Abstract
Papillary thyroid carcinoma (PTC), the most common malignancy of the endocrine system, is frequently driven by BRAFV600E mutation, which was reported in 35–60% cases in Western series. Numerous studies have recently emerged from Asian countries and regions; however sufficient summary is lacking to date. BRAF mutation serves as a diagnostic and prognostic tool in thyroid cancer, therefore establishing a rate of BRAF on the national scale could be of practical significance. We performed systematic reviews of available literature to investigate the prevalence of BRAF mutation in series of PTC from various Asian countries and regions. Out of the total 3,966 reports identified via initial screening, 138 studies encompassing over 40,000 PTCs were included for the final analysis. A vast majority (90.2%) of PTCs with known BRAF status were from East Asia, including China, South Korea, and Japan, with BRAF mutation rates of 71.2%, 75.5%, and 70.6%, respectively. Less abundant Indian and Saudi Arabian series found 45.6% and 46.3% prevalence of BRAFV600E in PTC, respectively. Much limited evidence was available from Thailand, Iran, Kazakhstan, Taiwan, Singapore, Indonesia, Hong Kong, Philippines, Vietnam, Iraq, and Myanmar. No relevant publications were found from other highly populated countries, such as Pakistan, Bangladesh, and Malaysia. After grouping by geographic region, we found that the highest rate of BRAFV600E was reported in the PTC series from East Asia (76.4%). Much lower rate (45–48%) was seen in PTC cohorts from South Asia, Central Asia, and the Middle East while the Southeast Asian series were in between (57%). Further subgroup analysis revealed that studies employing fresh frozen tissue and fine-needle aspirates showed higher rates of BRAF compared to those used formalin-fixed paraffin-embedded tissues. We found that the PTC series enrolled patients’ cohorts after 2010 demonstrated a higher rate of BRAF compared to the earlier series. Finally, pediatric PTCs had lower BRAF prevalence compared to the baseline rate for the country. In conclusion, despite considerable among and within countries heterogeneity, the Asian PTC series showed a higher prevalence of BRAFV600E mutation than that in Western series. Causes of geographic heterogeneity, whether genuine (etiology, genetics) or methodology-related should be further investigated.
Keywords: Asia, BRAFV600E, China, Japan, Korea, papillary thyroid carcinoma (PTC)
Introduction
Thyroid carcinoma is the most common endocrine malignancy that represents almost 2.1% of newly diagnosed cancer cases (1). Papillary thyroid carcinoma (PTC) is the most common histological type of differentiated thyroid carcinoma accounting for 75–85% cases, often characterized by low mortality rate and good response to radioiodine therapy (2). The 10-year overall survival rate for differentiated types of thyroid carcinoma is exceeding more than 90% (1).
Different types of thyroid cancer are characterized by different gene alterations. PTC development is closely linked to somatic point mutations in BRAF and rearrangements in RET/PTC1, RET/PTC3 and NTRK1/3 genes (2). Interestingly, driver gene aberrations in well-differentiated thyroid cancer are mutually exclusive. Rearranged during transfection (RET) gene encodes a single transmembrane tyrosine kinase receptor (3). RET/PTC fusions seem to be important in the early pathogenesis of PTC. These fusions are involved in development of 10–20% of PTC either it is sporadic or radiation-induced PTC (4,5). Other genetic alterations like RAS and PAX/PPARG are less involved in development of conventional PTC but more often associated with follicular thyroid carcinoma and follicular variant PTC (FVPTC) (2).
B-type Raf kinase (BRAF) proto-oncogene is the most common molecular target in PTC. BRAFV600E mutation activates the protein kinase domain of BRAF that results in constitutive initiation of mitogen-activated protein kinase pathway, which in turn promotes cell growth and proliferation (5,6). Up to 10–15% of all human cancers are reported to have BRAF mutation, with a high prevalence in PTC and melanoma (7). BRAFV600E is detected in about half of PTC cases, and may have higher rates in certain populations and histological types (2,5). In thyroid carcinoma, BRAFV600E mutation has been shown to be associated with high risk clinicopathological characteristics, tumor recurrence, metastasis, and reduced sensitivity to radioiodine therapy (8,9). Recently, BRAFV600E was introduced in the clinical guidelines to aid risk stratification of PTC (10).
Since BRAF mutation may serve as a diagnostic and prognostic tool, establishing a rate of BRAF on the national scale is of practical significance. Western countries have extensively reported on BRAFV600E mutation rates in the past decade. In particular, the prevalence of BRAFV600E mutation in the USA and Europe was ranging 35–60% (11-13). Numerous studies have recently emerged from Asian countries and regions; however, these were not sufficiently summarized to date except for showing overall high BRAFV600E mutation prevalence in Asian PTC compared to that in Western series (14,15).
Therefore, we performed a systematic review of available literature to investigate the prevalence of BRAF mutation in series of PTC originated from various Asian countries and regions.
Methods
Search strategy
We conducted a search within three electronic databases available for all coauthors (PubMed, Google Scholar, and Scopus). Relevant articles were identified using the following combination of keywords: PTC (or cancer) and BRAF combined with the name of each Asian country. The latter was searched in the authors' affiliation. Transcontinental countries, such as Russia and Turkey were disregarded. An additional manual search was done by screening references within included publications. Furthermore, if the search in the above databases was not successful, relevant local publications were queried from the members of the Asian Working Group in Thyroid Pathology (16). We followed the recommendations of Preferred Reporting Items for Systematic Review and Meta-analysis (PRISMA) statement (17).
Data extraction
Results of the search from all sources were imported into EndNote reference manager (Thompson Reuters, New York, NY) and two reviewers (F. R. and J. M.) independently screened the abstracts. Upon mutual agreement on eligibility of the study, both reviewers independently extracted data as per the predefined data collection sheet. The only target of our review was BRAFV600E, therefore, other BRAF mutations were excluded from the analysis. The following information was extracted: first author, year of publication, name of institution, city, country/region, tissue type used for nucleic acid extraction, technique to detect BRAF mutation, total number of PTCs, and number of cases positive for BRAF. Therefore, clear indication of all these parameters either in the abstract or full text was qualified as inclusion criteria for our systematic review. Additional information, such as histological type of PTC, age of the cohort (e.g., pediatric), and years where study cohort belongs to, was optional but not mandatory. Baseline and clinicopathological characteristics of the patients except specified above were not required. In addition, two reviewers were asked to score and record the quality and risk of bias of the included studies. Any discrepancies during data extraction were resolved after consultation with a supervisor (A. B.) of the study. Studies not qualified as per inclusion criteria were disregarded. More exclusion criteria were as follows: (I) less than 10 cases enrolled; (II) non-primary PTC (regional and distant metastases) only and thyroid tumors other than PTC; (III) experimental and animal studies, (IV) duplicated cohorts. The latter were decided based on the overlapping of the institution name and study cohort in several publications. If potential overlap was found, a study with the largest sample size was selected.
Statistics
Descriptive statistics were calculated with Microsoft Office Excel 2010 (Microsoft, CA, USA). Further statistical analysis was performed with SPSS 23.0 statistical software package (SPSS, Chicago, IL, USA). A χ2 test with Yates’s correction was applied for subgroup analysis. P value of less than 0.05 was considered to be statistically significant.
Results
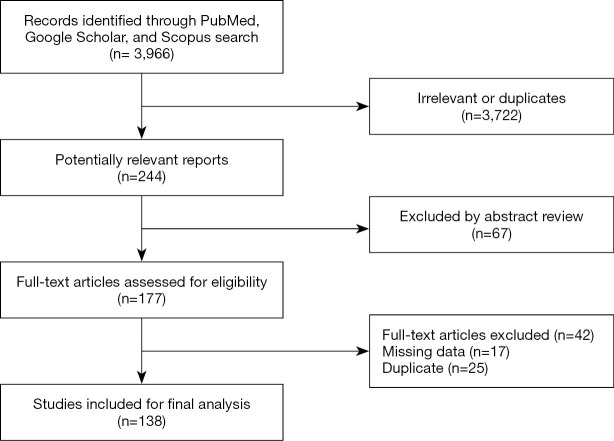
Out of the total 3,966 studies identified via search in electronic libraries, only 244 met our inclusion criteria and were selected for further evaluation. Finally, after reading abstracts and full texts, 138 studies were qualified as eligible for data extraction. A flow chart of the data selection process is shown in Figure 1.
Figure 1.
The PRISMA flowchart of study selection.
Asian studies classified by country and geographical region
We further separated 3 studies from Japan, China, and Saudi Arabia (18-20) in a subgroup, composed exclusively of pediatric PTCs, and focused on 135 adult or unselected series from Asian countries and regions including 40,371 PTCs. The largest datasets were provided by Japan (Table 1), South Korea (Table 2), China (Table 3), India (Table 4), and Saudi Arabia (Table 5). Summary of all 135 publications stratified by country and region of origin is shown in Table 6.
Table 1. Characteristics of included studies from Japan.
| ## | Author | Year | Institution | City | Study cohort | Tissue type | Technique | Total PTC | BRAF+ | BRAF rate (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Kondo T (21) | 2007 | University of Yamanashi | Yamanashi | n/s | FFPE | Seq | 31 | 13 | 42 |
| 2 | Kumagai A (22) | 2007 | Nagasaki University Hospital | Nagasaki | 2003–2006 | FNA | PCR-RFLP | 14 | 12 | 86 |
| 3 | Takahashi K (23) | 2007 | Radiation Effects Research Foundation | Hiroshima | 2003–2005 | FFPE | RFLP | 64 | 38 | 59 |
| 4 | Matsuse M (24) | 2011 | Kuma Hospital | Kobe | n/s | FFPE | Seq | 492 | 388 | 79 |
| 5 | Mitsutake N (18) | 2015 | Fukushima Medical University | Fukushima | 2013–2014 | Fresh frozen | Seq | 67 | 43 | 64 |
| 6 | Xing M (25) | 2015 | Kanagawa Cancer Center | Yokohama | n/s | Fresh frozen | Seq | 49 | 33 | 67 |
| 7 | Nasirden A (26) | 2016 | Juntendo University Hospital | Tokyo | 2009, 2017 | FFPE | Seq | 144 | 53 | 37 |
| 8 | Vuong HG (27) | 2016 | Yamanashi Hospital | Yamanashi | 2011–2014 | FFPE | AS-PCR | 67 | 55 | 82 |
| 9 | Oishi N (28) | 2017 | University of Yamanashi + Kuma Hospital | Yamanashi, Kobe | 1991–2013 | FFPE | AS-PCR + Seq | 172 | 121 | 70 |
| 10 | Bandoh N (29) | 2018 | Hokuto Hospital | Obihiro | 2014–2016 | FFPE | Seq | 34 | 27 | 79 |
AS-PCR, allele specific-polymerase chain reaction; FFPE, formalin fixed paraffin embedded tissues; FNA, fine needle aspiration; n/s, not specified; PCR-RFLP, polymerase chain reaction-restriction fragment length polymorphism; Seq, sequencing.
