol (−5.5%; P value not supplied). McEneny et al. (48) found a significant increase in both HDL2 (+0.84 mmol/L) and HDL3 (+0.08 mmol/L) cholesterol post–lycopene intervention compared with the control group (P < 0.001 and P < 0.001, respectively). This was the only study that reported on these specific outcomes; however, it was considered a good-quality study. A study conducted by Blum et al. (36) found that daily consumption of 300 g tomatoes resulted in a significant increase in HDL cholesterol (+7.3 mg/dL, P = 0.03) in participants who had low HDL-cholesterol concentrations at baseline. In an 8-wk intervention (7 mg lycopene/d via tomato extract capsule) by Gajendragadkar et al. (20), there were no changes in LDL cholesterol observed in subjects with stable CVD on statin therapy. Similarly, in a study by Thies et al. (18) in a population with MetS risk factors or moderate hypercholesterolemia, a diet high in tomato-based foods or lycopene delivered in a supplement (10 mg lycopene/d via lycopene capsule) over a 16-wk intervention resulted in no significant changes in lipid concentrations. The full outcome data for blood lipids can be seen in Table 1.
Effect of lycopene on OS markers and inflammatory markers
Of the 19 studies that reported an effect of lycopene on OS markers and inflammatory markers, 13 studies reported that lycopene interventions resulted in statistically significant improvements in ≥1 OS or inflammatory marker when compared with the control/placebo group (12, 24, 35, 37, 46, 47, 44, 51, 28, 29, 53, 54, 27). Despite this, only 5 studies showing improvement were deemed of good quality (24, 35, 37, 47, 28). There was also considerable heterogeneity among the OS outcome measures reported. The most frequently reported marker was malondialdehyde (MDA; μmol/mL), which was reported in 6 of the 18 studies (12, 23, 40, 53, 54, 27). There were significant reductions in MDA in 3 studies [reduction not reported (P = 0.01), −4.3 μmol/mL (P < 0.001), and −2.77 μmol/mL (P < 0.05), respectively] (12, 53, 27). Ghavipour et al. (12) reported significant improvements for OS outcomes (TAC, SOD, GPx, CAT) despite not reporting actual changes. MDA (μmol/mL) in overweight and/or obese females indicated that 330 mL/d of tomato juice for 20 days can reduce OS in overweight females. A study by Burton-Freeman et al. (35) showed that a healthy cohort consuming a tomato-containing diet can have a significant acute reduction in oxidized LDL cholesterol after 360 min compared with a control group. The effect of lycopene on C-reactive protein (CRP) was reported in 5 studies (24, 44, 50–52). Of these 5 studies, only Kim et al. (24) and Biddle et al. (50) saw significant reductions. Biddle et al. (50) found that a lycopene dose of 29.4 mg/d for 1 mo in patients with heart failure significantly reduced CRP concentrations in women but not in men (P value not supplied). Baseline CRP values for the intervention group were 3.4 ± 3.1 mg/L, not indicative of acute or active inflammation. A study by Colmán Martínez et al. (51) found that 400 mL of tomato juice 4 times/wk for 28 d resulted in significant improvements in intercellular adhesion molecules (ICAMs) and vascular cell adhesion molecule-1 (VCAM-1) (P < 0.001 and P < 0.001, respectively). While a large number of the studies found significant improvements for OS outcomes (12, 24, 35, 37, 46, 47, 44, 51, 28, 29, 53, 54, 27) there were many OS marker studies that showed no improvement (13, 14, 23, 34, 40, 42, 45, 49, 52, 39, 38, 32, 30, 41, 43). Thus, there is insufficient evidence to determine what the effect of lycopene is with regard to OS markers. Table 1 provides details of the OS outcomes for each study. A meta-analysis was not carried out for OS markers as only a few studies reported these outcome measures and, of those studies, there was substantial heterogeneity and a high number deemed of poor quality with incomplete outcome data.
Effect of lycopene on insulin-like growth factor
One study examined insulin-like growth factor I (IGF-I) and IGF binding protein 3 (IGFBP-3). Graydon et al. (31) showed that an increase in serum lycopene for the intervention group was not associated with a change in IGF-I and IGFBP-3 (P = 0.52 and P = 0.55, respectively). This was deemed a good-quality study. No conclusions can be drawn about the effect of lycopene on this measure. The effects of lycopene on insulin and glucose measures were inconsistent across the papers and have not been included in this review.
Quality assessment
The quality of all studies included in this review were assessed using the Cochrane Quality Assessment tool (Supplemental Table 1). Randomization was used in 30 of the 42 studies, with most of these reporting allocation concealments. Thirteen articles were deemed to be of negative quality (14, 25, 36, 37, 40, 49, 52, 39, 32, 30, 43, 27), 14 were neutral (12, 17, 21, 22, 45, 46, 51, 33, 31, 38, 41, 53, 54), and 17 were positive (3, 13, 16, 18–20, 23, 24, 34, 35, 42, 47, 50, 26, 28, 29, 48). Studies of negative and neutral quality often lacked information on intervening factors and, in some cases, biases and limitations of the studies.
Compliance was commonly not reported within studies, while drop-out rate was generally cited. All studies reported that participants were asked to maintain their usual lifestyle and dietary habits and excluded any other dietary supplements or habitual tomato consumption. All studies measured baseline characteristics; however, some neglected to report all outcome data. All studies deemed to be of a negative quality were included in the review as there were many and excluding them may skew the results and narrative conclusions. However, these negative-quality studies were excluded from the meta-analysis to prevent bias and to ensure only the highest-quality outcome data were reported.
