Abstract
Introduction
Rheumatoid arthritis is a common autoimmune disease and schizophrenia is a relatively common and debilitating neurological disorder. There are several common features between rheumatoid arthritis and schizophrenia. The inverse relationship between rheumatoid arthritis and schizophrenia has been replicated in several studies. Despite evidence for an inverse epidemiological relationship and negative correlations for risk between rheumatoid arthritis and schizophrenia, there are no biological data that directly support this inverse relationship.
Materials and Methods’
We meta‐analyzed the genome‐wide association studies to investigate the shared association loci between rheumatoid arthritis and schizophrenia at the genome‐wide scale. Rheumatoid arthritis‐ and schizophrenia‐associated loci in most recent genome‐wide association studies of rheumatoid arthritis and schizophrenia were tested. Genetic risk score analysis was also conducted to investigate the collective contribution of schizophrenia risk loci to rheumatoid arthritis risk.
Results
Rheumatoid arthritis and schizophrenia meta‐genome‐wide association study showed a significant peak at the major histocompatibility complex locus on chromosome 6 in both rheumatoid arthritis‐schizophrenia meta‐genome‐wide association study and inverted meta‐genome‐wide association study datasets. Testing rheumatoid arthritis‐ and schizophrenia‐associated loci outside the human leukocyte antigen region showed no association with both rheumatoid arthritis and schizophrenia at a genome‐wide level of significance. Weighted genetic risk scores showed no evidence for a statistically significant association between rheumatoid arthritis and schizophrenia.
Conclusion
The finding of our study is consistent with the role of the major histocompatibility complex locus in the genetic correlation between rheumatoid arthritis and schizophrenia, and suggests that either schizophrenia has an autoimmune basis and/or rheumatoid arthritis has an active neurological component.
Schizophrenia genetic risk scores explaining variance in RA status.

1. INTRODUCTION
Rheumatoid arthritis (RA; OMIM 180300) is a common autoimmune disease that causes chronic inflammation of the joints (Zamanpoor, 2019). Schizophrenia (OMIM 181500) is a relatively common and debilitating neurological disorder and characterized by chronic psychotic symptoms and psychosocial impairment (Zamanpoor, 2020). The etiologies of both RA and schizophrenia are best explained by a multi‐factorial polygenic threshold model that invokes multiple genetic risk factors modified by the environment (Malavia et al., 2017; Smolen, Aletaha, & McInnes, 2016; Zamanpoor, 2019, 2020). Both RA and schizophrenia have a similar estimated point prevalence of 0.46% for RA (Helmick et al., 2008) and lifetime prevalence of 0.48% for schizophrenia (Simeone, Ward, Rotella, Collins, & Windisch, 2015). There are some common etiological factors such as their link with the HLA complex genes and also their association with infectious agents such as T. gondii (Feigenson, Kusnecov, & Silverstein, 2014; Torrey & Yolken, 2001). There is an established negative association between RA and schizophrenia.
Epidemiological and clinical studies have consistently reported a lower prevalence of RA in patients with schizophrenia in comparison with the general population (Chen, Wang, Chiang, Hsu, & Shen, 2019; Ching Lok, Mok, Cheng, & Chi Cheung, 2010; W. Eaton et al., 2004; Feigenson et al., 2014; Gorwood et al., 2004; Malavia et al., 2017; Mittendorfer‐Rutz et al., 2019; Ohi et al., 2016; Oken & Schulzer, 1999; Sellgren, Frisell, Lichtenstein, Landen, & Askling, 2014). The risk for developing RA among schizophrenia patients has been estimated to be 30%–50% that of the control group (Feigenson et al., 2014). This has been supported by reduced rates of RA in families with multiple schizophrenia members (Feigenson et al., 2014). Similarly there is a lower risk of developing schizophrenia among individuals with RA (Gorwood et al., 2004). In a Swedish population the relative risks of developing RA in schizophrenia and schizoaffective disorder were significantly decreased (hazard ratio = 0.69; Sellgren et al., 2014).
The inverse relationship between RA and schizophrenia has been replicated in several studies (Chen et al., 2012; Ching Lok et al., 2010; Eaton, Hayward, & Ram, 1992; Gorwood et al., 2004; Mors, Mortensen, & Ewald, 1999; Oken & Schulzer, 1999; Sellgren et al., 2014). The RA and schizophrenia correlation has been highlighted in several publications, as shown in Table 1. Epidemiological studies are inconsistent on the relationship between schizophrenia and RA. Several studies showed less frequent occurrence of RA among schizophrenia cases than would be expected by chance, while other studies have failed to replicate this protective effect of schizophrenia on RA (Euesden, Breen, Farmer, McGuffin, & Lewis, 2015). Meta‐analysis across studies showed a significant reduction in prevalence of RA in schizophrenia cases (OR = 0.48, p < 0.0001; Euesden et al., 2015). Polygenic risk scoring in a RA case control study revealed a weak contribution of schizophrenia genetic risk to RA risk (Euesden et al., 2015).
TABLE 1.
The prevalence of rheumatoid arthritis in schizophrenic patients and controls. All control sets have another psychiatric illness except Eaton et al. (2006) in which controls were free from psychiatric illness.
| Data source | Location | Sample size | RA frequency, n (%) | Odds ratio/hazard ratio | p value | ||
|---|---|---|---|---|---|---|---|
| Cases | Controls | Cases | Controls | ||||
| Ross, Hay and Mcdowall (1950) | Canada | 800 | 808 | 0 | 4 (0.49) | 0.11 (0.006–2.08) | 0.14 |
| Pilkington (1956) | England | 130 | 188 | 1 (0.77) | 4 (2.66) | 0.28 (0.03–2.46) | 0.25 |
| Mellsop, Koadlow, Syme and Whittingham (1974) | Australia and New Zealand | 301 | 3,157 | 0 | 234 (7.41) | 0.02 (0.001–0.33) | 0.006 |
| Österberg (1978) | Sweden | 62,228 | 360,271 | 16 (0.047) | 299 (0.10) | 0.45 (0.28–0.72) | 0.001 |
| Baldwin (1980) | England | 2,314 | 5,404 | 2 (0.09) | 23 (0.43) | 0.20 (0.05–0.86) | 0.03 |
| Mohamed, Merskey, Kazarian and Disney (1982) | USA | 111 | 51 | 0 | 3 (5.88) | 0.06 (0.003–1.23) | 0.07 |
| Allebeck, Rodvall and Wistedt (1985) | Sweden | 1,190 | 16,617 | 2 (0.17) | 71 (0.41) | 0.41 (0.09–1.74) | 0.22 |
| Oken & Schulzer, 1999 | Canada | 27,630 | 202,342 | 101 (0.11) | 900 (0.44) | 0.24 (0.17–0.35) | 0.001 |
| Oken & Schulzer, 1999 | USA | 1,323 | 661 | 1 (0.08) | 2 (0.30) | 0.25 (0.02–2.75) | 0.26 |
| Eaton et al. (2006) | Denmark | 7,704 | 192,590 | 15 (0.19) | 336 (0.17) | 1.15 (0.055–2.5) | 0.24 |
| Chen et al., 2012 | Taiwan | 10,811 | 108,110 | 30 (0.28) | 507 (0.47) | 0.52 (0.35–0.76) | 0.007 |
| Benros et al. (2014) | Denmark | 39,364 | 3,790,636 | 82 (0.21) | 15,686 (0.41) | 0.50 (0.40–0.62) | — |
| Sellgren et al., 2014 | Sweden | 47,326 | — | 157 (0.33) | — | 0.69 (0.59–0.80) | — |
| Chen et al., 2019 | Taiwan | 58,847 | 235,382 | 21 (0.04) | 189 (0.08) | 0.48 (0.31–0.76) | — |
Several psychosocial and biochemical hypotheses such as abnormal tryptophan metabolism, prostaglandin deficiency, and consequences of treatment have been proposed to explain the RA‐schizophrenia relationship (Lee et al., 2015). The reduction in comorbidity between RA and schizophrenia and the strong genetic contribution to both disorders suggests that there might be a common genetic pathway responsible for susceptibility to one disorder and at the same time protecting against the other (de la Fontaine et al., 2006). Numerous candidate genes have been suggested for the association between RA and schizophrenia, but the HLA system has been the most studied locus (Gorwood et al., 2004).
