ABSTRACT
Observational studies regarding the putative associations between dietary intake of homocysteine metabolism-related B-vitamins (vitamin B-6, folate, and vitamin B-12) and stroke risk have yielded inconsistent results. Thus, we conducted a systematic meta-analysis of prospective studies in order to examine the relation between the dietary (from diet and supplements) intake of these B-vitamins and the risk of stroke. PubMed and Web of Science were searched for relevant articles published through to 25 February, 2020, and RR of stroke in relation to dietary intake of vitamin B-6, folate, and vitamin B-12 were pooled using a random-effects model. Eleven publications of 12 prospective studies comprising 389,938 participants and 10,749 cases were included in the final analysis. We found that dietary intake of vitamin B-6 and folate were associated with a reduced risk of stroke, and this inverse association remained significant in studies with >10 y of follow-up periods and among participants without a pre-existing stroke event. A dose-response analysis revealed a linear inverse association between folate and vitamin B-6 intake and the risk of stroke, with a pooled RR of 0.94 (95% CI: 0.90–0.98) and 0.94 (95% CI: 0.89–0.99) for each 100 μg/d increment in folate intake and 0.5 mg/d increment in vitamin B-6 intake, respectively. In contrast, we found no significant association between dietary vitamin B-12 intake and the risk of stroke, with an RR of 1.01 (95% CI: 0.97–1.06) per 3 μg/d increase. In conclusion, our findings suggest that increased intake of vitamin B-6 and folate is associated with a reduced risk of stroke, supporting the notion that increasing habitual folate and vitamin B-6 intake may provide a small but beneficial effect with respect to stroke.
Keywords: dietary intake, stroke, B-vitamins, dose-response, meta-analysis
Introduction
Stroke (also known as a cerebrovascular accident or CVA), is one of the leading causes of mortality and morbidity worldwide. In 2017, stroke accounted for 11% of all deaths and caused a global loss of 132 million disability-adjusted life years (1–3). Thus, stroke prevention is clearly an important and urgent public health issue. Among the variety of preventive strategies studied, homocysteine-lowering therapies have attracted considerable attention, as studies suggested that homocysteine can affect atherosclerosis (4–6); moreover, a growing body of epidemiological evidence suggests an association between elevated homocysteine concentrations and an increased risk of stroke (7–10).
B-vitamins, including vitamin B-6, folate (vitamin B-9), and vitamin B-12, are important regulators of homocysteine metabolism (11). Low intake of these nutrients is associated with increased blood homocysteine concentrations, and taking B-vitamins supplements or consuming foods rich in B-vitamins significantly reduces circulating homocysteine concentrations (12–17). These findings suggest that these B-vitamins might have a beneficial effect with respect to reducing the risk of stroke. Indeed, several systematic reviews of intervention studies provided evidence that taking supplements containing B-vitamins, particularly folic acid, may be associated with a decreased risk of stroke (18–23). Specifically, the most recent meta-analysis of 12 randomized controlled trials (RCTs) suggested that dietary supplementation with B-vitamins significantly reduces the risk of stroke by 10%, mainly among individuals who are at high risk of developing vascular disease (23).
Despite the results obtained from intervention trials, evidence obtained from observation studies is relatively scarce. Although several epidemiological studies examined the association between dietary intake of B-vitamins and the risk of stroke, the results to date are not entirely consistent (24–27). To the best of our knowledge, no systematic analysis has been performed regarding the putative association between the dietary intake of homocysteine metabolism-related B-vitamins and the risk of stroke in the general population. Thus, we conducted a comprehensive meta-analysis of all published relevant prospective studies in order to investigate this association.
Methods
This meta-analysis was performed in accordance with the Meta-analysis of Observational Studies in Epidemiology (MOOSE) protocol (28).
Search strategy
We systematically searched the PubMed and Web of Science databases for articles published through to 25 February, 2020. The following keywords were used in our search: (vitamin B OR B-vitamins OR vitamin B-6 OR pyridoxine OR vitamin B9 OR folate OR folic acid OR vitamin B-12 OR cobalamin) AND (cardiovascular OR cardiovascular disease OR stroke OR cerebrovascular accident OR cerebrovascular disease OR ischemic stroke OR hemorrhage OR cerebral infarct OR brain ischemic) AND (prospective OR prospectively OR cohort OR longitudinal OR follow-up). No restriction was imposed with respect to the language of the publications. The references cited within the retrieved relevant articles were also reviewed in order to identify additional publications.
Study selection
Studies that satisfied the following criteria were included in our meta-analysis: 1) community-based or population-based prospective design; 2) the exposure was the intake of dietary vitamin B-6, folate, and/or vitamin B-12; 3) the outcome of interest was stroke; and 4) the study reported (or it was possible to calculate) the risk estimate of stroke using an OR, HR, or RR, with the corresponding 95% CI for the highest versus the lowest category. We excluded meta-analyses, review articles, retrospective studies, case-control studies, studies involving nonhuman subjects, nonoriginal studies, and studies lacking sufficient data. To ensure that eligible studies were identified correctly, we used a 2-step selection process (29). Two independent investigators (authors LC and QL) performed an initial screening of all titles and/or abstracts and then evaluated all potentially relevant articles based on the full-text publications.
Data extraction and quality assessment
Data were extracted using a standardized data collection form. Two investigators (authors LC and QL) independently extracted the following information from eligible studies: the first author's last name, publication year, study location, study name, duration of follow-up, participants’ age and sex, sample size (i.e. the number of cases and/or participants), the percentage of participants with hypertension, diabetes, and/or pre-existing stroke, the method used to assess dietary B-vitamins intake, validation of B-vitamins in FFQs, exposure source and percentage of participants using dietary supplements, categories of intake, and the covariates adjusted for in the multivariable analysis. We extracted the risk estimates with the most adjustment.
The Newcastle–Ottawa scale was used to assess the risk of bias for each included cohort study (30). This scale assigns a maximum of 9 points to each study, with a score of 0–3, 4–6, and 7–9 indicating low, moderate, and high quality, respectively. To assess the validity and quality of the nutrition-related exposure, we modified the Newcastle–Ottawa scale “ascertainment of exposure” component (31). Specifically, we evaluated the risks of bias regarding information about the dietary assessment instrument, the use of supplements, and vitamin validation of the dietary assessment, using ratings of “A” and “B.” Detailed information regarding the modified instructions is presented in Supplemental Table 1. Any discrepancies were resolved through group discussion.
Statistical analysis
RR (with corresponding 95% CI) was used as the common effect size across studies; HRs and ORs were considered to approximate RR. We used the risk estimates of the original studies from multivariable models with the most complete adjustment for potential confounders. For studies that considered the highest category as the reference, we performed a transformation using the method described by Hamling et al. (32). Studies that stratified the data by sex, stroke subgroup, and/or race were treated as separate reports. We used a random-effects model to calculate the summarized RRs and their corresponding 95% CIs for the comparison between the highest and lowest categories of B-vitamins intake (33).
Given the various cut-off points among studies, we performed dose-response analyses for the increased intake of vitamin B-6, folate, and vitamin B-12 using the method recommended by Greenland and Longnecker and the publicly available Stata code written by Orsini et al. (34, 35). We extracted the mean intake (or range) of these B-vitamins in each category, the distribution of cases and participants (or person-years), and RR with 95% CI. The results of the linear dose-response shown in the forest plots are presented for each 100 μg/d increment in folate intake, 0.5 mg/d increment in vitamin B-6 intake, and 3 μg/d increment in vitamin B-12 intake. If the number of cases and/or person-years in each category was not reported, the variance-weighted least squares regression model was used to calculate the risk estimate (36, 37). If neither median nor mean intake per category were provided, the midpoint of the upper and lower boundaries in each category was used as the average intake. If the highest or lowest category was open-ended, the midpoint of the category was estimated by assuming that the width of the category was the same as the next adjacent category. In addition, we evaluated possible nonlinear associations between the intake of dietary B-vitamins and the risk of stroke using restricted cubic splines, with 3 knots at the 10th, 50th, and 90th percentiles of the distribution (38). The P value for nonlinearity was calculated by testing the null hypothesis that the coefficient of the second spline was equal to zero.
Heterogeneity among the studies was estimated using the I2 statistic, with values of 25%, 50%, and 75% representing low, moderate, and high degrees of heterogeneity, respectively (39). We also conducted subgroup analyses in order to explore the potential sources of heterogeneity, stratified by geographic location, sex, duration of follow-up, number of participants, stroke subtype, quality score, exclusion of pre-existing stroke, mean age, dietary assessment, the source of B-vitamins intake (foods only or foods and supplements combined), adjustment model (basic and multivariable), and adjustment for potential confounding factors (including age, BMI, physical activity, alcohol consumption, energy intake, level of education/income, hypertension/blood pressure, and diabetes). Heterogeneity between subgroups was evaluated by meta-regression analysis (40, 41). To test the robustness of each association, sensitivity analyses were conducted by omitting one study at a time.
Possible publication bias was assessed using Egger's test (42) and Begg's test (43), with the results considered to indicate publication bias at P < 0.10. In addition, we visually examined funnel plots for asymmetry. If possible, publication bias was indicated, we also used the trim-and-fill method to recalculate the pooled risk estimate (44). All data were analyzed using the statistical software program Stata, version 12.0 (STATA Corp.), and unless stated otherwise, a P value < 0.05 was considered significant.
Results
Literature search and study characteristics
The screening and selection process is shown in Figure 1. In brief, our search strategy initially identified 2233 articles in PubMed and 3054 articles in Web of Science. After we excluded duplicate publications and studies that did not meet the inclusion criteria, 11 publications (24–27, 45–51) were included in our meta-analysis. One publication (27) reported the results of 2 separate large-scale prospective cohort studies and was therefore regarded as 2 separate studies, resulting in a final analysis of 12 studies. One study (47) included male smokers who were previously enrolled in an RCT (52). These studies included a total of 389,938 participants and 10,749 stroke cases. Of the 12 included studies, 4 were conducted in the USA (24, 25, 50, 51), 3 were conducted in China (27, 48), 2 were conducted in Finland (45, 47), and 1 study each was conducted in Sweden (46), the Netherlands (26), and Japan (49). One study had a nested case-control design (46), and the remaining 11 studies were prospective cohort studies. The studies were published from 2002 through to 2019, and the follow-up period ranged from 4.2 to 19 y. One study (24) assessed dietary B-vitamins intake using 24-h dietary recall, one study (45) performed dietary history interviews, and the remaining 10 studies used a validated FFQ. Three studies used FFQs that validate for B-vitamins intake, whereas others did not. Information regarding the validation of FFQs is provided in Supplemental Table 2. Most studies excluded pre-existing stroke at baseline. All 12 studies provided risk estimates adjusted for cigarette smoking, and most studies also adjusted for other conventional risk factors, including BMI, energy intake, alcohol consumption, hypertension, and/or physical activity. Study quality scores ranged from 5 to 9; the mean quality score was 7.4 (Supplemental Table 3). The detailed characteristics regarding the studies included in our analysis are summarized in Tables 1 and 2.
FIGURE 1.

Flow chart depicting the literature search and selection strategy.
TABLE 1.
