ABSTRACT
Multiple sclerosis (MS) is a chronic inflammatory autoimmune disease of the central nervous system. The role of diet in the progression of MS and severity of symptoms remains unclear. Various systematic literature reviews (SRs) have reported the effects of single nutrients on MS progression or the role of dietary factors on specific symptoms of MS. Narrative reviews have examined the effects of various dietary patterns in MS populations. An umbrella review was undertaken to collate the findings from review articles and evaluate the strength of the scientific evidence of dietary interventions for people living with MS. Scientific databases including MEDLINE, PubMed, CINAHL, and The Cochrane Library were systematically searched up to April 2019. Review articles and meta-analyses were included if they examined the effect of any dietary intervention in adult populations with MS. Outcomes included MS progression indicated by relapses, disability, MRI activity and disease classification, and MS symptoms. Characteristics and findings from both review articles and their included primary studies were extracted and summarized. A total of 19 SRs and 43 narrative reviews were included. Vitamin D and PUFAs were the most commonly studied interventions. Across SR studies, vitamin D supplementation had no significant effect on relapses, MRI, or disability progression; however, an inverse association was found between vitamin D status and disability scores through observational studies. Effects of PUFA supplementation on major outcomes of MS progression were inconsistent across review articles. Other interventions less commonly studied included vitamin, mineral, and herbal supplementation and varying dietary patterns. Strong consistent evidence is lacking for dietary interventions in persons with MS. The body of evidence is primarily focused around the isolation of individual nutrients, many of which demonstrate no effect on major outcomes of MS progression. Stronger food-focused studies are required to strengthen the evidence.
Keywords: MS, diet, nutrition, disability, relapse, magnetic resonance imaging, fatigue, anxiety, depression, cognitive function
Introduction
Multiple sclerosis (MS) is a chronic inflammatory autoimmune disease affecting the central nervous system (1). MS currently affects >2.3 million people worldwide and is a major cause of disability in young adults (2). In adults, MS presents in various forms including relapsing remitting MS (RRMS), primary progressive (PPMS), relapsing progressive MS, and secondary progressive MS (SPMS) (1). RRMS is the most common subtype and is characterized by episodes of neurologic disfunction and worsening of symptoms separated by periods of relative clinical stability, referred to as relapses and remissions. Progressive MS is the result of chronic neurodegeneration associated with inflammation and other mechanistic processes (3). The etiology of MS is not completely understood; however, environmental factors including viral infections, nutrition, physical inactivity, childhood obesity, smoking, and low vitamin D concentrations are thought to play a role (4).
Many studies of varying quality have explored the effects of nutrients and dietary patterns in populations with MS; however, the role of diet in disease progression is not well understood (4). MS progression is assessed using varying measures of disease activity including disability progression, MRI outcomes, and MS relapses, as well as associated symptoms. To date, no review exists to collate all of the existing evidence nor has the quality of this evidence been addressed overall. An umbrella review as a methodological approach aims to summarize the evidence from review articles of the same population group investigating different interventions, different approaches to the application of the same intervention, or the same intervention where different outcomes are assessed. Therefore, an umbrella review will allow for the quality of multiple dietary interventions and their effects on different outcomes of MS progression to be evaluated and presented in 1 accessible review.
The aim of this umbrella review was to evaluate the scientific evidence for dietary interventions in people living with MS.
Methods
Protocol registration
This review was conducted in adherence with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) checklist (5). The methods were adapted from the Joanna Briggs Institute approach to umbrella reviews (6) and the Cochrane protocol for overviews of reviews (7). The protocol was registered 26 March 2019 (https://www.crd.york.ac.uk/PROSPERO; registration number: CRD42019123813).
Search strategy
A systematic search was conducted using the MEDLINE, PubMed, The Cochrane Library, and Cumulative Index to Nursing and Allied Health Literature (CINAHL) scientific databases, searched up to April 2019. A combination of search terms identified using Medical Subject Headings (MeSH) were developed through several pilot searches and adapted to the relevant databases (Supplemental Table 1). The review answered the primary question: “How does diet affect disease activity (relapses, changes on MRI) or progression of disability in people living with MS?” The following secondary question was also addressed: “How does diet affect MS symptoms?”
Eligibility criteria
Published reviews and meta-analyses were included if they met the following criteria:
Adults living with clinically definitive MS of any subtype (i.e., RRMS, PPMS, SPMS, or PRMS)
Study of dietary patterns, dietary supplements, nutrients, whole foods, drinks, or food groups
Primary outcomes reported as: the number of relapses during the study period or change in annualized relapse rates (ARRs); changes in disability status indicated by the Expanded Disability Status Scale (EDSS) (8) or Kurtzke Disability Status Scale (9); change in MRI parameters of disease activity including new lesions on T2-weighted images, pre-existing lesions enlarged on T2-weighted images, gadolinium-enhancing lesions in T1-weighted images, combined unique activity, or changes in brain volume; change in disease classification (i.e., progression from RRMS to progressive MS)
Secondary outcomes reported as: physical symptoms related to fatigue as measured by validated scales [e.g., Fatigue Severity Scale (FSS) (10), MS-specific FSS (11), Fatigue Impact Scale (FIS) (12), or Modified FIS (13)]; morbidity; the inflammatory marker C-reactive protein (CRP); measures of cognitive function (e.g., Stroop test).
No language restrictions or time frames of publications were implemented as recommended by the Cochrane Handbook for Systematic Reviews of Interventions (14). All foods were cross checked against compositional data (15).
Exclusion criteria
Studies were excluded if they met the following criteria:
Were related to MS onset or risk factors for developing MS
Involved pediatric onset MS or Clinically Isolated Syndrome populations
Involved animal or in vitro experimental studies
Study selection
All abstracts and citations of retrieved studies were exported to the reference management software EndNote X9 (Thompson Reuters). Study selection was conducted and recorded according to the PRISMA protocol (5) (Figure 1). All titles and abstracts of retrieved studies were reviewed independently by 1 review author (ART). A 10% random sample of full text studies were screened independently by a second review author (YCP) for quality assurance. All extracted data was reviewed by both authors. Any disagreements were resolved to consensus between the reviewers.
FIGURE 1.

PRISMA flow diagram for the umbrella review addressing MS and diet. MS, multiple sclerosis; NR, narrative review; PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses; SR, systematic literature review.
Data management and extraction
Data were extracted using predefined data extraction tables listing the primary studies referenced in each review as per the review protocol by Petrovskaya et al. (16). This approach allows for the elimination of duplicate primary studies to manage overlap of outcomes reported across reviews (17). Extracted data included characteristics of the included reviews, comprising objectives, search strategies, number of studies and participants, population, intervention, and comparator details. Characteristics and findings from primary studies were also extracted including study sample size, intervention details, outcome measures, and effects of interventions as reported by each review author. Where outcome data were missing, inadequately reported, or reported differently across reviews, these data were extracted directly from the primary studies cited within the review. Authors of identified reviews were also consulted where further details were required.
Assessment of risk of bias and certainty of evidence
The AMSTAR 2 tool (18) was used to assess the methodological quality of included systematic reviews (SRs). The risk-of-bias assessments of primary studies were extracted from included SRs. Where the risk of bias of included studies was not reported, this was determined directly from the primary studies using the Cochrane risk-of-bias tool (19) or epidemiological principles (20).
The Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach (21), was used to assess the certainty of the body of evidence. The certainty of evidence was classified as high, moderate, low, or very low. Where other methods of quality assessment were implemented, these were extracted and interpreted from the included review articles.
Data synthesis and analysis
Where multiple reviews reported overlapping studies, findings from the most recent, higher quality review with the largest data set were included. If these reviews, however, reported different primary or secondary outcomes, then both reviews were included. Findings from included narrative reviews were presented through a narrative synthesis along with visual depictions of the data. The certainty of the evidence determined from the underlying primary studies within the included reviews was structured around each dietary intervention area and the effects on multiple outcomes of MS disease progression.