Advances in technology provided a new opportunity to further investigate the genetic basis of the RA‐schizophrenia relationship (Lee et al., 2015). The meta‐analysis of three RA and two schizophrenia GWAS datasets genetically confirmed the small but significant negative correlation (Lee et al., 2015). Associated loci for both RA (101 loci) and schizophrenia (128 loci) exhibit more shared loci than expected by chance (Lee et al., 2015). This could be dominated by the positive correlation between variations in the major histocompatibility complex (MHC) region (Lee et al., 2015). The MHC region is the most associated region in GWAS of schizophrenia. This genetic evidence supports the immune hypothesis that variation within immune genes contributes to schizophrenia (Michel, Schmidt, & Mirnics, 2012; Pouget et al., 2016). HLA class II, containing the HLA‐DR4 (DRB1*04) allele of the HLA‐DRB1 gene, is the most frequently reported genetic allele in association with schizophrenia (Wright, Nimgaonkar, Donaldson, & Murray, 2001). Associated HLA class II antigens or alleles include HLA‐DRB1*0101 and HLA‐DRB1*04 (*0401, *0403, *0405, and *0406) that control antibody‐mediated immune responses (Wright et al., 2001). HLA‐DRB1, including the HLA‐DR4 serotype, is associated positively with RA and inversely with schizophrenia (Watanabe et al., 2009). HLA‐DRB1 has a well‐established effect in RA but its function is more modest in schizophrenia (Lee et al., 2015). This might suggest that HLA‐mediated antigen recognition has a different role in RA and schizophrenia (Lee et al., 2015). Therefore, a negative genetic correlation between RA and schizophrenia could be due to the different roles of variants in immune response pathways in different tissues and/or in response to different challenges (Lee et al., 2015).
Understanding this negative correlation between RA and schizophrenia may reveal novel insights into the etiology of both diseases. Identifying genes associated with these disorders increases the understanding of polygenic disease. The putative pathway responsible for the negative correlation between RA and schizophrenia has potential as a therapeutic target. It may be possible to manipulate the putative pathway in such a way that the body is deceived into believing one phenotype is active and thus protected against the other. It is, therefore, important to identify any genes that may be responsible for the link between the two disorders. Our study aimed to investigate genes that play a role in both the autoimmune and neurological systems to find variants responsible for the negative association between RA and schizophrenia.
2. MATERIALS AND METHODS
In this study, meta‐analysis of the large GWAS datasets were performed using METAL which provides a computationally efficient tool to combine large GWAS datasets (Willer, Li, & Abecasis, 2010). Associated loci of RA and schizophrenia, at a genome‐wide level of significance, in most recent GWAS of RA (Okada et al., 2014) and schizophrenia (Li et al., 2017) were tested.
Okada et al. (2014) RA GWAS included 29,880 RA cases and 73,758 controls of European and Asian ancestry. All RA patients were diagnosed by rheumatologists and met the criteria for the diagnosis of RA according to the 1987 American College of Rheumatology classification system. As a result, 42 novel RA‐associated loci was discovered and the total RA risk loci at a genome‐wide level of significance was increased to 101. The reported heritability for RA risk loci outside of the MHC region was 5.5% and 4.7% in Europeans and Asians, respectively (Okada et al., 2014). Li et al. (2017) schizophrenia GWAS included 43,175 cases and 65,166 controls in total, consisted of the 7,699 cases and 18,327 controls of Chinese ancestry in addition to the schizophrenia working group of the psychiatric genomics consortium (PGC2) data. The reported heritability of schizophrenia explained by GWAS loci was only 3.5% (Li et al., 2017).
Two inverse‐variance weighted fixed‐effects meta‐GWAS were performed to combine the RA and the schizophrenia GWAS datasets using the R package meta. The first meta‐GWAS was performed by combining the RA and schizophrenia datasets. In order to perform the second meta‐GWAS (inverted meta‐GWAS), the OR values of the schizophrenia data were inverted and then, combined with the RA data. Before meta‐analysis, the RA and the schizophrenia GWAS datasets underwent extensive quality control. Monomorphic SNPs and SNPs with minor allele frequency <0.01 and HWE p < 5 × 10−8 were filtered and the remaining SNPs were used for subsequent analyses. Consequently, data for 1,483,435 genotyped SNPs were available for meta‐analysis. Genome‐wide significance was defined as a p < 5 × 10−8 based on a Bonferroni correction for an assumed million independent variants in the human genome (Johnson et al., 2010). QQ and Manhattan plots were created using ggplot2 in R (Wickham, 2016).
Genetic risk score (GRS) analysis was conducted to investigate the collective contribution of schizophrenia risk loci to RA risk. Weighted GRS (wGRS) was performed in the RA (Okada et al., 2014) dataset, based on SNPs extracted from the schizophrenia (Li et al., 2017) dataset meeting p value thresholds of 0.01, 0.05, 0.1, 0.2, 0.3, 0.4, 0.5, 0.6, 0.7, 0.8, and 0.9. At each threshold, SNPs with schizophrenia association p value below the threshold were used to construct wGRS in the RA dataset by summing the number of risk alleles at each SNP weighted by the natural logarithm of its effect size on schizophrenia or RA as appropriate. GRS analysis was done using the GRS function implemented in the grs.summary module of the R package Genetics ToolboX (gtx‐package, version 0.0.8). In this method, Nagelkerke's R 2 m is the (pseudo) variance explained in the testing dataset from the likelihood ratio test statistic that can be obtained under the regression model. Chi‐square χ2 m test of independence was also used to determine all SNPs have independent effects. The grs.summary module implements the summary statistic method described by International Consortium for Blood Pressure Genome‐Wide Association et al., (2011) for approximating the regression of an outcome onto an additive GRS, using only single SNP association summary statistics extracted from GWAS results. This method is described in more detail in Dastani et al. (2012).
3. RESULTS
3.1. Meta‐GWAS combining both RA and schizophrenia datasets
QQ plots showed deviations of p values from the diagonal line in both RA‐schizophrenia meta‐GWAS and inverted meta‐GWAS datasets. This indicates that p values were smaller than expected by chance (Figure 1). The Manhattan plots showed a significant peak at the MHC locus on chromosome 6 (Figures 2 and 3) in both meta‐GWAS datasets. After filtering for minor allele frequency (MAF < 0.01) and Hardy–Weinberg equilibrium (HWE) p < 5 × 10−8, the MHC locus was the only locus found above the genome‐wide level of significant threshold in both RA‐schizophrenia meta‐GWAS and inverted meta‐GWAS datasets. Regions with the significant associated variants have been visualized (Figures 4 and 5) using LocusZoom tool (Pruim et al., 2010; available from http://locuszoom.sph.umich.edu/).
FIGURE 1.
