Abstract
Purpose
Pediatric acute liver failure (PALF) is a serious condition; however, data on PALF in developing countries are sparse, particularly concerning molecular diagnosis and liver transplantation (LT). This study aimed to determine the causes, outcomes, and prognostic factors of PALF.
Methods
We retrospectively reviewed the medical records of children (age <15 years) with PALF diagnosed using the American Association for the Study of Liver Diseases criteria at our center from 2011 to 2016. The collected data included laboratory results, complications, outcomes, and potential factors associated with death and LT.
Results
We included a total of 27 patients, with a median age of 2 years (interquartile range, 3 months to 4 years). Viral infection was the most common etiology (n=8, 30%), predominantly dengue infection (n=4). A total of 16 patients (59%) died and 11 patients survived (3 patients with LT). The prognostic factors associated with death or LT requirement were grade IV hepatic encephalopathy (p<0.01), hypotension (p=0.02), gastrointestinal bleeding (p=0.03), increased intracranial pressure (p=0.04), and higher peak serum lactate level (p=0.01). Peak serum lactate ≥6 mmoL/L had a sensitivity of 79% and a specificity of 88% for predicting mortality or the necessity of LT.
Conclusion
Viral infection was the most common cause of PALF. The mortality rate remained high, and a considerable number of patients required LT. In addition to several clinical factors, peak serum lactate could be a potential marker for predicting poor outcomes in PALF.
Keywords: Acute liver failure, Children, Hepatic encephalopathy, Liver transplantation
INTRODUCTION
Pediatric acute liver failure (PALF) is a serious condition [1] caused by infection, drug toxicity, metabolic liver diseases, and autoimmune hepatitis [2]. The identified etiologies of PALF vary across different studies. Although acetaminophen toxicity and viral infection are the two most common causes of PALF [2,3,4], acute liver failure in infants is predominantly caused by metabolic liver diseases [5]. Approximately half of the reported PALF cases had an unknown etiology [2].
Determining the causes of PALF is mandatory to provide a disease-specific approach, which may include liver transplantation (LT), a life-saving intervention for severe progressive PALF [6]. Making a decision to perform LT is challenging because the outcomes of PALF vary depending on the etiology [7]. Whereas indeterminate PALF and non-acetaminophen drug-induced PALF have a poor prognosis, the spontaneous recovery rate from acetaminophen-induced PALF is high [2]. The timing to perform LT and the preoperative evaluation are also complex issues [8]. Many patients have severe coagulopathy, intracranial bleeding, and secondary infection [9]; however, delayed LT could result in death or irreversible brain damage [9].
The prognostic factors for PALF are inconsistently reported in studies, including international normalized ratio (INR), hepatic encephalopathy (HE), factor V, and pediatric end-stage liver disease (PELD) score [2,7,9,10]. Moreover, the King's College Criteria have been developed to aid in making decisions in PALF [11]. Nevertheless, no reliable criteria exist for predicting death in non-acetaminophen drug-induced PALF [12].
Previous studies have revealed that PALF in developing countries has different etiologies from PALF in Europe and North America [13,14]. In Thailand, studies before the LT era have demonstrated that dengue infection is the most common etiology of PALF [15,16], with a mortality rate of 69%. Currently, our institution is a referral center with an active liver transplant program. For this reason, we aimed to identify the etiologies and outcomes of PALF in the LT era. We also planned to determine potential factors associated with mortality and/or the requirement for LT.
MATERIALS AND METHODS
Study population
From the medical records, we identified patients aged ≤15 years diagnosed with acute liver failure from 2011 to 2016. The diagnosis of PALF was reviewed according to the criteria from the American Association for the Study of Liver Diseases (AASLD), as follows: 1) a biochemical injury without evidence of chronic liver diseases and 2) coagulopathy uncorrected with vitamin K administration (INR >1.5 with HE or INR >2 regardless of HE) [2,6]. In addition, HE was defined according to clinical characteristics and categorized into four grades following the West Haven criteria [17]. Patients who did not fulfill the AASLD criteria were excluded from the study.
