Abstract
BACKGROUND
Subthalamic nucleus (STN) and globus pallidus interna (GPi) are the most effective targets in deep brain stimulation (DBS) treatment for Parkinson disease (PD). However, the individualized selection of targets remains a clinical challenge.
OBJECTIVE
To combine unilateral STN and contralateral GPi stimulation (STN DBS in one brain hemisphere and GPi DBS in the other) to maximize the clinical advantages of each target while inducing fewer adverse side effects in selected patients with PD because each target has its own clinical effects and risk profiles.
METHODS
We reviewed the clinical outcomes of 8 patients with idiopathic PD treated with combined unilateral STN and contralateral GPi DBS. Clinical outcome assessments, focusing on motor and nonmotor symptoms, were performed at baseline and 6-mo and 12-mo follow-up. We performed the assessments under the following conditions: medication on and off (bilateral stimulation on and off and unilateral STN stimulation on).
RESULTS
Patients showed a significant improvement in motor symptoms, as assessed by the Unified Parkinson Disease Rating Scale III (UPDRS-III) and Timed Up-and-Go Test (TUG), in the off-medication/on-stimulation state at 6-mo and 12-mo follow-up. Also, patients reported a better quality of life, and their intake of levodopa was reduced at 12-mo follow-up. In the on-medication condition, bilateral stimulation was associated with an improvement in axial symptoms, with a 64% improvement in measures of gait and falls at 12-mo follow-up. No irreversible adverse side effects were observed.
CONCLUSION
Our findings suggest that combined unilateral STN and contralateral GPi DBS could offer an effective and well-tolerated DBS treatment for certain PD patients.
Keywords: Deep brain stimulation, Globus pallidus interna, Parkinson disease, Subthalamic nucleus, Symptom-tailored
Graphical Abstract
Graphical Abstract.
ABBREVIATIONS
- BMI
body mass index
- CT
computed tomography
- DBS
deep brain stimulation
- EQ-5D-5 L
EuroQol 5 Dimensions Questionnaire
- GFQ
Gait and Falls Questionnaire
- GPi
globus pallidus interna
- HADS
Hospital Anxiety and Depression Scale
- LEDD
levodopa equivalent daily dose
- MMSE
Mini-Mental State Examination
- MRI
magnetic resonance imaging
- NMS
nonmotor symptom
- NMSQ
Non-Motor Symptoms Questionnaire
- STN
subthalamic nucleus
- PD
Parkinson disease
- PDQ-8
8-item Parkinson Disease Questionnaire
- QoL
quality of life
- UPDRS-III
Unified Parkinson Disease Rating Scale III
Deep brain stimulation (DBS) is a well-established option for the treatment of patients with advanced Parkinson disease (PD) who suffer from medication-resistant motor symptoms, motor fluctuations, or levodopa-induced dyskinesias.1 Over the years, different brain structures have been targeted in an effort to improve the motor outcomes and minimize side effects. Although the best DBS target for PD remains unclear, extensive long-term research has indicated that both the globus pallidus interna (GPi) and subthalamic nucleus (STN) are effective targets for achieving and maintaining control of motor symptoms.2 DBS of each target combined with medical treatment is more effective than the best medical treatment alone. However, target selection is still a widely debated issue.
Target selection depends on patient's medical history, stage of illness, and current clinical symptoms, which vary greatly between individual patients. In particular, substantial interindividual differences exist in symptom frequency, type, severity, and laterality, as well as in response to medications and DBS.3 Moreover, some patients with PD experience or develop cognitive deficits and/or psychiatric symptoms. Given that each target has its own clinical effects and risk profiles, selection of the appropriate DBS target for a given patient is of paramount importance.
Specifically, STN stimulation is particularly effective for patients with PD who need to use fewer dopaminergic medications.4 Also, although there is no evidence indicating that STN is significantly better for tremor than GPi, STN stimulation is often the preferred option in clinical practice.2 Nonetheless, GPi stimulation may be preferred when the primary therapeutic goals are to improve medication tolerability and to reduce levodopa-induced dyskinesias.4 Additionally, GPi appears to be a better target for patients with PD who present with or are at high risk for developing cognitive deficits or psychiatric problems.4,5 Moreover, GPi might be a better target for patients who show moderate levels of dysphagia, gait impairment, falls, dysarthria, or other axial symptoms and signs.6
Accordingly, GPi and STN stimulation may complement each other in DBS treatment for PD. This means that the specific target chosen does not necessarily have to be the same across or within patients. The same applies to the decision whether to use bilateral or unilateral DBS. Bilateral DBS has more often been used than unilateral DBS in PD treatment because it seems better suited to address the progressive nature of PD.7 However, unilateral DBS can also significantly improve PD symptoms while tending to produce fewer side effects, especially in patients with marked asymmetric motor symptoms.7 Nevertheless, most patients with initial unilateral DBS are likely to become candidates for a second surgery of bilateral DBS.8,9 Conceivably, a personalized and symptom-oriented approach is critical for initial target selection and subsequent DBS treatment to optimize individual clinical outcomes and to reduce adverse side effects.
In this study, we aimed to assess the utility of combined unilateral STN and contralateral GPi DBS (ie, STN stimulation in one hemisphere and GPi stimulation in the other) in the clinical management of advanced PD. To achieve this aim, we conducted a retrospective review of clinical outcomes (at 6 and 12 mo after surgery) of 8 patients with PD treated with this combined DBS treatment. We hypothesized that the combined DBS treatment would produce clinical benefits to the patients similar to those reported in previous studies using bilateral stimulation of one target, along with inducing fewer adverse side effects.
METHODS
Medical Ethics
The study was approved by the local ethics committee and carried out in accordance with the Declaration of Helsinki. All patients provided written informed consent, including consent to academic presentation without personally identifiable information.
