Abstract
Background and Objectives
Tuberculosis (TB) is a global public health issue. The emergence of multidrug-resistant (MDR) TB has further complicated the situation in the form of poor treatment outcomes and costs to individuals and health-care systems. We therefore aimed to measure the prevalence and associated risk factors of MDR TB among TB patients in Makkah city.
Patients and Methods
This was a cross-sectional study conducted at Al-Noor Specialist Hospital, a public-sector hospital in Makkah. We included records of 158 confirmed TB patients from the list of all patients admitted in the hospital from January 2009 to January 2019 by systematic random sampling. Data were collected on socio-demographics, clinical profile and drug resistance patterns. Analysis was done in SPSS version 21.0.
Results
The mean age of the participants was 43.4 ± 18.7 years, and two-thirds (66.5%) were male. About 40% of the patients had chronic disease while lung disease other than TB was present in 5% patients. About 13% of cases were extrapulmonary infections. Prevalence of drug resistance was found to be 17.1% among TB patients. Among the resistant cases, streptomycin (25.9%) and isoniazid (11.1%) were the drugs most commonly affected by resistance. Prevalence of MDR TB was 5% among TB patients. Age, smoking, lung disease and previous TB were significant factors associated with MDR TB.
Conclusion
Prevalence of MDR TB, although comparable to current national estimates, is higher compared to previous reports. There is a need to reduce this burden through strengthening TB control programs to prevent further emergence of a public health threat of MDR TB. History of previous TB was the strongest risk factor in this study. This calls physicians, program managers and policy makers to focus on counselling and support of TB patients for compliance with the regimen to complete treatment without interruption.
Keywords: MDR TB, prevalence, risk factors, Saudi Arabia
Introduction
Tuberculosis (TB) is a major health challenge globally and countries around the world are striving to tackle this using high standards of prevention and control measures. Across the globe, over 10 million new cases of TB in 2018 and 1.2 million deaths resulted from this disease.1 By the year 2035, the World Health Organization (WHO) aims to achieve a reduction in the incidence rate of TB by 90% and a reduction of its deaths by 95%, with the ultimate goal of total eradication of TB worldwide.2 The annual TB incidence rate was 10/100,000 population in 2018 in Saudi Arabia.1 The most important factors behind this continued burden of TB in the Kingdom of Saudi Arabia (KSA) include Hajj and Umrah pilgrimage and a large proportion of expatriates, the majority of which belongs to Asia and Africa where TB burden is high.3
Treatment failure may result in multidrug-resistant tuberculosis (MDR TB) which is defined as bacilli resistant to both isoniazid and rifampicin with or without the involvement of other drugs.4 Based on the drug susceptibility testing (DST), the MDR TB is classified as: mono-resistant TB (resistance to only one first-line anti-TB drug), polydrug-resistant TB (resistance to conventionally more than one first-line anti-TB drug and they are neither isoniazid nor rifampicin), multidrug-resistant TB (MDR TB) (resistance to minimally both isoniazid and rifampicin), rifampicin-resistant TB (RR-TB) (resistance to rifampicin); and extensively drug-resistant TB (XDR-TB), which is resistance to any fluoroquinolones and at least one of the three second-line injectable drugs (amikacin, capreomycin, and kanamycin) in addition to multidrug resistance.5
MDR TB is an important problem globally because of its serious social and economic consequences. Makkah, being the pilgrimage hub, has potential for the spread of communicable diseases like any other place in the world where there are mass gatherings. People are in close contact with each other and for longer durations and therefore the risk of spread of respiratory infections is high. The literature on MDR TB in KSA is scarce and to the best of the authors’ knowledge no study has been conducted in Makkah to measure the prevalence and associated risk factors of MDR TB. This study therefore aimed to measure the prevalence and associated risk factors of MDR TB among TB patients in Makkah city.
Patients and Methods
Study Design and Setting
This was a cross-sectional study conducted in Makkah Al-Mukarramah, which is located in the western part of the KSA. It is about 400 km away from Madinah and 72 km from Jeddah. It contains Almasjed Alharam (Sacred Mosque) and the Kaaba, which is the Qibla for Muslims in their prayer. The city of Makkah also includes holy places for Muslims to perform Hajj such as Muzdalifah, Mina and Arafat. This study was carried out in Al-Noor Specialist Hospital, a public-sector hospital in Makkah.
