Abstract
Aims
A proximal femur fracture (PFF) is a common orthopaedic presentation, with an incidence of over 25,000 cases reported in the Australian and New Zealand Hip Fracture Registry (ANZHFR) in 2018. Hip fractures are known to have high mortality. The purpose of this study was to determine the utility of the Clinical Frailty Scale (CFS) in predicting 30-day and one-year mortality after a PFF in older patients.
Methods
A retrospective review of all fragility hip fractures who met the inclusion/exclusion criteria of the ANZHFR between 2017 and 2018 was undertaken at a single large volume tertiary hospital. There were 509 patients included in the study with one-year follow-up obtained in 502 cases. The CFS was applied retrospectively to patients according to their documented pre-morbid function and patients were stratified into five groups according to their frailty score. The groups were compared using t-test, analysis of variance (ANOVA), and the chi-squared test. The discriminative ability of the CFS to predict mortality was then compared with American Society of Anaesthesiologists (ASA) classification and the patient’s chronological age.
Results
A total of 38 patients were deceased at 30 days and 135 patients at one year. The 30-day mortality rate increased from 1.3% (CFS 1 to 3; 1/80) to 14.6% (CFS ≥ 7; 22/151), and the one-year mortality increased from 3.8% (CFS 1 to 3; 3/80) to 41.7% (CFS ≥ 7; 63/151). The CFS was demonstrated superior discriminative ability in predicting mortality after PFF (area under the curve (AUC) 0.699; 95% confidence interval (CI) 0.651 to 0.747) when compared with the ASA (AUC 0.634; 95% CI 0.576 to 0.691) and chronological age groups (AUC 0.585; 95% CI 0.523 to 0.648).
Conclusion
The CFS demonstrated utility in predicting mortality after PFF fracture. The CFS can be easily performed by non-geriatricians and may help to reduce age related bias influencing surgical decision making.
Cite this article: Bone Joint Open 2020;1-8:443–449.
Keywords: proximal femur fracture, femur, hip fracture
Key points
The Clinical Frailty Scale (CFS) demonstrates superior discriminative ability for predicting mortality after proximal femur fracture (PFF) when compared with the American Society of Anaesthesiologists (ASA) score or chronological age.
Increasing degrees of frailty as defined by CFS were inversely related with a patient’s likelihood of discharge to private residence.
The CFS could be an accurate tool to identify a patient’s suitability for a total hip arthroplasty instead of a hemiarthroplasty.
Introduction
A proximal femur fracture (PFF) is a common orthopaedic presentation. The Australian and New Zealand Hip Fracture Registry (ANZHFR) reports proximal femur fractures to be the most serious and costly fall-related injury suffered by older people.1 The ANZHFR annual report 2019 documented more than 25,000 hip fractures across Australia and New Zealand, with an approximate cost of $1 billion to the economy each year. The vast majority of these fractures occurred in elderly patients in the setting of minimal trauma such as a multifactorial fall with comorbid osteoporosis.1 As life expectancy continues to rise, the number of people admitted to hospital with a proximal femur fracture will increase accordingly. Elderly patients are more likely to sustain proximal femur fractures due to primary or secondary osteoporosis and are more likely to have multiple medical comorbidities which contribute to their degree of frailty.2
Frailty is a well-established medical condition and a recognized factor in surgical and geriatric outcomes.3-5 It is defined as a state in which a vulnerable individual has a diminished physiological capacity to respond to external stressors such as trauma or infection.6 There are several validated clinical tools to estimate an individual’s frailty. Our tertiary orthogeriatrics service has adopted the Clinical Frailty Scale (CFS) as a rapid bedside frailty screening tool.