Characteristics of the prospective cohort studies included in the meta-analysis
| Author, year (ref) | Location | Study name | Gender | Age (mean), y | Follow-up, y | Cases, n | Participants, n | Participants with hypertension/diabetes/pre-existing stroke (%) |
|---|---|---|---|---|---|---|---|---|
| Al-Delaimy et al., 2004 (51) | USA | Nurses’ Health Study | F | 34–55 (44.5) | 18 | 1140 stroke cases | 83,272 | 15.4/2.0/0 |
| Bazzano et al., 2002 (24) | USA | NHANES I Epidemiologic Follow-up Study (NHEFS) | M/F | 25–74 (49) | 19 | 926 stroke cases | 9764 | 27/4.0/0 |
| Cui et al., 2010 (49) | Japan | Japan Collaborative Cohort Study | M/F | 40–79 (56) | 14 | 986 stroke cases | 58,730 | 19.6/4.5/0 |
| Dalmeijer et al., 2008 (26) | Dutch | Dutch PROSPECT-EPIC (European Prospective study Into Cancer and nutrition) cohort | F | 49–70 (57) | 8.1 | 227 CVA cases | 16,165 | 19.7/2.1/0 |
| He et al., 2004 (25) | USA | Health Professional Follow-up Study | M | 40–75 (52–55) | 14 | 725 stroke cases | 43,732 | 20.0/0/0 |
| Larsson et al., 20081 (47) | Finland | α-Tocopherol, β-carotene Cancer Prevention Study | M | 50–69 (57.1–58.1) | 13.6 | 2702 cerebral infarction cases; 383 intracerebral hemorrhage cases; 196 subarachnoid hemorrhage cases | 26,556 | N.A./6.3/0 |
| Luu et al., 2011 (50) | USA | Atherosclerosis Risk in Communities Study | M/F | 45–64 (54) | 16.5 | 594 stroke cases | 13,520 | 33.2/10.7/0 |
| Marniemi et al., 2005 (45) | Finland | Turku Health Survey | M/F | 65–99 (77) | 10 | 70 stroke cases | 755 | N.A./N.A./0 |
| Van Guelpen et al., 2005 (46) | Sweden | Northern Sweden Health and Disease Cohort | M/F | 25–74 (55.1) | 4.2 | 334 ischemic stroke cases; 62 hemorrhagic stroke cases | 1192 | 32.6/8.4/N.A. |
| Weng et al., 2008 (48) | China | Cardiovascular Disease risk Factor 2-township Study | M/F | 40–70 (56.6) | 10.6 | 132 stroke cases | 1772 | 23/10.0/0 |
| Zhao et al., 2019 (27) | China | Shanghai Men's Health Study, China | M | 40–74 (55.39) | 10.3 | 955 stroke cases | 59,746 | 29.4/6.0/3.8 |
| Zhao et al., 2019 (27) | China | Shanghai Women's Health Study, China | F | 40–70 (52.36) | 16.2 | 1317 stroke cases | 74,734 | 24.0/4.4/1.1 |
TABLE 2.
Characteristics of the prospective cohort studies included in the meta-analysis
| Author, year (ref) | Exposure source/percentage of participants using supplements (%) | Categories of intake | Exposure assessment | Adjustment for covariates | Quality score | |||
|---|---|---|---|---|---|---|---|---|
| Al-Delaimy et al., 2004 (51) | Foods + supplements; N.A. (folate) | Folate (μg/d): 30–210, 211–271, 272–354, 355–526, >526 | FFQ, 61 food items | Age, time period, smoking history, BMI, hormone use and menopausal status, currently taking aspirin, vitamin E supplements, physical activity, alcohol use, history of high blood pressure, history of diabetes, history of hypercholesterolemia, parental history of myocardial infarction at or before the age of 65 y, total caloric intake | 8 | |||
| Bazzano et al., 2002 (24) | Foods + supplements; 30% (multivitamin) | Folate (μg/d): <136, 136–203.7, 203.7–300.6, >300.6 | 24-h dietary recall | Age, race, sex, systolic blood pressure, serum cholesterol, BMI, history of diabetes, physical activity, level of education, regular alcohol consumption, current cigarette smoking, saturated fat intake, and total energy intake | 8 | |||
| Cui et al., 2010 (49) | Foods + supplements; 12.5% (multivitamin) | Folate (μg/d) | Vitamin B-6 (mg/d) | VitaminB-12 (μg/d) | FFQ, 33 food items | Age, BMI, history of hypertension and diabetes, smoking status, ethanol and energy intakes, as well as intakes of SFAs and n–3 and n–6 PUFAs | 7 | |
| <272 | <0.79 | <4.5 | ||||||
| 272–351 | 0.79–0.96 | 4.5–5.9 | ||||||
| 352–430 | 0.97–1.11 | 6.0–7.6 | ||||||
| 431–535 | 1.12–1.32 | 7.7–9.8 | ||||||
| >536 | >1.33 | >9.9 | ||||||
| Dalmeijer et al., 2008 (26) | Foods + supplements; 7.1% (multivitamin) | Folate (μg/d):<169169–191191–215>215Median intake | FFQ, 178 food items | Age, hypertension, cholesterolemia, mean systolic blood pressure, total physical activity, BMI, smoking, diabetes, intake of energy, proteins, saturated fats, monounsaturated fats, polyunsaturated fats, alcohol, vitamin B-2, vitamin B-6, vitamin B-12, betaine, and choline | 8 | |||
| He et al., 2004 (25) | Foods + supplements; 0%–50.5% (B-complex) | Folate (μg/d) | Vitamin B-6 (mg/d) | Vitamin B-12 (μg/d) | FFQ, 131 food items | Age, BMI, physical activity, history of hypertension and hypercholesterolemia, smoking status, aspirin use, alcohol, and total calorie intakes of fiber, potassium, and vitamin E | 9 | |
| 262 | 1.8 | 5.0 | ||||||
| 336 | 2.3 | 7.2 | ||||||
| 413 | 2.8 | 10.0 | ||||||
| 547 | 4.2 | 13.3 | ||||||
| 821 | 10.9 | 21.0 | ||||||
| Median intake | ||||||||
| Larsson et al., 20081 (47) | Foods; 0% | Folate (μg/d)262300330360410 | Vitamin B-6 (mg/d)1.92.22.52.73.0 | Vitamin B-12 (μg/d)6.68.510.212.316.2 | FFQ, 276 food items | Age, supplementation group, total number of cigarettes smoked daily, BMI, systolic and diastolic blood pressure, serum total cholesterol, serum HDL cholesterol, histories of diabetes and coronary heart disease, leisure-time physical activity, and intakes of alcohol and total energy | 8 | |
| Luu et al., 2011 (50) | Foods + supplements; N.A. (folic acid) | Folate (μg/d):0–155 | FFQ, 66 food items | Age, gender, current smoking status, diabetes, caloric intake, and hypertension | 7 | |||
| 156–211 | ||||||||
| 212–278 | ||||||||
| ≥279 | ||||||||
| Marniemi et al., 2005 (45) | Foods; N.A. (nutritional) | Lowest tertileMiddle tertile | Dietary history interview | Age, gender, smoking, functional capacity, and weight-adjusted energy intake | 5 | |||
| Highest tertile | ||||||||
| Van Guelpen et al., 2005 (46) | Foods + supplements; 17.4%–28.6% (multivitamin) | Folate (μg/103kcal*d) | Vitamin B-12 (μg/103kcal*d) | FFQ, 84 food items | BMI, current smoking, cholesterol, diabetes, and hypertension. Plasma homocysteine | 6 | ||
| <105 | <132 | <2.1 | <2.0 | |||||
| 105–134, | 132–166, | 2.1–2.8 | 2.0–2.7 | |||||
| 134–173, | 166–274, | 2.8–3.3 | 2.7–3.7 | |||||
| >173(M) | >274 (F) | >3.3(M) | >3.7(F) | |||||
| Weng et al., 2008 (48) | Foods; 0% | Folate (μg/d):<297.33297.33–369.45>396.45 | FFQ, 49 food items | Age, sex, area, hypertension, use of antihypertensive drugs, diabetes mellitus, central obesity, alcohol consumption habits, smoking habit, sex-smoking habit interaction, BMI, self-report heart disease, hypercholesterolemia, hypertriglyceridemia, physical activity, fibrinogen, apoB, and plasminogen | 9 | |||
| Zhao et al., 2019 (27) | Foods + supplements; 7.73%–13.93% (B-vitamins) | Median intake vitamin B-6 (mg/d):1.311.531.691.872.2 | FFQ, 81 food items | Age at baseline, energy intake, education, income, occupation, smoke, alcohol, BMI, waist-hip ratio, physical activity, history of diabetes, hypertension, coronary heart disease, and stroke, vitamin B supplements use, menopausal status, and hormone replacement therapy | 7 | |||
| Zhao et al., 2019 (27) | Foods + supplements; 7.85%–12.8% (B-vitamins) | Median intake vitamin B-6 (mg/d):1.241.451.621.792.11 | FFQ, 77 food items | Age at baseline, energy intake, education, income, occupation, smoke, alcohol, BMI, waist-hip ratio, physical activity, history of diabetes, hypertension, coronary heart disease, and stroke, vitamin B supplements use, menopausal status, and hormone replacement therapy | 7 | |||
Dietary vitamin B-6 intake and the risk of stroke
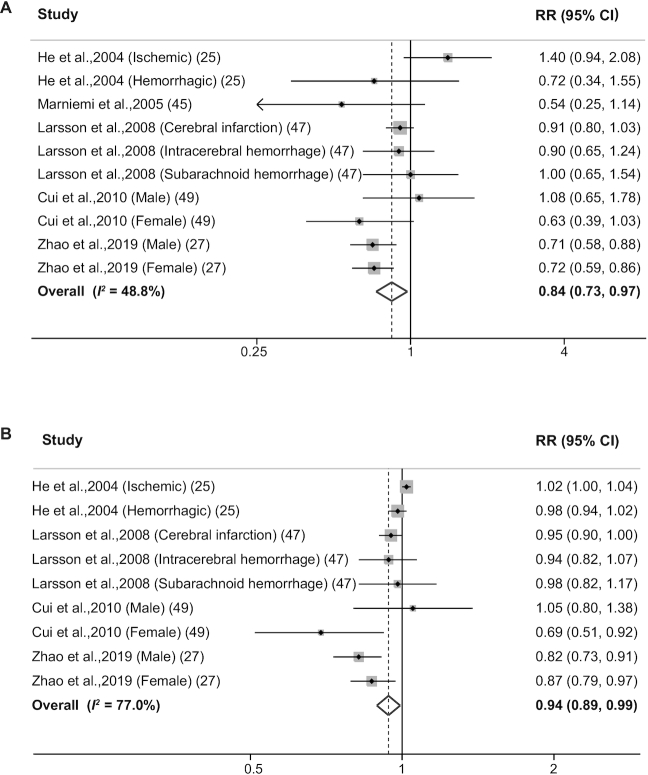
Ten reports in 5 publications (25, 27, 45, 47, 49) evaluated the association between dietary vitamin B-6 intake and the risk of stroke. A significant inverse association was observed for the highest versus the lowest category of dietary vitamin B-6 intake (RR: 0.84; 95% CI: 0.73–0.97; Figure 2A), with moderate heterogeneity (I2 = 48.8%). The RR was 0.91 (95% CI: 0.82–1.02; I2 = 0.0%) when intake from supplements was excluded and 0.80 (95% CI: 0.65–0.99; I2 = 54.1%) when intake from foods and supplements was taken into consideration. We found no evidence of publication bias using either Egger's test (P = 0.966) or Begg's test (P = 1.00), which is consistent with the funnel plot (Supplemental Figure 1A).
FIGURE 2.
Forest plots summarizing the RR with 95% CI of stroke between the highest and lowest categories of dietary vitamin B-6 intake (A) and each 0.5 mg/d increase in dietary vitamin B-6 intake (B).
One study (45) was considered ineligible for inclusion in the dose-response analysis due to a lack of information regarding the exposure of vitamin B-6 doses in each category. Nevertheless, our analysis based on the 9 reports in the other 4 studies (25, 27, 47, 49) revealed that each 0.5 mg/d increment in vitamin B-6 intake was associated with a 6% reduction in the risk of stroke (RR: 0.94; 95% CI: 0.89–0.99; Figure 2B), with high heterogeneity (I2 = 77.0%).