We reviewed the history and clinical progression of PALF. At our institution, the causes of PALF were initially determined from history taking and basic laboratory investigations (acetaminophen level; serology for hepatitis A/B/C, cytomegalovirus, Epstein-Barr virus, and other viruses; autoantibodies; metabolic screening; and other tests, as clinically indicated). If the cause of PALF was unidentified, further investigations included molecular virologic studies (i.e., polymerase chain reaction for herpesvirus family and enterovirus) followed by whole-exome sequencing for possible genetic/metabolic liver diseases. The recorded complications for the clinical course were seizure, increased intracranial pressure, gastrointestinal bleeding, hypotension, and hypoglycemia. We also collected potential prognostic laboratory data, such as initial and peak serum total bilirubin, direct bilirubin, INR, ammonia, lactate, and D-dimer. The initial and lowest fibrinogen levels, and the initial and peak PELD score during the admission were also examined.
The patients were categorized according to PALF outcomes, as follows: survived with the native liver, survived with LT, died without LT, and died after LT. The PALF complications and laboratory results were compared between a group of children who survived with the native liver and a group of children who died or required LT.
The study was approved by the Human Research Ethics Committee, Faculty of Medicine Ramathibodi Hospital, Mahidol University (Institutional Review Board No. MURA2017/474).
Statistical analysis
We used the STATA program, version 13.0 (Stata Co., College Station, TX, USA), for statistical analyses. To identify the association with a poor PALF prognosis (i.e., death or LT requirement), Fisher's exact test and the Mann–Whitney U-test were applied. A p-value of <0.05 was considered to indicate statistical significance. Receiver operating characteristic (ROC) curve analysis was performed to determine the optimal cutoff for a poor prognosis. The sensitivity, specificity, and area under the ROC curve were also calculated.
RESULTS
We identified 38 children diagnosed with acute liver failure. However, only 27 patients met the AASLD criteria for PALF and were included in the study. The median age was 2 years (interquartile range: 0.3–4 years), and 11 patients (40.7%) were aged <1 year. At the initial presentation, all patients were clinically diagnosed with HE, with the following severity grades: grade I (n=4, 14.8%), grade II (n=12, 44.4%), grade III (n=9, 33.3%), and grade IV (n=2, 7.4%). The peak grade of HE during the admission was grade IV in most patients (n=16, 59.3%), followed by grade III (9 patients, 33.3%). In addition, 25 patients (92.6%) required mechanical ventilation. The characteristics and laboratory results of all patients are summarized in Table 1.
Table 1. Characteristics of 27 children with pediatric acute liver failure.
| Patient | Age (mo) | Initial HE (grade) | Peak HE (grade) | Initial INR | Peak PELD score | Peak AST (U/L) | Peak ALT (U/L) | Peak TB (mg/dL) | Peak DB (mg/dL) | Peak INR | Peak serum lactate (mmoL/L) | Peak serum ammonia (μmoL/L) | Etiology | Outcome | Cause of death | Notable intervention |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 4 | 1 | 2 | 1.5 | 17 | 1,177 | 868 | 16 | 9 | 1.7 | 1 | NA | Unknown | Survived with the native liver | - | - |
| 2 | 7 | 3 | 4 | 3.3 | 20 | 13,948 | 9,356 | 2 | 1.9 | 3.8 | 13.2 | 30 | Unknown | Died w/o LT | SI | - |
| 3 | 11 | 4 | 4 | 1.7 | 7 | 3,359 | 4,757 | 0.8 | 0.4 | 1.7 | 2.7 | NA | Unknown | Died w/o LT | IH | TE |