Patients
We retrospectively reviewed the clinical outcomes of patients with idiopathic PD who underwent combined unilateral STN and contralateral GPi DBS at our hospital between August 2017 and March 2019. Eight patients with complete preoperative and 1-yr postoperative clinical assessment data were included in the present study. Patient selection criteria for combined unilateral STN and contralateral GPi DBS included (1) highly asymmetrical parkinsonism (asymmetry index higher than 0.25) or (2) prominent cardinal motor symptoms with severe axial symptoms, without cognitive deficits or psychiatric problems. In this cohort, 6 patients presented with significant symptom asymmetry and 2 patients had prominent motor symptoms with severe axial symptoms. The main demographic and clinical characteristics of each patient are presented in Table 1. Other study inclusion and exclusion criteria were the same as used for the general DBS surgery. The clinical decision regarding the specific DBS target used for each patient was made by an experienced multidisciplinary DBS team. Specifically, unilateral STN DBS was applied in an effort to treat the more severe side in 6 patients (patients 1 to 6) with significant symptom asymmetry. For the other 2 patients (patients 7 and 8), we applied unilateral STN DBS to the left hemisphere because there is evidence indicating that a left-hemispheric dominance exists for appendicular movements and a right-hemispheric dominance for axial motor control.3,10
TABLE 1.
Demographic and Clinical Characteristics of Each Patient
| Programming parameters (amplitude, frequency, and pulse width) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Gender | Education (yr) | Age at surgery (yr) | Disease duration at surgery (yr) | LEDD at surgery (mg) | Asymmetry Index (med off, med on) | Target selection criteria | Target | 6-mo follow-up | 12-mo follow-up | |
| Patient 1 | M | 12 | 63 | 11 | 731.25 | 0.28, 0.60 | Asymmetric symptoms | L-GPi | 2.55 V, 160 Hz, 70 μs | 2.80 V, 160 Hz, 70 μs |
| R-STN | 2.60 V, 160 Hz, 60 μs | 3.00 V, 160 Hz, 60 μs | ||||||||
| Patient 2 | M | 14 | 70 | 6 | 487.5 | 0.64, 0.52 | Asymmetric symptoms | L-GPi | 3.35 V, 170 Hz, 80 μs | 2.35 V, 170 Hz, 60 μs |
| R-STN | 2.35 V, 170 Hz, 60 μs | 3.35 V, 170 Hz, 80 μs | ||||||||
| Patient 3 | M | 9 | 71 | 18 | 1222.5 | –0.70, –0.60 | Asymmetric symptoms | L-STN | 2.35 V, 145 Hz, 60 μs | 2.35 V, 135 Hz, 60 μs |
| R-GPi | 2.75 V, 145 Hz, 70 μs | 3.15 V, 135 Hz, 70 μs | ||||||||
| Patient 4 | M | 9 | 59 | 6 | 977.5 | –0.29, –0.25 | Asymmetric symptoms | L-STN | 2.95 V, 160 Hz, 60 μs | 2.85 V, 160 Hz, 70 μs |
| R-GPi | 3.15 V, 160 Hz, 70 μs | 3.25 V, 160 Hz, 70 μs | ||||||||
| Patient 5 | M | 9 | 68 | 15 | 1000 | 0.30, 1 | Asymmetric symptoms | L-GPi | 3.50 V, 145 Hz, 70 μs | 3.60 V, 160 Hz, 70 μs |
| R-STN | 2.70 V, 145 Hz, 60 μs | 2.50 V, 160 Hz, 60 μs | ||||||||
| Patient 6 | M | 6 | 65 | 9 | 600 | –0.44, –0.23 | Asymmetric symptoms | L-STN | 3.45 V, 170 Hz, 90 μs | 3.55 V, 160 Hz, 70 μs |
| R-GPi | 2.00 V, 170 Hz, 60 μs | 2.75 V, 160 Hz, 50 μs | ||||||||
| Patient 7 | F | 2 | 69 | 9 | 500 | 0, 0 | Prominent cardinal motor symptoms with severe axial symptoms | L-STN | 2.65 V, 105 Hz, 70 μs | 2.60 V, 125 Hz, 60 μs |
| R-GPi | 3.25 V, 105 Hz, 70 μs | 2.75 V, 125 Hz, 60 μs | ||||||||
| Patient 8 | M | 17 | 74 | 7 | 700 | –0.11, 0 | Prominent cardinal motor symptoms with balance problem | L-STN | 2.95 V, 160 Hz, 60 μs | 3.25 V, 160 Hz, 70 μs |
| R-GPi | 2.75 V, 160 Hz, 60 μs | 3.00 V, 160 Hz, 70 μs | ||||||||
F, female; LEDD, levodopa equivalent daily dose; L-GPi, left unilateral stimulation of the globus pallidus interna; L-STN, left unilateral stimulation of the subthalamic nucleus M, male; R-GPi, right unilateral stimulation of the globus pallidus interna; R-STN, right unilateral stimulation of the subthalamic nucleus; TEED, total electrical energy delivered.
Mean age at surgery, 67.4 ± 4.8 yr; mean disease duration at surgery, 10.1 ± 4.4 yr; mean LEDD at surgery, 777.3 ± 264.1 mg.
The asymmetry index was calculated as the absolute difference between the total of the items for each side divided by the sum of the items for both sides ([left-right]/[left + right]). Higher asymmetry index indicated higher asymmetry in symptom severity or symptom types.
