Abstract
BACKGROUND
Obsessive compulsive disorder (OCD) is a complex neuropsychiatric disease characterized by obsessions and compulsions. Deep brain stimulation (DBS) has demonstrated efficacy in improving symptoms in medically refractory patients. Multiple targets have been investigated.
OBJECTIVE
To systematically review the current level and quality of evidence supporting OCD-DBS by target region with the goal of establishing a common nomenclature.
METHODS
A systematic literature review was performed using the PubMed database and a patient/problem, intervention, comparison, outcome search with the terms “DBS” and “OCD.” Of 86 eligible articles that underwent full-text review, 28 were included for review. Articles were excluded if the target was not specified, the focus on nonclinical outcomes, the follow-up period shorter than 3 mo, or the sample size smaller than 3 subjects. Level of evidence was assigned according to the American Association of Neurological Surgeons/Congress of Neurological Surgeons joint guideline committee recommendations. Quality of evidence was assessed using the Grading of Recommendations Assessment, Development and Evaluation approach.
RESULTS
Selected publications included 9 randomized controlled trials, 1 cohort study, 1 case-control study, 1 cross-sectional study, and 16 case series. Striatal region targets such as the anterior limb of the internal capsule, ventral capsule/ventral striatum, and nucleus accumbens were identified, but stereotactic coordinates were similar despite differing structural names. Only 15 of 28 articles included coordinates.
CONCLUSION
The striatal area is the most commonly targeted region for OCD-DBS. We recommend a common nomenclature based on this review. To move the field forward to individualized therapy, active contact location relative to stereotactic coordinates and patient specific anatomical and clinical variances need to be reported.
Keywords: Deep brain stimulation, Neuromodulation, Obsessive compulsive disorder, Striatum
ABBREVIATIONS
- ALIC
anterior limb of the internal capsule
- BDI
beck depression inventory
- BNST
bed nucleus of stria terminalis
- DBS
Deep brain stimulation
- fMRI
functional magnetic resonance imaging
- GAF
Global Assessment of Functioning Scale
- GPi
globus pallidus internus
- GRADE
Grading of Recommendations Assessment, Development and Evaluation
- HARS
Hamilton Anxiety Rating Scale
- HDRS
Hamilton Depression Rating Scale
- ITP
inferior thalamic peduncle
- MCP
midcommissural point
- NAc
nucleus accumbens
- OCD
Obsessive compulsive disorder
- PICO
patient/problem, intervention, comparison, outcome
- RCT
randomized controlled trial
- STN
subthalamic nucleus
- VC
ventral capsule
- VS
ventral striatum
- Y-BOCS
Yale-Brown Obsessive Compulsive Scale
Obsessive compulsive disorder (OCD) is a complex neuropsychiatric disease characterized by persistent and intrusive thoughts and dysfunctional, repetitive, and ritualized behaviors.1-4 OCD may be severely debilitating, inducing significant distress with impaired function and diminished quality of life. It has an estimated prevalence of 1% to 3% within the general population, and often occurs with comorbid conditions such as anxiety and depressive disorders.1-4 Conventional management includes pharmacotherapy and cognitive-behavioral therapy,3 although response to treatment may take months, and high medication dosages are often necessary. Only 40% to 60% of patients achieve significant improvement with these therapies, and 10% develop severe refractory symptoms unresponsive to multimodality treatment.2,5,6
Prior to neuromodulation, surgical intervention for treatment-refractory OCD consisted of ablative procedures such as subcaudate tractotomy,7 anterior capsulotomy,8 anterior cingulotomy,9 and limbic leucotomy.10 These procedures are hypothesized to interrupt abnormalities in the cortico-striatal-thalamic-cortical or medial and orbital frontal-basal ganglia circuitry, which are believed to underlie the pathophysiology of OCD.11 Deep brain stimulation (DBS) serves as an alternative to ablation through circuit modulation, with potential benefits of being partially reversible and adjustable, with purportedly similar efficacy. The adjustability of DBS allows for individualized treatment of a heterogenous disease.12,13
The first case of DBS for OCD was reported by Nuttin et al in 1999.14 The anterior limb of the internal capsule (ALIC) was targeted due to the authors’ previous experience with anterior capsulotomy.14-16 In 2009, DBS for OCD was granted a humanitarian exemption by the Food and Drug Administration.17 Multiple targets have since been utilized and continue to be investigated.14,18,19 The most commonly targeted structures are within the striatal region, comprised of the ALIC, ventral capsule (VC)/ventral striatum (VS), nucleus accumbens (NAc), and caudate nucleus. These structures have an important role in reward, addiction, motivation, and decision-making, with key pathways in cognitive and emotional processing. Additional targets include the subthalamic nucleus (STN), bed nucleus of stria terminalis (BNST), inferior thalamic peduncle (ITP), and globus pallidus internus (GPi).
Although evidence-based guidelines regarding the use of DBS in OCD have been established,19 there has been no consensus regarding the optimal target. Furthermore, it is becoming increasingly recognized that targets are nominally different but comprise similar regions.20 For example, the VC/VS is a region that includes the NAc, and targeting of this region is likely to involve stimulation of both anatomic structures. Previous studies and systematic reviews have emphasized defining the best target for neuromodulation. The goal of the current study is to systematically review the current level of evidence and Grading of Recommendations Assessment, Development and Evaluation (GRADE) criteria supporting DBS for OCD by target region, to evaluate the consistency of literature terminology, and establish a common nomenclature if warranted.
METHODS
Literature Search and Inclusion Criteria
This systematic review was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses for Protocols guidelines.21 PubMed was queried using the term “DBS and OCD.” The PICO (patient/problem, intervention, comparison, outcome) tool is a strategy developed to facilitate evidence-based practice literature searches.22 The PICO tool from the National Library of Medicine was utilized with the term “P(OCD)I(DBS)”. Only “P” and “I” were employed, with “C” and “O” tools excluded as to not limit the breadth of our query. Only original peer-reviewed clinical studies of DBS in humans whose results were published in the English language were considered for inclusion.
Articles were excluded if the DBS anatomical target was not specified or multiple targets were used and outcomes combined, the focus was on nonclinical outcomes, or the sample size was smaller than 3 subjects. Studies with fewer patients tended to be case series and were classified as having low-to-moderate quality of evidence. Minimum follow-up was 3 mo in order to minimize non-stimulation effects without excluding studies that may still provide valuable information, is in accordance with Schruers et al.23 The effects of DBS often improve with time; thus, early follow-up would not unduly influence the results apart from non-stimulation effects. References from previous systematic reviews and meta-analyses were evaluated for the inclusion of additional studies.