Dietary folate intake and the risk of stroke
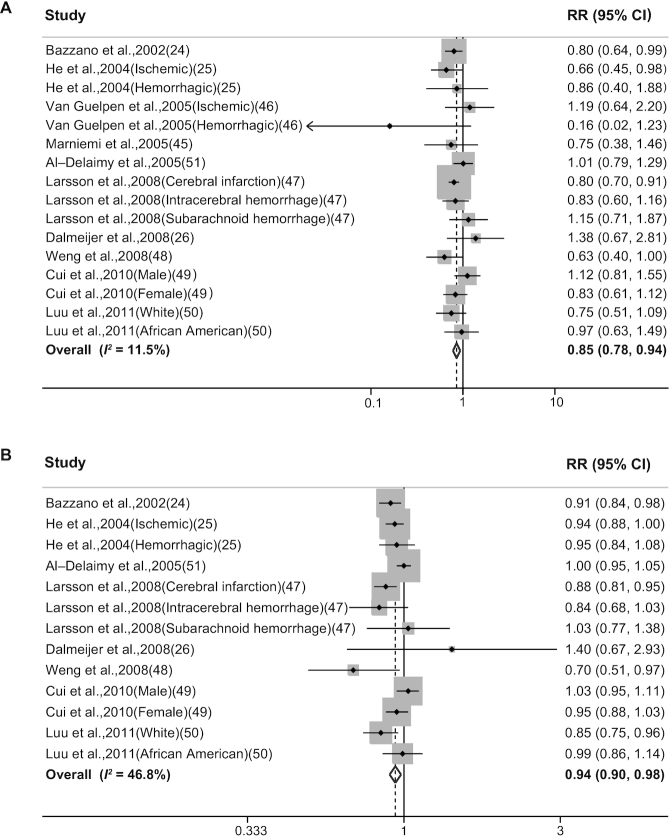
Sixteen reports in 10 publications (24–26, 45–51) evaluated the association between dietary folate intake and the risk of stroke. We found that the highest category of dietary folate intake was associated with a 15% lower risk of stroke compared with the lowest category of dietary folate intake (RR: 0.85; 95% CI, 0.78–0.94; Figure 3A), with low heterogeneity (I2 = 11.5%). Two studies conducted in the USA (25, 50) included both the prefortification and postfortification periods based on the mandatory fortification of folic acid introduced in 1998. After these 2 studies were excluded, the inverse association remained significant (RR: 0.88; 95% CI: 0.78–0.98; I2 = 24.2%). One study (24) used 24-h dietary recall to assess diet; after omitting this study, the RR was 0.87 (95% CI: 0.78–0.96). In addition, RR was 0.81 (95% CI: 0.71–0.92; I2 = 6.9%) for folate intake from foods and 0.88 (95% CI: 0.78–0.99; I2 = 11.9%) for intake from both foods and supplements. No evidence of publication bias was found when using either Egger's test (P = 0.76) or Begg's test (P = 0.96), and the funnel plot was basically symmetrical (Supplemental Figure 1B).
FIGURE 3.
Forest plots summarizing the RR with 95% CI of stroke between the highest and lowest categories of dietary folate intake (A) and each 100 μg/d increase in dietary folate intake (B).
A total of 13 reports (24–26, 47–51) were eligible for inclusion in the dose-response analysis, which revealed that for each 100 μg/d increase in dietary folate intake, the risk of stroke was reduced by 6% (RR: 0.94; 95% CI: 0.90–0.98; Figure 3B), with moderate heterogeneity (I2 = 46.8%).
Dietary vitamin B-12 intake and the risk of stroke
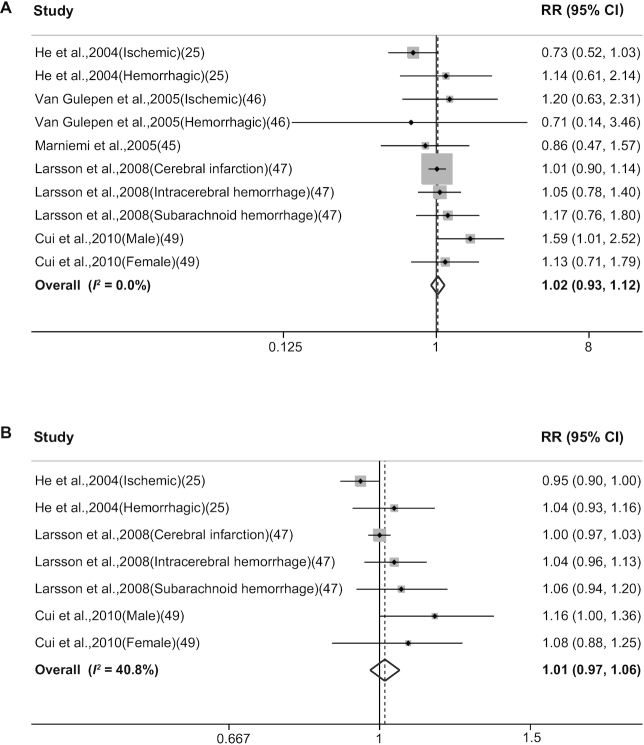
Ten reports in 5 publications (25, 45–47, 49) evaluated the association between dietary vitamin B-12 intake and the risk of stroke. Our analysis revealed no significant association between dietary vitamin B-12 intake and the risk of stroke, with a pooled RR for the highest versus the lowest category of vitamin B-12 intake of 1.02 (95% CI: 0.93–1.12; Figure 4A), with low heterogeneity (I2 = 8.8%). Similar results were obtained when we stratified the studies with various subgroups. The RR was 1.02 (95% CI: 0.92–1.13; I2 = 0.0%) for vitamin B-12 intake from foods and 1.07 (95% CI: 0.81–1.42; I2 = 37.8%) for B-12 intake from foods and supplements. We also performed a dose-response analysis and found that each 3 μg/d increase in dietary vitamin B-12 intake was not significantly associated with an increased risk of stroke (RR: 1.01; 95% CI: 0.97–1.06; Figure 4B), with moderate heterogeneity (I2 = 40.8%).
FIGURE 4.
Forest plots summarizing the RR with 95% CI of stroke between the highest and lowest categories of dietary vitamin B-12 intake (A) and each 3 μg/d increase in dietary vitamin B-12 intake (B).
Dose-response curve between dietary B-vitamins intake and stroke risk
We next assessed the dose-response relation between the intake of dietary B-vitamins and risk of stroke using a restricted cubic spine model. We observed linear associations between the risk of stroke and dietary vitamin B-6 intake (P = 0.18 for nonlinearity; Figure 5A) or dietary folate intake (P = 0.21 for nonlinearity; Figure 5B). In addition, no evidence of a nonlinear association was found between dietary vitamin B-12 intake and stroke risk (P = 0.87 for nonlinearity; Figure 5C).
FIGURE 5.
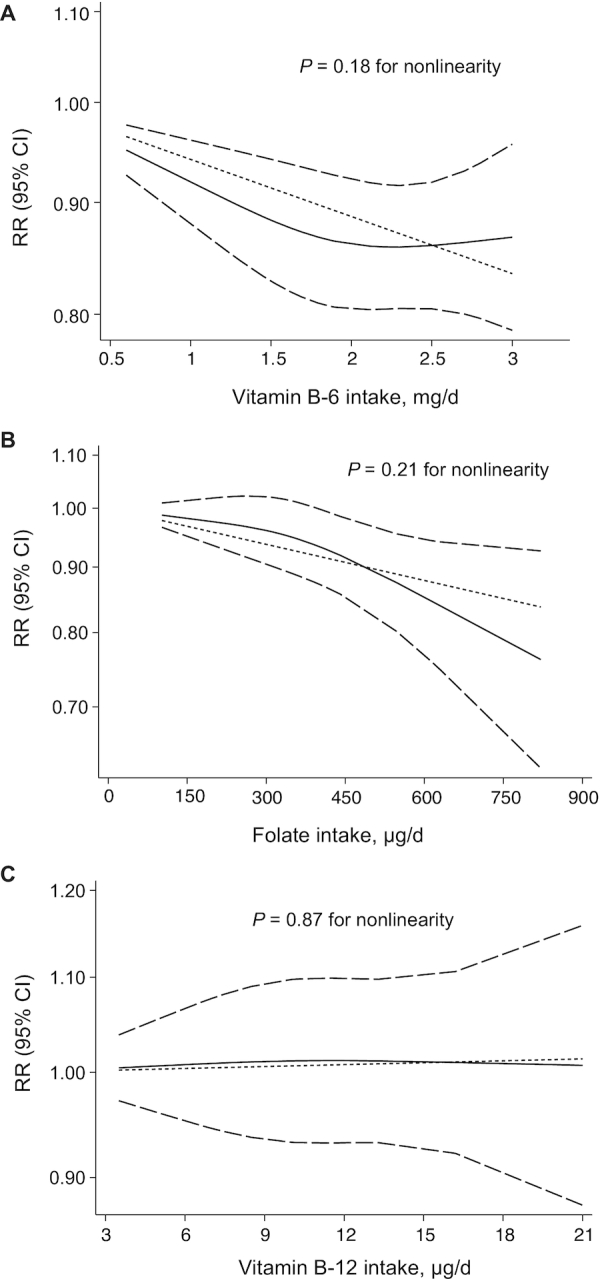
Dose-response analyses of the nonlinear association between the RR of stroke and vitamin B-6 intake (A), folate intake (B), and vitamin B-12 intake (C). Dashed lines indicate the 95% CI. In each panel, the solid line and dashed lines represent the estimated RR and 95% CI, respectively, and the dotted line represents the linear fit to the data.
Subgroup, sensitivity, and meta-regression analyses
Next, we performed several subgroup and meta-regression analyses to examine the possible sources of heterogeneity; the results of these analyses are summarized in Table 3. Overall, the inverse association between stroke risk and both folate and vitamin B-6 intake was evident in most subgroups. With respect to both folate and vitamin B-6 intake, this inverse association remained after adjusting for major confounding factors, including age, BMI, alcohol consumption, physical activity, energy intake, and diabetes. Cigerette smoking was adjusted for in all inculded studies; thus, no stratified results by smoking were reported. In addition, we found significant associations in studies with longer follow-up and studies without a pre-existing stroke event. When we stratified studies by mean age, we found no differences between age ≥55 y and age <55 y for folate (P = 0.98); as for vitamin B-6, we found a significant inverse association in the ≥55 y subgroup. When we stratified studies based on adjustment for hypertension/blood pressure, the RR was 0.86 (95% CI: 0.78–0.95) for folate and 0.86 (95% CI: 0.74–0.99) for vitamin B-6. When using FFQs to assess diet, the RR was 0.87 (95% CI: 0.78–0.97) for folate and 0.86 (95% CI: 0.74–0.99) for vitamin B-6. When using FFQs that validate for B-vitamins intake, the RR was 0.80 (95% CI: 0.71–0.89) for folate and 0.96 (95% CI: 0.83–1.10) for vitamin B-6. With respect to folate, our analyses revealed that folate intake was inversely associated with stroke risk among males and among US-based studies, although little evidence of heterogeneity was found between these subgroups. We also found that folate intake was associated with a reduced risk of ischemic stroke (RR: 0.84; 95% CI: 0.73–0.97; 4623 cases included in the analysis), but not hemorrhagic stroke (RR: 0.85; 95% CI: 0.61–1.19; 647 cases included in the analysis). In our analysis of vitamin B-6, we found evidence of heterogeneity when studies were stratified by participant number (P = 0.004), with a weaker association in studies that included a relatively smaller sample size (i.e. <50,000 participants). When stratified by geographic location, we found a stronger association among studies conducted in Asia compared with studies conducted in Europe and the USA. When stratified by stroke subtype, we found no significant association between vitamin B-6 intake and ischemic stroke (RR: 1.08; 95% CI: 0.72–1.64; 3157 cases included in the analysis) or hemorrhagic stroke (RR: 0.87; 95% CI: 0.65–1.17; 508 cases included in the analysis).