| 4 | 36 | 3 | 4 | 8.2 | 41 | 19,048 | 4,869 | 12.1 | 9 | 8.2 | 0.8 | 354 | Unknown | Died w/o LT | MOF | - |
| 5 | 60 | 2 | 3 | 7.4 | 19 | 21,200 | 12,000 | 5.4 | 4.9 | 7.5 | 4.4 | 68 | DHF | Survived with the native liver | - | - |
| 6 | 28 | 3 | 3 | 10.1 | 31 | 3,382 | 4,845 | 25 | 17 | 10.1 | 2 | 360 | Unknown | Survived with the native liver | - | NAC, TE |
| 7 | 3 | 1 | 4 | 12 | 50 | 4,455 | 1,643 | 20.2 | 9.4 | 12 | 6.8 | 122 | HLH | Died w/o LT | MOF | IVIG, TE |
| 8 | 3 | 2 | 4 | 9 | 48 | 751 | 317 | 16.3 | 5.5 | 9 | 17.5 | 713 | HLH | Died w/o LT | MOF | IVIG, TE |
| 9 | 660 | 3 | 4 | 2.2 | 21 | 25,765 | 2,308 | 33 | 16 | 2.3 | 13.8 | 209 | DHF | Died w/o LT | SI | CVVH, NAC, TE |
| 10 | 60 | 4 | 4 | 3.4 | 15 | 9,299 | 7,729 | 4.8 | 3.6 | 4.6 | 10.8 | 76 | Wilson's disease | Died w/o LT | MOF | CVVH, PE, CVVH |
| 11 | 36 | 2 | 3 | 8.5 | 46 | 3,937 | 3,204 | 30.7 | 26 | 8.5 | 8.6 | 136 | Parvovirus B19 | Survived with LT | - | - |
| 12 | 48 | 2 | 3 | 5.9 | 45 | 30,753 | 17,942 | 21.5 | 12.5 | 5.9 | 2.3 | 87 | NBAS deficiency | Survived with the native liver | - | - |
| 13 | 24 | 2 | 3 | 23 | 68 | 2,227 | 3,476 | 12.9 | 2.8 | 23 | 6 | 60 | Amanita spp. intoxication | Survived with LT | - | NAC, TE |
| 14 | 12 | 3 | 3 | 9 | 44 | 8,240 | 6,520 | 3 | 1.4 | 9 | 5.4 | 108 | NBAS deficiency | Survived with the native liver | - | TE |
| 15 | 120 | 3 | 3 | 3.7 | 27 | 1,123 | 964 | 31.7 | 19.3 | 3.8 | 3 | 120 | Unknown | Survived with LT | - | NAC |
| 16 | 36 | 3 | 3 | 4.5 | 30 | 1,079 | 1,475 | 1.6 | 0.8 | 4.5 | 1.8 | 98 | Citrullinemia type I | Survived with the native liver | - | - |
| 17 | 36 | 3 | 4 | 2.7 | 16 | 29,300 | 30,000 | 5.4 | 3 | 2.7 | 6.9 | 204 | DHF | Died w/o LT | MOF | CVVH, IVIG, TE |
| 18 | 4 | 2 | 2 | 2.9 | 28 | 1,012 | 348 | 37.5 | 2.92 | 4.8 | 0.8 | NA | Unknown | Survived with the native liver | - | - |
| 19 | 108 | 2 | 4 | 1.5 | 17 | 4,200 | 1,338 | 17.5 | 8.3 | 2.1 | 0.5 | 98 | HLH | Died w/o LT | MOF | - |
| 20 | 24 | 2 | 4 | 3.6 | 29 | 18,000 | 12,000 | 8.5 | 2.9 | 5.4 | 9.4 | 361 | Amanita spp. intoxication | Died after LT | HAT | NAC, TE |
| 21 | 0.5 | 2 | 4 | 12 | 48 | 18,683 | 1,411 | 11.8 | 3.8 | 12 | 14.8 | 202 | HSV type 1 | Died w/o LT | MOF | TE |
| 22 | 2 | 3 | 4 | 12 | 55 | 790 | 253 | 39 | 19 | 12 | 7.6 | 158 | CMV | Died w/o LT | SI | IVIG, TE |
| 23 | 0.2 | 1 | 4 | 5.3 | 37 | 6,756 | 998 | 9.8 | 1.2 | 5.6 | 6.8 | 130 | Enterovirus | Died w/o LT | MOF | IVIG, TE |
| 24 | 0.5 | 2 | 4 | 17.4 | 60 | 896 | 425 | 23.6 | 4.6 | 17.4 | 6.8 | 482 | NH | Died w/o LT | MOF | IVIG, TE |
| 25 | 108 | 2 | 4 | 4.9 | 28 | 22,357 | 3,441 | 13.7 | 6.6 | 4.9 | 15.8 | 142 | DHF | Died w/o LT | MOF | IVIG, PE |
| 26 | 24 | 2 | 3 | 3.1 | 27 | 1,177 | 1,452 | 17 | 9.8 | 3.1 | 7.9 | 103 | Cassia occidentalis intoxication | Survived with the native liver | - | NAC |
| 27 | 0.03 | 1 | 4 | 3.9 | 29 | 4,437 | 448 | 29.2 | 19.1 | 3.9 | 6.5 | NA | NH | Died w/o LT | MOF | TE |
HE: hepatic encephalopathy, INR: international normalized ratio, PELD: pediatric end-stage liver disease, AST: aspartate aminotransferase, ALT: alanine aminotransferase, TB: total bilirubin, DB: direct bilirubin, NA: not available, DHF: dengue hemorrhagic fever, HLH: hemophagocytic lymphohistiocytosis, HSV: herpes simplex virus, CMV: cytomegalovirus, NH: neonatal hemochromatosis, LT: liver transplantation, w/o: without, SI: secondary infection, IH: intracranial hemorrhage, MOF: multiorgan failure, HAT: hepatic artery thrombosis, TE: total exchange transfusion, NAC: N-acetylcysteine, CVVH: continuous venovenous hemofiltration, PE: plasma exchange, IVIG: intravenous immunoglobulin.