Results
A total of 3664 articles were retrieved. After applying the inclusion/exclusion criteria, 231 published reviews (n = 31 SRs, n = 200 narrative reviews) were eligible for inclusion (Figure 1). Following the data extraction process, review articles citing overlapping studies were excluded from the final analysis. This resulted in 19 SRs and 43 narrative reviews included for data analysis and synthesis.
Included SRs
Characteristics of the included SRs are summarized in Supplemental Table 2. The study designs consisted of randomized controlled trials (RCTs) (n = 46), uncontrolled or nonrandomized trials (n = 9), and prospective (n = 7) and retrospective (n = 3) cohort, case-control (n = 1), cross-sectional (n = 7), and survey (n = 2) studies. Overall, the SRs included 75 primary studies with a total of 10,497 participants ranging from 5 to 2469 participants. Included primary studies reported the effects of supplementation with vitamin D (n = 17, 1106 participants), PUFAs (n = 14, 3454 participants), α-lipoic acid (n= 1, 46 participants), multivitamins (n = 1, 45 participants), vitamin A (n = 2, 136 participants), B vitamins (n = 4, 300 participants), zinc sulfate (n = 1, 43 participants), carnitine (n = 1, 36 participants), antioxidants (n = 1, 9 participants), coenzyme Q10 (n = 1, 45 participants), ginkgo biloba (n = 4, 216 participants), ginseng (n = 2, 73 participants), and other herbal supplements (n = 4, 173 participants). Dietary patterns studied included a gluten-free diet (n = 1, 72 participants), a low-fat plant-based diet (n = 1, 61 participants), a modified Paleo diet (n = 1, 34 participants), high dietary sodium intake (n = 1, 70 participants), and higher-quality dietary patterns (based on fruit, vegetable, and PUFA intake) (n = 1, 2087 participants). Twenty included studies reported the effects of nutritional status on MS progression including serum vitamin D (n = 16, 2454 participants) and serum vitamin B-12 (n = 1, 37 participants).
Included narrative reviews
A total of 43 narrative reviews with a total of 33,244 participants across studies (excluding epidemiologic studies as sample sizes were not reported) ranging from 5 to 9881 participants were included in the qualitative synthesis. Overall, these narrative reviews included an additional 58 primary studies that were not already included from the SRs. Study designs consisted of RCTs (n = 14), nonrandomized and open-label trials (n = 11), prospective (n = 13) and retrospective (n = 2) cohort studies, cross-sectional studies (n = 4), population-based surveys (n = 9), case-control studies (n = 2), and ecological studies (n = 3; including populations from 1 to 36 countries). Included studies reported the effects of supplementation with vitamin D (n = 7, 5133 participants), vitamin A (n = 1, 101 participants), B vitamins (n = 4, 201 participants), antioxidants (n = 1, 37 participants), α-lipoic acid (n = 1, 37 participants), probiotics (n = 3, 70 participants), ginkgo biloba (n = 2, 147 participants), coenzyme Q10 (n = 1, 48 participants), carnitine (n = 1, 170 participants), and tryptophan (n = 1, 32 participants), as well as dietary intake and supplementation of PUFAs (n = 5, 5058 participants) on MS progression and symptoms. Dietary patterns studied included population dietary fat consumption (n = 3); saturated fat intake (n = 1, 144 participants); alcohol, coffee, and fish consumption (n = 6, 8401 participants); dietary sodium (n = 1, 400 participants); dietary intake of fruits, vegetables, dairy, fats, and whole grains (n = 3, 9545 participants); dietary PUFAs and vitamins (n = 1, 83 participants); dietary folate intake (n = 1, 101 participants); fasting (n = 3, 170 participants); a gluten-free diet (n = 1, 42 participants); and a modified Mediterranean diet (n = 1, 43 participants). Twenty-five studies reported the effects of nutritional status on MS progression including serum status of vitamin D (n = 7, 3021 participants), vitamin A (n = 3, 209 participants), folates (n = 1, 101 participants), and tocopherols (n = 1, 88 participants).
The distribution of 133 studies addressing MS progression and symptoms is shown in Figure 2; 75 of the primary studies were included within the SRs and an additional 58 within the narrative reviews. The proportion of included studies investigating dietary supplementation, dietary intake, and nutrient status and the related study designs of the 145 s33dies included across all of the review articles can be seen in Figures 3 and 4. Due to the number of studies, a summary of the effects of the dietary supplementation studies on outcomes of MS progression, across 4 SRs providing a combined analysis of results (22–25), is shown in Supplemental Tables 3 and 4.
FIGURE 2.

Categorization of primary study designs examining MS progression and symptoms in relation to diet. MS, multiple sclerosis; PUFA, polyunsaturated fatty acids.
FIGURE 3.

Proportion of primary studies examining the effect of dietary supplementation, dietary intake, and nutritional status on outcomes of MS progression and symptoms. MS, multiple sclerosis.
FIGURE 4.

Designs of primary studies included within all review articles addressing MS and diet. MS, multiple sclerosis.
Vitamin D
Supplementation
No effect of vitamin D supplementation, in doses of 20–40,000 IU/d, was found on ARR, EDSS scores, or MRI gadolinium-enhancing T1 lesions in combined analysis of results from 12 RCTs (22). Only 2 studies addressed fatigue as a symptom. One RCT reported a significant reduction in FIS scores with vitamin D supplementation for 26 wk, while the other trial found no effect of vitamin D supplementation for 96 wk on fatigue. Vitamin D supplementation, in doses of 1000–5000 IU/d, was found to decrease the number of relapses, relapse rates, and exacerbation rates when compared with a patient's own history in 3 open-label studies (26). One additional RCT found a significant effect of 10,000 IU of vitamin D supplementation for 12 wk on EDSS score (27).
When combining these results with findings from the narrative reviews, a total of 24 primary studies across 9 reviews (22, 26–33) addressed vitamin D supplementation. Doses ranged from 25 to 60,000 IU/d administered in varying frequencies (daily, weekly, or monthly) and forms [cholecalciferol (vitamin D3), ergocalciferol (vitamin D2), 1,25-dihydroxycholecalciferol, or cod liver oil]. Studies included RCTs (n = 13), open-label/nonrandomized trials (n = 8), and prospective cohort (n = 1) and survey (n = 2) studies. Results across all of the included reviews showed no differences in effects with higher doses ( Figure 5). A larger proportion of studies (57–86%) found no significant effects for all outcomes measured, except for fatigue. Fatigue was measured across only 3 studies, with 1 survey study included within 1 narrative review finding a significant effect (33).
FIGURE 5.

Findings of primary studies across all review articles examining the effect of vitamin D supplementation on outcomes of MS progression and symptoms. MRI, magnetic resonance imaging; MS, multiple sclerosis.
Serum concentrations
Three SRs included studies examining the association between serum vitamin D concentrations and outcomes of MS progression, including disability and relapses (26, 28, 34). Seven studies (4 cross-sectional, 3 cohort) found an inverse correlation between serum 25-hydroxycholecalciferol concentrations and disability scores (26, 35–40). The largest cohort found a dose-response with each 10-ng/mL increase in serum 25-hydroxycholecalciferol associated with lower subsequent disability (39). One other cohort study found no association between vitamin D and EDSS scores (41).
Two cohort studies found a significant reduction in relapse risk with higher vitamin D concentrations (38, 42), with one finding a significant 50% decrease in relapse risk among participants with high 25-hydroxycholecalciferol concentrations (>100 nmol/L) compared with those with low concentrations (<50 nmol/L) (42). One cohort study and 1 cross-sectional study found a significant reduction in relapse risk with each 10-nmol/L increase in vitamin D concentrations (38, 43), whereas 4 cohort studies found no association between vitamin D concentrations and risk of relapse, or relapse rates (39–41, 44).
Two cohort studies found that each 25-nmol/L increase in 25-hydroxycholecalciferol concentrations was associated with 12–15% lower risk of new T2 lesions (39, 41) and a 32% lower risk of gadolinium-enhancing lesions (39). However, 2 other studies found no correlation between vitamin D and MRI lesions (40, 45). Low serum vitamin D was not associated with fatigue scores (46).
