The global pandemic caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) (1) represents a one-in-a-lifetime, worldwide health crisis with 11,638,136 confirmed cases worldwide since the beginning of the epidemic and 538,266 fatalities (4.63% lethality) (data provided by the WHO Health Emergency Dashboard on May 10th, 10.00 am CET).
The key clinical features observed in patients affected by SARS-CoV-2 are related to lower respiratory tract illness with fever, dry cough, and dyspnea. Recent evidence shows that those patients have a disproportionately higher incidence of cerebral ischemic stroke and myocardial infarction (2-5).
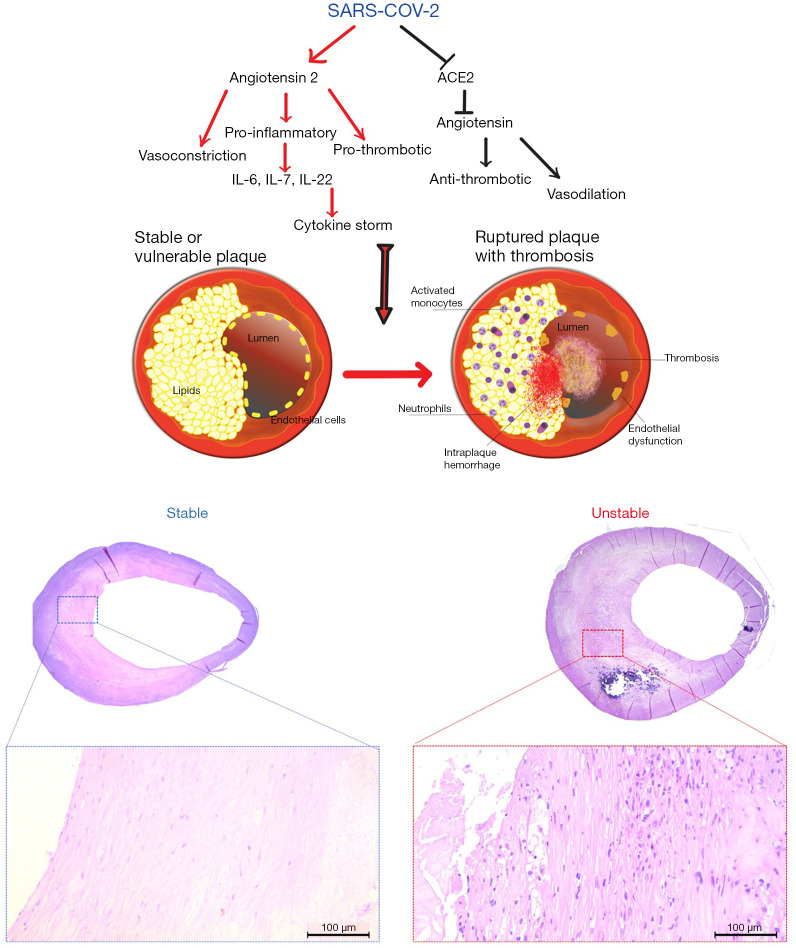
The pathophysiology of the SARS-CoV-2 is not yet fully understood but recent studies suggest that the virus gains entry into the host through the use of angiotensin-converting enzyme 2 (ACE2) as its cellular receptor. ACE2 is a membrane-bound mono-carboxypeptidase found ubiquitously in humans in the type II pneumocytes of the lungs, in the kidneys, in the intestine but also in cardiomyocytes, coronary pericytes and coronary endothelial cells (6). ACE2 plays a counterbalancing role in the renin angiotensin-converting system (RAAS) and is a carboxypeptidase that converts angiotensin II (Ang II) into angiotensin 1–7 (Ang 1–7). The loss of ACE2, caused by the SARS-CoV-2 at the cell surface, leads to (I) a decrease in the levels of cardioprotective (Ang 1–7) and (II) an increase in the levels of Ang II, which promotes endothelial dysfunction and inflammation, and promote atherogenesis (7). ACE2 plays an important role in the molecular pathways implicated in the development of carotid and coronary atherosclerotic plaques (8,9). In Figure 1, the consequences of the dysregulation of the renin-angiotensin system caused by the SARS-CoV-2 inside atherosclerotic plaques are illustrated: endothelial dysfunction, leading to thrombosis (10); enhanced permeability of the endothelial barrier, favouring the invasion of the plaque by inflammatory cells and the insurgence of intra-plaque haemorrhage (IPH); accumulation of inflammatory cells, including neutrophils, activated monocytes, lymphocytes and plasma cells in the plaque. The pro-inflammatory and pro-thrombotic activity of angiotensin II might transform a vulnerable plaque into a ruptured and complicated plaque. The ACE2 pathway alterations promote the development of vascular diseases associated with Ang-II-mediated vascular inflammation and activation of the c-Jun N-terminal kinase (JNK) signalling, leading to the notion that ACE2 demodulation reduces protection against vascular diseases (11,12).
