Abstract
Objective
Olfactory and taste dysfunction (OTD) is a potential neurological manifestation of coronavirus‐2019 (COVID‐19). We aimed to investigate the diagnostic value of symptoms of anosmia and dysgeusia for COVID‐19.
Methods
A comprehensive electronic search was conducted using PubMed, MEDLINE, Scopus, Cochrane database, and Google Scholar from 1 June 2020 to 12 June 2020. All studies reporting symptoms of anosmia and dysgeusia in COVID‐19‐positive patients were included. A total of 23 studies were included in the systematic review.
Results
Symptoms of anosmia and dysgeusia were frequently reported by COVID‐19‐positive patients. Symptoms were more common in females and in younger patients. There was no direct association between the severity of COVID‐19 and the presence of symptoms. However, some evidence was found for a longer duration of these symptoms and increased severity of COVID‐19 infection in young patients.
Conclusion
OTD is commonly reported by COVID‐19 patients. Due to limited literature on the association between OTD and COVID‐19, it is currently not possible to conclude that these symptoms alone can be used to diagnose COVID‐19. However, the presence of OTD can potentially be used as a screening tool for COVID‐19 especially in young and female patients. Further research is required to establish the true diagnostic value of these symptoms and efficacy as screening tools for COVID‐19 patients.
Keywords: anosmia, COVID‐19, dysgeusia
Neurological manifestations of COVID‐19 can be in any form, assessment of such symptoms should be taken into consideration when examining patients for possible COVID‐19 infection.
1. INTRODUCTION
Coronavirus disease 2019 (COVID‐19) is caused by severe acute respiratory syndrome coronavirus‐2 (SARS‐CoV‐2) (Yuki, Fujiogi, & Koutsogiannaki, 2020). Since the introduction of COVID‐19 to the human population in the Chinese city of Wuhan, it has spread rapidly across the globe and was officially considered a pandemic in March 2020 (World Health Organization, 2020; Yuki et al., 2020). As of 16 August 2020, there have been > 21 million cases with > 700,000 deaths globally (World Health Organisation, 2019). Coronaviruses (CoV) belongs to the Coronavirinae subfamily and are known for their microscopic crown‐like appearance (Chen, Liu, & Guo, 2020a; Ren et al., 2020). The COVID‐19 pathogen is a human RNA virus and belongs to the β‐CoVs in the CoV phylogenetic tree (Chen, Liu, et al., 2020). Genome examinations reveal that the novel SARS‐CoV‐2 is 87.99% genetically similar to bats SARS‐like coronavirus and genetically distant from the previously known SARS and MERS viruses (Lovato & Filippis, 2020; Ren et al., 2020). The virus is transmittable from person‐to‐person via respiratory droplets and has a basic reproductive number of approximately 1–3 (Flahault, 2020; Lovato & Filippis, 2020).
Studies report that COVID‐19 patients can present with fever, dry cough, dyspnea, and fatigue (Guan et al., 2020; Lovato & Filippis, 2020). In severe cases, the infection can cause viral pneumonia leading to severe acute respiratory distress syndrome or even death (Guan et al., 2020; Lovato & Filippis, 2020). Symptoms of pharyngodynia, nasal congestion, and rhinorrhoea have also been reported in infected patients (Lovato & Filippis, 2020). Sense of smell is controlled by the olfactory cranial nerve (Cranial & Nerve). The European Rhinology society reported that a significant number of COVID‐19 patients (20%–60%) appear to have loss of smell also known as anosmia (IMPORTANT INFO ON, 2020). Furthermore, reports from China, South Korea, and Italy also reported anosmia in COVID‐19 patients (Entuk.org., 2020; Guan et al., 2020; Kang, Cho, Lee, Kim, & Park, 2020; Lee, Min, Lee, & Kim, 2020). In the UK, anosmia is classed as an official symptom for COVID‐19 (Statement from the UK, 2020). Anosmia may be the first presenting symptom, preceding the occurrence of other COVID‐19 symptoms such as cough and fever (Kang et al., 2020). Interestingly, changes in sense of taste also known as dysgeusia have also been reported in infected patients (Carrillo‐Larco & Altez‐Fernandez, 2020).