Figure 6 shows the results of the 23 primary studies included across both narrative reviews and SRs (26, 28, 29, 34, 46–55). Of these studies, 78% found a significant effect on ≥1 outcome of MS progression (54% relapses, 77% disability progression, and 75% MRI lesions) with higher vitamin D concentrations and better disease outcomes. Increments of 10–50 nmol/L of serum 25-hydroxycholecalciferol were found to be associated with reduced relapse risk, lower disability scores, and lowered risk of increased or new T1 and T2 gadolinium-enhancing lesions.
FIGURE 6.

Findings of primary studies across all review articles examining the effect of serum vitamin D status on outcomes of MS progression and symptoms. MIRRI, megnetic resonance imaging; MS, multiple sclerosis.
Fatty acids
In total, 19 primary studies of PUFAs across 5 narrative reviews (56–60) and 7 SRs (23, 24, 28, 61–64) were included in this review (Figure 7). Omega-3 (ω-3) fatty acid (FA) supplementation was reported across 8 studies administered as fish oil, flaxseed oil, or capsules. Daily doses ranged from 0.5 to 6.0 g ω-3 FAs for 3 to 30 mo (23, 24, 28, 56–58, 60, 62). Two studies examined the effect of ω-3 FA supplementation in combination with multivitamin supplementation and a dietary intervention aimed at reducing saturated fat intake (63, 64). Six studies reported across 3 review articles examined the effects of ω-6 FA supplementation administered in the form of sunflower seed oil, capsules, or borage oil. Daily doses ranged from 2.9 to 17. 2 g ω-6 FAs for 18 to 30 mo (24, 28, 59). A combination of ω-3 FA and omega-6 FAs in doses of 18–21 g/d for a duration of 6 to 30 mo was studied in 3 RCTs. This was administered in the form of hempseed and evening primrose oil (9:1 ratio) in combination with a dietary intervention low in saturated fats (61, 62) or an oil formulation (1:1 ratio of ω-3 and ω-6 FAs) combined with a multivitamin supplement (23). All included studies whereby PUFA supplementation used in combination with a dietary intervention to address disability progression found significant improvements. In contrast, only 40% and 17% of studies found significant improvements for disability progression for isolated supplementation of ω-3 FA or ω-6 FAs, respectively.
FIGURE 7.

Findings of primary studies across all review articles examining the effect of fatty acid intake on MS relapses and disability progression. MS, multiple sclerosis; PUFA, polyunsaturated fatty acids.
Results of 1 RCT including 46 participants (65) showed no significant changes in EDSS scores with ɑ-lipoic acid supplementation at a dose of 1200 mg/d for 12 wk (66). An additional RCT of ɑ-lipoic acid supplementation was included in 1 narrative review (59). Similarly, results revealed no significant change in disability scores with 2400 mg/d ɑ-lipoic acid supplementation (67).
Vitamins and minerals
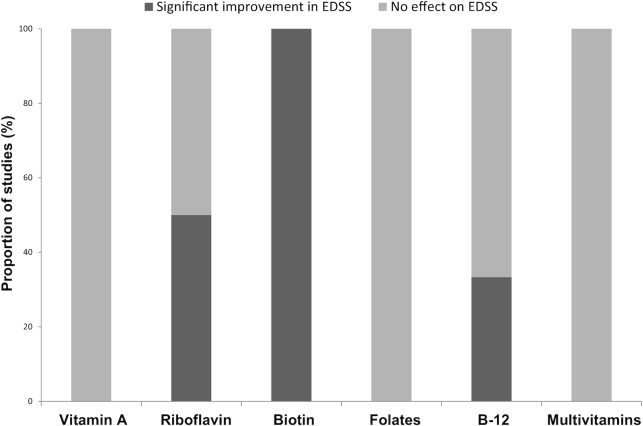
One SR (68) included an RCT studying the effects of supplementation with zinc sulfate and found a significant improvement in symptoms of depression in comparison to placebo (69). A total of 10 studies across 5 narrative reviews (56, 58, 70–72) studied the effects of vitamin A, tocopherols, and B vitamins (thiamin, riboflavin, biotin, folates, and vitamin B-12) and 8 studies from 6 SRs (23, 28, 62, 64, 68, 73) studied the effects of vitamin A, B vitamins (riboflavin, biotin, vitamin B-12), and multivitamin supplementation on MS progression and symptoms. Vitamin A (10,000–25,000 IU/d for 12 mo) and riboflavin supplementation (10 mg/d for 6 mo) studies showed no significant effect on relapse rates or disability scores in comparison to placebo (74–76). One study, however, found a significant reduction in disability scores with riboflavin supplementation at 5 mg/d (77). High-dose biotin supplementation reported across 2 SRs (62, 68) found a significant reduction in disability scores at doses ranging from 100 to 600 mg/d (78, 79), and 1 SR (28) reported a negative correlation between serum concentration of vitamin B-12 and disability scores (80).
Ten of the studies reported the effects of vitamins on disability progression (74, 76–84). Figure 8 shows the proportion of studies that found a significant improvement with either supplementation (n = 9) or increased serum concentrations and dietary intake (n = 1). Two observational studies, reported in a narrative review (70), found higher serum vitamin A (retinol) concentrations to be significantly asssociated with the reduced odds of new MRI lesions and improved symptoms of fatigue and depression (85, 86), while 2 other narrative reviews reported supplementation of vitamin B-12 in doses of 1.0–60 mg/d had no effect on disability progression (71, 72). A final narrative review (71) reported the effects of folates on disability progression and MS-related fatigue and found low dietary folate (mean: 268 ± 66.9 μg/d) to be correlated with increased fatigue (87); however, this was contradicted by an RCT of supplementation with 5 mg folic acid, which had no effect on disability scores (83).
FIGURE 8.
Findings of primary studies examining the effect of vitamin supplementation and serum vitamin levels on disability scores for MS. Disability measured using the EDSS. B-12, vitamin B-12; EDDS, Expanded Disability Status Scale; MS, multiple sclerosis.
Carnitine
One SR reported 1 randomized trial studying the effects of acetyl l-carnitine supplementation on fatigue and found no significant effect compared with standard treatment (25). One narrative review including an open-label study reported a significant reduction in fatigue with supplementation of 1–2 g/d of levocarnitine (88).
Herbal supplements and probiotics
Effects of ginkgo biloba supplementation on MS symptoms including fatigue and cognitive function were found to be inconsistent across 4 studies in 1 review (61). All studies found no significant improvements in MS symptoms. The review also reported supplementation with ginseng extract in doses of 100–500 mg/d on MS symptoms, with only 1 of the 2 included studies showing significant improvements in fatigue at higher doses of 500 mg/d. Another SR reported a significant reduction in fatigue scores through 1 RCT of coenzyme Q10 supplementation in doses of 500 mg/d (89).
Additional RCTs of ginkgo biloba (n = 2) and coenzyme Q10 (n = 1) supplementation were included within 2 narrative reviews (59, 90). Coenzyme Q10 supplementation was found to have no effect on disability scores (59), and ginkgo biloba supplementation was similarly found to have no effect on disability or fatigue (59). However, a significant effect of ginkgo biloba supplementation over placebo was found on cognitive function including the Stroop test attention and executive functions (90) in doses of 120 mg/d over a 12-wk period.
One review reported the effects of daily supplementation of 200 mg Lippia citriodora(Lemon verbena) in combination with a low-fat (≤30% of energy) diet and found a significant reduction in CRP markers (28). Four narrative reviews included studies of probiotic supplementation, with 2 reporting on open-label studies and 1 RCT (91–94). Probiotic supplementation containing 2500 Trichuris suis ova strains, for 12 wk had no significant effect on disability scores or MRI lesions.