The CFS, initially proposed by Rockwood et al,7 was a seven-point scale ranging from fit (CFS 1) to severely frail (CFS 7) based on self-reported medical comorbidities and the degree of assistance required for activities of daily living. Since 2005, the CFS has been revised to a nine-point scale adding an additional 2° of frailty for the very severely frail and the terminally ill8 (online supplementary figure 1). There are gerontological publications which have demonstrated the CFS as an independent predictor of inpatient mortality.6,9 More recently, the CFS has been applied to outcomes after transcatheter aortic valve arthroplasty and coronary artery bypass graft, and both studies have found the CFS to be a useful marker of mortality.10,11 In the orthopaedic community, the CFS has already been associated with increasing in-hospital complication rates and increasing length of stay after hip surgery.12
Given the prevalence of frailty in patients who sustained proximal femur fractures, this retrospective cohort study aimed to investigate the usefulness of the CFS for predicting the prognosis of patients who underwent surgery for proximal femur fractures at our tertiary referral centre. We hypothesized that increased clinical frailty scores would be associated with increased 30-day and one-year mortality.
Methods
We performed a retrospective cohort study of all minimal trauma hip fracture patients who presented to this tertiary referral centre over a period of one year and were identified prospectively as satisfying the inclusion/exclusion criteria for the ANZHFR.1 At this tertiary referral centre, orthopaedic trauma lists prioritizing patients with proximal femur fractures were available every day of the week, including weekends and public holidays. This tertiary hospital has a mature orthogeriatric service that sees all hip fracture patients and orthopaedic and anaesthetic care is consultant led. Each patient was examined on arrival by the orthopaedic team and a routine set of screening investigations was performed on all patients. Patients were reviewed by the consultant-led orthogeriatric team and assessed by allied health team members to optimise the patient’s health, mobility and environment prior to discharge or transfer to a rehabilitation bed. A total of 509 patients were identified for the ANZHFR between 1 November 2017 and 31 October 2018 and were included in this study. There were no bilateral fractures in this study period. In total, there were seven cases that were lost to follow-up (Table I), and all of these patients were overseas visitors with CFS ≤ 5. The mean age of this sample was 82.7 ± 9.1 years (mean ± standard deviation (SD)) and 73.5% of cases were female.
Table I.
Clinical Frailty Scale and patient characteristics and outcomes.
| Variable | CFS 1-3 (n = 80) | CFS 4 (n = 91) | CFS 5 (n = 117) | CFS 6 (n = 70) | CFS ≥ 7 (n = 151) | p-value |
|---|---|---|---|---|---|---|
| Mean ASA, grade (SD) | 2.6 (0.8) | 2.8 (0.6) | 3.0 (0.6) | 3.2 (0.5) | 3.0 (0.5) | < 0.001* |
| Mean age, yrs (SD) | 73.8 (8.8) | 80.3 (9.0) | 84.3 (8.3) | 84.7 (6.9) | 86.6 (7.3) | < 0.001* |
| Female sex, n (%) | 60 (75.0) | 71 (78.0) | 86 (73.5) | 50 (71.4) | 107 (70.9) | 0.783† |
| Admitted from residential care, n (%) | 6 (7.5) | 10 (11) | 11 (9.4) | 16 (22.9) | 115 (76.2) | < 0.001† |
| Mean acute LOS, days (SD) | 3.6 (1.5) | 3.7 (2.5) | 4.4 (3.7) | 4.8 (3.0) | 4.3 (2.0) | 0.027* |
| Discharged to private residence, n (%) | 27 (33.8) | 13 (14.3) | 8 (6.8) | 3 (4.3) | 3 (2.0) | < 0.001† |
| Discharged to rehabilitation, n (%) | 47 (58.8) | 68 (74.7) | 97 (82.9) | 55 (78.6) | 50 (33.1) | < 0.001† |
| Inpatient death, n (%) | 0 (0) | 0 (0) | 3 (2.6) | 1 (1.4) | 7 (4.6) | 0.077† |
| 30-day mortality, n (%) | 1 (1.3%) | 4 (4.4%) | 6 (5.1%) | 5 (7.1%) | 22 (14.6%) | 0.004† |
| One-year mortality, n (%) | 3 (3.8%) | 13 (14.3%) | 28 (23.9%) | 28 (40%) | 63 (41.7%) | < 0.001† |
| Unknown mortality, n (%) | 4 (5.0%) | 2 (2.2%) | 1 (0.9%) | 0 (0.0%) | 0 (0.0%) |
CFS, Clinical Frailty Scale; ASA, American Society of Anaesthesiologists; LOS, length of stay.