Data Extraction and Outcome Measures
Articles were evaluated and selected by title, abstract, and full-text review for data abstraction. Abstracted data included first author, year, diagnosis, study design, follow-up interval, DBS target, outcome, and main findings. Level of evidence was assigned to each article according to recommendations by the American Association of Neurological Surgeons/Congress of Neurological Surgeons joint guideline committee24 and was similar in implementation and in agreement with the results of a previous systematic review by Hamani et al.19 The levels of evidence (Levels I-III) were determined by evaluating study design, data collection and analysis, bias risk, and follow-up. The GRADE approach (Grading of Recommendations Assessment, Development and Evaluation) was also employed to assess the quality of evidence.25 The risk of bias (low/high/uncertain) was assessed using the Cochrane Handbook for Systematic Reviews of Interventions.26
Outcomes of interest included clinical measures related to OCD, depression, anxiety, daily functioning, and quality of life. The majority of studies to date utilized the Yale-Brown Obsessive Compulsive Scale (Y-BOCS).27 Responders to treatment are defined as a ≥35% reduction in Y-BOCS score from baseline, and partial responders as a reduction between 25% and 35%.1,28-30 Additional outcome measures included the Beck Depression Inventory (BDI), the Hamilton Depression Rating Scale (HDRS), the Hamilton Anxiety Rating Scale (HARS), and the Global Assessment of Functioning Scale (GAF).31
RESULTS
Study Selection
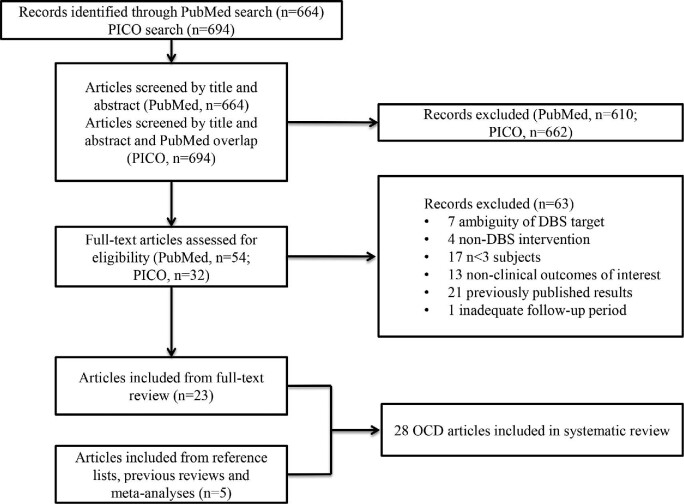
A total of 664 and 694 articles were identified through the PubMed and PICO database search, respectively, and underwent title, abstract, and full-text review (Figure). After screening by title and abstract to meet inclusion/exclusion criteria and removing duplicate publications, 86 articles underwent full-text review. A total of 63 articles were subsequently excluded. Reasons for exclusion included: ambiguity of DBS target (n = 7), non-DBS intervention (n = 4), nonclinical outcomes of interest, such as functional imaging papers that did not address clinical effects (n = 13), studies with fewer than 3 subjects (n = 17) or a follow-up period shorter than 3 mo (n = 1), and studies presenting previously published results (n = 21). Five additional articles were identified through a review of references. A total of 28 studies met inclusion criteria for this systematic review (Table 1), including 9 randomized controlled trials (RCTs), 1 cohort study, 1 case-control study, 1 cross-sectional study, and 16 case series.
FIGURE.
Systematic review.
TABLE 1.
Summary of Studies from Systematic Review
| Author | Study design (number of patients) | Follow-up (mo) | DBS target | Major findings | Level of Evidence | GRADECategory |
|---|---|---|---|---|---|---|
| Luyten 201630 | Double-blind RCT with cross-over (24) | 48-171 | BNST/ALIC | BNST/ALIC stimulation is safe and significantly decreased obsessions, compulsions, and associated anxiety and depressive symptoms, and improves global functioning in a blinded crossover trial (n = 17), after 4 yr (n = 18), and at last follow-up (up to 171 mo, n = 24). Only 17 of 24 patients participated in the randomized phase. | II | High |
| Mallet 200848 | Multicenter double-blind RCT with crossover (17) | 10 | STN | STN DBS had significantly lower Y-BOCS (19 ± 8 vs 28 ± 7; P = .01) and higher GAF (56 ± 14 vs 43 ± 8, P = .005) compared to sham. Depression and anxiety were not modified by stimulation. | I | High |
| Polosan 201949 | Double-blind RCT (10) | 5-71 | STN | Patients underwent double-blind, randomized on and off STN DBS and degrees of valence and arousal were assessed in response to images. STN stimulation increased positive ratings and decreased negative ratings. Postoperative Y-BOCS baseline scores at the time of the study were reduced by 41% ± 28% in this population when compared to preoperative scores. | III | High |
| Barcia 201834 | Double-blind RCT (7) | 3 | NAc (contacts 0-1) Caudate (contacts 2-3) | Six patients were responders, with median 50% symptomatic reduction from each patient's best contact; located at the caudate in 4 cases and NAc in 2 cases. The locus for best contact correlated with an index derived by combining fMRI responses to prevailing symptom provocation and prefronto-cortico-striatal projections defined by probabilistic tractography. | II | High |
| Tyagi 201928 | Double-blinded RCT (6) | 9 | STN or VC/VS | DBS at each site significantly and equivalently reduced OCD symptoms with little additional gain following combined stimulation. Anteromedial STN significantly improved cognitive flexibility, whereas VC/VS had a greater effect on mood. There was no further improvement following CBT, reflecting a floor effect of DBS on OCD. | III | High |
| Abelson 200546 | Double-blind RCT (4) | 7 | ALIC/NAc | The patients underwent a 12-wk double-blind testing phase consisting of 4 consecutive 3-wk blocks of randomized on-off stimulation (2 off, 2 on in a randomized order), followed by an open-ended, open-label stimulation period. One of four patients achieved significant improvement in symptoms during the double-blind phase, and an additional patient achieved significant improvement during the open phase. | III | High |
| Denys 201029 | Randomized, double-blind, cross-over study (16) | 21 | NAc | In the open phase, Y-BOCS decreased by 46%, from 33.7 ± 3.6 at baseline to 18.0 ± 11.4 after 8 mo (P = .001). 9/16 patients were responders, with Y-BOCS decrease of 23.7 ± 7.0 (72%). In the double-blind, sham-controlled phase (n = 14), Y-BOCS difference between active and sham was 8.3 ± 2.3 (25%; P = .004). Depression and anxiety decreased significantly. | II | High |
| Goodman 20101 | Randomized, double-blind, staggered-onset pilot study (6) | 12 | Ventral ALIC and VC | At 12 mo, 4 of 6 (66.7%) were found to be responders. Patients did not improve during sham. Depressive symptoms improved significantly in the group overall; global functioning improved in the 4 responders. Stimulation interruption led to rapid but reversible induction of depressive symptoms in 2 cases. | III | High |
| Tsai 201433 | Double-blind prospective observational study (4) | 15 | VC/VS | At 15 mo, there was a 33.06% decrease in OCD severity (Y-BOC score 24.3 ± 9.1, P = .001), 32.51% decrease in depression severity (HDRS score 24.5 ± 11.1, P = .005). WAIS-III was 106 at baseline and 102 at 12-mo follow-up. | II | Moderate |
| Greenberg 201041 | Multicenter case series (26) | 96 | VC/VS | Y-BOCS decreased to 20.9 ± 2.4 at 36 mo with improvement apparent by 3 mo (21.0 ± 1.8). On average, there was 12.5 ± 1.4 point decrease between baseline and treatment phases (c2 = 19.59; P < .001). Responder rate increased from 28% at 1 mo (7 of 25) to 61.5% (16 of 26) at last follow-up. Overall, 73% of patients had > 25% Y-BOCS improvement at last follow-up. | III | Moderate |
