Abstract
Introduction
Imidacloprid is the most commonly used neonicotinoid insecticide worldwide. Despite its reputation for safety, there is increasing evidence regarding its toxicity. This study characterized the clinical manifestations and outcomes of acute imidacloprid poisoning.
Methods
This was a retrospective study of patients with imidacloprid poisoning who were referred to the Ramathibodi Poison Center in Bangkok, Thailand between 2010 and 2018.
Results
A total of 163 patients with imidacloprid-only exposure were included. Most were exposed by ingestion (93.3%). The patients were predominantly male (55.8%), with a median age of 41.3 years. The common presenting features were gastrointestinal symptoms (63.8%) with no corrosive injuries and neurological effects (14.2%). The majority of medical outcomes was no (18.4%) to mild (76.1%) toxicity. One patient had symptoms mimicking cholinergic syndrome, three developed liver injury, and five died. Among the five deaths, two patients presented severe initial severity, and one presented moderate initial severity. Two of the patients who died initially presented only mild severity. The mortality rate was 3.1%. The estimated amount of ingestion, cardiovascular effects (especially tachycardia and cardiac arrest), central nervous system effects (especially coma), dyspnea, and diaphoresis were significantly associated with mortality. Patient management primarily included supportive and symptomatic care.
Conclusion
Most patients with imidacloprid poisoning developed only mild toxicity. The mortality rate was low, but a few patients with mild initial severity died. Patients who ingest a large amount or show these warning signs including cardiovascular effects, central nervous system effects, dyspnea, and diaphoresis at the initial presentation should be considered for close observation and monitoring.
Keywords: imidacloprid, neonicotinoid insecticide, poisoning, toxicity, humans
Introduction
Neonicotinoids were developed to replace older and more harmful insecticides.1–3 They are one of the most popular and widely used insecticides in the world.1–3 Many synthetic neonicotinoids are currently marketed including acetamiprid, clothianidin, dinotefuran, flonicamid, imidacloprid, nitenpyram, thiacloprid, and thiamethoxam.1,3 Among these, imidacloprid is the most commonly used.1,3 Imidacloprid functions as a nicotinic acetylcholine receptor (nAChR) agonist, particularly for the α4β2 subtype, and induces neuromuscular paralysis and death in insects.4,5 It is believed to be less toxic to humans owing to its higher affinity for insect nAChRs and its inability to penetrate the mammalian blood–brain barrier.4,5 Despite its safety profile, imidacloprid toxicity has been previously reported in many countries. The first case report was documented from Taiwan in 2001.6 Most cases later were reported in Taiwan,7 Sri Lanka,8 Korea,9 United Kingdom10 and the United States.11 In addition, several sporadic cases have been published such as four cases were in India,12–15 five cases in Taiwan,16–19 one case each in Iran,20 Turkey,21 Saudi Arabia,22 Japan23 and two cases in Portugal.24 The clinical manifestations of this toxicity include nausea, vomiting, diarrhea, abdominal discomfort, headache, and in severe cases, dyspnea/apnea, coma, tachycardia, and hypotension.8,19 Deaths resulting from neonicotinoid poisoning have mostly been caused by imidacloprid; however, fatality associated with imidacloprid is low ranging from 0% to 4.2%.7–9,11 Sporadic dead cases from acute imidacloprid poisoning are also reported in the literature.14,18–20,23,24 The management is mainly supportive.7,8,19
Imidacloprid was introduced into the Thai market in 1995.25 It is currently the most common neonicotinoid insecticide in Thailand.25 Studies of imidacloprid poisoning in Southeast Asia including Thailand are limited. Therefore, the objective of this study was to characterize the clinical manifestations and the clinical outcomes of acute imidacloprid poisoning in Thailand.
Materials and Methods
This was a retrospective study that reviewed cases of imidacloprid poisoning who were referred and consulted to the Ramathibodi Poison Center (RPC) between January 2010 and December 2018. The RPC is based in a tertiary teaching hospital and responds to inquiries and provides toxicological information for both healthcare professionals and the general public 24 hours a day, every day of the year. Most calls to the RPC are from medical personnel. There are typically 15,000–25,000 calls per year. Follow-up calls are periodically made to collect and monitor case progress, to provide ongoing treatment recommendations, and to determine the medical outcomes of cases. All cases are recorded in the RPC Toxic Surveillance System database and are verified by a team of information scientists and medical toxicologists. The primary outcomes were the clinical characteristics and outcomes of patients with imidacloprid poisoning. The secondary outcome was factors associated with mortality.
