Abstract
The expression of beta-lactamases in bacteris is a central cause of drug resistance. In this report, we present a beta-lactamase chemiluminescent probe, termed CCP, which can for the first time detect beta-lactamase activity via chemiluminescence and can detect beta lactamase with a sensitivity that is 4-orders of magnitude higher than the commercially available fluorescent lactamase substrate fluorocillin.
The expression of beta-lactamase in bacteria is a central cause of drug resistance, and chemical probes that can measure its concentration in tissue samples have the potential to significantly improve the treatment of drug resistant infections and their global monitoring. However, the concentration of beta-lactamase in patient samples is extremely low and developing lactam probes that have the sensitivity needed to detect lactamase directly from patient specimens remains a major challenge.1–3
A wide variety of beta-lactamase probes have been developed based on fluorescence sensing.4–12 For example, coumarin,5 fluorescein,4 and the Tokyo green fluorophores10,11 have all been conjugated to self-immolative cephalosporins, and can detect beta-lactamase via fluorescence. However, fluorescent based probes for beta-lactamase lack the sensitivity needed to directly detect beta-lactamase from patient samples, due to the inherent limitations of fluorescence sensing. Therefore, at present beta-lactamase expression in bacteria is still determined by culturing tissue specimens for several days, and thus requires a significant amount of time, cost, and infrastructure. Consequently, the vast majority of drug resistant infections go undetected, and are frequently treated with ineffective antibiotics. Chemical probes that can detect beta-lactamase directly from patient tissue samples have the potential to significantly improve the treatment of bacterial infections and there is a great need for new strategies for imaging beta-lactamase.
Dioxetane based chemiluminescence has great potential for increasing the sensitivity of enzyme based probes.13, 14 For example, chemiluminescent probes for a variety of enzymes, such as alkaline phosphatase,20 beta-galactosidase,16 neuraminidase,17 esterase,22 and caspase-315 have been developed using dioxetane chemiluminescence, which can detect femtomolar concentrations of their target enzymes, and are in general 3-4 orders of magnitude more sensitive than analogous fluorescent based probes.18, 19 Importantly, dioxetane based chemiluminescece is fairly versatile in its applications, any enzymatic reaction that generates a free phenolic anion can potentially be detected via dioxetane chemiluminescence.
However, developing dioxetane based probes for beta-lactamase poses several challenges. In particular, dioxetane chemiluminescence occurs most efficiently at a pH of 10, due to the pKa of the phenol, and the lactam ring in cephalosporins hydrolyze at this pH, which will cause a high background signal. In addition, the synthesis of cephalosporin-dioxetane probes is challenging, because the conjugation between a cephalosporin and a dioxetane requires strong basic conditions, which is incompatible with the cephalosporins, due to their propensity for double bond isomerization and opening of the lactam ring.
In this report, we present a dioxetane based cephalosporin probe, termed CCP, which can for the first time detect beta-lactamase activity via chemiluminescence. The chemical structure of CCP and the mechanism by which it detects beta-lactamase is shown in Figure 1. CCP is composed of a cephalosporin that is conjugated to a dioxetane via a thioether linkage (Figure 1). CCP is a substrate for beta-lactamase. In the presence of beta lactamases, CCP is hydrolysed into 2 and releases the chemiluminescent probe 3. The compound 3 spontaneously decomposes into the ketone 5 and a high-energy intermediate 6, which releases a photon, resulting in the detection of beta-lactamase. A thiophenol21 was selected as the leaving group in CCP, as opposed to the more common phenolic leaving group, because of the lower pKa of the thiophenol. The low pKa of the thiophenol enables CCP to generate chemiluminescence at neutral pH, as opposed to pH 10. In addition, CCP can also be synthesized under mild basic conditions, because the low pKa of the thio-phenol.
Figure 1:

A cephalosporin-chemiluminescent probe (CCP) increases the detection sensitivity of beta-lactamases. CCP is composed of a cephalosporin and a chemiluminescent probe conjugated together via a thioether linkage. CCP spontaneously hydrolyses in the presence of beta-lactamases. Subsequently, it undergoes a spontaneous 1,4-elimination to release the chemiluminescent probe 3. The chemiluminescent probe 3 degrades into a high-energy intermediate 6 that emits a photon and produces chemiluminescence.