| Liebrand 201935 | Case series (12) | 12 | Ventral ALIC | Active stimulation of the ventral ALIC closer to the medial forebrain bundle than the anterior thalamic radiation was associated with better treatment outcome. | III | Moderate |
| Hartmann 201637 | Case series (6) | 24 | NAc/ALIC | Two patients were responders, and two partial responders. Modulation of the right anterior middle frontal gyrus was associated with excellent response. In contrast, non-responders showed high activation in the orbital part of the right inferior frontal gyrus. | III | Moderate |
| Lee 201917 | Case series (5) | 12 | ITP | All patients were considered responders at 1 yr (52% Y-BOCS reduction; range 39%-73%) and last follow-up (54% improvement, range 38%-85%). | III | Moderate |
| Roh 201242 | Case series (4) | 24 | Ventral ALIC and VS | All 4 patients were responders with improvement of 59.7 ± 14.6% after 24 mo. At 3 mo, depression decreased ≥ 42% from baseline. At 24 mo, HDRS scores decreased by 50%. | III | Moderate |
| Park 201945 | Case series (4) | 72 | NAc or ALIC | ALIC and NAc were both found to be safe and effective targets for DBS in refractory OCD patient, though NAc was found to be superior in this study. The 2 NAc patients achieved reductions of 93% and 69% in Y-BOCS scores, while the ALIC patients experienced reductions of 55% and 50%. | III | Moderate |
| Nair 201452 | Case series (4) | 4 | Anteromedial GPi | All 4 patients experienced dramatic benefit in their motor and vocal tics. Two patients experienced complete resolution of their OCD symptoms with the other two having > 85% reduction in their OCI scores. | III | Moderate |
| Huff 201043 | Double-blind sham-controlled crossover study (10) | 12 | Unilateral right NAc (contacts 0-1) Ventral ALIC (contacts 2-3) | Y-BOCS decreased significantly from 32.2 ± 4.0 to 25.4 ± 6.7 after 12 mo (P = .012). 5/10 patients showed at least partial response (≥25%) and one had > 35% response. Depression, GAF, and QoL improved within 1 yr. | II | Low |
| Rauch 200632 | Case-control, cross-sectional study (6) | 3 | VC/VS | All six participants had decreased Y-BOCS: 28.4 ± 16.7%. Y-BOCS improvement was not correlated with the magnitude of PET activation | III | Low |
| Greenberg 200618 | Case series (8) | 36 | VC/VS | Y-BOCS decreased from 34.6 ± 0.6 to 22.3 ± 2.1 at 36 mo. 4/8 patients were responders. Two patients had Y-BOCS decline between 25% and 35%. GAF improved from 36.6 ± 1.5 to 53.8 ± 2.5 at 36 mo. Depression, anxiety, self-care, independent living, and work, school, and social functioning also improved. | III | Low |
| Mantione 201538 | Case-control study (16 DBS; 14 controls) | 8 | NAc | In the DBS group, OCD, anxiety and depressive symptoms improved significantly during the open phase. They experienced mean decreases of 15.7 ± 10.8 points on the Y-BOCS, 10.7 ± 8.1 points on the HARS, and 9.0 ± 6.2 points on the HDRS. In the control group (medical management), OCD, anxiety and depressive symptoms remained unchanged. | III | Low |
| Huys 201939 | Case series (20) | 12 | NAc/ALIC | ALIC-NAc DBS significantly decreased OCD symptoms (33% Y-BOCS reduction, 40% full responders) and improved global functioning without loss of efficacy over 1 yr. No significant changes were found in depressive or anxiety symptoms. 35% of patients reported a sudden increase in anxiety and anhedonia after acute cessation of stimulation. | III | Low |
| Farrand 201844 | Case series (7) | 31 (range: 8-54) | NAc or BNST | All patients showed improvement on symptom severity rating scales. Three responders with other four showing responses between 7% and 20%. | III | Low |
| Jimenez 201350 | Case series (6) | 12 | ITP | ITP DBS decreased Y-BOCS (51%) and increased GAF to 68% (P = .026) at 1 yr. HDRS for the only patient with major depression went from 42 to 6. | III | Low |
| Maarouf 201651 | Case series (3) | 3.7-34.6 | MD/VA | Continuous thalamic stimulation yielded no significant improvement in OCD symptom severity. Over the course of thalamic DBS, only one patient was a partial responder. BDI scores dropped 46% in the de novo group; anxiety symptoms improved by up to 34%. Of note, one patient had follow-up less than 3 mo and was excluded from analysis. | III | Low |
| Gabriels 200336 | Case series (3) | 12 | ALIC | Two patients with Y-BOCS decrease of 12 and 23 points. Total Maladjustment Score on BPRS reduced by 44 and 59%. | III | Low |
| Guehl 200847 | Case series (3) | 12 | Caudate Nucleus | Electrophysiological unit recordings were performed followed by implantation of a chronic DBS electrode in the caudate nucleus of 3 patients. DBS of the caudate nucleus produced a 35% to 60% reduction in Y-BOCS score. The findings of this study suggest that caudate hyperactivity contributes to the manifestation of obsessions. | III | Low |
| Baldermann 201940 | Case series (22) | 12 | NAc/ALIC | After 12 mo, Y-BOCS decreased significantly by 30.4 ± 20.1%. No significant correlation between age at surgery or preoperative baseline symptom severity with clinical outcome. Models of optimal connectivity successfully cross-predicted clinical outcomes. Degree of connectivity between stimulation sites and medial and lateral prefrontal cortices significantly predicted clinical improvement. | III | Very Low |
| Sturm 200312 | Case series (4) | 24-30 | NAc | Significant improvement in OCD and anxiety symptoms in 3 of 4 | III | Very Low |
Randomized controlled trial (RCT); Yale-Brown Obsessive Compulsive Scale (Y-BOCS); Responders (≥35% Y-BOCS reduction); Partial responders (25-35% Y-BOCS reduction); Brief Psychiatric Rating Scale (BPRS); Profile of Mood States (POMS); Hamilton Depression Rating Scale (HDRS); Hamilton Anxiety Rating Scale (HARS); Global Assessment of Functioning (GAF) Scale; Beck Depression Inventory (BDI); State and Trait Anxiety Inventory (STAI); Modular System of Quality of Life (MSLQ); quality of life (QoL); Clinical Global Impression (CGI); Short Form Health Survey (SF-36); Montgomery-Asberg Depression Rating Scale (MADRS); Yale Global Tic Severity Scale (YGTSS); Obsessive Compulsive Inventory (OCI); Wechsler Adult Intelligence Scale-Third Edition (WAIS-III); Montreal Cognitive Assessment (MoCA); Oxford Happiness Questionnaire (OHQ); Warwick-Edinburgh Mental Well-Being Scale (WEMWBS); Sheehan Disability Scale (SDS); HDRS; HAMS; Brown Assessment of Beliefs Scale (BABS).
Target Coordinates
Studies were examined for target coordinates. Of the 28 articles included in this systematic review, 15 included a description of coordinates. The remaining articles defined the anatomical target without a reference to coordinates, or referred to previous publications. Those articles with explicitly disclosed coordinates are presented in Table 2.
TABLE 2.