TABLE 3.
Subgroup analysis of the dietary intake of homocysteine metabolism-related B-vitamins and the risk of stroke1
| Vitamin B-6 | Folate | Vitamin B-12 | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | RR (95% CI) | I2 | P 2 | P 3 | N | RR (95% CI) | I2 | P 2 | P 3 | N | RR (95% CI) | I2 | P 2 | P 3 | |
| Overall | 10 | 0.84 (0.73, 0.97) | 48.8 | 0.041 | 16 | 0.85 (0.78, 0.94) | 11.5 | 0.322 | 10 | 1.03 (0.94, 1.13) | 0.4 | 0.434 | |||
| Location | 0.015 | 0.803 | 0.45 | ||||||||||||
| USA | 2 | 1.09 (0.58, 2.05) | 56.8 | 0.128 | 6 | 0.85 (0.74, 0.97) | 0 | 0.467 | 2 | 0.84 (0.56, 1.26) | 33.1 | 0.221 | |||
| Europe | 4 | 0.9 (0.81, 1.01) | 0 | 0.566 | 7 | 0.88 (0.73, 1.06) | 23.3 | 0.251 | 6 | 1.02 (0.51, 0.69) | 0 | 0.946 | |||
| Asia | 4 | 0.73 (0.64, 0.83) | 0 | 0.429 | 3 | 0.86 (0.64, 1.17) | 53.6 | 0.116 | 2 | 1.34 (0.96, 1.88) | 5.6 | 0.303 | |||
| Sex | 0.067 | 0.203 | 0.67 | ||||||||||||
| Male | 7 | 0.91 (0.78, 1.07) | 44.4 | 0.095 | 6 | 0.86 (0.74, 1) | 24.7 | 0.249 | 6 | 1.04 (0.88, 1.22) | 36.5 | 0.163 | |||
| Female | 2 | 0.71 (0.60, 0.84) | 0 | 0.615 | 3 | 0.96 (0.8, 1.16) | 1.5 | 0.362 | 1 | 1.13 (0.71, 1.79) | — | — | |||
| Follow-up | 0.266 | 0.371 | 0.84 | ||||||||||||
| >10 y | 9 | 0.86 (0.74, 0.99) | 50.6 | 0.04 | 12 | 0.84 (0.77, 0.92) | 2.7 | 0.418 | 7 | 1.04 (0.9, 1.2) | 25.6 | 0.234 | |||
| ≤10 y | 1 | 0.54 (0.25, 1.14) | — | — | 4 | 0.96 (0.57, 1.6) | 38.2 | 0.183 | 3 | 0.98 (0.64, 1.5) | 0 | 0.705 | |||
| Participants | 0.004 | 0.05 | 0.10 | ||||||||||||
| <50,000 | 6 | 0.94 (0.80, 1.11) | 26.3 | 0.237 | 13 | 0.81 (0.74, 0.89) | 0 | 0.511 | 8 | 1.00 (0.90, 1.10) | 0 | 0.688 | |||
| >50,000 | 4 | 0.73 (0.64, 0.83) | 0 | 0.429 | 3 | 0.98 (0.83, 1.15) | 0 | 0.395 | 2 | 1.34 (0.96, 1.88) | 5.6 | 0.303 | |||
| Stroke subtype | 0.43 | 0.978 | 0.62 | ||||||||||||
| Ischemic stroke | 2 | 1.08 (0.72, 1.64) | 75.6 | 0.043 | 7 | 0.84 (0.73, 0.97) | 33.9 | 0.169 | 3 | 0.94 (0.74, 1.19) | 42.5 | 0.176 | |||
| Hemorrhagic stroke | 2 | 0.87 (0.65, 1.17) | 0 | 0.596 | 4 | 0.85 (0.61, 1.19) | 11.1 | 0.338 | 3 | 1.05 (0.81, 1.37) | 0 | 0.863 | |||
| Mean age (y) | 0.73 | 0.98 | 0.11 | ||||||||||||
| ≥55 | 7 | 0.84 (0.73, 0.96) | 28.4 | 0.212 | 10 | 0.88 (0.76, 1.01) | 27.1 | 0.195 | 6 | 1.05 (0.95, 1.16) | 0 | 0.695 | |||
| <55 | 3 | 0.91 (0.51, 1.49) | 77.5 | 0.012 | 6 | 0.85 (0.74, 0.96) | 0 | 0.467 | 4 | 0.84 (0.56, 1.26) | 33.1 | 0.221 | |||
| Exposure assessment | 0.266 | 0.501 | 0.57 | ||||||||||||
| FFQ | 9 | 0.86 (0.74, 0.99) | 50.6 | 0.04 | 14 | 0.87 (0.78, 0.97) | 21.1 | 0.224 | 9 | 1.03 (0.92, 1.15) | 5.8 | 0.387 | |||
| Non-FFQ | 1 | 0.54 (0.25, 1.14) | — | — | 2 | 0.80 (0.65, 0.98) | 0 | 0.844 | 1 | 0.86 (0.47, 1.57) | — | — | |||
| Validation on B-vitamins | 0.03 | 0.04 | 0.56 | ||||||||||||
| Yes | 5 | 0.96 (0.83, 1.10) | 15.6 | 0.315 | 6 | 0.80 (0.71, 0.89) | 0 | 0.519 | 5 | 1.00 (0.90, 1.11) | 1.6 | 0.397 | |||
| No | 4 | 0.73 (0.64, 0.83) | 0 | 0.429 | 8 | 0.96 (0.83, 1.11) | 11.3 | 0.342 | 4 | 1.29 (0.96, 1.71) | 0 | 0.637 | |||
| Exposure source | 0.05 | 0.265 | 0.56 | ||||||||||||
| Foods + supplements | 7 | 0.80 (0.65, 0.99) | 54.1 | 0.041 | 12 | 0.89 (0.78, 0.99) | 11.9 | 0.328 | 6 | 1.07 (0.81, 1.42) | 37.8 | 0.154 | |||
| Foods | 3 | 0.91 (0.82, 1.02) | 0 | 0.914 | 4 | 0.81 (0.71, 0.92) | 6.9 | 0.359 | 4 | 1.02 (0.92, 1.13) | 0 | 0.862 | |||
| Quality | 0.266 | 0.846 | 0.84 | ||||||||||||
| <7 | 1 | 0.54 (0.25, 1.14) | — | — | 3 | 0.80 (0.40, 1.59) | 47.2 | 0.151 | 3 | 0.98 (0.64, 1.50) | 0 | 0.705 | |||
| ≥7 | 9 | 0.86 (0.74, 0.99) | 50.6 | 0.04 | 13 | 0.85 (0.78, 0.93) | 8.5 | 0.36 | 7 | 1.04 (0.90, 1.20) | 25.6 | 0.234 | |||
| Adjustments4 | |||||||||||||||
| Age | — | 0.56 | 0.783 | ||||||||||||
| Yes | 10 | 0.84 (0.73, 0.97) | 48.8 | 0.041 | 14 | 0.85 (0.78, 0.92) | 1.9 | 0.428 | 8 | 1.03 (0.91, 1.16) | 16.3 | 0.302 | |||
| No | 0 | — | — | — | 2 | 0.56 (0.08, 3.76) | 70.1 | 0.067 | 2 | 1.11 (0.61, 2.04) | 0 | 0.552 | |||
| BMI | 0.266 | 0.828 | 0.57 | ||||||||||||
| Yes | 9 | 0.86 (0.74, 0.99) | 50.6 | 0.04 | 13 | 0.86 (0.78, 0.96) | 25.1 | 0.19 | 9 | 1.03 (0.92, 1.15) | 5.8 | 0.387 | |||
| No | 1 | 0.54 (0.25, 1.14) | — | — | 3 | 0.82 (0.63, 1.07) | 0 | 0.646 | 1 | 0.86 (0.47, 1.57) | — | — | |||
| Physical activity | 0.503 | 0.36 | 0.84 | ||||||||||||
| Yes | 7 | 0.86 (0.74, 1.00) | 56.8 | 0.031 | 9 | 0.84 (0.75, 0.93) | 13.8 | 0.319 | 5 | 1.00 (0.90, 1.11) | 1.6 | 0.397 | |||
| No | 3 | 0.75 (0.50, 1.13) | 38.4 | 0.197 | 7 | 0.9 (0.76, 1.08) | 11.8 | 0.339 | 5 | 1.20 (0.92, 1.55) | 0 | 0.545 | |||
| Alcohol | 0.266 | 0.967 | 0.21 | ||||||||||||
| Yes | 9 | 0.86 (0.74, 0.99) | 50.6 | 0.04 | 11 | 0.85 (0.77, 0.95) | 19 | 0.263 | 7 | 1.04 (0.90, 1.20) | 25.6 | 0.234 | |||
| No | 1 | 0.54 (0.25, 1.14) | — | — | 5 | 0.85 (0.65, 1.11) | 12.9 | 0.332 | 3 | 0.98 (0.64, 1.50) | 0 | 0.705 | |||
| Energy intake | 0.512 | 0.78 | |||||||||||||
| Yes | 10 | 0.84 (0.73, 0.97) | 48.8 | 0.041 | — | 13 | 0.85 (0.79, 0.92) | 0 | 0.475 | 8 | 1.03 (0.91, 1.16) | 16.3 | 0.302 | ||
| No | 0 | — | — | — | 3 | 0.72 (0.36, 1.44) | 58.9 | 0.088 | 2 | 1.11 (0.61, 2.04) | 0 | 0.552 | |||
| Excluding pre-existing stroke | 0.266 | 0.705 | 0.57 | ||||||||||||
| Yes | 9 | 0.86 (0.74, 0.99) | 50.6 | 0.04 | 15 | 0.86 (0.78, 0.95) | 16.7 | 0.267 | 9 | 1.03 (0.92, 1.15) | 5.8 | 0.387 | |||
| No | 1 | 0.54 (0.25, 1.14) | — | — | 1 | 0.75 (0.38, 1.46) | — | — | 1 | 0.86 (0.47, 1.57) | — | — | |||
| Level of education | 0.004 | 0.572 | — | ||||||||||||
| Yes | 2 | 0.72 (0.62, 0.82) | 26.7 | 0.216 | 1 | 0.80 (0.64, 0.99) | — | — | 0 | — | — | — | |||
| No | 8 | 0.92 (0.79, 1.08) | 48.8 | 0.041 | 15 | 0.87 (0.78, 0.96) | 15.9 | 0.276 | 10 | 1.03 (0.94, 1.13) | 0.4 | 0.434 | |||
| Level of income | 0.004 | — | — | ||||||||||||
| Yes | 2 | 0.72 (0.62, 0.82) | 26.7 | 0.216 | 0 | — | — | — | 0 | — | — | — | |||
| No | 8 | 0.92 (0.79, 1.08) | 48.8 | 0.041 | 16 | 0.85 (0.78, 0.94) | 11.5 | 0.322 | 10 | 1.03 (0.94, 1.13) | 0.4 | 0.434 | |||
| Adjustment model | 0.517 | 0.565 | 0.763 | ||||||||||||
| Basic | 7 | 0.77 (0.65, 0.92) | 74.3 | <0.001 | 11 | 0.85 (0.76, 0.95) | 32.2 | 0.141 | 7 | 1.10 (0.96, 1.25) | 24.7 | 0.241 | |||
| Multivariable | 7 | 0.82 (0.72, 0.93) | 37.3 | 0.141 | 11 | 0.88 (0.78, 0.99) | 30.2 | 0.158 | 7 | 1.05 (0.95, 1.16) | 0 | 0.635 | |||
| Diabetes | 0.266 | 0.705 | 0.57 | ||||||||||||
| Yes | 9 | 0.86 (0.74, 0.99) | 50.6 | 0.04 | 15 | 0.86 (0.78, 0.95) | 16.7 | 0.267 | 9 | 1.03 (0.92, 1.15) | 5.8 | 0.387 | |||
| No | 1 | 0.54 (0.25, 1.14) | — | — | 1 | 0.75 (0.38, 1.46) | — | — | 1 | 0.86 (0.47, 1.57) | — | — | |||
| Hypertension/blood pressure | 0.266 | 0.705 | 0.57 | ||||||||||||
| Yes | 9 | 0.86 (0.74, 0.99) | 50.6 | 0.04 | 15 | 0.86 (0.78, 0.95) | 16.7 | 0.267 | 9 | 1.03 (0.92, 1.15) | 5.8 | 0.387 | |||
| No | 1 | 0.54 (0.25, 1.14) | — | — | 1 | 0.75 (0.38, 1.46) | — | — | 1 | 0.86 (0.47, 1.57) | — | — | |||
Pooled estimates were obtained using a random-effects model by comparing the highest category and lowest category.