Patient Assessment
Primary and Secondary Clinical Outcomes
The primary and secondary clinical outcome measures were obtained before surgery (baseline) and after 6 and/or 12 mo of continuous unilateral STN DBS and contralateral GPi DBS. The primary clinical outcome was motor symptom severity, as assessed by using the Unified Parkinson Disease Rating Scale III (UPDRS-III). To gain insight into the specific effects of the different targets, we classified the UPDRS-III subscales into 3 categories: (1) axial signs, as measured by scores on speech, facial expression, arising from chair, posture, gait, freezing of gait, and posture stability; scores could range from 0 (no axial signs) to 28 (severe axial signs); (2) STN-stimulated contralateral appendicular symptoms; and (3) GPi-stimulated contralateral appendicular symptoms. Appendicular symptom severity was measured by using the subscale scores of the corresponding limb on rigidity, finger tapping, hand movements, hand pronation supination, toe tapping, leg agility, posture tremor, kinetic tremor, and resting tremor amplitude; scores could range from 0 (no appendicular symptoms) to 52 (severe appendicular symptoms). The scores on resting tremor constancy and bradykinesia were added to the scores on the limbs with corresponding symptoms because not all symptoms were present in the limb of interest. Also, the patient's response to L-dopa was calculated by subtracting the total UPDRS-III score obtained in the on-medication condition from the corresponding score obtained in the off-medication condition. Additionally, we employed the Timed Up-and-Go Test (TUG) and the Gait and Falls Questionnaire (GFQ) as primary motor outcome measures; the GFQ scores can range from 0 to 64, with higher scores indicating more severe gait and fall problems.
We utilized 3 secondary clinical outcomes: (1) quality of life (QoL), as measured by using the 8-item Parkinson Disease Questionnaire (PDQ-8), UPDRS-I, UPDRS-II, EuroQol 5 Dimensions Questionnaire (EQ-5D-5 L), and the patient's body mass index (BMI); (2) nonmotor symptoms (NMS), as assessed by the Non-Motor Symptoms Questionnaire (NMSQ), which consists of 30 items covering 10 symptom domains, and the Hospital Anxiety and Depression Scale (HADS) and the Mini-Mental State Examination (MMSE), which provide more specific measures of the patient's emotional status and global cognitive function, respectively; and (3) motor complications, as measured by the UPDRS-IV and medication dose; the patient's daily dose of antiparkinsonian medication was converted into a levodopa equivalent daily dose (LEDD).11
Assessment Conditions at Baseline and 6-Month and 12-Month Follow-up
Before surgery, the patients’ baseline motor functions were assessed after an overnight (more than 12 h) withdrawal of medication and 1 h after they had received a suprathreshold dose of levodopa. Using the same protocol, clinical outcome assessments were performed at 6-mo and 12-mo follow-up. At 6-mo follow-up, we also evaluated the on-stimulation and off-stimulation conditions in the off-medication state (GPi–STN–Med– and GPi+STN+Med–). At 12-mo follow-up, we conducted the same assessments in the on-/off-stimulation conditions in both the on- and off-medication state (GPi–STN–Med–, GPi+STN+Med–, GPi–STN–Med+, and GPi+STN+Med+), as well as assessing the effect of unilateral STN stimulation in both medication conditions (GPi–STN+Med– and GPi–STN+Med+).
Stimulation off was defined as the situation in which the DBS had been turned off for more than 1 h. At 12-mo follow-up, the patients remained in the hospital for 2 d. Bilateral stimulation on and off were tested on the first day. After the patients stopped taking all drugs in the first night, unilateral STN stimulation on and contralateral GPi stimulation off (GPi–STN+Med– and GPi–STN+Med+) were tested on the second day. For each patient, the DBS programming parameters used were documented at the last follow-up.
Surgical Procedure and Contact Localization
A Leksell stereotactic frame (Elekta, Stockholm, Sweden) was mounted on the patient's head under local anesthesia prior to computed tomography (CT) scanning. The fusion image was achieved by merging the CT and 3.0 T magnetic resonance imaging (MRI) images using Surgiplan software (Elekta, Stockholm, Sweden). Bilateral leads (Medtronic 3387/3389 [Medtronic, Dublin, Ireland] or PINS L302 and PINS 302 [Beijing PINS Medical Co, Beijing, China]) were implanted simultaneously under general anesthesia, and intraoperative C-arm X-ray was applied to verify the depth of the electrodes. System impedance was tested before closing the incision. Postoperative brain imaging (CT or MRI) was performed to confirm the lead position and rule out hemorrhage and intracranial pneumatosis.
Statistical Analysis
Initial data inspection indicated that the continuous clinical outcome variables deviated significantly from normality, as confirmed by the Kolmogorov-Smirnov test, which precluded the use of parametric tests. Correspondingly, the (nonparametric) Wilcoxon matched-pairs signed-rank test was used to make pairwise comparisons of the clinical data at baseline and 6-mo and 12-mo follow-up. Pearson's chi-square tests were employed to compare the categorical baseline and follow-up data. Problems related to missing data were addressed by imputing the group mean. A 2-tailed probability (P) value of .05 or lower was considered statistically significant. All analyses were performed using SPSS 20.0 (IBM, Armonk, New York).
RESULTS
Initially, we describe the effects of the combined unilateral STN and contralateral GPi DBS treatment on the primary and secondary clinical outcome measures. Next, we report the effects of the medication and GPi–STN+ conditions.
Clinical Outcomes After DBS Treatment (Combined Unilateral STN and Contralateral GPi DBS)
Motor Symptoms
After treatment, patients showed a significant improvement in overall motor function, as measured by the UPDRS-III in the off-medication/on-stimulation state (GPi+STN+Med–). At 6-mo and 12-mo follow-up, the mean total UPDRS-III score was reduced by 45% and 43%, respectively, as compared with the corresponding baseline score (Table 2).
TABLE 2.