Dietary patterns
A total of 3 SRs with 5 studies examined the effects of dietary patterns on MS progression and symptoms (28, 63, 95). Results from a 2018 review suggested that consuming a low-fat plant-based diet, with complex carbohydrates as the main source of dietary energy, excluding animal products and vegetable oils or a modified Paleo diet excluding gluten, eggs, and dairy and increasing fruit and vegetable intake may improve self-reported fatigue levels (63). A diet containing <15% of energy from fat with the addition of fish-oil supplementation (6 g/d), however, was observed to worsen fatigue in another included study (63). One SR reported the effects of a gluten-free diet and found significant improvements in disability scores and MRI lesion activity in comparison to a regular diet (95). A traditional Iranian medicine “hot nature” diet addressing the warming effect of certain foods that focuses on reducing saturated fats, refined starch, and dairy products and increasing fruits, vegetables, and PUFAs, in addition to hempseed and evening primrose oil supplementation, was reported to have no significant effect on disability progression in comparison to a placebo (23).
A total of 25 primary studies across 17 narrative reviews and 3 SRs studied the effects of dietary intake of nutrients and dietary patterns on outcomes of MS progression and symptoms. Primary studies consisted of RCTs (n = 5), open-label/nonrandomized trials (n = 2), and prospective (n = 5) and retrospective cohort (n = 1), cross-sectional (n = 1), case-control (n = 1), ecological (n = 3), and survey/questionnaire (n = 7) studies. Five survey studies included within narrative reviews found moderate alcohol intake (≤30–40 g ethanol/d) was associated with reduced odds of disability progression compared with low or no alcohol intake (33, 56, 91, 96–98). However, 1 study showed this was associated with greater MRI lesion volume (97). Consumption of coffee was studied in 1 case-control and 1 survey study, with outcomes reported across 3 reviews (56, 91, 99). One study found a significant dose-response relation between coffee consumption and disability progression (99). Both studies found higher coffee consumption was associated with reduced odds of reaching a disability score ≥6. A larger study assessing sodium intake was included in 1 narrative review (100). This study found no association between sodium intake and relapses or MRI lesions. One SR reported that an increase in relapse rates and risk of developing new MRI lesions was associated with high amounts of sodium intake, at amounts >4.8 g sodium/d (28). An additional 2 included survey studies using the dietary habit questionnaires found lower levels of patient-determined disability with higher fruit and vegetable consumption and PUFA intake from fish, oils, and ω-3 FA supplementation (28). Three studies across 3 reviews found positive effects of regular fish intake on all reported outcomes (28, 56, 60), with 1 study finding a clear dose-response with patient-reported disability (28).
An additional 2 studies across 2 narrative reviews considered intake of fruits, vegetables, dairy, whole grains, and dietary fats to assess dietary quality and the effect on self-reported fatigue disability and relapses (60, 100). One study found an inverse correlation of intake of whole grains and dairy with disability severity (100). Higher diet-quality scores were associated with decreased odds of a higher disability score, while lower diet-quality scores increased participant odds of fatigue (60, 100). Two studies assessed fasting and found no effect on disability scores or relapses (101, 102). One RCT reported in a narrative review studied the effects of a ketogenic or fasting diet and found no effect on disability progression over 6 mo (56). Another RCT studied the effects of a modified Mediterranean diet, with a focus on vegetarian eating and a lower gluten content in addition to vitamin D, multivitamin, PUFA, and resveratrol supplementation. No significant effect was found on disability scores or fatigue over the duration of 7 mo (56). An open-label study of a gluten-free dietary pattern was reported in a narrative review and found that participants still experienced progression in disability after 2 y of adherence to the diet. A positive correlation was found between saturated fat intake and mortality rates in populations with MS in 1 cohort study and 1 ecological study reported across 2 narrative reviews (50, 103).
Methodological quality of included SRs
Overall, there was a considerable amount of bias present across the studies included within the SRs. The Cochrane risk-of-bias tool was commonly used. Upon applying the AMSTAR 2 tool (18) to assess the methodological quality of the 19 included SR articles (Supplemental Table 5), the overall confidence was appraised as high for 8 SRs (22, 26, 27, 34, 63, 89, 95, 104), moderate for 2 reviews (23, 46), low for 5 reviews (24, 25, 61, 62, 64), and critically low in 4 review articles (28, 66, 68, 73). The 4 reviews considered as critically low did not conduct any assessment of methodological quality and risk of bias within the included studies.
Quality of the evidence
SRs implementing the GRADE approach to assess the certainty of evidence classified findings from RCTs of vitamin D supplementation as “very low” regarding effects on disability and MRI lesions (22). The certainty of evidence of ω-3 FA and ω-6 FA supplementation, riboflavin supplementation, and the “hot nature diet” having no effect on disability progression was classified as “moderate” (23). The evidence for the effects of PUFA supplementation, vitamin D supplementation, and biotin was classified as moderately low risk and the evidence for supplementation of ginkgo biloba was classified as low risk; however, it was noted that there was limited or no replication of the findings (62).
Discussion
This umbrella review found differences between the outcomes reported by published SRs compared with narrative reviews in relation to dietary outcomes related to MS progression and symptoms. The greatest proportion of studies (60%) examined the effects of supplementing individual or combinations of nutrients or herbal supplements, while 19% studied the effects of dietary intake of foods and 21% the effect of nutritional status. The most commonly studied nutrients are vitamin D and PUFAs, which are captured across a range of SRs and narrative reviews.
Vitamin D was the most consistently studied, demonstrating that supplementation irrespective of dose had no effect on MS progress. The certainty of the evidence was very low due to the high risk of bias and wide variation in doses and study durations (22, 27). When combined with narrative review findings, vitamin D supplementation was found to still have no effect on these outcomes in a majority of studies. Higher-quality study designs, implementing consistent dosing regimens and outcome measures, are essential to examine whether vitamin D supplementation can improve outcomes of MS progression.
Observational studies found more consistent associations between serum vitamin D status and outcomes of disability progression. SRs have shown consistent effects of higher serum vitamin D with disability scores, while narrative reviews found significant reductions with higher serum vitamin D concentrations. Findings within the SRs, however, require consideration of reverse causality, while retrospective cohort designs presented limited confounding factors affecting the certainty of the relation. These findings highlight the significance of nutritional adequacy of vitamin D as opposed to a need for high-dose supplementation. Similar patterns were noted for vitamins A and B-12; higher serum concentrations were associated with improved disease progression. However, these studies found no effect on specific disease measures, again indicating the benefit of nutritional adequacy as opposed to high-dose supplementation. There are also risks of hepatotoxicity with high-dose vitamin A (75).
The PUFAs were the second most commonly studied nutrients in populations with MS presented across SRs and narrative reviews for improving progression and symptoms of MS; the evidence was also inconsistent. Many studies found no significant effect on MS relapses or disability progression with supplementation alone. The few studies assessing PUFA supplementation in combination with diet found significant beneficial effects on disability progression only. However, the positive effects may be due to the dietary approach or the addition of other vitamins providing greater nutritional adequacy. These findings highlight the potential of increasing PUFAs and decreasing saturated fats in combination with increased dietary vitamins to improve outcomes of MS progression.
Findings for ginkgo biloba and biotin supplementation should be interpreted cautiously as the overall confidence of the evidence was appraised as low. Therefore, no dietary recommendations should be concluded from this evidence. Similarly, supplementation with carnitine was found to be effective compared with placebo only on fatigue in a study that was not replicated. Only 2 RCTs investigated supplementation with ginseng extract and found contradicting results. No effects for studies with tryptophan, probiotics, or antioxidant supplementation were observed due to the limited number of studies.
Only 3 RCT studies involved food-based dietary interventions. Two RCTs of dietary patterns both found significant reductions in fatigue. However, the sample sizes were small and the studies contained a considerable risk of bias. Similarly, the 1 RCT studying the effects of a gluten-free diet found significant improvements in disability score and MRI lesions, although a moderate risk of bias and limited replication of results were found. The restrictive nature of these dietary patterns presents potential risks of excluding key nutrients unnecessarily; therefore, further studies are required to support these findings. Many of the interventions also focused on increasing plant-based foods, which contain other beneficial nutrients (105) and may contribute to the positive outcomes that were found.