P value for heterogeneity within each subgroup.
P value for heterogeneity between subgroups with meta-regression analysis.
Cigarette smoking was adjusted for in all studies; therefore, no stratified results by smoking are reported.
We also analyzed studies that provided RR values that reflect the least and greatest degree of covariate adjustments. With respect to folate, the pooled RR in the basic and multivariable adjustment model was 0.85 (95% CI: 0.76–0.95) and 0.88 (95% CI: 0.78–0.99), respectively; for vitamin B-6, the pooled RR was 0.77 (95% CI: 0.65–0.92) and 0.82 (95% CI: 0.72–0.93), respectively.
We also performed sensitivity analyses in order to further test the robustness of the associations. With respect to folate and vitamin B-12, the overall results were not changed substantially by excluding one study at a time. With respect to vitamin B-6, when we omitted either of the 2 studies published by Zhao et al. (27), the pooled RR for each 0.5 mg/d increase in vitamin B-6 intake increased to 0.96 (95% CI: 0.92‒1.01), although the heterogeneity decreased slightly to I2 = 69%. Excluding any other single study had no notable effect on overall RR.
Publication bias
We found little evidence of publication bias with respect to most of the analyses included in our study. Only for the dose-response analysis between dietary vitamin B-6 intake and stroke risk, we observed some indication of publication bias when using Egger's test (P = 0.02), which is consistent with the funnel plots shown in Supplemental Figures 1 and 2. However, use of the trim-and-fill method did not change the pooled risk estimate, suggesting that the results were unlikely to be affected by this publication bias (Supplemental Table 4).
Discussion
This systematic meta-analysis based on 11 prospective studies involving 389,938 participants and 10,749 cases of stroke revealed a clear correlation between dietary intake of homocysteine metabolism-related B-vitamins and the risk of stroke. Specifically, we found inverse associations between stroke risk and the intake of both folate and vitamin B-6. Moreover, our dose-response analysis revealed that either a 0.5 mg/d increase in vitamin B-6 intake or a 100 μg/d increase in folate intake reduces the risk of stroke by 6%. These inverse associations remained significant even after adjusting for conventional risk factors associated with cardiovascular disease (CVD), including BMI, smoking, physical activity, alcohol consumption, diabetes/hypertension, and energy intake. In contrast to our findings with vitamin B-6 and folate, we found no significant association between vitamin B-12 intake and stroke risk. To the best of our knowledge, our study is the first meta-analysis designed to evaluate the dose-response relation between dietary B-vitamins intake and the risk of stroke.
Vitamin B-6, folate, and vitamin B-12 are essential nutrients involved in the one-carbon metabolism pathway, which plays an important role in homocysteine metabolism in the human body (53). Specifically, vitamin B-6, folate, and vitamin B-12 act as coenzymes either to degrade homocysteine to form cysteine or to methylate homocysteine to form methionine (54). Previous RCTs showed that supplementation with folic acid, either alone or in combination with vitamin B-6 and/or vitamin B-12, significantly reduces blood homocysteine concentrations (16, 55–57). Early in 1969, McCully postulated that homocysteine affects atherosclerosis (58), and subsequent studies found that high concentrations of homocysteine can cause vascular damage, including endothelial dysfunction, increased intimal-medial thickness, and increased arterial stiffness (5, 59, 60), thus leading to atherosclerosis and vascular disease. Observational studies have provided evidence that a 3 μmol/L reduction in blood homocysteine concentrations is associated with an 11% reduction in the risk of ischemic heart disease and a 19% reduction in the risk of stroke (10). On the other hand, each 5 μmol/L increase in serum homocysteine concentration has been associated with a 32% higher risk of ischemic heart disease and a 59% higher risk of stroke (7).
The mechanism underlying the inverse association between folate intake and stroke risk remains speculative but is biologically plausible, given that folate serves as an essential methyl donor in the remethylation of homocysteine to form methionine (61). RCTs have shown that daily supplementation with 0.5–5 mg of folic acid reduces plasma homocysteine concentrations by ∼25% (55). Moreover, as discussed above, the adverse effects of high homocysteine concentrations on stroke risk are well known and have been summarized in a number of review articles. In addition to its effects on homocysteine metabolism, some studies have also suggested that folate may play a role in other antiatherosclerotic processes, such as delaying the development of atherosclerotic lesions (62), increasing plasma HDL cholesterol concentrations (63), and reverting endothelial nitric oxide synthase (eNOS) dysfunction by increasing the production of nitric oxide (64, 65).
Since 2007, several meta-analyses of clinical trials have been conducted regarding the intervention effect of folic acid on the risk of stroke (18–21, 23, 66), and nearly all of these studies suggested that folic acid supplementation can reduce the risk of stroke, with RR values ranging from 0.82 to 0.96. Nevertheless, our findings based on observational studies are not comparable with findings based on intervention studies, as several discrepancies exist. First, the RCTs were generally performed among individuals with pre-existing vascular disease, whereas the participants in epidemiological studies were typically healthier. Based on our results, we found robust inverse associations in studies that excluded participants with a pre-existing cerebrovascular disease, which suggests that folate may play a role in the primary prevention of stroke. Indeed, a previous study conducted among participants without diabetes or CVD found that lowering homocysteine with folate-based B-vitamins significantly reduced the progression of early-stage subclinical atherosclerosis (67). In addition, folate derived from food differs from that derived from supplementation in both type and amount. Intervention studies usually use high-dose folic acid, which may be associated with an increased risk of other chronic diseases, such as cancer (68, 69). In the present meta-analysis, we found that increasing folate intake at physiologically relevant daily amounts is associated with a reduced risk of stroke. Furthermore, the power to determine associations based on long-term follow-up in RCTs may be limited by the fact that the majority of these studies were designed with a relatively short time period. In the present meta-analysis, we observed a significant association in a subgroup of studies with a long follow-up duration (i.e. >10 y), suggesting that folate intake may have long-term health benefits in terms of reducing the risk of stroke.
With respect to vitamin B-6, we found that each 0.5 mg/d increase in vitamin B-6 intake reduces the risk of stroke by 6%. A recent meta-analysis by Jayedi and Zargar revealed that a higher intake of vitamin B-6 was associated with a decreased risk of ischemic heart disease in the general population (70). Combined with our findings, it is interesting to note that consuming foods rich in vitamin B-6 may have health benefits in the context of atherosclerosis. Other than homocysteine-lowering effects, vitamin B-6 may lower the risk of stroke via other vascular-protective properties. For example, vitamin B-6 can act as an antioxidant by scavenging oxygen radicals (71), reducing the production of lipid peroxides (72), and preventing LDL-induced vascular endothelial dysfunction (73). Moreover, vitamin B-6 is also involved in the inflammatory process (74), and inflammation has been reported to play a key role in the pathogenesis of stroke (75). Several epidemiological studies have suggested that plasma concentrations of PLP (pyridoxal 5’-phosphate, the bioactive form of vitamin B-6) are negatively correlated with certain inflammatory biomarkers, including high-sensitivity C-reactive protein (hs-CRP) and fibrinogen (76, 77).
Certain factors should be taken into consideration when attempting to analyze the relation between vitamin B-6 and stroke. One important concern is age. The aging process has been reported to result in a higher requirement for vitamin B-6 intake in humans (78). Although vitamin B-6 deficiency is relatively rare in young people due to the presence of vitamin B-6 in many foods, including animal products and vegetables (79), the relatively high prevalence of vitamin B-6 deficiency among the elderly cannot be ignored (80–82). This deficiency may be attributed to increased hydrolysis of PLP with aging (83). Thus, one's intake of vitamin B-6 should increase with age, particularly in populations with poor nutritional status. When we stratified the studies by geographic location, we observed a significant inverse association between dietary vitamin B-6 intake and the risk of stroke in Asian studies, a borderline inverse association in European studies, and no significant association in US-based studies. Notably, we found that the median values of the lowest category of vitamin B-6 intake in 2 Asian studies (0.6 and 1.24 mg/d) are both lower than the lowest category in US-based studies (1.8 mg/d) (25, 27, 49). Given that the amount of vitamin B-6 intake in the reference group approached the maximal benefit in US-based studies (25), where the RDA for vitamin B-6 is 2 mg/d (84), this may have limited our ability to detect an inverse association in the highest quintile. Importantly, as the rate of stroke tends to be higher in Asia compared with most Western countries, and given that the at-risk population in Asia is extremely large, the public health benefit associated with vitamin B-6 intake cannot be overstated (85, 86). Nevertheless, because the number of studies in each subgroup is relatively low, further studies are warranted in order to investigate this relation in various regions.
Interestingly, our analysis revealed that dietary vitamin B-12 intake does not appear to be associated with the risk of stroke. The reason for this difference between vitamin B-12 and both vitamin B-6 and folate is not clear; however, one possible explanation is that vitamin B-12 has a relatively mild effect on homocysteine concentrations. Indeed, a meta-analysis of RCTs suggested that supplementation with folic acid reduces homocysteine concentrations by ≤25%, and the addition of vitamin B-12 produced only a 7% further reduction in homocysteine concentrations (15). Another explanation is that a small percentage of study participants had vitamin B-12 deficiency; indeed, the median intake of the reference quintile in the included studies (5, 6.6, and 3.6 μg/d) were all above the RDA for vitamin B-12 (2.4 μg/d) (25, 47, 49). Vitamin B-12 deficiency is usually associated with impaired absorption (for example, due to atrophic gastritis) rather than low nutritional intake. However, in the study by Larsson et al. (47), when the authors excluded men with low serum pepsinogen I concentrations to exclude any potential bias due to impaired absorption, they still found no significant association. Thus, further studies are warranted to examine the relation between vitamin B-12 intake and the risk of stroke in vitamin B-12-deficient regions, such as India (87, 88).
Given the effect of B-vitamins on various types of vascular diseases, it is important to note that our results suggest that increasing habitual B-vitamins intake in healthy populations could be beneficial for stroke, although previous RCTs found little or no effect on the risk of IHD (23, 89). The precise mechanism underlying this apparent differential effect of B-vitamins on stroke and IHD is unknown; however, several factors may play a role in this difference. First, stroke and IHD have different etiologies and pathophysiologies. For example, IHD primarily affects large coronary vessels, whereas stroke often involves both large and small cerebral vessels. A previous study has reported that elevated homocysteine is a risk factor for cerebral small vessel disease (90); thus, the putative beneficial effect of B-vitamins in lowering homocysteine on stroke but not IHD may be attributed to reduced atherosclerosis primarily in small cerebral vessels.
