ABSTRACT
Anemia is a multifactorial condition arising from inadequate nutrition, infection, chronic disease, and genetic-related etiologies. Our aim was to assess the impact of nutrition-sensitive and nutrition-specific interventions on hemoglobin (Hb) concentrations and anemia to inform the prioritization and scale-up of interventions to address the multiple causes of anemia. We performed a meta-review synthesis of information by searching multiple databases for reviews published between 1990 and 2017 and used standard methods for conducting a meta-review of reviews, including double independent screening, extraction, and quality assessment. Quantitative pooling and narrative syntheses were used to summarize information. Hb concentration and anemia outcomes were pooled in specific population groups (children aged <5 y, school-age children, and pregnant women). Methodological quality of the systematic reviews was assessed using Assessing the Methodological Quality of Systematic Reviews (AMSTAR) criteria. Of the 15,444 records screened, we identified 118 systematic reviews that met inclusion criteria. Reviews focused on nutrition-specific interventions (96%). Daily and intermittent iron supplementation, micronutrient powders, malaria treatment, use of insecticide-treated nets (ITNs), and delayed cord clamping were associated with increased Hb concentration in children aged <5 y. Among children older than 5 y, daily and intermittent iron supplementation and deworming, and in pregnant women, daily iron-folic acid supplementation, use of ITNs, and delayed cord clamping, were associated with increased Hb concentration. Similar results were obtained for the reduced risk of anemia outcome. This meta-review suggests the importance of nutrition-specific interventions for anemia and highlights the lack of evidence to understand the influence of nutrition-sensitive and multifaceted interventions on the condition.
Keywords: anemia, hemoglobin concentration, meta-review, systematic review, nutrition interventions
This meta-review reports on significant impacts on anemia from nutrition-specific interventions and highlights the lack of evidence for nutrition-sensitive interventions on anemia.
Introduction
Anemia is one of the world's most common public health problems. Globally, 43% of children aged <5 y, 38% of pregnant women, and 29% of women of reproductive age are anemic, and relatively little progress has been made in reducing prevalence since 1995 (1). Anemia is associated with negative health consequences at every stage of the life cycle. Anemia in women of reproductive age increases the risk of having low-birthweight infants, preterm delivery, and newborn and maternal mortality (2, 3). Among children, anemia is associated with delayed physical, cognitive, and socioemotional development and general poor health. Iron deficiency anemia has been shown to account for a substantial portion of disability-adjusted life years lost, and there can be negative societal impacts arising from anemia burden, such as increased health care costs and lost economic productivity (3).
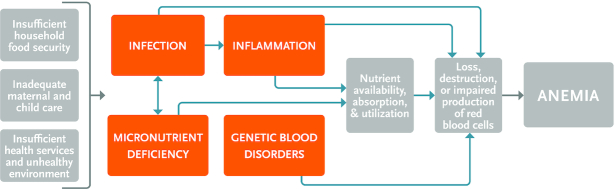
Anemia is a multifactorial condition arising from inadequate nutrition, infection, chronic disease, and genetic-related etiologies. Globally, the most important causes of anemia are iron deficiency, hookworm infestation, sickle-cell disorders, thalassemia, schistosomiasis, and malaria, although the contribution of the causal factor varies according to context (4). Given the complexity of the anemic condition, a range of interventions can be required. The Lancet’s Maternal and Child Nutrition Study Group defined 2 categories of nutritional interventions: nutrition-sensitive and nutrition-specific (5). Nutrition-specific interventions are those directed at the immediate causes of anemia, like diet and infection, whereas nutrition-sensitive interventions address underlying determinants of hemoglobin (Hb) concentration and anemia, such as food insecurity and insufficient maternal and child health care services. By considering both types of nutrition interventions, we identify the current state of the evidence on the impact of interventions that address the immediate and underlying determinants of anemia. To inform our search, we used a framework for the causal factors of anemia that was developed by the Biomarkers Reflecting Inflammation and Nutritional Determinants of Anemia (BRINDA) project and adapted by the Strengthening Partnerships, Results, and Innovations in Nutrition Globally (SPRING) project (Figure 1).
FIGURE 1.
Anemia causal framework.
A meta-review collates information from systematic reviews and synthesizes the results, with the intention of facilitating evidence-based decision-making by policy makers and other practitioners (6, 7). This meta-review aims to determine the effects of nutrition-specific and nutrition-sensitive interventions on the outcomes of Hb concentrations and anemia in the general population. This resource, which synthesizes the impact of nutrition interventions over nearly 3 decades, can be used by public health and nutrition program and policy practitioners to inform more effective interventions to reduce the burden of anemia among children and women.
Methods
Inclusion criteria
We performed a meta-review of systematic reviews to assess the effects of nutrition-specific and nutrition-sensitive interventions on Hb concentration or anemia. The criteria for inclusion were as follows: systematic reviews of randomized and nonrandomized controlled comparative studies that enrolled generally healthy target populations, included people aged 6 mo to 49 y, and measured Hb concentration or anemia as primary outcomes. We included studies of newborn children related to reviews on delayed cord clamping, because the outcomes are studied in this age group. We included studies published from 1990 to April 2017. We excluded all primary research studies, systematic reviews where a full text was not available, commentaries, and narrative reviews. As a starting point, we used an anemia causal framework to identify interventions targeting micronutrient deficiencies, infection, and genetic blood disorders (Figure 1). Classification of the interventions into the categories of nutrition-specific or nutrition-sensitive was based on definitions from the Lancet series on maternal and child nutrition applied to the causes in the anemia framework (5). Nutrition-specific interventions are targeted at immediate factors affecting micronutrient status and anemia: supplementation, fortification, infant and young child feeding, promotion of dietary diversity, delayed cord clamping, deworming, and malaria prevention and treatment [insecticide-treated nets (ITNs), indoor residual spraying (IRS), and treatment]. Nutrition-sensitive interventions are targeted at underlying factors and include agriculture, biofortification, child development, child protection, conditional cash transfers, education, family planning, maternal mental health, social safety nets, water, sanitation, and hygiene (WASH), and women's empowerment. The study arms with the above-listed interventions were compared with study arms that included control and accepted standards of care.
Search strategy
Keywords for the nutrition-specific and nutrition-sensitive interventions were identified and used for searching the following databases: MEDLINE (OVID), Embase, Cochrane Library, CINAHL, Global Health, and Scopus. A complete report of the search strategy, including databases, keywords, and dates, is available (Supplemental Table 1).
Data collection
We conducted double independent abstract screening using Abstrackr (8). Titles and abstracts were assigned to all coauthors, ensuring that 2 coauthors screened each title and abstract to assess its eligibility for inclusion in the review. We subsequently conducted a double independent screening of the full text of the articles for those screened-in titles. In both stages, all disagreements were resolved by consensus between the 2 authors or, if required, a third.
After full-text screening, we extracted data from the reviews into a template that included study identification details (review authors, title, journal, and year), study aims and design elements (systematic search strategy used, number of studies included, sample size, descriptions of intervention and comparison groups, and summary outcomes), and any subgroup analyses (age, sex, and population, among others). We used the standardized methods outlined in the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (9).
Data synthesis and analyses
Quantitative pooling and narrative syntheses were used to summarize information from reviews (6). For each predefined intervention, we listed the systematic reviews providing information on the impact on Hb concentration and anemia. Unique trials (and summary effect sizes) included in the reviews that qualified for quantitative pooling were then listed to ensure they were not double counted across multiple reviews. Studies that shared similarities across populations, interventions, comparators, and outcomes qualified for quantitative pooling. We did not use the unit less effect size metric to pool studies. The unit less effect size is useful for comparison across different settings, especially when there exists a possibility of the outcomes being measured using different methods. This is not the case with Hb concentration and anemia. The measurement of Hb is usually reliable, and the conversion of Hb concentration to anemia prevalence uses standardized metrics proposed by the WHO. Our rationale for not using the metric was the limitation in the translation of the practical significance of the effect size (Cohen d, Hedges g, etc.). Whereas many authors have proposed classification schemes that use cutoffs to interpreting effect sizes, in our case, the mean difference in Hb for different interventions was a more useful measure to evaluate practical impact of the interventions (10). Another limitation in calculating effect sizes was the unavailability of pooled SDs in the studies—the articles reported sample SDs, which can lead to bias if the samples are not representative of the general population. In the interest of interpretation, we decided to maintain the outcome metrics of mean difference.
The pooled analysis was conducted using random-effects modeling in OpenMeta[Analyst] software (11). Primary data from individual studies were extracted from reviews to avoid double-counting from studies included in >1 review, and to align outcome metrics and populations. Effect sizes using mean difference (MD) in Hb concentration (grams per deciliter) were pooled separately, as were those reporting RR for anemia between the intervention and control groups.
We carried out subgroup analyses by age defined by children aged 6–59 mo and those aged >5 y. Among women, we included those of reproductive age, who were pregnant, at or near term (37 wk), and those who were 6 wk postpartum. We performed our search with no limitation on sex of the population; however, reviews that included men did not meet the inclusion criteria. When it was not possible to pool the reviews quantitatively due to heterogeneity between studies, we synthesized them qualitatively in a narrative review. We judged the comprehensiveness of each review by examining included studies and noting overlaps of studies in other reviews evaluating the same intervention. We identified data gaps in the systematic reviews for the various interventions.
