Abstract
Purpose
Malnutrition is a common feature in critically ill children. Enteral nutrition (EN) is the main strategy to nutritionally support critical ill children, but its use can be hindered by the development of intolerance. The study aimed to assess the effectiveness and safety of amoxicillin/clavulanate (A/C) to treat EN intolerance.
Methods
We retrospectively evaluated patients admitted to the pediatric intensive care unit from October 2018 to October 2019. We conducted a case-control study: in the first 6 months (October 2018-April 2019) we implemented the nutritional protocol of our Institution with no drug, whereas in the second half (May 2019-October 2019) we employed A/C for 1 week at a dose of 10 mg/kg twice daily.
Results
Twelve cases were compared with 12 controls. At the final evaluation, enteral intake was significantly higher than that at baseline in the cases (from 2.1±3.7 to 66.1±27.4% of requirement, p=0.0001 by Wilcoxon matched-pairs signed rank test) but not in the controls (from 0.2±0.8 to 6.0±14.1% of the requirement, p=NS). Final gastric residual volume at the end of the observation was significantly lower in the cases than in the controls (p=0.0398). The drug was well tolerated as shown by the similar safety outcomes in both cases and controls.
Conclusion
Malnutrition exposes critically ill children to several complications that affect the severity of disease course, length of stay, and mortality; all may be prevented by early EN. The development of intolerance to EN could be addressed with the use of A/C. Future prospective clinical trials are needed to confirm these conclusions.
Keywords: Gastrointestinal motility, Amoxycillin clavulanic acid, Enteral nutrition, Nutrition disorder, Pediatric intensive care unit, Prokinetic, Amoxicillin
INTRODUCTION
Malnutrition is a common feature in patients admitted to the pediatric intensive care unit (PICU), ranging from 10 to 45% of patients; owing to an imbalance between energy requirements and caloric intake [1,2,3,4]. In this setting of care, malnutrition can be life-threatening and can affect the course of infections and the length of ventilation and hospitalization [1,2,3].
Enteral nutrition (EN) is the main strategy to nutritionally support critical ill children and to treat and prevent malnutrition. It has been suggested that EN should be started within the first 24 h of admission to the PICU and that EN withdrawal should be avoided in an attempt to quickly realize the predicted caloric goal [2,4,5]. However, in the PICU, the EN course can be hindered by many factors, in particular, delayed EN beginning or repeated EN interruptions owing to perceived intolerance or required fasting for procedures [2]. Even up to half of patients treated in the PICU may have a true or suspected EN intolerance [5,6,7], which would lead to the maintenance or start of parenteral nutrition (PN) regimens and withdrawal of EN [8,9]. Some treatments can improve EN intolerance, however unfortunately, they are contraindicated or not recommended in children or not available in the market in many countries [8,9]. Amoxicillin/clavulanate (A/C), available and easy to manage in the PICU, seems capable of promoting small intestinal motility, even if administered by the enteral route [10,11,12]. Notably, this drug, if administered into the small bowel lumen before ingestion of a meal, has been shown to induce the development of duodenal phase III-type contractions in the duodenum, with features similar to those present in the fasting state [11]. This observation suggests the potential application of A/C as a prokinetic agent [10,11,12].
The objective of the present study was to assess the effectiveness and safety of A/C to treat EN intolerance in critically ill children.
MATERIALS AND METHODS
Study design
We retrospectively evaluated patients admitted to the PICU from October 2018 to October 2019 and who were treated with the nutritional protocol implemented in our hospital since July 2018 (Fig. 1) and focused on critically ill children aged more than 1 month. The study period was divided into two half-year periods (October 2018–April 2019 and May 2019–October 2019) according to the start of the use of A/C and was approved by our Ethical Committee in April 2019 (see below). Therefore, we conducted a case-control study: in the first 6 months (October 2018–April 2019) we implemented the protocol with no drug that could improve intestinal tolerance, whereas in the second half (May 2019–October 2019) we employed A/C as a prokinetic agent in the cases with suspected EN intolerance. The treatment lasted 1 week at a dose of 10 mg/kg twice daily for 7 days and was administered by devices recommended for EN (see below).