All instances of human exposures to imidacloprid that were documented in the RPC Toxic Surveillance System database were retrieved. The diagnosis of imidacloprid poisoning was based on clinical data including a history of exposure to products that had either the trade name, generic name, or formula of imidacloprid on the container label. The collected data included demographic data, reason for exposure, amount of exposure, duration from exposure to arrival at a healthcare facility, clinical features, laboratory results, treatment modalities, initial severity, and clinical outcome. Clinical severity of poisoning was divided into none, minor, moderate, major severity, and death. The definitions and terms used in the database have been adopted from the International Programme on Chemical Safety (IPCS) INTOX Data Management System.26 Patients who had coingested imidacloprid together with other pesticides, illicit drugs or overdose of medications at the presentation, were excluded from the study.
The amount of imidacloprid exposure was calculated and is shown in grams. The ingested volume was estimated by 1 mouthful being equal to 25 milliliters (mL) in adults, 9 mL in children,7 and 1 cup equaling 250 mL. Imidacloprid-containing products in Thailand have many different concentrations and formulations.27,28 The size of bottles and sachets varies among different products or even different formulations.27,28 Many of the cases described their ingestion of imidacloprid, but there was no associated container or concentration information. In such cases, it was not possible to estimate the amount of exposure.
Hypertension was defined as blood pressure higher than 130 over 80 millimeters of mercury (mmHg),29 while hypotension was defined as systolic blood pressure less than 90 mmHg.30 A heart rate greater than 100 beats per minute was defined as tachycardia, and a heart rate less than 60 beats per minute was defined as bradycardia.31 The normal vital signs in pediatric patients were based on the normal values for each age.32 Acute liver injury was defined by increased serum alanine aminotransferase >5 times the upper limit of normal (ULN) and/or of alkaline phosphatase >2 times the ULN.33 Liver injury pattern was classified following the updated RUCAM (Roussel Uclaf Causality Assessment Method).33 Information regarding the total Thai population per year was obtained online via the published annual reports of the Strategy and Planning Division, Ministry of Public Health, Thailand.34
This study was approved by the Institutional Ethics Committee Board of the Ramathibodi Hospital Faculty of Medicine, Mahidol University (COA.MURA2019/6100, Date of Approval: July 11th, 2019). Patient consent was not required by the ethics committee board because this study used the preexisting confidential database from RPC and all results were reported anonymously.
Statistical Analysis
The data were analyzed with IBM SPSS Statistics for Windows, version 18 (IBM Corp., Armonk, NY, USA) and managed using Microsoft Excel. Descriptive statistics were performed to characterize the data. The mean, median, minimum, maximum, standard deviation, and interquartile range (IQR) were analyzed for continuous data, while the frequency and percentage were assessed for categorical data. Between-group comparisons were performed by Student’s t-test if the data were normally distributed and by the Mann–Whitney U-test if they were not normally distributed. Differences in categorical variables were evaluated by chi-squared analysis and Fisher’s exact test. A p-value of 0.05 was considered to be statistically significant.
Results
Demographic Data
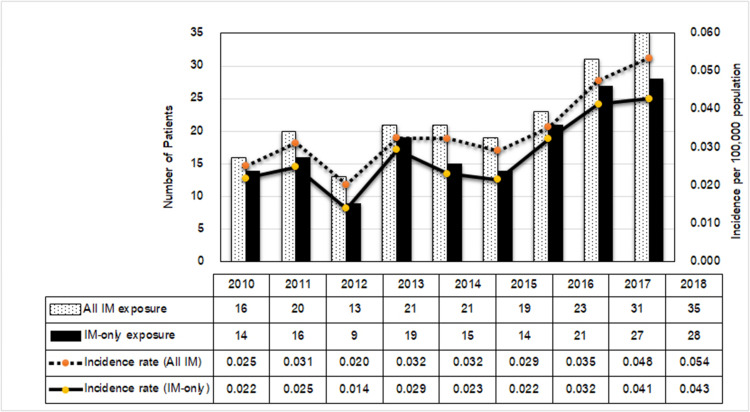
During the 9-year period, 199 cases of imidacloprid exposure were reported to the RPC. The median annual number of exposures was 21 cases (IQR17.5–27 cases), with an incidence rate ranging from 0.025 to 0.054 per 100,000 population. The reported cases and the incidence rate increased dramatically since 2016, as shown in Figure 1. Thirty-six patients were co-exposed to other chemicals; therefore, 163 patients with imidacloprid-only exposure were included in this study.