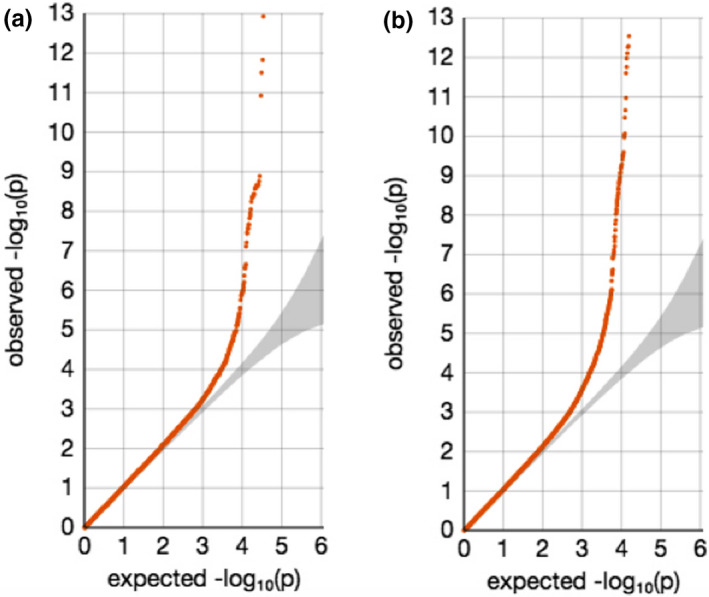
QQ plots for meta‐GWAS datasets using METAL. (a) QQ plot for the RA‐schizophrenia meta‐GWAS dataset. (b) QQ plot for the inverted meta‐GWAS analysis of RA and schizophrenia. Deviations from the diagonal line were observed in both RA‐schizophrenia meta‐GWAS dataset.
FIGURE 2.
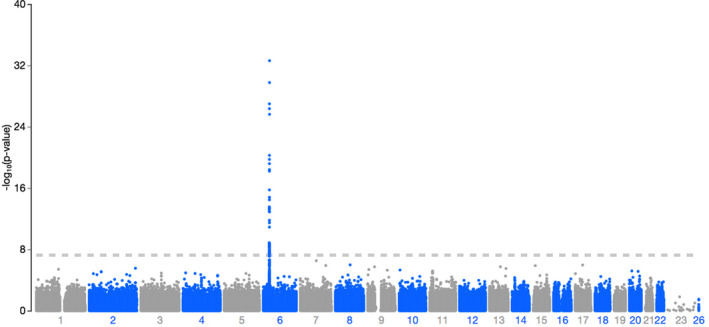
Manhattan plot of ‐log10 (p value) in the combined RA‐schizophrenia meta‐GWAS dataset. There was a significant peak on chromosome 6. Data are colored as gray and blue according to chromosome and base‐pair position.
FIGURE 3.
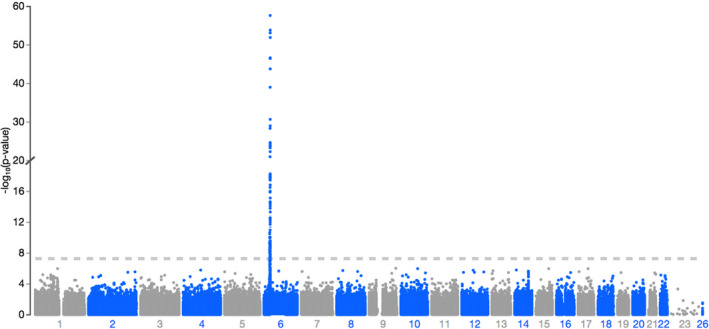
Manhattan plot of ‐log10 (p value) in the inverted meta‐GWAS of RA and schizophrenia. There was a significant peak on chromosome 6. Data are colored as gray and blue according to chromosome.
FIGURE 4.
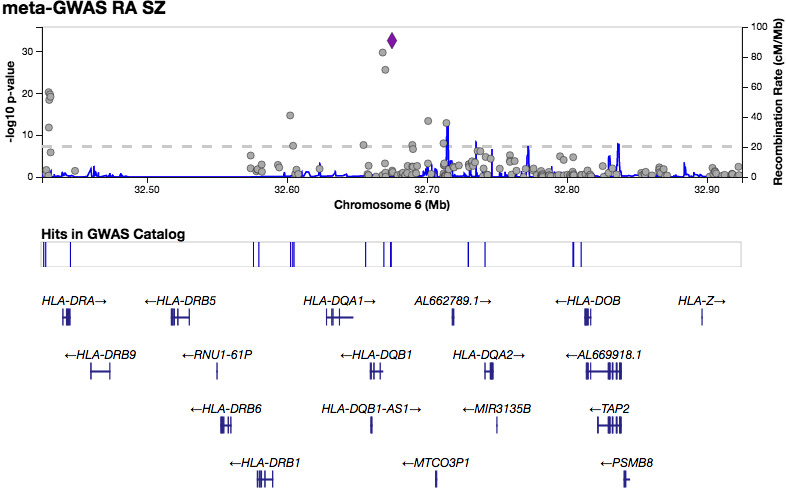
LocusZoom plot showing the most significant associated region within the MHC region on chromes 6 in RA‐schizophrenia meta‐GWAS dataset.
FIGURE 5.
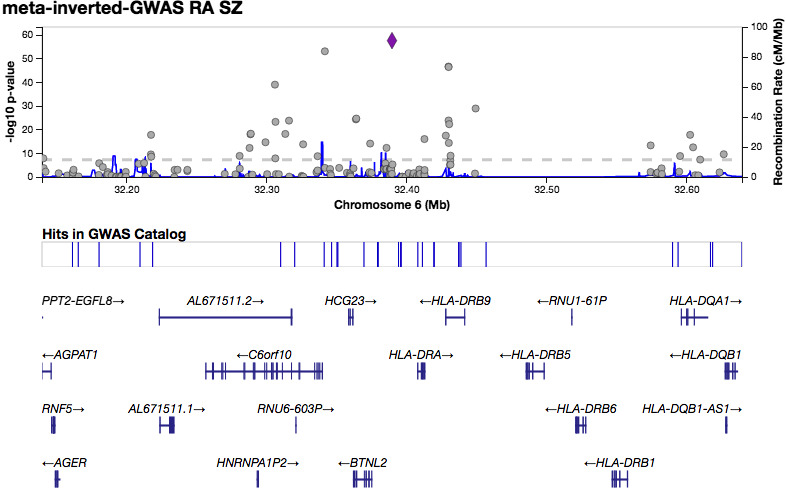
LocusZoom plot showing the most significant associated region within the MHC region on chromes 6 in RA‐schizophrenia inverted meta‐GWAS dataset.
3.2. Testing RA loci in schizophrenia GWAS datasets and schizophrenia loci in RA GWAS
No schizophrenia‐associated locus was found to be associated with RA at a genome‐wide level of significance (p < 5 × 10−8). However, 14 SNPs were nominally associated with RA (p < 0.05; Table 2).
TABLE 2.