Study Population
Officially registered confirmed TB patients al Al-Noor Specialist Hospital over the last 10 years, ie, January 2009 to January 2019.
We included all adult confirmed TB cases registered in the hospital. Those who were transferred out were excluded from the study.
Sample Size
According to the official statistics provided by Al-Noor Specialist Hospital administration, there was a total of 634 confirmed TB patients registered in the hospital records. We used an expected prevalence of MDR TB cases, reported to be 11.8%.6 At a confidence level of 95% and a tolerable error of 5%, the sample size for this study is calculated using the Raosoft statistical program to be 128 patients. The sample size was increased by 20% to compensate for any missing data. So the final sample size required was 154 participants.
Sampling Technique
After listing all 634 registered patients, participants in the Medical Record Numbers (MRN), systemic sampling technique was applied to it. The k-th number obtained was “4.11”, which we rounded off to “4” to avoid undersampling. Every fourth patient on the list was included in the sample population until the list was completed.
Data Collection Tool (Instrument) and Procedure
The researchers used a data collection sheet which was developed by the research team after review of literature and reviewed and approved by two infectious disease consultants. The data collection sheet had two sections. The first section collected information about socio-demographic and personal data including age, gender, nationality, place of residence, occupation, smoking, exercise, weight, height, and Body Mass Index (BMI). The second part included variables on clinical data including the presence of chronic diseases (diabetes, hypertension, asthma, hypercholesterolemia, among others), TB site, lung diseases, HIV, chronic renal failure, immunosuppression, previous TB treatment (whether completed or interrupted) and drug resistance.
All these clinical and laboratory data were extracted from the patients’ records. In practice, the diagnosis of TB infection is done through standardized sputum microscopy and chest X-ray. GeneXpert Polymerase Chain Reaction (PCR), which was introduced by Ministry of Health in KSA in 2015, as well as appropriate culture and sensitivity for all confirmed TB patients are done for drug resistance.
All selected files based on sampling strategy were assessed for eligibility. Once meeting the eligibility criteria, records were extensively reviewed by the researcher and data collectors. Anonymity of the participants was ensured by excluding from data collection any personal identifiers such as name, ID number or any other information that could reveal the identity of the participants. All the required data were documented in the data collection sheet. If there were any missing data with regard to cultures or needed investigations, a thorough search was done in the appropriate laboratory section in the hospital looking for the missing results. If the results were still missing, the participant’s file was replaced by the next one on the list.
Data Analysis
Data were entered and analyzed using the statistical package for social sciences (SPSS) version 24. Descriptive analysis was carried out to measure frequencies and proportions for categorical variables and means with standard deviations for continuous variables. Prevalence of primary and secondary outcomes, ie MDR TB and drug-resistant TB (resistant to any anti-TB drug), respectively, were determined. Univariate and multivariate logistic regression were used to assess the risk factors associated with outcome variables. Variables which had a p-value <0.25 in the univariate analysis or were biologically plausible were carried into multivariate models. Variables in the final multivariate models were retained based on the −2 log likelihood ratio and contribution in the overall model. We developed separate models for primary and secondary outcomes. A p-value less than 0.05 was considered statistically significant for all inferential analysis.
Ethical Considerations
This study was conducted in accordance with Declaration of Helsinki and approved from the IRB committee in Makkah Region (H-02-K-076-1119-219). All information was kept confidential and no personal identifiers were collected.
Results
A total of 158 records met our eligibility criteria and were included in the data analysis. The mean age of the participants was 43.4 ± 18.7 years and two-thirds (66.5%) were male. Almost half of the patients were Saudi (48.1%). Among non-Saudi participants the majority were from Pakistan (20.7%), followed by Myanmar and India with 12.2% and 9.8% respectively. The majority of the participants (84%) were resident in Makkah, and the remainder were visiting Makkah for pilgrimage. About 41% of the participants were ever smoker. Mean weight (kg) and height (cm) were 63.1 (±11.0) and 163.8 (±7.5), respectively. Mean BMI was 24.4 (±4.2) kg/m2. The proportion of underweight participants was 7.2% while 40.3% of the patients were overweight or obese. About 40% of the patients had chronic disease while lung disease other than TB was present in 5% patients. Only one participant (0.7%) was positive for HIV (Table 1).
Table 1.