Figure 1.
Number of reported imidacloprid (IM) exposures between all and single (IM-only) exposure, and the incidence rate (per 100,000 population). The total population in Thailand from 2010 to 2018 was 63,701,703–65,406,320 people.
Table 1 summarizes the demographic and exposure characteristics of patients with acute imidacloprid exposure. Most patients were male (55.8%) and were predominantly located in the northeastern region (35.0%) and central region (29.5%) of Thailand. The mean age was 41.3 years (range 2–88 years). The main circumstance of exposure was intentional or suicide attempts (62.6%), followed by accidental (35%) and occupational (2.4%). Ingestion (93.3%) was the major route of exposure. The median dose of ingestion was 2.5 grams (g) (IQR 1.4–5 g). The most common formulations were 70% water-dispersible granules or powder (49%) and 10% weight/volume soluble concentrates (19%).
Table 1.
Demographic and Exposure Characteristics of Patients with Acute Imidacloprid Exposure
| Characteristics | Number of Cases (%) |
|---|---|
| Sex | |
| Male | 91 (55.8) |
| Female | 72 (44.2) |
| Age in years, mean ± SD (min-max) | 41.3 ± 22.2 (2–88) |
| Region of exposure | |
| Northeast | 57 (35.0) |
| Central | 48 (29.5) |
| North | 22 (13.5) |
| East | 14 (8.6) |
| West | 12 (7.4) |
| South | 10 (6.1) |
| Route of exposure | |
| Oral | 152 (93.3) |
| Inhalation | 7 (4.3) |
| Dermal | 2 (1.2) |
| Ocular | 1 (0.6) |
| Dermal and inhalation | 1 (0.6) |
| Concentrations and formulations | |
| 70% WG/WS | 80 (49.0) |
| 10% w/v SL | 31 (19.0) |
| 5% w/v EC | 8 (4.9) |
| 35% w/v EC | 3 (1.8) |
| 10% WG | 3 (1.8) |
| 0.5% w/w | 3 (1.8) |
| 20% w/v EC | 1 (0.6) |
| 0.03% w/v | 1 (0.6) |
| Unknown | 33 (20.2) |
| aEstimated amount in grams, median (IQR); | 2.5 (1.4–5.0) |
| - Intentional exposure | 2.5 (1.4–5.6) |
| - Accidental exposure | 2.5 (1.4–3.75) |
| bTime to hospital in hours, median (IQR) | 1 (0.5–2.7) |
| Admitted to the hospital | |
| No | 28 (17.2) |
| Yes | 135 (82.8) |
| Length of stay in days, median (IQR) | 1 (1–2) |
Notes: aData on estimated amount of ingestion were available for 68 patients; intentional exposure for 55 patients, accidental exposure for 13 patients. bDuration from exposure to arrival at healthcare facility.
Abbreviations: WG/WS, water-dispersible granules/powders; w/v, weight by volume; SL, soluble concentrates; w/w, weight by weight; EC, emulsifiable concentrates.
Clinical Presentation and Medical Outcome
The median time from exposure to presentation at a hospital was 1 hour (IQR 0.5–2.7 hours). At the time of consultation with the RPC, the majority of the cases had mild initial severity (73.0%), with symptoms such as nausea/vomiting, abdominal pain, drowsiness, headache, or dizziness; 24.5% had no symptoms. Two patients initially presented with signs of moderate toxicity including hypotension/hypertension, brady/tachycardia, and dyspnea. The other two patients had severe initial effects, including cardiac arrest, coma, hypotension, tachycardia, or dyspnea requiring endotracheal intubation. The majority of the clinical outcomes were mild (76.1%) or no effect (18.4%). Four patients (2.5%) had moderate outcomes (Table 2). The first patient was a 55-year-old male who presented with vomiting, diaphoresis, hypotension (blood pressure 87/63 mmHg), and bradycardia (heart rate 56 beats/min) after the deliberate ingestion of 30 mL of 5% weight/volume imidacloprid emulsifiable concentrate (estimated 1.5 g) 1 hour prior to arriving at the hospital. This patient responded to fluid resuscitation and a dose of pralidoxime (2 g) and atropine (1.8 milligrams), which was administered because of the suspicion of organophosphorus insecticide poisoning. He was discharged home on the second day. The other three patients with moderate outcomes developed liver injury; these were patients 1, 2, and 4 as described in a previous publication by Sriapha et al.35 Among these three patients, the first two were given N-acetylcysteine, while the other received supportive treatment, and all recovered. Five patients died in this study, giving a mortality rate of 3.1% (Table 2). Additional details of the deaths are provided in Table 3.