Analysis of variance.
Chi-squared test.
All operations were performed at our tertiary referral centre. In order to ensure the highest level of accuracy, all data was gathered manually from the digital medical record, operation notes and radiology reports. Data collected included the age and sex of the patient, the American Society of Anesthesiologists (ASA) score, fracture type, the time of surgical intervention,the type of surgical intervention, the acute length of stay (LOS) after surgery, the patient’s residence at the time of admission and the patient’s discharge destination after the acute management of the fracture. The CFS score was applied retrospectively to cases according to the orthopaedic, orthogeriatric and allied health admission notes which detailed cognitive function, medical comorbidities, admission residence, premorbid fitness/activity, mobility aids used, and the degree of services and/or supports implemented in the .home/residential care facility.
To minimize variance, the SHARE-Clinical Frailty Scale algorithm was utilized to determine the CFS group (online supplementary figure 1). The CFS results were categorized into five groups as follows: non-frail (CFS 1 to 3), vulnerable (CFS 4), mildly frail (CFS 5), moderately frail (CFS 6), and severely frail (CFS ≥ 7). Mortality data was collected from a local database, which is updated by the Western Australian Registry of Births, Deaths and Marriages, to determine 30-day and one-year mortality rates. Patients from outside Western Australia were followed up by phone call.
Statistical analysis
All statistical analysis was calculated using SPSS statistics 22 (IBM, Armonk, New York, USA). Continuous variables were expressed as mean ± SD. Categorical data are expressed as percentages of the total. Comparisons among the five groups were made using χ2 tests for categorical covariates and one-way analysis of variance for continuous covariates that were listed as mean value and SD. The Kaplan-Meier method was used to estimate cumulative mortality rates in the five groups. Survival differences in each group were compared using log-rank tests. The discriminatory ability of the CFS was assessed by the receiver operating characteristics (ROC) curve test and this was compared with the ASA physical status classification system and the patient’s age group. Statistical tests were all two-sided, and values of p < 0.05 or a 95% confidence interval (CI) were considered statistically significant.
Ethics approval was obtained from our hospital’s office of ethics and research governance prior to commencement of the study.
Results
All patients were stratified into the five study groups, and Table I demonstrates the breakdown of patient characteristics and outcomes by the frailty score. Mean age increased with frailty scores (p < 0.001) and significant differences were observed in the admission residence (p < 0.001), mean acute LOS after surgery (p = 0.027) and mean ASA (p < 0.001) across the study groups (Table I).
CFS 1 to 3 had the highest frequency of discharge to home after the surgical admission documented in 27/80 cases. Patients who were part of the CFS 4, CFS 5, and CFS 6 groups were highly likely to require a period of inpatient rehabilitation documented in 68/91, 97/117, and 55/70 cases respectively (Table I). The CFS ≥ 7 group were least likely to discharge to a private residence (3/151) or a rehabilitation bed (50/151) after the acute admission, and this reflects the high proportion of patients who were living in residential care at the time of admission (115/151).
Overall, 30-day and one-year survival data was obtained in 502 (98.6%) cases. In all, 11 patients died during the admission, 38 patients at 30 days, and 135 patients at one year. Mortality increased with frailty at 30 days (p < 0.001) and one year (p < 0.001). Mortality was highest in the CFS ≥ 7 group reporting 22 (14.6%) patients deceased at 30 days and 63 (41.7%) patients deceased at one-year. Kaplan-Meier analysis of cumulative mortality of the CFS groups is demonstrated in Figure 1A and 1B demonstrates the cumulative mortality of patients stratified by their ASA classes for comparison, and mortality was observed to increase with the ASA score (p < 0.001).
Fig. 1.