Motor Symptom Severity of Patients (N = 8) Before and After Treatment as a Function of Stimulation (On and Off) in the Off-Medication Condition
| STN+GPi+ | STN+GPi+ | |||
|---|---|---|---|---|
| Baselinea | 6 mob | 1 yr | ||
| Total UPDRS-III | 43.8 ± 13.5 | 24.1 ± 10.1a | 24.8 ± 10.0a | |
| STN-stim contralateral limb | Tremor | 6.0 ± 5.0 | 1.0 ± 1.5a | 0.1 ± 0.4a |
| Rigidity | 4.5 ± 1.3 | 2.8 ± 2.1 | 3.6 ± 1.2 | |
| Bradykinesia | 11.6 ± 5.6 | 5.1 ± 2.0a | 5.6 ± 2.7a | |
| GPi-stim contralateral limb | Tremor | 1.1 ± 2.1 | 0.4 ± 0.7 | 0.3 ± 0.5 |
| Rigidity | 3.6 ± 1.8 | 3.5 ± 2.0 | 3.5 ± 1.1 | |
| Bradykinesia | 9.6 ± 4.4 | 5.0 ± 3.1a | 4.4 ± 4.0a | |
| Axial signs | Total axial score | 9.1 ± 4.5 | 7.4 ± 3.5 | 8.3 ± 4.7 |
| Arise from chair | 1.0 ± 1.1 | 0.3 ± 0.5 | 0.3 ± 0.5 | |
| Gait | 1.5 ± 0.8 | 0.9 ± 0.6 | 1.0 ± 0.9 | |
| Postural stability | 1.1 ± 1.0 | 0.6 ± 0.5 | 0.8 ± 1.2 | |
| Posture | 1.5 ± 1.1 | 1.5 ± 1.3 | 1.6 ± 0.9 | |
| TUG | 20.7 ± 10.8 | 12.2 ± 3.1a | 13.9 ± 4.1 | |
| GFQ | Total score | 13.6 ± 10.2 | 6.6 ± 6.7 | 4.9 ± 3.4a |
| Gait score | 11.5 ± 8.6 | 5.9 ± 6.8 | 3.5 ± 2.9a | |
| Falls score | 2.1 ± 2.7 | 0.7 ± 1.1 | 1.4 ± 2.1 | |
| STN–GPi– | STN–GP–- | |||
| Baselinea | 6 mob | 1 yr | ||
| Total UPDRS-III | 43.8 ± 13.5 | 41.1 ± 10.3 | 46.8 ± 13.6 | |
| STN-stim contralateral limb | Tremor | 6.0 ± 5.0 | 5.4 ± 4.9 | 6.0 ± 4.6 |
| Rigidity | 4.5 ± 1.3 | 5.1 ± 1.2 | 5.4 ± 1.6 | |
| Bradykinesia | 11.6 ± 5.6 | 9.9 ± 3.7 | 11.9 ± 4.2 | |
| GPi-stim contralateral limb | Tremor | 1.1 ± 2.1 | 0.5 ± 0.8 | 0.9 ± 2.1 |
| Rigidity | 3.6 ± 1.8 | 4.1 ± 1.4 | 4.5 ± 1.4 | |
| Bradykinesia | 9.6 ± 4.4 | 8.1 ± 4.1 | 9.1 ± 4.4 | |
| Axial signs | Total axial score | 9.1 ± 4.5 | 9.4 ± 3.9 | 11.6 ± 4.6b |
| Arise from chair | 1.0 ± 1.1 | 0.5 ± 0.5 | 1.0 ± 0.9 | |
| Gait | 1.5 ± 0.8 | 1.5 ± 0.8 | 1.4 ± 0.7 | |
| Postural stability | 1.1 ± 1.0 | 0.8 ± 1.0 | 0.9 ± 1.0 | |
| Posture | 1.5 ± 1.1 | 1.6 ± 1.1 | 1.8 ± 0.7 | |
| TUG | 20.7 ± 10.8 | 14.9 ± 4.9 | 20.7 ± 9.2 | |
GFQ, Gait and Fall Questionnaire; GPi, globus pallidus interna; GPi-STN–, without stimulation; GPi+STN+, with unilateral STN and contralateral GPi stimulation; med off, without medication; STN, the subthalamic nucleus; TUG, Timed Up-and-Go Test; UPDRS, Unified Parkinson Disease Rating Scale.
a,bThe letters a and b indicate a significant difference (P < .05) between 2 time points (Wilcoxon signed-rank test).
Values are presented as mean ± SD.
After treatment, the patients displayed no statistically significant changes in the severity of their axial symptoms, as indexed by the total UPDRS-III axial score, while being off medication (GPi+STN+Med–) at 6-mo and 12-mo follow-up. However, the score on the TUG was improved by 41% both at 6-mo and 12-mo follow-up compared with baseline (Table 2). Furthermore, the total GFQ score was improved by 64% at 12-mo follow-up (Table 2). At the GFQ subscale level, scores on gait items showed a greater improvement following treatment than scores on falls items.
Quality of Life
At 6-mo follow-up, the patients showed significant improvements in QoL (57% total score reduction on the PDQ-8 relative to baseline) and the nonmotor aspects (41% total score reduction on the UPDRS-I) and motor aspects (62% item score reduction on the UPDRS-II) of daily functioning. However, only the improvement of the nonmotor aspects of daily functioning was maintained at 12-mo follow-up. No significant changes in the patients’ BMI were observed over the 12-mo follow-up (Table 3).
TABLE 3.