The synthesis of CCP is shown in Figures 2 and 3 and was accomplished in 10-steps, via a convergent synthesis. The key building blocks in the synthesis were the allyl protected cephalosporin chloride 8 and the thiophenol adamantine vinyl ether 17. The protected CCP 9 was synthesized by coupling the chemiluminescent probe precursor 17 with the in-situ generated allylic chloride of the cephalosporin under mild basic conditions. The vinyl ether of 9 was converted into a 1,2-dioxetane via a singlet oxygen mediated transformation, which used methylene blue and O2 as the singlet oxygen source. Importantly, the methylene blue oxidation procedure is selective for the vinyl ether double bond and does not destroy the double bond on the lactam ring. Finally, the desired chemiluminescent probe 1 was achieved by Pd-catalyzed deprotection of the allylic ester 10 (Figure 2)
Figure 2.

Synthesis of cephalosporin-chemiluminescent probe (CCP). (a) BCl3, DCM (b) 17, acetone-water (8.5:1.5), NaHCO3, 1.5 h, rt, 63%; (c) 0.5% methylene blue, O2, DCM, −78 °C, 13%; (d) Pd(PPh3)4 (2 mol%), sodium hexanoate, DCM, 73%.
Figure 3.

Synthesis of chemiluminescent probe precursor 17: (a) Dimethylthiocarbamoyl chloride, THF, NaOH, 93%; (b) NMP, 225 °C, 67%; (c) 2,2-dimethoxy propane, pTSA, 70 °C; (d) DCM, TiCl3, trimethyl phosphite, −78 °C, 97%; (e) THF, LDA, −78 °C to rt, 18h, 53%; (f) 10% KOH in ethanol, 70 °C, 92%.
The thiophenol adamantine vinyl ether 17 was synthesized in six steps starting from 3-hydroxybenzaldehyde. The hydroxyl group was converted into an O-thiocarbamate and was subjected to a Newman-Kwart rearrangement to generate S-thiocarbamate 13. The S-Thiocarbamate 13 was converted into an acetal using 2,2 dimethoxy propane, to yield compound 14, which was asymmetrically hydrolyzed at low temperature in the presence of the nucleophilic trimethoxy phosphine to yield 15. Finally, the phosphonate ester was condensed with 2-adamantanone 5, generating the S-thiocarbamate adamantine vinyl ether 16, which was hydrolyzed in ethanolic KOH to achieve the thiophenol adamantine vinyl ether 17.
CCP needs to be a substrate for beta-lactamase in order to detect drug resistance. However, CCP contains a bulky hydrophobic adamantine group as its 3’-substiteunt, which can potentially cause weak binding at the active site of beta-lactamases. We therefore investigated the kinetics of CCP hydrolysis by the beta-lactamase TEM-1. CCP was either mixed wfith TEM-1, CTXM-15, NDM-1 and OXA-1 (1 nM) or with PBS and a chemiluminescent enhancer and the increase in luminescence was measured over a period of 60 min. Figure 4 and Supporting Figure 1 demonstrates that CCP is a universal substrate. Figure 4a demonstrated that TEM-1 beta-lactamases generated an increase in luminescence over a 60 min period, under these conditions. In contrast, CCP incubated in PBS alone, did not generate any chemiluminescence and there was a 20-fold (relative chemiluminescence intensity at 30 min) enhancement in chemiluminescence signal between CCP+TEM-1 versus CCP in PBS alone.