Socio-Demographic and Physical Characteristics of Tuberculosis Patients (n = 158)
| Variables | Frequency | Percentage |
|---|---|---|
| Age Mean (SD) |
43.4 (18.7) |
|
| Gender Male Female |
105 53 |
66.5 33.5 |
| Nationality Saudi Non-Saudi |
76 82 |
48.1 51.9 |
| Non-Saudi (n = 82) Pakistan Myanmar India Yamen Other |
17 10 8 7 40 |
20.7 12.2 9.8 8.5 48.8 |
| Residence Makkah Outside Makkah |
132 26 |
83.5 16.5 |
| Occupation (n = 35) Student Teacher Jobless House wife Laborer Driver Nurse Retired House maid |
12 5 5 3 3 2 2 2 1 |
34.3 13.9 13.9 8.6 8.6 5.7 5.7 5.7 2.9 |
| Smoking (n = 103) Non-smoker Ever smoker |
61 42 |
59.2 40.8 |
| Weight (n = 140) Mean (SD) |
63.1 (11.0) |
|
| Height (n = 139) Mean (SD) |
163.8 (7.5) |
|
| BMI (n = 139) Mean (SD) |
24.4 (4.2) |
|
| BMI (n = 139) Normal Underweight Overweight/bese |
73 10 56 |
52.5 7.2 40.3 |
| Chronic disease Yes No |
64 94 |
40.5 59.5 |
| Lung disease Yes No |
8 150 |
5.1 94.9 |
| HIV nfection (n = 139) Yes No |
0.7 99.3 |
1 138 |
The majority of cases were pulmonary TB while 13.3% were extrapulmonary infections. Of the extrapulmonary cases, the most common site was the spine (61.9%) followed by lymphadenitis and meningitis (14.3% each). Fourteen (8.9%) patients had a history of previous TB infection, of which 64.3% (9) patients had interrupted TB treatment. Prevalence of drug resistance was found to be 17.1% among TB patients. Streptomycin (25.9%) and isoniazid (11.1%) were the drugs most commonly affected by resistance. Nearly half (44%) of the drug resistances cases were of monoresistance. Multidrug resistance was present in about 30% of the drug resistance cases. Nineteen percent of the cases were polyresistant (Table 2).
Table 2.
Characteristics of Tuberculosis (n = 158)
| Variables | Frequency | Percentage |
|---|---|---|
| TB site Pulmonary Extrapulmonary |
137 21 |
86.7 13.3 |
| Extrapulmonary site (n = 21) Spine Lymphadenitis Meningitis Abdomen Bone |
13 3 3 1 1 |
61.9 14.3 14.3 4.8 4.8 |
| Previous TB treatment Yes No |
14 144 |
8.9 91.1 |
| Status of previous treatment (n = 14) Completed Interrupted |
5 9 |
35.7 64.3 |
| Drug resistance Yes No |
27 131 |
17.1 82.9 |
| Names of drug (n = 27) Streptomycin Isoniazid Ethambutol Rifampicin Streptomycin–isoniazid Streptomycin–rifampicin Rifampicin–isoniazid Streptomycin–isoniazid–ethambutol Rifampicin–isoniazid–ethambutol Rifampicin–isoniazid–ethambutol–streptomycin Rifampicin–isoniazid–ethambutol–pyrazinamide Rifampicin–isoniazid–ethambutol–streptomycin–pyrazinamide |
7 3 2 2 2 2 2 1 1 2 1 2 |
25.9 11.1 7.4 7.4 7.4 7.4 7.4 3.7 3.7 7.4 3.7 7.4 |
| Resistance class (n = 27) MDR Mono Rifampicinesistant Polyresistant |
8 12 2 5 |
29.6 44.4 7.4 18.5 |
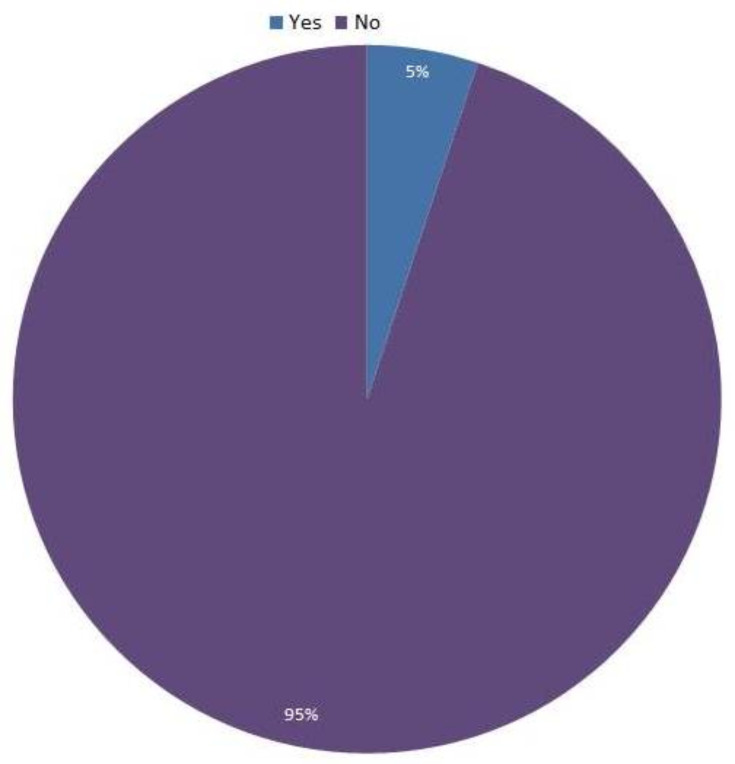