Hypertension has been reported to be an important risk factor for stroke. However, due to limited information, we were unable to conduct subgroup analysis among participants with hypertension and normotensive participants. Thus, we performed analyses based on adjustments for hypertension/blood pressure. Omitting the single study that did not make this adjustment (45) yielded generally similar results (the RRs changed from 0.85 to 0.86 for folate and from 0.84 to 0.86 for vitamin B-6), suggesting that hypertension may not significantly affect the relation between folate or vitamin B-6 intake and stroke risk.
Age is another major risk factor for stroke. To examine the effect of age on our overall results, we performed a sensitivity analysis by excluding one study that did not adjust for age (46) and found no significant change in the inverse relation between folate or vitamin B-6 and stroke risk. As none of the included studies provided risk estimates for different age intervals, we performed analysis using the mean/median age in each cohort and found that increasing vitamin B-6 intake may have an additional beneficial effect on stroke among older individuals (i.e. aged ≥55 y). Nevertheless, further studies are needed in order to determine the association in various age groups.
Our meta-analysis has several key strengths. First, because we based our analysis on prospective studies, our findings are not likely due to recall bias or selection bias. Moreover, most of the studies included in our analysis had a large sample size and long follow-up duration, which provided high statistical power to our assessment of the correlation between dietary vitamin-B intake and the risk of stroke. In addition, we also conducted subgroup analyses and found that the inverse associations persisted even after adjusting for major risk factors for CVD. Finally, in addition to comparing the highest and lowest categories of B-vitamins intake, we also generated dose-response curves for vitamin B-6, folate, and vitamin B-12 intake.
Despite these strengths, our analysis has several limitations that should be acknowledged when interpreting our findings. First, due to the observational nature of the included studies, we cannot exclude the potential bias effect of unmeasured or residual confounders, as subjects with a higher B-vitamins intake tend to have a healthier lifestyle, such as less smoking, a reduced incidence of overweight, higher physical activity, and lower consumption of alcohol (91–94), and they are also more likely to have a higher social-economic status. In order to estimate the potential impact of these factors on our results, we calculated the effect size using the RR values based on basic and multivariable adjustment models and found that these adjustments may contribute to ∼3% and 5% reduction in stroke risk for folate and vitamin B-6, respectively. Nevertheless, when we stratified the studies by adjustment for major lifestyle factors (e.g. smoking, alcohol consumption, physical activity, and BMI), these inverse associations remained in the majority of subgroups. With respect to social-economic status, we found that the inverse associations persist in studies adjusted for level of income/education. Another possible limitation with respect to evaluating the association between B-vitamins intake and stroke risk is that dietary factors may not have been considered to a sufficient degree. Among the studies included in our analysis, only a few (24–26, 49) adjusted for several dietary factors, including the intake of saturated fats/polyunsaturated fats, vitamin E, and/or dietary fiber; thus, we cannot exclude the possibility that other nutrients and/or dietary components correlated with dietary B-vitamins (e.g. fruits, vegetables, and/or whole grains) may have been responsible, at least in part, for the associations identified in our study. Further studies should therefore attempt to control for adequate dietary variables to minimize any bias associated with the conclusions.
Secondly, meta-analyses can result in significant heterogeneity due to differences in the characteristics of studies. In our study, we found either low evidence or no evidence of heterogeneity in the analysis of folate and vitamin B-12, and moderate heterogeneity in the analysis of vitamin B-6. We conducted several subgroup and meta-regression analyses to explore the potential source of heterogeneity. For both folate and vitamin B-6, we found that the significant inverse associations persisted in the subgroup of studies with relatively long follow-up (i.e. >10 y), which indicates that dietary intake of folate and vitamin B-6 may have beneficial effects on stroke in the long term, but not necessarily the short term, in the general population. In our analysis of vitamin B-6, we found significant between-subgroup heterogeneity when we stratified studies by participant number, with a stronger inverse association among studies with a relatively large number of participants (i.e. >50,000), as well as reduced heterogeneity within subgroups. We also found evidence of heterogeneity when we stratified studies by geographic location, with stronger inverse associations among Asian studies and no significant association among US studies. With respect to stroke subtypes, we found an inverse association between folate intake and the risk of ischemic stroke. In addition, we observed no evidence of heterogeneity between subgroups when we stratified the analyses by quality score or adjustment for confounding factors. Nevertheless, the overall heterogeneity appeared to be driven more by differences in the size of the association rather than by differences in the direction of the association, as the majority of subgroup analyses yielded an inverse association.
Thirdly, the potential impacts of measurement error need to be addressed. For example, the intake of B-vitamins may have changed during long follow-up periods, possibly without timely updates. Most of the studies included in our analysis assessed dietary B-vitamins intake at baseline, and only one study used repeated measurements in an attempt to reduce error (25). We found that when using baseline diet compared with that from cumulative average diet, the associations with stroke risk were only slightly weakened. In addition, the different methods used to assess diet will inevitably lead to some degree of heterogeneity. For example, Bazzano et al. (24) used a single 24-h recall, which may not be representative of the participants’ long-term intake. The exclusion of this study yielded a pooled RR of 0.87. Another study performed dietary history interviews (45); after omitting these 2 studies, the results were not materially changed, suggesting that our findings are generally robust with respect to measurements of assessing diet. In addition, we conducted analysis in studies with validation for B-vitamins intake, finding that folate intake was inversely associated with stroke risk, whereas vitamin B-6 was not. Notably, as only a few studies used FFQs that provide correlation for the B-vitamins under study, more studies need to be done on this issue.
Concern also arises from different ranges of folate and vitamin B-6 intake, which might be attributed to the differences in dietary habits, dietary assessments, and geographic locations across studies. The differences of intake categories could also result in heterogeneity and lead to some degree of insecurity on the risk estimates (36). Therefore, it is important to deal with the potential bias introduced by methodological differences that seem to be inherent in nutritional studies. Future studies would be better conducted with consistent methods estimating the intake and similar exposure levels to minimize the bias and enhance the accuracy of the overall results.
Another potential factor is the different sources of B-vitamins in the included studies. Some studies measured B-vitamins intake from foods, whereas other studies included participants who consumed B-vitamins from both food and supplements. To explore the potential impact of supplements on the results, we performed subgroup analysis stratified by the source of B-vitamins. For folate, similar inverse associations were observed across studies with or without supplements; as for vitamin B-6, a stronger association was observed in studies with supplements, suggesting that vitamin B-6 intake from supplements may have been a potential source of bias. Thus, future studies should attempt to evaluate the association among participants with or without vitamin supplements.
Another limitation that warrants discussion is the fact that in 1998, the US FDA required that flour and uncooked cereal-grain products be fortified with folic acid, which could have led to a misclassification of long-term folate intake (95). However, after we excluded 2 US studies that included both prefortification and postfortification periods (25, 50), the association between folate intake and stroke risk remained statistically significant, suggesting that the mandatory fortification of folate may not have affected the results.
In addition, our sensitivity analysis with respect to vitamin B-6 intake suggests that the result may not have been sufficiently stable. When we excluded the study by Zhao et al. (27), the pooled risk estimates remained inverse but were only marginally significant. As noted by the authors of that study, vitamin B-6 intake was lower than the reference nutrient intake in the cohorts for 36.5% and 40.3% of men and women, respectively; thus, the relatively low dose in the reference quintile may have contributed, at least in part, to the strong inverse association reported in their study. Thus, more studies are warranted in order to further elucidate the inverse relation between vitamin B-6 intake and stroke risk. Lastly, the number of studies in several of the subgroups is relatively small, which limits our analysis (when the studies were stratified by sex, geographic location, or stroke subtype). Thus, future studies should attempt to clarify the association between dietary B-vitamins intake and the risk of specific stroke subtypes in various regions and in each sex.
Conclusions
In conclusion, the results of our meta-analysis indicate that both vitamin B-6 and folate intake are inversely correlated with the risk of stroke; in contrast, vitamin B-12 intake does not appear to be associated with the risk of stroke. Our findings support the notion that increasing habitual vitamin B-6 and folate intake have a small, but beneficial effect on the risk of stroke. In the future, more prospective studies are needed to investigate the associations using consistent dietary assessment methods and comparable intake levels to reduce the impact of methodological issues. Further studies are also warranted to report results by different stroke subtypes and regions. Moreover, well-designed RCTs should help identify the putative causal role that B-vitamins play in reducing the risk of stroke.
Supplementary Material
ACKNOWLEDGEMENTS
The authors’ responsibilities were as follows—LC, JM, and FW: conceived and designed the study; LC and QL: performed the database search and checked the results against the inclusion and exclusion criteria; QL, XF, and XW: conducted the quality assessment of the included studies; LC: analyzed the data and wrote the initial draft of the manuscript; FW and JM: reviewed and edited the manuscript; and all authors read and approved the final manuscript.
Notes
This work was supported by research grants from the National Key Research and Development Program (2018YFA0507800 and 2018YFA0507801 to JM; 2018YFA0507802 to FW), the National Natural Science Foundation of China (31930057 and 31530034 to FW; 31570791 and 91542205 to JM; 31900835 to XF), and the China Postdoctoral Science Foundation (2019M650139 to XF).
Author disclosures: The authors report no conflicts of interest.
Supplemental Tables 1–4 and Supplemental Figures 1 and 2 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/advances/.
Abbreviations used: CVD, cardiovascular disease; IHD, ischemic heart disease; PLP, pyridoxal 5’-phosphate; RCT, randomized controlled trial.
Contributor Information
Liyun Chen, The First Affiliated Hospital, School of Public Health, Institute of Translational Medicine, Zhejiang University School of Medicine, Hangzhou, China.
Qianwen Li, Department of Nutrition, Precision Nutrition Innovation Center, School of Public Health, Zhengzhou University, Zhengzhou, China.
Xuexian Fang, The First Affiliated Hospital, School of Public Health, Institute of Translational Medicine, Zhejiang University School of Medicine, Hangzhou, China.
Xinhui Wang, The First Affiliated Hospital, School of Public Health, Institute of Translational Medicine, Zhejiang University School of Medicine, Hangzhou, China.
Junxia Min, The First Affiliated Hospital, School of Public Health, Institute of Translational Medicine, Zhejiang University School of Medicine, Hangzhou, China.
Fudi Wang, The First Affiliated Hospital, School of Public Health, Institute of Translational Medicine, Zhejiang University School of Medicine, Hangzhou, China.