We evaluated the methodological quality of the included systematic reviews using Assessing the Methodological Quality of Systematic Reviews (AMSTAR) criteria (9). This approach facilitates evaluating each review as “yes” or “no” for 11 criteria: a priori design, duplicate study selection and data extraction, comprehensive literature search, status of publication used as inclusion criteria, list of studies provided, characteristics of included studies, scientific quality of included studies assessed and documented, scientific quality of included studies used appropriately for formulating conclusions, methods used to combine appropriate findings, likelihood of publication bias assessed, and conflict of interest stated.
The protocol for this meta-review was registered on PROSPERO and can be accessed at http://www.crd.york.ac.uk/PROSPERO/display_record.asp?ID=CRD42016044120.s
Results
Search results
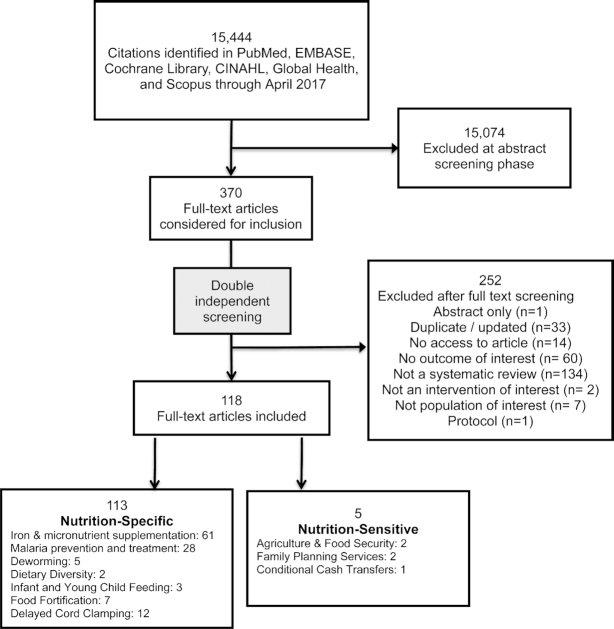
The search identified 15,444 records, each screened by title and abstract. After the first stage of review, 370 abstracts were selected for full-text screening (Figure 2). Of these, 252 articles were excluded. The remaining 118 reviews were extracted and provide the data for this analysis. Reviews were categorized into nutrition-specific (n = 113) or nutrition-sensitive (n = 5) interventions. Total numbers for each intervention type exceeded total reviews, because some systematic reviews provided information on >1 intervention (Supplemental Table 2).
FIGURE 2.
Flow diagram.
Quality of included studies (AMSTAR criteria)
All 118 systematic reviews met some or all of the AMSTAR criteria (Table 1). Those criteria met by >90% of the systematic reviews were: #3 (comprehensive literature search); #6 (characteristics of included studies); and #9 (methods used to combine findings appropriate). Those less frequently (<60%) met by the group of reviews we examined were: #5 (list of studies provided, both included and excluded); #8 (scientific quality of included studies used appropriately for formulating conclusions); #10 (likelihood of publication bias assessed); and #11 (conflict of interest stated) (Table 1). The complete quality rating for all the domains for all included studies is found in Supplemental Table 3.
TABLE 1.
Rating of included studies according to AMSTAR quality criteria1
| Criterion # | AMSTAR quality criteria | Yes n (%) | No n (%) |
|---|---|---|---|
| 1 | A priori design | 105 (89) | 13 (11) |
| 2 | Duplicate study selection and data extraction | 86 (73) | 32 (27) |
| 3 | Comprehensive literature search | 108 (92) | 10 (8) |
| 4 | Status of publication used as inclusion criteria (gray literature included) | 89 (75) | 29 (25) |
| 5 | List of studies provided, both included and excluded | 68 (58) | 50 (42) |
| 6 | Characteristics of included studies | 107 (91) | 11 (9) |
| 7 | Scientific quality of included studies assessed and documented | 88 (75) | 30 (25) |
| 8 | Scientific quality of included studies used appropriately for formulating conclusions | 69 (58) | 49 (42) |
| 9 | Methods used to combine findings appropriate | 109 (92) | 9 (8) |
| 10 | Likelihood of publication bias assessed | 63 (53) | 55 (47) |
| 11 | Conflict of interest stated | 49 (42) | 69 (58) |
1AMSTAR, Assessing the Methodological Quality of Systematic Reviews.
Quantitative pooling
Iron supplementation
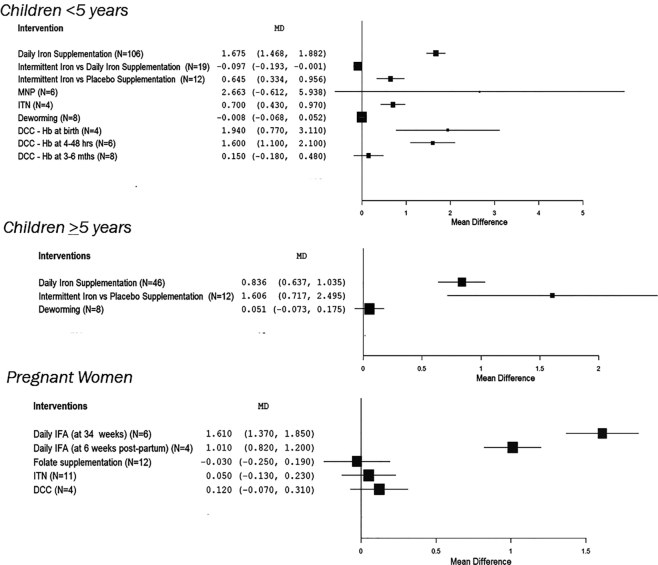
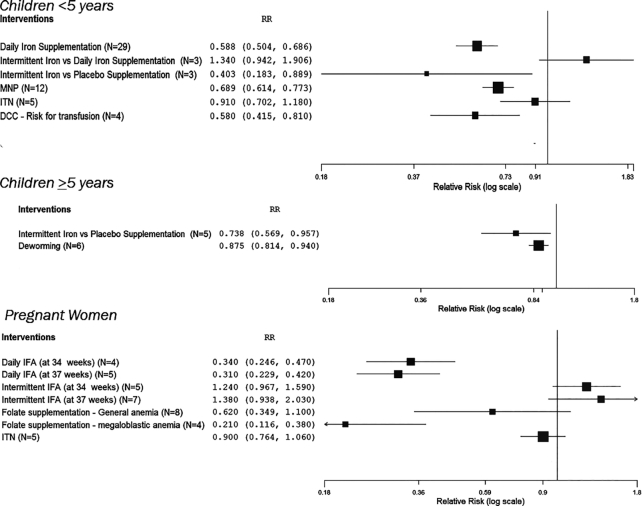
Daily iron supplementation compared with control increased Hb concentration by 1.7g/ dL in children aged <5 y, and by 0.8g/ dL in children aged >5 y (Table 2, Figures 3 and 4). It decreased the risk of anemia by 41% in children <5 y (Table 2, Figures 3 and 4). Trials comparing intermittent iron supplementation with a placebo in children <5 y and >5 y showed increases in Hb concentration but reported lower Hb concentrations when compared with children who received daily iron supplementation (Table 2, Figures 3 and 4). In daily iron supplementation studies, we found that Hb concentration showed larger increases if the children were anemic at baseline, compared with when their anemia status at baseline was unknown (Supplemental Table 4). There was only 1 age group—that of children between 6 and 23 mo of age—in which we could pool and compare outcomes between anemic children (n = 18) and nonanemic children (n = 3) and found that the increase in Hb concentration in anemic children at baseline was much larger than in nonanemic children (Supplemental Table 4). There were not enough studies on intermittent iron supplementation with baseline anemic status to draw any comparative conclusions (Table 2, Supplemental Table 4). There were no systematic reviews on providing iron without any other micronutrients in pregnant women that had baseline anemia status.
TABLE 2.
