ABSTRACT
Tea is one of the most widely consumed beverages, but its association with cancer risk remains controversial and unclear. We performed an umbrella review to clarify and determine the associations between tea consumption and various types of cancer by summarizing and recalculating the existing meta-analyses. Meta-analyses of observational studies reporting associations between tea consumption and cancer risk were searched on PubMed and Embase. Associations found to be statistically significant were further classified into levels of evidence (convincing, suggestive, or weak), based on P value, between-study heterogeneity, prediction intervals, and small study effects. Sixty-four observational studies (case-control or cohort) corresponding to 154 effect sizes on the incidence of 25 types of cancer were included. Forty-three (27.9%) results in 15 different types of cancer were statistically significant. When combining all studies on the same type of cancer, 19 results in 11 different types of cancer showed significant associations with lower risk of gastrointestinal tract organ cancer (oral, gastric, colorectal, biliary tract, and liver cancer), breast cancer, and gynecological cancer (endometrial and ovarian cancer) as well as leukemia, lung cancer, and thyroid cancer. Only the reduced risk of oral cancer in tea-consuming populations (OR = 0.62; 95% CI: 0.55, 0.72; P value < 10−6) was supported by convincing evidence. Suggestive evidence was found for 6 results on biliary tract, breast, endometrial, liver, and oral cancer. To summarize, tea consumption was shown to have protective effects on some types of cancer, particularly oral cancer. More well-designed prospective studies are needed with consideration of other factors that can cause biases.
Keywords: tea, cancer, oral cancer, meta-analysis, umbrella review
Introduction
Tea produced from the leaves of the plant Camellia sinensis has been cultivated and consumed for centuries, and is still one of the most widely consumed beverages worldwide (1). Tea components vary with factors such as tea variety, climate, season, agricultural practices, the age of the leaf, and manufacturing processes (2). Green tea manufacturing involves steaming or pan-frying fresh tea leaves, thereby rapidly inactivating enzymes and preventing the oxidation of polyphenols, mainly catechins (3). Black tea is made by rolling the tea leaves to promote oxidation, followed by fermenting the leaves, which forms compounds such as theaflavins and thearubigins (4).
Historically tea has been claimed to have various beneficial health benefits and used for medical purposes (5). The compounds of tea have been suggested to have cancer-preventive effects in several studies (6–8). However, there has been no clear consensus in epidemiological literature about whether tea consumption is beneficial to health or not, especially concerning cancer (8). Because a large population consumes tea regularly throughout adult life, potential minor health benefits or risks associated with its consumption can have profound health implications at the population level. There are multiple quantitative studies on the association between tea and different types of cancer; however, there is still a need for a comprehensive appraisal of uncertainty and/or biases in the claimed associations. Recently, a new quantitative approach called “umbrella reviews” has been developed to understand the epidemiological credibility of complex health areas such as cardiovascular diseases, cancer, and multiple health outcomes (9–11).
Using existing meta-analyses of observational studies, we conducted an umbrella review of the meta-analyses and critically appraised the strengths and breadth of claimed associations between tea consumption and risk of cancer. In this study, we summarized the results from previously published meta-analyses and also performed the most updated meta-analysis by combining individual studies or the same subject (same type of cancer). To the best of our knowledge, this study is the first umbrella review to consider the whole breadth of evidence concerning tea consumption and cancer incidence.
Methods
Data sources and searches
Three investigators (TLK, GHJ, and JIS) independently searched PubMed and Embase databases for meta-analyses on the effect of tea consumption on different types of cancers. Articles were limited to those written in English published up to April 30, 2019. Keywords used in the search were “(Tea) AND (cancer OR carcinoma OR tumor) AND (meta-analysis OR systematic review).” The articles found using the two databases were screened and selected for eligibility based on examination of titles, abstracts, and full texts. Meta-analyses included prospective cohort studies, case-control studies (hospital-based and population-based), or both study designs (hereinafter referred to combined observational studies). Studies of unrelated topics, letters, and case reports were excluded while screening by title.
Eligibility criteria and extraction of data
Only systematic reviews and meta-analyses investigating the association between tea consumption and cancer were eligible for inclusion. Studies that did not specifically include tea as an independent exposure, such as combined caffeine exposure or maté tea, were not included. Tea consumption was divided into consumption of 2 specific types of tea (green tea and black tea) or consumption of any tea (regardless of type). The comparison groups of tea exposure were subclassified as high compared with low, any compared with none, and increments of 1–3 cups/d. The definition of criteria of high compared with low consumption of tea and size of a cup followed that of the original meta-analysis included in our review. Only meta-analyses that reported outcomes with metrics that were relevant to the risk of cancer, such as RR, OR, or HR, were included.
From the eligible meta-analyses, the following data were extracted: title, first author, year of publication, number of studies included, type of study (case-control, cohort, or observational studies including both case-control and cohort), type of tea, comparison groups of tea consumption, type of cancer, number of cancer cases/total number of participants, type of outcome metrics (RR or OR), meta-analysis model, effect size and its 95% CI, and largest effect size among included studies from each meta-analysis.
Statistical analysis
The primary studies obtained from the original articles were recalculated to receive additional information to evaluate the evidence level of meta-analyses. Comprehensive Meta-Analysis (v. 3.3.070; Biostat) and Microsoft Excel (v. 16.0) were used for the recalculation. The summary effect size, 95% CI, and P values were calculated under both random- and fixed-effects models using the identical type of metrics used as in the original meta-analyses. The summary effect size (represented as RR, HR, or OR) and 95% CI were recalculated using meta-analysis with both random-effects and fixed-effects models.
The between-study heterogeneity was recalculated using the I2 statistic and the P value from the χ2-based Cochran Q test. The I2 statistic describes the percentage of variation among studies that is due to heterogeneity rather than due to chance. I2 <50% is considered as low-to-moderate heterogeneity between studies, whereas I2 > 50% is considered as large and I2 > 75% as very large heterogeneity, respectively (12). If the heterogeneity between studies was large or very large, the meta-analysis was re-examined to determine if the heterogeneity was due to differences in the size of the association or due to differences in the direction of the effect. Using the recalculated data, the 95% prediction interval (PI) was also estimated. A 95% PI represents the distribution of true effects in which 95% of new and unique studies on the same subject will fall (13). Therefore, 95% PI further signifies between-study heterogeneity, whereas a 95% CI of each meta-analysis represents the accuracy of the summary effect size (14).
The P value of the Egger regression test was also calculated to evaluate small study effects. The Egger test assumes that when meta-analyses are based on a limited number of small trials the results are more prone to bias than larger studies (15). The threshold for the implications of small study effects was P < 0.10 from the Egger test. The random-effects summary effect size of the largest component study of each meta-analysis was compared with the random-effect summary effect size of each recalculated meta-analysis to evaluate whether the 2 effect sizes were concordant or discordant. Moreover, within each meta-analysis, we recorded the number of component studies that were statistically significantly associated with decreased risk, not statistically significant, or statistically significantly associated with increased risk—D (decreased risk), N (no association), I (increased risk), respectively.
Determination of the level of evidence in meta-analyses
Associations between tea consumption and the risk of different types of cancer were classified into 5 levels of evidence strength in accordance with grading schemes applied in previously published umbrella reviews (16–18). Evidence of strong statistical significance using random-effects meta-analyses at P value <10−6 (19), magnitude of between-study heterogeneity (I2 < 50%), absence of small study effects (Egger P value >0.10), and 95% PI excluded the null.
The criteria for determining the level of evidence were as follows:
Nonsignificant association: random-effects P value did not meet the significance threshold (random-effects P value >0.05).
Weak evidence: result was significant (random-effects P value <0.05), but there was evidence of between-study heterogeneity (I2 > 50 and 95% PI included the null) or small study effect.
Suggestive evidence: result was significant (random-effects P value <0.05), and there was no evidence of both between-study heterogeneity (I2 < 50) and small study effect, number of cases >1000, but 95% PI failed to reject the null hypothesis.
Convincing evidence: result was highly significant for random-effects P value <10−6, low to moderate heterogeneity (I2 < 50), 95% PI rejected the null hypothesis, no evidence of small study effect, number of cases >1000, and the largest study was concordant in terms of statistical significance with the random-effects result.
In case of inadequate number of individual studies or unavailable information for calculating 95% PI, I2, and Egger P value, we determined that the evidence was insufficient to state conclusions (see Supplemental Table 1).
In addition, we performed random-effects meta-analysis under a credibility ceiling for associations that satisfy the criteria of convincing level of evidence to determine the robustness of the associations. Credibility ceilings account for inherent methodological bias that can result in spurious significant results of the meta-analyses due to reporting of exaggerated associations in small studies (20, 21). We checked whether statistical significance was retained under a credibility ceiling of 10%, which is considered to be relatively lenient, to adjust each study included in the meta-analysis so as not to exceed a maximum certainty of 90%.
Meta-analysis combining all individual studies of the meta-analyses
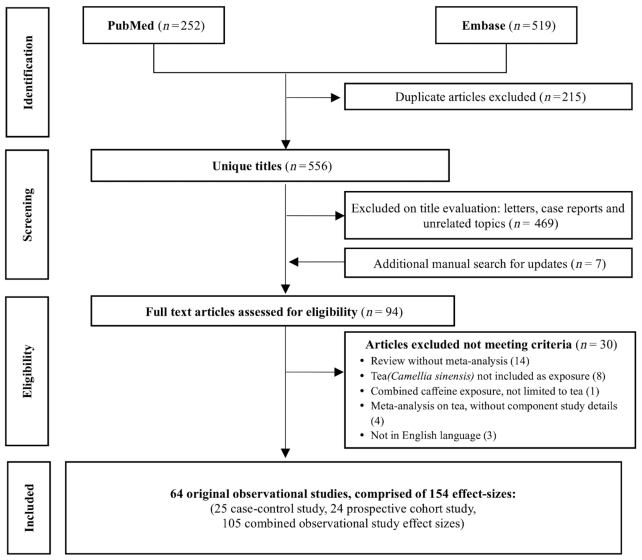
To account for the inconsistencies of the results between multiple meta-analyses studying the same subject (same type of cancer) but consisting of different individual studies, we combined all the individual studies of the meta-analyses of the same subjects and performed “the most updated” meta-analysis. While combining the meta-analyses, we identified and excluded the individual studies duplicated in >1 meta-analysis. If ≥2 individual studies based on identical population groups were identified, only the most recently published studies were included. We then meta-analyzed this new set of individual studies (the most updated meta-analysis) and evaluated the level of evidence of the associations. Finally, we performed subset analyses of case-control and cohort studies, with respect to the statistically significant results of meta-analyses. We also compared the results with those of meta-analyses of overall studies and cohort studies with the highest number of individual studies, respectively. The flowchart of the analysis is presented in Figure 1.