Quality of Life of Patients (N = 8) Before and After Treatment
| Baselinea | 6-mo follow-upb | 1-yr follow-up | |
|---|---|---|---|
| PDQ-8 | |||
| Total | 9.6 ± 5.4 | 4.1 ± 3.7a | 6.5 ± 4.5 |
| Mobility | 1.5 ± 0.9 | 1.0 ± 1.4 | 1.1 ± 1.1 |
| Activity of daily living | 1.5 ± 0.8 | 0.6 ± 1.1a | 0.8 ± 0.9 |
| Emotional well-being | 1.0 ± 1.1 | 0.3 ± 0.5 | 0.4 ± 0.7 |
| Stigma | 0.9 ± 1.1 | 0 ± 0 | 0.5 ± 0.8 |
| Social support | 0.6 ± 0.9 | 0 ± 0 | 0.8 ± 0.7b |
| Cognitions | 1.4 ± 1.1 | 0.3 ± 0.5 | 0.4 ± 0.7a |
| Communications | 1.0 ± 1.2 | 0.4 ± 1.1 | 1.8 ± 1.0a |
| Bodily discomfort | 1.8 ± 1.5 | 1.6 ± 1.0 | 0.9 ± 1.1 |
| EQ-5D-5L | |||
| Global health status | 72.5 ± 17.3 | 72.1 ± 20.4 | 83.1 ± 11.9 |
| Mobility | 1.0 ± 0.8 | 0.6 ± 0.5 | 0.6 ± 0.7 |
| Self-care | 1.3 ± 1.3 | 0.3 ± 0.5 | 0.4 ± 0.7a |
| Activities of daily living | 1.3 ± 1.6 | 0.6 ± 0.8 | 0.6 ± 0.9 |
| Pain or discomfort | 0.9 ± 0.8 | 0.4 ± 0.5 | 1.1 ± 1.0 |
| Anxiety or depression | 1.3 ± 1.0 | 0.1 ± 0.4a | 0.4 ± 0.5 |
| UPDRS-I | |||
| Total | 15.1 ± 7.6 | 8.9 ± 4.4a | 10.3 ± 6.9a |
| Mood status | 2.8 ± 3.1 | 0.4 ± 0.5a | 1.4 ± 1.3b |
| Other nonmotor functions | 12.4 ± 4.9 | 8.4 ± 4.2a | 8.9 ± 5.7a |
| UPDRS-II | |||
| Total | 16.4 ± 8.7 | 10.9 ± 5.1 | 10.5 ± 8.4a |
| Fine motor functions | 5.6 ± 4.1 | 5.6 ± 3.2 | 4.8 ± 4.0 |
| Tremor and eating task | 2.6 ± 1.8 | 1.0 ± 1.2a | 1.1 ± 1.4a |
| Complex motor functions | 8.1 ± 4.9 | 4.3 ± 2.9 | 4.6 ± 3.8a |
| BMI (kg/m²) | 26.9 ± 3.7 | n/a | 26.9 ± 3.8 |
BMI, body mass index; EQ-5D-5 L, 5-Level EuroQol-5 Dimensions; n/a, not applicable; PDQ, Parkinson Disease Questionnaire; UPDRS, Unified Parkinson Disease Rating Scale.
a,bThe letters a and b indicate a significant difference (P < .05) between 2 time points (Wilcoxon signed-ranks tests).
Values are presented as mean ± SD.
Nonmotor Symptoms
We used the NMSS to screen for NMS in an all-or-none manner. After treatment, the number of patients who experienced NMS did not significantly change over the 12-mo follow-up (Table 4). Additionally, the patients’ level of anxiety and depression (measured by the HADS) and global cognitive function (measured by the MMSE) did not show significant changes over the 12-mo follow-up (Table 4).
TABLE 4.
Nonmotor Symptoms of Patients (N = 8) Before and After Treatment
| Baselinea | 6-mo follow-upb | 1-yr follow-up | |
|---|---|---|---|
| NMSS | |||
| Total | 70/240 | 52/210 | 47/240 |
| Gastrointestinal tract (items 1-7 and 27) | 26/64 | 20/56 | 15/64a |
| Urinary tract (items 8-9) | 12/16 | 9/14 | 7/16 |
| Sexual function (items 18-19) | 6/16 | 2/14 | 0/16a |
| Cardiovascular (items 20-21) | 5/16 | 4/14 | 3/16 |
| Apathy/attention/memory (items 12, 13, and 15) | 8/24 | 7/21 | 10/24 |
| Hallucinations/delusions (items 14 and 30) | 1/16 | 2/14 | 0/16 |
| Depression/anxiety/anhedonia (items 16 and 17) | 6/16 | 3/14 | 6/16 |
| Pain (unrelated to other causes) (item 10) | 2/8 | 3/7 | 4/8 |
| Miscellaneous (items 11 and 29) | 4/16 | 2/14 | 2/16 |
| HADS | |||
| Total | 14.0 ± 8.3 | 11.4 ± 6.1 | 11.1 ± 7.1 |
| HADS-D | 7.4 ± 4.2 | 7.1 ± 4.2 | 6.4 ± 2.7 |
| HADS-A | 6.6 ± 4.7 | 3.3 ± 2.1 | 4.8 ± 5.0 |
| MMSE | 28.3 ± 2.2 | n/a | 28.1 ± 1.7 |
A, anxiety; D, depression; HADS, Hospital Anxiety and Depression Scale; MMSE, Mini-Mental State Examination. n/a, not applicable; NMSS, Non-Motor Symptoms Scale; one patient lost NMSS at 6-mo follow-up.
a,bThe letters a and b indicate a significant difference (P < .05) between 2 time points (Wilcoxon signed-ranks tests and Pearson's chi-square test).
Continuous variables are presented as mean (SD); categorical variables were summarized by counts of patients and ratios.
Motor Complications and Medication
After treatment, the patients showed a reduced overall level of motor complications and fluctuations, as indexed by the total UPDRS-IV score, at 6-mo and 12-mo follow-up. Additionally, the treatment achieved a 41% reduction in LEDD at 12-mo follow-up (Table 5). Of note, the total electrical energy delivered to patients did not differ significantly between the GPi and STN stimulations (Tables 1 and 5).