DBS Targets and Coordinates
| Author | DBS target | Coordinates | |||
|---|---|---|---|---|---|
| Sturm (2003)12* | NAc | 6.5l, 2.5a to AC, 4.5i to MCP | |||
| Rauch (2006)32* | VC/VS | 7.97 ± 1.24l, 3.31 ± 1.04a to AC, and 4.12 ± 1.85i to MCP | |||
| Greenberg* (2006)18* | VC/VS | 6.5l, 2.5a to AC, 4.5i to MCP | |||
| Mallet (2008)48 | STN | 11l, 2a to AC, 4i to MCP | |||
| Guehl (2008)47 | Caudate | 8l, 33.4a to PC, 2.5i | |||
| Huff (2010)43 | NAc/vALIC | Relative to AC in patients with ≥ 25% improvement in Y-BOCS | |||
| Contact | x Mean(SEM) | y Mean(SEM) | z Mean(SEM) | ||
| 0 | 7.1(0.30) | -0.98(0.60) | -3.88(0.52) | ||
| 1 | 8.64(0.30) | 0.48(0.71) | -1.72(0.43) | ||
| 2 | 10.32(0.32) | 1.98(0.84) | 0.30(0.43) | ||
| 3 | 11.76(0.38) | 3.36(1.01) | 2.36(0.23) | ||
| Distal contacts (0 and 1) located in NAc, proximal contacts (2 and 3) located in vALIC | |||||
| Goodman (2010)1 | vALIC/VC | Four responders relative to MCP | |||
| x Mean(SEM) | y Mean(SEM) | z Mean(SEM) | |||
| Right | 8.45(1.25) | 15.58(1.22) | -0.58(1.33) | ||
| Left | 10.03(1.30) | 15.63(1.11) | -0.48(2.08) | ||
| Denys (2010)29* | NAc | 7l, 3a to AC, 4i to MCP | |||
| Roh (2012)42 | ALIC/VS | 8l, 4a to AC, 2i to MCP. The proximal contacts (2, 3) at VC and IC | |||
| Jimenez (2013)50 | ITP | 2-3l to fornix, 3-5p to AC; no depth given | |||
| Tsai (2014)33* | VC/VS | Contact (MCP) | x Mean(SEM) | y Mean(SEM) | z Mean(SEM) |
| 0 | 7.4(0.41) | 15.53(1.03) | -3.63(0.74) | ||
| 1 | 8.50(0.56) | 16.70(1.03) | -0.80(0.75) | ||
| 2 | 10.30(0.58) | 17.58(0.94) | 1.80(0.92) | ||
| 3 | 12.13(.58) | 18.55(0.84) | 4.73(0.91) | ||
| Barcia (2019)34* | NAc/caudate | 6.5l, 2.5a, 4.5i to AC at NAc close to BNST | |||
| Liebrand (2019)35* | vALIC | 7l, 2a to AC, 4i to MCP | |||
| Lee (2019)17 | ITP | 6.5l, 3p to AC, 0.5i to MCP | |||
| Polosan (2019)49 | STN | 10l, 1a to MCP, 4i to AC | |||
All targets reported in mm. Lateral (l); anterior (a); inferior (i); posterior (p); midcommissural point (MCP); anterior commissure (AC). Coordinates for lead tip (distal contact) except when specified. * indicates similar targeting.
Assessment of Risk of Bias for the Included Studies
Details from the assessment of risk of bias are presented in Table 3. The majority of studies incorporated nonrandomized, unblinded designs that introduced potential for selection, allocation, and/or measurement bias. Of the RCTs, one study was single-blinded and seven did not report methodology describing random sequence generation for treatment allocation. Additionally, lack of washout periods in between crossover arms was present, which may lead to carryover effects (persistence of clinical benefits following stimulation).
TABLE 3.
Assessment of Risk of Bias
| Year | Authors | Random sequence generation | Allocation concealment | Blinding | Incomplete outcome data | Selective reporting | Other bias |
|---|---|---|---|---|---|---|---|
| 2003 | Gabriels36 | High | High | High | Low | Low | High |
| 2003 | Sturm12 | High | High | High | High | High | High |
| 2005 | Abelson46 | Low | Low | Low | Low | Low | High |
| 2006 | Rauch32 | High | High | High | Low | Low | Low |
| 2006 | Greenberg18 | High | High | High | Low | Low | Low |
| 2008 | Guehl47 | High | High | High | Low | Low | High |
| 2008 | Mallet48 | Low | Low | Low | Low | Low | Low |
| 2010 | Huff43 | High | Low | Low | Low | Low | Uncertain |
| 2010 | Greenberg41 | High | High | High | Low | Low | High |
| 2010 | Goodman1 | Uncertain | Low | Low | Low | Low | Low |
| 2010 | Denys29 | Low | Low | Low | Low | Low | High |
| 2012 | Roh42 | High | High | High | Low | Low | Low |
| 2013 | Jimenez50 | High | High | High | Low | High | Low |
| 2014 | Nair52 | High | High | High | Low | Low | High |
| 2014 | Tsai33 | High | High | Low | Low | Low | Low |
| 2015 | Mantione38 | High | High | High | Low | Low | Low |
| 2016 | Hartmann37 | High | High | High | Low | Low | Low |
| 2016 | Maarouf51 | High | High | High | Low | Low | High |
| 2016 | Luyten30 | Low | Low | Low | Low | Low | Low |
| 2018 | Farrand44 | High | High | High | Low | Low | High |
| 2018 | Barcia34 | Uncertain | Low | Low | Low | Low | Low |
| 2019 | Tyagi28 | Low | Low | Low | Low | Low | Low |
| 2019 | Huys39 | High | High | High | Low | Low | Low |
| 2019 | Baldermann40 | High | High | High | Low | Low | Low |
| 2019 | Liebrand35 | High | High | High | Low | Low | Uncertain |
| 2019 | Lee17 | High | High | High | Low | Low | Low |
| 2019 | Park45 | High | High | High | Low | Low | High |
| 2019 | Polosan49 | Low | Low | Low | Low | Uncertain | Uncertain |
Assessment of Reported Targets
Table 1 outlines a summary of the results from the systematic review. The following section highlights those studies in which targets nuances were discussed.
Ventral Capsule/Ventral Striatum
Target nomenclature was inconsistent, and the VC/VS overlaps anatomically with the ALIC and NAc. We found that similar targets were identified as varying structures (Table 2).12,18,29,32-35 The studies for which coordinates were unavailable were categorized according to the author's classification of the site.36-40 One Level II and six Level III publications addressed this region.
Greenberg et al reported the 3-yr outcomes of eight patients with bilateral VC/VS DBS, with overall improvement at 36-mo. A multicenter retrospective cohort study by the same group evaluated 26 patients with VC/VS DBS (aforementioned eight patients included).18 More posteriorly positioned leads were associated with improved outcomes and lower stimulation amplitude requirements.41
Rauch et al32 evaluated six patients with bilateral VC/VS DBS, and Liebrand et al35 performed bilateral ventral ALIC DBS in 12 patients, in addition to tractography analysis. Despite classification as separate targets, the coordinates were similar between these studies, and overall improvement was seen. In the latter study, patients with active stimulation closer to the medial forebrain bundle were more responsive.35
Two studies targeted VC/VS but utilized different coordinates.1,42 Goodman et al1 evaluated 6 patients with bilateral VC/VS DBS. The mean location of the active contact in responders was reported as 8.45 to 10.03 mm lateral, 15.58 to 15.63 mm anterior, and 0.48 to 0.58 mm inferior relative to midcommissural point (MCP). Four subjects (66.7%) were considered responders after 1 yr, with improved depressive symptoms in all patients. Roh et al42 evaluated 2-yr outcomes in 4 patients. These authors also reported different VC/VS coordinates, 8 mm lateral and 4 mm anterior to AC, and 2 mm inferior to MCP. All 4 patients (100%) were responders, in addition to a significant improvement in depressive symptoms.
Nucleus Accumbens
The NAc is the most ventral extension of the striatum and is located directly below the ALIC. Target nomenclature often overlaps with the VC/VS (6.5-7 mm lateral, 2.5-3 mm anterior, and 4-4.5 mm inferior to AC), with the active contact located at the bottom of the target.12,29,34 One study reported a different y-coordinate.43 The studies for which coordinates were unavailable were categorized according to the author's classification of the site.37-40,44-46 Three Level II and eight Level III studies addressed this region.
Barcia et al34 implanted electrodes in 7 patents along a striatal axis, with 2 contacts positioned in the NAc and caudate head each. Following randomized stimulation, each patient's “best contact” was determined and found to correlate with a site predicted by a predetermined index derived from functional magnetic resonance imaging (fMRI) and probabilistic tractography. Of 6 responders (86%), the “best contact” was located in the caudate in 4 patients and the NAc in 2 patients.