Etiologies of PALF
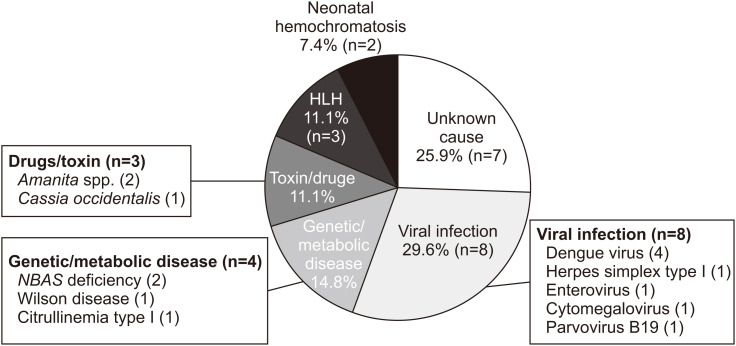
The etiologies of PALF are shown in a pie chart in Fig. 1. Viral infection was the most common cause (n=8, 29.6%), with half of the cases attributed to dengue infection (n=4, 14.8% of total). The second most common cause was genetic or metabolic disease (n=4, 14.8%). Drugs/toxin and hemophagocytic lymphohistiocytosis as causes of PALF were found in equal proportion (n=3, 11.1%). Despite extensive investigations, the cause of PALF was not identified in seven patients (25.9%).
Fig. 1. Etiologies of pediatric acute liver failure.
HLH, hemophagocytic lymphohistiocytosis.
Outcomes of PALF and prognostic factors
A total of 16 patients (59.3%) died (15 without LT and 1 with hepatic artery thrombosis after LT), whereas 11 patients survived (8 with the native liver and 3 with LT). The causes of death in non-LT patients were multiorgan failure (n=11, 40.7%), sepsis (n=6, 22.2%), intracranial hemorrhage (n=3, 11.1%), and increased intracranial pressure with brain herniation (n=1, 3.7%). In eight patients who survived with the native liver, the etiology of PALF was determined to be unknown (n=3, 11.1%), metabolic diseases (n=3, 11.1%), dengue hemorrhagic fever (n=1, 3.7%), and drugs/toxin (n=1, 3.7%).