Meta-analysis results
Effect of lycopene supplementation on BP
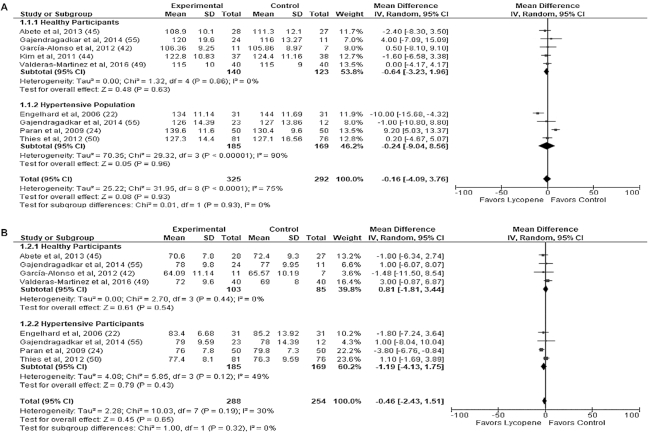
A meta-analysis of 9 trial arms from 8 studies (16, 18, 20–24, 54) including data from 617 participants were pooled to assess the effect of lycopene intervention versus control on SBP. There seemed to be no favorable group in this meta-analysis, with lycopene shown to have minimal impact on MD (−0.16 mmHg; 95% CI: −4.09, 3.76 mmHg; P = 0.93). Similarly, when trials were divided for subgroup analysis [healthy (−0.64 mmHg; 95% CI: −3.23, 1.96 mmHg; P = 0.63) vs. “hypertensive” (−0.24 mmHg; 95% CI: −9.04, 8.56 mmHg; P = 0.63)] there were no significant differences (I2 = 0%, P = 0.93) and heterogeneity was significantly high (I2 = 75%, P < 0.0001).
The effect of lycopene intervention on DBP did not have any significant effects when 8 trial arms from 7 studies (16, 18, 20–23, 54) including pooled data from 542 participants were assessed. Subgroup meta-analysis in hypertensive and healthy participants was also performed with no significant differences within groups (Figure 2B) and, similar to SBP, minimal effect was observed (−0.46 mmHg; 95% CI: −2.43, 1.51 mmHg; P = 0.65). Heterogeneity levels were reasonably low (I2 = 30%, P = 0.19) for the cohort and between-subgroup analysis (I2 = 0%, P = 0.32).
FIGURE 2.
Sub-group meta-analysis of selected studies on the effect of supplemental or dietary lycopene on systolic (A) and diastolic (B) blood pressure (mmHg) of adults by health status at baseline. IV, inverse variance.
Effect of lycopene on blood lipids
TC
A meta-analysis of 10 studies involving a total of 657 participants evaluated the impact of lycopene intervention on TC (3, 13, 18, 21–23, 34, 47, 53, 54). The meta-analysis including all trial arms showed no effect of lycopene intervention on TC compared with the control group (−0.31 mg/dL; 95% CI: −5.55, 4.93 mg/dL; P = 0.91) (A). Subgroup analyses in the hyperlipidemic population (5.21 mg/dL; 95% CI: −10.89, 21.30 mg/dL; P = 0.53) and the healthy population (−1.02 mg/dL; 95% CI: −6.72, 4.69 mg/dL; P = 0.63) were not significant. Heterogeneity was low between the studies included (I2 = 0%, P = 0.71) and for the subgroup analysis (I2 = 0%, P = 0.48) (Figure 3).
Figure 3.
Subgroup meta-analysis of selected studies on the effect of supplemental and dietary lycopene on blood lipids serum total cholesterol (A), LDL cholesterol (B), HDL cholesterol (C), and triglycerides (D) (mg/dL) of adults by health status at baseline. IV, inverse variance.
LDL cholesterol
A meta-analysis of 11 studies, with 13 trial arms (3, 13, 18, 20–23, 34, 47, 53, 54) evaluated the impact of lycopene intervention on LDL cholesterol (n = 727 participants). The findings favored the intervention group; however, they did not reach significance (−2.02 mg/dL; 95% CI: −5.90, 1.86 mg/dL; P = 0.31) (B). Subgroup analyses of the hyperlipidemic and healthy populations were not significant. The meta-analysis in the hyperlipidemic group had a larger MD (8.76 mg/dL; 95% CI: −26.36, 8.84 mg/dL; P = 0.33) when compared with the healthy group (−1.42 mg/dL; 95% CI: −5.45, 2.62 mg/dL; P = 0.49), although neither group reached significance. There was very low heterogeneity for studies included for LDL cholesterol (I2 = 2%, P = 0.43) and subgroup analysis (I2 = 0%, P = 0.43).
HDL cholesterol
A meta-analysis of 11 studies with 12 study arms (3, 13, 18, 20–23, 34, 47, 53, 54), involving 727 participants, assessed the effect of lycopene intervention on HDL cholesterol. Lycopene intervention had no effect on HDL cholesterol in the overall cohort, with an MD of +1.58 mg/dL (95% CI: −2.00, 5.16 mg/dL; P = 0.39) (C). MDs in subgroup analysis of healthy and hyperlipidemic participants were not significant ( I2 = 0%, P = 0.45). Heterogeneity levels assessed by the I2 test were significantly high at 72% (P < 0.0001).
TGs
Nine studies (3, 21–23, 34, 35, 47, 53, 54) with 375 participants were pooled together; the effect of lycopene intervention on TG concentrations was not significant. A subgroup analysis was also conducted, which included 8 of the 9 studies assessing the hyperlipidemic group. This showed a nonsignificant MD of 17.83 mg/dL (95% CI: −9.06, 44.73 mg/dL; P = 0.20) (D). Heterogeneity levels were significantly high (I2 = 78%, P < 0.0001) for the overall pooled data but not for the subgroup analysis on the hyperlipidemic population (I2 = 44.4%, P = 0.81).