Summary of intervention effect sizes for hemoglobin concentrations and anemia1
| Outcome: Hb, g/dL | Outcome: anemia | |||||
|---|---|---|---|---|---|---|
| Interventions | References | Population group | n | MD (95% CI; P value when NS) | n | RR (95% CI) |
| Daily iron supplementation | (13, 19–43) | Children <5 y | 106 | 1.675 (1.468, 1.882)*** | 29 | 0.588 (0.503, 0.686)*** |
| Children ≥5 y | 46 | 0.836 (0.638, 1.035; P = 0.101) | ||||
| Intermittent iron supplementation | (13, 19–43) | Children <5 y | 19 | −0.097 (−0.193, −0.001)* (vs. daily iron) | 3 | 1.340 (0.943, 1.906; P = 0.103) (vs. daily iron) |
| 12 | 0.645 (0.334, 0.956)*** (vs. placebo) | 3 | 0.403 (0.183, 0.889)* (vs. placebo) | |||
| Children ≥5 y | 6 | −0.044 (−0.217, −0.130; P = 0.088) (vs. daily iron) | 5 | 0.738 (0.569, 0.957)* (vs. placebo) | ||
| 10 | 1.606 (0.717, 2.495)*** (vs. placebo) | ND | ND | |||
| Daily IFA | (13, 27, 40, 12, 44–46) | Women at or near term (34 wk) | 6 | 1.61 (1.37, 1.85)*** | 4 | 0.34 (0.25, 0.47)*** |
| Mothers 6 wk postpartum | 4 | 1.01 (0.81, 1.2)*** | ND | |||
| Women at term (37 wk) | ND | ND | 5 | 0.31 (0.23, 0.42)*** | ||
| Intermittent IFA | (13, 27, 29, 40, 12, 44–46) | Women near or at term (34 wk) | ND | ND | 5 | 1.24 (0.96,1.59; P = 0.098) |
| Women at term (37 wk) | ND | 7 | 1.38 (0.94, 2.03; P = 0.1) | |||
| MNP | (47–51) | Children <5 y | 6 | 2.663 (−0.611, 5.938; P = 0.111) | 12 | 0.689 (0.615, 0.773)*** |
| Folate | (52) | Pregnant women | 12 | −0.03 (−0.25, 0.19)*** | 8 | General anemia: 0.62 (0.35, 1.10; P = 0.1) |
| ND | 4 | Megaloblastic anemia: 0.21 (0.11, 0.38)*** | ||||
| Malaria treatment | (53–57) | All ages | 6 | Day 28: 0.12 (0.03, 0.21)** | 3 | 0.93 (0.77, 1.12; P = 0.451) |
| 4 | Day 42: 0.26 (0.08, 0.44)** | ND | ND | |||
| IPTp | (58–67) | Pregnant women | 5 | 0.41 (0.27, 0.54)*** | 23 | 0.9 (0.84, 0.95)*** |
| ITN | (68–70) | Children <5 y | 4 | 0.7 (0.44, 0.97)*** | 5 | 0.91 (0.7, 1.18; P = 0.46) |
| Pregnant women | 11 | 0.05 (−0.12, 0.23; P = 0.539) | 5 | 0.9 (0.77, 1.06; P = 0.195) | ||
| Deworming | (71–75) | Children <5 y | 8 | −0.008 (−0.069, 0.052; P = 0.789) | ||
| Children ≥5 y | 8 | 0.051 (−0.072, 0.175; P = 0.415) | 6 | 0.875 (0.816, 0.940)*** | ||
| Delayed cord clamping | (76–87) | Full-term newborns | 4 | Hb at birth: 1.94 (0.76, 3.11)*** | 4 | Outcome is risk for transfusion: 0.58 (0.42, 0.81)*** |
| 6 | Hb at 4–48 h: 1.6 (1.1, 2.1)*** | ND | ||||
| 8 | Hb at 3–6 mo: 0.15 (−0.19, 0.48; P = 0.389) | ND | ||||
| Mothers | 4 | 0.12 (−0.06, 0.31; P = 0.182) | ND | |||
1 *,**,***Significant effect size: *P < 0.05, **P < 0.01, ***P < 0.001. Hb, hemoglobin; IFA, iron-folic acid; IPTp, intermittent preventive treatment in pregnancy; ITN, insecticide-treated nets; MD, mean difference; MNP, micronutrient powder; ND, no data; NS, not significant.
FIGURE 3.
Forest plots of intervention effects (and 95% CI) on hemoglobin concentration, by age groups: <5 y, ≥5 y, and pregnant women. DCC, delayed cord clamping; Hb, hemoglobin; IFA, iron-folic acid; ITN, insecticide-treated nets; MD, mean difference; MNP, micronutrient powders.
FIGURE 4.
Forest plots of intervention effects (and 95% CI) on anemia, by age groups: <5 y, ≥5 y, and pregnant women. DCC, delayed cord clamping; IFA, iron-folic acid; ITN, insecticide-treated nets; MNP, micronutrient powders.
Iron-folic acid or folate alone
Iron-folic acid (IFA) supplementation in pregnant women at 34 wks and mothers through 6 wk postpartum increased Hb concentration in the range 1.0–1.6 g/dL and decreased the risk of anemia by 66–69% (Table 2, Figures 3 and 4). Dosing was not provided for all studies, but in 1 study in a review (12) of IFA supplementation interventions the mean dosage was 124 mg. Daily folic acid supplementation (with or without iron) in pregnancy did not have an impact on predelivery anemia or mean Hb concentration, but it did reduce the incidence of megaloblastic anemia by 79% (Table 2, Figures 3 and 4). There were no systematic reviews on providing IFA or folic acid alone for children aged <5 y and >5 y.
Micronutrient powders
Micronutrient powders (MNPs) have been most frequently reviewed in children aged 6–23 mo, though there are reviews that include other age groups. MNP use in children aged <5 y is associated with increases in Hb concentration and reduction in the risk of anemia (Table 2, Figures 3 and 4).
Interventions for malaria
Treatment for malaria across all ages was associated with increases in Hb but showed no impact on anemia (Table 2). Use of intermittent preventive malaria treatment in pregnancy increased Hb concentration by 0.4 g/dL and reduced the risk of anemia by 10% (Table 2). Sleeping under ITNs was associated with increased Hb concentration in children <5 y (Table 2, Figures 3 and 4). ITN use did not reduce risk of anemia across any age group (Table 2, Figures 3 and 4). We did not have studies with baseline data to examine the impact of malaria prevention and treatment in anemic or nonanemic populations.
Helminth infection
Deworming did not increase Hb concentrations in children <5 y but it did reduce anemia in school-age children (Table 2, Figures 3 and 4). We did not have studies with baseline data on anemia status to examine the impact deworming in anemic or nonanemic populations.
Delayed cord clamping
The practice of delayed cord clamping increased Hb concentrations at birth and from 4 to 48 h after birth but not at 3–6 mo, when compared with those without delayed cord clamping (Table 2, Figures 3 and 4). The studies on delayed cord clamping also reported on hematocrit—which represents the proportion of RBCs in blood—in full-term and preterm newborns. In full-term newborns, those who received delayed cord clamping showed increased hematocrit at 48 h by 6% (95% CI: 1.8, 10.6%; P = 0.006); and at day 5 by 12% (95% CI: 8.5, 15.5%; P < 0.001). In preterm newborns, delayed cord clamping was associated with a hematocrit increase of 3.2% (95% CI: 1.8, 4.7%; P < 0.001) at birth; of 5.4% (95% CI: 3.3, 7.4%; P < 0.001) at 4 h; and of 3.3% (95% CI: 1.3, 5.2%; P < 0.001) at 24 h.
Narrative syntheses
Iron and other micronutrient supplementation
One review evaluating the impact of iron and other micronutrient supplementation on adolescent anemia included 13 studies. This review found a 31% reduction in anemia associated with IFA supplementation with or without other micronutrients (RR: 0.69; 95% CI: 0.62, 0.76). In a literature review, Fishman et al. (13) concluded that although provision of multivitamins across age groups (preschool and school children including adolescents) reported increases in Hb concentration and reductions in anemia, these effects were indistinguishable from those seen with iron alone. Allen et al. (14) reported similar increases in Hb concentrations when either multiple micronutrient supplements or iron (with or without folic acid) were given to pregnant women. Another review showed only small increases in Hb concentration with iron and micronutrient supplementation compared with iron supplementation alone in children aged from 5 mo to 19 y [weighted mean difference (WMD) = 0.14 g/dL; 95% CI: 0, 0.28 g/dL] (15). When iron and micronutrient supplements were compared with placebo in children aged 5 mo to 19 y, larger changes in Hb concentration were seen (WMD = 0.65 g/dL; 95% CI: 0.5, 0.8 g/dL). Haider and Bhutta (16) found no reduction in the risk of anemia associated with multiple micronutrient supplementation in pregnancy (RR = 0.98; 95% CI: 0.86, 1.11).
One review of neonatal vitamin A supplementation compared with placebo reported no impact on the risk of anemia (RR = 0.97; 95% CI: 0.87, 1.07) (17); another comparing pregnant women who received vitamin A supplementation with those who did not showing that vitamin A supplementation during pregnancy was associated with a reduced risk of anemia (RR = 0.64; 95% CI: 0.43, 0.94) (18).
We also included reviews that described results from supplementation trials with other micronutrients. In 1 review of vitamin B-12 supplementation with 3 studies (88), the authors reported an increase in Hb concentration in women and children who received a vitamin B-12 supplement compared with those who did not. One systematic review concluded that vitamin C supplementation increased Hb concentrations in children and nonpregnant women, but not in pregnant women (13). Vitamin E supplementation increased Hb concentrations in preterm infants (WMD = 0.46 g/dL; 95% CI: 0.24, 0.69 g/dL) and infants with very low birthweight (WMD = 0.43 g/dL; 95% CI: 0.09, 0.77 g/dL) (89). One review reported no difference in Hb concentrations in pregnant women given zinc supplements in pregnancy (44), which was also reported from another review of the effect of zinc supplementation on Hb in children ≤15 y of age (90) and children aged 6 mo to 12 y (91).