Table 2.
Medical Outcome by Severity of Initial Signs and Symptoms
| Initial Severity | Medical Outcome Number of Cases (%) |
Total Number of Cases (%) |
|||
|---|---|---|---|---|---|
| No Effects | Minor | Moderate | Death | ||
| No effects | 30 (75) | 8 (20) | 2 (5) | 0 | 40 (24.5) |
| Mild | 0 | 116 (97.5) | 1 (0.8) | 2 (1.7) | 119 (73) |
| Moderate | 0 | 0 | 1 (50) | 1 (50) | 2 (1.2) |
| Severe | 0 | 0 | 0 | 2 (100) | 2 (1.2) |
| Total | 30 (18.4) | 124 (76.1) | 4 (2.5) | 5 (3.1)a | 163 (100) |
Note: aThe mortality rate was 3.1%.
Table 3.
Details of Fatal Cases
| Patient No. |
Sex/Age (Year) | Formulation | Dose Ingested (Grams) |
Timea to Hospital (Hours) | Initial Severity | Clinical Manifestations | Timeb to Death |
|---|---|---|---|---|---|---|---|
| 1 | Male/47 | Unknown | NA | 2 | Severe | Coma and cardiopulmonary arrest | 2 days |
| 2 | Male/52 | 70% WG | 20 | NA | Severe | Nausea/vomiting, diaphoresis, hypotension, tachycardia, progressive coma, cardiovascular collapse. | 5 hours after arrival |
| 3 | Female/64 | 10% w/v SL | NA | 8 | Moderate | Dizziness, dyspnea, tachycardia and hypertension, respiratory failure and cardiac arrest | 13 hours |
| 4 | Male/88 | 35% w/v EC | 70 | 1 | Mild | Tachypnea, diaphoresis, mental status changes, hypotension, tachycardia, and prolonged shock | 22 hours |
| 5 | Female/49 | 10% w/v SL | 40 | 6 | Mild | Nausea/vomiting, burning sensation of throat, dyspnea, confusion, and cardiopulmonary arrest | 2 days |
Notes: aDuration from exposure to arrival at healthcare facility. b Time until patient died after ingestion.
Abbreviations: NA, not applicable; WG, water-dispersible granules; w/v, weight by volume; SL, soluble concentrates; EC, emulsifiable concentrates.
All patients who died ingested the substance intentionally, and all died within 2 days after ingestion. To determine the factors in the initial presentation that might be associated with mortality, we performed a subgroup analysis between the patients who survived and those who died (Table 4). The factors showing significant differences between the two groups were the estimated amount of ingestion, cardiovascular effects (especially tachycardia and cardiac arrest), central nervous system effects (especially coma), dyspnea, and diaphoresis at the initial presentation.
Table 4.
Subgroup Analysis of Clinical Manifestations Between Patients Who Survived and Those Who Died
| Clinical Manifestations | Survived (n = 158) |
Died (n = 5) |
p-value* |
|---|---|---|---|
| Number (%) male to female | 88:70 (55.7:44.3) | 3:2 (60:40) | 1.00 |
| Age in years, mean ± SD (min-max) | 40.7 ± 22.1(2–86) | 60 ± 16.9(47–88) | 0.056 |
| Number (%) of age in years | 0.496 | ||
| Less than 5 years | 18(11.4) | 0 | |
| 6–12 years | 2 (1.3) | 0 | |
| 13–19 years | 7 (4.4) | 0 | |
| 20–39 years | 43(27.2) | 0 | |
| 40–59 years | 54(34.2) | 3 (60) | |
| More than 60 years | 34 (21.5) | 2 (40) | |
| Time to hospital in hours, median (min-max); data available for 160 patients | 1 (0.17–72) n = 156 |
4 (1–8) n = 4 |
0.064 |
| Estimated ingestion amount in grams, median (min-max); data available for 68 patients | 2.5 (0.1–30) n = 65 |
40 (14–87.5) n = 3 |
0.004** |
| Number (%) of initial signs and symptomsa | |||
| Gastrointestinal: | 98 (62.0) | 2 (40) | 0.376 |
| Nausea/vomiting | 84 (53.2) | 2 (40) | 0.668 |
| Abdominal pain | 33 (20.9) | 0 | 0.584 |
| Burning sensation in throat | 11 (7.0) | 1 (20) | 0.321 |
| Cardiovascular: | 13 (8.2) | 3 (60) | 0.007** |
| Tachycardia | 3 (1.9) | 3 (60) | <0.001** |
| Bradycardia | 1 (0.6) | 0 (0) | 1.000 |
| Hypertension | 8 (5.1) | 1 (20) | 0.250 |
| Hypotension | 2 (1.3) | 1 (20) | 0.090 |
| Cardiac arrest | 0 | 1 (20) | 0.031** |
| Central nervous system: | 5 (3.2) | 3 (60) | 0.001** |
| Dizziness | 20 (12.7) | 1 (20) | 0.503 |
| Drowsiness | 5 (3.2) | 1 (20) | 0.173 |
| Headache | 4 (2.5) | 0 | 1.000 |
| Coma | 0 | 1 (20) | 0.031** |
| Respiratory: | |||