Figure 4.
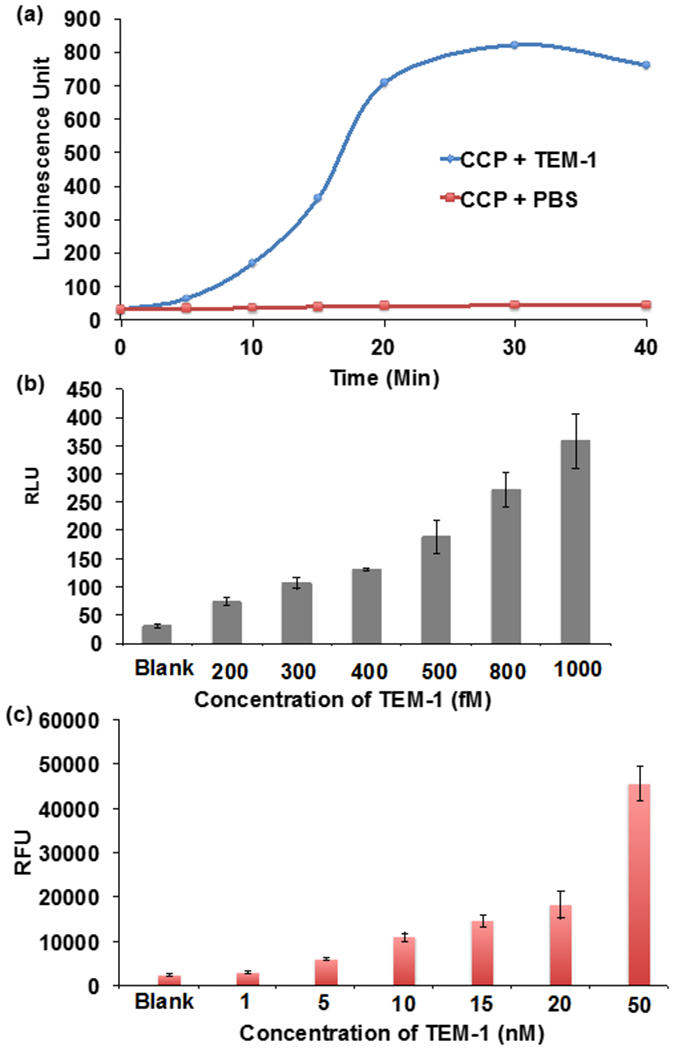
CCP is a beta-lactamase substrate and can detect low concentrations of beta-lactamase. (a) CCP (0.2 mM) was either mixed with TEM-1 (1 nM) beta-lactamase or PBS at RT and the chemiluminescence at 470 nm was measured over time. TEM-1 produces a 20-fold (relative chemiluminescence intensity at 30 min at 470 nm) higher chemiluminescence signal than CCP mixed with PBS. (b) CCP (0.2 mM) or (c) fluorocilin (0.2 mM) was mixed with various concentrations of TEM-1 beta lactamase at RT for 30 min. CCP has a detection sensitivity of 200 fM. It is 4-orders of magnitude more sensitive than fluorocilin.
The detection sensitivity of CCP is a key factor that will determine its potential use as a diagnostic for drug resistance. We therefore investigated the detection sensitivity of CCP and compared it against fluorocillin, a commercially available fluorescent probe for beta- lactamase. CCP or fluorocillin was mixed with various concentrations of TEM-1 and the resulting increase in signal was measured. Figure 4b demonstrates that CCP can detect TEM-1 with ultrasensitivity and could detect a 200 fM concentration of TEM-1. In contrast, Figure 4c demonstrates that fluorocillin could only detect a 5 nM concentration of beta-lactamase. CCP is therefore orders of magnitude more sensitive than fluorocillin at detecting TEM-1 and has the potential to accelerate the development of point of care assays for beta-lactamases.
We performed experiments to determine if CCP could distinguish between E.coli that is beta-lactamase positive from E.coli that is lactamase negative. 106 CFUs/mL of lactamase expressing E.coli lysates or wild type E.coli lysates were mixed with CCP and the resulting response in chemiluminescence was determined. Figure 5a demonstrates that CCP can distinguish beta-lactamase expressing E.coli from E.coli that are lactamase negative. For example, beta-lactamase expressing E.coli generated a 20-fold increase in chemiluminescence over E.coli that were lactamase negative. In addition, we determined the minimum number of bacteria that CCP could detect resistance from, to determine if it had potential as a clinical diagnostic. CCP was mixed with varying concentrations of bacteria and the resulting in increase in chemiluminescence was determined. Figure 5b demonstrates that CCP has a detection sensitivity of 105 CFUs/mL, which is the CFU level present in the majority of urinary tract infections (UTI). Additionally, we also investigated the detection sensitivity of fluorocillin (Supporting Figure 2) and compared it with the CCP based detection method. Fluorocillin had a detection sensitivity of 107 CFUs/mL, and CCP is therefore two orders of magnitude more sensitive than fluorocillin.
Figure 5.