Fig. 1. Enteral feeding management protocol. *Nutritional goal: 75% of the caloric intake according to national guidelines [13,14] at the 7th day following PICU admission. **Gastric tolerance evaluation was based on the GRV (mL/kg) assessment every 4 hours. GRV was extracted from PEG in all patients and it was discarded before the next dietary process. GRV has been considered suggestive for intolerance if it is ≥50% of the volume/h in continuous EN or if it is ≥50% of the bolus volume in intermittent EN over at least three consecutive evaluations following EN beginning. PICU: pediatric intensive care unit, GRV: gastric residual volume, EN: enteral nutrition, HF: hydrolysate formula, IVF: intravenous fluids, MEF: minimal enteral feeding, PN: parenteral nutrition, PF: polymeric formula.
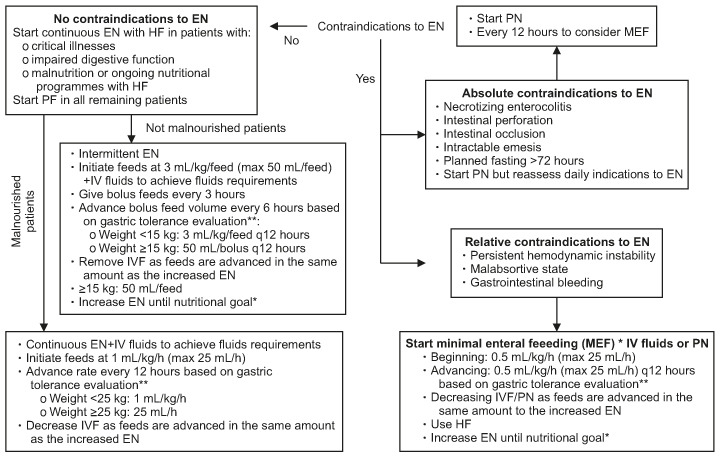
Inclusion criteria for the study were:
a) Age ranging from 1 month to 18 years,
b) Enteral intake lower than 10% of requirement after 7 days since admission to the PICU,
c) Neurological impairment,
d) Indications for admission to the PICU: respiratory failure, seizures, and post-surgery care,
e) Available naso-gastric tube (NGT) or gastrostomy (PEG)/gastro-jejunostomy (PEG-PEJ) for the nutritional treatment.
Exclusion criteria:
a) Known allergy to A/C,
b) Ongoing ketogenic diet,
c) Clinical contraindications to advance EN feeds.
Study protocol
We evaluated all patients (cases and controls) over 8 days; the baseline point was day 1 (for the cases, day 1 was the day before starting A/C) and the end point was day 8. At baseline, we collected the following data:
a) z-scores for weight, height, and body mass index;
b) EN intake (% of energy requirements); gastric residual volume (GRV) expressed in mL/kg of weight.
At the end point we assessed:
a) rate of weaning off mechanical ventilation (MV), death, overall treatments required (opiates, sedatives, muscle relaxants, and vasopressors), and diarrhea;
b) EN intake (% of energy requirements), GRV expressed in mL/kg of weight.
The main outcomes of effectiveness were:
1) Enteral intake of 50% of the requirements at the end point,
2) GRV reduced by at least 50% at the end point.
The main outcomes of safety were the prevalence of the following:
1) Weaning off MV,
2) Death.
3) Overall drugs required.
4) Development of diarrhea (defined as ≥3 liquid stools/day in patients with previous normal stool and/or increase of ≥50% of the number of liquid stools).
Nutritional requirements were assessed according to the Italian National Guidelines [13,14]. GRV, reported as mL/kg, was assessed every 4 h (according to our protocol, Fig. 1). GRV was extracted from PEG in all patients and it was discarded before the next dietary process.