| Dyspnea | 0 | 2 (40) | 0.001** |
| Other: | |||
| Muscle twitchingb | 2 (1.3) | 0 | 1.000 |
| Diaphoresis | 4 (2.5) | 2 (40) | 0.011** |
| Salivation | 6 (3.8) | 0 (0) | 1.000 |
| Paresthesiab | 3 (1.9) | 0 (0) | 1.000 |
Notes: aData from all routes of exposure. bSymptoms occurred only after dermal or inhalational exposures. * Comparisons between-group were performed by Student’s t-test if the data were normally distributed and by the Mann–Whitney U-test if they were not normally distributed. Differences in categorical variables were evaluated by chi-squared analysis and Fisher’s exact test. ** Statistically significant.
Management
Most patients (82.8%) were admitted to the hospital. The median length of hospital stay was 1 day (range 0.13–11 days). Management mainly included symptomatic and supportive care. Treatment modalities included intravenous fluids (67.5%), gastric lavage (63.8%), a single dose of activated charcoal (62.6%), and oxygen therapy (4.9%). Endotracheal intubation and inotropic drug infusion were performed in five (3.1%) dead patients. Atropine and/or pralidoxime were administered to two patients because they had diaphoresis and bradycardia, which led the treating physician to suspect organophosphorus or carbamate poisoning. N-acetylcysteine was used to treat two patients who developed liver injury.
Discussion
This study described the instances of imidacloprid exposures reported to the RPC. With 163 cases analyzed over a 9-year period, this is one of the largest studies to date on imidacloprid poisoning, especially regarding intentional exposure. The reported cases were indicated predominantly in the Central and the Northeastern regions of Thailand which is the agricultural areas of Thailand where pesticides are commonly applied.36 A highly potential risk of poisoning was found in adult male greater than female. This finding is consistent with previous publication. Tawatsin et al report the highest risk group of pesticide poisoning in male aged 45–54 years which is the main labor force in the agricultural sector of Thailand.36 In the present study, self-poisoning by ingestion was the major route of imidacloprid exposure. Poisonings in adults tend to be more severe and can even result in death, particularly in patients with the intention to self-harm. This might be explained by patients with intentional exposure or suicide attempts frequently expose by oral route and might receive higher doses when compared to other routes and accidental exposure.
The exposure incidence rate was quite low; however, the annual number of exposures increased over the studied time period, a finding similar to previous investigations that have analyzed data from poison centers.7,11
Imidacloprid and other neonicotinoid insecticides are theoretically safer than the anticholinesterase insecticides.7 Our findings confirm this claim because most exposures were non-toxic or asymptomatic, minimally toxic, or had at most a mild effect. The mortality rate was 3.1%, which is consistent with previous studies that have reported low mortality rates ranging from 0% to 4.2%.7–9,11 The main clinical features of acute imidacloprid poisoning found in this study were also similar to previous findings.7,8,10,11 Most patients with oral exposure had only minimal or no symptoms, but a small percentage developed more severe symptoms such as respiratory failure and coma. Therefore, oral exposure was the main route causing apparent systemic toxicity from imidacloprid. Because imidacloprid is an nAChR agonist, some patients exhibited symptoms mimicking cholinergic syndrome (eg, diaphoresis, salivation, and bradycardia). This finding has also been described in other previous reports.7,8,17,36 Interestingly, no patients in our study presented seizure or the nicotinic symptoms of neuromuscular junctions, such as muscle fasciculation or weakness. Additional study is needed to further clarify this finding.