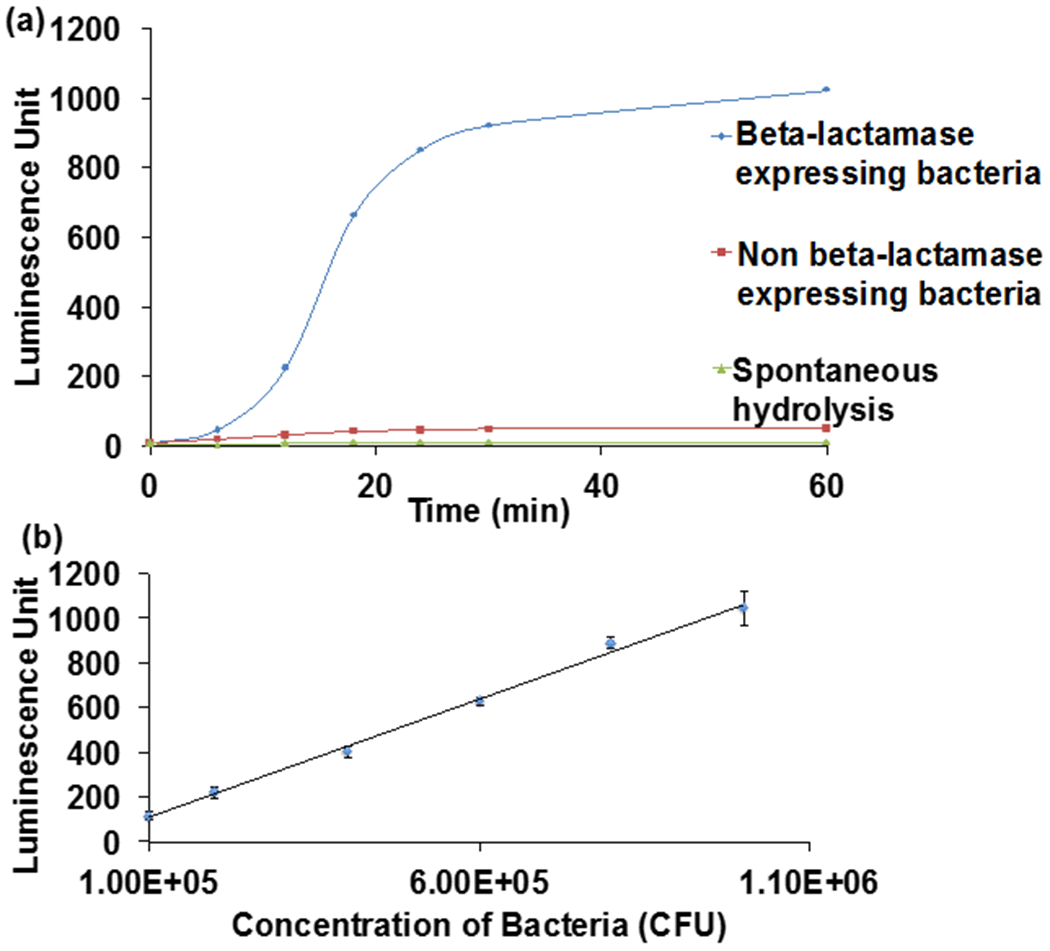
CCP can detect beta-lactamase activity from bacterial lysates. (a) CCP (0.2 mM) was mixed with lysates (88 μl of 106 CFU/mL) from beta-lactamase/non beta-lactamase expressing bacteria at RT for 30 min. Lysates from beta-lactamase expressing bacteria generate an 20 fold increase in chemiluminescence at 470 nm over lysates from non-beta lactamase expressing bacteria. (b) Various concentrations of bacteria (88 μL) were mixed with CCP (0.2 mM) at RT for 30 min and the detection sensitivity was determined. CCP has a detection sensitivity of 105 CFU/mL. (K12 E.coli)
We investigated if CCP could identify beta-lactamase from E.coli clinical isolates. The clinical isolates used in this study were collected from UTI patients and were genotyped to identify ampicillin susceptibility and resistance. Six susceptible and resistant strains (Supporting table 1) were used for this experiment. Bacteria at a concentration of 5 x 105 CFUs/mL were mixed with CCP and the relative luminescence was measured. We set a 100 units of luminescence as the cut-off point for identifying a sample as drug resistant, which was determined by taking the average chemiluminescence of the six ampicillin sucsceptible strains. Figure 6 demonstrates that CCP was able to identify 5/6 of the resistant strains, based on chemiluminescence. In addition, CCP was also able to identify 5/6 of the AmpC susceptible strains. CCP therefore has potential as a point care diagnostic for measuring bacterial drug resistance.
Figure 6.

CCP can detect ampicillin (AmpC) resistance from clinical bacterial samples. CCP (0.2 mM) was treated with 88 μL lysate of 5 x 105 CFUs/mL of either AmpC susceptible and resistant E.coli strains at RT for 30 min. CCP identified resistance in 5 out of 6 clinical samples. Resistance was confirmed by genotyping the bacteria.
Conclusions
In summary, we have developed a new chemical probe termed cephalosporin-chemiluminescent conjugate (CCP), which can detect beta-lactamase activity via chemiluminescence. CCP is 4-orders of magnitude more sensitive at detecting beta-lactamase than the fluorescent based probe fluorocillin. CCP was able to identify lactam resistance in E.coli clinical isolates and has great potential as diagnostic marker for drug resistance.