Table 2. Characteristics of included studies from South Korea.
| ## | Author | Year | Institution | City | Study cohort | Tissue type | Technique | Total PTC | BRAF+ | BRAF rate (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Kim KH (30) | 2005 | Eulji University Hospital, Chungnam National University Hospital | Daejeon | 2000–2003 | FFPE | PCR | 79 | 64 | 81 |
| 2 | Kim TY (31) | 2005 | Asan Medical Center | Seoul | 1997–2001 | FFPE | PCR | 60 | 31 | 52 |
| 3 | Jo YS (32) | 2006 | Chungnam National University Hospital | Daejeon | 2004–2005 | FFPE | IHC + Seq | 161 | 102 | 63 |
| 4 | Kim TY (33) | 2006 | Asan Medical Center | Seoul | n/s | FFPE | PCR + Seq | 203 | 149 | 73 |
| 5 | Lee JH (34) | 2006 | Korea University Guro Hospital | Seoul | 2000–2003 | FFPE | PCR | 100 | 58 | 58 |
| 6 | Park SY (35) | 2006 | Seoul National University Bundang Hospital | Seongnam | 2003–2005 | FFPE | PCR + Seq | 61 | 53 | 87 |
| 7 | Kim SK (36) | 2009 | Konkuk University Hospital | Seoul | 2005–2006 | FNA | Pyroseq | 101 | 88 | 87 |
| 8 | Kwak JY (37) | 2009 | Severance Hospital | Seoul | 2008 | FNA | PCR + Seq | 339 | 213 | 63 |
| 9 | Kim JH (38) | 2010 | Kosin University College of Medicine | Busan | 2007–2009 | Fresh frozen | Seq | 109 | 35 | 32 |
| 10 | Kim SW (39) | 2010 | Samsung Medical Center | Seoul | n/s | FNA | PCR + Seq | 263 | 221 | 84 |
| 11 | Lee HJ (40) | 2010 | Asan Medical Center | Seoul | 2008 | FNA | PCR, Seq, Pyroseq | 52 | 47 | 90 |
| 12 | Park YJ (41) | 2010 | Seoul National University Hospital | Seoul | 1983–2004 | FFPE | PCR | 230 | 153 | 67 |
| 13 | Ahn D (42) | 2012 | Kyungpook National University Hospital | Daegu | 2010 | FFPE | multiplex PCR | 107 | 85 | 79 |
| 14 | Chang H (43) | 2012 | Korea University Guro Hospital | Seoul | 2008–2009 | FNA | Seq + MCA | 126 | 96 | 76 |
| 15 | Joo JY (44) | 2012 | Chungnam National University | Daejeon | 2009–2011 | FNA | Seq | 148 | 79 | 53 |
| 16 | Kim SJ (45) | 2012 | Seoul National University Hospital | Seoul | 2009–2010 | FFPE | PCR + Seq | 547 | 381 | 70 |
| 17 | Moon WJ (46) | 2012 | Konkuk University Medical Center | Seoul | 2006–2008 | FNA | PCR+Seq | 164 | 140 | 85 |
| 18 | Choi SY (47) | 2013 | Dong-A Medical Center | Busan | 2011–2012 | FFPE | qPCR | 101 | 72 | 71 |
| 19 | Jeong D (48) | 2013 | Soonchunhyang University College of Medicine | Cheonan | n/s | FFPE | qPCR | 211 | 189 | 90 |
| 20 | Kang KH (49) | 2013 | Seoul National University Hospital | Seoul | n/s | Fresh frozen | PCR | 46 | 37 | 80 |
| 21 | Lim JY (50) | 2013 | Severance Hospital | Seoul | 2009–2012 | FFPE | RFLP + Seq | 3130 | 2313 | 74 |
| 22 | Min HS (51) | 2013 | Seoul National University Hospital | Seoul | 2009 | FFPE | Seq + IHC | 255 | 179 | 70 |
| 23 | Chai YJ (52) | 2014 | Seoul National University Hospital | Seoul | 2009–2013 | FFPE | Seq | 137 | 35 | 26 |
| 24 | Han SA (53) | 2014 | Kyung Hee University Hospital | Seoul | 2010–2012 | FFPE | qPCR | 499 | 353 | 71 |
| 25 | Hong AR (54) | 2014 | Seoul National University Hospital | Seoul | 1995–2003, 2009–2012 | FFPE | RFLP + Seq | 2624 | 1912 | 73 |
| 26 | Jung YY (55) | 2015 | Chung-Ang University Hospital | Seoul | 2011–2012 | FFPE | IHC + qPCR, RNA FISH | 467 | 402 | 86 |
| 27 | Lee SR (56) | 2015 | Ajou University | Suwon | 2012 | FFPE | IHC+ PCR, Seq | 163 | 143 | 88 |
| 28 | Na JI (57) | 2015 | Chonnam National University Hospital | Gwang-ju | 2005–2013 | FFPE | IHC + qPCR, Seq | 104 | 71 | 68 |
| 29 | Xing M (25) | 2015 | Ulsan University Hospital | Ulsan | n/s | FFPE | Seq | 197 | 144 | 73 |
| 30 | Kim SK (58) | 2016 | Samsung Medical Center | Seoul | 2008–2012 | FNA + FFPE | PCR + Seq | 3107 | 2530 | 81 |
| 31 | Kim S (59) | 2017 | Ajou University | Suwon | 2009–2013 | FFPE | PCR | 1503 | 1171 | 78 |
| 32 | Lee SE (60) | 2017 | Konkuk University Medical Center | Seoul | 2010–2014 | FNA | Pyroseq | 769 | 625 | 81 |
| 33 | Yeo MK (61) | 2017 | Chungnam National University School of Medicine | Daejeon | 2010 | FFPE | qPCR | 99 | 88 | 89 |
| 34 | Kim H (62) | 2018 | Pusan National University Hospital | Busan | 2011–2012 | FFPE | PCR | 1411 | 861 | 61 |
| 35 | Kim HJ (63) | 2018 | Samsung Medical Center | Seoul | 2010–2015 | FNA | PCR + Seq | 215 | 173 | 80 |
| 36 | Kim JK (64) | 2018 | Seoul National University Hospital | Seoul | 2013–2016 | FFPE | IHC + Seq | 697 | 627 | 90 |
| 37 | Oh HS (65) | 2018 | Asan Medical Center | Seoul | 2011–2013 | FFPE | Seq | 62 | 57 | 92 |
| 38 | Lee SM (66) | 2019 | Severance Hospital | Seoul | 2011–2012 | FNA | qPCR | 911 | 717 | 79 |
| 39 | Choden S (67) | 2020 | St. Mary’s Hospital | Seoul | 2008–2010 | FFPE | IHC + Seq | 514 | 436 | 85 |
| 40 | Yoon JH (68) | 2020 | Severance Hospital | Seoul | 2015–2017 | FFPE | PCR | 527 | 428 | 81 |
AS-PCR, allele specific-polymerase chain reaction; FFPE, formalin fixed paraffin embedded tissues; FISH, fluorescence in situ hybridization; FNA, fine needle aspiration; IHC, immunohistochemistry; MCA, melting curve analysis; n/s, not specified; PCR, polymerase chain reaction; PCR-RFLP, polymerase chain reaction-restriction fragment length polymorphism; Pyroseq, pyrosequencing; qPCR, quantitative polymerase chain reaction; Seq, sequencing.
Table 3. Characteristics of included studies from China.
| ## | Author | Year | Institution | City | Study cohort | Tissue type | Technique | Total PTC | BRAF+ | BRAF rate (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Gu LQ (69) | 2009 | Shanghai Jiaotong University School of Medicine + Yueqing People’s Hospital | Shanghai, Zhejiang | n/s | FFPE | PCR | 123 | 42 | 34 |
| 2 | Guan H (70) | 2009 | multisite | Shenyang, Shanghai, Binzhou, Heze, Qingdao | 1990–2007 | FFPE | Seq | 1032 | 639 | 62 |
| 3 | Feng L (71) | 2011 | Dalian Medical University | Dalian | 2006–2007 | FFPE | IHC | 70 | 42 | 60 |
| 4 | Wang W (72) | 2012 | The First Affiliated Hospital, Zhejiang University School of Medicine | Hangzhou | 2006–2008 | FFPE | PCR + Seq | 208 | 115 | 55 |
| 5 | Xia T (73) | 2012 | Affiliated Tumor Hospital of Tianjin Medical University | Tianjin | 2011 | Fresh frozen | PCR + Seq | 110 | 69 | 63 |
| 6 | Zheng X (74) | 2012 | Tianjin Medical University Cancer Institute and Hospital | Tianjin | 1995–2000 | FFPE | PCR | 512 | 263 | 51 |
| 7 | Zhou YL (75) | 2012 | First Affiliated Hospital of Wenzhou Medical College | Wenzhou | 2010–2011 | FNA | PCR | 100 | 31 | 31 |
| 8 | Gong RX (76) | 2013 | West China Hospital, Sichuan University | Chengdu | 2009–2011 | FFPE | PCR | 187 | 119 | 64 |
| 9 | Huang Y (77) | 2013 | The First Affiliated Hospital, Sun Yat-Sen University | Guangzhou | 2008–2010 | Fresh frozen | PCR | 69 | 33 | 48 |
| 10 | Guo HQ (78) | 2014 | Chinese Academy of Medical Science | Beijing | 2010–2011 | FNA | PCR | 63 | 41 | 65 |
| 11 | He G (79) | 2014 | West China Hospital, Sichuan University | Sichuan | 2009–2011 | FFPE | PCR | 187 | 119 | 64 |
| 12 | Huang FJ (80) | 2014 | Shanghai Jiaotong University | Shanghai | 2009–2011 | Fresh frozen | Seq | 214 | 147 | 69 |
| 13 | Liu S (81) | 2014 | Xian Jiaotong University Health Science Center | Xian | 2011–2014 | FNA | Pyroseq | 132 | 80 | 61 |
| 14 | Liu X (82) | 2014 | multisite | Shanghai, Shenyang, Qingdao, Heza, Binzhou | n/s | FFPE | PCR | 408 | 250 | 61 |
| 15 | Lu H (83) | 2014 | Chinese Academy of Medical Science | Beijing | 2010–2012 | FFPE | PCR + Seq | 292 | 190 | 65 |
| 16 | Shao H (84) | 2014 | Heze Municipal Hospital | Shandong | 2002–2006 | FFPE | Seq | 200 | 133 | 67 |
| 17 | Wei X (85) | 2014 | Tianjin Cancer Hospital | Tianjin | 2011–2013 | FFPE | IHC | 369 | 297 | 80 |
| 18 | Lu J (86) | 2015 | Peking Union Medical College Hospital (PUMCH) | Beijing | 2013–2014 | FFPE | ARMS PCR + qPCR | 150 | 121 | 81 |
| 19 | Qiu T (87) | 2015 | Chinese Academy of Medical Sciences | Beijing | 2010–2014 | FFPE | IHC + Seq | 127 | 102 | 80 |
| 20 | Shi C (88) | 2015 | Second Affiliated Hospital of Harbin Medical University | Harbin | n/s | FFPE | qPCR | 126 | 87 | 69 |
| 21 | Sun J (89) | 2015 | Peking Union Medical College Hospital (PUMCH) | Beijing | 2010–2012 | FFPE | IHC + Seq | 556 | 419 | 75 |
| 22 | Yang LB (90) | 2015 | West China Hospital | Sichuan | 2013–2014 | FFPE | Seq | 543 | 170 | 31 |
| 23 | Yu L (91) | 2015 | Hangzhou First Peoples Hospital | Hangzhou | 2012–2013 | Fresh frozen | PCR+ Seq | 65 | 40 | 62 |
| 24 | Zhao H (92) | 2015 | Chinese Academy of Medical Sciences | Beijing | 2010–2012 | FNA | PCR | 170 | 114 | 67 |
| 25 | Jin L (93) | 2016 | Wenzhou Medical University | Wenzhou | 2009–2014 | FFPE | Seq | 653 | 416 | 64 |
| 26 | Sun J (94) | 2016 | Peking Union Medical College Hospital (PUMCH) | Beijing | 2010–2013 | FFPE | Seq | 455 | 343 | 75 |
| 27 | Wen H (95) | 2016 | XinJiang Medical University | Urumqi | 2007–2011 | FFPE | Seq | 26 | 19 | 73 |
| 28 | Zhang B (96) | 2016 | Affiliated Hospital of the Academy of Military Medical Sciences | Beijing | 2011–2014 | FFPE | ARMS qPCR | 120 | 106 | 88 |
| 29 | Zheng L (97) | 2016 | First Affiliated Hospital of Anhui Medical University | Hefei | 2009–2012 | FFPE | PCR + Seq | 60 | 40 | 67 |
| 30 | Geng J (19) | 2017 | Beijing Children’s Hospital | Beijing | 1994–2014 | FFPE | qPCR | 48 | 17 | 35 |
| 31 | Li Q (98) | 2017 | Affiliated Cancer Hospital of Zhengzhou University | Zhengzhou | n/s | Fresh frozen | PCR + Seq | 34 | 18 | 53 |
| 32 | Zhang Q (99) | 2017 | Shanghai Tenth People’s Hospital of Tongji University School | Shanghai | 2015–2016 | FFPE | PCR | 438 | 379 | 87 |
| 33 | Guan Q (100) | 2018 | Fudan University Shanghai Cancer Center | Shanghai | 2012–2013 | FFPE | qPCR + Seq | 99 | 63 | 64 |
| 34 | Huang L (101) | 2018 | Wuhan Puai Hospital | Wuhan | 2010–2016 | FFPE | qPCR | 184 | 140 | 76 |
| 35 | Liang J (102) | 2018 | Beijing Cancer Hospital | Beijing | n/s | FFPE | DNA, RNA Seq | 355 | 257 | 72 |
| 36 | Liu Z (103) | 2018 | Shanghai Jiaotong University School of Medicine | Shanghai | 2016 | Fresh frozen | PCR + Seq | 145 | 81 | 56 |
| 37 | Ren H (104) | 2018 | Chongqing Medical University | Chongqing | 2016–2017 | Fresh frozen | Seq | 342 | 270 | 79 |
| 38 | Zheng B (105) | 2018 | Guangzhou Kingmed Diagnostics | Guangzhou | 2014–2016 | FNA | PCR | 55 | 37 | 67 |
| 39 | Zhou D (106) | 2018 | Inner Mongolia Peoples’ Hospital | Hohhot | 2016–2017 | FFPE | PCR | 50 | 37 | 74 |
| 40 | Chen B (107) | 2019 | Hospital of Jiangsu University | Jiangsu | 2014–2017 | FFPE | Seq | 116 | 70 | 60 |
| 41 | Gao J (108) | 2020 | The First Affiliated Hospital of USTC | Hefei | 2017–2018 | FFPE | ARMS qPCR | 60 | 39 | 65 |
| 42 | Huang M (109) | 2019 | Xijing Hospital | Xian | 2018–2019 | FFPE | Seq | 483 | 419 | 87 |
| 43 | Ji W (110) | 2019 | Beijing Shijitan Hospital | Beijing | 2012–2015 | FFPE | ARMS qPCR | 89 | 67 | 75 |
| 44 | Li X (111) | 2019 | Renji Hospital, Shanghai Jiaotong University | Shanghai | 2016–2018 | FNA | ARMS PCR | 777 | 674 | 87 |
| 45 | Li XJ (112) | 2019 | Jiangsu Province Hospital | Nanjing | 2016–2018 | FNA | qPCR | 333 | 304 | 91 |
| 46 | Lin ZM (113) | 2019 | Second Affiliated Hospital, Zhejiang University School of Medicine | Zhejiang | 2016–2018 | FNA | PCR | 1199 | 791 | 66 |
| 47 | Liu Y (114) | 2019 | Chongqing Medical University | Chongqing | 2016–2018 | FNA | PCR | 207 | 155 | 75 |
| 48 | Shen G (115) | 2019 | West China Hospital of Sichuan University | Chengdu | 2012–2015 | FFPE | PCR | 236 | 147 | 62 |
| 49 | Wang J (116) | 2019 | Beijing Hospital | Beijing | 2015–2018 | FFPE | qPCR | 444 | 384 | 86 |
| 50 | Yan C (117) | 2019 | Xijing Hospital | Shaanxi | 2015–2018 | FFPE | PCR | 2048 | 1715 | 84 |
| 51 | Yang T (118) | 2019 | West China Hospital, Sichuan University | Chengdu | n/s | FFPE | Seq | 326 | 269 | 83 |
| 52 | Zhou C (119) | 2019 | Liaocheng People's Hospital | Liaocheng | 2015–2016 | FFPE | qPCR | 162 | 135 | 83 |
ARMS-PCR, amplification refractory mutation system-polymerase chain reaction; FFPE, formalin fixed paraffin embedded tissues; FNA, fine needle aspiration; IHC, immunohistochemistry; n/s, not specified; PCR-RFLP, polymerase chain reaction-restriction fragment length polymorphism; Pyroseq, pyrosequencing; qPCR, quantitative polymerase chain reaction; Seq, sequencing.