The presence of anosmia and dysgeusia may help neurologists and otolaryngologists identify patients with COVID‐19 early, allowing prompt management and infection control procedures to be implemented. The current study aimed to systematically review the current literature on the clinical presentation of COVID‐19, specifically focussing on the symptoms of anosmia and dysgeusia and their diagnostic value in COVID‐19 patients.
2. METHODS
2.1. Electronic database search
A comprehensive literature search was conducted using PubMed, Medline, Scopus, Cochrane database, and Google Scholar using MeSH words including: “COVID‐19," “Coronavirus 2019,” “Anosmia,” “Dysgeusia.” The systematic review was carried out according to the Preferred Reporting Items for Systematic Reviews and Meta‐analysis (PRISMA) (Moher, Liberati, Tetzlaff, & Altman, 2009). We examined articles and abstracts available in the English language. Literature was screened for original data, and any related references were retrieved and checked manually for other relevant studies.
2.2. Inclusion and exclusion criteria
Studies were included if the following criteria were met: (a) Articles were original reports, (b) studies included laboratory‐confirmed COVID‐19 patients, (c) studies reported details of clinical presentation, and (d) the reports were published in the English language. Exclusion criteria included: (a) study design: case report, editorial, letter to the editor, or review; (b) studies reporting symptoms in children/infants.
2.3. Data extraction
All studies were screened by two authors independently (KP and SI). Any disagreements were resolved by discussion between the review team members. The extracted data were then cross‐checked by another author (SAZ) to validate its accuracy. Included studies were analyzed to extract all available data and assure eligibility for all patients. Description data of patients including age, sex, clinical symptoms, underlying medical conditions, and outcomes were extracted and recorded for all studies.
2.4. Methodological assessment of the studies
Quality Assessment of the included qualitative studies was conducted using the Newcastle‐Ottawa Scale (Table 1) (Wells et al., 2020). The scale was devised specifically to allow quality assessment of the nonrandomized studies included in the systematic review. The scale allows assessment of bias using a star‐based rating system with a maximum score of 9 indicating a low risk of bias and a minimum of 0 indicating the highest risk (Wells et al., 2020). Scores ≥ 7 generally represent a low risk of bias (Wells et al., 2020). The quality of included studies was rated by two of the authors (SAZ and SI).
Table 1.
Quality assessment of included studies using the Newcastle‐Ottawa Scale
| Author | Selection | Comparability | Outcomes | Quality | |||||
|---|---|---|---|---|---|---|---|---|---|
| Representation of patients with COVID−19 | Selection of patients with olfactory and gustatory dysfunction | Ascertainment of exposure | Demonstration that outcome of interest was not present at start of study | Assessment of outcomes | Follow‐up long enough for outcomes to occur | Adequate reporting of outcomes | |||
| Lee et al. (2020) | * | * | * | * | ** | * | * | * | Good |
| Hopkins, Surda, Whitehead, et al. (2020) | * | * | * | Poor | |||||