Food fortification
There was heterogeneity in reviews included for fortified food trials. Whereas all reported associated increases in Hb concentration, effects on anemia varied. Shah et al. (92) reviewed the effects of staples and commonly consumed foods fortified with a mix of micronutrients including iron, folic acid, and vitamin A, compared with similar unfortified foods and found no impact on anemia (RR = 0.89; 95% CI: 0.35, 2.28). A 2014 report showed that the Hb concentration of children who consumed fortified foods was higher than it was in those who did not, by 0.5 g/dL (95% CI: 0.3, 0.7 g/dL) (93). A similar finding was reported by Das et al. (94) when consumption of various iron-, zinc-, and vitamin A–fortified foods was compared with diets comprising unfortified foods (standardized mean difference = 0.5; 95% CI: 0.34, 0.76). Gera et al. in 2012 (95) reported an increase in Hb concentration (0.42 g/dL; 95% CI: 0.28, 0.56 g/dL) and a reduction in anemia (RR = 0.59; 95% CI: 0.48, 0.71) in the general population associated with iron-fortified foods. Eichler et al. (96) showed that the multiple micronutrient fortification of formula milk and cereals increased Hb concentration (weighted mean difference= 0.87 g/dL; 95% CI: 0.57, 1.16 g/dL) and reduced anemia (RR = 0.43; 95% CI: 0.26, 0.71) compared with iron fortification alone. In other reviews, Assuncao and Santos (97) and Hurrell et al. (98) presented individual results from their studies and reported that food fortification increased Hb concentration and reduced the risk of anemia. The authors did not present pooled results.
Malaria prevention and treatment
One review reported a pooled summary in which the risk of anemia was reduced by 21.3% (95% CI: 8.2, 32.5%) with intermittent malaria prophylaxis in infants (99). Another review of 24 studies on chemoprophylaxis in children aged ≤19 y reported increased Hb concentration (maximum change of 1 g/dL) and reduced risk of anemia, although anemia results were inconsistent across the included studies (100). The most recent review of IPTc did not report any difference in the risk of anemia (RR = 0.82; 95% CI: 0.65, 1.04) or in Hb concentration (MD = 0.03 g/dL; 95% CI: 0.08, 0.14 g/dL) when comparing children who received controls (101). One review did not report summary effects according to intervention category, but did report that multiple interventions, including ITNs, chemoprophylaxis, or a combination of the two increased Hb concentration (MD = 0.76 g/dL; 95% CI: 0.61, 0.91 g/dL) and reduced the risk of anemia (RR = 0.73; 95% CI: 0.27, 1.00) in children aged <5 y (102). Two groups of investigators, Pluess et al. (103) and Korenromp et al. (102) evaluated the effect of indoor residual spraying (IRS) in children 1 to 15 y of age. Korenromp et al. reported a change in mean Hb concentration of 1.6 g/dL in a 2-group assessment performed before and after spraying. Pluess et al. reported on 2 comparisons from randomized trials—IRS with no intervention and IRS with ITNs. In the former comparison they reported an increase of 0.85 g/dL with IRS, and an increase of 0.06 g/dL with IRS in the latter.
Feeding of infants and young children
A review that examined the promotion of complementary feeding behaviors (104) reported that nutrition education for parents of children aged <5 y had limited impact on anemia (increase of 0.4 g/dL in Hb and reduction of 5 percentage points in the prevalence of anemia), whereas the provision of fortified complementary foods resulted in 13–21% reduced risk of anemia. Another review looked at the timing of introduction of complementary feeding and concluded that infants who started feeding at 4 mo showed an average increase in Hb concentration that was 0.5 g/dL higher than it was among those who started feeding at 6 mo (105). A third review that evaluated the effect of supplementary feeding reported higher Hb concentrations and a reduced risk of anemia among infants and children aged <5 y who received supplementary food with standard care, compared with children who received only standard care (106).
Dietary diversity
Of the 7 studies cited in the 2 reviews that reported on change in Hb values from programs of dietary diversification targeted at families with children aged 0–17 y, 2 reported decreases in the prevalence of anemia in the intervention group that practiced any form of dietary diversification or modification compared with the control group (107). Among them were 2 animal-source food interventions with a decrease in anemia—one in nonpregnant women in Peru after development of dietary diverse recipes and meals, and the other in 4–12-mo-old children in China after a program of nutrition education, and counseling on breastfeeding and diverse complementary feeding. One review of studies examining the association between consumption of animal-source foods and the outcome of Hb concentration or anemia identified 8 experimental and 41 observational studies in adults aged >18 y (108). The review reported a positive association between animal-source food intake (85–300 g/d) and Hb concentrations.
Agriculture and food security
A 2011 review included 3 studies that evaluated the effect on anemia prevalence of agricultural interventions, which included home gardens, aquaculture and small fisheries, dairy development, and raising of livestock (109). Only 1 of 3 homestead food production studies in 4 different countries showed lower anemia prevalence in children in 2 of the 4 study sites. Among the 2 studies that were not statistically significant, one assessed anemia in women and children and the other only assessed anemia in children. A 2012 review of 2 studies that were not included in the 2011 review did not report any differences in rates of anemia in women and in children aged <5 y who had and had not been exposed to agricultural interventions (110).
Family planning
Two reviews assessed the impact of birth spacing on maternal anemia (111, 112). Two of the 5 studies in these reviews reported an increased risk of maternal anemia if the birth interval was either <6 mo (as evaluated in 1 study) or <24 mo (as in the second study). The other 3 studies did not report a significant association between birth interval—ranging from <6 mo to >75 mo—and maternal anemia.
Conditional cash transfers
A review evaluated the impact of 3 conditional cash programs/transfers (PROSPERA in Mexico, Bolsa Família in Brazil, and Más Familias en Acción in Colombia) on nutrition in young children, focusing on but not limited to children <5 y of age (113). The 2 studies of PROSPERA found that providing mothers with income support for health and education led to higher Hb concentrations (mean difference = 0.37 g/dL) and a 25% reduction in the likelihood of anemia in their children. The review of the effects of Bolsa Família did not find any difference regarding these same measures in the children of families comparing those who did or did not receive income support. The Colombian program did not report on Hb concentration or anemia.
Discussion
Key findings
Our meta-review of 118 systematic reviews summarizing the influence of interventions on Hb concentrations and anemia in children and pregnant women showed that a variety of nutrition-specific and nutrition-sensitive programs are associated with a positive impact. We found the greatest number of reviews summarized interventions with iron and other micronutrient supplementation (52%) and malaria prevention and treatment strategies (24%), with a limited number of reviews describing nutrition-sensitive interventions (4%). Quantitative pooling of data showed increases in Hb concentration with in children <5y daily and intermittent iron supplementation, ITNs, and delayed cord clamping in children aged <5 y seen on first 2 days of birth; and with daily and intermittent IFA supplementation, ITNs, and delayed cord clamping in pregnant women. Similarly, reduced risk of anemia was seen with daily and intermittent iron supplementation, MNPs, ITNs, and delayed cord clamping in children aged <5 y (as measured by risk of transfusion); intermittent iron supplementation and deworming in children aged >5 y; and daily IFA and folate supplementation and ITNs in pregnant women. We did see that the direction of change in the quantitative pooling was in line with the reported direction of effect in the narrative syntheses for iron supplementation. Owing to heterogeneity across studies, we were not able to quantitatively pool findings from trials of supplementation of vitamin A or other micronutrients, delivered without the inclusion of iron in the formulation. The findings from analyses of systematic reviews of MNPs and fortified foods also suggest the advantage of confronting nutrient anemias beyond iron deficiency anemia.
World Health Assembly nutrition targets
Anemia persists as a leading public health problem globally, and there has been limited progress in achieving reduction targets (114). Globally, of the 185 countries included in the World Health Assembly goal of reducing anemia in women of reproductive age by 50% by 2025, only 3 are on target (114). In view of anemia's long-term individual-level health implications and its impact on society more broadly, interventions to overcome this public health problem need to be identified. When considering the priority interventions, program managers should also consider the cost-effectiveness of the intervention on Hb: if expected changes in Hb from an intervention are smaller, we will need a larger investment of resources compared with interventions that generate large changes in Hb. In our review, change in Hb across a wide array of interventions ranged from 0.12 g/dL for treatment of malaria across all ages to 1.7 g/dL for iron supplementation in children were among the interventions that showed a statistically significant impact. These findings indicate that nutrition-specific interventions remain the most important set of interventions to improve Hb concentrations, with daily iron being more effective than intermittent iron for supplementation. Although MNPs were not found to nor reduce Hb concentration, this could potentially be because the types of studies that we included reflected the real-world conditions of multiple comorbidities challenges in the implementation of MNP programs; nevertheless, MNPs were still found to reduce anemia. Nutrition-sensitive interventions that were found to effectively contribute to sustained increases in Hb concentrations were malaria interventions but not deworming or delayed cord clamping (beyond the first two days of life). Additionally, although some reviews studied formula feeding and early introduction of complementary foods, they were constrained by methodological limitations and small effect sizes, with limited external validity. Similarly, iron and malaria prevention and treatment interventions were effective at increasing Hb concentrations in pregnant women when compared with standard of care, but with higher effect sizes. With the reported positive impact on anemia from a range of interventions in our review, the benefit of broadening the portfolio of nutrition interventions and tailoring to context when designing programs is evident.