Studies included within narrative reviews captured a greater range of food-based dietary studies and a greater range of study designs compared with the SRs. The narrative reviews demonstrated moderate alcohol and higher coffee consumption decreased the odds of disability progression, although these beverages do not appear to worsen disability progression. However, alcohol is known to affect neural pathways, with variability in its effects depending on the dose ingested (106). Further investigation of this dose-response relation is essential to determine safe amounts for consumption.
The RCTs reported across the included review articles varied from 2 wk to 30 months. A large proportion of these studies found no significant effects on MS progression. Due to the progressive nature of MS, it is apparent that outcomes of disability status, annualized relapse rate, and neurologic signs of disease activity may only be detected over longer durations, which may not be plausible in RCTs. This review has highlighted the need for studies using validated tools over longer periods of time. Dietary pattern studies in the varying subgroups of disease courses are also needed as evidence is lacking in populations with progressive MS.
This review was implemented in an attempt to minimize bias within the review process. However, reference lists of the included reviews were not hand-searched as this was not possible due to the large number of included review articles. The screening process was independently conducted by a second author for quality assurance, although data extraction was carried out individually by the primary author. Narrative review articles were included in the umbrella review to give a comprehensive view of the entire body of literature. The inclusion of narrative reviews captured a range of studies, interventions, and findings that were not included within SRs. However, the poor methodologic quality of these study designs and likely opinion of review authors influencing the reporting may affect the overall findings. Due to these limitations, the findings of the narrative reviews were not included in the overall grading of evidence. There are also limitations secondary to the RCTs within the included SRs, including the heterogeneity of baseline characteristics of the populations as well as variation in intervention doses and study durations affecting their interpretation.
In conclusion, it is evident that strong consistent evidence is lacking in the field of dietary interventions for persons with MS. The body of evidence is primarily focused around the isolation of individual nutrients in the form of supplements, many of which found no effect on relapses, disability progression, or MRI lesion activity in persons with MS. The most common nutrients studied in MS populations are vitamin D and PUFAs. This review has found evidence that serum vitamin D status is associated with improved outcomes of MS progression; however, no causal link was demonstrated. Evidence of both ω-3 FA and ω-6 FA supplementation is inconsistent, predominantly suggesting that supplementation has no benefit on major outcomes of MS progression. This review has highlighted the potential of food-based dietary interventions to affect outcomes of MS progression as well as the associated symptoms; however, limited evidence was obtained from high-quality SR articles. Finally, RCTs in this area have methodological limitations creating uncertainty, while observational studies lack a prospective longitudinal design and valid outcome measures, highlighting the necessity of future quality research studies in this area.
Supplementary Material
ACKNOWLEDGEMENTS
The authors’ responsibilities were as follows—YCP: was involved in the design, writing, and final content of the manuscript; ART: was involved in the conduct, writing, and review of the final content; and both authors: read and approved the final manuscript.
Notes
The authors reported no funding received for this study.
Author disclosures: YCP is a person with multiple sclerosis. ART reports no conflicts of interest.
Supplemental Tables 1–5 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/advances/.
Abbreviations used: ALA, ɑ-lipoic acid; ARR, annualized relapse rate; CRP, C-reactive protein; EDSS, Expanded Disability Status Scale; FA, fatty acid; FIS, Fatigue Impact Scale; FSS, Fatigue Severity Scale; GRADE, Grading of Recommendations Assessment, Development, and Evaluation; MS, multiple sclerosis; PPMS, primary progressive multiple sclerosis; PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses; RCT, randomized controlled trial; RRMS, relapsing remitting multiple sclerosis; SPMS, secondary progressive multiple sclerosis; SR, systematic literature review.
Contributor Information
Abbey R Tredinnick, School of Medicine, Faculty of Science Medicine and Health, University of Wollongong, Wollongong, New South Wales, Australia.
Yasmine C Probst, School of Medicine, Faculty of Science Medicine and Health, University of Wollongong, Wollongong, New South Wales, Australia; Illawarra Health and Medical Research Institute, University of Wollongong, Wollongong, New South Wales, Australia.
References
- 1. Macaron G, Ontaneda D. Diagnosis and management of progressive multiple sclerosis. Biomedicines. 2019;7(3):56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Browne P, Chandraratna D, Angood C, Tremlett H, Baker C, Taylor BV, Thompson AJ. Atlas of multiple sclerosis 2013: a growing global problem with widespread inequity. Neurology. 2014;83(11):1022–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Dendrou CA, Fugger L, Friese MA. Immunopathology of multiple sclerosis. Nat Rev Immunol. 2015;15(9):545–58. [DOI] [PubMed] [Google Scholar]
- 4. Penesová A, Dean Z, Kollár B, Havranová A, Imrich R, Vlček M, Rádiková Ž. Nutritional intervention as an essential part of multiple sclerosis treatment?. Physiol Res. 2018;67(4):521–33. [DOI] [PubMed] [Google Scholar]
- 5. Moher D, Liberati A, Tetzlaff J, Altman DG; The PRISMA Group. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Aromataris E, Fernandez RS, Godfrey C, Holly C, Khalil H, Tungpunkom P. Methodology for JBI umbrella reviews. In: Aromataris E. (Ed.) Joanna Briggs Institute Reviewers’ Manual: 2014 edition (supplement). South Australia: The Joanna Briggs Institute; 2014. p. 1–34. [Google Scholar]
- 7. Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA Cochrane handbook for systematic reviews of interventions version 6.0 [updated July 2019] [Internet]. Cochrane Collaboration; 2019; [cited 2020 Jan]. Available from: www.training.cochrane.org/handbook. [Google Scholar]
- 8. Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an Expanded Disability Status Scale (EDSS). Neurology. 1983;33(11):1444–52. [DOI] [PubMed] [Google Scholar]
- 9. Kurtzke JF. On the evaluation of disability in multiple sclerosis. Neurology. 1961;11:686–94. [DOI] [PubMed] [Google Scholar]
- 10. Krupp LB, LaRocca NG, Muir-Nash J, Steinberg AD. The Fatigue Severity Scale: application to patients with multiple sclerosis and systemic lupus erythematosus. Arch Neurol. 1989;46(10):1121–3. [DOI] [PubMed] [Google Scholar]