Figure 1 shows the prevalence of MDR TB among all TB patients, which was found to be 5%. The prevalence of MDR TB was significantly higher among patients who had TB previously (21.4%) compared to new cases, where the prevalence was 3.5%.
Figure 1.
Prevalence of MDR TB among TB patients in Makkah (n = 158).
Table 3 shows the factors associated with MDR TB among TB patients. In univariate analysis we found that MDR TB was associated with smoking [crude odds ratio (OR) 12.0 (95% CI: 1.42–101.62)] and previous TB [OR 7.6 (95% CI: 1.60–36.0)]. No association was found between MDR and age, gender, nationality, residence, BMI, presence of chronic disease, presence of lung disease and site of TB infection. Multivariate analysis showed that age was positively associated with risk of MDR TB [adjusted OR (aOR) 1.094 (1.016–1.179)]. Ever smoking was strongly associated with MDR TB [aOR 72.1 (95% CI: 2.41–2157.8)]. The presence of chronic disease was found to be protective against MDR TB [aOR 0.20 (95% CI: 0.001–0.37)]. The presence of lung disease and previous TB were also significant predictors of MDR TB in multivariate analysis.
Table 3.
Factors Associated with MDR TB and Anti-TB Drug Resistance in Makkah, KSA
| Variables | MDR Tuberculosis | Anti-TB Drug-Resistant TB | ||||||
|---|---|---|---|---|---|---|---|---|
| Crude OR (95% Confidence Interval) |
p-value |
Adjusted¥ OR (95% Confidence Interval) |
p-value |
Crude OR (95% Confidence Interval) |
p-value |
Adjusted¥ OR (95% Confidence Interval) |
p-value |
|
| Age | 1.01 (0.97–1.04) | 0.732 | 1.094 (1.016–1.179) | 0.017* | 1.01 (0.99–1.03) | 0.297 | 1.04 (1.004–1.071) | 0.029* |
| Gender Male Female |
1 0.65 (0.13–3.32) |
0.602 |
– |
– |
1 0.99 (0.41–2.38) |
0.980 |
– |
– |
| Nationality Saudi Non-Saudi |
1 1.58 (0.36–6.85) |
0.541 |
– |
– |
1 0.58 (0.25–1.35) |
0.206 |
– |
– |
| Residence Makkah Other |
1 0.00 (0.00–0.00) |
0.998 |
– |
– |
1 0.16 (0.02–1.26) |
0.082 |
– | – |
| Smoking Never Ever |
1 12.0 (1.42–101.62) |
0.023 |
1 72.1 (2.41–2157.8) |
0.014* |
1 1.83 (0.74–4.54) |
0.192 |
1 2.14 (0.79–5.82) |
0.136 |
| BMI Normal Underweight Overweight/obese |
1 2.59 (0.24–27.66) 1.80 (0.38–8.37) |
0.430 0.456 |
– |
– |
1 2.18 (0.49–9.64) 1.39 (0.57–3.37) |
0.305 0.471 |
1 3.90 (0.69–22.06) 1.57 (0.56–4.38) |
0.124 0.390 |
| Chronic disease No Yes |
1 0.20 (0.02–1.64) |
0.133 |
1 0.20 (0.001–0.37) |
0.009* |
1 0.56 (0.23–1.38) |
0.210 |
1 0.45 (0.14–1.46) |
0.184 |
| TB site Pulmonary Extrapulmonary |
1 2.30 (0.43–12.2) |
0.329 |
– |
– |
1 0.78 (0.21–2.88) |
0.715 |
– |
– |
| Lung disease No Yes |
1 2.92 (031–27.10) |
0.346 |
1 43.08 (1.06–1746.9) |
0.046* |
1 0.68 (0.08–5.78) |
0.725 |
– |
– |
| Previous TB No Yes |
1 7.6 (1.60–36.0) |
0.011 |
1 55.4 (3.26–941.4) |
0.005* |
1 1.36 (0.35–5.26) |
0.652 |
1 2.29 (0.46–11.36) |
0.310 |
Notes: ¥Mutually adjusted for each other. *p-value <0.05. – not included in the multivariate model.