Discussion
The present systematic review and meta-analyses evaluates the impact of lycopene from supplementation and/or diet on CVD risk factors. The cumulative descriptive results highlighted no consistent effects and the meta-analyses showed no significant improvement with lycopene intervention on BP and blood lipids when compared with control groups. Of potential significance to the translation of findings was the variability in how lycopene was delivered in the interventions, being either provided as a supplement with or without food, which included tomato juice/paste/raw product or combined with olive oil. The dose of lycopene also varied widely, ranging from 1.44 to 75 mg lycopene/d, and the duration of effect was also considerable (30 min to 6 mo), making it difficult to establish the optimal level.
There is an established consensus in the literature that improved control of BP leads to a reduction in the risks of cardiovascular events and death (56–59). A reduction in mean SBP/DBP to the treatment goal of <140/90 mmHg (60) or a 10% reduction is comparable to the effect of pharmacological treatments such as angiotensin-II receptor blockers and angiotensin-converting enzyme inhibitors for patients with elevated BP (61). Two of the 3 studies in this review that reported significant improvements in BP (16, 21) were in hypertensive participants and thus participants had notably higher values at baseline compared with healthy participants. Mean SBP at baseline was 11.61 mmHg greater in the trials that included participants with elevated BP and/or HT compared with studies with healthy participants. In the 6 studies that included healthy participants, differences reported were not significant. This pathological difference between study samples may explain why some studies saw significant improvements and others did not as there is a greater scope for improvement in individuals who already have elevated BP or HT (and medicated) at baseline. A similar trend has been observed in previous studies (62–64), where a greater treatment effect with lycopene was observed with higher baseline BP. Specifically, in Li and Xu (63), lycopene supplementation of >12 mg/d effectively decreased SBP; however, this effect was more pronounced in populations who had starting SBP values defined within the range of HT. These outcomes of the aforementioned studies are not consistent with the findings of the present meta-analysis, where no significant reduction in SBP was shown following lycopene intervention. The authors propose that the lycopene dose was not sufficient and the ability to reduce SBP in healthy participants did not translate. Due to the high level of variability within these studies and especially in the absence of significant results, it is hard to draw conclusions regarding appropriate recommendations for lycopene intake and/or supplementation with BP. More homogenous research, in particular with regard to dosage and duration of lycopene and the delivery through supplementation or food-based approaches, which are essential to establish a dose-response and to better understand the potential impact of bioavailability of lycopene in different populations (i.e., healthy alone vs. hypertensive alone), is needed.
A meta-analysis for the effects of lycopene intervention and LDL cholesterol in participants who are healthy or hyperlipidemic at baseline suggested that there was no significant effect in a pooled analysis (C). However, most studies were not powered to detect a change, and it could be postulated that perhaps in a more homogenous sample with adequate numbers differences may be observed. The other blood lipid parameters were also not affected by lycopene intervention; however, some improvement in TGs was observed for the control group in the meta-analysis (B). This may be reflective of background diet quality rather than the placebo/control food or supplement ingested as part of the study. The findings from our review are consistent with another review that evaluated the effect of lycopene supplementation and tomato products on cholesterol (65). The review concluded that while there is convincing evidence linking lycopene consumption with the regulation of cholesterol metabolism, there is a need for more human intervention studies to better establish the causal relation.
There were 5, 3, and 4 studies, respectively, that reported lycopene dosage within the interventions that had significant effects for OS (12, 24, 34, 35, 28, 29, 27), BP (16, 21, 24), and blood lipids (37, 52, 53, 48). Of the studies that showed significant improvements in outcomes with lycopene intervention, the dose provided was so variable that an optimal dose cannot be deduced. In addition, there were numerous studies (n = 11) that did not report lycopene dosage.
Furthermore, studies investigating lycopene in humans are fraught by the complexity associated with differentiating between lycopene and other antioxidants present within the diet. Another potential confounder is the baseline or habitual levels of lycopene in a person's diet. While some studies attempt to control for this, advising a low-lycopene diet or using a wash-out period, this is not consistent across studies and the overall effect is unclear. As a result, there is difficulty interpreting the benefits attributable to lycopene specifically and thus determining cause and effect when the intervention involves a whole of diet approach (24). The heterogeneity in the quality, quantity, and source of lycopene administered throughout the trials make it difficult to pool the results from this review. In addition to variances in dosage, the bioavailability of lycopene is improved with cooking and evidence shows that when lycopene is combined with other ingredients such as EVOO there is an increase in total antioxidant capacity; this way of eating is promoted by the Mediterranean dietary pattern and culture (66). These factors are ultimately difficult to assess and control for and pose a limitation to these intervention trials. In this review it appears from the studies identifying a significant effect of lycopene on CVD risk factors that a higher dosage of lycopene may have a greater treatment potential. In animal studies, where much work has been published in this area, higher doses of lycopene (9% in tomato paste) had a significant cholesterol-lowering effect on TC and LDL cholesterol, compared with lower doses of lycopene (3% in tomato paste) where there was no effect in hamsters (67). This is also supported by cell studies wherein a 14% reduction in LDL cholesterol after a 3-mo intervention using a daily dosage of 60 mg in tomato extract (68) was reported. Of note, among the human studies within this review that reported significant improvements in CVD risk factors with the provision of lycopene, there was a mean dosage of 22.2 mg/d.
Lycopene interventions that were more likely to produce improvements in CVD risk factors typically shared similar characteristics in that they were RCTs that were double-blinded and administered lycopene doses >15 mg/d. One such consideration is the feasibility of achieving a 15-mg dose of lycopene through dietary consumption, which would require individuals to consume ∼3–4 whole tomatoes, or 2 tablespoons of tomato paste daily, a quantity that may not be feasible for some people to consume. Therefore, lycopene supplementation may be a more realistic approach to achieve a level to facilitate benefits with respect to ameliorating CVD risk factors.