Table 4. Characteristics of included studies from India.
| ## | Author | Year | Institution | City | Study cohort | Tissue type | Technique | Total PTC | BRAF+ | BRAF rate (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Chakraborty A (120) | 2012 | Tata Memorial Hospital | Mumbai | 2002–2006 | Fresh frozen | PCR + Seq | 86 | 46 | 53 |
| 2 | Khan MS (121) | 2014 | Sher-I-Kashmir Institute of Medical Sciences | Srinagar | 2010–2012 | FFPE | PCR | 42 | 15 | 36 |
| 3 | Agarwal S (122) | 2016 | All India Institute of Medical Sciences | New Delhi | 2015–2016 | FFPE | Seq | 40 | 19 | 48 |
| 4 | Nair CG (123) | 2017 | Amrita Institute of Medical Sciences | Kochi | 2012 | FFPE | PCR | 59 | 30 | 51 |
| 5 | Ahmad F (124) | 2018 | Research and Development Division of SRL | Mumbai | n/s | FFPE | PCR + Seq | 70 | 35 | 50 |
| 6 | Fonseca D (125) | 2018 | Basavatarakam Indo American Cancer Hospital | Telangana | 2015–2018 | FFPE | IHC | 23 | 11 | 48 |
| 7 | George N (126) | 2018 | Sanjay Gandhi Postgraduate Institute | Lucknow | 2000–2014 | FFPE | PCR | 109 | 56 | 51 |
| 8 | Hemalatha R (127) | 2018 | Christian Medical College | Vellore | n/s | FNA | Seq | 53 | 19 | 36 |
| 9 | Krishnamurthy A (128) | 2018 | Cancer Institute (WIA) | Chennai | 2005–2006 | FFPE | IHC + qPCR | 79 | 25 | 32 |
IHC, immunohistochemistry; FFPE, formalin fixed paraffin embedded tissues; FNA, fine needle aspiration; n/s, not specified; qPCR, quantitative polymerase chain reaction; Seq, sequencing
Table 5. Characteristics of included studies from other Asian countries and regions.
| Country/region | ## | Author | Year | Institution | City | Study cohort | Tissue type | Technique | Total PTC | BRAF+ | BRAF rate (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Saudi Arabia | 1 | Abubaker J (129) | 2008 | King Faisal Specialist Hospital | Riyadh | 1988–2004 | FFPE | Seq | 296 | 153 | 52 |
| 2 | Schulten HJ (130) | 2012 | King Abdulaziz University + King Faisal Specialist Hospital | Jeddah | 1995–2011 | FFPE | PCR, Seq | 213 | 87 | 41 | |
| 3 | Zou M (131) | 2014 | King Faisal Specialist Hospital | Riyadh | 1987–2006 | Fresh frozen | Seq | 88 | 42 | 48 | |
| 4 | Qasem E (132) | 2015 | King Faisal Specialist Hospital | Riyadh | 2008–2011 | FFPE | Seq | 243 | 105 | 43 | |
| 5 | Murugan AK (133) | 2016 | King Faisal Specialist Hospital | Riyadh | n/s | FFPE | Seq | 201 | 95 | 47 | |
| 6 | Alzahrani AS (20) | 2017 | King Faisal Specialist Hospital | Riyadh | 1998–2015 | FFPE | Seq | 79 | 19 | 24 | |
| Iran | 1 | Mohammadi-Asl J (134) | 2009 | Tehran University of Medical Science | Tehran | 2007–2008 | FFPE | PCR-RFLP | 28 | 20 | 71 |
| 2 | Ranjbari N (135) | 2013 | Imam Khomeini Hospital | Ahvaz | 2000–2010 | FFPE | PCR-RFLP | 63 | 49 | 78 | |
| 3 | Daliri M (136) | 2014 | Ghaem Hospital | Mashhad | 1999–2014 | FFPE | PCR-RFLP | 69 | 28 | 41 | |
| 4 | Zarkesh M (137) | 2018 | Erfan Hopital + Atiyeh Hospital | Tehran | 2015–2016 | Fresh frozen | Seq | 60 | 24 | 40 | |
| 5 | Ghasemi M (138) | 2019 | Khalili Hospital | Shiraz | 2012–2017 | FFPE | PCR-RFLP | 79 | 65 | 82 | |
| Iraq | 1 | Salih A (139) | 2017 | Duhok Private Medical Laboratory + Vin Private Medical laboratory | Duhok | 2011–2015 | FFPE | qPCR | 47 | 12 | 26 |
| Kazakhstan | 1 | Kumagai A (22) | 2007 | Medical Institute of Semipalatinsk | Semipalatinsk | 2004–2006 | FNA | PCR-RFLP | 76 | 19 | 25 |
| 2 | Tlegenov AS (140) | 2018 | Kazakh Scientific Research Institute of Oncology and Radiology | Almaty | 2016–2017 | Fresh frozen | IHC | 92 | 62 | 67 | |
| Myanmar | 1 | Than MM (141) | 2017 | Yangon University of Medicine | Yangon | 2014–2016 | FFPE | PCR | 44 | 10 | 23 |
| Thailand | 1 | Choden S (142) | 2020 | Chulalongkorn University | Bangkok | 2007–2017 | FFPE | IHC | 476 | 290 | 61 |
| Vietnam | 1 | Vuong HG (27) | 2016 | Cho Ray Hospital | Ho Chi Minh | 2011–2014 | FFPE | AS-PCR | 53 | 44 | 83 |
| Indonesia | 1 | Brahma B (143) | 2013 | Mangunkusumo Hospital Medical Faculty University | Jakarta | 2010–2011 | FNA | PCR RFLP | 44 | 17 | 39 |
| 2 | Kristiani E (144) | 2016 | Siloam Hospitals Lippo Village | Tangerang | n/s | FFPE | IHC | 50 | 17 | 34 | |
| Singapore | 1 | Yang P (145) | 2015 | Singapore National University Hospital | Singapore | n/s | FFPE | IHC | 49 | 39 | 80 |
| 2 | Goh X (146) | 2019 | Singapore National University Hospital | Singapore | 2010–2012 | FFPE | Seq | 75 | 42 | 56 | |
| Taiwan | 1 | Liu RT (147) | 2005 | Chang Gung Memorial Hospital | Kaohsiung | 1997–2002 | FFPE | Seq | 105 | 49 | 47 |
| 2 | Chang YS (148) | 2013 | China Medical University Hospital | Taichung | n/s | Fresh frozen | Seq | 52 | 32 | 62 | |
| Hong Kong | 1 | Lo CC (149) | 2004 | University of Hong Kong | Hong Kong | 2001–2003 | FFPE | Seq | 34 | 17 | 50 |
| 2 | Law Y (150) | 2009 | The Hong Kong Polytechnic University | Hong Kong | n/s | FFPE | PCR RFLP | 50 | 24 | 48 | |
| Philippines | 1 | Navarro-Locsin CG (151) | 2016 | St. Luke’s Medical Center | Quezon City | 2010–2012 | FFPE | Seq + qPCR | 65 | 25 | 38 |
| 2 | Espiritu GAM (152) | 2019 | Makati Medical Center | Makati | 2016 | FFPE | Seq | 17 | 12 | 71 |
AS-PCR, allele specific-polymerase chain reaction; FFPE, formalin fixed paraffin embedded tissues; FNA, fine needle aspiration; IHC, immunohistochemistry; n/s, not specified; PCR-RFLP, polymerase chain reaction-restriction fragment length polymorphism; qPCR, quantitative polymerase chain reaction; Seq, sequencing
Table 6. Summary on BRAF rate in PTC from Asian countries and regions.
| Region | Country/region | No. of studies | Year of publication | Total PTC* | BRAF-positive | BRAF rate (%) |
|---|---|---|---|---|---|---|
| East Asia | Japan | 9 | 2007–2018 | 986 | 696 | 71 |
| South Korea | 40 | 2005–2020 | 20599 | 15558 | 76 | |
| China | 51 | 2009–2019 | 15509 | 11038 | 71 | |
| Taiwan | 2 | 2005, 2013 | 157 | 81 | 52 | |
| Hong Kong | 2 | 2004, 2009 | 84 | 41 | 49 | |
| South Asia | India | 9 | 2012–2018 | 561 | 256 | 46 |
| Central Asia | Kazakhstan | 2 | 2007, 2018 | 168 | 81 | 48 |
| Middle East | Saudi Arabia | 5 | 2008–2017 | 1041 | 482 | 46 |
| Iran | 5 | 2008–2017 | 346 | 198 | 57 | |
| Iraq | 1 | 2017 | 47 | 12 | 26 | |
| Southeast Asia | Myanmar | 1 | 2017 | 44 | 10 | 23 |
| Thailand | 1 | 2020 | 476 | 290 | 61 | |
| Vietnam | 1 | 2016 | 53 | 44 | 83 | |
| Indonesia | 2 | 2013, 2016 | 94 | 34 | 36 | |
| Singapore | 2 | 2015, 2019 | 124 | 81 | 65 | |
| The Philippines | 2 | 2016, 2019 | 82 | 37 | 45 |
*, pediatric cases were excluded if indicated in the original studies (one series from Japan, China, and Saudi Arabia). PTC, papillary thyroid carcinoma.
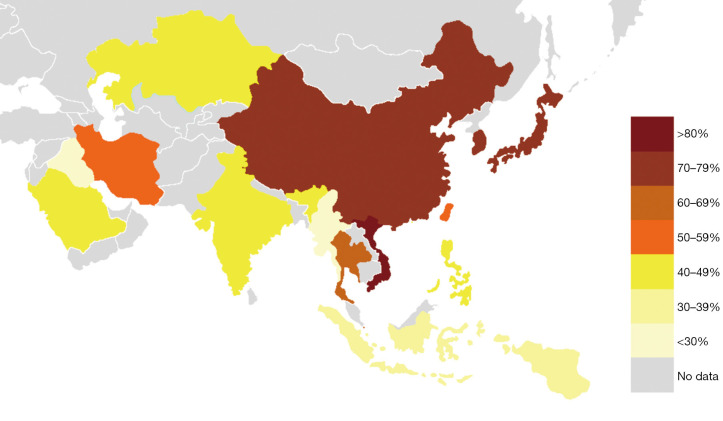
Nine Japanese studies included 986 PTCs with an average BRAF rate 70.6% (Table 1). Korean series were more extensive, encompassing 20,599 PTCs in 40 studies with resultant BRAF prevalence 75.5% (Table 2). The largest number of reports were from China (n=51; 15,509 PTCs), which showed 71.2% BRAF rate (Table 3). In contrast to the countries above, Indian institutions started to report their findings quite recently, so far providing results on 561 PTCs from 9 centers across the country, averaging 45.6% prevalence of BRAFV600E mutation (Table 4). Five studies from Saudi Arabia (mean BRAF rate 46.3%) along with less abundant reports from other Asian countries and regions, including Iran, Iraq, Kazakhstan, Myanmar, Thailand, Vietnam, Indonesia, Singapore, Taiwan, Hong Kong, and Philippines are shown in Table 5. Figure 2 summarizes BRAF prevalence on the color map. We could not find any relevant publications from such large and highly populated Asian countries as Pakistan, Bangladesh, and Malaysia.