Denys et al29 reported on 16 patients with bilateral NAc DBS, and coordinates were similar to those above reported for VC/VS. Nine of the sixteen patients (56%) were responders during the open phase of stimulation.
Huff et al43 performed unilateral right DBS in ten patients, with contacts in both the NAc (distal 2 contacts) and ventral ALIC (proximal 2 contacts). Five patients (50%) demonstrated partial response, with only one being a responder (10%). Park et al45 followed 4 patients, 2 with DBS leads implanted in the ALIC and NAc each; NAc was found to be the more effective target. In Abelson et al,46 4 patients were implanted with electrodes in the ALIC near its junction with the NAc. One patient achieved significant improvement during the double-blind phase and another patient during the open phase.
Farrand et al44 implanted 7 patients, 3 positioned in the NAc bilaterally, 3 in the BNST bilaterally, and 1 with unilateral stimulation of each target. One patient from each target group and the patient stimulated at both targets were responders (43%). Sturm et al12 reported significant improvement in OCD and anxiety with unilateral right NAc stimulation in 3 of 4 patients (75%), suggesting that bilateral stimulation may not be necessary.
Bed Nucleus of Stria Terminalis
The BNST lies several millimeters behind the NAc, posterior to the AC. Two studies targeted the BNST without explicit definition of the coordinates chosen. Luyten et al30 described the y-coordinate as 0 to 2 mm posterior to the posterior border of the AC; however, other coordinates were not defined. Farrand et al44 demonstrated this posterior placement radiographically only. Greenberg et al41 reported improved outcomes with more posterior lead placement in their VC/VS series, with the distal contact as traversing the BNST, within 1 to 2 mm of the posterior border of AC; however, the results are reported summatively as targeting the VC/VS. One Level II study addressed the role of BNST as a target in DBS for OCD.30 A Level III article that involved both the NAc and BNST is discussed above.44
Luyten et al30 reported on 24 patients who underwent bilateral DBS targeting the ALIC (n = 5), BNST (n = 5), and the remainder with variable placement in the BNST and IC/ALIC. When divided by area of primary stimulation, only 25% of ALIC patients demonstrated significant improvement, whereas 80% of BNST stimulated subjects achieved a sufficient response.
Caudate Nucleus
Only one Level III study that met inclusion criteria targeted the caudate nucleus specifically. Guehl et al47 defined this target as 8 mm lateral and 2.5 mm inferior to the AC-PC line, and 33.4 mm anterior to PC. These authors reported a 35% to 60% reduction in Y-BOCS score in all patients (n = 3) at 1-yr follow-up.
Of note, Barcia et al34 chose a trajectory that spanned both the caudate and NAc (discussed above), and coordinates were described for the NAc. This study found the most efficacious contact to be located in the caudate for 4 of 6 responders (67%). Furthermore, the NAc target was chosen so that the most distal contact would be close to the BNST.
Subthalamic Nucleus
The proposed OCD target is 1 mm medial and 2 mm anterior to the standard STN target.48 The literature is limited to 1 Level I and 2 Level III studies.
The Level I study by Mallet et al48 consisted of a double-blind, multicenter crossover trial evaluating eight patients with STN DBS. Six of the eight (75%) patients demonstrated improvement in OCD symptoms during active vs sham stimulation, although without a significant difference in mood.
A level III study by Polosan et al49 reported on 10 patients with bilateral STN DBS. Emotional valence and arousal were assessed in response to emotional images, and STN stimulation was associated with increased positive and decreased negative valuations. Tyagi et al28 compared VC/VS and anteromedial STN stimulation. Both targets significantly and equivalently alleviated OCD symptoms, with minimal gain from combined stimulation. Notably, STN affected cognitive flexibility to a greater degree, whereas VC/VS stimulation was more effective in mood enhancement. These findings support the hypothesis that different neural circuits are involved in the pathophysiology underlying OCD.
Inferior Thalamic Peduncle
The ITP is a white matter tract that interconnects the orbitofrontal cortex and ascending reticular activating system with the thalamus, which has been implicated in both OCD and major depression.50 Two studies targeted the ITP, with similar coordinates reported. Jimenez et al50 described the target as 2 to 3 mm lateral to the fornix (approximately 6 mm lateral to the midsagittal plane) and 3 to 5 mm posterior to AC; although a depth was not explicitly stated, the authors described 2 adjacent contacts needing to be placed immediately above and below the AC-PC line. Similarly, Lee et al17 reported their coordinates as 6.5 mm lateral to the midsagittal plane, 3 mm posterior to AC, and 0.5 mm below the AC-PC line. Both of these studies were Level III.
In the studies reported by Jimenez et al50 and Lee et al,17 all patients were classified as responders at 12-mo follow-up. Lee et al17 identified significant depressive improvements at 24 but not 12 mo. Maarouf et al51 performed DBS of the medial dorsal and ventral anterior nuclei of the thalamus in 4 patients, with neither target resulting in adequate symptom relief.
Globus Pallidus Internus
The anteromedial GPi has been identified as a target in the treatment of Tourette syndrome and has been proposed as a target for OCD.52 The target is at the anteroposterior level of the columns of the fornix, which is more dorsal than that for dystonia, approximately 6 mm anterior and 6 mm medial to the traditional motor target.52
The single Level III study targeting GPi evaluated 4 patients with Tourette syndrome and comorbid moderate-to-severe OCD. All patients experienced improvement in motor and vocal tics, with 2 patients achieving complete symptom resolution.52
DISCUSSION
DBS is being increasingly used in the treatment of intractable OCD with overall good results; however, some patients remain refractory. Most studies consist of small sample sizes (n < 22) with heterogeneous presentations and variable follow-up periods (0.5-171 mo). The most commonly targeted regions include the ALIC, NAc, and VC/VS. Follow-up ranged from 3 mo to 14 yr; however, just under half of studies had follow-up of 12 mo or less, and some studies were excluded due to follow-up of 1 mo or less.53 We suggest that follow-up should be 3 mo at minimum, and ideally 1 yr for optimal reporting. Parameters used to measure therapeutic efficacy varied with the exception of Y-BOCS scores, which were used in all but 3 articles; division into responders (≥35% improvement) and partial responders (25%-34% improvement) improves our understanding of treatment response. We also recommend the reporting of depression (BDI), anxiety (HARS), and quality of life (GAF) metrics.
DBS Efficacy of Common Targets
This review demonstrates the efficacy of common targets, with improved outcomes in patients previously considered to be refractory to treatment. Responder rates with ALIC, NAc, and VC/VS were high. Interestingly, responder rates were also high in those VC/VS studies using different coordinates,1,42 highlighting that efficacy was obtained on a patient-specific basis. Five studies had a follow-up of 36 mo or greater.18,30,41,45,49 Clinical improvement was typically apparent by 3 mo, although responses improved with time.18,45 Studies that followed patients for less than 12 mo were mostly high-quality, double-blind RCTs.34,38,46,48,52
Compared to the striatal region, there are considerably fewer data on other targets. STN DBS has responder rates of 40% to 75%28,48,54; however, its failure to demonstrate improvement in anxiety and depressive symptoms suggests that patients with more severe comorbidities may benefit from a different target.28,54 ITP demonstrated encouraging results, requiring further investigation.17,50 Good results were achieved with GPi; however, studies performed in patients with a primary diagnosis of OCD are necessary.52 Several nascent targets, including the dorsal anterior cingulate cortex and supero-lateral branch of the medial forebrain bundle, have shown promising results in case studies and merit further investigation.13,55
Interestingly, we observed considerable variation in the reporting of evidence. Specifically, there was poor agreement between assessment of the level and quality of evidence. This may be due in part to the heterogeneity introduced by variations in diagnosis, target, and clinical assessment; as such, proposing strong and generalizable conclusions is impractical.