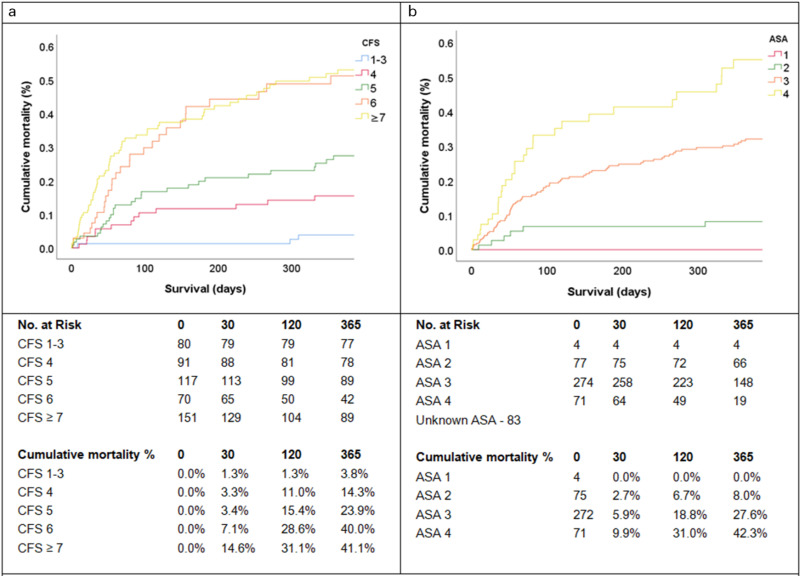
Kaplan-Meier analysis of all-cause mortality. a) Cumulative mortality across the CFS groups; and b) Cumulative mortality across the ASA classes. Statistically significant increases in mortality were demonstrated with increasing CFS score (p < 0.001) and ASA class (p < 0.001).
When examining the occurrence of death within one-year following a PFF using area under the curve (AUC) analysis, the CFS demonstrated the best discriminative ability (AUC 0.699; 95% CI 0.651 to 0.747) when compared with the ASA (AUC 0.634; 95% CI 0.576 to 0.691) and patient age groups (AUC 0.585; 95% CI 0.523 to 0.648). The corresponding ROC curve can be seen in Figure 2.
Fig. 2.
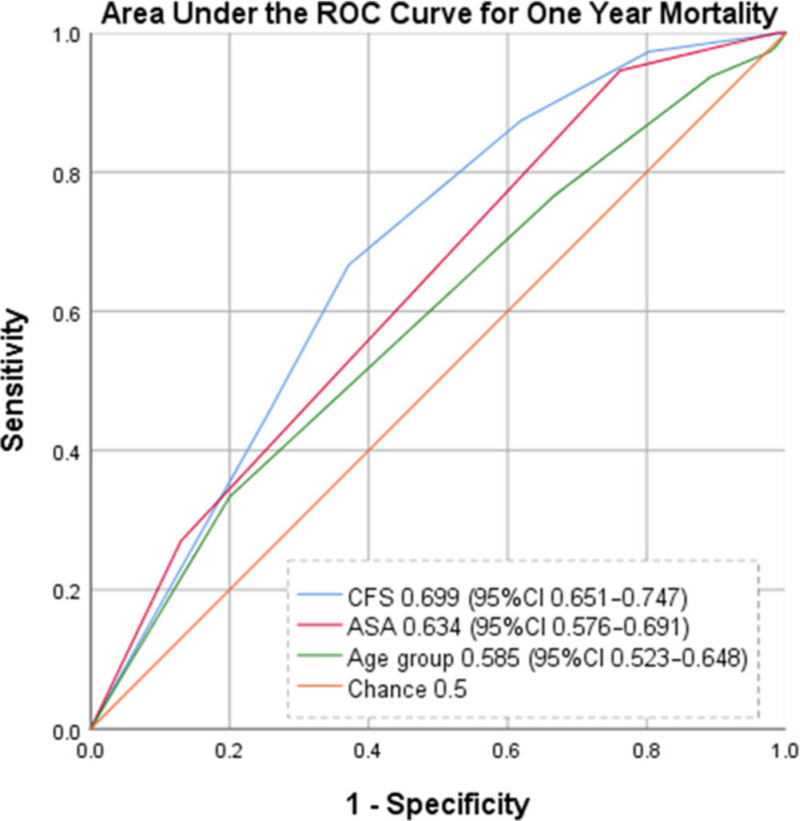
Receiver operating characteristic curve for one-year mortality after hip fracture.