FIGURE 1.
Flow diagram of our umbrella review.
Results
Characteristics of studies included in the final analyses
Initially 556 unique articles were screened, and 64 original articles corresponding to 154 effect sizes (25 case-control studies, 24 cohort studies, 105 combined observational study effect sizes) met the eligibility criteria, as shown in the flowchart in Figure 2. Of the 154 effect sizes including 25 different types of cancer, 25 (16.2%) effect sizes were estimated from case-control studies (hospital-based or population-based), 24 (15.6%) from prospective cohort studies, and 105 (68.2%) from both case-control and cohort studies (combined observational studies) (see Table 1 and Supplemental references).
FIGURE 2.
Flowchart of literature search.
TABLE 1.
Summary of individual effect sizes from original meta-analyses of the associations on tea consumption and risk of cancer included in the study
| Category | Number of effect sizes | Comparison details, % | n |
|---|---|---|---|
| Total | 154 | 100.0 | |
| By exposure (tea type) | |||
| Any tea | 78 | 50.7 | 38 Any vs. none 32 High vs. low 8 Increment of 1–3 cups/d |
| Black tea | 19 | 12.3 | 5 Any vs. none 11 High vs. low 3 Increment of 1–2 cups/d |
| Green tea | 57 | 37.0 | 12 Any vs. none 41 High vs. low 4 Increment of 1–2 cups/d |
| By study type | |||
| Case-control | 25 | 16.2 | |
| Cohort | 24 | 15.6 | |
| Observational (combined) | 105 | 68.2 | |
| By cancer type | |||
| Biliary tract cancer | 1 | 0.6 | |
| Bladder cancer | 7 | 4.5 | |
| Brain cancer | 3 | 1.9 | |
| Breast cancer | 31 | 20.1 | |
| Colorectal cancer | 8 | 5.2 | |
| Colon cancer | 1 | 0.6 | |
| Rectal cancer | 1 | 0.6 | |
| Endometrial cancer | 11 | 7.1 | |
| Esophageal cancer | 6 | 3.9 | |
| Gastric cancer | 17 | 11.0 | |
| Gallbladder cancer | 2 | 1.3 | |
| Glioma | 2 | 1.3 | |
| Renal cell carcinoma | 1 | 0.6 | |
| Liver cancer | 4 | 2.6 | |
| Lung cancer | 5 | 3.2 | |
| Leukemia (childhood) | 8 | 5.2 | |
| Leukemia (adult) | 2 | 1.3 | |
| Ovarian cancer | 12 | 7.8 | |
| Laryngeal cancer | 5 | 3.2 | |
| Oral cancer | 7 | 4.5 | |
| Oropharyngeal cancer | 3 | 1.9 | |
| Pharyngeal cancer | 3 | 1.9 | |
| Pancreatic cancer | 5 | 3.2 | |
| Prostate cancer | 7 | 4.5 | |
| Thyroid cancer | 1 | 0.6 | |
| Skin cancer (nonmelanoma) | 1 | 0.6 | |
| By level of evidence | |||
| Convincing | 2 | 1.3 | |
| Suggestive | 16 | 10.4 | |
| Weak | 25 | 16.2 | |
| Nonsignificant | 107 | 69.5 | |
| Not adequately assessed | 4 | 2.6 | |
Summary of individual meta-analyses under conventional interpretation of meta-analysis criteria (random-effects P value <0.05)
We evaluated 154 meta-analyses including tests for bias and heterogeneity (see Table 1 and Supplemental Tables 2 and 3). Under conventional thresholds of statistical significance (random-effects P value <0.05), 43 (27.9%) meta-analyses on 15 types of cancer were significant and adequately assessed, and 42 (27.2%) showed decreased associations between tea consumption and risks of cancer incidence. The only original meta-analysis that showed significant increased risk of cancer was for breast cancer (high compared with low black tea consumption). Within 43 significant associations, 7 (16.3%) meta-analyses were significant at P < 0.001 using random-effects model.
Results of meta-analyses combining all individual studies under conventional interpretation of meta-analysis criteria (random-effects P value <0.05)
The original studies from each of the meta-analyses were combined for a comprehensive umbrella review comprising all the studies that were on the comparison regarding tea consumption and type of cancer. This resulted in 66 results on 25 types of cancer comparing different patterns of tea consumption (see Table 2 and Supplemental Tables 3 and 4).
TABLE 2.
The results and the level of evidence of the effect of tea and risk of cancer1
| Outcome | Study type | Comparison | Type of tea | P value (random-effects)2 | 95% PI including null | Heterogeneity (I2)2 | Effect-size distribution (D/N/I)3 | Small study effect4 | Concordance | Metrics | Summary effect (random-effects)5 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Associations supported by convincing evidence | |||||||||||
| Oral cancer | CC | Any vs. none | Any tea | <10−6 | No | Not large | 6/0/0 | No | Yes | OR | 0.62 (0.55, 0.72) |
| Associations supported by suggestive evidence | |||||||||||
| Biliary tract cancer | Obs | Any vs. none | Any tea | <0.05 but >10−6 | Yes | Not large | 4/4/0 | No | Yes | RR | 0.77 (0.64, 0.92) |
| Breast cancer | CC | High vs. low | Green tea | <0.05 but >10−6 | Yes | Large6 | 6/5/0 | No | Yes | RR | 0.75 (0.61, 0.92) |
| Endometrial cancer | Obs | High vs. low | Green tea | <0.05 but >10−6 | Yes | Not large | 2/4/0 | No | Yes | RR | 0.78 (0.61, 1.00)7 |
| Liver cancer | Obs | High vs. low | Green tea | <0.05 but >10−6 | Yes | Not large | 2/9/0 | No | No | RR | 0.87 (0.78, 0.98) |
| Oral cancer | Obs | High vs. low | Green tea | <0.05 but >10−6 | Yes | Not large | 1/4/0 | No | No | RR | 0.82 (0.69, 0.96) |
| Oral cancer | Obs | High vs. low | Any tea | <0.05 but >10−6 | No | Not large | 5/26/0 | No | Yes | RR | 0.86 (0.80, 0.91) |
| Associations supported by weak evidence | |||||||||||
| Breast cancer | Obs | High vs. low | Green tea | <0.05 but >10−6 | Yes | Large | 6/10/0 | No | No | RR | 0.82 (0.71, 0.96) |
| Breast cancer | Obs | Any vs. none | Green tea | <0.05 but >10−6 | Yes | Large | 3/11/0 | No | Yes | OR | 0.87 (0.76, 0.99) |
| Breast cancer | Obs | Any vs. none | Any tea | <0.05 but >10−6 | Yes | Very large | 6/20/0 | No | No | RR | 0.81 (0.71, 0.94) |
| Colorectal cancer | Obs | High vs. low | Any tea | <0.05 but >10−6 | Yes | Not large | 6/45/2 | Yes | No | RR | 0.93 (0.87, 0.99) |
| Colorectal cancer | Obs | High vs. low | Green tea | <0.05 but >10−6 | Yes | Large | 4/11/0 | No | No | RR | 0.87 (0.75, 1.00)7 |
| Gastric cancer | Obs | Any vs. none | Any tea | <0.001 | Yes | Very large | 23/30/3 | Yes | No | RR | 0.78 (0.70, 0.86) |
| Leukemia | Obs | High vs. low | Any tea | <0.001 | No | Not large | 4/4/0 | Yes | No | RR | 0.55 (0.43, 0.72) |
| Leukemia | Obs | Any vs. none | Any tea | <0.05 but >10−6 | No | Not large | 1/7/0 | Yes | No | RR | 0.76 (0.65, 0.89) |
| Liver cancer | Obs | Any vs. none | Green tea | <0.05 but >10−6 | Yes | Large | 3/7/0 | No | No | RR | 0.65 (0.48, 0.88) |
| Lung cancer | Obs | Any vs. none | Any tea | <0.001 | Yes | Very large | 18/24/1 | No | Yes | RR | 0.76 (0.67, 0.86) |
| Ovarian cancer | Obs | Any vs. none | Any tea | <0.05 but >10−6 | Yes | Very large | 8/22/1 | No | No | RR | 0.82 (0.71, 0.94) |
| Thyroid cancer | Obs | High vs. low | Any tea | <0.05 but >10−6 | Yes | Not large | 1/13/0 | Yes | No | RR | 0.77 (0.61, 0.97) |
| Nonsignificant associations | |||||||||||
| Acute leukemia (childhood) | Obs | High vs. low | Any tea | >0.05 | Yes | Not large | 0/8/1 | No | Yes | RR | 0.93 (0.74, 1.18) |
| Acute leukemia (childhood) | Obs | Any vs. none | Any tea | >0.05 | Yes | Not large | 0/14/0 | No | No | RR | 0.93 (0.82, 1.05) |
| Bladder cancer | CC | High vs. low | Any tea | >0.05 | Yes | Large | 1/22/2 | No | Yes | RR | 0.97 (0.87, 1.09) |
| Bladder cancer | Obs | High vs. low | Any tea | >0.05 | Yes | Large | 2/29/2 | No | Yes | RR | 0.95 (0.86, 1.06) |
| Bladder cancer | Co | High vs. low | Any tea | >0.05 | Yes | Large | 1/7/0 | No | Yes | RR | 0.86 (0.65, 1.13) |
| Bladder cancer | Obs | High vs. low | Green tea | >0.05 | Yes | Not large | 0/5/0 | No | Yes | RR | 1.03 (0.82, 1.31) |
| Brain cancer | Obs | Any vs. none | Any tea | >0.05 | Yes | Not large | 2/6/0 | No | No | RR | 0.90 (0.74, 1.09) |
| Breast cancer | Co | High vs. low | Black tea | >0.05 | Yes | Not large | 0/15/0 | No | No | RR | 1.04 (0.97, 1.12) |
| Breast cancer | CC | High vs. low | Black tea | >0.05 | Yes | Large | 1/12/0 | No | No | RR | 0.91 (0.80, 1.03) |
| Breast cancer | Obs | High vs. low | Black tea | >0.05 | Yes | Large | 1/27/0 | No | Yes | RR | 0.98 (0.91, 1.06) |
| Breast cancer | Co | High vs. low | Green tea | >0.05 | Yes | Not large | 0/5/0 | No | Yes | RR | 0.99 (0.83, 1.77) |