References
- 1. Katan M, Luft A. Global burden of stroke. Semin Neurol. 2018;38(2):208–11. [DOI] [PubMed] [Google Scholar]
- 2. Collaborators GBDCoD. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392(10159):1736–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. DALYs GBD, Collaborators H. Global, regional, and national disability-adjusted life-years (DALYs) for 359 diseases and injuries and healthy life expectancy (HALE) for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392(10159):1859–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mujumdar VS, Aru GM, Tyagi SC. Induction of oxidative stress by homocyst(e)ine impairs endothelial function. J Cell Biochem. 2001;82(3):491–500. [DOI] [PubMed] [Google Scholar]
- 5. Tsai JC, Perrella MA, Yoshizumi M, Hsieh CM, Haber E, Schlegel R, Lee ME. Promotion of vascular smooth muscle cell growth by homocysteine: a link to atherosclerosis. Proc Natl Acad Sci. 1994;91(14):6369–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Welch GN, Loscalzo J. Homocysteine and atherothrombosis. N Engl J Med. 1998;338(15):1042–50. [DOI] [PubMed] [Google Scholar]
- 7. Wald DS, Law M, Morris JK. Homocysteine and cardiovascular disease: evidence on causality from a meta-analysis. BMJ. 2002;325(7374):1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fallon UB, Virtamo J, Young I, McMaster D, Ben-Shlomo Y, Wood N, Whitehead AS, Smith GD. Homocysteine and cerebral infarction in Finnish male smokers. Stroke. 2003;34(6):1359–63. [DOI] [PubMed] [Google Scholar]
- 9. Iso H, Moriyama Y, Sato S, Kitamura A, Tanigawa T, Yamagishi K, Imano H, Ohira T, Okamura T, Naito Y et al. Serum total homocysteine concentrations and risk of stroke and its subtypes in Japanese. Circulation. 2004;109(22):2766–72. [DOI] [PubMed] [Google Scholar]
- 10. Homocysteine Studies C. Homocysteine and risk of ischemic heart disease and stroke: a meta-analysis. JAMA. 2002;288(16):2015–22. [DOI] [PubMed] [Google Scholar]
- 11. Selhub J, Bagley LC, Miller J, Rosenberg IH. B vitamins, homocysteine, and neurocognitive function in the elderly. Am J Clin Nutr. 2000;71(2):614S–20S. [DOI] [PubMed] [Google Scholar]
- 12. Jacob RA, Wu MM, Henning SM, Swendseid ME. Homocysteine increases as folate decreases in plasma of healthy men during short-term dietary folate and methyl group restriction. J Nutr. 1994;124(7):1072–80. [DOI] [PubMed] [Google Scholar]
- 13. Chasan-Taber L, Selhub J, Rosenberg IH, Malinow MR, Terry P, Tishler PV, Willett W, Hennekens CH, Stampfer MJ. A prospective study of folate and vitamin B6 and risk of myocardial infarction in US physicians. J Am Coll Nutr. 1996;15(2):136–43. [DOI] [PubMed] [Google Scholar]
- 14. Brouwer IA, van Dusseldorp M, West CE, Meyboom S, Thomas CM, Duran M, van het Hof KH, Eskes TK, Hautvast JG, Steegers-Theunissen RP. Dietary folate from vegetables and citrus fruit decreases plasma homocysteine concentrations in humans in a dietary controlled trial. J Nutr. 1999;129(6):1135–9. [DOI] [PubMed] [Google Scholar]
- 15. Homocysteine Lowering Trialists C. Dose-dependent effects of folic acid on blood concentrations of homocysteine: a meta-analysis of the randomized trials. Am J Clin Nutr. 2005;82(4):806–12. [DOI] [PubMed] [Google Scholar]
- 16. Lonn E, Yusuf S, Arnold MJ, Sheridan P, Pogue J, Micks M, McQueen MJ, Probstfield J, Fodor G, Held C et al. Homocysteine lowering with folic acid and B vitamins in vascular disease. N Engl J Med. 2006;354(15):1567–77. [DOI] [PubMed] [Google Scholar]
- 17. Chait A, Malinow MR, Nevin DN, Morris CD, Eastgard RL, Kris-Etherton P, Pi-Sunyer FX, Oparil S, Resnick LM, Stern JS et al. Increased dietary micronutrients decrease serum homocysteine concentrations in patients at high risk of cardiovascular disease. Am J Clin Nutr. 1999;70(5):881–7. [DOI] [PubMed] [Google Scholar]
- 18. Wang X, Qin X, Demirtas H, Li J, Mao G, Huo Y, Sun N, Liu L, Xu X. Efficacy of folic acid supplementation in stroke prevention: a meta-analysis. Lancet. 2007;369(9576):1876–82. [DOI] [PubMed] [Google Scholar]
- 19. Lee M, Hong KS, Chang SC, Saver JL. Efficacy of homocysteine-lowering therapy with folic acid in stroke prevention: a meta-analysis. Stroke. 2010;41(6):1205–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Li Y, Huang T, Zheng Y, Muka T, Troup J, Hu FB. Folic acid supplementation and the risk of cardiovascular diseases: a meta-analysis of randomized controlled trials. J Am Heart Assoc. 2016;5(8):e003768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tian T, Yang KQ, Cui JG, Zhou LL, Zhou XL. Folic acid supplementation for stroke prevention in patients with cardiovascular disease. Am J Med Sci. 2017;354(4):379–87. [DOI] [PubMed] [Google Scholar]
- 22. Zhao M, Wu G, Li Y, Wang X, Hou FF, Xu X, Qin X, Cai Y. Meta-analysis of folic acid efficacy trials in stroke prevention: insight into effect modifiers. Neurology. 2017;88(19):1830–8. [DOI] [PubMed] [Google Scholar]
- 23. Jenkins DJA, Spence JD, Giovannucci EL, Kim YI, Josse R, Vieth R, Blanco Mejia S, Viguiliouk E, Nishi S, Sahye-Pudaruth S et al. Supplemental vitamins and minerals for CVD prevention and treatment. J Am Coll Cardiol. 2018;71(22):2570–84. [DOI] [PubMed] [Google Scholar]
- 24. Bazzano LA, He J, Ogden LG, Loria C, Vupputuri S, Myers L, Whelton PK. Dietary intake of folate and risk of stroke in US men and women: NHANES I Epidemiologic Follow-up Study. National Health and Nutrition Examination Survey. Stroke. 2002;33(5):1183–8. [DOI] [PubMed] [Google Scholar]
- 25. He K, Merchant A, Rimm EB, Rosner BA, Stampfer MJ, Willett WC, Ascherio A. Folate, vitamin B6, and B12 intakes in relation to risk of stroke among men. Stroke. 2004;35(1):169–74. [DOI] [PubMed] [Google Scholar]
- 26. Dalmeijer GW, Olthof MR, Verhoef P, Bots ML, van der Schouw YT. Prospective study on dietary intakes of folate, betaine, and choline and cardiovascular disease risk in women. Eur J Clin Nutr. 2008;62(3):386–94. [DOI] [PubMed] [Google Scholar]
- 27. Zhao LG, Shu XO, Li HL, Gao J, Han LH, Wang J, Fang J, Gao YT, Zheng W, Xiang YB. Prospective cohort studies of dietary vitamin B6 intake and risk of cause-specific mortality. Clin Nutr. 2019;38(3):1180–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker SB. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283(15):2008–12. [DOI] [PubMed] [Google Scholar]
- 29. Wang X, Ouyang Y, Liu J, Zhu M, Zhao G, Bao W, Hu FB. Fruit and vegetable consumption and mortality from all causes, cardiovascular disease, and cancer: systematic review and dose-response meta-analysis of prospective cohort studies. BMJ. 2014;349:g4490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25(9):603–5. [DOI] [PubMed] [Google Scholar]
- 31. Chung M, Zhao N, Wang D, Shams-White M, Karlsen M, Cassidy A, Ferruzzi M, Jacques PF, Johnson EJ, Wallace TC. Dose-response relation between tea consumption and risk of cardiovascular disease and all-cause mortality: a systematic review and meta-analysis of population-based studies. Adv Nutr. 2020;11(4):790–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hamling J, Lee P, Weitkunat R, Ambuhl M. Facilitating meta-analyses by deriving relative effect and precision estimates for alternative comparisons from a set of estimates presented by exposure level or disease category. Stat Med. 2008;27(7):954–70. [DOI] [PubMed] [Google Scholar]
- 33. DerSimonian R, Laird N. Meta-analysis in clinical trials revisited. Contemporary Clinical Trials. 2015;45(Pt A):139–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Greenland S, Longnecker MP. Methods for trend estimation from summarized dose-response data, with applications to meta-analysis. Am J Epidemiol. 1992;135(11):1301–9. [DOI] [PubMed] [Google Scholar]
- 35. Orsini N, Bellocco R, Greenland S. Generalized least squares for trend estimation of summarized dose-response data. Stata J. 2006;6(1):40–57. [Google Scholar]
- 36. Larsson SC, Giovannucci E, Wolk A. Folate and risk of breast cancer: a meta-analysis. J Natl Cancer Inst. 2007;99(1):64–76. [DOI] [PubMed] [Google Scholar]
- 37. Wang ZM, Zhou B, Wang YS, Gong QY, Wang QM, Yan JJ, Gao W, Wang LS. Black and green tea consumption and the risk of coronary artery disease: a meta-analysis. Am J Clin Nutr. 2011;93(3):506–15. [DOI] [PubMed] [Google Scholar]
- 38. Orsini N, Li R, Wolk A, Khudyakov P, Spiegelman D. Meta-analysis for linear and nonlinear dose-response relations: examples, an evaluation of approximations, and software. Am J Epidemiol. 2012;175(1):66–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Jackson D, White IR, Riley RD. Quantifying the impact of between-study heterogeneity in multivariate meta-analyses. Stat Med. 2012;31(29):3805–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Thompson SG, Higgins JPT. How should meta-regression analyses be undertaken and interpreted?. Stat Med. 2002;21(11):1559–73. [DOI] [PubMed] [Google Scholar]
- 42. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50(4):1088–101. [PubMed] [Google Scholar]
- 44. Duval S, Tweedie R. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56(2):455–63. [DOI] [PubMed] [Google Scholar]
- 45. Marniemi J, Alanen E, Impivaara O, Seppanen R, Hakala P, Rajala T, Ronnemaa T. Dietary and serum vitamins and minerals as predictors of myocardial infarction and stroke in elderly subjects. Nutr Metab Cardiovasc Dis. 2005;15(3):188–97. [DOI] [PubMed] [Google Scholar]
- 46. Van Guelpen B, Hultdin J, Johansson I, Stegmayr B, Hallmans G, Nilsson TK, Weinehall L, Witthoft C, Palmqvist R, Winkvist A. Folate, vitamin B12, and risk of ischemic and hemorrhagic stroke: a prospective, nested case-referent study of plasma concentrations and dietary intake. Stroke. 2005;36(7):1426–31. [DOI] [PubMed] [Google Scholar]
- 47. Larsson SC, Mannisto S, Virtanen MJ, Kontto J, Albanes D, Virtamo J. Folate, vitamin B6, vitamin B12, and methionine intakes and risk of stroke subtypes in male smokers. Am J Epidemiol. 2008;167(8):954–61. [DOI] [PubMed] [Google Scholar]
- 48. Weng LC, Yeh WT, Bai CH, Chen HJ, Chuang SY, Chang HY, Lin BF, Chen KJ, Pan WH. Is ischemic stroke risk related to folate status or other nutrients correlated with folate intake?. Stroke. 2008;39(12):3152–8. [DOI] [PubMed] [Google Scholar]
- 49. Cui R, Iso H, Date C, Kikuchi S, Tamakoshi A. Dietary folate and vitamin B6 and B12 intake in relation to mortality from cardiovascular diseases: Japan collaborative cohort study. Stroke. 2010;41(6):1285–9. [DOI] [PubMed] [Google Scholar]