TABLE 5.
Medication and Motor Complications of Patients (N = 8) Before and After Treatment Along With Total Electrical Energy Delivered
| Baselinea | 6-mo follow-upb | 1-yr follow-up | |
|---|---|---|---|
| LEDD (mg) | 777.3 ± 264.1 | 535.9 ± 125.3 | 457.8 ± 112.0a |
| TEED (μW) | |||
| (STN) TEED | n/a | 100 ± 37 | 98 ± 33 |
| (GPi) TEED | n/a | 78 ± 28 | 106 ± 32 |
| UPDRS IV | |||
| Total | 6.3 ± 3.5 | 0.3 ± 0.5a | 0.9 ± 1.5a |
| Dyskinesia | 0 ± 0 | 0 ± 0 | 0 ± 0 |
| Motor fluctuation | 5.6 ± 2.9 | 0 ± 0a | 0.6 ± 1.2a |
| Off-state pain | 0.6 ± 1.4 | 0.3 ± 0.5 | 0.3 ± 0.5 |
(GPi) TEED, total electrical energy delivered by unilateral stimulation of the globus pallidus interna; LEDD, levodopa equivalent daily dose; n/a, not applicable; (STN) TEED, total electrical energy delivered by unilateral stimulation of the subthalamic nucleus; TEED, total electrical energy delivered; UPDRS, Unified Parkinson Disease Rating Scale.
a, bThe letters a and b indicate a significant difference (P < .05) between 2 time points (Friedman test and Wilcoxon post hoc test).
Values are presented as mean (SD).
Effects of Medication (Baseline Med+ and Postoperative GPi–STN–Med+ Conditions and Baseline Med– and Postoperative GPi–STN–Med– Conditions)
We further assessed the differences between the medication on (Med+) and medication off (Med–) conditions while DBS was turned off at baseline and 6-mo and 12-mo follow-up. We found no significant differences in the total UPDRS-III scores between the baseline Med– and postoperative GPi–STN–Med– conditions at 6-mo and 12-mo follow-up. On the other hand, the patients’ response to L-dopa was reduced from 48% at baseline (Med– compared with Med+) to 26% at 12-mo follow-up (GPi–STN–Med– compared with GPi–STN–Med+). Finally, medication was associated with a 41% improvement in axial symptoms at baseline and showed a 15% improvement at 12-mo follow-up (Tables 2 and 6).
TABLE 6.
Motor Symptom Severity of Patients (N = 8) Before and After Treatment as a Function of Stimulation (On and Off) in the On-Medication Condition
| STN + GPi+ | STN-GPi- | |||
|---|---|---|---|---|
| Baselinea | 1 yr | 1 yr | ||
| Total UPDRS-III | 22.6 ± 16.8 | 21.9 ± 10.0 | 34.4 ± 19.2a | |
| STN-stim contralateral limb | Tremor | 1.1 ± 2.1 | 0 ± 0 | 2.1 ± 3.0 |
| Rigidity | 2.5 ± 2.4 | 3.1 ± 0.8 | 5.0 ± 2.1a | |
| Bradykinesia | 6.4 ± 4.7 | 4.3 ± 3.1 | 7.1 ± 5.5 | |
| GPi-stim contralateral limb | Tremor | 0.3 ± 0.5 | 0.1 ± 0.4 | 0 ± 0 |
| Rigidity | 2.1 ± 2.3 | 3.0 ± 1.5 | 4.6 ± 1.3a | |
| Bradykinesia | 5.5 ± 5.6 | 4.1 ± 4.1 | 7.3 ± 5.4 | |
| Axial signs | Total axial score | 5.4 ± 3.8 | 8.0 ± 3.7a | 9.9 ± 5.5a |
| Arise from chair | 0.3 ± 0.5 | 0.3 ± 0.5 | 0.8 ± 1.4 | |
| Gait | 0.8 ± 0.7 | 0.8 ± 0.7 | 1.4 ± 0.7 | |
| Postural stability | 0.5 ± 1.1 | 0.5 ± 0.8 | 1.0 ± 1.1 | |
| Posture | 1.0 ± 0.9 | 1.5 ± 0.8 | 1.5 ± 0.8 | |
| TUG | 11.8 ± 3.3 | 11.8 ± 3.8 | 15.0 ± 6.4a | |
GPi, globus pallidus interna; GPi–STN–, without stimulation; GPi+STN+, with unilateral STN and contralateral GPi stimulation; STN, the subthalamic nucleus; TUG, Timed Up-and-Go Test; UPDRS, Unified Parkinson Disease Rating Scale.
aThe letter a indicates a significant difference (P < .05) between 2 time points (Wilcoxon signed-rank test).
Values are presented as mean (SD).
Acute Effects of Unilateral STN Stimulation (Turning Off GPi Stimulation)
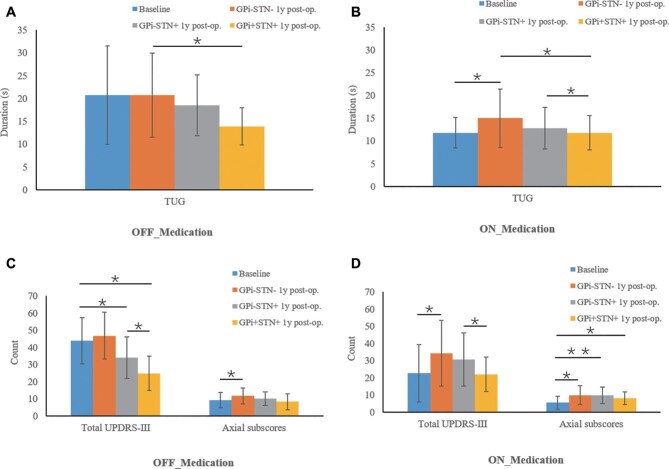
At 12-mo follow-up, we tested the effect of abruptly turning off the unilateral GPi stimulation while leaving the unilateral STN DBS turned on. This action resulted in an episode of acute worsening of the patients’ motor symptoms (measured by the UPDRS-III total score) compared with the immediately preceding stimulation-on condition, with a 37% symptom increase in the off-medication condition and a 40% symptom increase in the on-medication condition. Neither unilateral STN stimulation nor combined unilateral STN and contralateral GPi DBS affected the axial UPDRS-III scores in the on- and off-medication states (Figure).