Table II demonstrates the frequency of fracture types in each CFS group. The frequency of intertrochanteric fractures increased in association with frailty (p = 0.263) and was observed in 51.7% (78/151) of CFS ≥ 7 cases. Mortality by fracture type and CFS group is also shown in Table II. There was a total of 29 cases with subtrochanteric fractures in this study, which carried the highest mortality rate of 17.2% (5/29) at 30 days and 31.0% (9/29) at one year. For all fracture types, both 30-day (p = 0.219) and one-year mortality (p = 0.637) were generally observed to increase with frailty as demonstrated in Table II.
Table II.
Fracture types and mortality stratified by CFS groups where n denotes the total number of cases within the CFS group of each fracture type (p = 0.263). Mortality is indicated at 30 days (p = 0.219) and one year (p = 0.637).
| Intertrochanteric | Intracapsular (displaced) | Intracapsular (un-displaced) | Subtrochanteric | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | 30 days | One year | n | 30 days | One year | n | 30 days | One year | n | 30 days | One year | |
| CFS 1 to 3 | 29 | 0 | 2 | 30 | 1 | 1 | 15 | 1 | 1 | 6 | 0 | 0 |
| CFS 4 | 34 | 1 | 3 | 42 | 1 | 6 | 12 | 2 | 3 | 3 | 0 | 1 |
| CFS 5 | 46 | 2 | 9 | 41 | 1 | 12 | 23 | 1 | 4 | 7 | 2 | 3 |
| CFS 6 | 28 | 2 | 11 | 29 | 3 | 14 | 8 | 0 | 3 | 5 | 0 | 0 |
| CFS ≥ 7 | 78 | 9 | 31 | 52 | 10 | 21 | 13 | 0 | 5 | 8 | 3 | 5 |
| Overall | 214 | 14 | 56 | 194 | 16 | 54 | 71 | 4 | 16 | 29 | 5 | 9 |
CFS, Clinical Frailty Scale.
Hemiarthroplasty was the most frequent operation for management of the hip fractures accounting for 212 cases, followed closely by short intramedullary nail in 192 cases (Table III). Only 28 cases were managed with total hip arthroplasty and five cases were managed non-operatively. The 30-day and one-year mortality of each operation type tended to increase with frailty; however, no significant relationship was observed between the operation type, mortality and CFS score (Table III).
Table III.
Operative management and mortality stratified by CFS groups where ‘n’ denotes the total number of cases within the CFS group managed with each type of operation (p < 0.001). Mortality is indicated at 30 days (p = 0.137) and one-year (p = 0.161).
| Hemiarthroplasty | Intramedullary Nail | Sliding Hip Screw | THA | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | 30 days | One year | n | 30 days | One year | n | 30 days | One year | n | 30 days | One year | |
| CFS 1 to 3 | 23 | 1 | 1 | 29 | 0 | 1 | 13 | 0 | 1 | 15 | 1 | 1 |
| CFS 4 | 42 | 0 | 5 | 31 | 1 | 4 | 11 | 2 | 2 | 6 | 1 | 2 |
| CFS 5 | 55 | 1 | 15 | 35 | 3 | 8 | 21 | 1 | 3 | 5 | 0 | 1 |
| CFS 6 | 32 | 2 | 14 | 27 | 2 | 8 | 9 | 1 | 5 | 2 | 0 | 1 |
| CFS ≥ 7 | 60 | 8 | 23 | 70 | 10 | 30 | 18 | 1 | 6 | 0 | 0 | 0 |
| Overall | 212 | 12 | 57 | 192 | 16 | 51 | 72 | 5 | 17 | 28 | 2 | 5 |
THA; total hip arthroplasty; CFS, Clinical Frailty Scale.
Discussion
This retrospective review has demonstrated the potential prognostic value of the CFS grading tool as a risk stratification index before surgical management of proximal femur fracture in older patients. This study has found significant relationship between the degree of frailty (as defined by the CFS) and mortality. Although it is unsurprising that frailty was associated with mortality, the increased risk of death with each one-point increase in the CFS score, affirms that potential prognostic significance of frailty in this population.