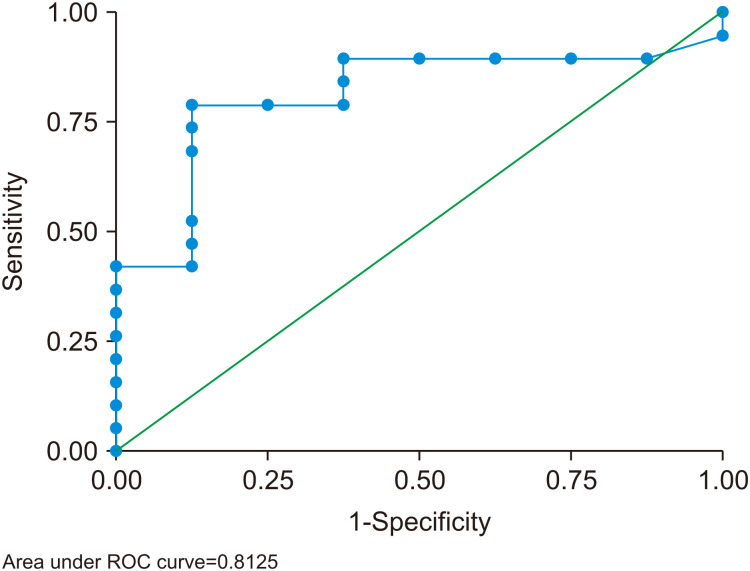
Comparisons between patients who survived with the native liver and patients who died or required LT are shown in Table 2. Factors associated with mortality or the requirement for LT were seizure, gastrointestinal bleeding, hypotension, and increased intracranial pressure. In addition, none of the patients who survived with the native liver had grade IV HE. Among the laboratory parameters, peak serum lactate was the only potential prognostic factor (p=0.01). Peak serum lactate ≥6 mmoL/L had a sensitivity of 79% and a specificity of 88% for predicting mortality or the necessity of LT. The area under the ROC curve was 0.81 (95% confidence interval, 0.64–0.99) (Fig. 2). Interventions, such as continuous venovenous hemofiltration, intravenous immunoglobulin, N-acetylcysteine, plasma exchange, and total exchange transfusion, were not different between the two groups.
Table 2. Comparison between patients who survived with the native liver and patients who died or required liver transplantation.
| Characteristics | Group | p-value | ||
|---|---|---|---|---|
| Survived with the native liver (n=8) | Died or required LT (n=19) | |||
| Peak HE during admission | ||||
| Grade IV | 0 | 16 | <0.01 | |
| Complications | ||||
| Seizure | 1 | 11 | 0.04 | |
| GI bleeding | 2 | 14 | 0.03 | |
| Hypotension | 2 | 15 | 0.02 | |
| Hypoglycemia | 1 | 10 | 0.09 | |
| Increase intracranial pressure | 1 | 11 | 0.04 | |
| Laboratory results | ||||
| Peak total bilirubin (mg/dL) | 16.5 (4.2, 23.3) | 13.7 (8.5, 29.2) | 0.85 | |
| Peak direct bilirubin (mg/dL) | 7 (2.2, 11.2) | 5.5 (2.9, 16) | 0.77 | |
| Peak INR | 5.3 (3.8, 8.2) | 5.4 (3.8, 12) | 0.69 | |
| Peak ammonia (μmoL/L) | 100.5 (87, 108) | 142 (120, 209) | 0.15 | |
| Peak lactate (mmoL/L) | 2.2 (1.4, 4.9) | 6.9 (6, 13.2) | 0.01 | |
| Peak D-dimer (mcg/L) | 4,385 (1,240, 18,580) | 17,417 (8,245, 25,475) | 0.48 | |
| Lowest fibrinogen (mg/dL) | 104.5 (81, 110) | 108.5 (69, 160) | 0.70 | |
| PELD score | ||||
| Initial | 27 (21, 37.5) | 29 (17, 48) | 0.73 | |
| Peak | 29 (23, 37.5) | 29 (20, 48) | 0.57 | |
Values are presented as number only or median (interquartile range).
LT: liver transplantation, HE: hepatic encephalopathy, GI: gastrointestinal, INR: international normalized ratio, PELD: pediatric end-stage liver disease.
Fig. 2. Receiver operating characteristic (ROC) curve of serum lactate ≥6 mmoL/L for predicting mortality or the necessity of liver transplantation. The area under the ROC curve was 0.81 (95% confidence interval, 0.64–0.99).
DISCUSSION
Our study showed that viral infection, with half of the cases caused by the dengue virus, was the most common etiology of PALF. In contrast, in previous reports, most of the PALF cases had an idiopathic etiology [1,2]. Although dengue remains the most commonly identified virus in Thailand, a high proportion of patients infected with other viruses has been noted. Poovorawan et al. [15] studied 45 Thai children from 14 hospitals in 2006, and found that PALF was predominantly caused by unknown etiologies (37%), followed by dengue infection (34%). The proportion of patients with an unknown cause of PALF was reduced in this study, accounting for 25% of the cases. This finding is likely due to the availability of PCR tests for several viruses at our center, which are not routinely used in regional or local Thai hospitals.