We also looked for the factors associated with any type of anti-TB drug resistance. In the univariate analysis we found no significant association of drug resistance with any of the studied variables. In the multivariate model age was significantly associated with drug resistance and it was found that each year increase in age led to a 4% increase in the risk of drug resistance [aOR 1.04 (95% CI: 1.004–1.071)]. Unlike MDR TB, chronic disease and previous TB were not significantly associated with drug resistance (Table 3).
Discussion
This is one of the few studies from the western region of Saudi Arabia to measure the burden and risk factors of MDR TB. We found that the prevalence of MDR TB was 5% among TB patients. The prevalence of any anti-TB drug resistance was found to be 17.1%. The prevalence of MDR TB was significantly higher among patients with a previous history of TB, 21.4% versus 3.5% in new cases. Age, smoking, chronic disease, lung disease other than TB and history of previous TB were factors significantly associated with MDR TB.
The prevalence of MDR TB (5%) reported in this study is comparable to national estimates, which have been reported to be around 4.4%.7 However, a previous national survey reported a lower prevalence (1.4%) of MDR TB in Saudi Arabia.8 A possible reason for this low prevalence could be low detection of MDR TB previously. Prevalence in this study is similar to a recent study from Madinah Almunawarrah, where it was reported to be 4.0%.9 However, a study from the Najran region of Saudi Arabia reported a very high prevalence (20.6%) of MDR TB.10 A possible reason for this high prevalence as mentioned by the authors is that the Najran region is close to the border of Yemen, which has the highest TB burden in the region and there was frequent movement of people across the border. Another possible reason for this high proportion of MDR TB could be the study setting from where the sample was drawn. They recruited participants from a chest clinic, which may have more severe/resistant cases than other settings. The prevalence of MDR TB in our study is also comparable to the estimated prevalence in Gulf Cooperation Countries (GCC) of 4%. However, it ranges from 1.7% to 6.3%.11
The prevalence of any drug resistance was 17% in our study, which is slightly higher than national estimates (15.5%).8 However, a higher prevalence of drug resistance was reported from Turkey (29%).12 Streptomycin and isoniazid were the drugs most commonly affected by resistance in our study. A similar pattern has been reported in national surveys from Saudi Arabia.3,7
The prevalence of MDR TB among patients with previous history of tuberculosis has been consistently reported to be high.3,11,13,14 We also found very high prevalence of MDR TB among patients with previous TB history. This prevalence in our study was higher, 21.4% compared to 15.9% reported previously from Saudi Arabia.3 Prevalence among newly diagnosed cases is higher in our study compared to that reported in previous studies from Saudi Arabia.3,7 These higher rates reported in our study compared to other studies from KSA could be due to fact that this study was conducted in the holy city of Makkah, which is the main pilgrimage center and millions of people visit the place year-round. This also includes people from high TB burden countries. It has been reported that during Hajj season, TB was the most common reason for hospitalization.15 Another study from Russia reported a very high prevalence (19.6%) of MDR among new cases.16
The association of age with risk of MDR TB is inconsistent and inconclusive in literature. We found that increasing age was associated with increased risk of MDR TB. This finding is in contrast to the findings of studies from Saudi Arabia,7 Ethiopia17 and Europe18 where they found a negative association between age and risk of MDR TB. However, other investigators did not find any significant association of age with risk of MDR TB.19–21 One study reported a positive association between age and MDR TB.22 This difference could be due to fact that the age range of the studied population varied from study to study. Moreover, an inadequate sample size could also explain this inconsistency of association of age with MDR TB. Smoking was found to be significantly associated with increased risk of MDR TB in our study. This finding is similar to a study from Russia.16 We found that the presence of chronic disease was protective against MDR TB. This finding is in contrast to other studies which have reported increased risk of MDR TB with chronic diseases such as chronic obstructive pulmonary disease and diabetes mellitus.23,24 There is the possibility that patients with chronic disease are more health-conscious and compliant with the treatment regimen because of their longstanding diseases.