Association analysis of schizophrenia loci at genome‐wide level of significance from Li et al. (2017) in RA GWAS (Okada et al., 2014).
| SNP | Chr | Position | Closest Gene | OR (SE) | p value | Disease | Effect direction |
|---|---|---|---|---|---|---|---|
| rs115329265 | 6 | 28,712,247 | NOP56P1, RPSAP2 | 1.210 (0.015) | 5.02E‐36 | SZ | Susceptible |
| 1.05 (0.040) | 0.029 | RA | Susceptible | ||||
| rs66691851 | 3 | 136,154,828 | STAG1 | 0.926 (0.010) | 4.17E‐15 | SZ | Protective |
| 0.96 (0.05) | 0.021 | RA | Protective | ||||
| rs1198589 | 1 | 98,550,411 | MIR137 | 0.907 (0.013) | 1.19E‐13 | SZ | |
| 0.95 (0.05) | 0.018 | RA | Protective | ||||
| rs2577831 | 3 | 52,628,056 | PBRM1 | 0.936 (0.009) | 4.53E‐13 | SZ | |
| 0.97 (0.03) | 0.05 | RA | Protective | ||||
| rs3814881 | 16 | 30,000,901 | TAOK2 | 0.936 (0.009) | 8.41E‐13 | SZ | Protective |
| 0.97 (0.03) | 0.028 | RA | Protective | ||||
| rs9607782 | 22 | 41,587,556 | EP300 | 1.090 (0.012) | 1.01E‐12 | SZ | Susceptible |
| 0.95 (0.05) | 0.042 | RA | Protective | ||||
| rs2970610 | 1 | 44,097,530 | PTPRF | 1.072 (0.010) | 1.24E‐12 | SZ | Susceptible |
| 1.05 (0.03) | 0.0029 | RA | Susceptible | ||||
| rs61882743 | 11 | 46,548,754 | AMBRA1 | 0.914 (0.013) | 6.99E‐12 | SZ | Protective |
| 0.95 (0.06) | 0.01 | RA | Protective | ||||
| rs112537273 | 8 | 38,248,306 | LETM2 | 1.077 (0.011) | 1.06E‐11 | SZ | Susceptible |
| 1.05 (0.04) | 0.041 | RA | Susceptible | ||||
| rs2381759 | 2 | 146,428,031 | TEX41 | 0.929 (0.013) | 8.63E‐09 | SZ | Protective |
| 0.93 (0.05) | 0.0046 | RA | Protective | ||||
| rs111782145 | 6 | 30,873,508 | GTF2H4 | 0.813 (0.037) | 1.80E‐08 | SZ | Protective |
| 1.18 (0.15) | 0.0051 | RA | Susceptible | ||||
| rs10894308 | 11 | 130,891,895 | SNX19 | 0.947 (0.010) | 2.09E‐08 | SZ | Protective |
| 1.04 (0.05) | 0.033 | RA | Susceptible | ||||
| rs2247870 | 5 | 90,151,589 | ADGRV1 | 1.052 (0.009) | 2.54E‐08 | SZ | Susceptible |
| 1.04 (0.04) | 0.048 | RA | Susceptible | ||||
| rs13266463 | 8 | 143,403,693 | TSNARE1 | 0.947 (0.010) | 2.25E‐08 | SZ | Protective |
| 1.04 (0.04) | 0.038 | RA | Susceptible |
Abbreviations: A1, minor allele; A2, major allele; Chr, chromosome; OR, odds ratio; SE, standard error; SZ, schizophrenia.
Examining the latest RA loci in schizophrenia GWAS (Li et al., 2017) showed no significant association at a genome‐wide level of significance (p < 5 × 10−8) between RA loci and schizophrenia. However, eight SNPs showed minimal association with schizophrenia (p < 0.05; Table 3).
TABLE 3.
Association analysis of RA loci at genome‐wide level of significance from Okada et al. (2014) in schizophrenia GWAS (Li et al., 2017).
| SNP | Chr | Position | Closest Gene | OR (SE) | p value | Disease | Effect direction |
|---|---|---|---|---|---|---|---|
| rs8032939 | 15 | 38,834,033 | RASGRP1 | 0.89 (0.03) | 2.40E−12 | RA | |
| 0.962 (0.011) | 0.0007 | SZ | Protective | ||||
| rs28411352 | 1 | 38,278,579 | MTF1, INPP5B | 1.11 (0.05) | 5.20E−09 | RA | Susceptible |
| 0.964 (0.011) | 0.0011 | SZ | Protective | ||||
| rs2228145 | 1 | 154,426,970 | IL6R | 1.07 (0.04) | 4.50E−06 | RA | Susceptible |
| 1.025 (0.01) | 0.0119 | SZ | Susceptible | ||||
| rs9826828 | 3 | 136,402,060 | STAG1, IL20RB | 1.42 (0.185) | 9.20E−08 | RA | Susceptible |
| 1.074 (0.036) | 0.0497 | SZ | Susceptible | ||||
| rs67250450 | 7 | 28,174,986 | JAZF1 | 1.10 (0.05) | 1.90E−06 | RA | Susceptible |
| 0.968 (0.013) | 0.0098 | SZ | Protective | ||||
| rs331463 | 11 | 36,501,787 | TRAF6, RAG1/2 | 0.90 (0.03) | 5.70E−06 | RA | Protective |
| 1.043 (0.014) | 0.0026 | SZ | Susceptible | ||||
| rs1950897 | 14 | 68,760,141 | RAD51B | 1.09 (0.04) | 2.50E−07 | RA | Susceptible |
| 0.969 (0.011) | 0.0049 | SZ | Protective | ||||
| chr21:35928240 | 21 | 35,928,240 | RCAN1 | 0.88 (0.04) | 2.90E−07 | RA | Protective |
| 0.967 (0.016) | 0.0364 | SZ | Protective |
Abbreviations: A1, minor allele; A2, major allele; Chr, chromosome; OR, odds ratio; SE, standard error; SZ, schizophrenia.
3.3. Genetic risk scores
To examine the genetic relationship between RA and schizophrenia, the weighted genetic risk scores (wGRS) were calculated using the published summary statistics of the Li et al. (2017) data for schizophrenia and the Okada et al. (2014) data for RA. Schizophrenia risk loci derived from Li et al. (2017) GWAS data, were examined in the Okada et al. (2014) data and similarly RA risk loci derived from Okada et al. (2014) were tested in the Li et al. (2017) GWAS data. The wGRS for schizophrenia and RA cases and controls were calculated using the panel of SNPs and fitted a logistic regression model. A series of p value thresholds (PT) were used to select RA and schizophrenia risk alleles for wGRS calculation for each set of SNPs. SNPs associated with schizophrenia at PT < 0.001 explain only about 0.03% of the variance in RA status (Table 4 and Figure 6). RA‐associated loci at PT < 0.001 explain only about 0.004% of the variance in schizophrenia status (Table 4 and Figure 7). The variance in RA and schizophrenia explained by wGRS was calculated separately as pseudo R 2.
TABLE 4.
Schizophrenia GRS across thresholds and variance in RA status explained, and RA GRS across thresholds and variance in schizophrenia status explained.
| p threshold | Number of SNPs | GRS for RA in schizophrenia | GRS for schizophrenia in RA | ||
|---|---|---|---|---|---|
| Pseudo R 2 | p value | Pseudo R 2 | p value | ||
| 0.0001 | 57 | 0.035 | 0.811 | 0.00439 | 0.795 |
| 0.001 | 135 | 0.032 | 0.635 | 0.00354 | 0.309 |
| 0.01 | 286 | 0.028 | 0.241 | 0.00466 | 0.475 |
| 0.05 | 4059 | 0.040 | 0.435 | 0.00243 | 0.373 |
| 0.1 | 5964 | 0.042 | 0.349 | 0.00301 | 0.514 |
| 0.2 | 7025 | 0.034 | 0.423 | 0.00381 | 0.685 |
| 0.3 | 8325 | 0.042 | 0.403 | 0.0029 | 0.248 |
| 0.4 | 11778 | 0.041 | 0.179 | 0.0033 | 0.428 |
| 0.5 | 15955 | 0.037 | 0.332 | 0.00392 | 0.575 |
| 0.6 | 17623 | 0.046 | 0.253 | 0.00362 | 0.353 |
| 0.7 | 19812 | 0.042 | 0.724 | 0.00421 | 0.470 |
| 0.8 | 21812 | 0.039 | 0.789 | 0.0043 | 0.884 |
| 0.9 | 24301 | 0.040 | 0.647 | 0.00504 | 0.377 |
FIGURE 6.