Figure 2.
Prevalence of BRAFV600E mutation in PTC from Asian countries and regions. PTC, papillary thyroid carcinoma.
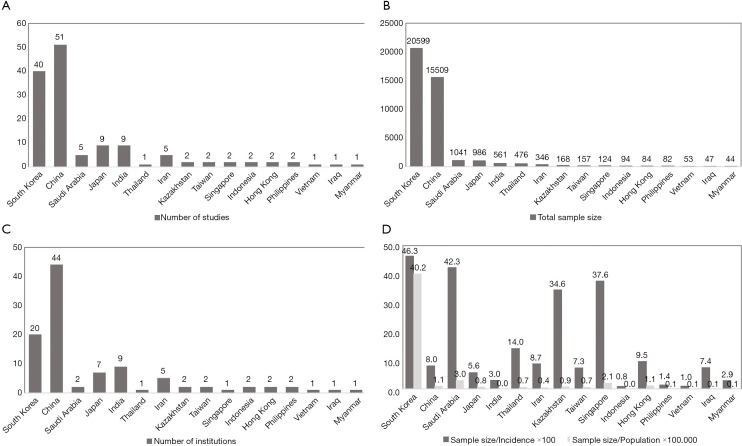
After grouping countries by geographic region, we found that the highest rate of BRAFV600E was reported in PTC series from East Asia (76.4%). Much lower rate (45–48%) was seen in PTC cohorts originated from South Asia, Central Asia, and the Middle East while the Southeast Asian series were in between (57%). At the same time, it should be noted that the level of evidence per country demonstrated by the number of studies, number of institutions, and the total sample size was highly heterogeneous (Figure 3A,B,C). For instance, 90.2% out of all PTCs with known BRAF status were reported from China, South Korea, and Japan—countries belonging to East Asia. After adjusting the sample size to such variables as the country’s population and incidence of thyroid carcinoma, we concluded that the South Korean series provided the best evidence on BRAF mutation prevalence at the national level (Figure 3D).
Figure 3.
Summary statistics of publications on BRAF mutation by country and region. (A) Total number of studies, (B) total sample size, (C) number of institutions, and (D) ratios of total sample size per annual incidence of thyroid carcinoma and total sample size per population.
Within country heterogeneity
As it could be seen from Tables 1-5 containing original data, there was a considerable heterogeneity of BRAF rate within most of the countries, the best illustrated in Japan (42–86%), South Korea (32–92%), and China (31–87%). Furthermore, such heterogeneity was found even within the same institution. For example, a group from Yamanashi, Japan reported two series of PTC with BRAF rate 42% and 82% (21,27). Similar discordances were found in studies from Sichuan, China (31% vs. 64%), Daejeon, Korea (53% vs. 89%), and others (31,44,61,65,79,90).
It is known that the detection rate of BRAF may depend on a large variety of factors. As per the data collection form, we were able to assess several of them. First, pediatric PTCs, where indicated, had lower BRAF prevalence compared to the baseline rate for the country; however this assumption was based only on a very limited number of studies with pediatric PTCs (18-20,28). Further analysis of factors potentially contributing to the within country heterogeneity was limited to China and South Korea, which had enough studies to be dichotomized by a variable of interest. We found that studies employed fresh frozen tissue and fine-needle aspirates showed a significantly higher rate of BRAF compared to those used formalin-fixed paraffin-embedded tissues (78% vs. 66% in Chinese series and 79% vs. 74% in Korean series; P<0.01). To evaluate a possibility of time trend, we divided studies into those enrolled samples before and after 2010 (only when this was indicated). It was found that the recent PTC series demonstrated a higher rate of BRAF—80% vs. 58% in Chinese series and 76% vs. 72% in Korean series (P<0.01).
Discussion
PTC is the most common malignancy of the endocrine system (153). The prevalence and annual incidence of PTC has approximately triplicated in the last three decades (154). Thyroid carcinoma ranks at the ninth place for incidence rates among all cancers (155). PTC constitutes up to 90% of thyroid carcinoma in contemporary series (156). The estimated age-standardized rate of thyroid cancer incidence in women is 3 times higher than that in men. Thyroid cancer was estimated to be the third most common malignant tumor in women in the USA and the fifth most common in Asia (157). Furthermore, the incidence rate in countries having a high human development index is 4–5 times greater than in those with the low index, while mortality rate does not differ between them (158). This is explained by the early diagnostics, advanced treatment options, and the development of the health care system in general. According to the GLOBOCAN 2018, Asia is the main continent contributing to the epidemiologic profile of thyroid cancer on a worldwide scale (157). For instance, Asia accounted for about 60% of thyroid carcinoma cases in terms of incidence, five-year prevalence, and mortality (157).
While Western opinion is dominating in the contemporary international guidelines on reporting and management of thyroid tumors, a wealth of evidence suggests that there are considerable differences between Western and Asian series of PTC, widely spanning from epidemiology and biology to specific practice patterns and treatment strategies (159-161). BRAFV600E mutation placed on the molecular end of the spectrum is a good example of such disparity. While European and American studies reported 30–60% rate of BRAF mutation in PTC, Asian series found it to be much more prevalent (11-15).
It is important that detection of BRAFV600E in PTC may have diagnostic significance in preoperative cytologic aspirates and also in surgical specimens (10,12,43,78,96,101,112). More recently BRAF was suggested as an important adjunct in predicting adverse prognosis in PTC, therefore getting wide recognition as a biomarker tailoring postoperative management of PTC patients (10,25). Furthermore, targeting of BRAF is considered as a promising strategy for patients with BRAF-mutant advanced thyroid cancer. In addition, recent studies found that concomitant BRAF and TERT promoter mutations in PTC patients were associated with a poor clinical outcome such as tumor aggressiveness and recurrence (162,163). Therefore, establishing a rate of BRAF on the regional and even institutional level is of practical significance. For instance, BRAF testing for rendering malignancy in preoperative thyroid fine-needle aspirates is much effective in regions with a high prevalence of BRAF mutation (39,164).
In this systematic review, we investigated a rate of BRAF mutation in a series of more than 40,000 PTCs originated from 16 Asian countries and regions, published in 2004–2020. The highest prevalence of BRAFV600E was reported in East Asian countries (>70%), followed by Southeast Asia (57%), and a region encompassing South Asia, Central Asia, and the Middle East (<50%) (Figure 2).
With this largest series to date, we could confirm that PTCs from Asian continent, particularly from East and Southeast Asia are much more saturated with BRAF mutation than those from Europe. Studies from Eastern (Poland, Czech Republic), Central (Germany), and Southern Europe (Italy, Spain, Portugal) consistently reported BRAF prevalence in PTC below 45% (14,165-172). Series from North (USA) and South America (Brazil) also showed BRAF rate lower than in Asia (168,173,174). Although we did not perform extensive search on Western series, existing evidence based on the above studies from leading thyroid cancer centers is sufficient to illustrate a substantial difference between Asian and Western PTC.
In addition to differences among geographic regions, we found a considerable within-country heterogeneity of BRAF rate. Causes of geographic heterogeneity are multifactorial, which could be due to different etiology or study methodology, including selection bias, detection techniques, and many more. There are several etiological factors associated with the development of thyroid carcinoma, of which ionizing radiation has been the well-documented environmental cause of PTC (175). Other factors include genetic predisposition via single nucleotide polymorphisms, hormonal influence, and dietary components, such as iodine and nitrates (24,176,177). The discrepancy identified in the BRAFV600E frequency among different regions of Asia might be due to the variation in the iodine intake. Dietary iodine intake varies in population from as low as 20 µg/d in iodine-deficient areas to as high as 1,000 µg/d in iodine-sufficient areas, where seaweed is rich in iodine, such as Japan and South Korea. Iodine intake is considered as a major risk factor for thyroid tumorigenesis especially in iodine-deficient regions (178). Thyroid follicular cells divide slowly in normal conditions but in the case of iodine deficiency, the proliferation rate of follicular cells increases due to growth of serum TSH level. Excessive proliferation of thyroid follicular cells makes their genome more susceptible to molecular alterations such as BRAFV600E mutation. The overall incidence of histological subtypes of thyroid cancer depends upon level of iodine intake, for instance, follicular thyroid carcinoma is more prevalent in iodine-deficient areas while PTC incidence is greater in high iodine intake areas (179). In China, BRAFV600E mutation is higher in regions where drinking water is rich in iodine compared to mildly deficient iodine intake (70). Interestingly, the most recent studies showed that both low iodine intake and excessive iodine intake served as a significant risk factor for the occurrence of BRAF mutations in the thyroid, therefore, may be risk factors for the development of PTC (63,180).
Nevertheless, we do not expect that our BRAF prevalence map (Figure 2) would match with the iodine status map due to a high complexity and interplay of all the factors that contributed to the variation of BRAF rate in different series of PTC. Our data collection protocol did not require extracting additional information about baseline characteristics of the PTC cohorts, such as age, gender, distribution of tumors by histological types, clinical stage, and other important clinicopathological variables. Further systematic review and meta-analysis studies should consider matching PTC series with the major characteristics of enrolled patients, which may help to elucidate certain tumor-specific parameters as a source of heterogeneity.
Apart from the above issues, technical aspects could greatly contribute to heterogeneity of results. We revealed that studies employed fresh frozen tissue and fine-needle aspirates showed significantly higher rates of BRAF compared to those used formalin-fixed paraffin-embedded tissues, which could be explained by the poorer DNA quality in the latter specimens. There was a wide variety of molecular methods used across the Asian studies, from a simple gel-based polymerase chain reaction (PCR) and routine Sanger sequencing to highly-sensitive real-time PCR, pyrosequencing, and next-generation sequencing. In addition, mutation-specific immunohistochemistry with VE1 antibody employed as an alternative for genotyping to detect BRAFV600E mutation got increasing adoption in the recent studies.
All the multitude of factors described above could contribute to the geographic heterogeneity of BRAF mutation whether within Asia or between Asian and Western series. From the pathologist’s standpoint, one of the potential reasons behind the difference is the inter-observer variability in encapsulated follicular lesions between Asian and Western practices (181). Most of Western FVPTCs are classified as benign follicular adenomas or follicular carcinomas in Asia. Follicular variant of PTC is associated with RAS driver mutation (2) and sharp increase of this mutation in PTC was documented in US (173) while RAS-mutated FVPTCs are rare in Asian series (14), making the BRAF mutation rate high in Asian PTC.
This study has several limitations, which are inherently coupled with drawbacks of the original studies, including lack of data about clinicopathological characteristics of patients and histological type of PTC. Despite of the huge amount of data accumulated on BRAF mutation prevalence in Asia, more than 90% of PTCs were reported from Japan, South Korea, and China. The evidence from other countries and regions of Asia is very limited (Figure 3). Many of the studies were performed on less than 100 samples, which could not be considered sufficient to draw relevant conclusions about the nationwide rate of BRAF mutation. Our estimates suggest that 300–400 PTC cases should be enrolled to qualify a well-powered study. However, dealing with a relatively large amount of samples may be challenging in the limited resources settings, which is a case of most Asian countries. Recently we developed and validated a low-cost testing algorithm to estimate the prevalence of BRAFV600E in large cohort studies based on mutation-specific immunostaining applied to small-sized specimens (67,142,182).
Conclusions
Our study found that the highest rate of BRAFV600E was reported in the PTC series from East Asia (76.4%), contributed by South Korea (75.5%), China (71.2%), and Japan (70.6%). Much lower rate (45–48%) based on the limited number of studies was seen in PTC cohorts originated from South Asia, Central Asia, and the Middle East while the Southeast Asian series were in between (57%). Asian series demonstrated considerable among and within countries heterogeneity regarding the prevalence of BRAFV600E mutation in PTC. Pooled Asian series of PTC showed a higher prevalence of BRAFV600E than in Western series. Causes of geographic heterogeneity, whether genuine (etiology, genetics) or methodology-related (selection bias, detection techniques, and more) should be further investigated.