Meta-review and methods
We conducted a meta-review of reviews, considered one of the higher-order methods of study designs for synthesizing results on intervention efficacy (115, 116). Anemia has been studied extensively, thereby the use of this type of analysis allows for synthesis of a broader range of interventions, which would normally take much longer when compared with conducting a systematic review of primary studies for each intervention. To our knowledge, this is the first time evidence on the impact of nutrition-specific and nutrition-sensitive interventions to address anemia has been synthesized using a systematic review of reviews approach. We were able to quantitatively synthesize the effects on the outcome not only for nutrition-specific supplementation but also for malaria prevention and treatment and deworming—both of which address key anemia causal factors. Regrettably, there were limited systematic reviews on other nutrition-sensitive interventions (e.g., agriculture-based) highlighting that knowledge in this area is lacking.
The meta-review can serve as an important tool for decision-makers in the design of programs and can present information to assist in the allocation of resources. Findings synthesize and integrate information about different intervention options for a single health outcome like anemia. When we examined the evidence using the AMSTAR criteria, however, many reviews fell short in terms of dissemination and translation into programs and policies. For example, only 58% of the reviews used results for appropriately formulating conclusions (6).
There were limitations in our meta-review. Importantly, meta-reviews are reliant on the studies included in the systematic reviews, which can introduce reporting and selection biases. Deworming trials, for example, were underrepresented relative to reviews of nutrient supplementation or malaria prevention and treatment. There could also have been an imbalance in the age group studied or context. These are 2 crucial factors driving Hb concentration and risk of anemia. External validity can be compromised as a result of this limitation. We also found that only 53% of the reviews assessed the likelihood of publication bias.
Another challenge posed by the meta-review design and the evidence base for anemia is heterogeneity in the primary studies in the individual systematic reviews. For example, there were differences in the use of outcome metrics like standardized mean difference and mean difference. These had to be assessed separately. There could be other differences between the reviews, apart from those variables that we attempted to control, such as population, intervention, and comparator variables that we used to assess qualifications for pooling. We found that anemia was a secondary outcome in a small subset of studies within reviews, which could present problems for interpretation. We were also unable to assess the effect of intervention duration on outcomes. Finally, as is the case with systematic reviews, the retrospective analysis in meta-reviews presents limitations in terms of the directness and relevance of research questions and the availability of required data.
It is widely established that anemia is a condition that arises from multiple etiologies related to nutrition, infection, chronic disease, and genetics. Our review demonstrated the relative abundance of reviews and studies focused on supplementation with iron and other micronutrients, followed by the treatment of malaria and helminth infection. By contrast, those falling into the nutrition-sensitive category were few. This could be due to the diminished attention given to these interventions and the logistics of conducting nutrition-sensitive interventions in experimental trials.
Future research
We identified several areas for future research in anemia while conducting our review. First, there is a need to build the evidence base for the contribution of hemoglobinopathies and other genetic conditions to anemia globally, and to design and test public health interventions for these problems. Second, we did not identify any reviews examining anemia in men, which could similarly be contributing significantly to the global burden of anemia. Finally, although we identified nutrition-specific and nutrition-sensitive interventions, we did not identify integrated approaches that deliver both types as a package.
Conceptually using a multisectoral approach to address anemia is appealing but there remains little empirical evidence to support this approach. Qualitative research on the use of a multisectoral approach has demonstrated opportunities for building political will and enacting change but also has highlighted challenges, such as disparate institutional mandates and the potential for ineffective time management (117–119). More research is needed on the extent to which anemia reduction will benefit from such an approach. Perhaps even more important is to better understand how interventions interact to have synergistic or antagonistic effects on anemia. Unfortunately, because most studies have focused on a single intervention, it hinders the use of a systematic review approach to generate evidence on multisectoral interventions. Evidence in this area is especially needed because there are concerns about the safe delivery of anemia reduction interventions exemplified by excessive amounts of micronutrients delivered through multiple channels or negative interactions between infections and iron in the absence of disease control. Thus, this demonstrates the need to consider multiple interventions delivered in combination (120, 121). Our study group conducted a series of trials in Haiti that provided evidence of the potential for integrated approaches to anemia reduction. The first trial showed that a fortified peanut butter paste had reduced the odds of anemia by 28% (adjusted OR: 0.72; 95% CI: 0.57, 0.91; P < 0.001) and had no effect on Hb concentration (122). In a subsequent trial that used the same fortified paste delivered along with systematic deworming in both the control and intervention groups, Hb concentration increased by 0.62 ± 0.27 g/dL (P = 0.001) and the odds of anemia decreased by 88% (P = 0.02) (123). In summary, in view of the multidimensionality of anemia, more research is needed for integrated approaches.
Conclusions
This comprehensive meta-review of 118 systematic reviews suggests the ongoing importance of nutrition-specific interventions, especially iron supplementation, for addressing anemia. It also points to the need for more research on nutrition-sensitive interventions that address underlying, systemic causes of anemia. This could require impact pathway analyses to examine intervention effects. Anemia remains an intractable public health problem globally, contributing markedly to problems of maternal mortality; low birth weight and poor growth and development among young children; and lost economic productivity. This compilation of information reinforces the need for more support to develop and evaluate multiple interventions to reduce anemia.
Supplementary Material
ACKNOWLEDGEMENTS
The authors’ responsibilities were as follows—DM, SN, RM: conducted the research; DM: analyzed the data; DM, SN, RM, LI: wrote the manuscript; DM: had primary responsibility for the final content; and all authors: designed the research, and read and approved the final manuscript.
Notes
This report is made possible by the generous support of the American people through the United States Agency for International Development (USAID) under the terms of the Cooperative Agreement No. AID-OAA-A-11-00031 (SPRING), managed by JSI Research & Training Institute, Inc (JSI). The contents are the responsibility of JSI, and do not necessarily reflect the views of USAID or the US government.
Author disclosures: The authors report no conflicts of interests.
Supplemental Tables 1–4 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/advances/.
Abbreviations used: AMSTAR, Assessing the Methodological Quality of Systematic Reviews; Hb, hemoglobin; IFA, iron-folic acid; IPTc, intermittent preventive treatment in children; IRS, indoor residual spraying; ITN, insecticide-treated net; MD, mean difference; MNP, micronutrient powder; SPRING, Strengthening Partnerships, Results, and Innovations in Nutrition Globally; WASH, water, sanitation, and hygiene; WMD, weighted mean difference.
Contributor Information
Denish Moorthy, USAID Advancing Nutrition (USAID AN), Arlington, VA, USA.
Rebecca Merrill, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD, USA.
Sorrel Namaste, The Demographic and Health Survey Program, ICF, Rockville, MD, USA.
Lora Iannotti, Brown School, Institute for Public Health, Washington University in St Louis, MO, USA.