Statistical analysis
Categorical variables were summarized as percentage and continuous variables as mean±standard deviation. Differences in caloric intake by EN and in GRV from baseline to the end point between cases and controls were compared using the Wilcoxon matched-pairs signed rank test. Differences in baseline and final caloric intake by EN, baseline and final GRV, and prevalence of overall treatments between cases and controls were evaluated using the Mann-Whitney-U-test. Categorical measures (MV dependency, death, and development of diarrhea) were assessed using the Fisher's exact test. A p-values<0.05 were considered statistically significant. Statistical evaluation and analyses were performed using Graph Pad Prism 6 for Windows (GraphPad Software, Inc., La Jolla, CA, USA).
Ethics
The Ethical Committee of “Bambino Gesù” Children's Hospital approved the present study (Protocol N° OPBG_1790/2019). Written informed consent was obtained from a parent or surrogate decision maker.
RESULTS
Twenty-four patients in total, 12 cases and 12 controls, were included (Table 1 for details). As shown, indications for admission to the PICU were similar between cases and controls. Age ranges were very wide, and no patient was severely malnourished [15]; access routes for long-term EN were present in 58% of the cases and in 42% of the controls. As shown by the rate of perinatal events, 33% of all patients were affected by cerebral palsy as the primary neurologic disease.
Table 1. Overview of the characteristics of cases and controls.
| Patient characteristics | Cases (n=12) | Controls (n=12) | p-value | |
|---|---|---|---|---|
| Age | 5.5 (0.67–14) | 4 (1–17) | NS | |
| Male/female | 4/8 | 6/6 | NS | |
| Weight* (z-score) | 0.6 (−3.3–1.2) | 0.9 (−0.02–1.5) | NS | |
| Height* (z-score) | −1.4 (−3.4–1.0) | 0.3 (−0.6–1.1) | NS | |
| Body mass index* (z-score) | 0.7 (−1.4–1.8) | 1.1 (−0.7–2.0) | NS | |
| Indication for admission to PICU | ||||
| Respiratory failure | 5 | 5 | ||
| Seizures | 5 | 5 | ||
| Post-surgery care | 3 | 3 | ||
| Perinatal events | 4 | 4 | NS | |
| Tracheostomy | 6 | 4 | NS | |
| NGT | 5 | 7 | NS | |
| PEG/PEJ | 7 | 5 | NS | |
Values are presented as mean (range), number only, or median (interquartile range).
PICU: pediatric intensive care unit, NGT: nasogastric tube, PEG: percutaneous endoscopic gastrostomy, PEJ: percutaneous endoscopic jejunostomy, NS: not significant.
*Reference 15.
Effectiveness measures
At final evaluation, in the case group, enteral intake resulted in significantly higher levels than at baseline (from 2.1±3.7 to 66.1±27.4% of the requirement, p=0.0001 by Wilcoxon matched-pairs signed rank test), but not in the control group (from 0.2±0.8 to 6.0±14.1% of the requirement, p=NS). At the end point, 9 out of 12 cases and none from the control achieved more than 50% of the EN requirements (p=0.0003).
Regarding the GRV at final evaluation, in comparison to the baseline, a 50% reduction was observed in the case group, although the change was not significant (4.1±4.6 and 1.8±3.9 mL/kg, respectively). In the control group, GRV at baseline and at the end point of the study was 5.7±6.7 and 6.9±7.6 mL/kg (p=NS), respectively. Overall, at the end of the observation, GRV was significantly lower in the cases than that in the controls (p=0.0398). Details are presented in Figs. 2, 3, 4.
Fig. 2. Daily trend in caloric intake by enteral nutrition and gastric residual volume. (A) Average daily trend of increase in caloric intake percent by enteral nutrition in patients treated with amoxicillin/clavulanate. (B) Average daily trend of increase in caloric intake percent by enteral nutrition in controls. (C) Average daily trend of gastric residual volume (mL/kg) in patients treated with amoxicillin/clavulanate. (D) Average daily trend of gastric residual volume (mL/kg) in controls.