Supplementary Material
Acknowledgements
The authors acknowledge support from the NIH, grants NIH R21 AI119115-01, NIH R01AI117064, NIH R61DA048444-01.
Footnotes
Electronic Supplementary Information (ESI) available: [details of any supplementary information available should be included here]. See DOI: 10.1002/mrm.28246
Conflicts of interest
There are no conflicts to declare
Notes and references
- 1.Eiamphungporn W, Schaduangrat N and Malik AA, Nantasenamat C, Int J. Mol. Sci, 2018, 19, 2222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bush K, Antimicrobial Agents and Chemotherapy, 2018, 62, e01076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Majiduddin FK, Materon IC and Palzkil TG, Int. J. Medicinal Microbiol, 2002, 292, 127. [DOI] [PubMed] [Google Scholar]
- 4.Xing B, Khanamiryan A and Rao J, J. Am. Chem. Soc, 2005, 127, 4158. [DOI] [PubMed] [Google Scholar]
- 5.Gao W, Xing B, Tsien RY and Rao J, J. Am. Chem. Soc, 2003, 125, 11146. [DOI] [PubMed] [Google Scholar]
- 6.Campbell RE, Trends in Biotechnology, 2004, 22, P208. [DOI] [PubMed] [Google Scholar]
- 7.Mizukami S, Watanabe S, Hori Y and Kikuchi K, J. Am. Chem. Soc, 2009, 131, 5016. [DOI] [PubMed] [Google Scholar]
- 8.Galarneau A, Primeau M, Trudeau L-E and Michnick SW, Nat. Biotech, 2002, 20, 619. [DOI] [PubMed] [Google Scholar]
- 9.Kong Y, Yao H, Ren H, Subbian S, Cirillo SLG, Sacchettini JC, Rao J and Cirillo JD, Pro. Nat. Acad. Sci, 2010, 107, 12239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen Y, Xianyu Y, Wu J, Zheng W, Rao J and Jiang Anal X. Chem. 2016, 88, 5605. [DOI] [PubMed] [Google Scholar]
- 11.Xie HX, Mire J, Kong Y, Chang MH, Hassounah HA, Thornton CN, Sacchetini JC, Cirillo JD and Rao J, Nat. Chem, 2012, 4, 802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cheng YF, Xie HX, Sule P, Hassounah H, Graviss EA, Kong Y, Cirillo JD and Rao J, Angew Chem. Int. Ed, 2014, 53, 9360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bronstein I, Edwards B and Voyta JC, J. Biolumin. Chemilumin, 1989, 4, 99. [DOI] [PubMed] [Google Scholar]
- 14.Adam W, Bronstein I, Edwards B, Engel T, Reinherdt D, Schneider FW, Trofimov AV, Vasilev RF, J. Am. Chem. Soc, 1996, 118, 10400. [Google Scholar]
- 15.Richard J-A, Jean L, Romieu A, Massoneau M, Noak-Fraissings P, Renard P-Y, Org. Lett, 2007, 9 4853. [DOI] [PubMed] [Google Scholar]
- 16.Jain VK and Magrath IT, Analytical Biochem., 1991, 199, 119. [DOI] [PubMed] [Google Scholar]
- 17.Wetherall NT, Trivedi T, Zeller J, Hodges-Savola C, Mckimm-Breschkim JL, Zambon M and Heyden J FG. Clin. Microbiol 2003, 41, 742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Roth-Konforti M, Green O, Hupfeld M, Fieseler L, Heinrich N, Ihssen J, Vorberg R, Wick L, Spitz U and Shabat D, Angew. Chem. Int. Ed. 2019, 58, 10361. [DOI] [PubMed] [Google Scholar]
- 19.Son S, Won M, Green O, Hananya N, Sharma A, Jeon Y, Kwak JH, Sessler JL, Shabat D and Kim JS Angew Chem Int. Ed, 2019, 58, 1739. [DOI] [PubMed] [Google Scholar]
- 20.Bronstein I, Voyta JC, Thorpe GHG, Cricka LJ and Armstrong G Clin Chem 1989, 35, 1441. [PubMed] [Google Scholar]
- 21.Sabelle S, Renard PY, Pecorella K, de Suzzoni-Dezard S, Créminon C, Grassi J and Mioskowski C J. Am. Chem. Soc 2002, 124, 4874–4880. [DOI] [PubMed] [Google Scholar]
- 22.M da Fonseca L, Yavo B, Catalani LH, Falcao RP, Brunetti IL and Campa J A. Biolumin Chemilumin 1998, 13, 195. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