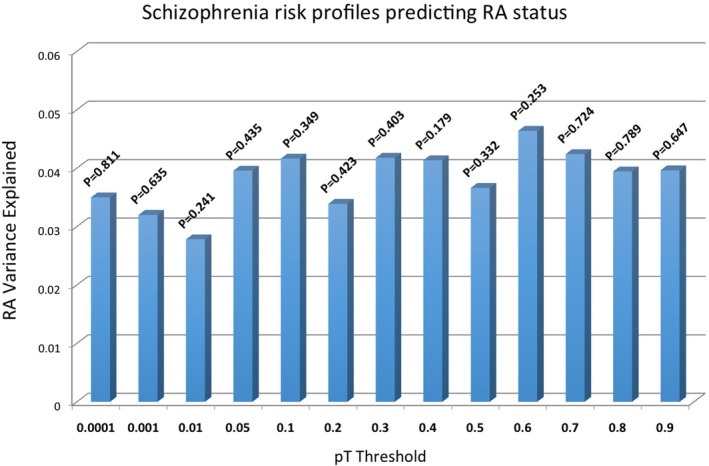
Schizophrenia genetic risk scores explaining variance in RA status in an independent test cohort. Scores are calculated across cutoff thresholds (PT).
FIGURE 7.
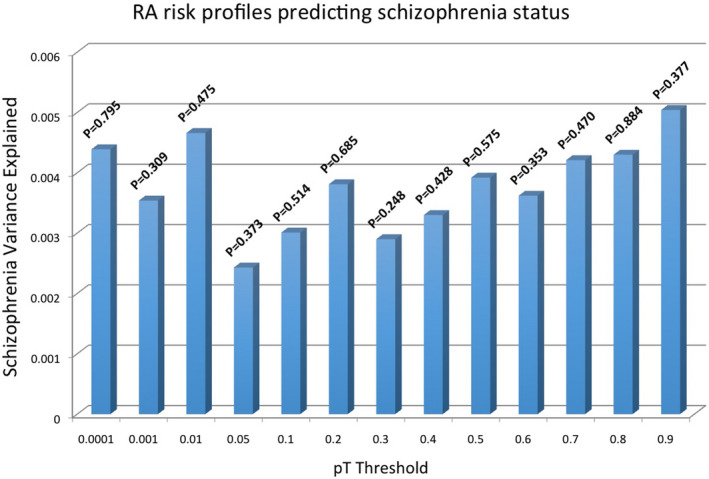
RA genetic risk scores explaining variance in schizophrenia status in an independent test cohort. Scores are calculated across cutoff thresholds (PT).
Logistic regression analysis was performed to examine the associations with wGRS at the different significance thresholds of SNPs list. First, we tested whether the schizophrenia GRS predicted variance in RA disease state in a logistic model and then, we tested whether the RA GRS predicted variance in schizophrenia disease state in a logistic model. There was no evidence for statistically significant association (p > 0.179 and p > 0.248, respectively), and, therefore, the wGRS between RA and schizophrenia is not stronger than it would be expected by chance.
4. DISCUSSION
Meta‐GWAS was performed to investigate shared association loci between RA and schizophrenia at the genome‐wide scale. Two different meta‐GWAS were performed. The first meta‐GWAS consisted of the whole RA GWAS data combined with the schizophrenia GWAS dataset. This aimed to identify RA‐schizophrenia shared association loci with the similar effects at the genome‐wide scale. Another (inverted) meta‐GWAS was performed to provide a new opportunity to identify RA‐schizophrenia shared association loci with opposite effects at the genome‐wide scale. Interestingly, significantly associated loci in both meta‐GWAS analyses were within the MHC region on the chromosome 6. However, stronger association was found in the inverse‐meta‐GWAS analysis. This observed strengthening evidence for the association of MHC region in the combined RA‐schizophrenia data with the inverted‐effect supporting a genetic basis to the inverse correlation between RA and schizophrenia.
The finding of this study is consistent with the well‐established role of the MHC in the etiology of both RA and schizophrenia. The MHC locus is best known for its role in immunity but the genes and molecular mechanisms accounting for this association need to be identified. Implication of the HLA‐DRB1 gene in schizophrenia supports the role of the immune system in the development of schizophrenia (Feigenson et al., 2014). Sekar et al. (2016) reported the distinct association of schizophrenia with variation in the MHC locus involving structurally diverse alleles of the complement component 4 (C4) genes that affect the expression of C4A and C4B genes in the brain (Sekar et al., 2016). Lee et al. (2015) reported that variants in immune response pathways such as HLA loci could play different roles in different tissues and/or in response to different challenges (Lee et al., 2015). The result of meta‐GWAS conducted in this study is also supported by the report of Watanabe et al. (2009; Watanabe et al., 2009) that HLA‐DRB1 is associated with both RA and schizophrenia. Given the strong association of the MHC locus with RA, achieving genome‐wide significance in combined RA‐schizophrenia is not that remarkable and is likely driven by the strong association between RA and the MHC locus. We recognize that genes and haplotypes mapped within the MHC region have a complex linkage structure and given that their structures and functions are highly divers, the investigation of their role in autoimmune‐ and neurological‐mediated complex diseases such as RA and schizophrenia have proved to be challenging (Lokki & Paakkanen, 2019; Muniz‐Castrillo, Vogrig, & Honnorat, 2020). Combining the RA and schizophrenia datasets increased the statistical power of meta‐analysis by maximizing the sample size but it would also create a more heterogeneous cohort due to the combination of patients with RA and schizophrenia. Our results of meta‐GWAS are consistent with the role of the MHC region in the genetic etiology y of both RA and schizophrenia disorders (Zamanpoor, 2019, 2020). This suggests that either schizophrenia has an autoimmune basis and/or RA has an active neurological component (Kipnis, Cardon, Strous, & Schwartz, 2006; Torrey & Yolken, 2001). Several studies have reported the contribution of an active immunological system in the etiology of schizophrenia (de la Fontaine et al., 2006). Common inflammatory mechanisms as part of the common pathogenetic hypothesis between RA and schizophrenia are supported by the role of immune dysregulation and alterations in neuroinflammatory pathways in schizophrenia (Altamura, Buoli & Pozzoli, 2014; Malavia et al., 2017). This might be explained by the genetically modulated inflammatory reactions such as dopamine‐induced activation of autoimmune T cells in the brain tissue and/or immune system (de la Fontaine et al., 2006). Several studies suggest the role of cytokines as a mediator of metabolic/brain changes associated with clinical symptoms of schizophrenia (Altamura et al., 2014; Gallego et al., 2018; Janicijevic, Dejanovic & Borovcanin, 2018; Kronfol & Remick, 2000; Monji, Kato & Kanba, 2009). Elevated levels of pro‐inflammatory markers and cytokines have been found in the peripheral blood, cerebrospinal fluid, and prefrontal cortex neurons of schizophrenic patients (Altamura et al., 2014; Malavia et al., 2017). Cytokines play a critical role in infectious and inflammatory processes by mediating between immune abnormalities and neurodevelopment (Altamura et al., 2014). Cytokines interact with monoaminergic system mediators such as dopamine, serotonin and glutamate, and the autonomic nervous system (Altamura et al., 2014; Kim, Jung, Myint, Kim & Park, 2007). Both schizophrenia and RA are associated with an imbalance in inflammatory cytokines suggesting a possible target for pharmacological treatments (Altamura et al., 2014). The positive effect of nonsteroidal anti‐inflammatory drugs to reduce psychotic symptom severity, supports the possibility of inflammatory mechanisms underlying schizophrenia pathogenesis (Malavia et al., 2017). This immune‐based anti‐inflammatory therapeutic approach opens interesting perspectives for immune therapy in schizophrenia (Müller, Weidinger, Leitner & Schwarz, 2016).