A previous history of TB has been widely reported as risk factor of MDR TB. We found a strong association between previous TB and MDR TB. This finding is consistent with studies from Saudi Arabia and other parts of the world.3,7,17,25,26 This finding has important implications for all the stakeholders in TB control, including policy makers, program implementers, physicians and other health workers and patients that compliance to the treatment is an essential part in TB management. Policy makers and implementers should ensure adequate and uninterrupted supply of anti-TB drugs. Physicians and health-care workers should counsel and educate patients and their families about their treatment regimen and ensure close follow-up of the patients. The patients should comply with the advice of health-care providers and seek regular follow-ups.
This study, to the best of our knowledge, is the only study from Makkah to report prevalence and risk factors of MDR TB. This study was done in a large public-sector hospital of Makkah and included confirmed cases of TB. However, certain limitations need to be considered while interpreting the findings of this study. First, this study was done in a single center which may limit its generalizability. However, the pattern of socio-demographic characteristics of the sample is similar to other studies from KSA. Second, we did a power calculation for the prevalence only. The sample may not be adequately powered for the risk factors assessed in this study. This is also evident from the very wide confidence intervals. Third, the cross-sectional nature of the study does not allow establishing a temporal relationship between exposure and outcome variables. Lastly, any comparison with the findings of other studies should be interpreted cautiously as there are variations in the study designs, settings, populations and methods used.
Conclusion
The prevalence of MDR TB in this study is comparable to estimates from Saudi Arabia. However, there is still a need to reduce this burden through strengthening TB control programs to prevent further emergence of a public health threat of MDR TB. We also investigated putative risk factors of MDR TB. Increasing age, smoking, and previous TB were factors associated with higher risk of MDR TB. History of previous TB was the strongest risk factor in this study. This calls physicians, programs managers and policy makers to focus on counselling and supporting TB patients for compliance with the regimen to complete treatment without interruption.
Funding Statement
There is no funding to report.
Disclosure
The author reports no conflicts of interest in this work.
References
- 1.WHO. Global Tuberculosis Report 2019. Geneva: World Health Organization; 2019. [Google Scholar]
- 2.Kumar K, Kon O. Diagnosis and treatment of tuberculosis: latest developments and future priorities. Ann Res Hosp. 2017;10. [Google Scholar]
- 3.Al-Hajoj S, Varghese B, Shoukri MM, et al. Epidemiology of antituberculosis drug resistance in Saudi Arabia: findings of the first national survey. Antimicrob Agents Chemother. 2013;57(5):2161–2166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Control C. Prevention. Primary multidrug-resistant tuberculosis–Ivanovo Oblast, Russia, 1999. MMWR Morb Mortal Wkly Rep. 1999;48(30):661. [PubMed] [Google Scholar]
- 5.Tuberculosis I, Disease L. Field guide for the management of drug-resistant tuberculosis. Int Union Against Tuberculosis Lung Dis. 2018;254. [Google Scholar]
- 6.Abu-Amero KK. Review of the current status of drug-resistant tuberculosis in Saudi Arabia. Ann Saudi Med. 2002;22(3–4):236–238. [DOI] [PubMed] [Google Scholar]