Acknowledgments
Funding: None.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
Footnotes
Provenance and Peer Review: This article was commissioned by the Guest Editor (Kennichi Kakudo) for the series “Asian and Western Practice in Thyroid Pathology: Similarities and Differences” published in Gland Surgery. The article was sent for external peer review organized by the Guest Editor and the editorial office.
Peer Review File: Available at http://dx.doi.org/10.21037/gs-20-430
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/gs-20-430). The series “Asian and Western Practice in Thyroid Pathology: Similarities and Differences” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
References
- 1.Bikas A, Burman KD. Epidemiology of thyroid cancer. In: Luster M, Duntas L, Wartofsky L. editors. The Thyroid and Its Diseases. Cham, Switzerland: Springer, 2019:541-7. [Google Scholar]
- 2.Fagin JA, Wells SA., Jr Biologic and Clinical Perspectives on Thyroid Cancer. N Engl J Med 2016;375:1054-67. 10.1056/NEJMra1501993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Romei C, Ciampi R, Elisei R. A comprehensive overview of the role of the RET proto-oncogene in thyroid carcinoma. Nat Rev Endocrinol 2016;12:192-202. 10.1038/nrendo.2016.11 [DOI] [PubMed] [Google Scholar]
- 4.Mulligan LM. RET revisited: expanding the oncogenic portfolio. Nat Rev Cancer 2014;14:173-86. 10.1038/nrc3680 [DOI] [PubMed] [Google Scholar]
- 5.Xing M. BRAF mutation in papillary thyroid cancer: pathogenic role, molecular bases, and clinical implications. Endocr Rev 2007;28:742-62. 10.1210/er.2007-0007 [DOI] [PubMed] [Google Scholar]
- 6.Niault TS, Baccarini M. Targets of Raf in tumorigenesis. Carcinogenesis 2010;31:1165-74. 10.1093/carcin/bgp337 [DOI] [PubMed] [Google Scholar]
- 7.Dhomen N, Marais R. New insight into BRAF mutations in cancer. Curr Opin Genet Dev 2007;17:31-9. 10.1016/j.gde.2006.12.005 [DOI] [PubMed] [Google Scholar]
- 8.Kim TH, Park YJ, Lim JA, et al. The association of the BRAF(V600E) mutation with prognostic factors and poor clinical outcome in papillary thyroid cancer: a meta-analysis. Cancer 2012;118:1764-73. 10.1002/cncr.26500 [DOI] [PubMed] [Google Scholar]
- 9.Tufano RP, Teixeira GV, Bishop J, et al. BRAF mutation in papillary thyroid cancer and its value in tailoring initial treatment: a systematic review and meta-analysis. Medicine 2012;91:274-86. 10.1097/MD.0b013e31826a9c71 [DOI] [PubMed] [Google Scholar]
- 10.Haugen BR, Alexander EK, Bible KC, et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 2016;26:1-133. 10.1089/thy.2015.0020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yip L, Nikiforova MN, Carty SE, et al. Optimizing surgical treatment of papillary thyroid carcinoma associated with BRAF mutation. Surgery 2009;146:1215-23. 10.1016/j.surg.2009.09.011 [DOI] [PubMed] [Google Scholar]
- 12.Xing M, Clark D, Guan H, et al. BRAF mutation testing of thyroid fine-needle aspiration biopsy specimens for preoperative risk stratification in papillary thyroid cancer. J Clin Oncol 2009;27:2977-82. 10.1200/JCO.2008.20.1426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cancer Genome Atlas Research Network Integrated genomic characterization of papillary thyroid carcinoma. Cell 2014;159:676-90. 10.1016/j.cell.2014.09.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Song YS, Lim JA, Park YJ. Mutation Profile of Well-Differentiated Thyroid Cancer in Asians. Endocrinol Metab (Seoul) 2015;30:252-62. 10.3803/EnM.2015.30.3.252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bychkov A. Prevalence of BRAF(V600E) mutation in Asian patients with thyroid cancer. Malays J Pathol 2017;39:95-6. [PubMed] [Google Scholar]
- 16.Bychkov A, Kakudo K, Hong S. Current Practices of Thyroid Fine-Needle Aspiration in Asia: A Missing Voice. J Pathol Transl Med 2017;51:517-20. 10.4132/jptm.2017.09.27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009;6:e1000097. 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mitsutake N, Fukushima T, Matsuse M, et al. BRAF(V600E) mutation is highly prevalent in thyroid carcinomas in the young population in Fukushima: a different oncogenic profile from Chernobyl. Sci Rep 2015;5:16976. 10.1038/srep16976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Geng J, Wang H, Liu Y, et al. Correlation between BRAF (V600E) mutation and clinicopathological features in pediatric papillary thyroid carcinoma. Sci China Life Sci 2017;60:729-38. 10.1007/s11427-017-9083-8 [DOI] [PubMed] [Google Scholar]
- 20.Alzahrani AS, Murugan AK, Qasem E, et al. Single Point Mutations in Pediatric Differentiated Thyroid Cancer. Thyroid 2017;27:189-96. 10.1089/thy.2016.0339 [DOI] [PubMed] [Google Scholar]
- 21.Kondo T, Nakazawa T, Murata S, et al. Enhanced B-Raf protein expression is independent of V600E mutant status in thyroid carcinomas. Hum Pathol 2007;38:1810-8. 10.1016/j.humpath.2007.04.014 [DOI] [PubMed] [Google Scholar]
- 22.Kumagai A, Namba H, Akanov Z, et al. Clinical implications of pre-operative rapid BRAF analysis for papillary thyroid cancer. Endocr J 2007;54:399-405. 10.1507/endocrj.K06-194 [DOI] [PubMed] [Google Scholar]
- 23.Takahashi K, Eguchi H, Arihiro K, et al. The presence of BRAF point mutation in adult papillary thyroid carcinomas from atomic bomb survivors correlates with radiation dose. Mol Carcinog 2007;46:242-8. 10.1002/mc.20277 [DOI] [PubMed] [Google Scholar]
- 24.Matsuse M, Takahashi M, Mitsutake N, et al. The FOXE1 and NKX2-1 loci are associated with susceptibility to papillary thyroid carcinoma in the Japanese population. J Med Genet 2011;48:645-8. 10.1136/jmedgenet-2011-100063 [DOI] [PubMed] [Google Scholar]
- 25.Xing M, Alzahrani AS, Carson KA, et al. Association between BRAF V600E mutation and recurrence of papillary thyroid cancer. J Clin Oncol 2015;33:42-50. 10.1200/JCO.2014.56.8253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nasirden A, Saito T, Fukumura Y, et al. In Japanese patients with papillary thyroid carcinoma, TERT promoter mutation is associated with poor prognosis, in contrast to BRAF (V600E) mutation. Virchows Arch 2016;469:687-96. 10.1007/s00428-016-2027-5 [DOI] [PubMed] [Google Scholar]
- 27.Vuong HG, Kondo T, Oishi N, et al. Genetic alterations of differentiated thyroid carcinoma in iodine-rich and iodine-deficient countries. Cancer Med 2016;5:1883-9. 10.1002/cam4.781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Oishi N, Kondo T, Nakazawa T, et al. Frequent BRAF (V600E) and Absence of TERT Promoter Mutations Characterize Sporadic Pediatric Papillary Thyroid Carcinomas in Japan. Endocr Pathol 2017;28:103-11. 10.1007/s12022-017-9470-y [DOI] [PubMed] [Google Scholar]
- 29.Bandoh N, Akahane T, Goto T, et al. Targeted next-generation sequencing of cancer-related genes in thyroid carcinoma: A single institution's experience. Oncol Lett 2018;16:7278-86. 10.3892/ol.2018.9538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim KH, Suh KS, Kang DW, et al. Mutations of the BRAF gene in papillary thyroid carcinoma and in Hashimoto's thyroiditis. Pathol Int 2005;55:540-5. 10.1111/j.1440-1827.2005.01866.x [DOI] [PubMed] [Google Scholar]
- 31.Kim TY, Kim WB, Song JY, et al. The BRAF mutation is not associated with poor prognostic factors in Korean patients with conventional papillary thyroid microcarcinoma. Clinical endocrinology 2005;63:588-93. 10.1111/j.1365-2265.2005.02389.x [DOI] [PubMed] [Google Scholar]
- 32.Jo YS, Li S, Song JH, et al. Influence of the BRAF V600E mutation on expression of vascular endothelial growth factor in papillary thyroid cancer. J Clin Endocrinol Metab 2006;91:3667-70. 10.1210/jc.2005-2836 [DOI] [PubMed] [Google Scholar]
- 33.Kim TY, Kim WB, Rhee YS, et al. The BRAF mutation is useful for prediction of clinical recurrence in low-risk patients with conventional papillary thyroid carcinoma. Clinical endocrinology 2006;65:364-8. 10.1111/j.1365-2265.2006.02605.x [DOI] [PubMed] [Google Scholar]
- 34.Lee JH, Lee ES, Kim YS, et al. BRAF mutation and AKAP9 expression in sporadic papillary thyroid carcinomas. Pathology 2006;38:201-4. 10.1080/00313020600696264 [DOI] [PubMed] [Google Scholar]
- 35.Park SY, Park YJ, Lee YJ, et al. Analysis of differential BRAF(V600E) mutational status in multifocal papillary thyroid carcinoma: evidence of independent clonal origin in distinct tumor foci. Cancer 2006;107:1831-8. 10.1002/cncr.22218 [DOI] [PubMed] [Google Scholar]
- 36.Kim SK, Song KH, Lim SD, et al. Clinical and pathological features and the BRAF(V600E) mutation in patients with papillary thyroid carcinoma with and without concurrent Hashimoto thyroiditis. Thyroid 2009;19:137-41. 10.1089/thy.2008.0144 [DOI] [PubMed] [Google Scholar]
- 37.Kwak JY, Kim EK, Chung WY, et al. Association of BRAFV600E mutation with poor clinical prognostic factors and US features in Korean patients with papillary thyroid microcarcinoma. Radiology 2009;253:854-60. 10.1148/radiol.2533090471 [DOI] [PubMed] [Google Scholar]
- 38.Kim JH, Choi JY. Relationship between BRAF Mutations in Papillary Thyroid Carcinomas and Clinicopathologic Factors. Korean J Endocr Surg 2010;10:147-51. 10.16956/kjes.2010.10.3.147 [DOI] [Google Scholar]
- 39.Kim SW, Lee JI, Kim JW, et al. BRAFV600E mutation analysis in fine-needle aspiration cytology specimens for evaluation of thyroid nodule: a large series in a BRAFV600E-prevalent population. J Clin Endocrinol Metab 2010;95:3693-700. 10.1210/jc.2009-2795 [DOI] [PubMed] [Google Scholar]
- 40.Lee HJ, Choi J, Hwang TS, et al. Detection of BRAF mutations in thyroid nodules by allele-specific PCR using a dual priming oligonucleotide system. Am J Clin Pathol 2010;133:802-8. 10.1309/AJCPO3F2ENKMDTUS [DOI] [PubMed] [Google Scholar]
- 41.Park YJ, Kim YA, Lee YJ, et al. Papillary microcarcinoma in comparison with larger papillary thyroid carcinoma in BRAF(V600E) mutation, clinicopathological features, and immunohistochemical findings. Head Neck 2010;32:38-45. [DOI] [PubMed] [Google Scholar]
- 42.Ahn D, Park JS, Sohn JH, et al. BRAFV600E mutation does not serve as a prognostic factor in Korean patients with papillary thyroid carcinoma. Auris Nasus Larynx 2012;39:198-203. 10.1016/j.anl.2011.07.011 [DOI] [PubMed] [Google Scholar]
- 43.Chang H, Lee H, Yoon SO, et al. BRAF(V600E) mutation analysis of liquid-based preparation-processed fine needle aspiration sample improves the diagnostic rate of papillary thyroid carcinoma. Hum Pathol 2012;43:89-95. 10.1016/j.humpath.2011.04.010 [DOI] [PubMed] [Google Scholar]
- 44.Joo JY, Park JY, Yoon YH, et al. Prediction of occult central lymph node metastasis in papillary thyroid carcinoma by preoperative BRAF analysis using fine-needle aspiration biopsy: a prospective study. J Clin Endocrinol Metab 2012;97:3996-4003. 10.1210/jc.2012-2444 [DOI] [PubMed] [Google Scholar]
- 45.Kim SJ, Lee KE, Myong JP, et al. BRAF V600E mutation is associated with tumor aggressiveness in papillary thyroid cancer. World J Surg 2012;36:310-7. 10.1007/s00268-011-1383-1 [DOI] [PubMed] [Google Scholar]
- 46.Moon WJ, Choi N, Choi JW, et al. BRAF mutation analysis and sonography as adjuncts to fine-needle aspiration cytology of papillary thyroid carcinoma: their relationships and roles. AJR Am J Roentgenol 2012;198:668-74. 10.2214/AJR.11.7185 [DOI] [PubMed] [Google Scholar]