As other studies have suggested the treatment effect of lycopene may be impacted upon by baseline levels (63), as those with higher starting levels have a great capacity for reduction, perhaps deviating towards the mean. Subgroup meta-analyses assessing results from healthy participants and those who were hypertensive or hyperlipidemic participants were carried out for each CVD outcome to try to overcome this potential barrier.
There have been an increasing number of reviews emerging that assess the impact of lycopene on CVD risk factors (69–72), all of which found a positive association between lycopene and ≥1 measure of CVD. In particular, a systematic review and meta-analysis by Cheng et al. (69) assessed the relation between lycopene supplementation and CVD risk factors and reported increased lycopene intake to have a positive effect on BP, blood lipids, and EF. The outcomes of our systematic review, which included a larger number of studies, are not consistent with these findings, and improvement in EF was less prominent. This outcome is also fraught by the lack of good quality studies assessing EF and OS. The 22 articles included in the review by Cheng et al. (69) were also captured in our review, although several were omitted from the meta-analysis due to being of poor quality. A meta-analysis by Senkus et al. (73) published in 2018 investigated the relation between lycopene and MetS and found that lycopene consumption appeared to positively influence MetS components, although 8 of the 11 included studies of this review were of a cross-sectional study-design nature. Furthermore, a meta-analysis by Song et al. (70) evaluated the impact of lycopene intake and CVD risk factors through observational studies and reported that higher lycopene intake is inversely associated with a lower risk of CVD. In a systematic review that looked at diet and supplementation of lycopene (74), it was found that, while available evidence is limited, lycopene supplementation is favored as a strategy in lowering BP among hypertensive individuals; however, improvements for other outcomes were inconclusive. These results are in agreement with our review with the disparity in the findings presented.
There is a plethora of research looking at the relation between various OS markers and the provision of lycopene; the findings from these studies are conflicting. A recent meta-analysis and systematic review (75) that involved 13 RCTs looking at OS found that lymphocyte DNA tail length significantly decreased with lycopene supplementation. Despite this positive effect, the paper concluded that more parallel research is required to gain a better understanding of the mechanisms behind lycopene's antioxidative effect and capacity to eliminate OS. It also needs to be considered that antioxidant enzymes may not be appropriate markers for oxidative stress as changes may not reflect changes of free radical derived products during enhanced OS processes. The studies captured in this review that reported on the effect of lycopene on OS outcomes, while yielding significant results, were excluded from a meta-analysis as there were not enough good-quality studies reporting on any single outcome. Due to varying methods in randomization and blinding and incomplete reporting of outcome data, the majority of the OS studies were deemed overall of poor quality.
The present systematic review has many strengths, the first of which is that only RCTs were included (76). This review is also, to the authors’ knowledge, the largest and most comprehensive systematic review and meta-analysis assessing the effect of lycopene intervention on risk factors. The strengths of this review also extend themselves to the meta-analyses, which only included studies of either neutral or positive quality. It is also a strength that subgroup meta-analyses were carried out to compare healthy participants with those who had increased baseline values for CVD risk factors.
Limitations of this review include the heterogeneity of the included studies, in particular when looking at how lycopene was administered across the interventions. It is difficult to make direct comparisons and draw conclusions, especially from the meta-analysis, based on lycopene provision when there is substantial heterogeneity between the source of lycopene, dose, duration, and possible interference from baseline concentrations and other micronutrients. Lycopene supplementation varied in concentration, volume, and administration and in whole-diet interventions often the effect of other nutrients is difficult to control for and the treatment effect in some cases may not be absolutely attributable to the lycopene alone. The heterogeneity within this review also meant that subgroup analysis could only be conducted in a small sample size, which makes it difficult to draw conclusions from the studies.
Another limitation of this review is that 11 studies (3, 22, 25, 34–36, 45, 46, 47, 44, 51) neglected to report lycopene dosage and, as a result, it is difficult to ascertain the true dose-response required for a potential significant or clinically meaningful effect. An additional limitation is that the included studies, and thus this review, does not address the variability in lycopene metabolism based on genetic influences.
Future research should focus on investigating lycopene using larger sample sizes and longer intervention periods in a homogenous sample, controlling for obvious confounders (baseline lycopene concentrations/habitual dietary intake and health status), in order to produce higher-quality results and demonstrate sustainability of improvements and potential clinical implications. Within each study, multiple CVD risk factors should be included to establish a clearer link between lycopene and improvements in CVD as a whole. Additional high-quality studies that can assist in establishing a clearer link of the therapeutic effects of lycopene will help to develop recommendations for lycopene delivery and/or supplementation to prevent and manage CVD risk factors.
Conclusions
This review of RCTs has demonstrated that lycopene interventions for cardiovascular risk factors are highly variable in both the dosage provided and mode of delivery (supplement or food based). As such, there are conflicting findings regarding the efficacy of lycopene to improve cardiovascular risk factors. There is a clear need for more consistent research to be conducted to establish a dose-response for lycopene for these measures. Currently, there is not enough evidence to recommend a therapeutic dose for the prevention or management of cardiovascular risk factors. However, given lycopene's known antioxidant properties, lycopene-rich foods should still be encouraged as part of a healthy, varied diet.
Supplementary Material
ACKNOWLEDGEMENTS
The authors’ responsibilities were as follows—ACT: critically reviewed the manuscript and contributed to all aspects of the review; CER: conducted the review analysis and drafted the manuscript; LMB: conducted the review; ESG: conceptualized the study, contributed and reviewed all aspects of the review; and all authors: read and approved the final manuscript.
Notes
The authors reported no funding received for this study.
Author disclosures: The authors report no conflicts of interest.
Supplemental Table 1 is available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at: https://academic.oup.com/advances.