Prediction of poor outcome after hip fracture helps inform the treatment decision and communication with patients and their carers. At present, there are many scores which have been studied as predictors of mortality after hip fracture. Scores such as the Charlson Comorbidity Index (CCI) and the Modified Frailty Index (MFI) have been demonstrated to predict morbidity and mortality after hip fracture; however, their clinical utilization is limited due to the many variables and time required for calculation.13,14
The ASA score which attempts to quantify the physiological reserve of a patient before surgery has been associated with poor patient prognosis through various regressions in numerous orthopaedic interventions.15–18 The ANZHFR collects the patients ASA score as a general measure of physical health or comorbidity.1 The 2019 annual report demonstrated that > 50% of patients with hip fracture were classified as ASA grade 3.1 Our study has documented 274 (65%) ASA grade 3 of the total 421 cases with documented ASA score. Figure 3 highlights ASA grade 3 as the most frequent score for all CFS groups with exception to CFS 1 to 3 where ASA grade 2 was most common. The CFS provides a more accurate prognosis for mortality than ASA by better differentiating patients into smaller groups.
Fig. 3.
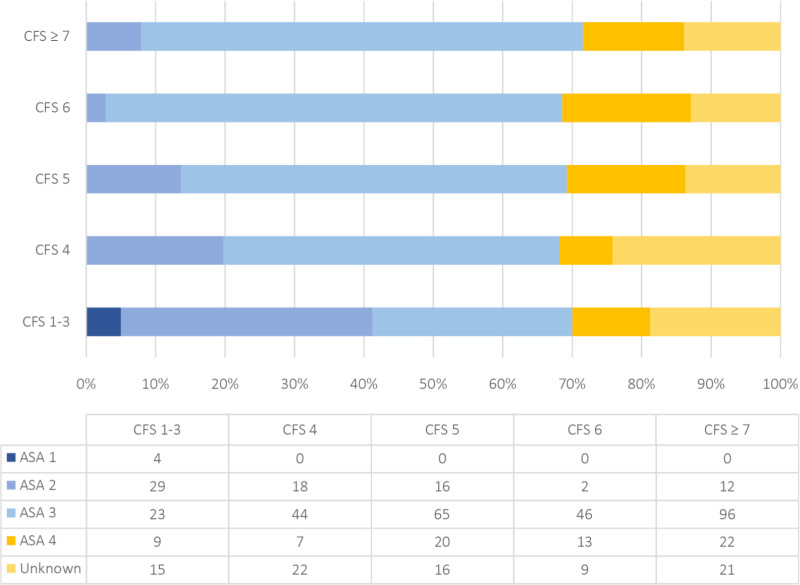
Frequency of ASA scores stratified by Clinical Frailty Scale groups.
While this study adds to the literature confirming the association between increasing ASA score and mortality after hip fracture (Figure 1B), this study demonstrates superior discriminative ability of the CFS (AUC 0.699; 95% CI 0.651 to 0.747) in predicting mortality over ASA (AUC 0.634; 95% CI 0.576 to 0.691) and the patient’s age (AUC 0.585; 95% CI 0.523 to 0.648). Interestingly, neither CFS, ASA, nor the patient’s age met the threshold of what is considered to be fair discrimination (AUC ≥ 0.7). However, at AUC 0.699 the CFS demonstrates potential for discrimination in predicting mortality after hip fracture, and further evaluation of the discriminative ability of the CFS should be explored with prospective assignment of CFS score in future studies.
To the knowledge of the authors there are no other validation studies exploring the discriminative ability of the CFS to predict mortality after a hip fracture. A gerontological secondary analysis of the Survey of Health, Ageing and Retirement in Europe (SHARE) studied the discriminative ability of the CFS to predict all-cause mortality at two years and five years and reported a similar AUC of 0.73 (95% CI 0.70 to 0.75) and 0.70 (95% CI 0.68 to 0.71) respectively.19
Regarding fracture type, this study did not find any statistically significant relationship between the fracture type and the patient’s degree of frailty. Notably, of the 38 patients CFS 1 to 3 who were managed with hemiarthroplasty (23/80) or total hip arthroplasty (15/80), only two were deceased at one year. Given patients within the CFS 1 to 3 group are non-frail and are active with at least routine walking (by definition), these patients would be in the group of patients where total hip arthroplasty could be considered. The CFS could be an accurate tool to identify a patient’s suitability for a total hip arthroplasty instead of a hemiarthroplasty.