| Yan et al. (2020) | * | * | * | * | * | * | Poor | ||
| Lechien et al. (2020) | * | * | * | * | * | * | * | * | Poor |
| Lechien et al. (2020) | * | * | * | * | * | * | * | * | Poor |
| Lechien et al. (2020) | * | * | * | * | * | * | * | * | Poor |
| Vaira et al. (2020) | * | * | * | * | * | * | * | * | Poor |
| Vaira et al. (2020) | * | * | * | * | ** | * | * | * | Good |
| Spinato et al. (2020) | * | * | * | * | * | * | * | * | Poor |
| Carignan et al. (2020) | * | * | * | * | ** | * | * | * | Good |
| Iravani et al. (2020) | * | * | * | Poor | |||||
| Boscolo‐Rizzo et al. (2020) | * | * | * | Poor | |||||
| Giacomelli et al. (2020) | * | * | * | * | * | * | * | * | Poor |
| Wee et al. (2020) | * | * | * | * | ** | * | * | * | Good |
| Bénézit et al. (2020) | * | * | * | * | * | * | * | Poor | |
| Beltrán‐Corbellini et al. (2020) | * | * | * | * | ** | * | * | * | Good |
| Moein et al. (2020) | * | * | * | * | * | * | * | * | Poor |
| Klopfenstein et al. (2020) | * | * | * | * | * | * | * | Poor | |
| Kaye et al. (2020) | * | * | * | * | * | * | * | * | Good |
| Mao et al. (2020) | * | * | * | * | * | ** | * | * | Good |
| Hopkins, Surda, Whitehead, et al. (2020) | * | * | * | Poor | |||||
| Levinson et al. (2020) | * | * | * | * | * | * | * | Poor | |
| Kosugi et al. (2020) | * | * | * | * | * | * | * | * | Poor |
Good quality: 3 or 4 stars (*) in selection domain AND 1 or 2 stars in comparability domain AND 2 or 3 stars in outcome domain; Fair quality: 2 stars in selection domain AND 1 or 2 stars in comparability domain AND 2 or 3 stars in outcome domain; Poor quality: 0 or 1 star in selection domain OR 0 stars in comparability domain OR 0 or 1 stars in outcome domain.
2.5. Statistical analysis
It was not possible to do pooled analysis due to high heterogeneity in study design and assessment.
3. RESULTS
3.1. Retrieving studies
A search was started on 10 June 2020, and the last search was on 20 June 2020. A total of 150 articles were retrieved from different databases. After removing duplicates and screening titles and abstracts of studies, we identified 35 studies potentially relevant to the topic. The full text screening of the articles allowed exclusion of 12 studies that did not meet our inclusion criteria. The remaining 23 studies were considered to be eligible for our systematic review. Figure 1 shows a summary of the selection process and criteria.
Figure 1.

PRISMA flow chart showing the selection process for included studies
3.2. Assessment of the studies
All 23 studies included were relevant for the review topic. (Beltrán‐Corbellini et al., 2020; Bénézit et al., 2020; Boscolo‐Rizzo et al., 2020; Carignan et al., 2020; Giacomelli et al., 2020; Hopkins, Surda, & Kumar, 2020; Hopkins, Surda, Whitehead, & Kumar, 2020; Iravani et al., 2020; Kaye, Chang, Kazahaya, Brereton, & Denneny, 2020; Klopfenstein et al., 2020; Kosugi et al., 2020; Lechien, Cabaraux, & Chiesa‐Estomba et al., 2020a, 2020b; Lechien et al., 2020; Lee et al., 2020; Levinson et al., 2020; Mao et al., 2020; Moein et al., 2020; Spinato et al., 2020; Vaira et al., 2020; Vaira, Salzano, Deiana, & De Riu, 2020; Wee et al., 2020; Yan, Faraji, Prajapati, Ostrander, & DeConde, 2020) There were no randomized control trials, and studies were mainly retrospective (20) or prospective (3) case‐series, case–control, cohort, cross‐sectional studies (Beltrán‐Corbellini et al., 2020; Bénézit et al., 2020; Boscolo‐Rizzo et al., 2020; Carignan et al., 2020; Giacomelli et al., 2020; Hopkins, Surda, & Kumar, 2020; Hopkins, Surda, Whitehead, et al., 2020; Iravani et al., 2020; Kaye et al., 2020; Klopfenstein et al., 2020; Kosugi et al., 2020; Lechien, Cabaraux, & Chiesa‐Estomba et al., 2020a, 2020b; Lechien et al., 2020; Lee et al., 2020; Levinson et al., 2020; Mao et al., 2020; Moein et al., 2020; Spinato et al., 2020; Vaira, Hopkins, Salzano et al., 2020; Vaira, Salzano, Deiana, & De Riu, 2020, 2020; Wee et al., 2020; Yan et al., 2020). Quality of the studies included in the review was assessed using the Newcastle‐Ottawa Scale (Table 1) (Wells et al., 2020). A “good” quality score required 3 or 4 stars in selection, 1 or 2 stars in comparability, and 2 or 3 stars in outcomes (Wells et al., 2020). A “fair” quality score required 2 stars in selection, 1 or 2 stars in comparability, and 2 or 3 stars in outcomes (Wells et al., 2020). A “poor” quality score reflected 0 or 1 star(s) in selection, or 0 stars in comparability, or 0 or 1 star(s) in outcomes (Wells et al., 2020). Most studies were poor quality, with only 6 studies identified to be of good quality. There is high risk of reporting bias as most studies utilized self‐report questionnaires. These questionnaires are subjective, and it may be possible that some patients exaggerated or over reported their symptoms. Confounders such as the presence of pre‐existing respiratory or otolaryngology disease were not universally accounted for.
3.3. Patient background
We included results from 12,314 patients in this review (Table 2) (Beltrán‐Corbellini et al., 2020; Bénézit et al., 2020; Boscolo‐Rizzo et al., 2020; Carignan et al., 2020; Giacomelli et al., 2020; Hopkins, Surda, & Kumar, 2020; Hopkins, Surda, Whitehead, et al., 2020; Iravani et al., 2020; Kaye et al., 2020; Klopfenstein et al., 2020; Kosugi et al., 2020; Lechien, Cabaraux, & Chiesa‐Estomba et al., 2020a, 2020b; Lechien et al., 2020; Lee et al., 2020; Levinson et al.,2020;Mao et al., 2020;Moein et al., 2020; Spinato et al., 2020; Vaira et al., 2020, 2020; Wee et al., 2020; Yan et al., 2020). Patient demographics were not reported by all studies but in those that had, most patients were male with the age of the patients ranging from 32 to 60 years (Beltrán‐Corbellini et al., 2020; Carignan et al., 2020; Giacomelli et al., 2020; Hopkins, Surda, Whitehead, et al., 2020; Kaye et al., 2020; Klopfenstein et al., 2020; Kosugi et al., 2020;Lechien, Cabaraux, & Chiesa‐Estomba et al., 2020a,2020b;Lechien et al., 2020; Levinson et al., 2020; Mao et al., 2020; Moein et al., 2020; Spinato et al., 2020; Vaira et al., 2020, 2020; Yan et al., 2020) The presence of comorbidities especially cardiovascular disease, cerebrovascular disease, and diabetes mellitus has been associated with increased risk of COVID‐19 infection and increased severity of the infection. Comorbidities were also not reported by most studies, but in those that had, hypertension (4.7%–28.3%) was the most common disease found in COVID‐19 patients. (Klopfenstein et al., 2020; Kosugi et al., 2020; Lechien, Cabaraux, & Chiesa‐Estomba et al., 2020a, 2020b; Lechien et al., 2020; Levinson et al., 2020; Mao et al., 2020; Moein et al., 2020; Spinato et al., 2020; Yan et al., 2020).
Table 2.