Interestingly, none of our patients had viral hepatitis A or B. Previous studies on PALF in Thailand also reported only small numbers of viral hepatitis cases [15,16], whereas other studies from Southeast Asia showed significant numbers of children with these viruses. Hepatitis A virus is the most common cause of PALF in the Philippines [18]. Similarly, Tran et al. [19] have reported that hepatitis A and B are the major causes of liver disease in Vietnamese children. The relatively low prevalence of viral hepatitis A and B in Thailand probably resulted from improvements in sanitation and immunization. The incidence of hepatitis A virus infection decreased from 2,839 to 897 per 100,000 Thai people in 1950 and 2000, respectively [20]. After the implementation of the universal hepatitis B virus immunization program, the percentage of Thai hepatitis B carriers and anti-hepatitis B core seropositive individuals decreased from 1.4% and 5.5% in 2004 to 0.4% and 1.6% in 2014, respectively [21].
Similar to previous studies, genetic and metabolic liver diseases were more common in young children in this study [2,5].
Interestingly, none of the patients with PALF in this study had acetaminophen toxicity, although we routinely investigated the acetaminophen level in every patient who received this medication. This low incidence was in contrast to a report from the United States [6,22]. Acetaminophen toxicity is also the most commonly identified cause of PALF in a multicenter study of 348 North American and British children [2]. This difference could be attributed to the fact that children with acetaminophen-induced PALF are not routinely referred to our institution, as they can be treated with N-acetylcysteine without requiring LT. In addition, two of our patients experienced Amanita phalloides intoxication, the most common cause of mushroom-induced PALF. Similar reports of this intoxication have been published in Europe, Asia, and northeastern Thailand [23,24].
The mortality rate in our patients was lower than that in the previous PALF study in Thailand (59% vs. 69%) [15]. This could be due to the different hospital settings, particularly the category of having facility for LT. Moreover, low mortality rate of children with PALF in the LT era is as low as 13% in other report [25].
Dhawan et al. [7] reported that poor outcomes or the requirement for LT depend on the etiologies of PALF. We could not find any relationship between etiologies and outcomes, probably owing to the small sample size. However, we noted that none of the patients who survived with the native liver had grade IV HE. Our results were consistent with a study by Squire et al. [2], in which a low spontaneous recovery rate of 22% was reported in patients with grade IV HE.
In this study, higher peak serum lactate level was associated with mortality or the requirement for LT, with an area under the ROC curve of 0.81. Similarly, high blood lactate level has been reported to be associated with poor outcomes in adults with acute liver failure and acute-on-chronic liver failure [26,27,28]. Hyperlactatemia is likely due to increased lactate production from persistent liver injury, multiorgan dysfunction, and tissue hypoperfusion, as well as impaired lactate clearance caused by poor hepatic metabolism [26,27]. Other poor prognostic factors have been proposed in other reports. Dhawan et al. [7] have demonstrated that INR ≥4, serum bilirubin ≥235 μmoL/L, age <2 years, and total white blood cells >9×109/L are poor prognostic factors, with an area under the ROC curve of 0.79, 0.76, 0.74, and 0.78, respectively. Rajanayagam et al. [10] also noted that the PELD score during admission is correlated with the prognosis of PALF, which was in contrast to our result. Devictor et al. [9] revealed that a factor V level of <25% suggests poor outcomes and could indicate the necessity of LT. Unfortunately, we did not routinely investigate factor V level in patients with PALF. Other laboratory tests that were associated with poor outcomes included blood glucose <45 mg/dL, serum bilirubin >10 mg/dL, and blood pH <7.35 or >7.45 [29]. However, the association between poor outcomes and these tests could not be demonstrated in this study.
Our report had some limitations owing to the small sample size and the retrospective design that could be prone to missing data. Further, we could not identify the definite time of HE progression, as it was diagnosed clinically, and electroencephalography was not performed in every case. Moreover, a referral bias may exist because our institution is an active liver transplant center to which severe cases of PALF are likely to be referred.
This study found that viral infection was the most common cause of PALF. The proportion of cases with an unknown cause seemed to decrease with advanced laboratory investigations. The mortality rate of PALF was also reduced in the LT era. Although further studies with larger sample sizes are required to confirm the identified potential prognostic factors, we propose peak serum lactate as a marker for predicting poor outcomes in PALF.
ACKNOWLEDGEMENTS
We thank our colleagues from Ramathibodi Hospital, including Mrs. Umaporn Udomsubpayakul, Division of Clinical Epidemiology and Biostatistics, for her advice on statistical analyses, and the Ramathibodi Liver Transplant Team for their support during the study.