Imidacloprid-containing products have many different concentrations and formulations. The solvent, N-methyl-2-pyrrolidone (NMP), which is present in imidacloprid available in Taiwan and Sri Lanka, has been postulated as the etiology of corrosive injuries, such as oral ulcers, dysphagia, and odynophagia.6–8 These reports are in contrast to our findings. A burning sensation in the throat and abdominal pain were the only local irritation symptoms that were reported by patients in the current study. This discrepancy might be explained by the different formulations and solvents in the products sold in different countries. The most common forms of imidacloprid that our patients were exposed to were water-dispersible granules and soluble liquid concentrates rather than a solvent-based formulation.
There were three patients in this study that developed liver injury. Liver injury patterns included hepatocellular, cholestatic, and mixed. Additional details are described in Sriapha et al.35
All of our dead cases were adult or elderly patients with deliberate ingestion. Among the five deaths, two patients had severe toxic effects at presentation. Curiously, the remaining three fatalities initially had only mild to moderate clinical signs and symptoms, but a few hours later, they developed serious effects and eventually died. Because no other conditions or complications during hospitalization were described as the cause of death in the patients who died, imidacloprid toxicity was likely the main contributor to the death of these five patients. Huang et al reported recurrent ventricular fibrillation in a woman with coronary artery disease and imidacloprid poisoning.18 Life-threatening arrhythmia was indicated as a cause of death for this patient.18 Unfortunately, electrocardiography and cardiac biomarkers during cardiopulmonary resuscitation were not noted in the records of these fatal cases. Imidacloprid poisoning is reported to cause severe neurological effects.19,21 Neurological depression that decreases airway protection, cardiac depression that aggravates the respiratory load, respiratory depression, and muscle paralysis might contribute to the development of respiratory failure.7,19 Accordingly, a longer observation period is necessary regardless of the patient’s initial severity.
There are many factors during the initial presentation that are significantly associated with mortality. In this study, the presence of cardiovascular effects (especially tachycardia and cardiac arrest), central nervous system symptoms (particularly coma), dyspnea, diaphoresis, and a large amount of substance ingestion are warning signs of mortality in acute imidacloprid poisoning. Most of these factors are similar to those identified in a previous study,19 except diaphoresis and the amount of ingestion. Previous studies have found that the severity of imidacloprid poisoning is not proportional to the amount of ingestion or the plasma concentration.7,8,19 This differs from our study as well. Hence, patients with acute imidacloprid poisoning who exhibit these significant life-threatening warning signs should be considered for close monitoring, observation, and aggressive management.
This study has some limitations. First, it is not mandatory to report potentially adverse exposures to imidacloprid insecticides to the RPC. Thus, not all exposures are reported, especially the negligible and mild cases. In addition, it is possible that the true rate of severe poisoning, liver injury, and mortality may be different. Second, the retrospective study design may have resulted in missing or incomplete data. Medical history was obtained from patients, which they recognized, reported to the medical personnel; therefore, sometimes this might not be clearly or completely accurate. If the symptom was not recorded in our database, it would not be included in our results in this study. Third, the diagnosis was based on a history of exposure, but not all histories may have been clear or completely accurate. Forth, there were a small number of dead patients in this study. So, this might limit the statistical analysis. Finally, there was no laboratory confirmation of imidacloprid exposure.
Conclusions
Cases of acute imidacloprid poisoning are mostly mild. Gastrointestinal symptoms and minor neurological presentations are common, while the mortality rate is low. In addition to a large amount of ingestion, the primary presence of cardiovascular effects, central nervous system effects, dyspnea, and diaphoresis are associated with death. Close monitoring and observation are indicated for acute imidacloprid-exposed patients who present with these signs.
Acknowledgments
The authors express sincere thanks to Mrs. Umaporn Udomsubpayakul from the section for Clinical Epidemiology and Biostatistics, Research Center, Faculty of Medicine Ramathibodi Hospital, Mahidol University for her help with statistical analysis.
Funding Statement
We declare no funding for this study.
Data Sharing Statement
The data are not available for public access because of patient privacy concerns, but they are available from the corresponding author on reasonable request.
Disclosure
The authors declare no conflicts of interest. The authors alone are responsible for the content and writing of this paper.