A growing list of the confirmed non‐HLA susceptibility loci is associated with RA and schizophrenia at a genome‐wide level of significance. In this study, RA‐associated variants were tested for association in schizophrenia GWAS data (Li et al., 2017) and schizophrenia‐associated variants were tested for association in RA GWAS data (Okada et al., 2014). However, there was no evidence of association for confirmed non‐HLA susceptibility loci with both RA and schizophrenia in our study. This suggests that the MHC locus might be mainly responsible for the inverse relationship between RA and schizophrenia and also that non‐HLA loci have a weaker effect that these GWAS datasets were still underpowered to detect. Although there were some loci with minimal association with both RA and schizophrenia (listed in Tables 2 and 3), these loci confer a weaker effect (0.67 < OR < 1.5) and, therefore, were of less interest. However, this might be worthy of further investigation.
The wGRS analysis in this study has shown that variance in RA status cannot be explained or predicted by schizophrenia risk alleles. Results of the wGRS analysis in this study provided no evidence for the contribution of schizophrenia risk loci to RA risk and, therefore, would not support the contribution of shared genetic factors in inverse correlation between schizophrenia and RA. The findings of our study are consistent with that of Euesden et al. (2015) and conclude that GRS cannot provide any genetic evidence to support the inverse correlation between schizophrenia and RA. Our finding is also consistent with several lines of evidence in recent linkage disequilibrium score regression studies, conducted by Pouget et al., 2019 (Pouget et al., 2019; rg = −0.032, p = 0.78) and Tylee et al., (2018) (rg = −0.0301, p = 0.68), reporting that there is no significant genome‐wide genetic correlations between schizophrenia and RA.
It is likely that other nongenetic and environmental factors may contribute to the inverse relationship between RA and schizophrenia, which is beyond the scope of this study. As suggested by Euesden et al. (2015), one of such factors could be the effect of medication. Most of antipsychotic medications have an anti‐inflammatory effect. Given that patients with schizophrenia have a much earlier age at onset in comparison to RA patients, it is likely that by age at onset for RA, schizophrenia patients are medicated which could have a protective effect on development of RA later in life. These findings would warrant further investigation for the causal effects between RA and schizophrenia using Mendelian randomization analysis. Another possible factor could be that many patients with schizophrenia might die before age of onset of RA due to shortened life expectancy in patients with schizophrenia.
There are some limitations in our study similar to previous studies investigated the genetic association between RA and schizophrenia, as it was discussed above, there is a substantial difference in the age of onset between RA and schizophrenia and the lack of adjustment for age among RA and schizophrenia patients might be partly accountable for confounding the association of RA and schizophrenia (Benros et al., 2014; Euesden et al., 2015). We analyzed data generated from different studies using different microarray platforms and this might also present another limitation. The study's power that is based on the sample size of any given study can affect the ability to detect the true correlation between genetic effects in GRS analysis (Euesden et al., 2015). As we discussed earlier, the most significantly associated loci were among the MHC region, which were excluded in our study, and this might be an important caveat in this study.
CONFLICT OF INTEREST
Authors declare that there is no conflict of interest.
AUTHOR CONTRIBUTION
MZ carried out the experiment and wrote the manuscript. HG and MDO verified the analytical methods and reviewed the manuscript drafts. All authors discussed the results and significantly contributed to the final manuscript.
ACKNOWLEDGMENT
This research was supported by University of Otago, New Zealand. Authors thank Professors Tony Merriman and Ian Morison from University of Otago, New Zealand for their scientific advice in this study.
How to cite this article: Zamanpoor M, Ghaedi H, Omrani MD. The genetic basis for the inverse relationship between rheumatoid arthritis and schizophrenia. Mol Genet Genomic Med. 2020;8:e1483. 10.1002/mgg3.1483
DATA AVAILABILITY STATEMENT
The data that support the findings of this study were derived from the following resources available in the public domain: http://gwas.bio-x.cn/, http://plaza.umin.ac.jp/~yokada/datasource/software.htm, and https://www.wtccc.org.uk/info/access_to_data_samples.html.
REFERENCE
- Altamura, A. C. , Buoli, M. , & Pozzoli, S. (2020). Role of immunological factors in the pathophysiology and diagnosis of bipolar disorder: comparison with schizophrenia. Psychiatry Clin Neurosci, 68, 21–36. [DOI] [PubMed] [Google Scholar]
- Allebeck, P. , Rodvall, Y. , & Wistedt, B. (1985). Incidence of rheumatoid arthritis among patients with schizophrenia, affective psychosis and neurosis. Acta Psychiatrica Scandinavica, 71, 615–619. [DOI] [PubMed] [Google Scholar]
- Baldwin, J. A. (1980). Schizophrenia and physical disease: a preliminary analysis of the data from the Oxford Record Linkage Study In Lancaster H. G. (Ed.), The Biochemistry of Schizophrenia and Addiction. Search of a Common Factor, (pp. 297‐318). Dordrecht, the Netherlands: MTP. [Google Scholar]
- Benros, M. E. , Pedersen, M. G. , Rasmussen, H. , Eaton, W. W. , Nordentoft, M. , & Mortensen, P. B. (2014). A nationwide study on the risk of autoimmune diseases in individuals with a personal or a family history of schizophrenia and related psychosis. American Journal of Psychiatry, 171(2), 218–226. 10.1176/appi.ajp.2013.13010086 [DOI] [PubMed] [Google Scholar]
- Chen, S.‐J. , Chao, Y.‐L. , Chen, C.‐Y. , Chang, C.‐M. , Wu, E.‐H. , Wu, C.‐S. , … Tsai, H.‐J. (2012). Prevalence of autoimmune diseases in in‐patients with schizophrenia: nationwide population‐based study. The British Journal of Psychiatry, 200(5), 374–380. [DOI] [PubMed] [Google Scholar]
- Chen, S. F. , Wang, L. Y. , Chiang, J. H. , Hsu, C. Y. , & Shen, Y. C. (2019). Assessing whether the association between rheumatoid arthritis and schizophrenia is bidirectional: A nationwide population‐based cohort study. Scientific Reports, 9(1), 1–9. 10.1038/s41598-018-38149-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ching Lok, E. Y. , Mok, C. C. , Cheng, C. W. , & Chi Cheung, E. F. (2010). Prevalence and determinants of psychiatric disorders in patients with rheumatoid arthritis. Psychosomatics, 51(4), 338–338.e8. 10.1176/appi.psy.51.4.338 [DOI] [PubMed] [Google Scholar]