Development of a Common Nomenclature
The description of targets can be unclear, with inconsistent or ambiguous labeling between studies or even within the same study. For example, the multicenter study reported by Greenberg et al41 described their collective experience targeting the VC/VS. However, the authors described improved results with a more posterior target, often with the distal contact in the NAc or BNST. They, of course, were the first group to venture into this target, and the nuances were unlikely to have been appreciated at that time. The VC/VS is technically a region and not a single structure, and the NAc is its most ventral extension. We observed significant overlap between the reporting of coordinates for VC/VS and NAc, despite classification of these as separate targets. A typical DBS electrode will traverse multiple structures.20 Indeed, some studies have described multiple targets along the same axis, such as both ALIC and NAc,37,39,40,43,45,46 ALIC and BNST,30 or caudate and NAc.34
Even within a defined target structure, the optimal location for stimulation remains variable.35,41 The inconsistent nomenclature of this region may indicate that one striatal target is superior to the others; however, its effects have been diluted by ambiguous classification. As such, it is necessary to standardize the reporting of results in the OCD literature. We propose labeling of VC/VS, NAc, ALIC, and BNST as “strial region” targets, distinct from STN, pallidal, and ITP. Clear reporting of the target location and active contacts relative to stereotactic coordinates is essential. This specific nomenclature is required, particularly in the striatum, where multiple targets can be traversed with a single electrode and different targets stimulated, depending on the active contact.
Improving DBS Outcomes
The variability to response can also be attributed to patient- or disease-specific factors.56 OCD is a markedly heterogeneous disease that can differ substantially in presentations of “obsessions” and “compulsions,”57 including variations in associated comorbidities, level of functioning, and response to medications and therapy. OCD often occurs in conjunction with other comorbid conditions, such as a 67% lifetime prevalence of major depression.58 As such, the variations in treatment response to DBS may not reflect an inconsistent response to therapy based on a target, but instead an inconsistent response due to the phenotypic diversity within the psychiatric diagnosis. Identification of core OCD symptoms and their associations with other symptoms may aid in characterizing disease heterogeneity, which can further inform treatment models.59 For example, negative appraisals of intrusive thoughts have been identified as a central symptom, which strongly predicts comorbid anxiety and depression.59 Different pathways may underlie OCD and its comorbidities, and target selection may need to consider the severity of these ailments. The heterogeneity of this disease may require more precise patient-specific targeting using advanced imaging techniques.34
It is becoming increasingly recognized that targets are only nominally different, and due to their anatomical overlap, multiple targets are likely stimulated based on the volume of tissue activated. Conversely, an optimal target may be stimulated by multiple electrode trajectories. As such, the previous research emphasis on identifying the best target is likely not possible. In order to advance the field, it is necessary to better understand the functional subanatomy of the striatal region by evaluating different studies and identifying commonalities, as opposed to focusing on single targets. It may be necessary to determine optimal electrode placement based on patient-specific anatomy or factors, which may be determined by advanced imaging techniques such as structural connectivity. The clinical effects of DBS are at least in part due to the activation of adjacent white matter fiber tracts, which can be visualized using diffusion-weighted imaging and tractography.60 The use of individualized tractography has been described in the use of DBS for depression; high responder rates were seen when contacts for chronic stimulation were selected by matching preoperative deterministic with postoperative probabilistic tractography.61 This “connectomic approach” may hold promise for future innovations in DBS for OCD.
Predictors of outcome may prove valuable in guiding DBS implantation and optimizing stimulation parameters. Transient hypomania with initiation of DBS, as well as both intraoperative and postoperative stimulation-induced smile and laughter has been shown to predict long-term response to DBS.33,62 Furthermore, surgery in cases of moderate OCD, particularly with significant comorbidities, may be beneficial for patients, as even moderate symptoms may considerably affect functionality and quality of life.
Limitations
Although the benefits of DBS include its partial reversibility and adjustable nature, it is important to recognize that there are specific risks with therapy. DBS surgery requires the chronic implantation of hardware, including the need for future generator replacements. Complications have been reported in up to 20% of cases.63,64 Furthermore, novel indications may be associated with unique or unknown risks,65 and require specialized centers that have expertise in implantation and programming. As such, surgery requires a detailed understanding of potential risks and a close therapeutic relationship.
CONCLUSION
It has been a decade since DBS for OCD received approval for a humanitarian device exemption. Despite numerous studied targets, the response to treatment is variable and likely attributable to phenotypic diversity within the psychiatric diagnosis. Given the heterogeneity in diagnosis and treatment, it is not possible to conclusively propose an ideal target that would benefit each individual patient. As such, future work should focus on individualizing therapy with respect to patient- or disease-specific factors. Patient-specific anatomy may be relevant in certain OCD subtypes, and certain striatal regions may be more effective targets; however, labeling remains inconsistent, and we suggest using a common nomenclature for striatal stimulation with the reporting of active contacts relative to stereotactic coordinates.
Disclosures
The authors have no personal, financial, or institutional interest in any of the drugs, materials, or devices described in this article. Dr Pilitsis is a consultant for Boston Scientific, Nevro, TerSera, and Abbott and receives grant support from Medtronic, Boston Scientific, Abbott, Nevro, TerSera, SBIR 1R43NS107076-01A1, NIH 2R01CA166379-06, and NIH U44NS115111. She is a medical advisor for AIM Medical Robotics and Karuna and has stock equity.
COMMENT
The authors provide a comprehensive and systematic review of trials of deep brain stimulation for obsessive compulsive disorder with a focus on assessing the relative value of different reported targets. Importantly, this work highlights a suboptimally reported literature, with different terminologies used for what are likely similar targets. The authors should be applauded for highlighting the discrepancies in the literature and offering this concise review that highlights the need to more precisely understand the anatomy, localization, and interconnections of the variously named targets in order to advance the field. Nomenclature and segmentation is ultimately a human simplification of the complex anatomy of the human brain. As the authors suggest and demonstrate, we must move past advocating for specific targets and move towards a more integrated understanding by using common nomenclatures, common reporting, and standardized outcomes. Given the current emphasis on understanding neurological and psychiatric diseases and deep brain stimulation therapy at the brain network level, it may be increasingly important to also standardize the imaging to maximize our learning from the collective experience in order to move the field forward. Collaboration and common understanding will significantly accelerate the impact that neurosurgery can have on expanding indications, especially for psychiatric diagnoses.