| Breast cancer | Co | Any vs. none | Green tea | >0.05 | Yes | Not large | 0/9/0 | No | Yes | OR | 0.94 (0.83, 1.05) |
| Breast cancer | CC | Any vs. none | Green tea | >0.05 | Yes | Very large | 3/2/0 | No | No | OR | 0.83 (0.62, 1.10) |
| Breast cancer | Co | High vs. low | Any tea | >0.05 | Yes | Not large | 0/12/2 | No | Yes | RR | 1.03 (0.96, 1.10) |
| Breast cancer | CC | High vs. low | Any tea | >0.05 | Yes | Large | 1/8/0 | No | Yes | OR | 0.90 (0.75, 1.10) |
| Breast cancer | Obs | High vs. low | Any tea | >0.05 | Yes | Large | 1/20/2 | No | Yes | RR | 0.98 (0.90, 1.06) |
| Colon cancer | Obs | High vs. low | Green tea | >0.05 | Yes | Not large | 1/9/1 | Yes | Yes | RR | 0.98 (0.85, 1.12) |
| Colorectal cancer | Obs | High vs. low | Black tea | >0.05 | Yes | Large | 2/14/4 | No | Yes | RR | 0.99 (0.87, 1.13) |
| Endometrial cancer | Obs | High vs. low | Any tea | >0.05 | Yes | Large | 3/12/1 | Yes | Yes | RR | 0.90 (0.75, 1.09) |
| Endometrial cancer | Obs | Increment of 1cup/d | Any tea | >0.05 | Yes | Large | 0/4/1 | No | Yes | RR | 1.04 (0.98, 1.10) |
| Endometrial cancer | Obs | High vs. low | Green tea | >0.05 | Yes | Large | 1/8/1 | No | No | RR | 0.99 (0.79, 1.23) |
| Esophageal cancer | Obs | High vs. low | Green tea | >0.05 | Yes | Large | 9/11/2 | No | Yes | RR | 0.81 (0.62, 1.06) |
| Gallbladder cancer | Obs | High vs. low | Any tea | >0.05 | Yes | Very large | 2/2/0 | No | No | RR | 0.57 (0.25, 1.30) |
| Gallbladder cancer | Obs | Any vs. none | Any tea | >0.05 | Yes | Very large | 3/3/0 | No | No | RR | 0.67 (0.40, 1.12) |
| Gastric cancer | Obs | High vs. low | Green tea | >0.05 | Yes | Large | 3/25/2 | Yes | Yes | RR | 0.93 (0.84, 1.04) |
| Gastric cancer | Obs | High vs. low | Black tea | >0.05 | Yes | Not large | 0/4/1 | No | Yes | RR | 1.18 (0.79, 1.77) |
| Gastric cancer | Obs | Increment of 3cups/d | Any tea | >0.05 | Yes | Not large | 0/5/0 | No | Yes | RR | 0.98 (0.89, 1.08) |
| Glioma | Obs | Any vs. none | Any tea | >0.05 | Yes | Very large | 0/4/0 | No | No | RR | 0.67 (0.40, 1.12) |
| Glioma | Obs | High vs. low | Any tea | >0.05 | Yes | Very large | 1/3/0 | No | No | RR | 0.57 (0.25, 1.30) |
| Laryngeal cancer | Obs | High vs. low | Any tea | >0.05 | Yes | Large | 2/5/1 | No | Yes | RR | 0.91 (0.67, 1.23) |
| Liver cancer | Obs | Any vs. none | Any tea | >0.05 | Yes | Very large | 3/9/0 | No | No | RR | 0.77 (0.57, 1.03) |
| Lung cancer | Obs | High vs. low | Green tea | >0.05 | Yes | Very large | 4/7/1 | No | Yes | RR | 0.78 (0.61, 1.01) |
| Lung cancer | Obs | High vs. low | Black tea | >0.05 | Yes | Large | 4/10/0 | No | Yes | RR | 0.86 (0.70, 1.05) |
| Oropharyngeal cancer | Obs | Any vs. none | Any tea | >0.05 | Yes | Large | 2/4/0 | No | Yes | RR | 0.68 (0.45, 1.03) |
| Ovarian cancer | Obs | Any vs. none | Green tea | >0.05 | Yes | Very large | 3/5/1 | No | Yes | RR | 0.76 (0.57, 1.02) |
| Ovarian cancer | Obs | Any vs. none | Black tea | >0.05 | Yes | Large | 4/12/0 | Yes | Yes | RR | 0.90 (0.78, 1.04) |
| Pancreatic cancer | Obs | High vs. low | Any tea | >0.05 | Yes | Not large | 1/20/1 | No | Yes | RR | 0.97 (0.85, 1.10) |
| Pancreatic cancer | Obs | Any vs. none | Any tea | >0.05 | Yes | Large | 2/25/2 | No | Yes | RR | 0.99 (0.89, 1.10) |
| Pancreatic cancer | Obs | High vs. low | Green tea | >0.05 | Yes | Large | 1/6/1 | No | Yes | RR | 0.99 (0.78, 1.25) |
| Pharyngeal cancer | Obs | Any vs. none | Any tea | >0.05 | Yes | Not large | 0/4/0 | No | Yes | RR | 0.88 (0.74, 1.04) |
| Prostate cancer | Obs | High vs. low | Green tea | >0.05 | Yes | Very large | 3/6/0 | Yes | Yes | RR | 0.73 (0.51, 1.06) |
| Prostate cancer | Obs | High vs. low | Any tea | >0.05 | Yes | Large | 6/15/2 | Yes | Yes | RR | 0.86 (0.71, 1.04) |
| Prostate cancer | Obs | Any vs. none | Any tea | >0.05 | Yes | Large | 7/20/2 | Yes | Yes | RR | 0.87 (0.75, 1.01) |
| Prostate cancer | Obs | High vs. low | Black tea | >0.05 | Yes | Not large | 1/9/1 | No | Yes | RR | 0.99 (0.82, 1.20) |
| Rectal cancer | Obs | High vs. low | Green tea | >0.05 | Yes | Large | 3/6/0 | No | Yes | RR | 0.97 (0.77, 1.22) |
| Renal cell carcinoma | Obs | Any vs. none | Any tea | >0.05 | Yes | Very large | 1/11/0 | No | Yes | RR | 1.03 (0.88, 1.21) |
| Skin cancer (non-melanoma) | Obs | Any vs. none | Any tea | >0.05 | Yes | Not large | 4/4/0 | No | Yes | OR | 0.88 (0.76, 1.02) |
CC, case-control studies; Co, cohort studies; Obs, observational studies; PI, prediction interval.
Heterogeneity is defined as “Very large” when I2 > 75%, “Large” when 50% < I2 < 75%, and “Not large” when I2 < 50%.
Number of individual studies of effect size with statistical significance in direction of decreased cancer risk(D)/no association(N)/increased cancer risk(I).
The presence of small study effects is determined if the Egger P value is <0.10.
Summary effect with 95% CI value obtained from umbrella review combining meta-analyses of the same comparison.
Although heterogeneity is large, the distribution of the effect sizes was considered over the I2 metrics.
The value is rounded up (to 2 decimal places), and hence is statistically significant.
Within 66 results, 19 (28.8%) showed significant results (random-effects P value <0.05) between tea consumption and decreased risk of 11 different types of cancer. The 19 statistically significant results were as follows: biliary tract cancer (any tea, any compared with none), breast cancer (green tea, any compared with none; green tea, high compared with low; any tea, any compared with none), colorectal cancer (green tea, high compared with low; any tea, high compared with low), endometrial cancer (green tea, high compared with low), gastric cancer (any tea, any compared with none), leukemia (any tea, high compared with low; any tea, any compared with none), liver cancer (green tea, any compared with none; green tea, high compared with low), lung cancer (any tea, any compared with none), oral cancer (green tea, high compared with low; any tea, high compared with low; any tea, any compared with none), ovarian cancer (any tea, any compared with none), and thyroid cancer (any tea, high compared with low) (see Table 2).
Level of evidence
After recalculating the data by considering heterogeneity between estimates and biases in the literature, 2 results (1.3%) were supported by convincing evidence. Sixteen results (10.4%) were supported by suggestive evidence, 25 results (16.2%) showed weak evidence, 107 results (69.5%) were nonsignificant, and 4 results (2.6%) were not adequately assessed due to insufficient information (see Supplemental Table 2).
From the 19 statistically significant results of updated meta-analyses combining all the individual studies, reduction in the incidence of oral cancer was found to have convincing evidence for any compared with none (OR = 0.62; 95% CI: 0.55, 0.72; P < 10−6) consumption of any type of tea. Under the consideration of credibility ceilings, the result with convincing level of evidence preserved statistical significance with a ceiling of 10%. Six results were found to have suggestive levels of evidence. Consumption of any type of tea showed a lowered risk of biliary tract cancers (RR = 0.77; 95% CI: 0.64, 0.92; P = 0.004) compared with no tea consumption. Also, the reduced risk of oral cancer with a high dose of tea consumption (RR = 0.86; 95% CI: 0.79, 0.93; P = 0.00024) showed a suggestive level of evidence. High consumption compared with low green tea consumption significantly lowered the risk of breast cancer (RR = 0.75; 95% CI: 0.61, 0.92; P = 0.006), liver cancer (RR = 0.87; 95% CI: 0.78, 0.98; P = 0.026), and oral cancer (RR = 0.82; 95% CI: 0.69, 0.96; P = 0.015). High consumption of green tea reduced the risk of endometrial cancer (RR = 0.78; 95% CI: 0.61, 1.00; P = 0.046) compared with low consumption of green tea. Twelve results associated with breast cancer, colorectal cancer, gastric cancer, leukemia, liver cancer, lung cancer, ovarian cancer, and thyroid cancer were classified to have weak evidence.
