Abstract
Background
MicroRNAs (miRs) have been suggested to be biomarkers to inform the diagnosis of major depressive disorder (MDD). We have previously shown that exosome-derived miR-139-5p had potential in differentiating between patients with MDD and healthy control (HC) subjects.
Materials and Methods
To validate the potential of exosome-derived miR-139-5p as a biomarker for MDD, here we recruited 30 patients with MDD and 30 HC subjects, and used TaqMan probes to detect serum exosomal miR-139-5p levels.
Results
The data showed that patients with MDD had significantly increased exosomal miR-139-5p levels when compared with controls. Correlation analysis suggested that sex, age, and body mass index did not significantly affect blood exosomal miR-139-5p levels in the tested subjects. The ROC curve showed that serum-derived miR-139-5p had reasonable performance in discriminating patients with MDD and HC subjects, with a sensitivity of 0.867 and specificity of 0.767, and the AUC was 0.807.
Discussion
Taken together, these results demonstrated that patients with MDD were accompanied by significantly increased blood exosomal miR-139-5p levels, and exosomal miR-139-5p is a promising biomarker for the diagnosis of MDD.
Keywords: major depressive disorder, exosome, miR-139-5p, biomarker
Introduction
Major depressive disorder (MDD) is one of the most common psychiatric disorders which leads to a significant increase in morbidity and mortality.13 Currently, the diagnosis of MDD solely relies on subjective evaluations made by clinicians, and approximately half of the patients failed to respond to the first-line anti-depressants.7,20 The recent approval of ketamine by the FDA as a treatment for MDD is exciting in the field, given that this drug works faster and better than traditional anti-depressants.22 However, the effect of ketamine on patients with MDD wears off quickly,16 and the therapy was reported to have serious side-effects.17 In this context, there is a clear need to better understand the cellular and molecular pathways underlying the pathophysiology of MDD, which is important for novel and effective drug designs for treatment of the disease. Additionally, early and accurate detection has been suggested to result in better outcome for treatment of MDD.19
A number of biomarker candidates have been proposed as the targets for the diagnosis and treatment of MDD, and these include inflammatory cytokines,3 brain-derived neurotrophic factors,15 and oxidative stress markers.4 However, the usefulness of these potential biomarkers is uncertain due to the inconsistent results and/or paucity of replicated studies. Recently, microRNAs (miRNAs) are gaining increasing attention in the field of neuropsychiatric diseases, given the important regulatory roles of miRNAs in cell growth, neural differentiation, and synaptic plasticity.19 Additionally, miRNAs are suitable for biomarkers because they are abundant in various body fluids (such as blood, saliva, and urine),5 and these body fluids can be collected easily and non-invasively. In fact, a number of studies have shown blood miRNA aberrations in patients with MMD when compared with healthy control (HC) subjects, and several blood miRNAs have been proposed as potential biomarkers for the diagnosis of MDD.22 More recently, we used a genome-wide analysis of miRNA expression in serum exosomes of patients with MDD in comparison with HC subjects, and revealed a set of miRNAs were differentially expressed in patients with MDD. Furthermore, the top differentially expressed serum exosomal miRNA in patients with MDD was miR-139-5p, and this miRNA had a reasonable performance in discriminating between patients with MDD and controls, suggesting a potential of miR-139-5p as a biomarker to inform the diagnosis of MDD.21
In this study, we aimed to validate the potential of exosome-derived miR-139-5p as a biomarker for MDD. We recruited 30 patients with MDD and 30 HC subjects, and we used Taqman probes to analyze serum exosomal miR-139-5p levels. We further used a receiver operating characteristic (ROC) curve to evaluate the accuracy of exosome-derived miR-139-5p in differentiating between patients with MDD and HC subjects.