- 11. Schwartz JE, Jandorf L, Krupp LB. The measurement of fatigue: a new instrument. J Psychosom Res. 1993;37(7):753–62. [DOI] [PubMed] [Google Scholar]
- 12. Shahid A, Wilkinson K, Marcu S, Shapiro CM. Fatigue Impact Scale (FIS). In: Shahid A, Wilkinson K, Marcu S, Shapiro CM STOP, THAT and one hundred other sleep scales. New York: Springer New York; 2012. p. 163–5. [Google Scholar]
- 13. Larson RD. Psychometric properties of the modified fatigue impact scale. Int J MS Care. 2013;15(1):15–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Higgins JPT, Green S Cochrane handbook for systematic reviews of interventions version 5.1.0 [updated March 2011]. The Cochrane Collaboration; 2011; [cited 2019 Aug 1] [Internet]. Available from: www.handbook.cochrane.org. [Google Scholar]
- 15. Food Standards Australia New Zealand. AUSNUT 2011–13 Australian Food, Supplement and Nutrient Database [Internet]. Canberra (Australia): FSANZ; 2014; [updated 2014 Sep] [cited 2020 Jan]. Available from: www.foodstandards.gov.au. [Google Scholar]
- 16. Petrovskaya O, Lau F, Antonio M. Synthesising evidence on patient portals: a protocol for an umbrella review. BMJ Open. 2019;9(3):e024469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pollock A, Campbell P, Brunton G, Hunt H, Estcourt L. Selecting and implementing overview methods: implications from five exemplar overviews. Syst Rev. 2017;6(1):145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Shea BJ, Reeves BC, Wells G, Thuku M, Hamel C, Moran J, Moher D, Tugwell P, Welch V, Kristjansson E et al. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ. 2017;358:j4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Higgins JPT, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, Savović J, Schulz KF, Weeks L, Sterne JAC. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Khan KS, Ball E, Fox CE, Meads C. Systematic reviews to evaluate causation: an overview of methods and application. Evid Based Med. 2012;17(5):137–41. [DOI] [PubMed] [Google Scholar]
- 21. Atkins D, Briss PA, Eccles M, Flottorp S, Guyatt GH, Harbour RT, Hill S, Jaeschke R, Liberati A, Magrini N et al. Systems for grading the quality of evidence and the strength of recommendations II: pilot study of a new system. BMC Health Serv Res. 2005;5(1):25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jagannath VA, Filippini G, Di Pietrantonj C, Asokan GV, Robak EW, Whamond L, Robinson SA. Vitamin D for the management of multiple sclerosis. Cochrane Database Syst Rev. 2018;9:CD008422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hempel S, Graham GD, Fu N, Estrada E, Chen AY, Miake-Lye I, Miles JN, Shanman R, Shekelle PG, Beroes JM et al. A systematic review of the effects of modifiable risk factor interventions on the progression of multiple sclerosis. Mult Scler. 2017;23(4):513–24. [DOI] [PubMed] [Google Scholar]
- 24. Farinotti M, Vacchi L, Simi S, Di Pietrantonj C, Brait L, Filippini G. Dietary interventions for multiple sclerosis. Cochrane Database Syst Rev. 2012;12:Cd004192. [DOI] [PubMed] [Google Scholar]
- 25. Tejani AM, Wasdell M, Spiwak R, Rowell G, Nathwani S. Carnitine for fatigue in multiple sclerosis. Cochrane Database Syst Rev. 2012;5:CD007280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ganesh A, Apel S, Metz L, Patten S. The case for vitamin D supplementation in multiple sclerosis. Mult Scler Relat Disord. 2013;2(4):281–306. [DOI] [PubMed] [Google Scholar]
- 27. Berezowska M, Coe S, Dawes H. Effectiveness of vitamin D supplementation in the management of multiple sclerosis: a systematic review. Int J Mol Sci. 2019;20(6):E1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bagur MJ, Murcia MA, Jimenez-Monreal AM, Tur JA, Bibiloni MM, Alonso GL, Martinez-Tome M. Influence of diet in multiple sclerosis: a systematic review. Adv Nutr. 2017;8(3):463–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sintzel MB, Rametta M, Reder AT. Vitamin D and multiple sclerosis: a comprehensive review. Neurol Ther. 2018;7(1):59–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Luque-Cordoba D, Luque de Castro MD. Metabolomics: a potential way to know the role of vitamin D on multiple sclerosis. J Pharm Biomed Anal. 2017;136:22–31. [DOI] [PubMed] [Google Scholar]
- 31. Brown SJ. The role of vitamin D in multiple sclerosis. Ann Pharmacother. 2006;40(6):1158–61. [DOI] [PubMed] [Google Scholar]
- 32. Goldsmith JR. Vitamin D as an immunomodulator: risks with deficiencies and benefits of supplementation. Healthcare (Basel). 2015;3(2):219–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Veauthier C, Paul F. [Therapy of fatigue in multiple sclerosis: a treatment algorithm.]. Nervenarzt. 2016;87(12):1310–21.(in German). [DOI] [PubMed] [Google Scholar]
- 34. McKay KA, Jahanfar S, Duggan T, Tkachuk S, Tremlett H. Factors associated with onset, relapses or progression in multiple sclerosis: a systematic review. Neurotoxicology. 2017;61:189–212. [DOI] [PubMed] [Google Scholar]
- 35. Shahbeigi S, Pakdaman H, Fereshtehnejad SM, Nikravesh E, Mirabi N, Jalilzadeh G. Vitamin D3 concentration correlates with the severity of multiple sclerosis. Int J Prev Med. 2013;4(5):585. [PMC free article] [PubMed] [Google Scholar]
- 36. Harandi AA, Shahbeigi S, Pakdaman H, Fereshtehnejad SM, Nikravesh E, Jalilzadeh R. Association of serum 25(OH) vitamin D3 concentration with severity of multiple sclerosis. Iran J Neurol. 2012;11(2):54–8. [PMC free article] [PubMed] [Google Scholar]
- 37. Weinstock-Guttman B, Zivadinov R, Qu J, Cookfair D, Duan X, Bang E, Bergsland N, Hussein S, Cherneva M, Willis L et al. Vitamin D metabolites are associated with clinical and MRI outcomes in multiple sclerosis patients. J Neuro Neurosurg Psychiatry. 2011;82(2):189–95. [DOI] [PubMed] [Google Scholar]
- 38. Smolders J, Menheere P, Kessels A, Damoiseaux J, Hupperts R. Association of vitamin D metabolite levels with relapse rate and disability in multiple sclerosis. Mult Scler. 2008;14(9):1220–4. [DOI] [PubMed] [Google Scholar]
- 39. Mowry EM, Waubant E, McCulloch CE, Okuda DT, Evangelista AA, Lincoln RR, Gourraud PA, Brenneman D, Owen MC, Qualley P et al. Vitamin D status predicts new brain magnetic resonance imaging activity in multiple sclerosis. Ann Neurol. 2012;72(2):234–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Neau JP, Artaud-Uriot MS, Lhomme V, Bounaud JY, Lebras F, Boissonnot L, Moinot N, Ciron J, Larrieu D, Mathis S et al. [Vitamin D and multiple sclerosis. A prospective survey of patients of Poitou-Charentes area.]. Rev Neurol (Paris). 2011;167(4):317–23.(in French). [DOI] [PubMed] [Google Scholar]
- 41. Loken-Amsrud KI, Holmoy T, Bakke SJ, Beiske AG, Bjerve KS, Bjornara BT, Hovdal H, Lilleas F, Midgard R, Pedersen T et al. Vitamin D and disease activity in multiple sclerosis before and during interferon-beta treatment. Neurology. 2012;79(3):267–73. [DOI] [PubMed] [Google Scholar]
- 42. Runia TF, Hop WCJ, de Rijke YB, Buljevac D, Hintzen RQ. Lower serum vitamin D levels are associated with a higher relapse risk in multiple sclerosis. Neurology. 2012;79(3):261. [DOI] [PubMed] [Google Scholar]
- 43. Simpson SJ, Taylor B, Blizzard L, Ponsonby AL, Pittas F, Tremlett H, Dwyer T, Gies P, van der Mei I. Higher 25-hydroxyvitamin D is associated with lower relapse risk in multiple sclerosis. Ann Neurol. 2010;68(2):193–203. [DOI] [PubMed] [Google Scholar]