Figure 1.
Diagram showing the molecular interactions that links the COVID19 and plaque’s vulnerability and an example of stable plaque and unstable plaque. Histological sections were obtained with H&E.
Recent exploration of the pulmonary immunopathology and microvascular coagulopathy associated with SARS-CoV-2 infection also suggest an enhanced activation of the immune system similar to the macrophage-activation syndrome that may unmask subclinical cardiovascular disease (13), potentially also promoting destabilizing intraplaque inflammation. Although the precise stimuli that trigger atherosclerotic plaque instability have not yet been identified, ‘Kounis syndrome’, myocardial infarction from massive activation of inflammation in anaphylaxis, exemplifies another association between an (extreme) inflammatory stimuli, plaque instability and atherothrombosis (14). COVID appears to be associated with often very vigorous inflammatory response/storm and clinical intervention in patients with COVID-19 has demonstrated a strong upregulation of cytokine production in those subjects who are critically ill with SARS-CoV2-induced pneumonia. The secretion of multiple cytokines, also termed Cytokine Release Syndrome (CRS), is closely related to development of clinical symptoms. In particular, the interferons (IFNs) that play the central role in innate immunity to viruses and other microbial pathogens, the interleukins (ILs) that regulate the immune cell differentiation and activation, and the chemokines that bind to one or more of 21 G-protein-coupled receptors and constitute the pathophysiologic substrate of Kounis anaphylaxis associated syndrome could be new therapeutic targets of COVID-19 infection
Based on these pathophysiological mechanisms, it appears possible that subjects infected with SARS-CoV-2 suffer an increased risk of conversion from asymptomatic, subclinical, atherosclerotic disease (15) into an unstable state with vulnerable plaques in the carotid and/or coronary arteries due to the immunopathology associated with the viral infection. Interestingly, Europe and US hospitals have seen a drop in admissions for acute myocardial infarction. Some EU countries showed a reduction of cardiac catheterization procedures of 48%, with a reduction of 40% for primary angioplasty and similar results have been reported in the USA (16,17). However, this is matter of debate and it is hypothesized that it is due to the delay in seeking for care by the patients.
This hypothesis would also explain the increased prevalence of ischemic events12 in young subjects, asymptomatic for lower respiratory tract illness, who have developed unexpected occurrence of ischemic events consistent with large-vessel stroke.
In conclusion, we hypothesize that SARS-CoV-2 has the potential to trigger cellular and molecular processes in coronary and carotid atherosclerotic lesions promote an increased vulnerability with subsequent increased risk of cerebral ischemic stroke or myocardial infarction. If this hypothesis would be demonstrated, it could suggest using protective approaches for plaque protection in subjects affected by SARS-CoV-2 with special attention to medical therapy for cardiovascular disease, including an antithrombotic preventive approach (18) and potentially also therapy targeting immune pathways operating in the disease, once these have been unravelled (19).
Moreover, this would have a significant clinical implication for high-risk category patients in whom COVID-19 may be severe or fatal. These include the elderly (>60 years old), obese patients, diabetic patients, smokers and patients with hypertension and cardiovascular disease, all categories at higher risk for developing atherosclerosis too. These patients, if positive for SARS-CoV-2 even if asymptomatic, could be at increased risk of developing cerebral ischemic strokes, and myocardial infarction due to increased instability of coronary and carotid plaques.
Acknowledgments
Funding: None.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
Footnotes
Provenance and Peer Review: This article was a free submission to the journal. The article was sent for external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/cdt-20-561). The authors have no conflicts of interest to declare.