Nader Pouratian
Los Angeles, California
REFERENCES
- 1. Goodman WK, Foote KD, Greenberg BD et al. Deep brain stimulation for intractable obsessive compulsive disorder: pilot study using a blinded, staggered-onset design. Biol Psychiatry. 2010;67(6):535-542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Liu Q, Tan B, Zhou J, Zheng Z, Li L, Yang Y. Pathophysiology of refractory obsessive-compulsive disorder. Medicine (Baltimore). 2017;96(1):e5655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Brakoulias V, Starcevic V, Albert U et al. Treatments used for obsessive-compulsive disorder-An international perspective. Hum Psychopharmacol Clin Exp. 2019;34(1):e2686. [DOI] [PubMed] [Google Scholar]
- 4. de Haan S, Rietveld E, Stokhof M, Denys D. Effects of deep brain stimulation on the lived experience of obsessive-compulsive disorder patients: in-depth interviews with 18 patients. PLoS One. 2015;10(8):e0135524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mulders AEP, Plantinga BR, Schruers K et al. Deep brain stimulation of the subthalamic nucleus in obsessive-compulsive disorder: neuroanatomical and pathophysiological considerations. Eur Neuropsychopharmacol. 2016;26(12):1909-1919. [DOI] [PubMed] [Google Scholar]
- 6. Hirschtritt ME, Bloch MH, Mathews CA. Obsessive-Compulsive disorder. JAMA. 2017;317(13):1358-1367. [DOI] [PubMed] [Google Scholar]
- 7. Knight G. Stereotactic tractotomy in the surgical treatment of mental illness. J Neurol Neurosurg Psychiatry. 1965;28 (4):304-310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Laitinen LV. Psychosurgery. Stereotact Funct Neurosurg. 2001;76(3-4):239-242. [DOI] [PubMed] [Google Scholar]
- 9. Whitty CW, Duffield JE, Tov PM, Cairns H. Anterior cingulectomy in the treatment of mental disease. Lancet North Am Ed. 1952;1(6706):475-481. [DOI] [PubMed] [Google Scholar]
- 10. Kelly D, Richardson A, Mitchell-Heggs N. Stereotactic limbic leucotomy: neurophysiological aspects and operative technique. Br J Psychiatry. 1973;123(573):133-140. [DOI] [PubMed] [Google Scholar]
- 11. Dougherty DD, Chou T, Corse AK et al. Acute deep brain stimulation changes in regional cerebral blood flow in obsessive-compulsive disorder. J Neurosurg. 2016;125(5):1087-1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sturm V, Lenartz D, Koulousakis A et al. The nucleus accumbens: a target for deep brain stimulation in obsessive–compulsive- and anxiety-disorders. J Chem Neuroanat. 2003;26(4):293-299. [DOI] [PubMed] [Google Scholar]
- 13. Coenen VA, Schlaepfer TE, Goll P et al. The medial forebrain bundle as a target for deep brain stimulation for obsessive-compulsive disorder. CNS Spectr. 2017;22(3):282-289. [DOI] [PubMed] [Google Scholar]
- 14. Nuttin B, Cosyns P, Demeulemeester H, Gybels J, Meyerson B. Electrical stimulation in anterior limbs of internal capsules in patients with obsessive-compulsive disorder. Lancet North Am Ed. 1999;354(9189):1526. [DOI] [PubMed] [Google Scholar]
- 15. Nuttin BJ, Gabriels LA, Cosyns PR et al. Long-term electrical capsular stimulation in patients with obsessive-compulsive disorder. Neurosurgery. 2003;52(6):1263-1274; discussion 1272-1264. [DOI] [PubMed] [Google Scholar]
- 16. Kohl S, Baldermann JC. Progress and challenges in deep brain stimulation for obsessive-compulsive disorder. Pharmacol Ther. 2018;186:168-175. [DOI] [PubMed] [Google Scholar]
- 17. Lee DJ, Dallapiazza RF, De Vloo P et al. Inferior thalamic peduncle deep brain stimulation for treatment-refractory obsessive-compulsive disorder: a phase 1 pilot trial. Brain Stimul. 2019;12(2):344-352. [DOI] [PubMed] [Google Scholar]
- 18. Greenberg BD, Malone DA, Friehs GM et al. Three-Year outcomes in deep brain stimulation for highly resistant obsessive–compulsive disorder. Neuropsychopharmacol. 2006;31(11):2384-2393. [DOI] [PubMed] [Google Scholar]
- 19. Hamani C, Pilitsis J, Rughani AI et al. Deep brain stimulation for obsessive-compulsive disorder: systematic review and evidence-based guideline sponsored by the american society for stereotactic and functional neurosurgery and the congress of neurological surgeons (CNS) and endorsed by the CNS and american association of neurological surgeons. Neurosurgery. 2014;75(4):327-333. [DOI] [PubMed] [Google Scholar]
- 20. Park YS, Sammartino F, Young NA, Corrigan J, Krishna V, Rezai AR. Anatomic review of the ventral capsule/ventral striatum and the nucleus accumbens to guide target selection for deep brain stimulation for obsessive-compulsive disorder. World Neurosurg. 2019;126:1-10. [DOI] [PubMed] [Google Scholar]
- 21. Liberati A, Altman DG, Tetzlaff J et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Brown D. A review of the pubmed PICO tool: using evidence-based practice in health education. Health Promot Pract. published online: 2019. (doi:10.1177/1524839919893361). [DOI] [PubMed] [Google Scholar]
- 23. Schruers K, Baldi S, van den Heuvel T et al. The effects of deep-brain non-stimulation in severe obsessive-compulsive disorder: an individual patient data meta-analysis. Transl Psychiatry. 2019;9(1):183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Robinson PD, Kalkanis SN, Linskey ME, Santaguida PL. Methodology used to develop the AANS/CNS management of brain metastases evidence-based clinical practice parameter guidelines. J Neurooncol. 2010;96(1):11-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Balshem H, Helfand M, Schunemann HJ et al. GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol. 2011;64(4):401-406. [DOI] [PubMed] [Google Scholar]
- 26. Higgins JP, Altman DG, Gotzsche PC et al. The cochrane collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Goodman WK, Price LH, Rasmussen SA et al. The yale-brown obsessive compulsive scale. Arch Gen Psychiatry. 1989;46(11):1006-1011. [DOI] [PubMed] [Google Scholar]
- 28. Tyagi H, Apergis-Schoute AM, Akram H et al. A randomized trial directly comparing ventral capsule and anteromedial subthalamic nucleus stimulation in obsessive-compulsive disorder: clinical and imaging evidence for dissociable effects. Biol Psychiatry. 2019;85(9):726-734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Denys D, Mantione M, Figee M et al. Deep brain stimulation of the nucleus accumbens for treatment-refractory obsessive-compulsive disorder. Arch Gen Psychiatry. 2010;67(10):726-734. [DOI] [PubMed] [Google Scholar]
- 30. Luyten L, Hendrickx S, Raymaekers S, Gabriels L, Nuttin B. Electrical stimulation in the bed nucleus of the stria terminalis alleviates severe obsessive-compulsive disorder. Mol Psychiatry. 2016;21(9):1272-1280. [DOI] [PubMed] [Google Scholar]
- 31. Figee M, Luigjes J, Smolders R et al. Deep brain stimulation restores frontostriatal network activity in obsessive-compulsive disorder. Nat Neurosci. 2013;16(4):386-387. [DOI] [PubMed] [Google Scholar]
- 32. Rauch SL, Dougherty DD, Malone D et al. A functional neuroimaging investigation of deep brain stimulation in patients with obsessive–compulsive disorder. J Neurosurg. 2006;104(4):558-565. [DOI] [PubMed] [Google Scholar]
- 33. Tsai HC, Chang CH, Pan JI et al. Acute stimulation effect of the ventral capsule/ventral striatum in patients with refractory obsessive-compulsive disorder - a double-blinded trial. Neuropsychiatr Dis Treat. 2014;10:63-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Barcia JA, Avecillas-Chasin JM, Nombela C et al. Personalized striatal targets for deep brain stimulation in obsessive-compulsive disorder. Brain Stimul. 2019;12(3):724-734. [DOI] [PubMed] [Google Scholar]