- 44. Langer-Gould A, Huang S, Van Den Eeden SK, Gupta R, Leimpeter AD, Albers KB, Horst R, Hollis B, Steinman L, Nelson LM. Vitamin D, pregnancy, breastfeeding, and postpartum multiple sclerosis relapses. Arch Neurol. 2011;68(3):310–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Soilu-Hänninen M, Laaksonen M, Laitinen I, Erälinna JP, Lilius EM, Mononen I. A longitudinal study of serum 25-hydroxyvitamin D and intact parathyroid hormone levels indicate the importance of vitamin D and calcium homeostasis regulation in multiple sclerosis. J Neurol Neurosurg Psychiatry. 2008;79(2):152–7. [DOI] [PubMed] [Google Scholar]
- 46. Rodenas Esteve I, Wanden-Berghe C, Sanz-Valero J. [Effects of nutritional status on the multiple sclerosis disease: systematic review.]. Nutr Hosp. 2018;35(1):211–23.(in Spanish). [DOI] [PubMed] [Google Scholar]
- 47. Burton JM, Costello FE. Vitamin D in multiple sclerosis and central nervous system demyelinating disease—a review. J Neuroophthalmol. 2015;35(2):194–200. [DOI] [PubMed] [Google Scholar]
- 48. Kočovská E, Gaughran F, Krivoy A, Meier UC. Vitamin-D deficiency as a potential environmental risk factor in multiple sclerosis, schizophrenia, and autism. Front Psychiatry. 2017;8:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Hanwell HE, Banwell B. Assessment of evidence for a protective role of vitamin D in multiple sclerosis. Biochim Biophys Acta. 2011;1812(2):202–12. [DOI] [PubMed] [Google Scholar]
- 50. D'Hooghe M B, Nagels G, Bissay V, De Keyser J. Modifiable factors influencing relapses and disability in multiple sclerosis. Mult Scler. 2010;16(7):773–85. [DOI] [PubMed] [Google Scholar]
- 51. Eikelenboom MJ, Killestein J, Kragt JJ, Uitdehaag BM, Polman CH. Gender differences in multiple sclerosis: cytokines and vitamin D. J Neurol Sci. 2009;286(1-2):40–2. [DOI] [PubMed] [Google Scholar]
- 52. Coyle PK. Symptom management and lifestyle modifications in multiple sclerosis. Continuum. 2016;22(3):815–36. [DOI] [PubMed] [Google Scholar]
- 53. Carlson NG, Rose JW. Vitamin D as a clinical biomarker in multiple sclerosis. Expert Opin Med Diagn. 2013;7(3):231–42. [DOI] [PubMed] [Google Scholar]
- 54. Hewer S, Lucas R, Van der Mei I, Taylor BV. Vitamin D and multiple sclerosis. J Clin Neurosci. 2013;20(5):634–41. [DOI] [PubMed] [Google Scholar]
- 55. Munger KL, Ascherio A. Prevention and treatment of MS: studying the effects of vitamin D. Mult Scler. 2011;17(12):1405–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Mische LJ, Mowry EM. The evidence for dietary interventions and nutritional supplements as treatment options in multiple sclerosis: a review. Curr Treat Options Neurol. 2018;20(4):8. [DOI] [PubMed] [Google Scholar]
- 57. Cendrowski W. [Importance of lipids in pathological mechanisms of multiple sclerosis.]. Neurol Neurochir Pol. 1975;9(2):253–8.(in Polish). [PubMed] [Google Scholar]
- 58. Crabtree-Hartman E. Advanced symptom management in multiple sclerosis. Neurol Clin. 2018;36(1):197–218. [DOI] [PubMed] [Google Scholar]
- 59. Schmitz K, Barthelmes J, Stolz L, Beyer S, Diehl O, Tegeder I “Disease modifying nutricals” for multiple sclerosis. Pharmacol Ther. 2015;148:85–113. [DOI] [PubMed] [Google Scholar]
- 60. Altowaijri G, Fryman A, Yadav V. Dietary interventions and multiple sclerosis. Curr Neurol Neurosci Rep. 2017;17(3):28. [DOI] [PubMed] [Google Scholar]
- 61. Farzaei MH, Shahpiri Z, Bahramsoltani R, Nia MM, Najafi F, Rahimi R. Efficacy and tolerability of phytomedicines in multiple sclerosis patients: a review. CNS Drugs. 2017;31(10):867–89. [DOI] [PubMed] [Google Scholar]
- 62. Claflin SB, van der Mei IAF, Taylor BV. Complementary and alternative treatments of multiple sclerosis: a review of the evidence from 2001 to 2016. J Neurol Neurosurg Psychiatry. 2018;89(1):34–41. [DOI] [PubMed] [Google Scholar]
- 63. Pommerich UM, Brincks J, Christensen ME. Is there an effect of dietary intake on MS-related fatigue? A systematic literature review. Mult Scler Relat Disord. 2018;25:282–91. [DOI] [PubMed] [Google Scholar]
- 64. Plemel JR, Juzwik CA, Benson CA, Monks M, Harris C, Ploughman M. Over-the-counter anti-oxidant therapies for use in multiple sclerosis: a systematic review. Mult Scler. 2015;21(12):1485–95. [DOI] [PubMed] [Google Scholar]
- 65. Khalili M, Azimi A, Izadi V, Eghtesadi S, Mirshafiey A, Sahraian MA, Motevalian A, Norouzi A, Sanoobar M, Eskandari G et al. Does lipoic acid consumption affect the cytokine profile in multiple sclerosis patients: a double-blind, placebo-controlled, randomized clinical trial. Neuro Immuno Modulation. 2014;21(6):291–6. [DOI] [PubMed] [Google Scholar]
- 66. de Sousa CNS, da Silva Leite CMG, da Silva Medeiros I, Vasconcelos LC, Cabral LM, Patrocinio CFV, Patrocinio MLV, Mouaffak F, Kebir O, Macedo D et al. Alpha-lipoic acid in the treatment of psychiatric and neurological disorders: a systematic review. Metab Brain Dis. 2019;34(1):39–52. [DOI] [PubMed] [Google Scholar]
- 67. Yadav V, Marracci G, Lovera J, Woodward W, Bogardus K, Marquardt W, Shinto L, Morris C, Bourdette D. Lipoic acid in multiple sclerosis: a pilot study. Mult Scler. 2005;11(2):159–65. [DOI] [PubMed] [Google Scholar]
- 68. Sanadgol N, Zahedani SS, Sharifzadeh M, Khalseh R, Barbari GR, Abdollahi M. Recent updates in imperative natural compounds for healthy brain and nerve function: a systematic review of implications for multiple sclerosis. Curr Drug Targets. 2017;18(13):1499–517. [DOI] [PubMed] [Google Scholar]
- 69. Salari S, Khomand P, Arasteh M, Yousefzamani B, Hassanzadeh K. Zinc sulphate: a reasonable choice for depression management in patients with multiple sclerosis. A randomized, double-blind, placebo-controlled clinical trial. J Pharmacol Rep. 2015;67(3):606–9. [DOI] [PubMed] [Google Scholar]
- 70. Fragoso YD, Stoney PN, McCaffery PJ. The evidence for a beneficial role of vitamin A in multiple sclerosis. CNS Drugs. 2014;28(4):291–9. [DOI] [PubMed] [Google Scholar]
- 71. Nemazannikova N, Mikkelsen K, Stojanovska L, Blatch GL, Apostolopoulos V. Is there a link between vitamin B and multiple sclerosis?. Med Chem. 2018;14(2):170–80. [DOI] [PubMed] [Google Scholar]
- 72. Khosravi-Largani M, Pourvali-Talatappeh P, Rousta AM, Karimi-Kivi M, Noroozi E, Mahjoob A, Asaadi Y, Shahmohammadi A, Sadeghi S, Shakeri S et al. A review on potential roles of vitamins in incidence, progression, and improvement of multiple sclerosis. eNurologicalSci. 2018;10:37–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Naghashpour M, Jafarirad S, Amani R, Sarkaki A, Saedisomeolia A. Update on riboflavin and multiple sclerosis: a systematic review. Iran J Basic Med Sci. 2017;20(9):958–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Bitarafan S, Saboor-Yaraghi A, Sahraian M, Nafissi S, Togha M, Beladi Moghadam N, Roostaei T, Siassi F, Eshraghian MR, Ghanaati H et al. Impact of vitamin a supplementation on disease progression in patients with multiple sclerosis. Arch Iran Med. 2015;18:435–40. [PubMed] [Google Scholar]