- Dastani, Z. , Hivert, M.‐F. , Timpson, N. , Perry, J. R. B. , Yuan, X. , Scott, R. A. , … Richards, J. B. (2012). Novel loci for adiponectin levels and their influence on type 2 diabetes and metabolic traits: a multi‐ethnic meta‐analysis of 45,891 individuals. PLoS Genetics, 8(3), e1002607 10.1371/journal.pgen.1002607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Fontaine, L. , Schwarz, M. J. , Riedel, M. , Dehning, S. , Douhet, A. , Spellmann, I. , … Müller, N. (2006). Investigating disease susceptibility and the negative correlation of schizophrenia and rheumatoid arthritis focusing on MIF and CD14 gene polymorphisms. Psychiatry Research, 144(1), 39–47. 10.1016/j.psychres.2006.01.006 [DOI] [PubMed] [Google Scholar]
- Eaton, W. W. , Byrne, M. , Ewald, H. , Mors, O. , Chen, C. Y. , Agerbo, E. , & Mortensen, P. B. (2006). Association of schizophrenia and autoimmune diseases: linkage of Danish national registers. American Journal of Psychiatry, 163, 521. [DOI] [PubMed] [Google Scholar]
- Eaton, W. W. , Hayward, C. , & Ram, R. (1992). Schizophrenia and rheumatoid arthritis: a review. Schizophrenia Research, 6(3), 181–192. 10.1016/0920-9964(92)90001-L [DOI] [PubMed] [Google Scholar]
- Eaton, W. , Mortensen, P. , Agerbo, E. , Byrne, M. , Mors, O. , & Ewald, H. (2004). Coeliac disease and schizophrenia: Population based case control study with linkage of Danish national registers. British Medical Journal, 328(7437), 438 10.1136/bmj.328.7437.438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Euesden, J. , Breen, G. , Farmer, A. , McGuffin, P. , & Lewis, C. M. (2015). The relationship between schizophrenia and rheumatoid arthritis revisited: genetic and epidemiological analyses. American Journal of Medical Genetics. Part B, Neuropsychiatric Genetics, 168B(2), 81–88. 10.1002/ajmg.b.32282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feigenson, K. A. , Kusnecov, A. W. , & Silverstein, S. M. (2014). Inflammation and the two‐hit hypothesis of schizophrenia. Neuroscience and Biobehavioral Reviews, 38, 72–93. S0149 -7634(13)00275 -3[pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallego, J. A. , Blanco, E. A. , Husain‐Krautter, S. , Madeline Fagen, E. , Moreno‐Merino, P. , Del ojo‐Jimenez, J. A. , … Malhotra, A. K. (2018). Cytokines in cerebrospinal fluid of patients with schizophrenia spectrum disorders: New data and an updated meta‐analysis. Schizophr Res. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorwood, P. , Pouchot, J. , Vinceneux, P. , Puechal, X. , Flipo, R. M. , De Bandt, M. , … Rhumatisme, C. (2004). Rheumatoid arthritis and schizophrenia: a negative association at a dimensional level. Schizophrenia Research, 66(1), 21–29. 10.1016/s0920-9964(03)00017-3 [DOI] [PubMed] [Google Scholar]
- Gorwood, P. , Pouchot, J. , Vinceneux, P. , Puechal, X. , Flipo, R. M. , De Bandt, M. , & Ades, J. (2004). Rheumatoid arthritis and schizophrenia: a negative association at a dimensional level. Schizophrenia Research, 66(1), 21–29. [DOI] [PubMed] [Google Scholar]
- Helmick, C. G. , Felson, D. T. , Lawrence, R. C. , Gabriel, S. , Hirsch, R. , Kwoh, C. K. , … Stone, J. H. (2008). Estimates of the prevalence of arthritis and other rheumatic conditions in the United States: Part I. Arthritis and Rheumatism, 58(1), 15–25. 10.1002/art.23177 [DOI] [PubMed] [Google Scholar]
- International Consortium for Blood Pressure Genome‐Wide Association, S , Ehret, G. B. , Munroe, P. B. , Rice, K. M. , Bochud, M. , Johnson, A. D. … Johnson, T. (2011). Genetic variants in novel pathways influence blood pressure and cardiovascular disease risk. Nature, 478(7367), 103–109. 10.1038/nature10405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janicijevic, S. , Dejanovic, S. , & Borovcanin, M. (2018). Interplay of brain‐derived neurotrophic factor and cytokines in schizophrenia. Serbian Journal of Experimental and Clinical Research (published online ahead of print 2018). 10.1515/sjecr-2017-0031 [DOI] [Google Scholar]
- Johnson, R. C. , Nelson, G. W. , Troyer, J. L. , Lautenberger, J. A. , Kessing, B. D. , Winkler, C. A. , & O'Brien, S. J. (2010). Accounting for multiple comparisons in a genome‐wide association study (GWAS). BMC Genomics, 11, 724 10.1186/1471-2164-11-724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, Y. K. , Jung, H. G. , Myint, A. M. , Kim, H. , & Park, S. H. (2007). Imbalance between pro‐inflammatory and anti‐inflammatory cytokines in bipolar disorder. J Affect Disord, 104, 91–5. [DOI] [PubMed] [Google Scholar]
- Kipnis, J. , Cardon, M. , Strous, R. D. , & Schwartz, M. (2006). Loss of autoimmune T cells correlates with brain diseases: possible implications for schizophrenia? Trends in Molecular Medicine, 12(3), 107–112. 10.1016/j.molmed.2006.01.003 [DOI] [PubMed] [Google Scholar]
- Kronfol, Z. , & Remick, D. G. (2000). Cytokines and the brain: implications for clinical psychiatry. Am J Psychiatry, 157, 683–94. [DOI] [PubMed] [Google Scholar]
- Lee, S. H. , Byrne, E. M. , Hultman, C. M. , Kähler, A. , Vinkhuyzen, A. A. E. , Ripke, S. , … van Riel, P. (2015). New data and an old puzzle: the negative association between schizophrenia and rheumatoid arthritis. International Journal of Epidemiology, 44(5), 1706–1721. 10.1093/ije/dyv136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, Z. , Chen, J. , Yu, H. , He, L. , Xu, Y. , Zhang, D. , … Shi, Y. (2017). Genome‐wide association analysis identifies 30 new susceptibility loci for schizophrenia. Nature Genetics, 49(11), 1576–1583. 10.1038/ng.3973 [DOI] [PubMed] [Google Scholar]
- Lokki, M. L. , & Paakkanen, R. (2019). The complexity and diversity of major histocompatibility complex challenge disease association studies. HLA, 93(1), 3–15. 10.1111/tan.13429 [DOI] [PubMed] [Google Scholar]
- Malavia, T. A. , Chaparala, S. , Wood, J. , Chowdari, K. , Prasad, K. M. , McClain, L. , … Nimgaonkar, V. L. (2017). Generating testable hypotheses for schizophrenia and rheumatoid arthritis pathogenesis by integrating epidemiological, genomic, and protein interaction data. NPJ Schizophr, 3, 11 10.1038/s41537-017-0010-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellsop, G. W. , Koadlow, L. , Syme, J. , & Whittingham, S. (1974). Absence of rheumatoid arthritis in schizophrenia. Aust N Z J Med, 4, 247–52. [DOI] [PubMed] [Google Scholar]
- Michel, M. , Schmidt, M. J. , & Mirnics, K. (2012). Immune system gene dysregulation in autism and schizophrenia. Dev Neurobiol, 72(10), 1277–1287. 10.1002/dneu.22044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittendorfer‐Rutz, E. , Rahman, S. , Tanskanen, A. , Majak, M. , Mehtälä, J. , Hoti, F. , … Tiihonen, J. (2019). Burden for parents of patients with schizophrenia‐a nationwide comparative study of parents of offspring with rheumatoid arthritis, multiple sclerosis, epilepsy, and healthy controls. Schizophrenia Bulletin, 45(4), 794–803. 10.1093/schbul/sby130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohamed, S. N. , Merskey, H. , Kazarian, S. , & Disney, T. F. (1982). An investigation of the possible inverse relationships between the occurrence of rheumatoid arthritis, osteoarthritis and schizophrenia. Can J Psychiatry, 27, 381–383. [DOI] [PubMed] [Google Scholar]