- 50. Luu HN, Kingah PL, North K, Boerwinkle E, Volcik KA. Interaction of folate intake and the paraoxonase Q192R polymorphism with risk of incident coronary heart disease and ischemic stroke: the Atherosclerosis Risk in Communities study. Ann Epidemiol. 2011;21(11):815–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Al-Delaimy WK, Rexrode KM, Hu FB, Albert CM, Stampfer MJ, Willett WC, Manson JE. Folate intake and risk of stroke among women. Stroke. 2004;35(6):1259–63. [DOI] [PubMed] [Google Scholar]
- 52. The alpha-tocopherol, beta-carotene lung cancer prevention study: design, methods, participant characteristics, and compliance. The ATBC Cancer Prevention Study Group. Ann Epidemiol. 1994;4(1):1–10. [DOI] [PubMed] [Google Scholar]
- 53. Rosenberg IH. B vitamins, homocysteine, and neurocognitive function. Nutr Rev. 2001;59(8 Pt 2):S69–73.; discussion S73–4. [DOI] [PubMed] [Google Scholar]
- 54. Selhub J. Homocysteine metabolism. Annu Rev Nutr. 1999;19:217–46. [DOI] [PubMed] [Google Scholar]
- 55. Lowering blood homocysteine with folic acid based supplements: meta-analysis of randomised trials. Homocysteine Lowering Trialists' Collaboration. BMJ. 1998;316(7135):894–8. [PMC free article] [PubMed] [Google Scholar]
- 56. Bonaa KH, Njolstad I, Ueland PM, Schirmer H, Tverdal A, Steigen T, Wang H, Nordrehaug JE, Arnesen E, Rasmussen K et al. Homocysteine lowering and cardiovascular events after acute myocardial infarction. N Engl J Med. 2006;354(15):1578–88. [DOI] [PubMed] [Google Scholar]
- 57. Huang T, Chen Y, Yang B, Yang J, Wahlqvist ML, Li D. Meta-analysis of B vitamin supplementation on plasma homocysteine, cardiovascular and all-cause mortality. Clin Nutr. 2012;31(4):448–54. [DOI] [PubMed] [Google Scholar]
- 58. McCully KS. Vascular pathology of homocysteinemia: implications for the pathogenesis of arteriosclerosis. Am J Pathol. 1969;56(1):111–28. [PMC free article] [PubMed] [Google Scholar]
- 59. Mangoni AA, Jackson SH. Homocysteine and cardiovascular disease: current evidence and future prospects. Am J Med. 2002;112(7):556–65. [DOI] [PubMed] [Google Scholar]
- 60. Rolland PH, Friggi A, Barlatier A, Piquet P, Latrille V, Faye MM, Guillou J, Charpiot P, Bodard H, Ghiringhelli O et al. Hyperhomocysteinemia-induced vascular damage in the minipig. Captopril-hydrochlorothiazide combination prevents elastic alterations. Circulation. 1995;91(4):1161–74. [DOI] [PubMed] [Google Scholar]
- 61. Scott JM, Weir DG. Folic acid, homocysteine and one-carbon metabolism: a review of the essential biochemistry. J Cardiovasc Risk. 1998;5(4):223–7. [PubMed] [Google Scholar]
- 62. Carnicer R, Navarro MA, Arbones-Mainar JM, Acin S, Guzman MA, Surra JC, Arnal C, de Las Heras M, Blanco-Vaca F, Osada J. Folic acid supplementation delays atherosclerotic lesion development in apoE-deficient mice. Life Sci. 2007;80(7):638–43. [DOI] [PubMed] [Google Scholar]
- 63. Villa P, Perri C, Suriano R, Cucinelli F, Panunzi S, Ranieri M, Mele C, Lanzone A. L-folic acid supplementation in healthy postmenopausal women: effect on homocysteine and glycolipid metabolism. J Clin Endocrinol Metab. 2005;90(8):4622–9. [DOI] [PubMed] [Google Scholar]
- 64. Stroes ES, van Faassen EE, Yo M, Martasek P, Boer P, Govers R, Rabelink TJ. Folic acid reverts dysfunction of endothelial nitric oxide synthase. Circ Res. 2000;86(11):1129–34. [DOI] [PubMed] [Google Scholar]
- 65. Stanhewicz AE, Kenney WL. Role of folic acid in nitric oxide bioavailability and vascular endothelial function. Nutr Rev. 2017;75(1):61–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Clarke R, Halsey J, Lewington S, Lonn E, Armitage J, Manson JE, Bonaa KH, Spence JD, Nygard O, Jamison R et al. Effects of lowering homocysteine levels with B vitamins on cardiovascular disease, cancer, and cause-specific mortality: meta-analysis of 8 randomized trials involving 37 485 individuals. Arch Intern Med. 2010;170(18):1622–31. [DOI] [PubMed] [Google Scholar]
- 67. Hodis HN, Mack WJ, Dustin L, Mahrer PR, Azen SP, Detrano R, Selhub J, Alaupovic P, Liu CR, Liu CH et al. High-dose B vitamin supplementation and progression of subclinical atherosclerosis: a randomized controlled trial. Stroke. 2009;40(3):730–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Figueiredo JC, Grau MV, Haile RW, Sandler RS, Summers RW, Bresalier RS, Burke CA, McKeown-Eyssen GE, Baron JA. Folic acid and risk of prostate cancer: results from a randomized clinical trial. J Natl Cancer Inst. 2009;101(6):432–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Ebbing M, Bonaa KH, Nygard O, Arnesen E, Ueland PM, Nordrehaug JE, Rasmussen K, Njolstad I, Refsum H, Nilsen DW et al. Cancer incidence and mortality after treatment with folic acid and vitamin B12. JAMA. 2009;302(19):2119–26. [DOI] [PubMed] [Google Scholar]
- 70. Jayedi A, Zargar MS. Intake of vitamin B6, folate, and vitamin B12 and risk of coronary heart disease: a systematic review and dose-response meta-analysis of prospective cohort studies. Crit Rev Food Sci Nutr. 2019;59(16):2697–707. [DOI] [PubMed] [Google Scholar]
- 71. Kannan K, Jain SK. Effect of vitamin B6 on oxygen radicals, mitochondrial membrane potential, and lipid peroxidation in H2O2-treated U937 monocytes. Free Radic Biol Med. 2004;36(4):423–8. [DOI] [PubMed] [Google Scholar]
- 72. Mahfouz MM, Zhou SQ, Kummerow FA. Vitamin B6 compounds are capable of reducing the superoxide radical and lipid peroxide levels induced by H2O2 in vascular endothelial cells in culture. Int J Vitam Nutr Res. 2009;79(4):218–29. [DOI] [PubMed] [Google Scholar]
- 73. Ji Y, Diao J, Han Y, Huang Y, Bai H, Chen Q, Fan L, Ferro A. Pyridoxine prevents dysfunction of endothelial cell nitric oxide production in response to low-density lipoprotein. Atherosclerosis. 2006;188(1):84–94. [DOI] [PubMed] [Google Scholar]
- 74. Lotto V, Choi SW, Friso S. Vitamin B6: a challenging link between nutrition and inflammation in CVD. Br J Nutr. 2011;106(2):183–95. [DOI] [PubMed] [Google Scholar]
- 75. Chamorro A. Role of inflammation in stroke and atherothrombosis. Cerebrovasc Dis. 2004;17:(Suppl 3):1–5. [DOI] [PubMed] [Google Scholar]
- 76. Friso S, Jacques PF, Wilson PW, Rosenberg IH, Selhub J. Low circulating vitamin B(6) is associated with elevation of the inflammation marker C-reactive protein independently of plasma homocysteine levels. Circulation. 2001;103(23):2788–91. [DOI] [PubMed] [Google Scholar]
- 77. Friso S, Girelli D, Martinelli N, Olivieri O, Lotto V, Bozzini C, Pizzolo F, Faccini G, Beltrame F, Corrocher R. Low plasma vitamin B-6 concentrations and modulation of coronary artery disease risk. Am J Clin Nutr. 2004;79(6):992–8. [DOI] [PubMed] [Google Scholar]
- 78. Spinneker A, Sola R, Lemmen V, Castillo MJ, Pietrzik K, Gonzalez-Gross M. Vitamin B6 status, deficiency and its consequences – an overview. Nutr Hosp. 2007;22(1):7–24. [PubMed] [Google Scholar]
- 79. Ueland PM, Ulvik A, Rios-Avila L, Midttun O, Gregory JF. Direct and functional biomarkers of vitamin B6 status. Annu Rev Nutr. 2015;35:33–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Haller J, Lowik MR, Ferry M, Ferro-Luzzi A. Nutritional status: blood vitamins A, E, B6, B12, folic acid and carotene. Euronut SENECA investigators. Eur J Clin Nutr. 1991;45:(Suppl 3):63–82. [PubMed] [Google Scholar]
- 81. Herrmann W, Knapp JP. Hyperhomocysteinemia: a new risk factor for degenerative diseases. Clin Lab. 2002;48(9–10):471–81. [PubMed] [Google Scholar]
- 82. Bates CJ, Pentieva KD, Prentice A, Mansoor MA, Finch S. Plasma pyridoxal phosphate and pyridoxic acid and their relationship to plasma homocysteine in a representative sample of British men and women aged 65 years and over. Br J Nutr. 1999;81(3):191–201. [PubMed] [Google Scholar]
- 83. Ribaya-Mercado JD, Russell RM, Sahyoun N, Morrow FD, Gershoff SN. Vitamin B-6 requirements of elderly men and women. J Nutr. 1991;121(7):1062–74. [DOI] [PubMed] [Google Scholar]
- 84. National Research C. Recommended dietary allowances: National Academy of Sciences, 1974. [Google Scholar]
- 85. Collaborators GBDLRoS, Feigin VL, Nguyen G, Cercy K, Johnson CO, Alam T, Parmar PG, Abajobir AA, Abate KH, Abd-Allah F et al. Global, regional, and country-specific lifetime risk of stroke, 1990–2016. N Engl J Med. 2018;379(25):2429–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Strong K, Mathers C, Bonita R. Preventing stroke: saving lives around the world. Lancet Neurol. 2007;6(2):182–7. [DOI] [PubMed] [Google Scholar]
- 87. Refsum H, Yajnik CS, Gadkari M, Schneede J, Vollset SE, Orning L, Guttormsen AB, Joglekar A, Sayyad MG, Ulvik A et al. Hyperhomocysteinemia and elevated methylmalonic acid indicate a high prevalence of cobalamin deficiency in Asian Indians. Am J Clin Nutr. 2001;74(2):233–41. [DOI] [PubMed] [Google Scholar]
- 88. Antony AC. Vegetarianism and vitamin B-12 (cobalamin) deficiency. Am J Clin Nutr. 2003;78(1):3–6. [DOI] [PubMed] [Google Scholar]
- 89. Marti-Carvajal AJ, Sola I, Lathyris D, Dayer M. Homocysteine-lowering interventions for preventing cardiovascular events. Cochrane Database Syst Rev. 2017;8:CD006612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Hassan A, Hunt BJ, O'Sullivan M, Bell R, D'Souza R, Jeffery S, Bamford JM, Markus HS, Homocysteine is a risk factor for cerebral small vessel disease, acting via endothelial dysfunction, Brain. 2004;127(1):212–9. [DOI] [PubMed] [Google Scholar]
- 91. Sanchez-Villegas A, Doreste J, Schlatter J, Pla J, Bes-Rastrollo M, Martinez-Gonzalez MA. Association between folate, vitamin B(6) and vitamin B(12) intake and depression in the SUN cohort study. J Hum Nutr Diet. 2009;22(2):122–33. [DOI] [PubMed] [Google Scholar]
- 92. Sellers TA, Kushi LH, Cerhan JR, Vierkant RA, Gapstur SM, Vachon CM, Olson JE, Therneau TM, Folsom AR. Dietary folate intake, alcohol, and risk of breast cancer in a prospective study of postmenopausal women. Epidemiology. 2001;12(4):420–8. [DOI] [PubMed] [Google Scholar]
- 93. Larsson SC, Giovannucci E, Wolk A. Methionine and vitamin B6 intake and risk of pancreatic cancer: a prospective study of Swedish women and men. Gastroenterology. 2007;132(1):113–8. [DOI] [PubMed] [Google Scholar]
- 94. Rimm EB, Willett WC, Hu FB, Sampson L, Colditz GA, Manson JE, Hennekens C, Stampfer MJ. Folate and vitamin B6 from diet and supplements in relation to risk of coronary heart disease among women. JAMA. 1998;279(5):359–64. [DOI] [PubMed] [Google Scholar]
- 95. Crider KS, Bailey LB, Berry RJ. Folic acid food fortification – its history, effect, concerns, and future directions. Nutrients. 2011;3(3):370–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.