References
- 1.Jeschke P, Nauen R, Schindler M, et al. Overview of the status and global strategy for neonicotinoids. J Agric Food Chem. 2011;59(7):2897–2908. doi: 10.1021/jf101303g [DOI] [PubMed] [Google Scholar]
- 2.Van der Sluijs J, Simon-Delso N, Goulson D, et al. Neonicotinoids, bee disorders and the sustainability of pollinator services. Curr Opin Environ Sustain. 2013;5(3–4):293–305. doi: 10.1016/j.cosust.2013.05.007 [DOI] [Google Scholar]
- 3.Thompson DA, Lehmler HJ, Kolpin DW, et al. A critical review on the potential impacts of neonicotinoid insecticide use: current knowledge of environmental fate, toxicity, and implications for human health. Environ Sci Process Impacts. 2020;22(6):1315–1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tomizawa M, Casida JE. Neonicotinoid insecticide toxicology: mechanisms of selective action. Annu Rev Pharmacol Toxicol. 2005;45(1):247–268. doi: 10.1146/annurev.pharmtox.45.120403.095930 [DOI] [PubMed] [Google Scholar]
- 5.Tomizawa M, Casida JE. Selective toxicity of neonicotinoids attributable to specificity of insect and mammalian nicotinic receptors. Annu Rev Entomol. 2003;48:339–364. doi: 10.1146/annurev.ento.48.091801.112731 [DOI] [PubMed] [Google Scholar]
- 6.Wu IW, Lin JL, Cheng ET. Acute poisoning with the neonicotinoid insecticide imidacloprid in N-methyl pyrrolidone. J Toxicol Clin Toxicol. 2001;39(6):617–621. doi: 10.1081/CLT-100108494 [DOI] [PubMed] [Google Scholar]
- 7.Phua DH, Lin CC, Wu ML, et al. Neonicotinoid insecticides: an emerging cause of acute pesticide poisoning. Clin Toxicol (Phila). 2009;47(4):336–341. doi: 10.1080/15563650802644533 [DOI] [PubMed] [Google Scholar]
- 8.Mohamed F, Gawarammana I, Robertson TA, et al. Acute human self-poisoning with imidacloprid compound: a neonicotinoid insecticide. PLoS One. 2009;4(4):e5127. doi: 10.1371/journal.pone.0005127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim JC, So BH, Kim HJ, et al. Clinical characteristics of patients with neonicotinoid insecticide poisoning. J Korean Soc Clin Toxicol. 2010;8:24–29. [Google Scholar]
- 10.Adams RD, Perry L, Bennett A, et al. The NPIS pesticide surveillance project neonicotinoids: comparison of toxicity against other insecticide classes. Clin Toxicol. 2013;51(4):353. [Google Scholar]
- 11.Forrester MB. Neonicotinoid insecticide exposures reported to six poison centers in Texas. Hum Exp Toxicol. 2014;33(6):568–573. [DOI] [PubMed] [Google Scholar]
- 12.David D, George IA, Peter JV. Toxicology of the newer neonicotinoid insecticides: imidacloprid poisoning in a human. Clin Toxicol (Phila). 2007;45(5):485–486. [DOI] [PubMed] [Google Scholar]
- 13.Panigrahi AK, Subrahmanyam DK, Mukku KK. Imidacloprid poisoning: a case report. Am J Emerg Med. 2009;27(2):256 e5–6. doi: 10.1016/j.ajem.2008.06.035 [DOI] [PubMed] [Google Scholar]
- 14.Iyyadurai R, George IA, Peter JV. Imidacloprid poisoning-newer insecticide and fatal toxicity. J Med Toxicol. 2010;6(1):77–78. doi: 10.1007/s13181-010-0041-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kumar A, Verma A, Kumar A. Accidental human poisoning with a neonicotinoid insecticide, imidacloprid: a rare case report from rural India with a brief review of literature. Egypt J Forensic Sci. 2013;3(4):123–126. doi: 10.1016/j.ejfs.2013.05.002 [DOI] [Google Scholar]
- 16.Hung YM, Meier KH. Acute® Confidor (imidacloprid-N-methyl pyrrolidone) insecticides intoxication with mimicking cholinergic syndrome. Toxicol Ind Health. 2005;21:137–140. doi: 10.1191/0748233705th217oa [DOI] [Google Scholar]
- 17.Hung YM, Lin SL, Chou KJ, et al. Imidacloprid-N-methyl pyrrolidone insecticides poisoning mimicking cholinergic syndrome. Clin Toxicol (Phila). 2006;44(5):771–772. [Google Scholar]
- 18.Huang NC, Lin SL, Chou CH, et al. Fatal ventricular fibrillation in a patient with acute imidacloprid poisoning. Am J Emerg Med. 2006;24(7):883–885. doi: 10.1016/j.ajem.2006.03.008 [DOI] [PubMed] [Google Scholar]