- 47.Choi SY, Park H, Kang MK, et al. The relationship between the BRAF(V600E) mutation in papillary thyroid microcarcinoma and clinicopathologic factors. World J Surg Oncol 2013;11:291. 10.1186/1477-7819-11-291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jeong D, Jeong Y, Park JH, et al. BRAF (V600E) mutation analysis in papillary thyroid carcinomas by peptide nucleic acid clamp real-time PCR. Ann Surg Oncol 2013;20:759-66. 10.1245/s10434-012-2494-0 [DOI] [PubMed] [Google Scholar]
- 49.Kang KH. Osteopontin expression in papillary thyroid carcinoma and its relationship with the BRAF mutation and tumor characteristics. J Korean Surg Soc 2013;84:9-17. 10.4174/jkss.2013.84.1.9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lim JY, Hong SW, Lee YS, et al. Clinicopathologic implications of the BRAF(V600E) mutation in papillary thyroid cancer: a subgroup analysis of 3130 cases in a single center. Thyroid 2013;23:1423-30. 10.1089/thy.2013.0036 [DOI] [PubMed] [Google Scholar]
- 51.Min HS, Lee C, Jung KC. Correlation of immunohistochemical markers and BRAF mutation status with histological variants of papillary thyroid carcinoma in the Korean population. J Korean Med Sci 2013;28:534-41. 10.3346/jkms.2013.28.4.534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chai YJ, Kim SJ, Kim SC, et al. BRAF mutation in follicular variant of papillary thyroid carcinoma is associated with unfavourable clinicopathological characteristics and malignant features on ultrasonography. Clin Endocrinol (Oxf) 2014;81:432-9. 10.1111/cen.12433 [DOI] [PubMed] [Google Scholar]
- 53.Han SA, Park WS, Jang JH, et al. BRAF mutation may predict higher necessity of postoperative radioactive iodine ablation in papillary thyroid cancer. Ann Surg Treat Res 2014;87:174-9. 10.4174/astr.2014.87.4.174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hong AR, Lim JA, Kim TH, et al. The Frequency and Clinical Implications of the BRAF(V600E) Mutation in Papillary Thyroid Cancer Patients in Korea Over the Past Two Decades. Endocrinol Metab (Seoul) 2014;29:505-13. 10.3803/EnM.2014.29.4.505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jung YY, Yoo JH, Park ES, et al. Clinicopathologic correlations of the BRAFV600E mutation, BRAF V600E immunohistochemistry, and BRAF RNA in situ hybridization in papillary thyroid carcinoma. Pathol Res Pract 2015;211:162-70. 10.1016/j.prp.2014.10.005 [DOI] [PubMed] [Google Scholar]
- 56.Lee SR, Yim H, Han JH, et al. VE1 antibody is not highly specific for the BRAF V600E mutation in thyroid cytology categories with the exception of malignant cases. Am J Clin Pathol 2015;143:437-44. 10.1309/AJCPOBI5CUZIBMO1 [DOI] [PubMed] [Google Scholar]
- 57.Na JI, Kim JH, Kim HJ, et al. VE1 immunohistochemical detection of the BRAF V600E mutation in thyroid carcinoma: a review of its usefulness and limitations. Virchows Arch 2015;467:155-68. 10.1007/s00428-015-1773-0 [DOI] [PubMed] [Google Scholar]
- 58.Kim SK, Lee JH, Woo JW, et al. BRAF V600E mutation: Differential impact on central lymph node metastasis by tumor size in papillary thyroid carcinoma. Head Neck 2016;38 Suppl 1:E1203-9. 10.1002/hed.24192 [DOI] [PubMed] [Google Scholar]
- 59.Kim S, Lee J, Soh EY. The Clinical Significance of the BRAF Mutation in Patients with Papillary Thyroid Cancer. J Endocr Surg 2017;17:175 10.16956/jes.2017.17.4.175 [DOI] [Google Scholar]
- 60.Lee SE, Hwang TS, Choi YL, et al. Molecular Profiling of Papillary Thyroid Carcinoma in Korea with a High Prevalence of BRAF(V600E) Mutation. Thyroid 2017;27:802-10. 10.1089/thy.2016.0547 [DOI] [PubMed] [Google Scholar]
- 61.Yeo MK, Jung MK, Lee SY, et al. The usefulness of a novel fully automated PCR-based Idylla test for detection of the BRAF V600E mutation in thyroid tissue: comparison with PNA-clamping PCR, real-time PCR and pyrosequencing. J Clin Pathol 2017;70:260-5. 10.1136/jclinpath-2016-204025 [DOI] [PubMed] [Google Scholar]
- 62.Kim H, Kim BH, Kim YK, et al. Prevalence of BRAF(V600E) Mutation in Follicular Variant of Papillary Thyroid Carcinoma and Non-Invasive Follicular Tumor with Papillary-Like Nuclear Features (NIFTP) in a BRAF(V600E) Prevalent Area. J Korean Med Sci 2018;33:e75. 10.3346/jkms.2018.33.e75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kim HJ, Park HK, Byun DW, et al. Iodine intake as a risk factor for BRAF mutations in papillary thyroid cancer patients from an iodine-replete area. Eur J Nutr 2018;57:809-15. 10.1007/s00394-016-1370-2 [DOI] [PubMed] [Google Scholar]
- 64.Kim JK, Seong CY, Bae IE, et al. Comparison of Immunohistochemistry and Direct Sequencing Methods for Identification of the BRAF(V600E) Mutation in Papillary Thyroid Carcinoma. Ann Surg Oncol 2018;25:1775-81. 10.1245/s10434-018-6460-3 [DOI] [PubMed] [Google Scholar]
- 65.Oh HS, Kwon H, Park S, et al. Comparison of Immunohistochemistry and Direct Sanger Sequencing for Detection of the BRAF(V600E) Mutation in Thyroid Neoplasm. Endocrinol Metab (Seoul) 2018;33:62-9. 10.3803/EnM.2018.33.1.62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lee SM, Lee CR, Kang SW, et al. Association between BRAFV600E Mutations and Clinicopathological Features of Papillary Thyroid Microcarcinoma (PTMC). J Endocr Surg 2019;19:7 10.16956/jes.2019.19.3.76 [DOI] [Google Scholar]
- 67.Choden S, Keelawat S, Jung CK, et al. VE1 Immunohistochemistry Improves the Limit of Genotyping for Detecting BRAF(V600E) Mutation in Papillary Thyroid Cancer. Cancers (Basel) 2020;12:596. 10.3390/cancers12030596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yoon JH, Han K, Lee E, et al. Radiomics in predicting mutation status for thyroid cancer: A preliminary study using radiomics features for predicting BRAFV600E mutations in papillary thyroid carcinoma. PloS One 2020;15:e0228968. 10.1371/journal.pone.0228968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gu LQ, Li FY, Zhao L, et al. BRAFV600E mutation and X-linked inhibitor of apoptosis expression in papillary thyroid carcinoma. Thyroid 2009;19:347-54. 10.1089/thy.2008.0246 [DOI] [PubMed] [Google Scholar]
- 70.Guan H, Ji M, Bao R, et al. Association of high iodine intake with the T1799A BRAF mutation in papillary thyroid cancer. J Clin Endocrinol Metab 2009;94:1612-7. 10.1210/jc.2008-2390 [DOI] [PubMed] [Google Scholar]
- 71.Feng L, Li M, Zhang QP, et al. Utility of BRAF protein overexpression in predicting the metastasis potential of papillary thyroid carcinoma. Oncol Lett 2011;2:59-63. 10.3892/ol.2010.219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wang W, Zhao W, Wang H, et al. Poorer prognosis and higher prevalence of BRAF (V600E) mutation in synchronous bilateral papillary thyroid carcinoma. Ann Surg Oncol 2012;19:31-6. 10.1245/s10434-011-2096-2 [DOI] [PubMed] [Google Scholar]
- 73.Xia T, Hu C, Zha J, et al. BRAFV600E Mutation in Papillary Thyroid Carcinoma. Chin Clin Oncol 2012;39:3. [Google Scholar]
- 74.Zheng X, Xia T, Lin L, et al. BRAFV600E status and clinical characteristics in solitary and multiple papillary thyroid carcinoma: experience of 512 cases at a clinical center in China. World J Surg Oncol 2012;10:104. 10.1186/1477-7819-10-104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhou YL, Zhang W, Gao EL, et al. Preoperative BRAF mutation is predictive of occult contralateral carcinoma in patients with unilateral papillary thyroid microcarcinoma. Asian Pac J Cancer Prev 2012;13:1267-72. 10.7314/APJCP.2012.13.4.1267 [DOI] [PubMed] [Google Scholar]
- 76.Gong RX, Gong YP, Yang J, et al. Efficient detection of the V600E mutation of the BRAF gene in papillary thyroid carcinoma using multiplex allele-specific polymerase chain reaction combined with denaturing high-performance liquid chromatography. Genet Mol Res 2013;12:4990-7. 10.4238/2013.October.24.11 [DOI] [PubMed] [Google Scholar]
- 77.Huang Y, Liao D, Pan L, et al. Expressions of miRNAs in papillary thyroid carcinoma and their associations with the BRAFV600E mutation. Eur J Endocrinol 2013;168:675-81. 10.1530/EJE-12-1029 [DOI] [PubMed] [Google Scholar]
- 78.Guo HQ, Zhao H, Zhang ZH, et al. Impact of molecular testing in the diagnosis of thyroid fine needle aspiration cytology: data from mainland China. Dis Markers 2014;2014:912182. 10.1155/2014/912182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.He G, Zhao B, Zhang X, et al. Prognostic value of the BRAF V600E mutation in papillary thyroid carcinoma. Oncol Lett 2014;7:439-43. 10.3892/ol.2013.1713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Huang FJ, Fang WY, Ye L, et al. BRAF mutation correlates with recurrent papillary thyroid carcinoma in Chinese patients. Curr Oncol 2014;21:e740-7. 10.3747/co.21.2029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Liu S, Zhang B, Zhao Y, et al. Association of BRAFV600E mutation with clinicopathological features of papillary thyroid carcinoma: a study on a Chinese population. Int J Clin Exp Pathol 2014;7:6922-8. [PMC free article] [PubMed] [Google Scholar]
- 82.Liu X, Qu S, Liu R, et al. TERT promoter mutations and their association with BRAF V600E mutation and aggressive clinicopathological characteristics of thyroid cancer. J Clin Endocrinol Metab 2014;99:E1130-6. 10.1210/jc.2013-4048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lu H, Qiu T, Ying J, et al. [Correlation between BRAF V600E mutation and clinicopathologic features of papillary thyroid carcinoma]. Zhonghua Bing Li Xue Za Zhi 2014;43:794-8. [PubMed] [Google Scholar]
- 84.Shao H, Yu X, Wang C, et al. Midkine expression is associated with clinicopathological features and BRAF mutation in papillary thyroid cancer. Endocrine 2014;46:285-91. 10.1007/s12020-013-0068-y [DOI] [PubMed] [Google Scholar]
- 85.Wei X, Li Y, Zhang S, et al. Prediction of thyroid extracapsular extension with cervical lymph node metastases (ECE-LN) by CEUS and BRAF expression in papillary thyroid carcinoma. Tumour Biol 2014;35:8559-64. 10.1007/s13277-014-2119-2 [DOI] [PubMed] [Google Scholar]
- 86.Lu J, Gao J, Zhang J, et al. Association between BRAF V600E mutation and regional lymph node metastasis in papillary thyroid carcinoma. Int J Clin Exp Pathol 2015;8:793-9. [PMC free article] [PubMed] [Google Scholar]
- 87.Qiu T, Lu H, Guo L, et al. Detection of BRAF mutation in Chinese tumor patients using a highly sensitive antibody immunohistochemistry assay. Sci Rep 2015;5:9211. 10.1038/srep09211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Shi C, Qin H, Ding C, et al. [Association between BRAF V600E mutation and central lymph node metastasis in patients with papillary thyroid carcinoma]. Zhonghua Zhong Liu Za Zhi 2015;37:123-7. [PubMed] [Google Scholar]
- 89.Sun J, Zhang J, Lu J, et al. Immunohistochemistry is highly sensitive and specific for detecting the BRAF V600E mutation in papillary thyroid carcinoma. Int J Clin Exp Pathol 2015;8:15072-8. [PMC free article] [PubMed] [Google Scholar]