References
- 1. Stevens GA, Finucane MM, De-Regil LM, Paciorek CJ, Flaxman SR, Branca F, Peña-Rosas JP, Bhutta ZA, Ezzati M. Global, regional, and national trends in haemoglobin concentration and prevalence of total and severe anaemia in children and pregnant and non-pregnant women for 1995–2011: a systematic analysis of population-representative data. Lancet Glob Health. 2013;1:e16–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Black MM, Baqui AH, Zaman K, Persson LA, Arifeen SE, Le K, McNary SW, Parveen M, Hamadani JD, Black RE. Iron and zinc supplementation promote motor development and exploratory behavior among Bangladeshi infants. Am J Clin Nutr. 2004;80:903–10. [DOI] [PubMed] [Google Scholar]
- 3. Balarajan Y, Ramakrishnan U, Ozaltin E, Shankar AH, Subramanian SV. Anaemia in low-income and middle-income countries. Lancet. 2011;378:2123–35. [DOI] [PubMed] [Google Scholar]
- 4. Kassebaum NJ, Jasrasaria R, Naghavi M, Wulf SK, Johns N, Lozano R, Regan M, Weatherall DJ, Chou DP, Eisele TP et al. A systematic analysis of global anemia burden from 1990 to 2010. Blood. 2014;123:615–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ruel MT, Alderman H. Nutrition-sensitive interventions and programmes: how can they help to accelerate progress in improving maternal and child nutrition?. Lancet. 2013;382:536–51. [DOI] [PubMed] [Google Scholar]
- 6. Hartling L, Vandermeer B, Fernandes RM. Systematic reviews, overviews of reviews and comparative effectiveness reviews: a discussion of approaches to knowledge synthesis. Evid Based Child Health. 2014;9:486–94. [DOI] [PubMed] [Google Scholar]
- 7. Thomson D, Russell K, Becker L, Klassen T, Hartling L. The evolution of a new publication type: steps and challenges of producing overviews of reviews. Res Synth Methods. 2010;1:198–211. [DOI] [PubMed] [Google Scholar]
- 8. Wallace B, Small K, Brodley CE, Lau J, Trikalinos TA. Deploying an interactive machine learning system in an evidence-based practice center: abstrackr. In: Proceedings of the ACM International Health Informatics Symposium (IHI). Miami, FL: Association for Computing Machinery; 2012. p. 819–24. [Google Scholar]
- 9. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JPA, Clarke M, Devereaux PJ, Kleijnen J, Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Maher JM, Markey JC, Ebert-May D. The other half of the story: effect size analysis in quantitative research. CBE Life Sci Educ. 2013;12:345–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wallace BC, Dahabreh IJ, Trikalinos TA, Lau J, Trow P, Schmid CH. Closing the gap between methodologists and end-users: R as a computational back-end. J Sta Soft. 2012;49:1–15. [Google Scholar]
- 12. Kulier R, Onis M de, Gulmezoglu AM, Villar J. Nutritional interventions for the prevention of maternal morbidity. Int J Gynecol Obstet. 1998;63:231–46. [DOI] [PubMed] [Google Scholar]
- 13. Fishman SM, Christian P, West KP. The role of vitamins in the prevention and control of anaemia. Public Health Nutr. 2000;3:125–50. [DOI] [PubMed] [Google Scholar]
- 14. Allen LH, Peerson JM, Olney DK. Provision of multiple rather than two or fewer micronutrients more effectively improves growth and other outcomes in micronutrient-deficient children and adults. J Nutr. 2009;139:1022–30. [DOI] [PubMed] [Google Scholar]
- 15. Gera T, Sachdev HP, Nestel P. Effect of combining multiple micronutrients with iron supplementation on Hb response in children: systematic review of randomized controlled trials. Public Health Nutr. 2009;12:756–73. [DOI] [PubMed] [Google Scholar]
- 16. Haider BA, Bhutta ZA. Multiple-micronutrient supplementation for women during pregnancy. Cochrane Database Syst Rev. 2015;2015(11):CD004905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Haider BA, Sharma R, Bhutta ZA. Neonatal vitamin A supplementation for the prevention of mortality and morbidity in term neonates in low and middle income countries. Cochrane Database Syst Rev. 2017;2:CD006980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. McCauley ME, van den Broek N, Dou L, Othman M. Vitamin A supplementation during pregnancy for maternal and newborn outcomes. Cochrane Database Syst Rev. 2015;(10):CD008666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sloan NL, Jordan E, Winikoff B. Effects of iron supplementation on maternal hematologic status in pregnancy. Am J Public Health. 2002;92:288–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Prinsen Geerligs PD, Brabin BJ, Omari AAA. Food prepared in iron cooking pots as an intervention for reducing iron deficiency anaemia in developing countries: a systematic review. J Hum Nutr Diet. 2003;16:275–81. [DOI] [PubMed] [Google Scholar]
- 21. Ramakrishnan U, Aburto N, McCabe G, Martorell R. Multimicronutrient interventions but not vitamin A or iron interventions alone improve child growth: results of 3 meta-analyses. J Nutr. 2004;134:2592–602. [DOI] [PubMed] [Google Scholar]
- 22. Iannotti LL, Tielsch JM, Black MM, Black RE. Iron supplementation in early childhood: health benefits and risks. Am J Clin Nutr. 2006;84:1261–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tarun G, Sachdev HPS, Nestel P, Sachdev SS. Effect of iron supplementation on haemoglobin response in children: systematic review of randomised controlled trials. J Pediatr Gastroenterol Nutr. 2007;44:468–86. [DOI] [PubMed] [Google Scholar]
- 24. Wang B, Zhan S, Xia Y, Lee L. Effect of sodium iron ethylenediaminetetra-acetate (NaFeEDTA) on haemoglobin and serum ferritin in iron-deficient populations: a systematic review and meta-analysis of randomised and quasi-randomised controlled trials. Br J Nutr. 2008;100:1169–78. [DOI] [PubMed] [Google Scholar]
- 25. De-Regil LM, Jefferds MED, Sylvetsky AC, Dowswell T. Intermittent iron supplementation for improving nutrition and development in children under 12 years of age. Cochrane Database Syst Rev. 2011;2011(12):CD009085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Fernández-Gaxiola AC, De-Regil LM. Intermittent iron supplementation for reducing anaemia and its associated impairments in menstruating women. Cochrane Database Syst Rev. 2011;(12):CD009218. [DOI] [PubMed] [Google Scholar]
- 27. Reveiz L, Gyte G ML, Cuervo G, Casasbuenas A. Treatments for iron-deficiency anaemia in pregnancy. Cochrane Database Syst Rev. 2011;(10):CD003094. [DOI] [PubMed] [Google Scholar]
- 28. Casgrain A, Collings R, Harvey LJ, Hooper L, Fairweather-Tait SJ. Effect of iron intake on iron status: a systematic review and meta-analysis of randomized controlled trials. Am J Clin Nutr. 2012;96:768–80. [DOI] [PubMed] [Google Scholar]
- 29. Long H, Yi JM, Hu PL, Li ZB, Qiu WY, Wang F, Zhu S. Benefits of iron supplementation for low birth weight infants: a systematic review. BMC Pediatr. 2012;12:99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Osungbade KO, Oladunjoye AO. Preventive treatments of iron deficiency anaemia in pregnancy: a review of their effectiveness and implications for health system strengthening. J Pregnancy. 2012;2012:454601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Abdullah K, Kendzerska T, Shah P, Uleryk E, Parkin PC. Efficacy of oral iron therapy in improving the developmental outcome of pre-school children with non-anaemic iron deficiency: a systematic review. Public Health Nutr. 2013;16:1497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Pasricha S-R, Drakesmith H, Black J, Hipgrave D, Biggs B-A. Control of iron deficiency anemia in low- and middle-income countries. Blood. 2013;121:2607–17. [DOI] [PubMed] [Google Scholar]
- 33. Domellöf M. Iron and other micronutrient deficiencies in low-birthweight infants. Nestle Nutr Inst Workshop Ser. 2013;74:197–206. [DOI] [PubMed] [Google Scholar]
- 34. Greig AJ, Patterson AJ, Collins CE, Chalmers KA. Iron deficiency, cognition, mental health and fatigue in women of childbearing age: a systematic review. J Nutr Sci. 2013;2:e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Haider BA, Olofin I, Wang M, Spiegelman D, Ezzati M, Fawzi WW. Anaemia, prenatal iron use, and risk of adverse pregnancy outcomes: systematic review and meta-analysis. BMJ. 2013;346:f3443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Low M, Farrell A, Biggs BA, Pasricha SR. Effects of daily iron supplementation in primary-school-aged children: systematic review and meta-analysis of randomized controlled trials. Can Med Assoc J. 2013;185:E791–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Thompson J, Biggs BA, Pasricha SR. Effects of daily iron supplementation in 2- to 5-year-old children: systematic review and meta-analysis. Pediatrics. 2013;131:739–53. [DOI] [PubMed] [Google Scholar]
- 38. Cantor AG, Bougatsos C, Dana T, Blazina I, McDonagh M. Routine iron supplementation and screening for iron deficiency anemia in pregnancy: a systematic review for the U.S. Preventive Services Task Force. Ann Intern Med. 2015;162:566–76. [DOI] [PubMed] [Google Scholar]
- 39. McDonagh MS, Blazina I, Dana T, Cantor A, Bougatsos C. Screening and routine supplementation for iron deficiency anemia: a systematic review. Pediatrics. 2015;135:723–33. [DOI] [PubMed] [Google Scholar]
- 40. Peña-Rosas JP, De-Regil LM, Gomez Malave H, Flores-Urrutia MC, Dowswell T. Intermittent oral iron supplementation during pregnancy. Cochrane Database Syst Rev. 2012;7(7):CD009997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Petry N, Olofin I, Boy E, Donahue Angel M, Rohner F. The effect of low dose iron and zinc intake on child micronutrient status and development during the first 1000 days of life: a systematic review and meta-analysis. Nutrients. 2016;8(12):773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Neuberger A, Okebe J, Yahav D, Paul M. Oral iron supplements for children in malaria-endemic areas. Cochrane Database Syst Rev. 2016;2(2):CD006589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Low MSY, Speedy J, Styles CE, De-Regil LM, Pasricha S-R. Daily iron supplementation for improving anaemia, iron status and health in menstruating women. Cochrane Database Syst Rev. 2016;4:CD009747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Villar J, Merialdi M, Gulmezoglu AM, Abalos E, Carroli G, Kulier R, Onis M de. Nutritional interventions during pregnancy for the prevention or treatment of maternal morbidity and preterm delivery: an overview of randomized controlled trials. J Nutr. 2003;133(5 Suppl 2):1606S–25S. [DOI] [PubMed] [Google Scholar]