- 35. Liebrand LC, Caan MWA, Schuurman PR et al. Individual white matter bundle trajectories are associated with deep brain stimulation response in obsessive-compulsive disorder. Brain Stimul. 2019;12(2):353-360. [DOI] [PubMed] [Google Scholar]
- 36. Gabriels L, Cosyns P, Nuttin B, Demeulemeester H, Gybels J. Deep brain stimulation for treatment-refractory obsessive-compulsive disorder: psychopathological and neuropsychological outcome in three cases. Acta Psychiatr Scand. 2003;107(4):275-282. [PubMed] [Google Scholar]
- 37. Hartmann CJ, Lujan JL, Chaturvedi A et al. Tractography activation patterns in dorsolateral prefrontal cortex suggest better clinical responses in OCD DBS. Front Neurosci. 2015;9:519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Mantione M, Nieman D, Figee M, van den Munckhof P, Schuurman R, Denys D. Cognitive effects of deep brain stimulation in patients with obsessive-compulsive disorder. J Psychiatry Neurosci. 2015;40(6):378-386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Huys D, Kohl S, Baldermann JC et al. Open-label trial of anterior limb of internal capsule–nucleus accumbens deep brain stimulation for obsessive-compulsive disorder: insights gained. J Neurol Neurosurg Psychiatry. 2019;90(7):805-812. [DOI] [PubMed] [Google Scholar]
- 40. Baldermann JC, Melzer C, Zapf A et al. Connectivity profile predictive of effective deep brain stimulation in obsessive-compulsive disorder. Biol Psychiatry. 2019;85(9):735-743. [DOI] [PubMed] [Google Scholar]
- 41. Greenberg BD, Gabriels LA, Malone DA Jr et al. Deep brain stimulation of the ventral internal capsule/ventral striatum for obsessive-compulsive disorder: worldwide experience. Mol Psychiatry. 2010;15(1):64-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Roh D, Chang WS, Chang JW, Kim CH. Long-term follow-up of deep brain stimulation for refractory obsessive-compulsive disorder. Psychiatry Res. 2012;200(2-3):1067-1070. [DOI] [PubMed] [Google Scholar]
- 43. Huff W, Lenartz D, Schormann M et al. Unilateral deep brain stimulation of the nucleus accumbens in patients with treatment-resistant obsessive-compulsive disorder: outcomes after one year. Clin Neurol Neurosurg. 2010;112(2):137-143. [DOI] [PubMed] [Google Scholar]
- 44. Farrand S, Evans AH, Mangelsdorf S et al. Deep brain stimulation for severe treatment-resistant obsessive-compulsive disorder: an open-label case series. Aust N Z J Psychiatry. 2018;52(7):699-708. [DOI] [PubMed] [Google Scholar]
- 45. Park HR, Kim IH, Kang H et al. Electrophysiological and imaging evidence of sustained inhibition in limbic and frontal networks following deep brain stimulation for treatment refractory obsessive compulsive disorder. PLoS One. 2019;14(7):e0219578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Abelson JL, Curtis GC, Sagher O et al. Deep brain stimulation for refractory obsessive-compulsive disorder. Biol Psychiatry. 2005;57(5):510-516. [DOI] [PubMed] [Google Scholar]
- 47. Guehl D, Benazzouz A, Aouizerate B et al. Neuronal correlates of obsessions in the caudate nucleus. Biol Psychiatry. 2008;63(6):557-562. [DOI] [PubMed] [Google Scholar]
- 48. Mallet L, Polosan M, Jaafari N et al. Subthalamic nucleus stimulation in severe obsessive–compulsive disorder. N Engl J Med. 2008;359(20):2121-2134. [DOI] [PubMed] [Google Scholar]
- 49. Polosan M, Droux F, Kibleur A et al. Affective modulation of the associative-limbic subthalamic nucleus: deep brain stimulation in obsessive–compulsive disorder. Transl Psychiatry. 2019;9(1):73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Jimenez F, Nicolini H, Lozano AM, Piedimonte F, Salin R, Velasco F. Electrical stimulation of the inferior thalamic peduncle in the treatment of major depression and obsessive compulsive disorders. World Neurosurg. 2013;80(3-4):S30.e17-S30.e25. [DOI] [PubMed] [Google Scholar]
- 51. Maarouf M, Neudorfer C, El Majdoub F, Lenartz D, Kuhn J, Sturm V. Deep brain stimulation of medial dorsal and ventral anterior nucleus of the thalamus in OCD: a retrospective case series. PLoS One. 2016;11(8):e0160750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Nair G, Evans A, Bear RE, Velakoulis D, Bittar RG. The anteromedial GPi as a new target for deep brain stimulation in obsessive compulsive disorder. J Clin Neurosci. 2014;21(5):815-821. [DOI] [PubMed] [Google Scholar]
- 53. Okun MS, Mann G, Foote KD et al. Deep brain stimulation in the internal capsule and nucleus accumbens region: responses observed during active and sham programming. J Neurol Neurosurg Psychiatry. 2007;78(3):310-314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Le Jeune F, Verin M, N’Diaye K et al. Decrease of prefrontal metabolism after subthalamic stimulation in obsessive-compulsive disorder: a positron emission tomography study. Biol Psychiatry. 2010;68(11):1016-1022. [DOI] [PubMed] [Google Scholar]
- 55. De Ridder D, Leong SL, Manning P, Vanneste S, Glue P. Anterior cingulate implant for obsessive-compulsive disorder. World Neurosurg. 2017;97:754.e7-754.e16. [DOI] [PubMed] [Google Scholar]
- 56. Staudt MD, Herring EZ, Gao K, Miller JP, Sweet JA. Evolution in the treatment of psychiatric disorders: from psychosurgery to psychopharmacology to neuromodulation. Front Neurosci. 2019;13:108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Mataix-Cols D, Rosario-Campos MC, Leckman JF. A multidimensional model of obsessive-compulsive disorder. Am J Psychiatry. 2005;162(2):228-238. [DOI] [PubMed] [Google Scholar]
- 58. Aouizerate B, Cuny E, Martin-Guehl C et al. Deep brain stimulation of the ventral caudate nucleus in the treatment of obsessive–compulsive disorder and major depression. J Neurosurg. 2004;101(4):682-686. [DOI] [PubMed] [Google Scholar]
- 59. Olatunji BO, Christian C, Brosof L, Tolin DF, Levinson CA. What is at the core of OCD? A network analysis of selected obsessive-compulsive symptoms and beliefs. J Affect Disord. 2019;257:45-54. [DOI] [PubMed] [Google Scholar]
- 60. Staudt MD, Ridge S, Sweet JA. Computational modeling and tractography for DBS targeting. In: Anderson WS, ed. Deep Brain Stimulation: Techniques and Practices. New York, Stuttgart: Thieme Publishers; 2019:37-42. [Google Scholar]
- 61. Riva-Posse P, Choi KS, Holtzheimer PE et al. A connectomic approach for subcallosal cingulate deep brain stimulation surgery: prospective targeting in treatment-resistant depression. Mol Psychiatry. 2018;23(4):843-849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Haq IU, Foote KD, Goodman WG et al. Smile and laughter induction and intraoperative predictors of response to deep brain stimulation for obsessive-compulsive disorder. Neuroimage. 2011;54(Suppl 1):S247-S255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Hamani C, Lozano AM. Hardware-related complications of deep brain stimulation: a review of the published literature. Stereotact Funct Neurosurg. 2006;84(5-6):248-251. [DOI] [PubMed] [Google Scholar]
- 64. Fenoy AJ, Simpson RK Jr Risks of common complications in deep brain stimulation surgery: management and avoidance. JNS. 2014;120(1):132-139. [DOI] [PubMed] [Google Scholar]
- 65. Maslen H, Cheeran B, Pugh J et al. Unexpected complications of novel deep brain stimulation treatments: ethical issues and clinical recommendations. Neuromodulation. 2018;21(2):135-143. [DOI] [PMC free article] [PubMed] [Google Scholar]