FIGURE.
Acute effects of unilateral STN stimulation on TUG performance and UPDRS total score and axial score at 12-mo follow-up. Patients were assessed at baseline and 12-mo follow-up using GPi+STN+/GPi–STN+/GPi–STN– stimulation and medication on/off. Means are plotted with the error bar representing the standard deviation. A, Effects of stimulation on TUG performance in off-medication condition. B, Effects of stimulation on TUG performance in on-medication condition. C, Effects of stimulation on UPDRS total score and axial score in off-medication condition. D, Effects of stimulation on UPDRS total score and axial score in on-medication condition.*P < .05 and **P < .01 indicate significant difference between conditions. This figure has been previously presented at the 2019 Annual Meeting of the Congress of Neurological Surgeons in San Francisco, California in association with presentation of the abstract by Zhang et al.17
We further evaluated the effects of turning off the GPi stimulation on the TUG score at 12-mo follow-up. The result indicated that bilateral stimulation was associated with a larger improvement in motor function than unilateral stimulation, as evidenced by the comparison between GPi–STN+Med+ (12.8 s) and GPi+STN+Med+ (11.8 s) in the on-medication condition, and the comparison between GPi–STN+Med– (10.1 s) and GPi+STN+Med– (8.3 s) in the off-medication condition (Figure).
Side Effects
One patient (13%) developed transient postoperative confusion and hallucinations. Two patients (25%) developed mild dysarthria after continuous stimulation for 6 mo. No other side effects were reported.
DISCUSSION
Key Results
In this cohort, combined unilateral STN and contralateral GPi DBS treatment was associated with significant improvements in patients’ motor symptoms at 12-mo follow-up. Also, the patients’ QoL and daily functioning were markedly improved after DBS treatment. Moreover, the daily dose of medication and motor complications, especially motor fluctuations, were substantially reduced following treatment. By contrast, the treatment affected neither the severity of axial signs nor the patients’ body weight or NMS, including global cognitive function, anxiety, and depression. Finally, the treatment was not associated with significant adverse side effects.
Interpretation
The extent of the observed improvement in overall motor symptom severity (43%-45% improvement) is comparable to the effect sizes (30%-60% improvement) found in previous studies using bilateral DBS of the STN or GPi.12 Also, even though we used unilateral STN DBS, the reduction in LEDD observed in the present study (41% reduction) is comparable to the extent of medication reduction (around 50% reduction) after 1 yr of bilateral STN DBS reported in previous studies.12 Accordingly, the present results indicate that medication reduction can be achieved by unilateral surgery, which may be particularly relevant to target selection for patients who have a pressing need for medication reduction and suffer from contralateral dyskinesia, mood disorders, or worsening cognition.5 In line with our hypothesis, these findings indicate that combined unilateral STN and contralateral GPi DBS treatment exploited the clinical gains produced by both targets with an acceptable safety profile. Notably, the patients treated in our study experienced few adverse effects on cognition and mood. In addition, bilateral STN-specific complications, including dyskinesias, weight gain, and depression,13 did not occur following the combined unilateral STN and contralateral GPi DBS treatment employed in the present study.
Unilateral DBS for PD has been found to improve unilateral motor symptoms, regardless of laterality.14 In the present study, we compared the clinical effects between bilateral stimulation and unilateral STN. As expected, bilateral stimulation was overall superior to unilateral STN stimulation. This is not completely surprising because many patients initially treated with unilateral DBS are not satisfied with the motor outcomes and ultimately require a second surgery to implant bilateral DBS.8,9
We hypothesized that the use of asymmetric leads could reduce adverse side effects in select cases of advanced PD. If this hypothesis were valid, the use of asymmetric leads would be a more forgiving treatment option, with potentially new patterns of symptom improvement, except for patients with specific conditions, such as brittle dyskinesia, which clearly require bilateral GPi DBS. Unfortunately, the present study yielded insufficient evidence for this hypothesis, potentially because of the study limitations, particularly the small sample size and short follow-up.
Limitations
As indicated, this study has several limitations. First, the study is observational in nature. Consequently, the presence of a biased patient sample and confounding variables cannot be excluded. Also, the study involves a small case series with a short-term follow-up period. The small sample size implies that the statistical power was sufficient to detect relatively large clinical effects but was insufficient to discern small and subtle effects. Similarly, it remains unclear whether the clinical improvements after treatment are maintained or whether other clinical effects emerge beyond the 1-yr study follow-up. In addition, the assessment of axial symptoms was restricted to gait, falls, and posture, without including speech and swallowing functions. Furthermore, we did not include a control group of patients treated with bilateral STN DBS, patients with bilateral GPi DBS, or conditions of unilateral GPi DBS. Finally, we utilized rather broad measures of cognitive function and psychiatric symptoms.