- 45. Yakoob MY, Bhutta ZA. Effect of routine iron supplementation with or without folic acid on anemia during pregnancy. BMC Public Health. 2011;11:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Salam RA, Faqqah A, Sajjad N, Lassi ZS, Das JK, Kaufman M, Bhutta ZA. Improving adolescent sexual and reproductive health: a systematic review of potential interventions. J Adolesc Health. 2016;59:S11–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ramakrishnan U, Goldenberg T, Allen LH. Do multiple micronutrient interventions improve child health, growth, and development?. J Nutr. 2011;141:2066–75. [DOI] [PubMed] [Google Scholar]
- 48. Dewey KG, Yang Z, Boy E. Systematic review and meta-analysis of home fortification of complementary foods. Matern Child Nutr. 2009;5:283–321. [Google Scholar]
- 49. De-Regil L M, Suchdev P S, Vist G E, Walleser S, Peña-Rosas J P. Home fortification of foods with multiple micronutrient powders for health and nutrition in children under two years of age. Cochrane Database Syst Rev. 2011;(9):CD008959. [DOI] [PubMed] [Google Scholar]
- 50. Salam RA, MacPhail C, Das JK, Bhutta ZA. Effectiveness of micronutrient powders (MNP) in women and children. BMC Public Health. 2013;13:S22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Suchdev P S, Peña-Rosas J P, De-Regil L M. Multiple micronutrient powders for home (point-of-use) fortification of foods in pregnant women. Cochrane Database Syst Rev. 2015;(6):CD011158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Lassi Z S, Salam R A, Haider B A, Bhutta Z A. Folic acid supplementation during pregnancy for maternal health and pregnancy outcomes. Cochrane Database Syst Rev. 2013;(3):CD006896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Sinclair D, Zani B, Donegan S, Olliaro P, Garner P. Artemisinin-based combination therapy for treating uncomplicated malaria. Cochrane Database Syst Rev. 2009;2009(3):CD007483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Okwundu C I, Nagpal S, Musekiwa A, Sinclair D. Home- or community-based programmes for treating malaria. Cochrane Database Syst Rev. 2013;2013(5):CD009527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Zani B, Gathu M, Donegan S, Olliaro Piero L, Sinclair D. Dihydroartemisinin-piperaquine for treating uncomplicated Plasmodium falciparum malaria. Cochrane Database Syst Rev. 2014;2014(1):CD010927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Graves PM, Gelband H, Garner P. Primaquine or other 8-aminoquinoline for reducing Plasmodium falciparum transmission. Cochrane Database Syst Rev. 2015;(2):CD008152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Isba R, Zani B, Gathu M, Sinclair D. Artemisinin-naphthoquine for treating uncomplicated Plasmodium falciparum malaria. Cochrane Database Syst Rev. 2015;2015(2):CD011547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Matangila JR, Mitashi P, Inocêncio da Luz RA, Lutumba PT, Van Geertruyden J-P. Efficacy and safety of intermittent preventive treatment for malaria in schoolchildren: a systematic review. Malar J. 2015;14:450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Salam RA, Das JK, Lassi ZS, Bhutta ZA. Impact of community-based interventions for the prevention and control of malaria on intervention coverage and health outcomes for the prevention and control of malaria. Infect Dis Poverty. 2014;3:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Radeva-Petrova D, Kayentao K, ter Kuile FO, Sinclair D, Garner P. Drugs for preventing malaria in pregnant women in endemic areas: any drug regimen versus placebo or no treatment. Cochrane Database Syst Rev [Internet]. 2014;2014(10):CD000169 Available from: 10.1002/14651858.CD000169.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. McClure EM, Goldenberg RL, Dent AE, Meshnick SR. A systematic review of the impact of malaria prevention in pregnancy on low birth weight and maternal anemia. Int J Gynaecol Obstet. 2013;121:103–9. [DOI] [PubMed] [Google Scholar]
- 62. Kayentao K, Garner P, van Eijk AM, Naidoo I, Roper C, Mulokozi A, MacArthur JR, Luntamo M, Ashorn P, Doumbo OK et al. Intermittent preventive therapy for malaria during pregnancy using 2 vs 3 or more doses of sulfadoxine-pyrimethamine and risk of low birth weight in Africa: systematic review and meta-analysis. JAMA. 2013;309:594–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. ter Kuile FO, van Eijk AM, Filler SJ. Effect of sulfadoxine-pyrimethamine resistance on the efficacy of intermittent preventive therapy for malaria control during pregnancy: a systematic review. JAMA. 2007;297:2603–16. [DOI] [PubMed] [Google Scholar]
- 64. Kalanda GC, Hill J, Verhoeff FH, Brabin BJ. Comparative efficacy of chloroquine and sulphadoxine-pyrimethamine in pregnant women and children: a meta-analysis. Trop Med Int Health. 2006;11:569–77. [DOI] [PubMed] [Google Scholar]
- 65. Newman RD, Parise ME, Slutsker L, Nahlen B, Steketee RW. Safety, efficacy and determinants of effectiveness of antimalarial drugs during pregnancy: implications for prevention programmes in Plasmodium falciparum-endemic sub-Saharan Africa. Trop Med Int Health. 2003;8:488–506. [DOI] [PubMed] [Google Scholar]
- 66. Garner P, Gulmezoglu AM. Drugs for preventing malaria-related illness in pregnant women and death in the newborn. Cochrane Database Syst Rev. 2003;CD000169 doi:10.1002/14651858.CD000169. [DOI] [PubMed] [Google Scholar]
- 67. Garner P, Brabin B. A review of randomized controlled trials of routine antimalarial drug prophylaxis during pregnancy in endemic malarious areas. Bull World Health Organ. 1994;72:89–99. [PMC free article] [PubMed] [Google Scholar]
- 68. Lengeler C. Insecticide-treated bed nets and curtains for preventing malaria. Cochrane Database Syst Rev. 2004;(2):CD000363. [DOI] [PubMed] [Google Scholar]
- 69. Gamble C L, Ekwaru J P, ter Kuile F O. Insecticide-treated nets for preventing malaria in pregnancy. Cochrane Database Syst Rev. 2006;2006(2):CD003755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Gamble C, Ekwaru PJ, Garner P, ter Kuile FO. Insecticide-treated nets for the prevention of malaria in pregnancy: a systematic review of randomised controlled trials. PLoS Med. 2007;4:e107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Gulani A, Nagpal J, Osmond C, Sachdev HPS. Effect of administration of intestinal anthelmintic drugs on haemoglobin: systematic review of randomised controlled trials. BMJ. 2007;334:1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Brooker S, Akhwale W, Pullan R, Estambale B, Clarke SE, Snow RW, Hotez PJ. Epidemiology of plasmodium-helminth co-infection in Africa: populations at risk, potential impact on anemia, and prospects for combining control. Am J Trop Med Hyg. 2007;77:88–98. [PMC free article] [PubMed] [Google Scholar]
- 73. Smith JL, Brooker S. Impact of hookworm infection and deworming on anaemia in non-pregnant populations: a systematic review. Trop Med Int Health. 2010;15:776–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Salam RA, Haider BA, Humayun Q, Bhutta ZA. Effect of administration of antihelminthics for soil-transmitted helminths during pregnancy. Cochrane Database Syst Rev. 2015;(6):CD005547. [DOI] [PubMed] [Google Scholar]
- 75. Taylor-Robinson DC, Maayan N, Soares-Weiser K, Donegan S, Garner P. Deworming drugs for soil-transmitted intestinal worms in children: effects on nutritional indicators, haemoglobin, and school performance. Cochrane Database Syst Rev. 2015;(7):CD000371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Mercer JS. Current best evidence: a review of the literature on umbilical cord clamping. J Midwifery Womens Health. 2001;46:402–14. [DOI] [PubMed] [Google Scholar]
- 77. Rabe H, Reynolds G, Diaz-Rossello J. Early versus delayed umbilical cord clamping in preterm infants. Cochrane Database Syst Rev. 2004;(4):CD003248. [DOI] [PubMed] [Google Scholar]
- 78. van Rheenen P, Brabin BJ. Late umbilical cord-clamping as an intervention for reducing iron deficiency anaemia in term infants in developing and industrialised countries: a systematic review. Ann Trop Paediatr. 2004;24:3–16. [DOI] [PubMed] [Google Scholar]
- 79. Lainez Villabona B, Bergel Ayllon E, Cafferata Thompson ML, Belizan Chiesa JM. ¿Pinzamiento precoz o tardío del cordón umbilical? Una revisión sistemática de la literatura médica [Early or late umbilical cord clamping? A systematic review of the literature]. An Pediatr. 2005;63:14–21.. Spanish. [DOI] [PubMed] [Google Scholar]
- 80. van Rheenen PF, Gruschke S, Brabin BJ. Delayed umbilical cord clamping for reducing anaemia in low birthweight infants: implications for developing countries. Ann Trop Paediatr. 2006;26:157–67. [DOI] [PubMed] [Google Scholar]
- 81. Hutton EK, Hassan ES. Late vs early clamping of the umbilical cord in full-term neonates: systematic review and meta-analysis of controlled trials. JAMA. 2007;297:1241–52. [DOI] [PubMed] [Google Scholar]
- 82. Garofalo M, Abenhaim HA. Early versus delayed cord clamping in term and preterm births: a review. J Obstet Gynaecol Can. 2012;34:525–31. [DOI] [PubMed] [Google Scholar]
- 83. Rabe H, Diaz-Rossello J L, Duley L, Dowswell T. Effect of timing of umbilical cord clamping and other strategies to influence placental transfusion at preterm birth on maternal and infant outcomes. Cochrane Database Syst Rev. 2012;(8):CD003248. [DOI] [PubMed] [Google Scholar]