References
- 1.Guan WJ, Ni ZY, Hu Y, et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med 2020;382:1708-20. 10.1056/NEJMoa2002032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Helms J, Kremer S, Merdji H, et al. Neurologic Features in Severe SARS-CoV-2 Infection. N Engl J Med 2020;382:2268-70. 10.1056/NEJMc2008597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shi S, Qin M, Shen B, et al. Association of Cardiac Injury with Mortality in Hospitalized Patients with COVID-19 in Wuhan, China. JAMA Cardiol 2020;5:802-10. 10.1001/jamacardio.2020.0950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guo T, Fan Y, Chen M, et al. Cardiovascular Implications of Fatal Outcomes of Patients With Coronavirus Disease 2019 (COVID-19). JAMA Cardiol 2020;5:811-8. 10.1001/jamacardio.2020.1017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mahammedi A, Saba L, Vagal A, et al. Imaging in Neurological Disease of Hospitalized COVID-19 Patients: An Italian Multicenter Retrospective Observational Study. Radiology 2020;297:E270-3. 10.1148/radiol.2020201933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hoffmann M, Kleine-Weber H, Schroeder S, et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell 2020;181:271-80.e8. 10.1016/j.cell.2020.02.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang C, Zhao YX, Zhang YH, et al. Angiotensin-converting enzyme 2 attenuates atherosclerotic lesions by targeting vascular cells. Proc Natl Acad Sci U S A 2010;107:15886-91. 10.1073/pnas.1001253107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.da Silva AR, Fraga-Silva RA, Stergiopulos N, et al. Update on the role of angiotensin in the pathophysiology of coronary atherothrombosis. Eur J Clin Invest 2015;45:274-87. 10.1111/eci.12401 [DOI] [PubMed] [Google Scholar]
- 9.Fraga-Silva RA, Savergnini SQ, Montecucco F, et al. Treatment with Angiotensin-(1-7) reduces inflammation in carotid atherosclerotic plaques. Thromb Haemost 2014;111:736-47. 10.1160/TH13-06-0448 [DOI] [PubMed] [Google Scholar]
- 10.Prieto-Lobato A, Ramos-Martínez R, Vallejo-Calcerrada N, et al. A Case Series of Stent Thrombosis During the COVID-19 Pandemic. JACC Case Rep 2020;2:1291-6. 10.1016/j.jaccas.2020.05.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sahara M, Ikutomi M, Morita T, et al. Deletion of angiotensin-converting enzyme 2 promotes the development of atherosclerosis and arterial neointima formation. Cardiovasc Res 2014;101:236-46. 10.1093/cvr/cvt245 [DOI] [PubMed] [Google Scholar]
- 12.Oxley TJ, Mocco J, Majidi S, et al. Large-Vessel Stroke as a Presenting Feature of Covid-19 in the Young. The N Engl J Med 2020;382:e60. 10.1056/NEJMc2009787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McGonagle D, O’Donnell JS, Sharif K, et al. Immune mechanisms of pulmonary intravascular coagulopathy in COVID-19 pneumonia. Lancet Rheumatol 2020;2:E437-45. 10.1016/S2665-9913(20)30121-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kounis NG, Mazarakis A, Tsigkas G, et al. Kounis syndrome: A new twist on an old disease. Future Cardiol 2011;7:805-24. 10.2217/fca.11.63 [DOI] [PubMed] [Google Scholar]
- 15.Saba L, Saam T, Jäger HR, et al. Imaging biomarkers of vulnerable carotid plaques for stroke risk prediction and their potential clinical implications. Lancet Neurol 2019;18:559-72. 10.1016/S1474-4422(19)30035-3 [DOI] [PubMed] [Google Scholar]
- 16.Negreira Caamaño M, Piqueras Flores J, Mateo Gómez C. Impact of COVID-19 pandemic in cardiology admissions. Med Clin (Barc) 2020;155:179-80. 10.1016/j.medcli.2020.05.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Garcia S, Albaghdadi MS, Meraj PM, et al. Reduction in ST-Segment Elevation Cardiac Catheterization Laboratory Activations in the United States During COVID-19 Pandemic. J Am Coll Cardiol 2020;75:2871-2. 10.1016/j.jacc.2020.04.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marongiu F, Grandone E, Barcellona D. Pulmonary thrombosis in 2019-nCoV pneumonia? J Thromb Haemost 2020;18:1511-3. 10.1111/jth.14818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hedin U, Matic LP. Recent advances in therapeutic targeting of inflammation in atherosclerosis. J Vasc Surg 2019;69:944-51. 10.1016/j.jvs.2018.10.051 [DOI] [PubMed] [Google Scholar]