Abbreviations used: BP, blood pressure; CRP, C-reactive protein; CVD, cardiovascular disease; DBP, diastolic blood pressure; EF, endothelial function; EVOO, extra-virgin olive oil; HT, hypertension; IGF-I, insulin-like growth factor I; IGFBP3, insulin-like growth factor binding protein 3; MD, mean difference; MDA, malondialdehyde; MetS, metabolic syndrome; NHMRC, National Health and Medical Research Council; OS, oxidative stress; RCT, randomized controlled trial; SBP, systolic blood pressure; TC, total cholesterol; TG, triglyceride; T2D, type 2 diabetes.
Contributor Information
Audrey C Tierney, School of Allied Health and Health Implementation Science and Technology Group, Health Research Institute, University of Limerick, Limerick, Ireland; School of Allied Health, La Trobe University, Bundoora, Victoria, Australia.
Chloe E Rumble, School of Allied Health, La Trobe University, Bundoora, Victoria, Australia.
Lauren M Billings, School of Allied Health, La Trobe University, Bundoora, Victoria, Australia.
Elena S George, School of Allied Health, La Trobe University, Bundoora, Victoria, Australia; Institute for Physical Activity and Nutrition, School of Exercise and Nutrition Sciences, Deakin University, Geelong, Victoria, Australia.
References
- 1. Roger VL, Go AS, Lloyd-Jones DM, Benjamin EJ, Berry JD, Borden WB, Bravata DM, Dai S, Ford ES, Fox CS et al. Heart disease and stroke statistics—2012 update: a report from the American Heart Association. Circulation. 2012;125(1):e2–e220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zahra A, Lee EW, Sun LY, Park JH. Cardiovascular disease and diabetes mortality, and their relation to socio-economical, environmental, and health behavioural factors in worldwide view. Public Health. 2015;129(4):385–95. [DOI] [PubMed] [Google Scholar]
- 3. Cuevas-Ramos D, Almeda-Valdes P, Chavez-Manzanera E, Meza-Arana CE, Brito-Cordova G, Mehta R, Perez-Mendez O, Gomez-Perez FJ. Effect of tomato consumption on high-density lipoprotein cholesterol level: a randomized, single-blinded, controlled clinical trial. Diabetes Metab Syndr Obes. 2013;6:263–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Australian Institute of Health and Welfare Health care expenditure on cardiovascular diseases. Canberra: Australian Government; 2014. [Google Scholar]
- 5. World Health Organization Global status report on noncommunicable diseases 2010. Geneva (Switzerland): World Health Organization; 2011. [Google Scholar]
- 6. Wallstrom P, Sonestedt E, Hlebowicz J, Ericson U, Drake I, Persson M, Gullberg B, Hedblad B, Wirfalt E. Dietary fiber and saturated fat intake associations with cardiovascular disease differ by sex in the Malmo Diet and Cancer Cohort: a prospective study. PLoS One. 2012;7(2):e31637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Siri-Tarino PW, Sun Q, Hu FB, Krauss RM. Saturated fat, carbohydrate, and cardiovascular disease. Am J Clin Nutr. 2010;91(3):502–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Michel de Lorgeril PS, Martin J-L, Monjaud I, Delaye J, Mamelle N. Mediterranean diet, traditional risk factors, and the rate of cardiovascular complications after myocardial infarction. Clin Trial. 1999;99:779–85. [DOI] [PubMed] [Google Scholar]
- 9. Estruch R, Ros E, Salas-Salvadó J, Covas M-I, Corella D, Arós F, Gómez-Gracia E, Ruiz-Gutiérrez V, Fiol M, Lapetra J et al. Primary prevention of cardiovascular disease with a Mediterranean diet. N Engl J Med. 2013;368(14):1279–90. [DOI] [PubMed] [Google Scholar]
- 10. Sofi F, Macchi C, Abbate R, Gensini GF, Casini A. Mediterranean diet and health status: an updated meta-analysis and a proposal for a literature-based adherence score. Public Health Nutr. 2014;17(12):2769–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bach-Faig A, Berry EM, Lairon D, Reguant J, Trichopoulou A, Dernini S, Medina FX, Battino M, Belahsen R, Miranda G et al. Mediterranean diet pyramid today: science and cultural updates. Public Health Nutr. 2011;14(12A):2274–84. [DOI] [PubMed] [Google Scholar]
- 12. Ghavipour M, Sotoudeh G, Ghorbani M. Tomato juice consumption improves blood antioxidative biomarkers in overweight and obese females. Clin Nutr. 2015;34(5):805–9. [DOI] [PubMed] [Google Scholar]
- 13. Devaraj S, Mathur S, Basu A, Aung HH, Vasu VT, Meyers S, Jialal I. A dose-response study on the effects of purified lycopene supplementation on biomarkers of oxidative stress. J Am Coll Nutr. 2008;27(2):267–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Collins JK, Arjmandi BH, Claypool PL, Perkins-Veazie P, Baker RA, Clevidence BA. Lycopene from two food sources does not affect antioxidant or cholesterol status of middle-aged adults. Nutr J. 2004;3:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: the PRISMA statement. J Clin Epidemiol. 2009;62(10):1006–12. [DOI] [PubMed] [Google Scholar]
- 16. Paran E, Novack V, Engelhard YN, Hazan-Halevy I. The effects of natural antioxidants from tomato extract in treated but uncontrolled hypertensive patients. Cardiovasc Drugs Ther. 2009;23(2):145–51. [DOI] [PubMed] [Google Scholar]