Summary of meta-analyses separated by study design
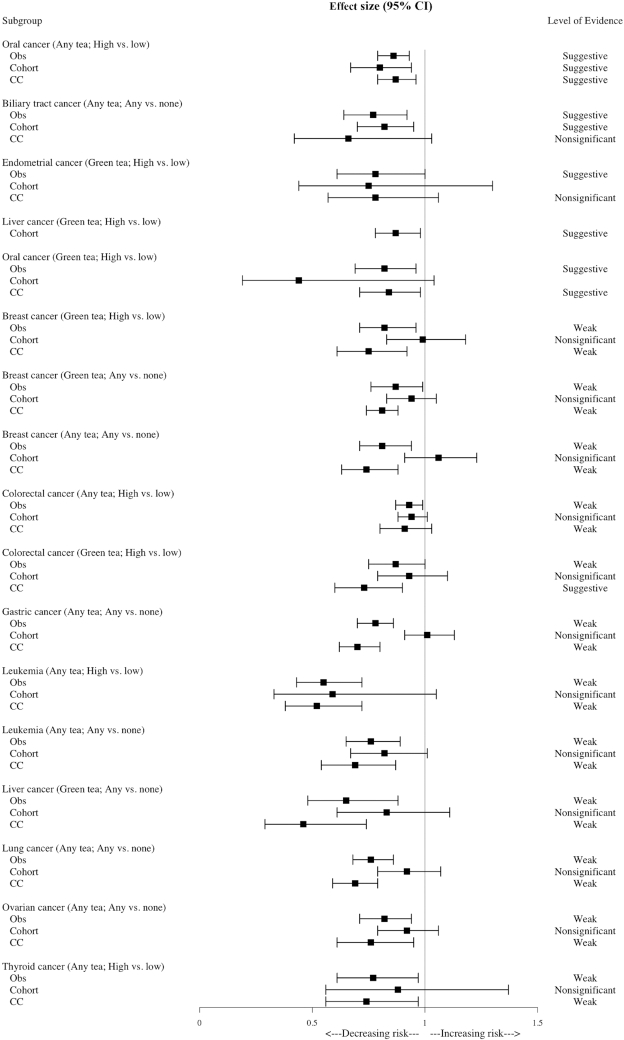
In the case of oral cancer, high consumption of any kind of tea showed suggestive evidence in observational studies due to the threshold P value being unsatisfied; also, the outcomes in both case-control and cohort studies showed suggestive evidence because their 95% PI included null. Further, the meta-analysis with the largest number of individual studies showed suggestive evidence. Among the 5 results with suggestive evidence, results on biliary tract cancer showed suggestive evidence in cohort studies but failed to show significance in case-control studies. In case of endometrial cancer, both cohort and case-control studies were not statistically significant. Besides the case of colorectal cancer with high compared with low tea consumption, all results that showed weak evidence presented nonsignificant results in cohort studies but showed significance in case-control studies (1 suggestive, 10 weak). In case of colorectal cancer with high compared with low tea consumption, the result of meta-analyses with both cohort and case-control studies failed to show its significance (see Table 3 and Figure 3).
TABLE 3.
Summary of results of the associations on tea consumption and risk of cancer outlined by study design, largest meta-analysis of observational studies, and largest meta-analysis of cohort studies1
| Cancer type | Study design | Metrics | Summary effect (random-effects)6 | P value (random-effects) | 95% PI including null | Heterogeneity (I2)2 | Small study effect3 | Concordance | Level of evidence4 |
|---|---|---|---|---|---|---|---|---|---|
| Oral cancer (any tea; any vs. none) | CC | OR | 0.62 (0.55, 0.72) | <0.001 | No | Not large | No | Yes | Convincing |
| Oral cancer (any tea; high vs. low) | Obs | RR | 0.86 (0.79, 0.93) | <0.001 | No | Not large | No | Yes | Suggestive |
| Co | RR | 0.80 (0.67, 0.94) | 0.007 | Yes | Not large | No | Yes | Suggestive | |
| CC | RR | 0.87 (0.79, 0.96) | 0.004 | Yes | Not large | No | No | Suggestive | |
| Largest MA (Obs) | RR | 0.84 (0.75, 0.94) | 0.002 | Yes | Not large | No | No | Suggestive | |
| Largest MA (Co) | NR | ||||||||
| Biliary tract cancer (any tea; any vs. none) | Obs | RR | 0.77 (0.64, 0.92) | 0.004 | Yes | Not large | No | Yes | Suggestive |
| Co | RR | 0.82 (0.70, 0.95) | 0.008 | Yes | Not large | No | Yes | Suggestive | |
| CC | RR | 0.66 (0.42, 1.03) | 0.068 | Yes | Not large | No | No | Nonsignificant | |
| Largest MA (Obs) | RR | 0.77 (0.64, 0.92) | 0.004 | Yes | Not large | No | Yes | Suggestive | |
| Largest MA (Co) | NR | ||||||||
| Endometrial cancer (green tea; high vs. low) | Obs | RR | 0.78 (0.61, 1.00)5 | 0.046 | Yes | Not large | No | No | Suggestive |
| Co | RR | 0.75 (0.44, 1.30) | 0.298 | N/A | N/A | N/A | N/A | Nonsignificant | |
| CC | RR | 0.78 (0.57, 1.06) | 0.108 | Yes | Not large | No | Yes | Nonsignificant | |
| Largest MA (Obs) | RR | 0.78 (0.61, 1.00)5 | 0.046 | Yes | Not large | No | No | Suggestive | |
| Largest MA (Co) | NR | ||||||||
| Liver cancer (green tea; high vs. low) | Obs | RR | 0.87 (0.78, 0.98) | 0.026 | Yes | Not large | No | No | Suggestive |
| Co | RR | 0.87 (0.78, 0.98) | 0.026 | Yes | Not large | No | No | Suggestive | |
| CC | NR | ||||||||
| Largest MA (Obs) | RR | 0.87 (0.78, 0.98) | 0.026 | Yes | Not large | No | No | Suggestive | |
| Largest MA (Co) | RR | 0.87 (0.78, 0.98) | 0.026 | Yes | Not large | No | No | Suggestive | |
| Oral cancer (green tea; high vs. low) | Obs | RR | 0.82 (0.69, 0.96) | 0.015 | Yes | Not large | No | No | Suggestive |
| Co | RR | 0.44 (0.19, 1.04) | 0.058 | N/A | N/A | N/A | N/A | N/A | |
| CC | RR | 0.84 (0.72, 0.98) | 0.030 | Yes | Not large | No | No | Suggestive | |
| Largest MA (Obs) | RR | 0.82 (0.69, 0.96) | 0.015 | Yes | Not large | No | No | Suggestive | |
| Largest MA (Co) | NR | ||||||||
| Breast cancer (green tea; high vs. low) | Obs | RR | 0.82 (0.71, 0.96) | 0.015 | Yes | Large | No | No | Weak |
| Co | RR | 0.99 (0.83, 1.18) | 0.895 | Yes | Not large | No | Yes | Nonsignificant | |
| CC | RR | 0.75 (0.61, 0.92) | 0.006 | Yes | Large | No | Yes | Weak | |
| Largest MA (Obs) | RR | 0.81 (0.67, 0.98) | <0.001 | Yes | Large | No | Yes | Weak | |
| Largest MA (Co) | RR | 0.98 (0.83, 1.16) | 0.821 | Yes | Not large | No | Yes | Nonsignificant | |
| Breast cancer (green tea; any vs. none) | Obs | OR | 0.87 (0.76, 0.99) | 0.040 | Yes | Very large | No | Yes | Weak |
| Co | OR | 0.94 (0.83, 1.05) | 0.278 | Yes | Not large | No | Yes | Nonsignificant | |
| CC | OR | 0.83 (0.62, 1.10) | 0.196 | Yes | Very large | No | No | Nonsignificant | |
| Largest MA (Obs) | OR | 0.87 (0.76, 0.99) | 0.040 | Yes | Very large | No | Yes | Weak | |
| Largest MA (Co) | OR | 0.94 (0.83, 1.05) | 0.278 | Yes | Not large | No | Yes | Nonsignificant | |
| Breast cancer (any tea; any vs. none) | Obs | RR | 0.81 (0.71, 0.94) | <0.001 | Yes | Very large | No | No | Weak |
| Co | RR | 1.06 (0.91, 1.23) | 0.451 | Yes | Not large | No | Yes | Nonsignificant | |
| CC | RR | 0.74 (0.63, 0.88) | <0.001 | Yes | Very large | No | No | Weak | |
| Largest MA (Obs) | RR | 0.79 (0.65, 0.95) | 0.012 | Yes | Very large | No | No | Weak | |
| Largest MA (Co) | NR | ||||||||
| Colorectal cancer (any tea; high vs. low) | Obs | RR | 0.93 (0.87, 0.99) | 0.031 | Yes | Not large | Yes | No | Weak |
| Co | RR | 0.94 (0.88, 1.01) | 0.108 | Yes | Not large | No | Yes | Nonsignificant | |
| CC | RR | 0.91 (0.80, 1.03) | 0.117 | Yes | Large | Yes | Yes | Nonsignificant | |
| Largest MA (Obs) | RR | 0.93 (0.87, 0.99) | 0.031 | Yes | Not large | Yes | No | Weak | |
| Largest MA (Co) | RR | 0.94 (0.88, 1.01) | 0.108 | Yes | Not large | No | Yes | Nonsignificant | |
| Colorectal cancer (green tea; high vs. low) | Obs | RR | 0.87 (0.75, 1.00)5 | 0.050 | Yes | Large | No | No | Weak |
| Co | RR | 0.93 (0.79, 1.10) | 0.408 | Yes | Large | No | Yes | Nonsignificant | |
| CC | RR | 0.73 (0.60, 0.90) | 0.003 | Yes | Not large | No | Yes | Weak | |
| Largest MA (Obs) | RR | 0.95 (0.81, 1.11) | 0.493 | Yes | Very large | Yes | Yes | Nonsignificant | |
| Largest MA (Co) | NR | ||||||||
| Gastric cancer (any tea; any vs. none) | Obs | RR | 0.78 (0.70, 0.86) | <0.001 | Yes | Very large | Yes | No | Weak |
| Co | RR | 1.01 (0.91, 1.13) | 0.791 | Yes | Not large | No | Yes | Nonsignificant | |
| CC | RR | 0.70 (0.62, 0.80) | <0.001 | Yes | Very large | Yes | Yes | Weak | |
| Largest MA (Obs) | RR | 0.76 (0.72, 0.80) | 0.010 | Yes | Very large | Yes | Yes | Weak | |
| Largest MA (Co) | NR | ||||||||
| Leukemia (any tea; high vs. low) | Obs | RR | 0.55 (0.43, 0.72) | <0.001 | No | Not large | No | No | Weak |
| Co | NR | ||||||||
| CC | RR | 0.52 (0.38, 0.72) | <0.001 | No | Not large | No | No | Weak | |
| Largest MA (Obs) | RR | 0.55 (0.43, 0.72) | <0.001 | No | Not large | No | No | Weak | |
| Largest MA (Co) | NR | ||||||||
| Leukemia (any tea; any vs. none) | Obs | RR | 0.76 (0.65, 0.89) | <0.001 | No | Not large | No | No | Weak |
| Co | NR | ||||||||
| CC | RR | 0.69 (0.54, 0.87) | 0.002 | No | Not large | No | No | Weak | |
| Largest MA (Obs) | RR | 0.76 (0.65, 0.89) | <0.001 | No | Not large | No | No | Weak | |
| Largest MA (Co) | NR | ||||||||
| Liver cancer (green tea; any vs. none) | Obs | RR | 0.65 (0.48, 0.88) | 0.004 | Yes | Large | No | No | Weak |
| Co | RR | 0.83 (0.61, 1.11) | 0.205 | Yes | Large | No | Yes | Nonsignificant | |