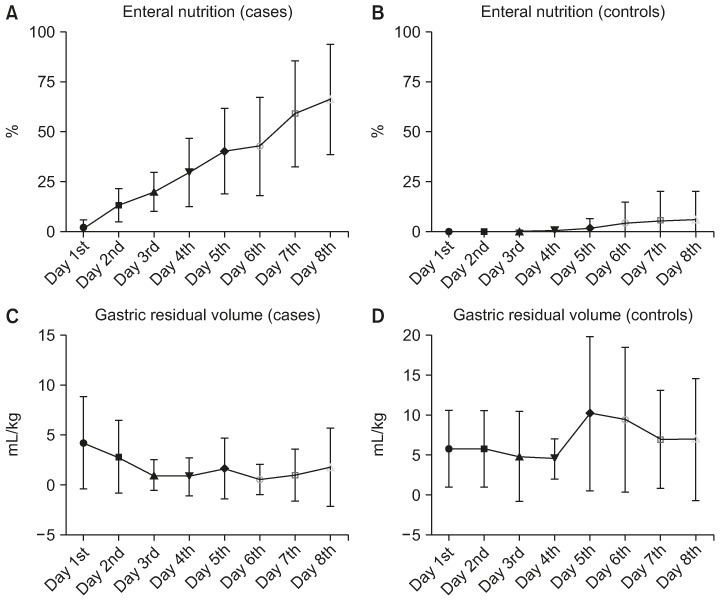
Fig. 3. Baseline and final assessment of caloric intake by enteral nutrition and gastric residual volume by group (Wilcoxon matched-pairs signed rank test). (A) Trend in caloric intake increase by enteral nutrition (%) from baseline to final assessment patients by patients in cases treated with amoxicilline/clavulanate; p=0.0005, by Wilcoxon matched-pairs signed rank test. (B) Trend in caloric intake increase by enteral nutrition (%) from baseline to final assessment patients by patients in controls. (C) Gastric residual volume (mL/kg) trend from baseline to final assessment patients by patients in cases treated with amoxicilline/clavulanate. (D) Gastric residual volume (mL/kg) trend from baseline to final assessment patients by patients in controls.
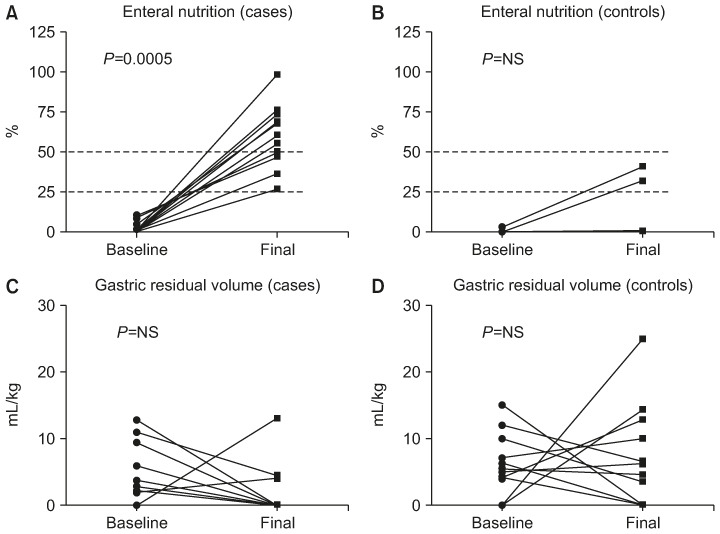
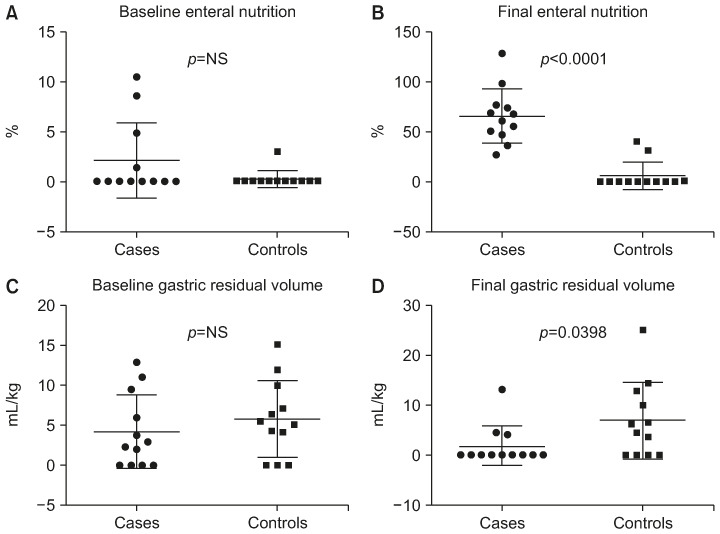
Fig. 4. Paired baseline and final assessment of caloric intake by enteral nutrition and gastric residual volume by group. (A) Comparison between the mean distribution of caloric intake by enteral nutrition (%) at baseline in the two groups (treated patients versus control patients). (B) Comparison between the mean distribution of caloric intake by enteral nutrition (%) at final assessment in the two groups (treated patients versus control patients). (C) Comparison between the mean gastric residual volume (mL/kg) distribution at baseline in the two groups (treated patients versus control patients). (D) Comparison between the mean gastric residual volume (mL/kg) distribution at final assessment in the two groups (treated patients versus control patients).