Footnotes
Conflict of Interest: The authors have no financial conflicts of interest.
References
- 1.Mouzaki M, Ng VL. Acute liver failure in children. Clin Pediatr Emerg Med. 2010;11:198–206. [Google Scholar]
- 2.Squires RH, Jr, Shneider BL, Bucuvalas J, Alonso E, Sokol RJ, Narkewicz MR, et al. Acute liver failure in children: the first 348 patients in the pediatric acute liver failure study group. J Pediatr. 2006;148:652–658. doi: 10.1016/j.jpeds.2005.12.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arora NK, Nanda SK, Gulati S, Ansari IH, Chawla MK, Gupta SD, et al. Acute viral hepatitis types E, A, and B singly and in combination in acute liver failure in children in north India. J Med Virol. 1996;48:215–221. doi: 10.1002/(SICI)1096-9071(199603)48:3<215::AID-JMV1>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 4.Escorsell A, Mas A, de la Mata M Spanish Group for the Study of Acute Liver Failure. Acute liver failure in Spain: analysis of 267 cases. Liver Transpl. 2007;13:1389–1395. doi: 10.1002/lt.21119. [DOI] [PubMed] [Google Scholar]
- 5.Durand P, Debray D, Mandel R, Baujard C, Branchereau S, Gauthier F, et al. Acute liver failure in infancy: a 14-year experience of a pediatric liver transplantation center. J Pediatr. 2001;139:871–876. doi: 10.1067/mpd.2001.119989. [DOI] [PubMed] [Google Scholar]
- 6.Polson J, Lee WM. AASLD position paper: the management of acute liver failure. Hepatology. 2005;41:1179–1197. doi: 10.1002/hep.20703. [DOI] [PubMed] [Google Scholar]
- 7.Dhawan A, Cheeseman P, Mieli-Vergani G. Approaches to acute liver failure in children. Pediatr Transplant. 2004;8:584–588. doi: 10.1111/j.1399-3046.2004.00292.x. [DOI] [PubMed] [Google Scholar]
- 8.Mendizabal M, Silva MO. Liver transplantation in acute liver failure: a challenging scenario. World J Gastroenterol. 2016;22:1523–1531. doi: 10.3748/wjg.v22.i4.1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Devictor D, Desplanques L, Debray D, Ozier Y, Dubousset AM, Valayer J, et al. Emergency liver transplantation for fulminant liver failure in infants and children. Hepatology. 1992;16:1156–1162. [PubMed] [Google Scholar]
- 10.Rajanayagam J, Coman D, Cartwright D, Lewindon PJ. Pediatric acute liver failure: etiology, outcomes, and the role of serial pediatric end-stage liver disease scores. Pediatr Transplant. 2013;17:362–368. doi: 10.1111/petr.12083. [DOI] [PubMed] [Google Scholar]
- 11.O'Grady JG, Alexander GJ, Hayllar KM, Williams R. Early indicators of prognosis in fulminant hepatic failure. Gastroenterology. 1989;97:439–445. doi: 10.1016/0016-5085(89)90081-4. [DOI] [PubMed] [Google Scholar]
- 12.Sundaram V, Shneider BL, Dhawan A, Ng VL, Im K, Belle S, et al. King's College Hospital Criteria for non-acetaminophen induced acute liver failure in an international cohort of children. J Pediatr. 2013;162:319–23.e1. doi: 10.1016/j.jpeds.2012.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aydoğdu S, Ozgenç F, Yurtsever S, Akman SA, Tokat Y, Yağci RV. Our experience with fulminant hepatic failure in Turkish children: etiology and outcome. J Trop Pediatr. 2003;49:367–370. doi: 10.1093/tropej/49.6.367. [DOI] [PubMed] [Google Scholar]
- 14.Alam S, Khanna R, Sood V, Lal BB, Rawat D. Profile and outcome of first 109 cases of paediatric acute liver failure at a specialized paediatric liver unit in India. Liver Int. 2017;37:1508–1514. doi: 10.1111/liv.13370. [DOI] [PubMed] [Google Scholar]