| CC | RR | 0.46 (0.29, 0.74) | 0.001 | Yes | Large | No | Yes | Weak | |
| Largest MA (Obs) | RR | 0.65 (0.48, 0.88) | 0.004 | Yes | Large | No | No | Weak | |
| Largest MA (Co) | NR | ||||||||
| Lung cancer (any tea; any vs. none) | Obs | RR | 0.76 (0.67, 0.86) | <0.001 | Yes | Very large | No | Yes | Weak |
| Co | RR | 0.91 (0.76, 1.08) | 0.273 | Yes | Very large | No | No | Nonsignificant | |
| CC | RR | 0.69 (0.59, 0.79) | <0.001 | Yes | Very large | No | No | Weak | |
| Largest MA (Obs) | RR | 0.77 (0.68, 0.88) | <0.001 | Yes | Very large | No | No | Weak | |
| Largest MA (Co) | NR | ||||||||
| Ovarian cancer (any tea; any vs. none) | Obs | RR | 0.82 (0.71, 0.94) | 0.006 | Yes | Very large | No | No | Weak |
| Co | RR | 0.92 (0.79, 1.06) | 0.260 | Yes | Not large | Yes | Yes | Nonsignificant | |
| CC | RR | 0.76 (0.61, 0.95) | 0.014 | Yes | Very large | No | No | Weak | |
| Largest MA (Obs) | RR | 0.89 (0.80, 1.00)5 | 0.045 | Yes | Not large | Yes | Yes | Weak | |
| Largest MA (Co) | RR | 0.71 (0.55, 0.93) | 0.013 | Yes | Not large | No | Yes | Suggestive | |
| Thyroid cancer (any tea; high vs. low) | Obs | RR | 0.76 (0.61, 0.96) | 0.024 | Yes | Not large | Yes | No | Weak |
| Co | RR | 0.88 (0.56, 1.37) | 0.568 | N/A | N/A | N/A | Yes | N/A | |
| CC | RR | 0.74 (0.56, 0.97) | 0.029 | Yes | Large | Yes | No | Weak | |
| Largest MA (Obs) | RR | 0.76 (0.61, 0.96) | 0.024 | Yes | Not large | Yes | No | Weak | |
| Largest MA (Co) | NR | ||||||||
CC, case-control studies; Co, cohort studies; MA, meta-analysis; N/A, not applicable; NR, not reported; Obs, observational studies; PI, prediction interval.
Heterogeneity is defined as “Very large” when I2 > 75%, “Large” when 50% < I2 < 75%, and “Not large” when I2 < 50%.
The presence of small study effects is determined if the Egger P value is <0.10.
The definition of each category of the level of evidence is presented in Supplemental Table 1.
The value is rounded up (to 2 decimal places), and hence is statistically significant.
Summary effect with 95% CI value obtained from umbrella review combining meta-analyses of the same comparison.
FIGURE 3.
Statistically significant associations between cancer and tea exposure from umbrella review outlined by study design. The definition of each category of the level of evidence is presented in Supplemental Table 1. CC, case-control studies; Obs, observational studies.
Discussion
In this study, we summarized and analyzed original meta-analyses to critically appraise the strength and breadth of claimed associations between tea consumption and risk of cancer incidence. We found that consumption of any type of tea was associated with a lower risk of 11 types of cancer (oral, biliary tract, breast, colorectal, endometrial, gastric, leukemia, liver, lung, ovarian, and thyroid cancer). However, only the association between tea consumption and lower risk of oral cancer was supported by convincing evidence. Suggestive evidence was found for lowering risk of biliary tract, breast, endometrial, liver, and oral cancer.
The negative associations between tea and the risk of specific cancers can be explained by several biological mechanisms. In vitro and in vivo studies have suggested that tea polyphenols have preventive effects against several types of cancer, including oral (22), biliary tract (23), breast (24), endometrial (25), liver (26), colorectal (27), gastric (28), leukemia (29), lung (30), ovarian (31), and thyroid cancer (32). As key antioxidants in tea, polyphenols or tea catechins are thought to contribute to reducing the risk of some cancers, acting as scavengers of reactive oxygen species and potentially affecting transcription factors and enzyme activities (33). Some important polyphenols are (−)-epigallocatechin gallate (EGCG), (−)-epigallocatechin, (−)-epicatechin gallate, and (−)-epicatechin (34). EGCG is the most abundant tea catechin and is thought to play the most important role in inhibiting cancer initiation and progression (35). Tea polyphenols are thought to suppress the growth of cancer cells by various proposed mechanisms, such as inducing the apoptosis of cancer cells (36), suppression of receptor-dependent signaling pathways and angiogenesis (37), silencing genes related to epigenetic mechanisms such as methylation of DNA (38), and inhibiting the activities of enzymes (39). However, additional mechanistic studies and more in-depth analyses focusing on molecular changes are needed.
We found a total of 19 significant meta-analyses with combined individual studies comprising 11 types of cancer. Specific findings of our outcome must be interpreted with caution. In case of some cancers such as endometrial cancer, a suggestive level of evidence in combined observational studies (cohort and case-control) was found, whereas the results were nonsignificant in both cohort and case-control, respectively. The combination of different study designs possibly has an impact on the results due to the heterogeneity between studies. The potential heterogeneity in nutritional epidemiology comes from the difference in the definition of the consumption amounts and follow-up periods. To conclude, because the outcomes were nonsignificant in both study designs, the outcome with suggestive evidence of endometrial cancer could overestimate the true effect and could thus be reconsidered. In addition, a convincing level of evidence was derived from a single meta-analysis of oral cancer including 8 individual case-control studies only. In general, this is a small number for umbrella review, further underlining our concern related to the level of evidence.
In our findings, meta-analyses of cohort studies tended to show null results whereas those of case-control studies were statistically significant. Cohort studies are usually thought to have higher levels of evidence than case-control studies. In general, case-control studies are prone to biases, including the possibility of recall bias and the presence of selection bias. Thus, we can assume that there might be a spurious association in meta-analyses of case-control studies.
Furthermore, we compared the relative risks and level of evidence from our study with reports published by the WHO International Agency for Research on Cancer (IARC) and the World Cancer Research Fund Network/American Institute for Cancer Research (WCRF/AICR). The IARC report states that there is inadequate evidence for the carcinogenicity of tea consumption in humans, and hence states that tea is not classifiable as to its carcinogenicity (40, 41). Our study is in line with this statement, because no result showed that tea consumption was associated with an increased risk of cancer. Moreover, the WCRF/AICR reports have stated that the evidence is limited and no firm conclusions can be drawn for any type of cancer (see Table 4). This includes all cancer types that were found to have decreased associations in our study (42). Especially for oral cancer, where our analyses revealed convincing evidence, the WCRF states there is no evidence for this association. Also, the limited suggestive evidence reported by the WCRF for reduced risk of bladder cancer by tea consumption (RR = 0.94; 95% CI: 0.89, 0.98, for 1 cup/d increment) was not reproduced in our analyses, because only 1 meta-analysis included in our study was significant and our final meta-analysis remained nonsignificant in this context.
TABLE 4.