Furthermore, we compared PN, EN, and GRV at baseline with those at the end point according to the access route for EN. Among the12 cases, 5 were fed by NGT, 6 by PEG, and only 1 by PEG-PEJ; among the controls, 7 patients had an NGT and 5 patients had a PEG. As shown in Table 2, we did not find any significant differences in PN, EN, and GRV trends according to the access route. The only patient to be fed by PEG-PEJ never showed a positive GRV.
Table 2. Outcomes according to the access route for enteral nutrition.
| Baseline/end point | Cases | Controls | ||
|---|---|---|---|---|
| PEG | NGT | PEG | NGT | |
| PN at baseline | 47.3±25.6 | 33.0±26.3 | 35.6±28.1 | 20.0±18.3 |
| PN at end point | 17.7±26.7 | 29.0±30.8 | 36.4±19.3 | 49.7±25.0 |
| EN at baseline | 10.6±7.1* | 6.1±5.7** | 0.4±0.9 | 0 |
| EN at end point | 43.5±20.9* | 32.4±10.8** | 10.0±13.9 | 0.9±1.6 |
| GRV at baseline | 26.7±30.8 | 115.0±153.5 | 263.0±361.7 | 224.3±373.4 |
| GRV at end point | 8.3±20.4 | 122.0±199.8 | 166.0±219.7 | 169.2±140.3 |
Values are presented as mean±standard deviation.
PEG: percutaneous endoscopic gastrostomy, NGT: naso-gastric tube, PN: parenteral nutrition, EN: enteral nutrition, GRV: gastric residual volume.
*p=0.0022; **p=0.0159; Mann-Whitney-U-test.
Safety measures
Administration of A/C was well tolerated in patients and the safety outcomes were not significantly different between cases and controls. In particular, weaning off MV occurred in six cases and eight controls, death occurred in one case and in none of the controls, and no patient developed diarrhea during observation. The drugs (midazolam, morphine, and dopamine) required for the overall treatment of the condition was not significantly different between the groups. Notably, eight out of the 12 cases vs. all the controls (p=0.0932) and six out of the 12 cases vs. seven out of the controls (p=1.000) received midazolam/morphine/dopamine at the beginning and the end of the study period, respectively.
DISCUSSION
Nutritional deterioration during hospitalization can worsen the clinical outcome of patients. Clinical guidelines suggest that almost two thirds of the daily caloric requirements should be achieved [16]. The enteral route is the recommended mode to achieve energy requirements for medical and surgical patients who are critically ill [16]. However, EN management is a challenge in several cases, owing to the low tolerance, in particular, in patients with neurological impairment [16,17,18].
Children with neurological impairment can experience poor feeding tolerance owing to hypothalamus or autonomic dysfunction (central and peripheral nervous systems) that can affect gastrointestinal motility resulting in visceral pain, bowel dysmotility, vomiting, and gastrointestinal distention [17,18,19].