- 17. Massa NM, Silva AS, Toscano LT, Silva JD, Persuhn DC, Goncalves Mda C. Watermelon extract reduces blood pressure but does not change sympathovagal balance in prehypertensive and hypertensive subjects. Blood Press. 2016;25(4):244–8. [DOI] [PubMed] [Google Scholar]
- 18. Thies F, Masson LF, Rudd A, Vaughan N, Tsang C, Brittenden J, Simpson WG, Duthie S, Horgan GW, Duthie G. Effect of a tomato-rich diet on markers of cardiovascular disease risk in moderately overweight, disease-free, middle-aged adults: a randomized controlled trial. Am J Clin Nutr. 2012;95(5):1013–22. [DOI] [PubMed] [Google Scholar]
- 19. Ried K, Frank OR, Stocks NP. Dark chocolate or tomato extract for prehypertension: a randomised controlled trial. BMC Complement Altern Med. 2009;8:9–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gajendragadkar PR, Hubsch A, Maki-Petaja KM, Serg M, Wilkinson IB, Cheriyan J. Effects of oral lycopene supplementation on vascular function in patients with cardiovascular disease and healthy volunteers: a randomised controlled trial. PLoS One. 2014;9(6):e99070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Engelhard YN, Gazer B, Paran E. Natural antioxidants from tomato extract reduce blood pressure in patients with grade-1 hypertension: a double-blind, placebo-controlled pilot study. Am Heart J. 2006;151(1):100. [DOI] [PubMed] [Google Scholar]
- 22. Valderas-Martinez P, Chiva-Blanch G, Casas R, Arranz S, Martinez-Huelamo M, Urpi-Sarda M, Torrado X, Corella D, Lamuela-Raventos RM, Estruch R. Tomato sauce enriched with olive oil exerts greater effects on cardiovascular disease risk factors than raw tomato and tomato sauce: a randomized trial. Nutrients. 2016;8(3):170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Abete I, Perez-Cornago A, Navas-Carretero S, Bondia-Pons I, Zulet MA, Martinez JA. A regular lycopene enriched tomato sauce consumption influences antioxidant status of healthy young-subjects: a crossover study. J Funct Foods. 2013;5(1):28–35. [Google Scholar]
- 24. Kim JY, Paik JK, Kim OY, Park HW, Lee JH, Jang Y, Lee JH. Effects of lycopene supplementation on oxidative stress and markers of endothelial function in healthy men. Atherosclerosis. 2011;215(1):189–95. [DOI] [PubMed] [Google Scholar]
- 25. Arranz S, Martinez-Huelamo M, Vallverdu-Queralt A, Valderas-Martinez P, Illan M, Sacanella E, Escribano E, Estruch R, Lamuela-Raventos RM. Influence of olive oil on carotenoid absorption from tomato juice and effects on postprandial lipemia. Food Chem. 2015;168:203–10. [DOI] [PubMed] [Google Scholar]
- 26. Massa NM, Silva AS, de Oliveira CV, Costa MJ, Persuhn DC, Barbosa CV, Goncalves MD. Supplementation with watermelon extract reduces total cholesterol and LDL cholesterol in adults with dyslipidemia under the influence of the MTHFR C677T polymorphism. J Am Coll Nutr. 2016;35(6):514–20. [DOI] [PubMed] [Google Scholar]
- 27. Sarkar PD, Sahu A, Gupta T. Lycopene—tomato's secret weapon against oxidative stress. Bangladesh J Med Sci. 2011;10(4):275–9. [Google Scholar]
- 28. MacKinnon ES, Rao AV, Josse RG, Rao LG. Supplementation with the antioxidant lycopene significantly decreases oxidative stress parameters and the bone resorption marker N-telopeptide of type i collagen in postmenopausal women. Osteoporos Int. 2011;22(4):1091–101. [DOI] [PubMed] [Google Scholar]
- 29. Xaplanteris P, Vlachopoulos C, Pietri P, Terentes-Printzios D, Kardara D, Alexopoulos N, Aznaouridis K, Miliou A, Stefanadis C. Tomato paste supplementation improves endothelial dynamics and reduces plasma total oxidative status in healthy subjects. Nutr Res. 2012;32(5):390–4. [DOI] [PubMed] [Google Scholar]
- 30. Briviba K, Schnabele K, Rechkemmer G, Bub A. Supplementation of a diet low in carotenoids with tomato or carrot juice does not affect lipid peroxidation in plasma and feces of healthy men. J Nutr. 2004;134(5):1081–3. [DOI] [PubMed] [Google Scholar]
- 31. Graydon R, Gilchrist SE, Young IS, Obermuller-Jevic U, Hasselwander O, Woodside JV. Effect of lycopene supplementation on insulin-like growth factor-1 and insulin-like growth factor binding protein-3: a double-blind, placebo-controlled trial. Eur J Clin Nutr. 2007;61(10):1196–200. [DOI] [PubMed] [Google Scholar]
- 32. Hininger IA, Meyer-Wenger A, Moser U, Wright A, Southon S, Thurnham D, Chopra M, Van den Berg H, Olmedilla B, Favier AE et al. No significant effects of lutein, lycopene or β-carotene supplementation on biological markers of oxidative stress and LDL oxidizability in healthy adult subjects. J Am Coll Nutr. 2001;20(3):232–8. [DOI] [PubMed] [Google Scholar]
- 33. Neyestani TR, Shariatzadeh N, Gharavi A, Kalayi A, Khalaji N. Physiological dose of lycopene suppressed oxidative stress and enhanced serum levels of immunoglobulin M in patients with type 2 diabetes mellitus: a possible role in the prevention of long-term complications. J Endocrinol Invest. 2007;30(10):833–8. [DOI] [PubMed] [Google Scholar]