- 75. Jafarirad S, Siassi F, Harirchian MH, Amani R, Bitarafan S, Saboor-Yaraghi A. The effect of vitamin a supplementation on biochemical parameters in multiple sclerosis patients. Iran Red Crescent Med J. 2013;15(3):194–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Naghashpour M, Majdinasab N, Shakerinejad G, Kouchak M, Haghighizadeh MH, Jarvandi F, Hajinajaf S. Riboflavin supplementation to patients with multiple sclerosis does not improve disability status nor is riboflavin supplementation correlated to homocysteine. Int J Vitam Nutr Res. 2013;83(5):281–90. [DOI] [PubMed] [Google Scholar]
- 77. Bisaga GN, Odinak MM, Boĭko AN, Mel'nik IB, Popova NF [Possibilities of treatment of multiple sclerosis exacerbations without corticosteroids: a role of metabolic and antioxidant therapy.] Zh Nevrol Psikhiatr Im S S Korsakova. 2011;111(2):44–8.(in Russian). [PubMed] [Google Scholar]
- 78. Sedel F, Papeix C, Bellanger A, Touitou V, Lebrun-Frenay C, Galanaud D, Gout O, Lyon-Caen O, Tourbah A. High doses of biotin in chronic progressive multiple sclerosis: a pilot study. Mult Scler Relat Disord. 2015;4(2):159–69. [DOI] [PubMed] [Google Scholar]
- 79. Tourbah A, Lebrun-Frenay C, Edan G, Clanet M, Papeix C, Vukusic S, De Sèze J, Debouverie M, Gout O, Clavelou P et al. MD1003 (high-dose biotin) for the treatment of progressive multiple sclerosis: a randomised, double-blind, placebo-controlled study. Mult Scler. 2016;22(13):1719–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Saka M, Saka M, Koseler E, Metin S, Bilen S, Aslanyavrusu M, Ak F, Kiziltan G. Nutritional status and anthropometric measurements of patients with multiple sclerosis. Saudi Med J. 2012;33(2):160–6. [PubMed] [Google Scholar]
- 81. Shinto L, Calabrese C, Morris C, Yadav V, Griffith D, Frank R, Oken BS, Baldauf-Wagner S, Bourdette D. A randomized pilot study of naturopathic medicine in multiple sclerosis. J Altern Complement Med. 2008;14(5):489–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Wade DT, Young CA, Chaudhuri KR, Davidson DLW. A randomised placebo controlled exploratory study of vitamin B-12, lofepramine, and L-phenylalanine (the “Cari Loder regime”) in the treatment of multiple sclerosis. J Neurol Neurosurg Psychiatry. 2002;73(3):246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Likosky WH, Fireman B, Elmore R, Eno G, Gale K, Goode GB, Ikeda K, Laster J, Mosher C, Rozance J et al. Intense immunosuppression in chronic progressive multiple sclerosis: the Kaiser study. J Neurol Neurosurg Psychiatry. 1991;54(12):1055–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Kira J, Tobimatsu S, Goto I. Vitamin B12 metabolism and massive-dose methyl vitamin B12 therapy in Japanese patients with multiple sclerosis. Intern Med. 1994;33(2):82–6. [DOI] [PubMed] [Google Scholar]
- 85. Besler HT, Comoğlu S, Okçu Z. Serum levels of antioxidant vitamins and lipid peroxidation in multiple sclerosis. Nutr Neurosci. 2002;5(3):215–20. [DOI] [PubMed] [Google Scholar]
- 86. Løken-Amsrud KI, Myhr K-M, Bakke SJ, Beiske AG, Bjerve KS, Bjørnarå BT, Hovdal H, Lilleås F, Midgard R, Pedersen T et al. Retinol levels are associated with magnetic resonance imaging outcomes in multiple sclerosis. Mult Scler. 2013;19(4):451–7. [DOI] [PubMed] [Google Scholar]
- 87. Bitarafan S, Harirchian M-H, Nafissi S, Sahraian M-A, Togha M, Siassi F, Saedisomeolia A, Alipour E, Mohammadpour N, Chamary M et al. Dietary intake of nutrients and its correlation with fatigue in multiple sclerosis patients. Iran J Neurol. 2014;13(1):28–32. [PMC free article] [PubMed] [Google Scholar]
- 88. Ayache SS, Chalah MA. Fatigue in multiple sclerosis—insights into evaluation and management. Neurophysiol Clin. 2017;47(2):139–71.. French. [DOI] [PubMed] [Google Scholar]
- 89. Mehrabani S, Askari G, Miraghajani M, Tavakoly R, Arab A. Effect of coenzyme Q10 supplementation on fatigue: a systematic review of interventional studies. Complement Ther Med. 2019;43:181–7. [DOI] [PubMed] [Google Scholar]
- 90. Yadav V, Bourdette D. Complementary and alternative medicine: is there a role in multiple sclerosis?. Curr Neurol Neurosci Rep. 2006;6(3):259–67. [DOI] [PubMed] [Google Scholar]
- 91. von Geldern G, Mowry EM. The influence of nutritional factors on the prognosis of multiple sclerosis. Nat Rev Neurol. 2012;8(12):678–89. [DOI] [PubMed] [Google Scholar]
- 92. Dolan KE, Finley HJ, Burns CM, Gasta MG, Gossard CM, Parker EC, Pizano JM, Williamson CB, Lipski EA. Probiotics and disease: a comprehensive summary—part 1, mental and neurological health. Integr Med (Encinitas). 2016;15(5):46–58. [PMC free article] [PubMed] [Google Scholar]
- 93. de Oliveira GLV, Leite AZ, Higuchi BS, Gonzaga MI, Mariano VS. Intestinal dysbiosis and probiotic applications in autoimmune diseases. Immunology. 2017;152(1):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Labuschagne IL, Blaauw R. An anti-inflammatory approach to the dietary management of multiple sclerosis: a condensed review. South Afr J Clin Nutr. 2018;31(3):67–73. [Google Scholar]
- 95. Thomsen HL, Jessen EB, Passali M, Frederiksen JL. The role of gluten in multiple sclerosis: a systematic review. Mult Scler Relat Disord. 2019;27:156–63. [DOI] [PubMed] [Google Scholar]
- 96. D'Hooghe MB, Nagels G, Bissay V, De Keyser J. Modifiable factors influencing relapses and disability in multiple sclerosis. Mult Scler. 2010;16(7):773–85. [DOI] [PubMed] [Google Scholar]
- 97. Wahls TL, Chenard CA, Snetselaar LG. Review of two popular eating plans within the multiple sclerosis community: low saturated fat and modified Paleolithic. Nutrients. 2019;11(2):352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Feinstein A, Magalhaes S, Richard J-F, Audet B, Moore C. The link between multiple sclerosis and depression. Nat Rev Neurol. 2014;10(9):507–17. [DOI] [PubMed] [Google Scholar]
- 99. Sharif K, Watad A, Bragazzi NL, Adawi M, Amital H, Shoenfeld Y. Coffee and autoimmunity: more than a mere hot beverage!. Autoimmun Rev. 2017;16(7):712–21. [DOI] [PubMed] [Google Scholar]
- 100. Katz Sand I. The role of diet in multiple sclerosis: mechanistic connections and current evidence. Curr Nutr Rep. 2018;7(3):150–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Jahromi SR, Sahraian MA, Ashtari F, Ayromlou H, Etemadifar M, Ghaffarpour M, Mohammadianinejad E, Nafissi S, Nickseresht A, Shaygannejad V et al. Islamic fasting and multiple sclerosis. BMC Neurol. 2014;14:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Moss B, Rensel M, Hersh C, Moss BP, Rensel MR, Hersh CM. Wellness and the role of comorbidities in multiple sclerosis. Neurotherapeutics. 2017;14(4):999–1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Swank RL, Goodwin J. Review of MS patient survival on a Swank low saturated fat diet. Nutrition. 2003;19(2):161–2. [DOI] [PubMed] [Google Scholar]
- 104. McLaughlin L, Clarke L, Khalilidehkordi E, Butzkueven H, Taylor B, Broadley SA. Vitamin D for the treatment of multiple sclerosis: a meta-analysis. J Neurol. 2018;265(12):2893–905. [DOI] [PubMed] [Google Scholar]
- 105. Mihrshahi S, Mishra GD. Challenges and opportunities with communicating the results of studies related to fruit and vegetable consumption for general wellbeing and mental health. Australasian Epidemiologist. 2014;21(1):8–11. [Google Scholar]
- 106. World Health Organization. Global status report on alcohol and health 2018. Geneva (Switzerland): World Health Organization; 2018. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.