At our Institution, a nutritional rehabilitation protocol for the PICU was released in July 2018. Therefore, in October 2018 we started to re-feed patients admitted in the PICU according to the new protocol. However, we observed that some patients with neurological impairments experienced difficulties in progressing to EN because of delayed gastric emptying. We then applied to our Hospital's Ethics Committee for approval to use A/C as a prokinetic agent to support nutritional rehabilitation in patients with neurological impairments admitted to the PICU. In the present study, although the cases and controls were included in two consecutive semesters, the selected sample was homogeneous for primary disease and for nutritional treatment.
Thus, we found that in patients with neurological disability, A/C improves EN tolerance and it does not cause side effects. Notably, the EN intake at final assessment was 50% more than the basal level in 75% of the cases and in only 17% of the controls. Regarding GRV, although it resulted in the end point being reduced by 50% in 42% of patients of both the case and control groups, it was significantly different between the two groups. This finding justifies the differences in the amount of EN tolerated at the end point between the groups. Concerning safety outcomes, one patient treated with A/C died; he was a 1-year-old baby affected by neurological disability due to a rare metabolic disease. Death occurred 20 days following A/C discontinuation; drug administration was discontinued because of the worsening of neurological complaints and the development of pulmonary hemorrhage. In fact, the patient received anticoagulant therapy for thrombosis of a central vein as a complication of CVC placement. No patient developed diarrhea even when intravenous antibiotic therapy was started (in three cases and five controls) owing to the appearance of fever and suspected infectious disease.
In adults, there is vast experience on the use of different prokinetics (erythromycin, domperidone, metoclopramide, azithromycin, and neostigmine); however, many of these agents cannot be used in pediatric populations owing to side effects. Acute extra-pyramidal reactions, dyskinesia, and QT interval corrected on ECG prolongation are side effects that do not suggest the prescription of dopamine-2 receptor antagonists (metoclopramide and domperidone) to increase enteral feeding tolerance in the pediatric population.
Neostigmine and pyridostigmine have been demonstrated to be effective in improving enteral feeding tolerance in patients with intestinal pseudo-obstruction [20,21,22].
Erythromycin has been studied with contrasting results and side effects (increased QT interval corrected on ECG and pyloric stenosis) owing to different protocols, sample size, dosing, and time of administration [23].
A/C showed prokinetic effects in a group of seven healthy adults, where A/C administration increased small bowel motility [10]; in the pediatric population, A/C also showed the ability to increase duodenal motility and small bowel transit, probably because of the stimulation local receptors [11].
An important restriction on the use of A/C as a prokinetic agent may be the potential induction of bacterial resistance [24]. In PICUs, the incidence of antimicrobial resistance for Klebsiella and Escherichia coli is approximately 6.7% and 3.5% of the tested isolates, respectively [24]. However, the development of bacterial resistance is related to duration and therapeutic dosages of antibiotics [24]. Considering all these reasons, we limited our trial to only one week of treatment and to a dosage below the standard prescribed.
To the best of our knowledge, this is the first study on the use of A/C, by enteral administration, as a prokinetic for pediatric critically ill patients with neurological impairment. However, some limitations should be highlighted. The small number and the clinical characteristics of patients (all with neurological impairment) do not allow extension of the results of this study to the whole paediatric population. Furthermore, the risk of developing bacterial resistance suggests that this treatment should be limited to selected severe and intractable EN intolerance to avoid PN. In clinical practice, it may also be useful to conduct microbiological investigations before and after treatment with A/C to test for possible development of bacterial resistance. Another limitation could be the lack of any motility evaluation; indeed, the clinically unstable conditions of the patients, prevented us from performing manometry or scintigraphy, because in our Institution, the areas designed for these evaluations are very far from the PICU.
In conclusion, malnutrition exposes critically ill children to several complications that affect the severity of disease course, length of stay, and mortality; all may be prevented by early EN. The development of intolerance to EN could be addressed with the use of A/C. Future prospective clinical trials are needed to confirm these conclusions.
Footnotes
Conflict of Interest: The authors have no financial conflicts of interest.