After operative management of the hip fracture, increasing degrees of frailty (as defined by CFS) were inversely related with a patient’s likelihood of discharge to private residence. Patients who did not discharge to private residence were either transferred to a rehabilitation bed or returned to residential care. Notably, there was a significantly higher frequency of patients admitted from residential care within the CFS ≥ 7 group as compared with the CFS 6 group, 76.2% (115/151) vs 22.9% (16/70) respectively. The majority of patients within the CFS 6 group were discharged after operative management to a rehabilitation bed (55/70); however, there was no data collected regarding complications during the rehabilitation admission and the patient’s likelihood of discharge to private residence after the rehabilitation admission. Given the degree of frailty and the reduction in function associated with hip fractures, collection and analysis of this data in future study may help tailor prognostic advice and facilitate patient expectation management.
The management of frail patients who sustain a hip fracture can be challenging and requires a multidisciplinary model of care. These patients often have multiple medical co-morbidities and pose a significant health burden that is predicted to increase in the future with an increasingly aged and comorbid population.20 The concept of frailty being an independent risk factor for mortality, morbidity, LOS, and readmission rate is not a new finding; however, with the use of the CFS, frailty can now be easily applied to hip fractures by all members of the multidisciplinary team including the surgeon.6,9–12
Furthermore, the CFS is a useful tool to help guide the most appropriate procedures (i.e. relatively quicker operations with shorter anaesthetic time or hemiarthroplasty over total hip arthroplasty in frail patients). Its ease of application and prognostic guidance supports its use in the orthopaedics and the orthogeriatric model of care. The CFS helps to reduce age-related bias regarding surgical management decision making by also identifying independent, robust elderly patients.
Limitations
The main limitation of this study is the retrospective application of the CFS score. The scores were calculated from the allied health documentation which details patient medical history, as well as cognitive and physical function prior to admission, however best practice for future studies would include the recording of the CFS score at the time of admission. This study did not explore the duration of the rehabilitation admission, complication rates and readmission rates according to CFS score.
Conclusion
This study demonstrates that frailty, rather than ASA or chronological age, better predicts mortality after proximal femur fracture. Identifying frailty in the acute setting represents a major challenge; however, the CFS proves a quick and reliable tool for accurate assessment by non-geriatricians and we recommend its incorporation into the orthopaedic admission and surgical decision-making process.
Supplementary material
SHARE-ClinicalFrailty Scale (Theou et al, 2013).
Acknowledgements
The authors thank Lisa Welthy (Clinical Nurse Specialist) for her significant contribution to data collection.
Footnotes
Author contributions: S. Narula: Collected the data, Undertook statistical analysis, Authored the manuscript.
A. Lawless: Collected the data, Undertook statistical analysis, Authored the manuscript.
P. D’Alessandro: Interpreted the data, Edited the manuscript.
C. W. Jones: Interpretted the data, edited the manuscript.
P. Yates: Interpreted the data, Edited the manuscript.
H. Seymour: Interpreted the data, Edited the manuscript.
Funding statement: No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
ICMJE COI statement: The authors have no financial or competing interests to disclose.
Ethical review statement: This study received full approval from our local Institutional Review Board.
Twitter: @SidNarula
References
- 1. Australian and New Zealand Hip Fracture Registry ANZHFR Annual Report of Hip Fracture Care, 2019. Available. https://anzhfr.org/wp-content/uploads/2019/09/2019-ANZHFR-Annual-Report-FINAL.pdf (date last accessed 15 July 2020).
- 2. ANZCA Anaesthesia and hip fracture; a review of current literature. http://www.anzca.edu.au/documents/aa2007-anaesthesia-and-hip-fracture.pdf (date last accessed 15 July 2020).