Materials and Methods
Subjects
Thirty patients with MDD and 30 HC subjects were recruited from the Third People’s Hospital of Foshan, Foshan, China. The diagnoses of patients with MDD were made by trained psychiatrists according to the International Classification of Diseases 10 and the Structured Clinical Interview for DSM-5. We further used the Montgomery-Asberg Depression Rating Scale and Hamilton Depression Rating Scale to evaluate the disease severity of patients with MDD. It should be noted that the patients and controls were completely different groups, as in our previously published paper.21
We obtained written informed consent from all patients and controls before they were included in this study. The study protocol was approved by the Ethics Committee at The Third People’s Hospital of Foshan, Foshan, China, and the experiments were conducted following the declaration of Helsinki.
RNA Extraction and qRT-PCR
Serum exosomes were isolated and validated as described previously.2 We extracted exosomal RNA by Trizol reagent (ThermoFisher, Waltham, MA, US), and the values of OD260/280 were between 1.8 to 2.0 for the extracted RNAs. Then the TaqManTM MicroRNA Reverse Transcription kit was used to convert RNA to cDNA according to the protocol from the manufacturer (ThermoFisher). Exosomal miR-139-5p levels were quantified using Taqman® MicroRNA Assay (including primers and probes, ThermoFisher), and miR-16 was used as an internal control.14 We used a LightCycler® 96 Real-Time PCR Detection System (Roche, Basel, Switzerland) to perform all PCR reactions.
Statistical Analysis
Data were presented as mean±standard error of mean. Serum exosomal miR-139-5p level difference between cases and controls was compared using Mann–Whitney U-test. Pearson’s correlation analysis was used to determine whether age and BMI affected miR-139-5p levels. Additionally, Crosstabs analysis (two-way chi-square test) was performed to assess the influence of sex on the miR-139-5p levels. We utilized a ROC curve to assess the accuracy of serum exosomal miR-139-5p in differentiating between patients with MDD and controls, and the accuracy of the test was calculated by area under the curve (AUC). P<0.05 was considered statistically significant in this study.
Results
We recruited 30 patients with MDD and 30 HC subjects in this study, and the demographic and clinical characteristics of the MDD patients and HC subjects are presented in Supplementary Table 1.
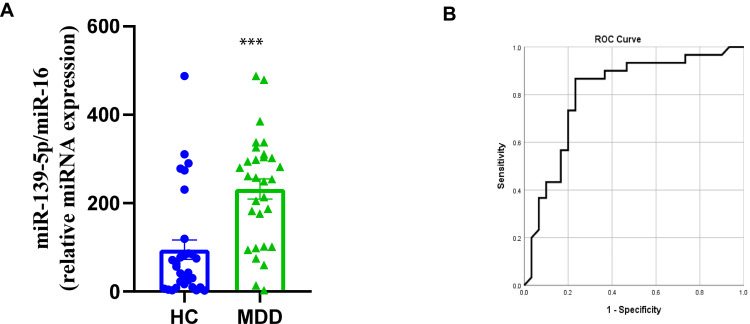
After exosome isolation from the included subjects, we used qRT-PCR to analyze the exosomal miR-139-5p levels. The results demonstrated that serum exosomal miR-139-5p levels were significantly increased in patients with MDD when compared with HC subjects (Figure 1A). Given the significant difference between cases and controls for serum exosomal miR-139-5p levels, we next explored whether miR-139-5p could be used as a biomarker for MDD. The ROC curve showed that serum exosomal miR-139-5p levels had reasonable performance in discriminating between patients with MDD and controls, with a sensitivity of 0.867 and specificity of 0.767, and the AUC was 0.807 (Figure 1B). These results suggested that patients with MDD were accompanied by increased blood exosomal miR-139-5p levels, and serum exosome-derived miR-139-5p is a candidate biomarker for MDD.
Figure 1.
Serum exosomal miR-139-5p as a biomarker for major depressive disorder. (A) Serum exosomal miR-139-5p levels in patients with major depressive disorder and control subjects. (B) A ROC curve was utilized to evaluate the accuracy of serum exosome-derived miR-139-5p in differentiating between patients with MDD and controls. *** P<0.001.