References
- 1.Mehta NM, Duggan CP. Nutritional deficiencies during critical illness. Pediatr Clin North Am. 2009;56:1143–1160. doi: 10.1016/j.pcl.2009.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kim H, Stotts NA, Froelicher ES, Engler MM, Porter C. Why patients in critical care do not receive adequate enteral nutrition? A review of the literature. J Crit Care. 2012;27:702–713. doi: 10.1016/j.jcrc.2012.07.019. [DOI] [PubMed] [Google Scholar]
- 3.de Menezes FS, Leite HP, Nogueira PC. What are the factors that influence the attainment of satisfactory energy intake in pediatric intensive care unit patients receiving enteral or parenteral nutrition? Nutrition. 2013;29:76–80. doi: 10.1016/j.nut.2012.04.003. [DOI] [PubMed] [Google Scholar]
- 4.Braegger C, Decsi T, Dias JA, Hartman C, Kolacek S, Koletzko B, et al. Practical approach to paediatric enteral nutrition: a comment by the ESPGHAN committee on nutrition. J Pediatr Gastroenterol Nutr. 2010;51:110–122. doi: 10.1097/MPG.0b013e3181d336d2. [DOI] [PubMed] [Google Scholar]
- 5.Canarie MF, Barry S, Carroll CL, Hassinger A, Kandil S, Li S, et al. Risk factors for delayed enteral nutrition in critically ill children. Pediatr Crit Care Med. 2015;16:e283–9. doi: 10.1097/PCC.0000000000000527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Meyer R, Harrison S, Sargent S, Ramnarayan P, Habibi P, Labadarios D. The impact of enteral feeding protocols on nutritional support in critically ill children. J Hum Nutr Diet. 2009;22:428–436. doi: 10.1111/j.1365-277X.2009.00994.x. [DOI] [PubMed] [Google Scholar]
- 7.Tillman EM, Smetana KS, Bantu L, Buckley MG. Pharmacologic treatment for pediatric gastroparesis: a review of the literature. J Pediatr Pharmacol Ther. 2016;21:120–132. doi: 10.5863/1551-6776-21.2.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.European Medicines Agency. European Medicines Agency recommends changes to the use of metoclopramide [Internet] London: European Medicines Agency; 2013. Jul 26, [cited 2019 May 21]. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/Press_release/2013/07/WC500146614.pdf. [Google Scholar]
- 9.Tomomasa T, Kuroume T, Arai H, Wakabayashi K, Itoh Z. Erythromycin induces migrating motor complex in human gastrointestinal tract. Dig Dis Sci. 1986;31:157–161. doi: 10.1007/BF01300701. [DOI] [PubMed] [Google Scholar]
- 10.Caron F, Ducrotte P, Lerebours E, Colin R, Humbert G, Denis P. Effects of amoxicillin-clavulanate combination on the motility of the small intestine in human beings. Antimicrob Agents Chemother. 1991;35:1085–1088. doi: 10.1128/aac.35.6.1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gomez R, Fernandez S, Aspirot A, Punati J, Skaggs B, Mousa H, et al. Effect of amoxicillin/clavulanate on gastrointestinal motility in children. J Pediatr Gastroenterol Nutr. 2012;54:780–784. doi: 10.1097/MPG.0b013e31824204e4. [DOI] [PubMed] [Google Scholar]
- 12.Mehta NM, Skillman HE, Irving SY, Coss-Bu JA, Vermilyea S, Farrington EA, et al. Guidelines for the provision and assessment of nutrition support therapy in the pediatric critically ill patient: Society of Critical Care Medicine and American Society for Parenteral and Enteral Nutrition. Pediatr Crit Care Med. 2017;18:675–715. doi: 10.1097/PCC.0000000000001134. [DOI] [PubMed] [Google Scholar]
- 13.Società Italiana di Nutrizione Umana. Fabbisogno energetico medio (AR) nell'intervallo d'età 6-12 mesi [Internet] Milano: Società Italiana di Nutrizione Umana; 2014. [cited 2014 Oct 20]. Available from: https://sinu.it/2019/07/09/fabbisogno-energetico-medio-ar-nellintervallo-deta-6-12-mesi/ Italian. [Google Scholar]