- 3. Collard RM, Boter H, Schoevers RA, Oude Voshaar RC. Prevalence of frailty in community-dwelling older persons: a systematic review. J Am Geriatr Soc. 2012;60(8):1487–1492. [DOI] [PubMed] [Google Scholar]
- 4. Makary MA, Segev DL, Pronovost PJ, et al. Frailty as a predictor of surgical outcomes in older patients. J Am Coll Surg. 2010;210(6):901–908. [DOI] [PubMed] [Google Scholar]
- 5. Morley JE, Vellas B, van Kan GA, et al. Frailty consensus: a call to action. J Am Med Dir Assoc. 2013;14(6):392–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Basic D, Shanley C. Frailty in an older inpatient population: using the clinical frailty scale to predict patient outcomes. J Aging Health. 2015;27(4):670–685. [DOI] [PubMed] [Google Scholar]
- 7. Rockwood K, Song X, MacKnight C, et al. A global clinical measure of fitness and frailty in elderly people. CMAJ. 2005;173(5):489–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cheung A, Haas B, Ringer TJ, et al. Canadian study of health and aging clinical frailty scale: does it predict adverse outcomes among geriatric trauma patients? J Am Coll Surg. 2017;225(5):658–665. [DOI] [PubMed] [Google Scholar]
- 9. Wallis SJ, Wall J, Biram RWS, Romero-Ortuno R. Association of the clinical frailty scale with hospital outcomes. QJM. 2015;108(12):943–949. [DOI] [PubMed] [Google Scholar]
- 10. Reichart D, Rosato S, Nammas W, et al. Clinical frailty scale and outcome after coronary artery bypass grafting. Eur J Cardiothorac Surg. 2018;54(6):1102–1109. [DOI] [PubMed] [Google Scholar]
- 11. Shimura T, Yamamoto M, Kano S, et al. Impact of the clinical frailty scale on outcomes after transcatheter aortic valve replacement. Circulation. 2017;135(21):2013–2024. [DOI] [PubMed] [Google Scholar]
- 12. Wang HT, Fafard J, Ahern S, et al. Frailty as a predictor of hospital length of stay after elective total joint replacements in elderly patients. BMC Musculoskelet Disord. 2018;19(1):14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ondeck NT, Bovonratwet P, Ibe IK, et al. Discriminative ability for adverse outcomes after surgical management of hip fractures: a comparison of the Charlson comorbidity index, Elixhauser comorbidity measure, and modified frailty index. J Orthop Trauma. 2018;32(5):231–237. [DOI] [PubMed] [Google Scholar]
- 14. Kirkland LL, Kashiwagi DT, Burton MC, et al. The Charlson comorbidity index score as a predictor of 30-day mortality after hip fracture surgery. Am J Med Qual. 2011;26(6):461–467. [DOI] [PubMed] [Google Scholar]
- 15. Nanjayan SK, John J, Swamy G, et al. Predictors of change in ‘discharge destination’ following treatment for fracture neck of femur. Injury. 2014;45(7):1080–1084. [DOI] [PubMed] [Google Scholar]
- 16. Michel JP, Klopfenstein C, Hoffmeyer P, et al. Hip fracture surgery: is the pre-operative American Society of Anesthesiologists (ASA) score a predictor of functional outcome? Aging Clin Exp Res. 2002;14(5):389–394. [DOI] [PubMed] [Google Scholar]
- 17. Bjorgul K, Novicoff WM, Saleh KJ. American Society of anesthesiologist physical status score may be used as a comorbidity index in hip fracture surgery. J Arthroplasty. 2010;25(6 Suppl):134–137. [DOI] [PubMed] [Google Scholar]
- 18. Kastanis G, Topalidou A, Alpantaki K, et al. Is the ASA score in geriatric hip fractures a predictive factor for complications and readmission? Scientifica. 2016;2016:7096245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Theou O, Brothers TD, Mitnitski A, Rockwood K. Operationalization of frailty using eight commonly used scales and comparison of their ability to predict all-cause mortality. J Am Geriatr Soc. 2013;61(9):1537–1551. [DOI] [PubMed] [Google Scholar]
- 20. Cesari M, Prince M, Thiyagarajan JA, et al. Frailty: an emerging public health priority. J Am Med Dir Assoc. 2016;17(3):188–192. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