Studies evaluating symptoms of anosmia and dysgeusia in COVID‐19 patients
| Author | Study design | Country | Cohort size | How was anosmia studied? | Patient demographics | Comments |
|---|---|---|---|---|---|---|
| Lee et al. (2020) | Prospective (cohort) | South Korea | 3,191 |
Telephone interview |
|
15.3% (488) reported anosmia or ageusia in early stages and 15.7% (367) reported it in asymptomatic‐to‐mild severity. Prevalence was significantly higher in younger individuals and women. Mean recovery time of anosmia and ageusia was 3 weeks. |
| Hopkins, Surda, Whitehead, et al. (2020) | Retrospective (Cross‐sectional) | United Kingdom | 2,428 | Survey distributed online | Does not report on COVID patients directly—only uses those with self‐reported symptoms | 17% did not report other COVID−19 symptoms and of those who did, 51% complained of fever or cough. No direct confirmation of COVID−19 test positive, instead relies on the presence of other COVID−19 symptoms |
| Yan et al. (2020) | Retrospective (Cross‐sectional) | USA | 262 |
Patient‐reported symptoms |
Age in years: 40–49 (median) Female: 29 (49.2%) Comorbidities
|
Smell and taste loss in COVID−19‐positive patients were seen in 68% and 71%, respectively, and impairment in both was independently associated with positive COVID−19 test. |
| Lechien et al. (2020) | Prospective (Cross‐sectional) | Belgium | 78 | Identification Test of the “Sniffin Sticks” test |
Age in years: 41.7 (mean) Female: 56 (65.1%) Comorbidities
|
Patients with initial sudden olfactory anosmia (ISOA) were separated into two groups based on duration of greater or less than 12 days. They were swabbed for COVID−19 and completed psychophysical olfactory evaluation. Complete cohort evaluation was limited by travel restriction but overall in the group with symptoms less than 12 days: 87.5% were positive for COVID−19 |
| Lechien et al. (2020) | Retrospective (case series) | Belgium | 86 |
Subjective assessment using SNOT−22 and impact on life with the sQOD‐NS with further psychophysical evaluation using identification Test of the “Sniffin Sticks” test |
|
Total loss of smell was reported by 61.4% in direct contrast to 47.7% identified as anosmic on objective testing. Of 9 anosmic patients to repeat the Sniffin Stick test—5 had improved. |
| Leichen et al. (2020) | Retrospective (Cross‐sectional) | Europe | 417 | Self‐report questionnaires—smell and taste section of the National Health and Nutrition Examination Survey and the short version of Questionnaire of Olfactory Disorders‐Negative Statements |
|
85.6% (356) reported olfactory dysfunction and 88.0% (367) reported gustatory dysfunction. Olfactory dysfunction (OD) appeared before other symptoms in 11.8% (49). Women were significantly more likely to report dysfunction. |
| Vaira et al. (2020) |
Retrospective (case series) |
Italy | 72 | CCCRC test |
|
Olfactory assessment showed 83.3% with hyposmia and 2.8% with anosmia. Gustatory assessment reported hypogeusia in 47.1% and ageusia in 1.4%. No correlation between the presence of OTD and COVID−19 severity. |
| Vaira et al. (2020) | Retrospective (cohort) | Italy | 256 | CCCRC test |
|
Multicentre study that conducted objective chemosensitive evaluation, 30% of those who did not report symptoms displayed objective hyposmia, suggesting the prevalence is under reported in questionnaires. No correlation between the presence of OTD and COVID−19 severity. |
| Spinato et al. (2020) | Retrospective (cross‐sectional) | Italy | 202 |
Telephone interview using SNOT−22 following positive nasopharyngeal and throat swabs |
|
OTD alteration was reported in 64.4% (130), of whom 34.6% (30) reported a blocked nose. 11.9% (29) reported change in taste and smell occurred before other symptoms and it was significantly more frequent among women. Using the SNOT−22 criteria, the median symptom level was “severe.” |
| Carignan et al. (2020) | Retrospective (case–control) | Canada | 134 | Telephone interview using the Self‐MOQ |
|
Independent presence of anosmia/dysgeusia or both present together were significant predictors of COVID−19 test positivity and may even serve as indicators for testing. |
| Iravani et al. (2020) | Retrospective (cohort) | Sweden | 2,440 | Online questionnaire distributed to Swedish population |
|