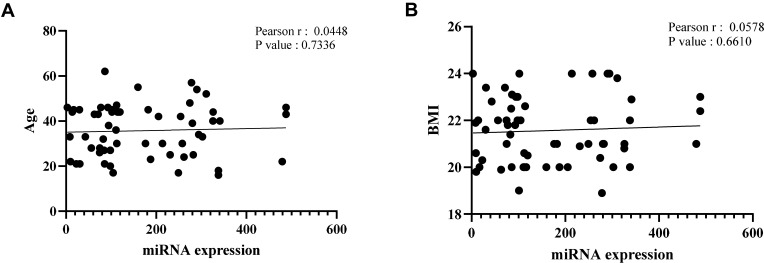
We next analyzed whether sex, age, and BMI were confounding factors for blood exosomal miR-139-5p levels in the tested subjects. The Pearson’s correlation analysis showed that age (Figure 2A) and BMI (Figure 2B) did not significantly associate with serum exosomal miR-139-5p levels. We also used Crosstabs analysis to evaluate whether sex influenced serum exosomal miR-139-5p levels. The results suggested that sex did not significantly affect exosomal miR-139-5p levels in the case group (P=0.538) and control group (P=0.441). These results suggested that sex, age, and BMI were unlikely to affect the accuracy of exosomal miR-139-5p as a biomarker for MDD.
Figure 2.
Correlation between serum exosomal miR-139-5p level and age (A, r=0.0448, P=0.7336) or body mass index (B, r=0.0578, P=0.661) as determined by Pearson’ correlation test.
Discussion
In this study, we used Taqman probes to analyze the miR-139-5p levels in serum exosome of patients with MDD, and showed patients with MDD had significantly increased serum exosomal miR-139-5p levels when compared with HC subjects, and this is consistent with our previous study.21 The previous study also demonstrated that mice under chronic unpredictable mild stress (CUMS) had significantly increased brain and peripheral blood exosomal miR-139-5p levels. Further investigations revealed that inhibition of miR-139-5p expression in CUMS mice alleviated their depressive-like behaviors, and the impaired hippocampal neurogenesis in the CUMS mice was restored by miR-139-5p antagomir treatment. Moreover, the in vitro data indicated that miR-139-5p was a negative regulator for neural stem cell proliferation and neuronal differentiation.21 Therefore, the above data suggested that exosomal miR-139-5p mediated depressive-like behaviors in CUMS mice with involvement of hippocampal neurogenesis. In supportive of a critical role of exosomes in stress-induced depression, a very recent study by Li et al6 found exosomes from natural killer cells alleviated depressive-like behaviors in mice under chronic mild stress. Their findings further suggested that the antidepressant-like effects of exosomes from natural killer cells in stressed mice were mediated by exosomal miR-207, which targeted Tril to inhibit NF-κB signaling in astrocytes. Taken together, these results revealed that exosome-derived miRNAs were dysregulated in patients with MDD, and these miRNAs have a potential to be served as novel targets for treatment of MDD.