- 19.Lin PC, Lin HJ, Liao YY, et al. Acute poisoning with neonicotinoid insecticides: a case report and literature review. Basic Clin Pharmacol Toxicol. 2013;112(4):282–286. doi: 10.1111/bcpt.12027 [DOI] [PubMed] [Google Scholar]
- 20.Shadnia S, Moghaddam HH. Fatal intoxication with imidacloprid insecticide. Am J Emerg Med. 2008;26(5):634 e1–4. doi: 10.1016/j.ajem.2007.09.024 [DOI] [PubMed] [Google Scholar]
- 21.Karatas AD. Severe central nervous system depression in a patient with acute imidacloprid poisoning. Am J Emerg Med. 2009;27(9):1171.e5-7. doi: 10.1016/j.ajem.2009.01.006 [DOI] [PubMed] [Google Scholar]
- 22.Agha A, Bella A, Aldosary B, et al. Imidacloprid poisoning presenting as leukoclastic vasculitis with renal and hepatic dysfunction. Saudi J Kidney Dis Transpl. 2012;23(6):1300–1303. [DOI] [PubMed] [Google Scholar]
- 23.Fuke C, Nagai T, Ninomiya K, et al. Detection of imidacloprid in biological fluids in a case of fatal insecticide intoxication. Leg Med (Tokyo). 2014;16(1):40–43. doi: 10.1016/j.legalmed.2013.10.007 [DOI] [PubMed] [Google Scholar]
- 24.Proenca P, Teixeira H, Castanheira F, et al. Two fatal intoxication cases with imidacloprid: LC/MS analysis. Forensic Sci Int. 2005;153(1):75–80. doi: 10.1016/j.forsciint.2005.04.015 [DOI] [PubMed] [Google Scholar]
- 25.Department of Agriculture, Ministry of Agriculture and Cooperatives. Annual Report of Imported Pesticides to Thailand 2018.
- 26.International Programme on Chemical Safety (IPCS). IPCS INTOX data management system. Available from: https://www.who.int/ipcs/poisons/guide lines_data_entry.pdf. Accessed May12, 2020.
- 27.Department of Agriculture, Ministry of Agriculture and Cooperatives. Registered pesticide products in Thailand. Available from: http://www.doa.go.th/ard/wp-content/uploads/2020/02/register-2554-2563-updat-jan.pdf. Accessed September6, 2020.
- 28.Core Research Unit on Chemical Management, Center of Excellence on Hazardous Substance Management. Chemical knowledge platform. Available from: http://www.chemtrack.org/default.asp. Accessed September6, 2020.
- 29.Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension. 2018;71(6):1269–1324. [DOI] [PubMed] [Google Scholar]
- 30.Nicks BA, Gaillard J. Approach to shock In: Tintinalli JE, Stapczynski JS, Ma OJ, Yealy DM, Meckler GD, Cline DM, editors. Tintinalli’s Emergency Medicine: A Comprehensive Study Guide. 8th ed. New York, NY: McGraw-Hill Education; 2016. [Google Scholar]
- 31.Michaud GF, Stevenson WG. Physiologic and nonphysiologic sinus tachycardia In: Jameson JL, Fauci AS, Kasper DL, Hauser SL, Longo DL, Loscalzo J, editors. Harrison’s Principles of Internal Medicine. 20th ed. New York, NY: McGraw-Hill Education; 2018. Available from:: https://accessmedicine.mhmedical.com/content.aspx?bookid=2129§ionid=188731358. Accessed April7, 2020. [Google Scholar]
- 32.Lewis S, Nelson LS, Howland MA, et al. Initial evaluation of the patient: vital signs and toxic syndromes In: Nelson LS, Howland MA, Lewin NA, Smith SW, Goldfrank LR, Hoffman RS, editors. Goldfrank’s Toxicologic Emergencies, 11th ed. New York: McGraw Hill Education; 2019:28–31. [Google Scholar]
- 33.Danan G, Teschke R. RUCAM in drug and herb induced liver injury: the update. Int J Mol Sci. 2015;17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Strategy and Planning Division, Ministry of Public Health. Thai population 2010 –2018. Available from: http://bps.moph.go.th/new_bps/. Accessed January12, 2020.
- 35.Sriapha C, Trakulsrichai S, Intaraprasong P, et al. Imidacloprid poisoning case series: potential for liver injury. Clin Toxicol (Phila). 2020;58(2):136–138. doi: 10.1080/15563650.2019.1616091 [DOI] [PubMed] [Google Scholar]
- 36.Tawatsin A, Thavara U, Siriyasatien P. Pesticides used in Thailand and toxic effects to human health. Med Res Arch. 2015;3:1–10. [Google Scholar]