- Monji, A. , Kato, T. , & Kanba, S. (2009). Cytokines and schizophrenia: Microglia hypothesis of schizophrenia. Psychiatry Clin Neurosci, 63, 257–65. [DOI] [PubMed] [Google Scholar]
- Mors, O. , Mortensen, P. B. , & Ewald, H. (1999). A population‐based register study of the association between schizophrenia and rheumatoid arthritis. Schizophrenia Research, 40(1), 67–74. S0920-9964(99)00030-4[pii] [DOI] [PubMed] [Google Scholar]
- Müller, N. , Weidinger, E. , Leitner, B. , & Schwarz, M. (2016). The Role of Inflammation and the Immune System in Schizophrenia. The Neurobiology of Schizophrenia: Elsevier. [Google Scholar]
- Muniz‐Castrillo, S. , Vogrig, A. , & Honnorat, J. (2020). Associations between HLA and autoimmune neurological diseases with autoantibodies. Auto Immun Highlights, 11(1), 2 10.1186/s13317-019-0124-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohi, K. , Kikuchi, M. , Ikeda, M. , Yamamori, H. , Yasuda, Y. , Fujimoto, M. , … Hashimoto, R. (2016). Polygenetic components for schizophrenia, bipolar disorder and rheumatoid arthritis predict risk of schizophrenia. Schizophrenia Research, 175(1–3), 226–229. 10.1016/j.schres.2016.04.009 [DOI] [PubMed] [Google Scholar]
- Okada, Y. , Wu, D. , Trynka, G. , Raj, T. , Terao, C. , Ikari, K. , … Plenge, R. M. (2014). Genetics of rheumatoid arthritis contributes to biology and drug discovery. Nature, 506(7488), 376–381. 10.1038/nature12873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oken, R. J. , & Schulzer, M. (1999). At issue: schizophrenia and rheumatoid arthritis: The negative association revisited. Schizophrenia Bulletin, 25(4), 625–638. 10.1093/oxfordjournals.schbul.a033407 [DOI] [PubMed] [Google Scholar]
- Österberg, E. (1978). Schizophrenia and rheumatic disease. Acta Psychiatrica Scandinavica, 58, 339–359. [DOI] [PubMed] [Google Scholar]
- Pilkington, T. L. (1956). The coincidence of rheumatoid arthritis and schizophrenia. The Journal of Nervous and Mental Disease, 124, 604. [DOI] [PubMed] [Google Scholar]
- Pouget, J. G. , Goncalves, V. F. , Schizophrenia Working Group of the Psychiatric Genomics, C. , Spain, S. L. , Finucane, H. K. , Raychaudhuri, S. … Knight, J. (2016). Genome‐wide association studies suggest limited immune gene enrichment in schizophrenia compared to 5 autoimmune diseases. Schizophrenia Bulletin, 42(5), 1176–1184. 10.1093/schbul/sbw059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pouget, J. G. , Schizophrenia Working Group of the Psychiatric Genomics, C. , Han, B. , Wu, Y. , Mignot, E. , Ollila, H. M. … Knight, J. (2019). Cross‐disorder analysis of schizophrenia and 19 immune‐mediated diseases identifies shared genetic risk. Human Molecular Genetics, 28(20), 3498–3513. 10.1093/hmg/ddz145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruim, R. J. , Welch, R. P. , Sanna, S. , Teslovich, T. M. , Chines, P. S. , Gliedt, T. P. , … Willer, C. J. (2010). LocusZoom: regional visualization of genome‐wide association scan results. Bioinformatics, 26(18), 2336–2337. 10.1093/bioinformatics/btq419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross, W. D. , Hay, J. , & Mcdowall, M. F. (1950). The incidence of certain vegetative disturbances in relation to psychosis. Psychosomatic Medicine, 12, 179. [DOI] [PubMed] [Google Scholar]
- Sekar, A. , Bialas, A. R. , de Rivera, H. , Davis, A. , Hammond, T. R. , Kamitaki, N. , … McCarroll, S. A. (2016). Schizophrenia risk from complex variation of complement component 4. Nature, 530(7589), 177–183. 10.1038/nature16549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sellgren, C. , Frisell, T. , Lichtenstein, P. , Landen, M. , & Askling, J. (2014). The association between schizophrenia and rheumatoid arthritis: A nationwide population‐based swedish study on intraindividual and familial risks. Schizophrenia Bulletin, 40(6), 1552–1559. 10.1093/schbul/sbu054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simeone, J. C. , Ward, A. J. , Rotella, P. , Collins, J. , & Windisch, R. (2015). An evaluation of variation in published estimates of schizophrenia prevalence from 1990 horizontal line 2013: a systematic literature review. BMC Psychiatry, 15, 193 10.1186/s12888-015-0578-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smolen, J. S. , Aletaha, D. , & McInnes, I. B. (2016). Rheumatoid arthritis. The Lancet, 388(10055), 2023–2038. 10.1016/S0140-6736(16)30173-8 [DOI] [PubMed] [Google Scholar]
- Torrey, E. F. , & Yolken, R. H. (2001). The Schizophrenia‐Rheumatoid Arthritis Connection: Infectious, Immune, or Both? Brain Behavior and Immunity, 15(4), 401–410. [DOI] [PubMed] [Google Scholar]
- Tylee, D. S. , Sun, J. , Hess, J. L. , Tahir, M. A. , Sharma, E. , Malik, R. , … Glatt, S. J. (2018). Genetic correlations among psychiatric and immune‐related phenotypes based on genome‐wide association data. American Journal of Medical Genetics. Part B, Neuropsychiatric Genetics, 177(7), 641–657. 10.1002/ajmg.b.32652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe, Y. , Nunokawa, A. , Kaneko, N. , Muratake, T. , Arinami, T. , Ujike, H. , … Someya, T. (2009). Two‐stage case–control association study of polymorphisms in rheumatoid arthritis susceptibility genes with schizophrenia. Journal of Human Genetics, 54(1), 62–65. 10.1038/jhg.2008.4 [DOI] [PubMed] [Google Scholar]
- Wickham, H. (2016). ggplot2: elegant graphics for data analysis. Springer. [Google Scholar]
- Willer, C. J. , Li, Y. , & Abecasis, G. R. (2010). METAL: fast and efficient meta‐analysis of genomewide association scans. Bioinformatics, 26(17), 2190–2191. 10.1093/bioinformatics/btq340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright, P. , Nimgaonkar, V. L. , Donaldson, P. T. , & Murray, R. M. (2001). Schizophrenia and HLA: a review. Schizophrenia Research, 47(1), 1–12. 10.1016/S0920-9964(00)00022-0 [DOI] [PubMed] [Google Scholar]
- Zamanpoor, M. (2019). The genetic pathogenesis, diagnosis and therapeutic insight of rheumatoid arthritis. Clinical Genetics, 95(5), 547–557. 10.1111/cge.13498 [DOI] [PubMed] [Google Scholar]
- Zamanpoor, M. (2020). Schizophrenia in a genomic era: a review from the pathogenesis, genetic and environmental etiology to diagnosis and treatment insights. Psychiatric Genetics, 30(1), 1–9. 10.1097/YPG.0000000000000245 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study were derived from the following resources available in the public domain: http://gwas.bio-x.cn/, http://plaza.umin.ac.jp/~yokada/datasource/software.htm, and https://www.wtccc.org.uk/info/access_to_data_samples.html.