- 15.Poovorawan Y, Hutagalung Y, Chongsrisawat V, Boudville I, Bock HL. Dengue virus infection: a major cause of acute hepatic failure in Thai children. Ann Trop Paediatr. 2006;26:17–23. doi: 10.1179/146532806X90565. [DOI] [PubMed] [Google Scholar]
- 16.Poovorawan Y, Chongsrisawat V, Shafi F, Boudville I, Liu Y, Hutagalung Y, et al. Acute hepatic failure among hospitalized Thai children. Southeast Asian J Trop Med Public Health. 2013;44:50–53. [PubMed] [Google Scholar]
- 17.Ferenci P, Lockwood A, Mullen K, Tarter R, Weissenborn K, Blei AT. Hepatic encephalopathy--definition, nomenclature, diagnosis, and quantification: final report of the working party at the 11th World Congresses of Gastroenterology, Vienna, 1998. Hepatology. 2002;35:716–721. doi: 10.1053/jhep.2002.31250. [DOI] [PubMed] [Google Scholar]
- 18.Bravo LC, Gregorio GV, Shafi F, Bock HL, Boudville I, Liu Y, et al. Etiology, incidence and outcomes of acute hepatic failure in 0-18 year old Filipino children. Southeast Asian J Trop Med Public Health. 2012;43:764–772. [PubMed] [Google Scholar]
- 19.Tran HTM, Vo HTD, Tran AD, Nguyen TT. P0261 Liver disease in Vietnamese children. J Pediatr Gastroenterol Nutr. 2004;39(Suppl 1):S157. [Google Scholar]
- 20.Van Effelterre T, Marano C, Jacobsen KH. Modeling the hepatitis A epidemiological transition in Thailand. Vaccine. 2016;34:555–562. doi: 10.1016/j.vaccine.2015.11.052. [DOI] [PubMed] [Google Scholar]
- 21.Posuwan N, Wanlapakorn N, Sa-Nguanmoo P, Wasitthankasem R, Vichaiwattana P, Klinfueng S, et al. The success of a universal hepatitis B immunization program as part of Thailand's EPI after 22 years' implementation. PLoS One. 2016;11:e0150499. doi: 10.1371/journal.pone.0150499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cranswick N, Coghlan D. Paracetamol efficacy and safety in children: the first 40 years. Am J Ther. 2000;7:135–141. doi: 10.1097/00045391-200007020-00010. [DOI] [PubMed] [Google Scholar]
- 23.Santi L, Maggioli C, Mastroroberto M, Tufoni M, Napoli L, Caraceni P. Acute liver failure caused by Amanita phalloides poisoning. Int J Hepatol. 2012;2012:487480. doi: 10.1155/2012/487480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chaiear K, Limpaiboon R, Meechai C, Poovorawan Y. Fatal mushroom poisoning caused by Amanita virosa in Thailand. Southeast Asian J Trop Med Public Health. 1999;30:157–160. [PubMed] [Google Scholar]
- 25.Lee WM, Squires RH, Jr, Nyberg SL, Doo E, Hoofnagle JH. Acute liver failure: summary of a workshop. Hepatology. 2008;47:1401–1415. doi: 10.1002/hep.22177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bernal W. Lactate is important in determining prognosis in acute liver failure. J Hepatol. 2010;53:209–210. doi: 10.1016/j.jhep.2010.02.017. [DOI] [PubMed] [Google Scholar]
- 27.Bernal W, Donaldson N, Wyncoll D, Wendon J. Blood lactate as an early predictor of outcome in paracetamol-induced acute liver failure: a cohort study. Lancet. 2002;359:558–563. doi: 10.1016/S0140-6736(02)07743-7. [DOI] [PubMed] [Google Scholar]
- 28.Cardoso FS, Abraldes JG, Sy E, Ronco JJ, Bagulho L, Mcphail MJ, et al. Lactate and number of organ failures predict intensive care unit mortality in patients with acute-on-chronic liver failure. Liver Int. 2019;39:1271–1280. doi: 10.1111/liv.14083. [DOI] [PubMed] [Google Scholar]
- 29.Kaur S, Kumar P, Kumar V, Sarin SK, Kumar A. Etiology and prognostic factors of acute liver failure in children. Indian Pediatr. 2013;50:677–679. doi: 10.1007/s13312-013-0189-7. [DOI] [PubMed] [Google Scholar]