Summary and comparison of individual meta-analysis articles, our umbrella review, and the WCRF report on associations of tea and cancer1
| Meta-analyses from original articles | Umbrella review | WCRF | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cancer type | Tea type | No. of meta-analyses | D/N/I | Evidence C/S/W/N/X | Comparison | No. of studies | D/N/I | RR (95% CI) | Level of evidence2 | Level of evidence |
| Biliary tract | Any | 1 | 1/0/0 | 0/1/0/0/0 | Any vs. none | 8 | 4/4/0 | 0.77 (0.64, 0.92) | Suggestive | N/A |
| Brain cancer | Any | 1 | 0/1/0 | 0/0/0/1/0 | Any vs. none | 8 | 2/6/0 | 0.89 (0.76, 1.05) | Nonsignificant | N/A |
| Breast | Any | 3 | 1/2/0 | 0/0/1/2/0 | Any vs. none | 26 | 6/20/0 | 0.81 (0.71, 0.94) | Weak | Limited—no conclusion |
| High vs. low | 23 | 1/20/2 | 0.98 (0.90, 1.06) | Nonsignificant | ||||||
| Black | 9 | 0/8/1 | 0/0/1/8/0 | High vs. low | 28 | 1/27/0 | 0.98 (0.91, 1.06) | Nonsignificant | ||
| High vs. low (cohort) | 15 | 0/15/0 | 1.04 (0.97, 1.12) | Nonsignificant | ||||||
| High vs. low (CC) | 13 | 1/12/0 | 0.91 (0.80, 1.03) | Nonsignificant | ||||||
| Green | 15 | 7/8/0 | 0/3/3/8/1 | High vs. low (CC) | 11 | 6/5/0 | 0.75 (0.61, 0.92) | Suggestive | ||
| High vs. low | 16 | 6/10/0 | 0.82 (0.71, 0.96) | Weak | ||||||
| High vs. low (cohort) | 5 | 0/5/0 | 0.99 (0.83, 1.77) | Nonsignificant | ||||||
| Any vs. none (CC) | 5 | 3/2/0 | 0.94 (0.83, 1.05) | Nonsignificant | ||||||
| Any vs. none | 14 | 3/11/0 | 0.87 (0.76, 0.99) | Weak | ||||||
| Any vs. none (cohort) | 9 | 0/9/0 | 0.83 (0.62, 1.10) | Nonsignificant | ||||||
| Bladder | Any | 5 | 0/5/0 | 0/0/0/5/0 | High vs. low (CC) | 25 | 1/22/2 | 0.97 (0.87, 1.09) | Nonsignificant | Limited—suggestive: decreases risk RR = 0.94 (95% CI: 0.89, 0.98) |
| High vs. low (cohort) | 8 | 1/7/0 | 0.86 (0.65, 1.13) | Nonsignificant | ||||||
| High vs. low | 33 | 2/29/2 | 0.95 (0.86, 1.06) | Nonsignificant | ||||||
| Green | 2 | 1/1/0 | 0/1/0/1/0 | High vs. low | 5 | 0/5/0 | 1.03 (0.82, 1.31) | Nonsignificant | ||
| Colorectal | Any | 4 | 1/3/0 | 0/0/1/3/0 | High vs. low | 53 | 6/45/2 | 0.93 (0.87, 0.99) | Weak | Limited—no conclusion |
| Black | 1 | 0/1/0 | 0/0/0/1/0 | High vs. low | 20 | 2/14/4 | 0.99 (0.87, 1.13) | Nonsignificant | ||
| Green | 3 | 1/2/0 | 0/0/1/2/0 | High vs. low | 15 | 4/11/0 | 0.87 (0.75, 1.00)3 | Weak | ||
| Colon | Green | 1 | 0/1/0 | 0/0/0/1/0 | High vs. low | 11 | 1/9/1 | 0.98 (0.85, 1.12) | Nonsignificant | Limited—no conclusion |
| Rectal | Green | 1 | 0/1/0 | 0/0/0/1/0 | High vs. low | 3/6/0 | 0.97 (0.77, 1.22) | Nonsignificant | Limited—no conclusion | |
| Endometrial | Any | 5 | 3/2/0 | 0/3/0/2/0 | High vs. low | 16 | 3/12/1 | 0.90 (0.75, 1.09) | Nonsignificant | Limited—no conclusion |
| Increment of 1 cup/d | 5 | 0/4/1 | 1.04 (0.98, 1.10) | Nonsignificant | ||||||
| Black | 3 | 0/3/0 | 0/0/0/3/0 | High vs. low | 6 | 2/4/0 | 0.78 (0.61, 1.00)3 | Suggestive | ||
| Green | 3 | 3/0/0 | 0/3/0/0/0 | High vs. low | 10 | 1/8/1 | 0.99 (0.79, 1.23) | Nonsignificant | ||
| Esophageal | Green | 6 | 1/5/0 | 0/0/1/5/0 | High vs. low | 22 | 9/11/2 | 0.81 (0.62, 1.06) | Nonsignificant | N/A |
| Gastric | Any | 2 | 1/1/0 | 0/0/1/1/0 | Any vs. none | 56 | 23/30/3 | 0.78 (0.70, 0.86) | Weak | Limited—no conclusion |
| Increment of 3 cup/d | 5 | 0/5/0 | 0.98 (0.89, 1.08) | Nonsignificant | ||||||
| Black | 1 | 0/1/0 | 0/0/0/1/0 | High vs. low | 5 | 0/4/1 | 1.18 (0.79, 1.77) | Nonsignificant | ||
| Green | 14 | 4/10/0 | 0/1/3/10/0 | High vs. low | 30 | 3/25/2 | 0.93 (0.84, 1.04) | Nonsignificant | ||
| Gallbladder | Any | 2 | 0/2/0 | 0/0/0/2/0 | High vs. low | 4 | 2/2/0 | 0.57 (0.25, 1.30) | Nonsignificant | Limited—no conclusion |
| Any vs. none | 6 | 3/3/0 | 0.67 (0.40, 1.12) | Nonsignificant | ||||||
| Glioma | Any | 2 | 1/1/0 | 0/0/1/1/0 | Any vs. none | 4 | 0/4/0 | 0.67 (0.40, 1.12) | Nonsignificant | N/A |
| High vs. low | 4 | 1/3/0 | 0.57 (0.25, 1.30) | Nonsignificant | ||||||
| Renal cell carcinoma | Any | 1 | 0/1/0 | 0/0/0/1/0 | Any vs. none | 12 | 1/11/0 | 1.03 (0.88, 1.21) | Nonsignificant | Limited—no conclusion |
| Liver | Any | 2 | 0/2/0 | 0/0/0/2/0 | Any vs. none | 12 | 3/9/0 | 0.77 (0.57, 1.03) | Nonsignificant | N/A |
| Green | 2 | 2/0/0 | 0/1/1/0/0 | High vs. low | 11 | 2/9/0 | 0.87 (0.78, 0.98) | Suggestive | ||
| Any vs. none | 10 | 3/7/0 | 0.65 (0.48, 0.88) | Weak | ||||||
| Lung | Any | 1 | 1/0/0 | 0/0/1/0/0 | Any vs. none | 44 | 18/24/2 | 0.76 (0.68, 0.86) | Weak | Limited—no conclusion |
| Black | 2 | 0/2/0 | 0/0/0/2/0 | High vs. low | 14 | 4/10/0 | 0.86 (0.70, 1.05) | Nonsignificant | ||
| Green | 2 | 1/1/0 | 0/0/1/1/0 | High vs. low | 12 | 4/7/1 | 0.78 (0.61, 1.01) | Nonsignificant | ||
| Leukemia (in childhood) | Any | 8 | 0/8/0 | 0/0/0/8/0 | High vs. low | 9 | 0/8/1 | 0.93 (0.74, 1.18) | Nonsignificant | N/A |
| Any vs. none | 14 | 0/14/0 | 0.93 (0.82, 1.05) | Nonsignificant | ||||||
| Leukemia | Any | 2 | 2/0/0 | 0/0/2/0/0 | High vs. low | 8 | 4/4/0 | 0.55 (0.43, 0.72) | Weak | N/A |
| Any vs. none | 8 | 1/7/0 | 0.76 (0.65, 0.89) | Weak | ||||||
| Ovarian | Any | 9 | 5/4/0 | 0/1/4/4/0 | Any vs. none | 21 | 8/22/1 | 0.82 (0.71, 0.94) | Weak | Limited—no conclusion |
| Black | 1 | 0/1/0 | 0/0/0/1/0 | Any vs. none | 16 | 4/12/0 | 0.90 (0.78, 1.04) | Nonsignificant | ||
| Green | 2 | 1/1/0 | 0/0/1/1/0 | Any vs. none | 9 | 3/5/1 | 0.76 (0.57, 1.02) | Nonsignificant | ||
| Laryngeal | Any | 5 | 0/5/0 | 0/0/0/4/1 | High vs. low | 8 | 2/5/1 | 0.91 (0.67, 1.23) | Nonsignificant | Limited—no conclusion |
| Oral | Any | 1 | 1/0/0 | 1/0/0/0/0 | Any vs. none | 6 | 6/0/0 | 0.62 (0.55, 0.72) | Convincing | Limited—no conclusion |
| Any | 5 | 2/2/0 | 0/2/0/2/0 | High vs. low | 31 | 5/26/0 | 0.86 (0.80, 0.91) | Suggestive | ||
| Green | 1 | 1/0/0 | 0/1/0/0/0 | High vs. low | 5 | 1/4/0 | 0.82 (0.69, 0.96) | Suggestive | ||
| Oropharyngeal | Any | 3 | 1/2/0 | 0/0/1/1/1 | Any vs. none | 6 | 2/4/0 | 0.68 (0.45, 1.03) | Nonsignificant | Limited—no conclusion |
| Pharyngeal | Any | 3 | 0/3/0 | 0/0/0/2/1 | Any vs. none | 4 | 0/4/0 | 0.88 (0.74, 1.04) | Nonsignificant | Limited—no conclusion |
| Pancreatic | Any | 4 | 0/4/0 | 0/0/0/4/0 | High vs. low | 22 | 1/20/1 | 0.97 (0.85, 1.10) | Nonsignificant | Limited—no conclusion |
| Any vs. none | 29 | 2/25/2 | 0.99 (0.89, 1.10) | Nonsignificant | ||||||
| Green | 1 | 0/1/0 | 0/0/0/1/0 | High vs. low | 8 | 1/6/1 | 0.99 (0.78, 1.25) | Nonsignificant | ||
| Prostate | Any | 1 | 0/1/0 | 0/0/0/1/0 | High vs. low | 23 | 6/15/2 | 0.86 (0.71, 1.04) | Nonsignificant | Limited—no conclusion |
| Any vs. none | 29 | 7/20/2 | 0.87 (0.75, 1.01) | Nonsignificant | ||||||
| Black | 2 | 0/2/0 | 0/0/0/2/0 | High vs. low | 11 | 1/9/1 | 0.99 (0.82, 1.20) | Nonsignificant | ||
| Green | 4 | 0/4/0 | 0/0/0/4/0 | High vs. low | 9 | 3/6/0 | 0.73 (0.51, 1.06) | Nonsignificant | ||
| Thyroid | Any | 1 | 1/0/0 | 0/0/1/0/0 | High vs. low | 14 | 1/13/0 | 0.77 (0.61, 0.97) | Weak | N/A |
| Skin (nonmelanoma) | Any | 1 | 0/1/0 | 0/0/0/1/0 | Any vs. none | 8 | 4/4/0 | 0.88 (0.76, 1.02) | Nonsignificant | N/A |
CC, case-control studies; C/S/W/N/D, convincing/suggestive/weak/nonsignificant/not adequately assessed; D/N/I, decrease in risk/no association/increase in risk; N/A, not applicable; WCRF, World Cancer Research Fund network.
The definition of each category of the level of evidence is presented in Supplemental Table 1.
The value is rounded up (to 2 decimal places), and hence is statistically significant.
There are several reasons why our results differ from those of the WCRF. First, the criteria for grading evidence are different. According to the WCRF criteria, the evidence level is determined by the presence of between-study heterogeneity, the quality of studies, biological rationale, and the number of cohort studies included. However, except for the statistical heterogeneity, the rest were not included as criteria of our study. Second, the WCRF largely relied on prospective cohort studies, whereas our review included both cohort and case-control studies. Finally, the WCRF attempted a dose–response meta-analysis of cohort studies whenever possible and presented summary estimates in continuous scale (e.g., 1 cup/d). However, we used the effect estimates from each meta-analysis, which were largely based on categorical comparisons of high compared with low or any compared with none intakes instead of a continuous scale of tea intake.