There was a significant difference in odor intensity being reported between patients who had other symptoms of COVID−19 compared to those with no symptoms. This study did not confirm the diagnosis of COVID−19, rather used symptoms that match the clinical picture and had an uncontrolled method of testing smell. |
| Boscolo‐Rizzo et al. (2020) |
Retrospective (cross‐sectional) |
Italy | 179 | Telephone survey of contacts of self‐isolating nonhospitalized COVID−19 patients |
|
Of the 296 contacts, 175 were not tested and 38.3% (33) reported typical COVID−19 symptoms with 4.0% (17) reporting loss of taste and smell. The prevalence of taste and smell loss in those testing negatives was significantly lower than those who tested positive. |
| Giacomelli et al. (2020) | Retrospective (cross‐sectional) | Italy | 59 | Survey of hospitalized patients |
|
34% (20) reported at least 1 olfactory/taste disorder and 18.6% (11) reported both. Taste alterations were more frequently present before hospitalization—but alterations to smell were common in hospital. |
| Wee et al. (2020) |
Retrospective (cross‐sectional) |
Singapore | 870 |
Questionnaire presented to patients in the emergency department |
|
Presence of olfactory and taste disorder (OTD) had high specificity (98.7%) but low sensitivity (22.7%) as a screening criterion, which is roughly similar to the sensitivity and specificity as history of close contact with COVID−19. Of admitted in‐patients with PCR proven respiratory viruses, COVID−19 patients were significantly more likely to develop OTD. |
| Bénézit et al. (2020) | Retrospective (cohort) | France—multicentre | 259 | Web‐based questionnaire |
|
Hypogeusia and hyposmia, either independently or together, were strongly associated with COVID−19 positivity. Hypogeusia was reported by 24% (63), hyposmia in 20% (51) and both in 17% (43). However, the anonymous nature of the survey meant that accuracy of COVID−19 infection cannot be ratified. |
| Beltrán‐Corbellini et al. (2020) | Retrospective (case–control | Spain | 79 | Telephone interview |
|
Multicentre study comparing COVID−19‐positive patients with influenza‐positive patients for olfactory and gustatory symptoms. New‐onset olfactory and gustatory symptoms were significantly more common in COVID−19 patients, in those patients who did report symptoms were significantly younger than those who did not. |
| Moein et al. (2020) | Retrospective (case–control) | Iran | 60 |
USPIT |
|
All but one of the patients had some level of olfactory dysfunction—the mean scoring of the USPIT was 20.98 indicating severe hyposmia. 58% (35) were anosmic and 33% (20) being severely hyposmic. |
| Klopfenstein et al. (2020) | Retrospective (cohort) | France | 114 | Unprompted patient reports |
|
47% (54) reported anosmia and 85% (46) had dysgeusia. Mean age was 47 and 67% were female. 98% recovered within 28 days—80% had recovered by 14 days. |
| Kaye et al. (2020) | Prospective (cohort) | USA | 237 |
COVID−19 Anosmia Reporting Tool for Clinicians |
|
73% (173) of all patients had anosmia prior to diagnosis and was the initial symptom in more than a quarter. 27% (46) reported improvement in anosmia, taking an average of 7.2 days, while 85% improved in 10 days. |
| Mao et al. (2020) |
Retrospective (case‐series) |
China | 214 | Interview |
|
Anosmia/ageusia had no bearing on severity of COVID−19, but were the most common PNS symptoms. |
| Hopkins, Surda, Whitehead, et al. (2020) |
Retrospective (cohort) |
UK | 382 | Online survey |
|
Anosmia and hyposmia reported in the majority of completed surveys, with significant improvements seen following one week—complete resolution of olfactory disorders within 11.5% of the 330 reporting issues. |
| Levinson et al. (2020) |
Retrospective (cohort) |
Israel | 42 | Online survey combined with further telephone interview following discharge |
|
Of the 35.7% of cohort reporting anosmia, median recovery time was 7.6 days in those that had recovered. |
| Kosugi et al. (2020) |
Retrospective (cohort) |
Brazil | 253 | Online survey |
|
Most of the patients reported sudden anosmia as opposed to hyposmia. However, in patients with hyposmia—this tended to recover sooner. |
Abbreviations: CCCRC, Connecticut Chemosensory Clinical Research Centre; CKD, chronic kidney disease; DM, diabetes mellitus; HTN, hypertension; OTD, olfactory and taste dysfunction; Self‐MOQ, Self‐reported Mini Olfactory Questionnaire; SNOT‐22, Sino‐nasal Outcome test; sQOD‐NS, Questionnaire of olfactory disorders—negative statements; USPIT, University of Pennsylvania Smell Identification test.