- 34. Tsitsimpikou C, Tsarouhas K, Kioukia-Fougia N, Skondra C, Fragkiadaki P, Papalexis P, Stamatopoulos P, Kaplanis I, Hayes AW, Tsatsakis A et al. Dietary supplementation with tomato-juice in patients with metabolic syndrome: a suggestion to alleviate detrimental clinical factors. Food Chem Toxicol. 2014;74:9–13. [DOI] [PubMed] [Google Scholar]
- 35. Burton-Freeman B, Talbot J, Park E, Krishnankutty S, Edirisinghe I. Protective activity of processed tomato products on postprandial oxidation and inflammation: a clinical trial in healthy weight men and women. Mol Nutr Food Res. 2012;56(4):622–31. [DOI] [PubMed] [Google Scholar]
- 36. Blum A, Merei M, Karem A, Blum N, Ben-Arzi S, Wirsansky I, Khazim K. Effects of tomatoes on the lipid profile Clin Invest Med 2006;29(5):298–300. [PubMed] [Google Scholar]
- 37. Silaste M-L, Alfthan G, Aro A, Kesaniemi YA, Horkko S. Tomato juice decreases LDL cholesterol levels and increases LDL resistance to oxidation. Br J Nutr. 2007;98(6):1251–8. [DOI] [PubMed] [Google Scholar]
- 38. Riso P, Visioli F, Grande S, Guarnieri S, Gardana C, Simonetti P, Porrini M. Effect of a tomato-based drink on markers of inflammation, immunomodulation, and oxidative stress. J Agric Food Chem. 2006;54(7):2563–6. [DOI] [PubMed] [Google Scholar]
- 39. Porrini M, Riso P, Oriani G. Spinach and tomato consumption increases lymphocyte DNA resistance to oxidative stress but this is not related to cell carotenoid concentrations. Eur J Nutr. 2002;41(3):95–100. [DOI] [PubMed] [Google Scholar]
- 40. Rao AV, Shen H. Effect of low dose lycopene intake on lycopene bioavailability and oxidative stress. Nutr Res. 2002;22(10):1125–31. [Google Scholar]
- 41. Stangl V, Kuhn C, Hentschel S, Jochmann N, Jacob C, Bohm V, Frohlich K, Muller L, Gericke C, Lorenz M. Lack of effects of tomato products on endothelial function in human subjects: results of a randomised, placebo-controlled cross-over study. Br J Nutr. 2011;105(2):263–7. [DOI] [PubMed] [Google Scholar]
- 42. Carroll YL, Corridan BM, Morrissey PA. Lipoprotein carotenoid profiles and the susceptibility of low density lipoprotein to oxidative modification in healthy elderly volunteers. Eur J Clin Nutr. 2000;54(6):500–7. [DOI] [PubMed] [Google Scholar]
- 43. Sarkar PD, Gupta T, Sahu A. Comparative analysis of lycopene in oxidative stress. J Assoc Physicians India. 2012;60(7):17–9. [PubMed] [Google Scholar]
- 44. Blum A, Monir M, Khazim K, Peleg A, Blum N. Tomato-rich (Mediterranean) diet does not modify inflammatory markers. CIM. 2007;30(2):70. [DOI] [PubMed] [Google Scholar]
- 45. Steinberg FM, Chait A. Antioxidant vitamin supplementation and lipid peroxidation in smokers [corrected] [published erratum appears in Am J Clin Nutr 1999;69(6): 1293]. Am J Clin Nutr. 1998;68(2):319–27. [DOI] [PubMed] [Google Scholar]
- 46. Upritchard JE, Sutherland WHF, Mann JI. Effect of supplementation with tomato juice, vitamin E, and vitamin C on LDL oxidation and products of inflammatory activity in type 2 diabetes. Diabetes Care. 2000;23(6):733–8. [DOI] [PubMed] [Google Scholar]
- 47. Samaras A, Tsarouhas K, Paschalidis E, Giamouzis G, Triposkiadis F, Tsitsimpikou C, Becker AT, Goutzourelas N, Kouretas D. Effect of a special carbohydrate-protein bar and tomato juice supplementation on oxidative stress markers and vascular endothelial dynamics in ultra-marathon runners. Food Chem Toxicol. 2014;69:231–6. [DOI] [PubMed] [Google Scholar]
- 48. McEneny J, Wade L, Young IS, Masson L, Duthie G, McGinty A, McMaster C, Thies F. Lycopene intervention reduces inflammation and improves HDL functionality in moderately overweight middle-aged individuals. J Nutr Biochem. 2013;24(1):163–8. [DOI] [PubMed] [Google Scholar]
- 49. Agarwal S, Rao AV. Tomato lycopene and low density lipoprotein oxidation: a human dietary intervention study. Lipids. 1998;33(10):981–4. [DOI] [PubMed] [Google Scholar]
- 50. Biddle MJ, Lennie TA, Bricker GV, Kopec RE, Schwartz SJ, Moser DK. Lycopene dietary intervention: a pilot study in patients with heart failure. J Cardiovasc Nurs. 2015;30(3):205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Colmán Martínez M, Martinez-Huelamo M, Valderas-Martinez P, Arranz-Martinez S, Almanza-Aguilera E, Corella D, Estruch R, Lamuela-Raventos RM. trans-Lycopene from tomato juice attenuates inflammatory biomarkers in human plasma samples: an intervention trial. Mol Nutr Food Res. 2017;61(11). [DOI] [PubMed] [Google Scholar]
- 52. Jacob K, Periago MJ, Bohm V, Berruezo GR. Influence of lycopene and vitamin C from tomato juice on biomarkers of oxidative stress and inflammation. Br J Nutr. 2008;99(1):137–46. [DOI] [PubMed] [Google Scholar]
- 53. Misra R, Mangi S, Joshi S, Mittal S, Gupta SK, Pandey RM. LycoRed as an alternative to hormone replacement therapy in lowering serum lipids and oxidative stress markers: a randomized controlled clinical trial. J Obstet Gynaecol Res. 2006;32(3):299–304. [DOI] [PubMed] [Google Scholar]
- 54. García-Alonso FJ, Jorge-Vidal V, Ros G, Periago MJ. Effect of consumption of tomato juice enriched with N-3 polyunsaturated fatty acids on the lipid profile, antioxidant biomarker status, and cardiovascular disease risk in healthy women. Eur J Nutr. 2012;51(4):415–24. [DOI] [PubMed] [Google Scholar]