Despite the above differences, our study has several strong points compared with the results from the WCRF. First, our study not only summarized the existing meta-analyses of the same subject but also performed the most updated meta-analyses with combined primary studies. This made it possible to understand the effects of tea consumption over a wider range and expanded statistical power due to the inclusion of overall studies. Second, the WCRF separately evaluated different sorts of tea (green and black tea), whereas we included any of type of tea in our analyses. Therefore, studies reporting the results of green or black tea but not tea overall contributed to “any” tea in our review but were excluded in the review performed by the WCRF. Again, this might have increased statistical power for the evaluation of tea.
The main strength of our umbrella review is the comprehensive summary and assessment of the level of evidence of tea consumption and cancer risk by including 64 original meta-analyses and 25 cancer sites. The umbrella review conducted in this study used standardized methods including the use of random-effects analysis and various measures of heterogeneity and publication bias. When studies reached the conventional threshold of statistical significance (P value <0.05), we further evaluated the results using the criteria for the level of evidence. The strength of classifying the level of evidence further provides information on the extent to which the different results are supported by evidence even though the standard significance threshold was reached. Also, we re-evaluated the results with a convincing level of evidence by applying the method of credibility ceilings. The aim of an umbrella review is to find out the trustworthy associations from prevailed significant result. In addition, the confirmation process of any significant results, such as by using credibility ceilings, has recently been suggested by some researchers. Otherwise, testing for excess significance bias has been proposed to evaluate the noteworthiness of statistically significant results. However, we did not evaluate ES due to lack of the data for calculating; also some authors opine that testing for ES has limited power, so it has not been recommended (43).
Generally, RR with 95% CI is used for determining associations between exposures and outcomes, but such associations must be questioned if the studies show high heterogeneity or publication bias (16). To overcome this issue, we used multiple criteria to estimate the results from the meta-analyses. In addition to 95% CI, 95% PI has been suggested in multiple umbrella reviews to yield robust conclusions (44). Moreover, we classified the I2 metrics to differentiate from conventional meta-analyses. If the heterogeneity using I2 metrics was large, the results were re-examined by considering the distribution of the effect size of the studies included. If more than half of the total number of studies were in the same direction, the analysis was not considered to have high heterogeneity. The rationale behind this decision is that I2 statistic can be biased in small meta-analyses and might not be useful in estimating heterogeneity with much precision in small studies (45). This was applied in the case of breast cancer (high compared with low green tea consumption) being classified as suggestive evidence despite showing very large heterogeneity (I2 > 75%) (see Supplemental Table 3).
However, several limitations to this study can be considered. First, we carried out recalculation and meta-analysis only with data that were available, therefore some individual studies could have been missed. Second, factors that could be relevant to the incidence of cancer, such as gender, ethnicity, age group, or smoking status, were not considered for the umbrella review. Some studies did not provide information needed to perform subgroup analysis. Third, the application of heterogeneity, publication bias, and 95% PI in the criteria for level of evidence might not be definitive. We included meta-analyses with both case-control studies and cohort studies. Because of the potential biases that can affect case-control studies, such as recall bias and selection bias, further prospective studies are needed before firm conclusions can be drawn. Furthermore, the summary effects of the meta-analyses about the same question might have variations due to multiple reasons (46). Also, evaluating any discrepancies or errors of individual meta-analyses was beyond the scope of our review. Another problem is that the summary effect size could be from a combination of studies with different measures, such as OR, RR, and HR. OR is statistically similar to RR when the outcome is uncommon (47). Moreover, the main comparisons for tea exposure used in this study (high compared with low, any compared with none) can vary over a wide range. The exact amount of tea polyphenol intake cannot be determined, because it can be affected by multiple factors such as individual tea preferences, the size of a cup, addition of sugar, different cultural practices, natural variability in polyphenol concentration in tea sorts, and other possible organic influencing factors.
Regardless of these limitations, the findings of this study show health implications that could be beneficial to individuals and populations. The association between tea consumption and the risk of oral cancer was supported by convincing evidence. It is possible that tea consumption can reduce the risk of some other cancers, but further prospective and mechanistic studies are needed before more robust conclusions can be made.
Supplementary Material
ACKNOWLEDGEMENTS
The authors’ responsibilities were as follows—TLK, GHJ, KHL, A Kronbichler, G Grosso, HJvdV, G Gamerith, FG, DA, JYK, BS, A Koyanagi, MS, SHH, ED, EC, LFMdR, ELG, JIS: contributed to the concept and design of the study; TLK, GHJ, JIS: acquired, collected, and analyzed the data; JIS: had final responsibility for the decision to submit for publication; and all authors: had full access to all the study data, participated in drafting the manuscript, and read and approved the final manuscript.
Notes
The authors reported no funding received for this study. BS is supported by a Clinical Lectureship (ICA-CL-2017-03-001) jointly funded by Health Education England (HEE) and the National Institute for Health Research (NIHR). BS is part funded by the NIHR Biomedical Research Centre at South London and Maudsley NHS Foundation Trust. BS is also supported by the Maudsley Charity, King's College London, and the NIHR South London Collaboration for Leadership in Applied Health Research and Care (CLAHRC) funding.
Author disclosures: The authors report no conflicts of interest.
This article presents independent research. The views expressed in this publication are those of the authors and not necessarily those of the acknowledged institutions.
Supplemental Tables 1–4 and Supplemental References are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/advances.
TLK and GHJ contributed equally to this work.
Abbreviations used: AICR, American Institute for Cancer Research; EGCG, epigallocatechin gallate; ES, excess of significance; IARC, International Agency for Research on Cancer; PI, prediction interval; WCRF, World Cancer Research Fund Network.
Contributor Information
Tai Lim Kim, Yonsei University College of Medicine, Severance Hospital, Seoul, Korea.
Gwang Hun Jeong, College of Medicine, Gyeongsang National University, Jinju, Korea.
Jae Won Yang, Department of Nephrology, Yonsei University Wonju College of Medicine, Wonju, Korea.
Keum Hwa Lee, Department of Pediatrics, Yonsei University College of Medicine, Seoul, Korea; Division of Pediatric Nephrology, Severance Children's Hospital, Seoul, Korea.
Andreas Kronbichler, Department of Internal Medicine IV (Nephrology and Hypertension), Medical University Innsbruck, Innsbruck, Austria.
Hans J van der Vliet, Department of Medical Oncology, Amsterdam UMC, VU University, Cancer Center Amsterdam, Amsterdam, The Netherlands.
Giuseppe Grosso, Department of Biomedical and Biotechnological Science, School of Medicine, University of Catania, Catania, Italy.
Fabio Galvano, Department of Biomedical and Biotechnological Science, School of Medicine, University of Catania, Catania, Italy.
Dagfinn Aune, Department of Epidemiology and Biostatistics, School of Public Health, Imperial College London, London, UK; Department of Nutrition, Bjørknes University College, Oslo, Norway; Department of Endocrinology, Morbid Obesity and Preventive Medicine, Oslo University Hospital, Oslo, Norway.
Jong Yeob Kim, Yonsei University College of Medicine, Severance Hospital, Seoul, Korea.
Nicola Veronese, National Research Council, Neuroscience Institute, Aging Branch, Padova, Italy.
Brendon Stubbs, Department of Psychological Medicine, Institute of Psychiatry, Psychology and Neuroscience, King's College London, London, UK; South London and Maudsley NHS Foundation Trust, London, UK; Positive Ageing Research Institute, Faculty of Health, Social Care, Medicine and Education, Anglia Ruskin University, Chelmsford, UK.
Marco Solmi, Department of Neuroscience, University of Padova, Padova, Italy.
Ai Koyanagi, Parc Sanitari Sant Joan de Déu/CIBERSAM, Universitat de Barcelona, Barcelona, Spain; ICREA, Barcelona, Spain.
Sung Hwi Hong, Yonsei University College of Medicine, Severance Hospital, Seoul, Korea; Department of Global Health and Population, Harvard T.H. Chan School of Public Health, Boston, MA, USA.
Elena Dragioti, Pain and Rehabilitation Centre, and Department of Health, Medicine and Caring Sciences, Linköping University, Linköping, Sweden.
Eunyoung Cho, Department of Dermatology, The Warren Alpert Medical School, Brown University, Providence, RI, USA; Channing Division of Network Medicine, Department of Medicine, Brigham and Women's Hospital and Harvard Medical School, Boston, MA, USA.
Leandro F M de Rezende, Universidade Federal de São Paulo, Escola Paulista de Medicina, Departamento de Medicina Preventiva, São Paulo, Brazil.
Edward L Giovannucci, Channing Division of Network Medicine, Department of Medicine, Brigham and Women's Hospital and Harvard Medical School, Boston, MA, USA; Department of Nutrition, Harvard T.H. Chan School of Public Health, Boston, MA, USA.
Jae Il Shin, Department of Pediatrics, Yonsei University College of Medicine, Seoul, Korea; Division of Pediatric Nephrology, Severance Children's Hospital, Seoul, Korea.
Gabriele Gamerith, Internal Medicine V, Department of Hematology & Oncology, Medical University Innsbruck, Innsbruck, Austria.