- 7.Al Ammari M, Al Turaiki A, Al Essa M, Kashkary AM, Eltigani SA, Ahmed AE. Drug resistant tuberculosis in Saudi Arabia: an analysis of surveillance data 2014–2015. Antimicrob Resist Infect Control. 2018;7:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ali Chaudhry L, Rambhala N, Al-Shammri AS, Al-Tawfiq JA. Patterns of antituberculous drug resistance in Eastern Saudi Arabia: a 7-year surveillance study from 1/2003 to 6/2010. J Epidemiol Glob Health. 2012;2(1):57–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Elhassan MM, Hemeg HA, Elmekki MA, Turkistani KA, Abdul-Aziz AA. Burden of Multidrug Resistant Mycobacterium tuberculosis Among New Cases in Al-Madinah Al-Monawarah, Saudi Arabia. Infect Disord Drug Targets. 2017;17(1):14–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Asaad AM, Alqahtani JM. Primary anti-tuberculous drugs resistance of pulmonary tuberculosis in Southwestern Saudi Arabia. J Infect Public Health. 2012;5(4):281–285. doi: 10.1016/j.jiph.2012.03.005 [DOI] [PubMed] [Google Scholar]
- 11.Areeshi MY, Bisht SC, Mandal RK, Haque S. Prevalence of drug resistance in clinical isolates of tuberculosis from GCC: a literature review from January 2002 to March 2013. J Infect Dev Countries. 2014;8(09):1137–1147. [DOI] [PubMed] [Google Scholar]
- 12.Department of TB Control. The 2012 Report of Fighting Tuberculosis in Turkey. Ankara, Turkey: Ministry of Health; 2013. [Google Scholar]
- 13.Ahmed MM, Velayati AA, Mohammed SH. Epidemiology of multidrug-resistant, extensively drug resistant, and totally drug resistant tuberculosis in Middle East countries. Int j Mycobacteriology. 2016;5(3):249–256. [DOI] [PubMed] [Google Scholar]
- 14.World Health Organization. Global Tuberculosis Report 2015 2015. Geneva, Switzerland: WHO; 2015. [Google Scholar]
- 15.Alzeer A, Mashlah A, Fakim N, et al. Tuberculosis is the commonest cause of pneumonia requiring hospitalization during Hajj (pilgrimage to Makkah). J Infect. 1998;36(3):303–306. [DOI] [PubMed] [Google Scholar]
- 16.Ruddy M, Balabanova Y, Graham C, et al. Rates of drug resistance and risk factor analysis in civilian and prison patients with tuberculosis in Samara Region, Russia. Thorax. 2005;60(2):130–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mulu W, Mekkonnen D, Yimer M, Admassu A, Abera B. Risk factors for multidrug resistant tuberculosis patients in Amhara National Regional State. Afr Health Sci. 2015;15(2):368–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Faustini A, Hall AJ, Perucci CA. Risk factors for multidrug resistant tuberculosis in Europe: a systematic review. Thorax. 2006;61(2):158–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Demile B, Zenebu A, Shewaye H, Xia S, Guadie A. Risk factors associated with multidrug-resistant tuberculosis (MDR-TB) in a tertiary armed force referral and teaching hospital, Ethiopia. BMC Infect Dis. 2018;18(1):249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mekonnen F, Tessema B, Moges F, Gelaw A, Eshetie S, Kumera G. Multidrug resistant tuberculosis: prevalence and risk factors in districts of metema and west armachiho, Northwest Ethiopia. BMC Infect Dis. 2015;15(1):461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Metanat M, Sharifi-Mood B, Shahreki S, Dawoudi S. Prevalence of multidrug-resistant and extensively drug-resistant tuberculosis in patients with pulmonary tuberculosis in zahedan, southeastern iran. Iran Red Crescent Med J. 2012;14(1):53. [PMC free article] [PubMed] [Google Scholar]
- 22.Mehari K, Asmelash T, Hailekiros H, et al. Prevalence and Factors Associated with Multidrug-Resistant Tuberculosis (MDR-TB) among Presumptive MDR-TB Patients in Tigray Region, Northern Ethiopia. Canadian J Infect Dis Med Microbiol. 2019;2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tegegne BS, Habtewold TD, Mengesha MM, Burgerhof JGM. Association between diabetes mellitus and multi-drug-resistant tuberculosis: a protocol for a systematic review and meta-analysis. Syst Rev. 2017;6(1):6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhao J-N, Zhang X-X, He X-C, et al. Multidrug-resistant tuberculosis in patients with chronic obstructive pulmonary disease in China. PLoS One. 2015;10:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gomes M, Correia A, Mendonça D, Duarte R. Risk factors for drug-resistant tuberculosis. J Tuberculosis Res. 2014;2014. [Google Scholar]
- 26.Stosic M, Vukovic D, Babic D, et al. Risk factors for multidrug-resistant tuberculosis among tuberculosis patients in Serbia: a case-control study. PLoS One. 2018;18(1):1114. [DOI] [PMC free article] [PubMed] [Google Scholar]