- 14.Società Italiana di Nutrizione Umana. Fabbisogno energetico medio (AR) nell'intervallo d'età 1-17 anni [Internet] Milano: Società Italiana di Nutrizione Umana; 2014. [cited 2014 Oct 20]. Available from: https://sinu.it/2019/07/09/fabbisogno-energetico-medio-ar-nellintervallo-deta-1-17-anni/ Italian. [Google Scholar]
- 15.Becker P, Carney LN, Corkins MR, Monczka J, Smith E, Smith SE, et al. Consensus statement of the Academy of Nutrition and Dietetics/American Society for Parenteral and Enteral Nutrition: indicators recommended for the identification and documentation of pediatric malnutrition (undernutrition) Nutr Clin Pract. 2015;30:147–161. doi: 10.1177/0884533614557642. [DOI] [PubMed] [Google Scholar]
- 16.Fivez T, Kerklaan D, Mesotten D, Verbruggen S, Wouters PJ, Vanhorebeek I, et al. Early versus late parenteral nutrition in critically ill children. N Engl J Med. 2016;374:1111–1122. doi: 10.1056/NEJMoa1514762. [DOI] [PubMed] [Google Scholar]
- 17.Hauer J. Feeding intolerance in children with severe impairment of the central nervous system: strategies for treatment and prevention. Children (Basel) 2017;5:1. doi: 10.3390/children5010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thapar N, Saliakellis E, Benninga MA, Borrelli O, Curry J, Faure C, et al. Paediatric intestinal pseudoobstruction: evidence and consensus-based recommendations from an ESPGHAN-led expert group. J Pediatr Gastroenterol Nutr. 2018;66:991–1019. doi: 10.1097/MPG.0000000000001982. [DOI] [PubMed] [Google Scholar]
- 19.Romano C, van Wynckel M, Hulst J, Broekaert I, Bronsky J, Dall'Oglio L, et al. European Society for Paediatric Gastroenterology, Hepatology and Nutrition guidelines for the evaluation and treatment of gastrointestinal and nutritional complications in children with neurological impairment. J Pediatr Gastroenterol Nutr. 2017;65:242–264. doi: 10.1097/MPG.0000000000001646. [DOI] [PubMed] [Google Scholar]
- 20.Mehta NM, Corkins MR, Lyman B, Malone A, Goday PS, Carney LN, et al. Defining pediatric malnutrition: a paradigm shift toward etiology-related definitions. JPEN J Parenter Enteral Nutr. 2013;37:460–481. doi: 10.1177/0148607113479972. [DOI] [PubMed] [Google Scholar]
- 21.World Health Organization. The Treatment of diarrhoea: a manual for physicians and other senior health workers [Internet] Geneva: World Health Organization; 2005. [cited 2020 Mar 10]. Available from: http://whqlibdoc.who.int/publications/2005/9241593180.pdf. [Google Scholar]
- 22.Manini ML, Camilleri M, Grothe R, Di Lorenzo C. Application of pyridostigmine in pediatric gastrointestinal motility disorders: a case series. Paediatr Drugs. 2018;20:173–180. doi: 10.1007/s40272-017-0277-6. [DOI] [PubMed] [Google Scholar]
- 23.Ericson JE, Arnold C, Cheeseman J, Cho J, Kaneko S, Wilson E, et al. Use and safety of erythromycin and metoclopramide in hospitalized infants. J Pediatr Gastroenterol Nutr. 2015;61:334–339. doi: 10.1097/MPG.0000000000000792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lake JG, Weiner LM, Milstone AM, Saiman L, Magill SS, See I. Pathogen distribution and antimicrobial resistance among pediatric healthcare-associated infections reported to the National Healthcare Safety Network, 2011-2014. Infect Control Hosp Epidemiol. 2018;39:1–11. doi: 10.1017/ice.2017.236. [DOI] [PMC free article] [PubMed] [Google Scholar]