References
- 1. Weisburger JH. Tea and health: a historical perspective. Cancer Lett. 1997;114(1-2):315–7. [DOI] [PubMed] [Google Scholar]
- 2. Graham HN. Green tea composition, consumption, and polyphenol chemistry. Prev Med. 1992;21(3):334–50. [DOI] [PubMed] [Google Scholar]
- 3. Lin YS, Tsai YJ, Tsay JS, Lin JK. Factors affecting the levels of tea polyphenols and caffeine in tea leaves. J Agric Food Chem. 2003;51(7):1864–73. [DOI] [PubMed] [Google Scholar]
- 4. Graham HN. Tea: the plant and its manufacture; chemistry and consumption of the beverage. Prog Clin Biol Res. 1984;158:29–74. [PubMed] [Google Scholar]
- 5. Khan N, Mukhtar H. Tea polyphenols for health promotion. Life Sci. 2007;81(7):519–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mukhtar H, Ahmad N. Tea polyphenols: prevention of cancer and optimizing health. Am J Clin Nutr. 2000;71(6 Suppl):1698S–702S.; discussion 703S–4S. [DOI] [PubMed] [Google Scholar]
- 7. Yuan JM. Cancer prevention by green tea: evidence from epidemiologic studies. Am J Clin Nutr. 2013;98(6 Suppl):1676S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Boehm K, Borrelli F, Ernst E, Habacher G, Hung SK, Milazzo S, Horneber M. Green tea (Camellia sinensis) for the prevention of cancer. Cochrane Database Syst Rev. 2009(3):CD005004 doi:10.1002/14651858.CD005004.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Poole R, Kennedy OJ, Roderick P, Fallowfield JA, Hayes PC, Parkes J. Coffee consumption and health: umbrella review of meta-analyses of multiple health outcomes. BMJ. 2017;359:j5024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Li X, Meng X, Timofeeva M, Tzoulaki I, Tsilidis KK, Ioannidis JP, Campbell H, Theodoratou E. Serum uric acid levels and multiple health outcomes: umbrella review of evidence from observational studies, randomised controlled trials, and Mendelian randomisation studies. BMJ. 2017;357:j2376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Khan SU, Khan MU, Riaz H, Valavoor S, Zhao D, Vaughan L, Okunrintemi V, Riaz IB, Khan MS, Kaluski E et al. Effects of nutritional supplements and dietary interventions on cardiovascular outcomes: an umbrella review and evidence map. Ann Intern Med. 2019;171(3):190–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Riley RD, Higgins JP, Deeks JJ. Interpretation of random effects meta-analyses. BMJ. 2011;342:d549. [DOI] [PubMed] [Google Scholar]
- 14. Michael Borenstein LVH, Julian P, Higgins T, Rothstein HR. Introduction to meta-analysis. Wiley; 2009. [Google Scholar]
- 15. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Belbasis L, Bellou V, Evangelou E, Ioannidis JP, Tzoulaki I. Environmental risk factors and multiple sclerosis: an umbrella review of systematic reviews and meta-analyses. Lancet Neurol. 2015;14(3):263–73. [DOI] [PubMed] [Google Scholar]
- 17. Bellou V, Belbasis L, Tzoulaki I, Middleton LT, Ioannidis JPA, Evangelou E. Systematic evaluation of the associations between environmental risk factors and dementia: an umbrella review of systematic reviews and meta-analyses. Alzheimers Dement. 2017;13(4):406–18. [DOI] [PubMed] [Google Scholar]
- 18. He Y, Li X, Gasevic D, Brunt E, McLachlan F, Millenson M, Timofeeva M, Ioannidis JPA, Campbell H, Theodoratou E. Statins and multiple noncardiovascular outcomes: umbrella review of meta-analyses of observational studies and randomized controlled trials. Ann Intern Med. 2018;169(8):543–53. [DOI] [PubMed] [Google Scholar]
- 19. Fusar-Poli P, Radua J. Ten simple rules for conducting umbrella reviews. Evid Based Mental Health. 2018;21(3):95–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Salanti G, Ioannidis JP. Synthesis of observational studies should consider credibility ceilings. J Clin Epidemiol. 2009;62(2):115–22. [DOI] [PubMed] [Google Scholar]
- 21. Papatheodorou SI, Tsilidis KK, Evangelou E, Ioannidis JP. Application of credibility ceilings probes the robustness of meta-analyses of biomarkers and cancer risk. J Clin Epidemiol. 2015;68(2):163–74. [DOI] [PubMed] [Google Scholar]
- 22. Babich H, Krupka ME, Nissim HA, Zuckerbraun HL. Differential in vitro cytotoxicity of (-)-epicatechin gallate (ECG) to cancer and normal cells from the human oral cavity. Toxicol In Vitro. 2005;19(2):231–42. [DOI] [PubMed] [Google Scholar]
- 23. Wang J, Pan Y, Hu J, Ma Q, Xu Y, Zhang Y, Zhang F, Liu Y. Tea polyphenols induce S phase arrest and apoptosis in gallbladder cancer cells. Braz J Med Biol Res. 2018;51(4):e6891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chen X, Li Y, Lin Q, Wang Y, Sun H, Wang J, Cui G, Cai L, Dong X. Tea polyphenols induced apoptosis of breast cancer cells by suppressing the expression of survivin. Sci Rep. 2015;4:4416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Manohar M, Fatima I, Saxena R, Chandra V, Sankhwar PL, Dwivedi A. (-)-Epigallocatechin-3-gallate induces apoptosis in human endometrial adenocarcinoma cells via ROS generation and p38 MAP kinase activation. J Nutr Biochem. 2013;24(6):940–7. [DOI] [PubMed] [Google Scholar]
- 26. Liang J, Li F, Fang Y, Yang W, An X, Zhao L, Xin Z, Cao L, Hu Q. Cytotoxicity and apoptotic effects of tea polyphenol-loaded chitosan nanoparticles on human hepatoma HepG2 cells. Mater Sci Eng C Mater Biol Appl. 2014;36:7–13. [DOI] [PubMed] [Google Scholar]
- 27. Sanchez-Tena S, Alcarraz-Vizan G, Marin S, Torres JL, Cascante M. Epicatechin gallate impairs colon cancer cell metabolic productivity. J Agric Food Chem. 2013;61(18):4310–7. [DOI] [PubMed] [Google Scholar]
- 28. Park JS, Khoi PN, Joo YE, Lee YH, Lang SA, Stoeltzing O, Jung YD. EGCG inhibits recepteur d'origine nantais expression by suppressing Egr-1 in gastric cancer cells. Int J Oncol. 2013;42(3):1120–6. [DOI] [PubMed] [Google Scholar]
- 29. Zhang L, Chen QS, Xu PP, Qian Y, Wang AH, Xiao D, Zhao Y, Sheng Y, Wen XQ, Zhao WL. Catechins induced acute promyelocytic leukemia cell apoptosis and triggered PML-RARalpha oncoprotein degradation. J Hematol Oncol. 2014;7:75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gu Q, Hu C, Chen Q, Xia Y. Tea polyphenols prevent lung from preneoplastic lesions and effect p53 and bcl-2 gene expression in rat lung tissues. Int J Clin Exp Pathol. 2013;6(8):1523–31. [PMC free article] [PubMed] [Google Scholar]
- 31. Chen H, Landen CN, Li Y, Alvarez RD, Tollefsbol TO. Epigallocatechin gallate and sulforaphane combination treatment induce apoptosis in paclitaxel-resistant ovarian cancer cells through hTERT and Bcl-2 down-regulation. Exp Cell Res. 2013;319(5):697–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. De Amicis F, Perri A, Vizza D, Russo A, Panno ML, Bonofiglio D, Giordano C, Mauro L, Aquila S, Tramontano D et al. Epigallocatechin gallate inhibits growth and epithelial-to-mesenchymal transition in human thyroid carcinoma cell lines. J Cell Physiol. 2013;228(10):2054–62. [DOI] [PubMed] [Google Scholar]
- 33. Higdon JV, Frei B. Tea catechins and polyphenols: health effects, metabolism, and antioxidant functions. Crit Rev Food Sci Nutr. 2003;43(1):89–143. [DOI] [PubMed] [Google Scholar]
- 34. Yang CS, Maliakal P, Meng X. Inhibition of carcinogenesis by tea. Annu Rev Pharmacol Toxicol. 2002;42:25–54. [DOI] [PubMed] [Google Scholar]
- 35. Ju J, Lu G, Lambert JD, Yang CS. Inhibition of carcinogenesis by tea constituents. Semin Cancer Biol. 2007;17(5):395–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Yang GY, Liao J, Kim K, Yurkow EJ, Yang CS. Inhibition of growth and induction of apoptosis in human cancer cell lines by tea polyphenols. Carcinogenesis. 1998;19(4):611–6. [DOI] [PubMed] [Google Scholar]
- 37. Shimizu M, Deguchi A, Lim JT, Moriwaki H, Kopelovich L, Weinstein IB. (-)-Epigallocatechin gallate and polyphenon E inhibit growth and activation of the epidermal growth factor receptor and human epidermal growth factor receptor-2 signaling pathways in human colon cancer cells. Clin Cancer Res. 2005;11(7):2735–46. [DOI] [PubMed] [Google Scholar]
- 38. Fang MZ, Wang Y, Ai N, Hou Z, Sun Y, Lu H, Welsh W, Yang CS. Tea polyphenol (-)-epigallocatechin-3-gallate inhibits DNA methyltransferase and reactivates methylation-silenced genes in cancer cell lines. Cancer Res. 2003;63(22):7563–70. [PubMed] [Google Scholar]
- 39. Yang CS, Wang X, Lu G, Picinich SC. Cancer prevention by tea: animal studies, molecular mechanisms and human relevance. Nat Rev Cancer. 2009;9(6):429–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Coffee, tea, mate, methylxanthines and methylglyoxal IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Lyon, February 27 to March 6, 1990. IARC Monogr Eval Carcinog Risks Hum. 1991;51:1–513. [PMC free article] [PubMed] [Google Scholar]
- 41. WCRF/AICR Continuous update project expert report. Judging the evidence. Washington, DC; World Cancer Research Fund/American Institute for Cancer Research; 2018. [Google Scholar]
- 42. WCRF/AIRC Continuous update project expert report. Non-alcoholic drinks and the risk of cancer. Washington, DC; World Cancer Research Fund/American Institute for Cancer Research; 2018. [Google Scholar]
- 43. Higgins JPT, Green S. Cochrane handbook for systematic reviews of interventions version 5.1.0 [Internet]. Cochrane Training; updated March 2011. Available from: www.handbook.cochrane.org. [Google Scholar]
- 44. Graham PL, Moran JL. Robust meta-analytic conclusions mandate the provision of prediction intervals in meta-analysis summaries. J Clin Epidemiol. 2012;65(5):503–10. [DOI] [PubMed] [Google Scholar]
- 45. von Hippel PT. The heterogeneity statistic I(2) can be biased in small meta-analyses. BMC Med Res Methodol. 2015;15:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Khamis AM, El Moheb M, Nicolas J, Iskandarani G, Refaat MM, Akl EA. Several reasons explained the variation in the results of 22 meta-analyses addressing the same question. J Clin Epidemiol. 2019;113:147–58. [DOI] [PubMed] [Google Scholar]
- 47. Woodward M. Epidemiology: study design and data analysis. 3rd ed. Chapman and Hall/CRC; 2014. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.