- 90.Yang LB, Sun LY, Jiang Y, et al. The Clinicopathological Features of BRAF Mutated Papillary Thyroid Cancers in Chinese Patients. Int J Endocrinol 2015;2015:642046. 10.1155/2015/642046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Yu L, Ma L, Tu Q, et al. Clinical significance of BRAF V600E mutation in 154 patients with thyroid nodules. Oncol Lett 2015;9:2633-8. 10.3892/ol.2015.3119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zhao H, Zhang ZH, Zhou B, et al. Detection of BRAF c.1799T > A (p.V600E) mutation using residual routine fine-needle aspiration specimens of papillary thyroid carcinoma. Diagn Cytopathol 2015;43:786-90. 10.1002/dc.23302 [DOI] [PubMed] [Google Scholar]
- 93.Jin L, Chen E, Dong S, et al. BRAF and TERT promoter mutations in the aggressiveness of papillary thyroid carcinoma: a study of 653 patients. Oncotarget 2016;7:18346-55. 10.18632/oncotarget.7811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sun J, Zhang J, Lu J, et al. BRAF V600E and TERT Promoter Mutations in Papillary Thyroid Carcinoma in Chinese Patients. PloS One 2016;11:e0153319. 10.1371/journal.pone.0153319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wen H, Aizezi A, Yasenjiang M, et al. Clinicopathological significance of BRAFV600E mutation in Uyghur Chinese patients with papillary thyroid carcinoma. Int J Clin Exp Pathol 2016;9:200-7. [Google Scholar]
- 96.Zhang B, Xu CW, Wu YF, et al. Diagnostic significance of the BRAF V600E mutation in conventional papillary thyroid carcinomas. Int J Clin Exp Med 2016;9:8296-303. [Google Scholar]
- 97.Zheng L, Zhao M, Hu X. Clinical significance of HBME-1, Galectin-3, and CK19 expression and the status of BRAF mutation in papillary thyroid carcinoma. Oncol Transl Med 2016;2:174-8. [Google Scholar]
- 98.Li Q, Yuan J, Wang Y, et al. Association between the BRAF V600E mutation and ultrasound features of the thyroid in thyroid papillary carcinoma. Oncol Lett 2017;14:1439-44. 10.3892/ol.2017.6276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zhang Q, Liu BJ, Ren WW, et al. Association between BRAF V600E Mutation and Ultrasound Features in Papillary Thyroid Carcinoma Patients with and without Hashimoto's Thyroiditis. Sci Rep 2017;7:4899. 10.1038/s41598-017-05153-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Guan Q, Wang Y, Liao T, et al. Overexpression of trophoblast cell surface antigen 2 is associated with BRAF V600E mutation and aggressive behavior in papillary thyroid cancer. Int J Clin Exp Pathol 2018;11:4130-9. [PMC free article] [PubMed] [Google Scholar]
- 101.Huang L, Wang X, Huang X, et al. Diagnostic significance of CK19, galectin-3, CD56, TPO and Ki67 expression and BRAF mutation in papillary thyroid carcinoma. Oncol Lett 2018;15:4269-77. 10.3892/ol.2018.7873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Liang J, Cai W, Feng D, et al. Genetic landscape of papillary thyroid carcinoma in the Chinese population. J Pathol 2018;244:215-26. 10.1002/path.5005 [DOI] [PubMed] [Google Scholar]
- 103.Liu Z, Lv T, Xie C, et al. BRAF V600E Gene Mutation Is Associated With Bilateral Malignancy of Papillary Thyroid Cancer. Am J Med Sci 2018;356:130-4. 10.1016/j.amjms.2018.04.012 [DOI] [PubMed] [Google Scholar]
- 104.Ren H, Shen Y, Hu D, et al. Co-existence of BRAF(V600E) and TERT promoter mutations in papillary thyroid carcinoma is associated with tumor aggressiveness, but not with lymph node metastasis. Cancer Manag Res 2018;10:1005-13. 10.2147/CMAR.S159583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Zheng B, Zarka MA, Chen C, et al. The largest CAP-certified Chinese reference laboratory experience with the Bethesda system for reporting thyroid cytopathology: correlation with histologic and BRAF data. J Am Soc Cytopathol 2018;7:16-21. 10.1016/j.jasc.2017.07.002 [DOI] [PubMed] [Google Scholar]
- 106.Zhou D, Li Z, Bai X. BRAF V600E and RET/PTC Promote the Activity of Nuclear Factor-kappaB, Inflammatory Mediators, and Lymph Node Metastasis in Papillary Thyroid Carcinoma: A Study of 50 Patients in Inner Mongolia. Med Sci Monit 2018;24:6795-808. 10.12659/MSM.909205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Chen B, Zhang Z, Wang K, et al. Association of BRAFV600E mutation with ultrasonographic features and clinicopathologic characteristics of papillary thyroid microcarcinoma: A retrospective study of 116 cases. Clin Hemorheol Microcirc 2019;73:545-52. 10.3233/CH-190568 [DOI] [PubMed] [Google Scholar]
- 108.Gao J, Ma XP, Deng FS, et al. Associations of the BRAF V600E Mutation and PAQR3 Protein Expression with Papillary Thyroid Carcinoma Clinicopathological Features. Pathol Oncol Res 2020;26:1833-41. 10.1007/s12253-019-00779-x [DOI] [PubMed] [Google Scholar]
- 109.Huang M, Yan C, Xiao J, et al. Relevance and clinicopathologic relationship of BRAF V600E, TERT and NRAS mutations for papillary thyroid carcinoma patients in Northwest China. Diagn Pathol 2019;14:74. 10.1186/s13000-019-0849-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ji W, Xie H, Wei B, et al. Relationship between BRAF V600E gene mutation and the clinical and pathologic characteristics of papillary thyroid microcarcinoma. Int J Clin Exp Pathol 2019;12:3492-9. [PMC free article] [PubMed] [Google Scholar]
- 111.Li X, Li E, Du J, et al. BRAF mutation analysis by ARMS-PCR refines thyroid nodule management. Clinical endocrinology 2019;91:834-41. 10.1111/cen.14079 [DOI] [PubMed] [Google Scholar]
- 112.Li XJ, Mao XD, Chen GF, et al. High BRAFV600E mutation frequency in Chinese patients with papillary thyroid carcinoma increases diagnostic efficacy in cytologically indeterminate thyroid nodules. Medicine 2019;98:e16343. 10.1097/MD.0000000000016343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lin ZM, Yan CX, Song Y, et al. The features of contrast enhanced ultrasound and BRAF V600E in papillary thyroid carcinoma. J Thorac Dis 2019;11:5071-8. 10.21037/jtd.2019.11.78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Liu Y, He L, Yin G, et al. Association analysis and the clinical significance of BRAF gene mutations and ultrasound features in papillary thyroid carcinoma. Oncol Lett 2019;18:2995-3002. 10.3892/ol.2019.10641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Shen G, Kou Y, Liu B, et al. The BRAFV600E mutation in papillary thyroid microcarcinoma with intermediate-risk to high-risk features: does the mutation have an effect on clinical response to radioiodine therapy? Nucl Med Commun 2019;40:8-13. 10.1097/MNM.0000000000000930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Wang J, Liu LT, Cui D, et al. [The co-relation of BRAF V600E mutation and factors affecting occurrence and prognosis of papillary thyroid carcinoma]. Zhonghua Bing Li Xue Za Zhi 2019;48:288-92. [DOI] [PubMed] [Google Scholar]
- 117.Yan C, Huang M, Li X, et al. Relationship between BRAF V600E and clinical features in papillary thyroid carcinoma. Endocr Connect 2019;8:988-96. 10.1530/EC-19-0246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Yang T, Chen C, Pan NF, et al. [BRAF V600E Mutation and TERT Promoter Mutation in Papillary Thyroid Carcinomas and Their Association with Clinicopathological Characteristics]. Sichuan Da Xue Xue Bao Yi Xue Ban 2019;50:919-24. [PubMed] [Google Scholar]
- 119.Zhou C, Li J, Wang Y, et al. Association of BRAF gene and TSHR with cervical lymph node metastasis of papillary thyroid microcarcinoma. Oncol Lett 2019;17:183-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Chakraborty A, Narkar A, Mukhopadhyaya R, et al. BRAF V600E mutation in papillary thyroid carcinoma: significant association with node metastases and extra thyroidal invasion. Endocr Pathol 2012;23:83-93. 10.1007/s12022-011-9184-5 [DOI] [PubMed] [Google Scholar]
- 121.Khan MS, Pandith AA, Azad N, et al. Impact of molecular alterations of BRAF in the pathogenesis of thyroid cancer. Mutagenesis 2014;29:131-7. 10.1093/mutage/get066 [DOI] [PubMed] [Google Scholar]
- 122.Agarwal S, Sharma MC, Karak AK, et al. BRAF mutation may predict higher risk of incomplete response to radioactive iodine ablation in papillary thyroid carcinoma. Indian J Endocrinol Metab 2016;21:1. [Google Scholar]
- 123.Nair CG, Babu M, Biswas L, et al. Lack of Association of B-type Raf Kinase V600E Mutation with High-risk Tumor Features and Adverse Outcome in Conventional and Follicular Variants of Papillary Thyroid Carcinoma. Indian J Endocrinol Metab 2017;21:329-33. 10.4103/ijem.IJEM_353_16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Ahmad F, Nathani R, Venkat J, et al. Molecular evaluation of BRAF gene mutation in thyroid tumors: Significant association with papillary tumors and extra thyroidal extension indicating its role as a biomarker of aggressive disease. Experimental and molecular pathology 2018;105:380-6. 10.1016/j.yexmp.2018.11.002 [DOI] [PubMed] [Google Scholar]
- 125.Fonseca D, Murthy SS, Tagore R, et al. BRAF status in the variants of papillary thyroid carcinoma. Int J Head Neck Pathol 2018;1:41-7. 10.4103/JHNP.JHNP_1_19 [DOI] [Google Scholar]
- 126.George N, Agarwal A, Kumari N, et al. Mutational Profile of Papillary Thyroid Carcinoma in an Endemic Goiter Region of North India. Indian J Endocrinol Metab 2018;22:505-10. 10.4103/ijem.IJEM_441_17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Hemalatha R, Pai R, Manipadam MT, et al. Presurgical Screening of Fine Needle Aspirates from Thyroid Nodules for BRAF Mutations: A Prospective Single Center Experience. Indian J Endocrinol Metab 2018;22:785-92. 10.4103/ijem.IJEM_126_18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Krishnamurthy A, Ramshankar V, Murhekar K, et al. Clinical utility of immunohistochemistry using the novel anti-BRAF V600E antibody (clone RM8) for detection of the BRAF V600E mutant protein in papillary thyroid cancers. Int J Mol Immuno Oncol 2018;3:28 10.18203/issn.2456-3994.IntJMolImmunoOncol20180471 [DOI] [Google Scholar]
- 129.Abubaker J, Jehan Z, Bavi P, et al. Clinicopathological analysis of papillary thyroid cancer with PIK3CA alterations in a Middle Eastern population. J Clin Endocrinol Metab 2008;93:611-8. 10.1210/jc.2007-1717 [DOI] [PubMed] [Google Scholar]
- 130.Schulten HJ, Salama S, Al-Mansouri Z, et al. BRAF mutations in thyroid tumors from an ethnically diverse group. Hered Cancer Clin Pract 2012;10:10. 10.1186/1897-4287-10-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Zou M, Baitei EY, Alzahrani AS, et al. Concomitant RAS, RET/PTC, or BRAF mutations in advanced stage of papillary thyroid carcinoma. Thyroid 2014;24:1256-66. 10.1089/thy.2013.0610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Qasem E, Murugan AK, Al-Hindi H, et al. TERT promoter mutations in thyroid cancer: a report from a Middle Eastern population. Endocr Relat Cancer 2015;22:901-8. 10.1530/ERC-15-0396 [DOI] [PubMed] [Google Scholar]
- 133.Murugan AK, Qasem E, Al-Hindi H, et al. Classical V600E and other non-hotspot BRAF mutations in adult differentiated thyroid cancer. J Transl Med 2016;14:204. 10.1186/s12967-016-0958-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Mohammadi-Asl J, Larijani B, Khorgami Z, et al. Prevalance of BRAFV600E Mutation in Iranian Patients with Papillary Thyroid Carcinoma: A Single-Center Study. J Appl Sci 2009;9:3593-7. 10.3923/jas.2009.3593.3597 [DOI] [Google Scholar]
- 135.Ranjbari N, Almasi S, Mohammadi-Asl J, et al. BRAF mutations in Iranian patients with papillary thyroid carcinoma. Asian Pac J Cancer Prev 2013;14:2521-3. 10.7314/APJCP.2013.14.4.2521 [DOI] [PubMed] [Google Scholar]