- 55. Steinberg FM, Chait A. Antioxidant vitamin supplementation and lipid peroxidation in smokers. Am J Clin Nutr. 1998;68(2):319–27. [DOI] [PubMed] [Google Scholar]
- 56. Estacion RO, Jeffers BW, Gifford N, Schrier RW. Effect of blood pressure control on diabetic microvascular complications in patients with hypertension and type 2 diabetes. J Diabetes Care. 2000;23:54–64. [PubMed] [Google Scholar]
- 57. Hansson L, Zanchetti A, Carruthers SG, Dahlöf B, Elmfeldt D, Julius S, Ménard J, Rahn KH, Wedel H, Westerling S. Effects of intensive blood-pressure lowering and low-dose aspirin in patients with hypertension: principal results of the Hypertension Optiomal Treatment (HOT) randomised trial. Lancet North Am Ed. 1998;351:1755–62. [DOI] [PubMed] [Google Scholar]
- 58. Wang J, Staessen JA, Gong L. Chinese trial on isolated systolic hypertension in the elderly. Arch Intern Med. 2000;160(2):211–20. [DOI] [PubMed] [Google Scholar]
- 59. Curb DJ, Pressel SL, Cutler JA, Savage PJ, Applegate WB, Black H, Camel G, Davis BR, Frost PH, Gonzalez N et al. Effect of diuretic-based antihypertensive treatment on cardiovascular disease risk in older diabetic patients with isolated systolic hypertension. JAMA. 1996;276(23):1886–92. [PubMed] [Google Scholar]
- 60. Düsing R. Blood pressure treatment goals in hypertension. Ther Adv Cardiovasc Dis. 2016;10(6):332–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Vijan S, Hayward RA. Treatment of hypertension in type 2 diabetes mellitus: blood pressure goals, choice of agents, and setting priorities in diabetes care. Ann Intern Med. 2003;138(7):593–602. [DOI] [PubMed] [Google Scholar]
- 62. Ried K, Fakler P. Protective effect of lycopene on serum cholesterol and blood pressure: meta-analyses of intervention trials. Maturitas. 2011;68(4):299–310. [DOI] [PubMed] [Google Scholar]
- 63. Li X, Xu J. Lycopene supplement and blood pressure: an updated meta-analysis of intervention trials. Nutrients. 2013;5(9):3696–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Taubert D, Berkels R, Roesen R, Klaus W. Chocolate and blood pressure in elderly individuals with isolated systolic hypertension. JAMA. 2003;290(8):1029–130. [DOI] [PubMed] [Google Scholar]
- 65. Palozza P, Catalano A, Simone RE, Mele MC, Cittadini A. Effect of lycopene and tomato products on cholesterol metabolism. Ann Nutr Metab. 2012;61(2):126–34. [DOI] [PubMed] [Google Scholar]
- 66. Fielding JM, Rowley KG, Cooper P, O'Dea K. Increases in plasma lycopene concentration after consumption of tomatoes cooked with olive oil. Asia Pac J Clin Nutr. 2005;14(2):131. [PubMed] [Google Scholar]
- 67. Hsu YM, Lai CH, Chang CY, Fan CT, Chen CT, Wu CH. Characterizing the lipid-lowering effects and antioxidant mechanisms of tomato paste. Biosci Biotechnol Biochem. 2008;72(3):677–85. [DOI] [PubMed] [Google Scholar]
- 68. Fuhrman B, Ellis A, Aviram M. Hypocholesterolemic effect of lycopene and b-carotene is related to suppression of cholesterol synthesis and augmentation of LDL receptor activity in macrophages. Biochem Biophys Res Commun. 1997;233(3):658–62. [DOI] [PubMed] [Google Scholar]
- 69. Cheng HM, Koutsidis G, Lodge JK, Ashor A, Siervo M, Lara J. Tomato and lycopene supplementation and cardiovascular risk factors: a systematic review and meta-analysis. Atherosclerosis. 2017;257:100–8. [DOI] [PubMed] [Google Scholar]
- 70. Song B, Liu K, Gao Y, Zhao L, Fang H, Li Y, Pei L, Xu Y. Lycopene and risk of cardiovascular diseases: a meta-analysis of observational studies. Mol Nutr Food Res. 2017;61(9). [DOI] [PubMed] [Google Scholar]
- 71. Baumgartner S, Ras RT, Trautwein EA, Mensink RP, Plat J. Plasma fat-soluble vitamin and carotenoid concentrations after plant sterol and plant stanol consumption: a meta-analysis of randomized controlled trials. Eur J Nutr. 2017;56(3):909–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Thies F, Mills LM, Moir S, Masson LF. Cardiovascular benefits of lycopene: fantasy or reality?. Proc Nutr Soc. 2017;76(2):122–9. [DOI] [PubMed] [Google Scholar]
- 73. Senkus KE, Tan L, Crowe-White KM. Lycopene and metabolic syndrome: a systematic review of the literature. Adv Nutr. 2018;10:(1):19–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Burton-Freeman BM, Sesso HD. Whole food versus supplement: comparing the clinical evidence of tomato intake and lycopene supplementation on cardiovascular risk factors. Adv Nutr. 2014;5(5):457–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Chen J, Song Y, Zhang L. Effect of lycopene supplementation on oxidative stress: an exploratory systematic review and meta-analysis of randomized controlled trials. J Med Food. 2013;16(5):361–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. National Health and Medical Research Council NHMRC additional levels of evidence and grades for recommendations for developers of guidelines. Canberra: Australian Government; 2009. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.