Generalizability
Also, the current findings cannot be automatically generalized to the whole population of PD patients treated with DBS given that the patients included do not constitute a randomly selected representative patient sample. It remains to be determined, therefore, whether the same or similar results will be obtained with different patient samples. Notwithstanding, at the explanatory level as opposed to the descriptive level, we confidently expect that the unilateral STN and contralateral GPi DBS approach to treatment used in this study will be beneficial for most patients with PD who are eligible for bilateral STN or GPi DBS in general, yet whether this approach is superior needs to be further investigated. Our approach could also serve as a new option for patients with PD who have already received a period of bilateral DBS but need to have their target replaced because of a poor clinical response,15,16 as well as for patients who have initially received unilateral DBS but require a second surgery for the continuing management of their clinical symptoms.8
CONCLUSION
To our knowledge, these preliminary findings provide the first evidence that combined unilateral STN and contralateral GPi DBS could offer an effective and well-tolerated DBS treatment option for select patients with advanced PD. We propose that a patient-specific and hemibody-specific approach should be adopted when choosing a DBS target rather than focusing on a single target applied routinely to both sides. The GPi and STN targets complement each other within the spectrum of therapeutic options for patients with PD. Hemisphere-specific target choice seems to be a reasonable first step toward a personalized DBS treatment for PD patients. Large, well-controlled clinical trials are required to confirm, refine, or refute the present results.
Disclosures
The authors have no personal, financial, or institutional interest in any of the drugs, materials, or devices described in this article.
Acknowledgments
We appreciate the support from Yun Peng and thank all the participants.
REFERENCES
- 1. Deep-Brain Stimulation for Parkinson's Disease Study Group Obeso JA, Olanow CW et al. Deep-brain stimulation of the subthalamic nucleus or the pars interna of the globus pallidus in Parkinson's disease. N Engl J Med. 2001;345(13):956-963. [DOI] [PubMed] [Google Scholar]
- 2. Ramirez-Zamora A, Ostrem JL. Globus pallidus interna or subthalamic nucleus deep brain stimulation for Parkinson disease a review. JAMA Neurol. 2018;75(3):367-372. [DOI] [PubMed] [Google Scholar]
- 3. Lizarraga K, Luca C, De Salles A, Gorgulho A, Lang A, Fasano A. Asymmetric neuromodulation of motor circuits in Parkinson's disease: the role of subthalamic deep brain stimulation. Surg Neurol Int. 2017;8:261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Follett KA, Weaver FM, Stern M et al. Pallidal versus subthalamic deep-brain stimulation for Parkinson's disease. N Engl J Med. 2010;362(22):2077-2091. [DOI] [PubMed] [Google Scholar]
- 5. Odekerken VJJ, Boel JA, Geurtsen GJ et al. Neuropsychological outcome after deep brain stimulation for Parkinson disease. Neurology. 2015;84(13):1355-1361. [DOI] [PubMed] [Google Scholar]
- 6. Rughani A, Schwalb JM, Sidiropoulos C et al. Congress of neurological surgeons systematic review and evidence-based guideline on subthalamic nucleus and globus pallidus internus deep brain stimulation for the treatment of patients with Parkinson's disease: executive summary. Neurosurgery. 2018;82(6):753-756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Petraglia FW, Farber SH, Han JL et al. Comparison of bilateral vs. staged unilateral deep brain stimulation (DBS) in Parkinson's disease in patients under 70 years of age. Neuromodulation. 2016;19(1):31-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ehm G, Kim H-J, Kim J-Y et al. Effect of unilateral subthalamic deep brain stimulation in highly asymmetrical Parkinson's disease: 7-year follow-up. J Neurosurg. published online: November 1, 2018. (doi:10.3171/2018.5.JNS172006). [DOI] [PubMed] [Google Scholar]
- 9. Taba HA, Wu SS, Foote KD et al. A closer look at unilateral versus bilateral deep brain stimulation: results of the National Institutes of Health COMPARE cohort. J Neurosurg. 2010;113(6):1224-1229. [DOI] [PubMed] [Google Scholar]
- 10. Serrien DJ, Ivry RB, Swinnen SP. Dynamics of hemispheric specialization and integration in the context of motor control. Nat Rev Neurosci. 2006;7(2):160-166. [DOI] [PubMed] [Google Scholar]
- 11. Romito LM, Contarino MF, Vanacore N, Bentivoglio AR, Scerrati M, Albanese A. Replacement of dopaminergic medication with subthalamic nucleus stimulation in Parkinson's disease: long-term observation. Mov Disord. 2009;24(4):557-563. [DOI] [PubMed] [Google Scholar]
- 12. Krack P, Martinez-Fernandez R, del Alamo M, Obeso JA. Current applications and limitations of surgical treatments for movement disorders. Mov Disord. 2017;32(1):36-52. [DOI] [PubMed] [Google Scholar]
- 13. Okun MS, Gallo BV, Mandybur et al. Subthalamic deep brain stimulation with a constant-current device in Parkinson's disease: an open-label randomised controlled trial. Lancet Neurol. 2012;11(2):140-149. [DOI] [PubMed] [Google Scholar]
- 14. Shemisa K, Hass CJ, Foote KD et al. Unilateral deep brain stimulation surgery in Parkinson's disease improves ipsilateral symptoms regardless of laterality. Parkinsonism Relat Disord. 2011;17(10):745-748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Shin D, Hilliard JD, Foote KD. DBS revision surgery: indications and nuances. In: Goodman RR, ed. Surgery for Parkinson's Disease. Cham: Springer International Publishing; 2019:91-104. [Google Scholar]
- 16. Zhang C, Pan Y, Wang L et al. Globus pallidus internus deep brain stimulation improves axial symptoms of Parkinson patients after long-term subthalamic nucleus stimulation: a case series study. Interdiscip Neurosurg. 2019;18:100516. [Google Scholar]
- 17. Zhang C, Wang L, Almeida, L, Sun B, Li D. Asymmetric deep brain stimulation electrodes for Parkinson's disease: a pilot study of symptom-tailored stimulation. Neurosurgery. 2019;66(Suppl. 1): nyz310_506. [Google Scholar]