In addition to our efforts of searching MDD biomarkers from blood exosomes, a number of studies have analyzed circulating (serum, plasma, or blood) miRNA levels in patients with MDD and control subjects, in hope of a better understanding of the etiology of MDD and finding biomarkers to inform the diagnosis and/or treatment response for MDD. It has been reported that miR-132 expression was up-regulated in the blood of patients with MDD, and serum BDNF levels were negatively correlated with the miR-132/miR-182 levels in depressed patients.9 Another study showed that serum miR-30e, miR-132, miR-185, and miR-212 levels were significantly increased in patients with MDD when compared with controls,10 and antidepressant treatment further increased serum miR-212 levels in patients with MDD.11 Additionally, clinical data on circulating miRNA levels in patients with MDD were summarized by a systematic review article, which included 23 studies and identified more than a hundred miRNAs that were differentially expressed in patients with MDD when compared with controls.22 However, biomarker implication for MDD from these results was unclear since few miRNAs were consistently dysregulated in patients with MMD across studies, and there was overall paucity of replicated studies assessing individual miRNA dysregulations in MDD. Interestingly, the up-regulation of miR-132 in patients with MDD was replicated in four studies,1,8,12,18 suggesting the potential of circulating miR-132 as a biomarker for MDD. Unfortunately, the accuracy of circulating miR-132 as a biomarker to diagnose MDD was not evaluated, making it difficult to ascertain the potential miR-132 as a biomarker to inform the diagnosis of MDD. Here, our study showed that miR-139-5p from serum exosomes had reasonable performance in discriminating between MDD patients and controls, with a sensitivity of 0.867 and specificity of 0.767, and the AUC was 0.807. Therefore, results from the present study replicated the findings of our previous study,21 and validated the potential of serum exosomal miR-139-5p as a biomarker to inform the diagnosis of SCZ. Nevertheless, there is increasing awareness that combining biomarkers reflective of different molecular pathways associated with pathophysiology of MDD would lead to a correct and better diagnosis. Therefore, further investigations are necessary to translate the findings from the previous and present studies into the benefits of patients with MDD.
Acknowledgments
This study was supported by the National Natural Science Foundation of China (82071676, 81703492), Beijing Natural Science Foundation (7182092), High-Level Hospital Development Program for Foshan “Climbing” Project.
Disclosure
The authors declare that there is no conflict of interest involved in this work.
References
- 1.Bocchio-Chiavetto L, Maffioletti E, Bettinsoli P, et al. Blood microRNA changes in depressed patients during antidepressant treatment. Eur Neuropsychopharmacol. 2013;23(7):602–611. doi: 10.1016/j.euroneuro.2012.06.013 [DOI] [PubMed] [Google Scholar]
- 2.Du Y, Yu Y, Hu Y, et al. Genome-wide, integrative analysis implicates exosome-derived MicroRNA dysregulation in schizophrenia. Schizophr Bull. 2019;45:1257–1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goldsmith DR, Rapaport MH, Miller BJ. A meta-analysis of blood cytokine network alterations in psychiatric patients: comparisons between schizophrenia, bipolar disorder and depression. Mol Psychiatry. 2016;21:1696–1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jimenez-Fernandez S, Gurpegui M, Diaz-Atienza F, Perez-Costillas L, Gerstenberg M, Correll CU. Oxidative stress and antioxidant parameters in patients with major depressive disorder compared to healthy controls before and after antidepressant treatment: results from a meta-analysis. J Clin Psychiatry. 2015;76:1658–1667. [DOI] [PubMed] [Google Scholar]
- 5.Layne TR, Green RA, Lewis CA, et al. microRNA detection in blood, urine, semen, and saliva stains after compromising treatments. J Forensic Sci. 2019;64:1831–1837. [DOI] [PubMed] [Google Scholar]