3.4. Prevalence of anosmia and dysgeusia
The prevalence of olfactory and taste dysfunction (OTD) in COVID‐19 varies widely in the literature (Table 2). A large multicentre study from Europe reports over 85% of patients who have been confirmed positive for COVID‐19 report OTD (Lechien et al., 2020). This proportion remains consistently high in the absence of nasal obstruction (Hopkins, Surda, & Kumar, 2020). Beltrán‐Corbellini et al. conducted a case–control study comparing the prevalence of olfactory and gustatory dysfunction in patients with COVID‐19 compared to those with other influenza‐like diseases (Beltrán‐Corbellini et al., 2020). The results corroborated that of Leichen et al., with over 80% reporting smell disorders and over 90% reporting taste dysfunction (Lechien, Cabaraux, & Chiesa‐Estomba et al., 2020a). Both symptoms were significantly more likely to occur whether the patient was COVID‐19‐positive. In contrast, a smaller study done by Giamecello et al. sets a smaller prevalence, with 34% of their sample reporting at least one form of taste and smell dysfunction (Giacomelli et al., 2020).
OTD exists on a spectrum, from hyposmia and hypogeusia to anosmia and ageusia. True absence of taste or smell is rarer than dysfunction. Vaira et al. reported the results of a case series of 72 COVID‐19 patients who underwent objective olfactory and gustatory testing. An assessment revealed 83.3% and 47.1% had hyposmia and hypogeusia, respectively. This contrasts with anosmia (2.8%) and ageusia (1.4%) (Vaira et al., 2020). Klopfenstein et al. conducted a retrospective cohort study that set the prevalence of anosmia at 47% and dysgeusia at 85% in its sample (Klopfenstein et al., 2020). It is important to regard nasal obstruction as a possible explanation for OTD. Lechien et al. and Spinato et al. report a minority of patients with concomitant nasal obstruction, but this aspect is not widely accounted for in the literature (Lechien, Cabaraux, & Chiesa‐Estomba et al., 2020b; Spinato et al., 2020). A consistent feature in the evidence base is that OTD is significantly more common in younger patients and those who are female. (Klopfenstein et al., 2020; Lechien, Cabaraux, & Chiesa‐Estomba et al., 2020b; Spinato et al., 2020; Vaira et al., 2020)
3.5. Other symptoms
In most studies that reported olfactory or gustatory dysfunction, a range of other symptoms such as fever, cough, headache, fatigue, and arthralgia/myalgia were also reported (Table 3) (Carignan et al., 2020; Giacomelli et al., 2020; Klopfenstein et al., 2020; Kosugi et al., 2020; Lechien, Cabaraux, & Chiesa‐Estomba et al., 2020a, 2020b; Lechien et al., 2020; Mao et al., 2020; Moein et al., 2020; Spinato et al., 2020; Vaira et al., 2020; Wee et al., 2020; Yan et al., 2020). The most commonly reported symptom in the majority of the studies was fever with a few reporting cough to be the most common. In contrast, Yan et al. reported fatigue as the most common symptom with 81.4% of patients with the symptom (Yan et al., 2020) This is consistent with Klopenstein et al. which reported 93% of COVID‐19 patients had fatigue (Klopfenstein et al., 2020; Yan et al., 2020). Interestingly, studies have also reported anorexia as a symptom. (Carignan et al., 2020; Kosugi et al., 2020; Mao et al., 2020; Moein et al., 2020; Spinato et al., 2020) The presence of anorexia varies between 3% and 56% (Carignan et al., 2020; Kosugi et al., 2020; Mao et al., 2020; Moein et al., 2020; Spinato et al., 2020). It is important to note that dysfunction in a sense of taste or smell could cause changes in appetite. Therefore, an examination of olfactory and gustatory function may be warranted in patients with anorexia.