- 136.Daliri M, Abbaszadegan MR, Bahar MM, et al. The role of BRAF V600E mutation as a potential marker for prognostic stratification of papillary thyroid carcinoma: a long-term follow-up study. Endocr Res 2014;39:189-93. 10.3109/07435800.2013.879169 [DOI] [PubMed] [Google Scholar]
- 137.Zarkesh M, Zadeh-Vakili A, Azizi F, et al. The Association of BRAF V600E Mutation With Tissue Inhibitor of Metalloproteinase-3 Expression and Clinicopathological Features in Papillary Thyroid Cancer. Int J Endocrinol Metab 2018;16:e56120. 10.5812/ijem.56120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Ghasemi M, Behbahani AB, Farhadi A, et al. Prevalence of BRAFV600E Mutation and Human Parvovirus B19 Infection in Thyroid Cancer. Shiraz E Med J. 2019 doi: 10.5812/semj.84207. [Epub ahead of print] [DOI] [Google Scholar]
- 139.Salih A, Naqshabandi M, Hassan N, et al. Braf Gene Mutation and CD56 Immunoexpression in Papillary Thyroid Carcinoma in Duhok-Iraq. Journal of Sulaimani Medical College 2017. doi: 10.17656/jsmc.10126. [DOI]
- 140.Tlegenov AS, Abylaiuly Z, Adilbay DG, et al. Prevalence of Mutant BRAFV600E in the Papillary Thyroid Carcinoma in Patients from Kazakhstan and Its Correlation with Clinical-Morphological Tumor Characteristic. Medical News of North Caucasus 2018. doi: 10.14300/mnnc.2018.13079. [DOI] [Google Scholar]
- 141.Than MM. BRAF(V600E) Mutation in Papillary Thyroid Carcinoma. Yangon, Republic of the Union of Myanmar.: University of Medicine. 2017. [Google Scholar]
- 142.Choden S, Keelawat S, Jung CK, et al. An affordable immunohistochemical approach to estimate the prevalence of BRAFV600E in large cohort studies—establishing the baseline rate of BRAF mutation in an institutional series of papillary thyroid carcinoma from Thailand. Gland Surg 2020. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Brahma B, Yulian ED, Ramli M, et al. Surgical perspective of T1799A BRAF mutation diagnostic value in papillary thyroid carcinoma. Asian Pac J Cancer Prev 2013;14:31-7. 10.7314/APJCP.2013.14.1.31 [DOI] [PubMed] [Google Scholar]
- 144.Kristiani E, Makes B, Hardjolukito A, et al. BRAF V600E immunoexpression in papillary thyroid carcinoma and its association with prognostic factors and histopathologic variant. Virchows Arch 2016;469:S76. [Google Scholar]
- 145.Yang P, Lum H, Quek J, et al. Braf Immunohistochemistry Score Predicts BRAF V600E Mutational Status in Classical Papillary Thyroid Cancer. Thyroid 2015;25 Suppl 1:A356-83.26465259 [Google Scholar]
- 146.Goh X, Lum J, Yang SP, et al. BRAF mutation in papillary thyroid cancer-Prevalence and clinical correlation in a South-East Asian cohort. Clin Otolaryngol 2019;44:114-23. 10.1111/coa.13238 [DOI] [PubMed] [Google Scholar]
- 147.Liu RT, Chen YJ, Chou FF, et al. No correlation between BRAFV600E mutation and clinicopathological features of papillary thyroid carcinomas in Taiwan. Clinical endocrinology 2005;63:461-6. 10.1111/j.1365-2265.2005.02367.x [DOI] [PubMed] [Google Scholar]
- 148.Chang YS, Lin IL, Yeh KT, et al. Rapid detection of K-, N-, H-RAS, and BRAF hotspot mutations in thyroid cancer using the multiplex primer extension. Clin Biochem 2013;46:1572-7. 10.1016/j.clinbiochem.2013.06.005 [DOI] [PubMed] [Google Scholar]
- 149.Lo CC. A Study of BRAF and RAS genes in Papillary Thyroid. Hong Kong: University of Hong Kong, 2004. [Google Scholar]
- 150.Law Y. Analysis of BRAF V600E Mutation in Thyroid Aspirates for Establishing a Pre-operative Diagnosis of Thyroid Tumours. Hong Kong: Hong Kong Polytechnic University, 2009. [Google Scholar]
- 151.Navarro-Locsin CG, Chang AM, Daroy ML, et al. Clinical and histopathological profile of BRAF V600E mutation in conventional papillary thyroid carcinoma in a Filipino population. Malays J Pathol 2016;38:141-8. [PubMed] [Google Scholar]
- 152.Espiritu GAM, Malana JT, Dumasis A, et al. High Preponderance of BRAF V600E Mutation in Papillary Thyroid Carcinoma Among Filipinos: A Clinicopathologic Study. J Glob Oncol 2019;5:1-6. 10.1200/JGO.18.00085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Mao Y, Xing M. Recent incidences and differential trends of thyroid cancer in the USA. Endocr Relat Cancer 2016;23:313-22. 10.1530/ERC-15-0445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Pellegriti G, Frasca F, Regalbuto C, et al. Worldwide increasing incidence of thyroid cancer: update on epidemiology and risk factors. J Cancer Epidemiol 2013;2013:965212. 10.1155/2013/965212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394-424. 10.3322/caac.21492 [DOI] [PubMed] [Google Scholar]
- 156.Bychkov A, Jung CK, Liu Z, et al. Noninvasive Follicular Thyroid Neoplasm with Papillary-Like Nuclear Features in Asian Practice: Perspectives for Surgical Pathology and Cytopathology. Endocr Pathol 2018;29:276-88. 10.1007/s12022-018-9519-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Ferlay J, Colombet M, Soerjomataram I, et al. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int J Cancer 2019;144:1941-53. 10.1002/ijc.31937 [DOI] [PubMed] [Google Scholar]
- 158.Simard EP, Ward EM, Siegel R, et al. Cancers with increasing incidence trends in the United States: 1999 through 2008. CA Cancer J Clin 2012;62:118-28. 10.3322/caac.20141 [DOI] [PubMed] [Google Scholar]
- 159.Kakudo K, Higuchi M, Hirokawa M, et al. Thyroid FNA cytology in Asian practice-Active surveillance for indeterminate thyroid nodules reduces overtreatment of thyroid carcinomas. Cytopathology 2017;28:455-66. 10.1111/cyt.12491 [DOI] [PubMed] [Google Scholar]
- 160.Bychkov A, Hirokawa M, Jung CK, et al. Low Rate of Noninvasive Follicular Thyroid Neoplasm with Papillary-Like Nuclear Features in Asian Practice. Thyroid 2017;27:983-4. 10.1089/thy.2017.0079 [DOI] [PubMed] [Google Scholar]
- 161.Kakudo K, Bychkov A, Abelardo A, et al. Malpractice Climate Is a Key Difference in Thyroid Pathology Practice Between North America and the Rest of the World. Arch Pathol Lab Med 2019;143:1171. 10.5858/arpa.2019-0228-LE [DOI] [PubMed] [Google Scholar]
- 162.Vuong HG, Altibi AMA, Duong UNP, et al. Prognostic implication of BRAF and TERT promoter mutation combination in papillary thyroid carcinoma-A meta-analysis. Clinical endocrinology 2017;87:411-7. 10.1111/cen.13413 [DOI] [PubMed] [Google Scholar]
- 163.Song YS, Yoo SK, Kim HH, et al. Interaction of BRAF-induced ETS factors with mutant TERT promoter in papillary thyroid cancer. Endocr Relat Cancer 2019;26:629-41. 10.1530/ERC-17-0562 [DOI] [PubMed] [Google Scholar]
- 164.Kleiman DA, Sporn MJ, Beninato T, et al. Preoperative BRAF(V600E) mutation screening is unlikely to alter initial surgical treatment of patients with indeterminate thyroid nodules: a prospective case series of 960 patients. Cancer 2013;119:1495-502. 10.1002/cncr.27888 [DOI] [PubMed] [Google Scholar]
- 165.Melo M, Gaspar da Rocha A, Batista R, et al. TERT, BRAF, and NRAS in Primary Thyroid Cancer and Metastatic Disease. J Clin Endocrinol Metab 2017;102:1898-907. 10.1210/jc.2016-2785 [DOI] [PubMed] [Google Scholar]
- 166.Xing M. BRAF V600E mutation and papillary thyroid cancer. JAMA 2013;310:535. 10.1001/jama.2013.8592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Sykorova V, Dvorakova S, Ryska A, et al. BRAFV600E mutation in the pathogenesis of a large series of papillary thyroid carcinoma in Czech Republic. J Endocrinol Invest 2010;33:318-24. 10.1007/BF03346593 [DOI] [PubMed] [Google Scholar]
- 168.Xing M, Alzahrani AS, Carson KA, et al. Association between BRAF V600E mutation and mortality in patients with papillary thyroid cancer. JAMA 2013;309:1493-501. 10.1001/jama.2013.3190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Musholt TJ, Fottner C, Weber MM, et al. Detection of papillary thyroid carcinoma by analysis of BRAF and RET/PTC1 mutations in fine-needle aspiration biopsies of thyroid nodules. World J Surg 2010;34:2595-603. 10.1007/s00268-010-0729-4 [DOI] [PubMed] [Google Scholar]
- 170.Lupi C, Giannini R, Ugolini C, et al. Association of BRAF V600E mutation with poor clinicopathological outcomes in 500 consecutive cases of papillary thyroid carcinoma. J Clin Endocrinol Metab 2007;92:4085-90. 10.1210/jc.2007-1179 [DOI] [PubMed] [Google Scholar]
- 171.Basolo F, Torregrossa L, Giannini R, et al. Correlation between the BRAF V600E mutation and tumor invasiveness in papillary thyroid carcinomas smaller than 20 millimeters: analysis of 1060 cases. J Clin Endocrinol Metab 2010;95:4197-205. 10.1210/jc.2010-0337 [DOI] [PubMed] [Google Scholar]
- 172.Riesco-Eizaguirre G, Gutierrez-Martinez P, Garcia-Cabezas MA, et al. The oncogene BRAF V600E is associated with a high risk of recurrence and less differentiated papillary thyroid carcinoma due to the impairment of Na+/I- targeting to the membrane. Endocr Relat Cancer 2006;13:257-69. 10.1677/erc.1.01119 [DOI] [PubMed] [Google Scholar]
- 173.Jung CK, Little MP, Lubin JH, et al. The increase in thyroid cancer incidence during the last four decades is accompanied by a high frequency of BRAF mutations and a sharp increase in RAS mutations. J Clin Endocrinol Metab 2014;99:E276-85. 10.1210/jc.2013-2503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Oler G, Cerutti JM. High prevalence of BRAF mutation in a Brazilian cohort of patients with sporadic papillary thyroid carcinomas: correlation with more aggressive phenotype and decreased expression of iodide-metabolizing genes. Cancer 2009;115:972-80. 10.1002/cncr.24118 [DOI] [PubMed] [Google Scholar]
- 175.Iglesias ML, Schmidt A, Ghuzlan AA, et al. Radiation exposure and thyroid cancer: a review. Arch Endocrinol Metab 2017;61:180-7. 10.1590/2359-3997000000257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Rogounovitch TI, Bychkov A, Takahashi M, et al. The common genetic variant rs944289 on chromosome 14q13.3 associates with risk of both malignant and benign thyroid tumors in the Japanese population. Thyroid 2015;25:333-40. 10.1089/thy.2014.0431 [DOI] [PubMed] [Google Scholar]
- 177.Orim F, Bychkov A, Shimamura M, et al. Thyrotropin signaling confers more aggressive features with higher genomic instability on BRAF(V600E)-induced thyroid tumors in a mouse model. Thyroid 2014;24:502-10. 10.1089/thy.2013.0038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Harach HR, Ceballos GA. Thyroid cancer, thyroiditis and dietary iodine: a review based on the Salta, Argentina model. Endocr Pathol 2008;19:209-20. 10.1007/s12022-008-9038-y [DOI] [PubMed] [Google Scholar]
- 179.Zimmermann MB, Boelaert K. Iodine deficiency and thyroid disorders. Lancet Diabetes Endocrinol 2015;3:286-95. 10.1016/S2213-8587(14)70225-6 [DOI] [PubMed] [Google Scholar]
- 180.Lee JH, Song RY, Yi JW, et al. Case-Control Study of Papillary Thyroid Carcinoma on Urinary and Dietary Iodine Status in South Korea. World J Surg 2018;42:1424-31. 10.1007/s00268-017-4287-x [DOI] [PubMed] [Google Scholar]
- 181.Hirokawa M, Carney JA, Goellner JR, et al. Observer variation of encapsulated follicular lesions of the thyroid gland. Am J Surg Pathol 2002;26:1508-14. 10.1097/00000478-200211000-00014 [DOI] [PubMed] [Google Scholar]
- 182.Bychkov A, Jain D. Multiple sections per slide for immunohistochemistry: A cost-effective alternative for research in resource-limited settings. Anal Quant Cytol Histol 2018;40:211-2. [Google Scholar]