- 6.Li D, Wang Y, Jin X, et al. NK cell-derived exosomes carry miR-207 and alleviate depression-like symptoms in mice. J Neuroinflammation. 2020;17(1):126. doi: 10.1186/s12974-020-01787-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li Y, Liu J, Liu X, et al. Antidepressant-like action of single facial injection of botulinum Neurotoxin A is associated with augmented 5-HT levels and BDNF/ERK/CREB pathways in mouse brain. Neurosci Bull. 2019;35:661–672. doi: 10.1007/s12264-019-00367-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li YJ, Xu M, Gao ZH, et al. Alterations of serum levels of BDNF-related miRNAs in patients with depression. PLoS One. 2013;8(5):e63648. doi: 10.1371/journal.pone.0063648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lin CC, Huang TL. Brain-derived neurotrophic factor and mental disorders. Biomed J. 2020;43(2):134–142. doi: 10.1016/j.bj.2020.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lin CC, Lee CT, Sun MH, Huang TL. Increased levels of miR-30e, miR-132, miR-185, and miR-212 at baseline and increased brain-derived neurotrophic factor protein and mRNA levels after treatment in patients with major depressive disorder. Neuropsychiatry. 2017;7:920–926. [Google Scholar]
- 11.Lin CC, Tsai MC, Lee CT, Sun MH, Huang TL. Antidepressant treatment increased serum miR-183 and miR-212 levels in patients with major depressive disorder. Psychiatry Res. 2018;270:232–237. doi: 10.1016/j.psychres.2018.09.025 [DOI] [PubMed] [Google Scholar]
- 12.Liu Y, Yang X, Zhao L, Zhang J, Li T, Ma X. Increased miR-132 level is associated with visual memory dysfunction in patients with depression. Neuropsychiatr Dis Treat. 2016;12:2905–2911. doi: 10.2147/NDT.S116287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lopez JP, Kos A, Turecki G. Major depression and its treatment: microRNAs as peripheral biomarkers of diagnosis and treatment response. Curr Opin Psychiatry. 2018;31(1):7–16. doi: 10.1097/YCO.0000000000000379 [DOI] [PubMed] [Google Scholar]
- 14.Occhipinti G, Giulietti M, Principato G, Piva F. The choice of endogenous controls in exosomal microRNA assessments from biofluids. Tumour Biol. 2016;37(9):11657–11665. doi: 10.1007/s13277-016-5164-1 [DOI] [PubMed] [Google Scholar]
- 15.Peng S, Li W, Lv L, Zhang Z, Zhan X. BDNF as a biomarker in diagnosis and evaluation of treatment for schizophrenia and depression. Discov Med. 2018;26:127–136. [PubMed] [Google Scholar]
- 16.Schwartz J, Murrough JW, Iosifescu DV. Ketamine for treatment-resistant depression: recent developments and clinical applications. Evid Based Ment Health. 2016;19:35–38. doi: 10.1136/eb-2016-102355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Short B, Fong J, Galvez V, Shelker W, Loo CK. Side-effects associated with ketamine use in depression: a systematic review. Lancet Psychiatry. 2018;5:65–78. doi: 10.1016/S2215-0366(17)30272-9 [DOI] [PubMed] [Google Scholar]
- 18.Su M, Hong J, Zhao Y, Liu S, Xue X. MeCP2 controls hippocampal brain-derived neurotrophic factor expression via homeostatic interactions with microRNA132 in rats with depression. Mol Med Rep. 2015;12:5399–5406. doi: 10.3892/mmr.2015.4104 [DOI] [PubMed] [Google Scholar]
- 19.Tavakolizadeh J, Roshanaei K, Salmaninejad A, et al. MicroRNAs and exosomes in depression: potential diagnostic biomarkers. J Cell Biochem. 2018;119:3783–3797. doi: 10.1002/jcb.26599 [DOI] [PubMed] [Google Scholar]
- 20.Undurraga J, Baldessarini RJ. Randomized, placebo-controlled trials of antidepressants for acute major depression: thirty-year meta-analytic review. Neuropsychopharmacology. 2012;37(4):851–864. doi: 10.1038/npp.2011.306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wei ZX, Xie GJ, Mao X, et al. Exosomes from patients with major depression cause depressive-like behaviors in mice with involvement of miR-139-5p-regulated neurogenesis. Neuropsychopharmacology. 2020;45(6):1050–1058. doi: 10.1038/s41386-020-0622-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yuan H, Mischoulon D, Fava M, Otto MW. Circulating microRNAs as biomarkers for depression: many candidates. Few Finalists, J Affect Disord. 2018;233:68–78. doi: 10.1016/j.jad.2017.06.058 [DOI] [PubMed] [Google Scholar]