- 84. Ghavam S, Batra D, Mercer J, Kugelman A, Hosono S, Oh W, Rabe H, Kirpalani H. Effects of placental transfusion in extremely low birthweight infants: meta-analysis of long- and short-term outcomes. Transfusion (Paris). 2014;54:1192–8. [DOI] [PubMed] [Google Scholar]
- 85. McDonald SJ, Middleton P, Dowswell T, Morris PS. Effect of timing of umbilical cord clamping of term infants on maternal and neonatal outcomes. Cochrane Database Syst Rev. 2013;2013(7):CD004074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Al-Wassia H, Shah PS. Efficacy and safety of umbilical cord milking at birth: a systematic review and meta-analysis. JAMA Pediatr. 2015;169:18–25. [DOI] [PubMed] [Google Scholar]
- 87. Dang D, Zhang C, Shi S, Mu X, Lv X, Wu H. Umbilical cord milking reduces need for red cell transfusions and improves neonatal adaptation in preterm infants: meta-analysis. J Obstet Gynaecol Res. 2015;41:890–5. [DOI] [PubMed] [Google Scholar]
- 88. Swaminathan S, Thomas T, Kurpad AV. B-vitamin interventions for women and children in low-income populations. Curr Opin Clin Nutr Metab Care. 2015;18:295–306. [DOI] [PubMed] [Google Scholar]
- 89. Brion LP, Bell EF, Raghuveer TS. Vitamin E supplementation for prevention of morbidity and mortality in preterm infants. Cochrane Database Syst Rev. 2003;(4)CD003665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Dekker LH, Villamor E. Zinc supplementation in children is not associated with decreases in hemoglobin concentrations. J Nutr. 2010;140:1035–40. [DOI] [PubMed] [Google Scholar]
- 91. Mayo-Wilson E, Imdad A, Junior J, Dean S, Bhutta ZA. Preventive zinc supplementation for children, and the effect of additional iron: a systematic review and meta-analysis. BMJ Open. 2014;4:e004647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Shah D, Sachdev HS, Gera T, De-Regil LM, Pena-Rosas JP. Fortification of staple foods with zinc for improving zinc status and other health outcomes in the general population. Cochrane Database Syst Rev. 2016;(6):CD010697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Ramesh A, Rao MVV, Nair KM. Impact of iron-fortified foods on Hb concentration in children (<10 years): a systematic review and meta-analysis of randomized controlled trials. Public Health Nutr. 2014;17:579–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Das JK, Salam RA, Kumar R, Bhutta ZA. Micronutrient fortification of food and its impact on woman and child health: a systematic review. Syst Rev. 2013;2:67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Gera T, Sachdev HS, Boy E. Effect of iron-fortified foods on hematologic and biological outcomes: systematic review of randomized controlled trials. Am J Clin Nutr. 2012;96:309–24. [DOI] [PubMed] [Google Scholar]
- 96. Eichler K, Wieser S, Ruthemann I, Brugger U. Effects of micronutrient fortified milk and cereal food for infants and children: a systematic review. BMC Public Health. 2012;12:506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Assuncao MCF, Santos IS. Effect of food fortification with iron on childhood anemia: a review study. Cad Saude Publica. 2007;23:269–81. [DOI] [PubMed] [Google Scholar]
- 98. Hurrell R, Ranum P, de Pee S, Biebinger R, Hulthen L, Johnson Q, Lynch S. Revised recommendations for iron fortification of wheat flour and an evaluation of the expected impact of current national wheat flour fortification programs. Food Nutr Bull. 2010;31:S7–21. [DOI] [PubMed] [Google Scholar]
- 99. Aponte JJ, Schellenberg D, Egan A, Breckenridge A, Carneiro I, Critchley J, Danquah I, Dodoo A, Kobbe R, Lell B et al. Efficacy and safety of intermittent preventive treatment with sulfadoxine-pyrimethamine for malaria in African infants: a pooled analysis of six randomised, placebo-controlled trials. Lancet. 2009;374:1533–42. [DOI] [PubMed] [Google Scholar]
- 100. Geerligs PDP, Brabin BJ, Eggelte TA. Analysis of the effects of malaria chemoprophylaxis in children on haematological responses, morbidity and mortality. Bull World Health Organ. 2003;81:205–16. [PMC free article] [PubMed] [Google Scholar]
- 101. Meremikwu M M, Donegan S, Sinclair D, Esu E, Oringanje C. Intermittent preventive treatment for malaria in children living in areas with seasonal transmission. Cochrane Database Syst Rev. 2012;2012(2):CD003756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Korenromp EL, Armstrong-Schellenberg JR, Williams BG, Nahlen BL, Snow RW. Impact of malaria control on childhood anaemia in Africa—a quantitative review. Trop Med Int Health. 2004;9:1050–65. [DOI] [PubMed] [Google Scholar]
- 103. Pluess B, Tanser F C, Lengeler C, Sharp B L. Indoor residual spraying for preventing malaria. Cochrane Database Syst Rev. 2010;2010(4):CD006657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Dewey KG, Adu-Afarwuah S. Systematic review of the efficacy and effectiveness of complementary feeding interventions in developing countries. Matern Child Nutr. 2008;4(Suppl 1):24–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Qasem W, Fenton T, Friel J. Age of introduction of first complementary feeding for infants: a systematic review. BMC Pediatr. 2015;15:107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Kristjansson E, Francis D K, Liberato S, Benkhalti J M, Welch V, Batal M, Greenhalgh T, Rader T, Noonan E, Shea B et al. Food supplementation for improving the physical and psychosocial health of socio-economically disadvantaged children aged three months to five years. Cochrane Database Syst Rev. 2015;2015(3):CD009924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Gibson RS, Anderson VP. A review of interventions based on dietary diversification or modification strategies with the potential to enhance intakes of total and absorbable zinc. Food Nutr Bull. 2009;30:S108–43. [DOI] [PubMed] [Google Scholar]
- 108. Jackson J, Williams R, McEvoy M, MacDonald-Wicks L, Patterson A. Is higher consumption of animal flesh foods associated with better iron status among adults in developed countries? A systematic review. Nutrients. 2016;8:89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Masset E, Haddad L, Cornelius A, Isaza-Castro J. A systematic review of agricultural interventions that aim to improve nutritional status of children. London: EPPI-Centre, SSRU, Institute of Education, University of London; 2011. [Google Scholar]
- 110. Girard AW, Self JL, McAuliffe C, Olude O. The effects of household food production strategies on the health and nutrition outcomes of women and young children: a systematic review. Paediatr Perinat Epidemiol. 2012;26:205–22. [DOI] [PubMed] [Google Scholar]
- 111. Conde-Agudelo A, Rosas-Bermudez A, Kafury-Goeta AC. Effects of birth spacing on maternal health: a systematic review. Am J Obstet Gynecol. 2007;196:297–308. [DOI] [PubMed] [Google Scholar]
- 112. Dewey KG, Cohen RJ. Does birth spacing affect maternal or child nutritional status? A systematic literature review. Matern Child Nutr. 2007;3:151–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Segura-Pérez S, Grajeda R, Pérez-Escamilla R. Conditional cash transfer programs and the health and nutrition of Latin American children. Pan Am J Public Health. 2016;40:124–37. [PubMed] [Google Scholar]
- 114. International Food Policy Research Institute Global nutrition report 2016. From promise to impact: ending malnutrition by 2030. [Internet] Washington (DC): IFPRI; 2016. [cited 2018 Jan 30]. Available from: http://ebrary.ifpri.org/utils/getfile/collection/p15738coll2/id/130354/filename/130565.pdf. [Google Scholar]
- 115. Smith V, Devane D, Begley CM, Clarke M. Methodology in conducting a systematic review of systematic reviews of healthcare interventions. BMC Med Res Methodol. 2011;11:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Aromataris E, Fernandez R, Godfrey CM, Holly C, Khalil H, Tungpunkom P. Summarizing systematic reviews: methodological development, conduct and reporting of an umbrella review approach. Int J Evid Based Healthc. 2015;13:132–40. [DOI] [PubMed] [Google Scholar]
- 117. Pelletier D, Gervais S, Hafeez-Ur-Rehman H, Sanou D, Tumwine J. Boundary-spanning actors in complex adaptive governance systems: the case of multisectoral nutrition. Int J Health Plann Manage. 2018;33:e293–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Sarkar D, Murphy H, Fisseha T, Koroma AS, Hodges MH, Adero N, Ngalombi S, Nabakooza J, Wun J, Namaste SML. Understanding the process of strengthening multi-sectoral efforts for anemia reduction: qualitative findings from Sierra Leone and Uganda. Int J Health Plann Manage. 2018;33:1024–44. [DOI] [PubMed] [Google Scholar]
- 119. Tangcharoensathien V, Srisookwatana O, Pinprateep P, Posayanonda T, Patcharanarumol W. Multisectoral actions for health: challenges and opportunities in complex policy environments. Int J Health Policy Manag. 2017;6:359–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Stoltzfus RJ. Iron and malaria interactions: programmatic ways forward. Adv Nutr. 2012;3:579–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Martorell R, de Romaña DL. Components of successful staple food fortification programs: lessons from Latin America. Food Nutr Bull. 2017;38:384–404. [DOI] [PubMed] [Google Scholar]
- 122. Iannotti LL, Henretty NM, Delnatus JR, Previl W, Stehl T, Vorkoper S, Bodden J, Maust A, Smidt R, Nash ML et al. Ready-to-use supplementary food increases fat mass and BMI in Haitian school-aged children. J Nutr. 2015;145:813–22. [DOI] [PubMed] [Google Scholar]
- 123. Iannotti L, Dulience SJ-L, Joseph S, Cooley C, Tufte T, Cox K, Eaton J, Delnatus JR, Wolff PB. Fortified snack reduced anemia in rural school-aged children of Haiti: a cluster-randomized, controlled trial. PLoS One. 2016;11:e0168121. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.