Table 3.
Other COVID‐19 associated symptoms
| Author | Study design | Cohort Size | Other Symptoms | |||||
|---|---|---|---|---|---|---|---|---|
| Fever | Cough | Headache | Arthralgia/Myalgia | Fatigue | Anorexia | |||
| Yan et al. (2020) | Retrospective (Cross‐sectional) | 262 | 41 (69.5%) | 39 (66.1%) | 25 (42.4%) | 37 (62.7%) | 48 (81.4%) | ‐ |
| Leichien et al. (2020) | Prospective (Cross‐sectional) | 78 | 62 (72.9%) | 42 (48.6%) | 52 (60.0%) | 36 (42.9%) | ‐ | ‐ |
| Leichien et al. (2020) | Retrospective (Cross‐sectional) | 417 | 48% | 78% | 45% | 31% | ‐ | ‐ |
| Vaira et al. (2020) | Retrospective (case series) | 72 | 69 (95.8%) | 60 (83.3%) | 30 (41.6%) | ‐ | 48 (66.7%) | ‐ |
| Spinato et al. (2020) | Retrospective (cross‐sectional) | 202 | 113 (55.9%) | 122 (60.4%) | 86 (42.6%) | 90 (44.6%) | ‐ | 110 (54.5%) |
| Carignan et al. (2020) | Retrospective (case–control) | 134 | 50 (37.3%) | 90 (72.4%) | 87 (64.9%) | 76 (56.7%) | ‐ | 75 (56.0%) |
| Giacomelli et al. (2020) | Retrospective (cross‐sectional) | 59 | 43 (72.8%) | 22 (37.3%) | 2 (3.4%) | 3 (5.1%) | ‐ | ‐ |
| Wee et al. (2020) | Retrospective (cross‐sectional) | 870 | 21 (60%) | 10 (28.5%) | ‐ | ‐ | ‐ | ‐ |
| Moein et al. (2020) | Retrospective (case–control) | 60 | 46 (77%) | 35 (58%) | 22 (37%) | 5 (8%) | ‐ | 2 (3%) |
| Klopfenstein et al. (2020) | Retrospective (cohort) | 114 | 40 (74%) | 47 (87%) | 44 (82%) | 40 (74%) | 50 (93%) | ‐ |
| Mao et al. (2020) | Retrospective (case–control) | 214 | 132 (61.7%) | 107 (50%) | 28 (13.1%) | ‐ | ‐ | 68 (31.8%) |
| Kosugi et al. (2020) | Retrospective (cohort) | 253 | 16 (42.1%) | 83 (57.2%) | 20(52.6%) | 8(21%) | 7 (18.4%) | 2 (5.2%) |
3.6. Severity
There is significant variability in the reported severity of symptoms (Table 2). In a cohort of 72 patients, administering the Connecticut Chemosensory Clinical Research Centre Orthonasal Olfaction test (CCCRC) revealed mild to moderate hyposmia as the most common presentation of OTD, while complete anosmia was only present in 2.8% of the cohort. (Vaira et al., 2020) There is similar variability in the presentation of taste dysfunction. In the same cohort, mild to moderate hypogeusia were common while there was only one patient with complete ageusia (complete loss of taste sensation). (Vaira et al., 2020) In another assessment of OTD, a cohort of 283 patients in Italy were assessed using a telephone interview after positive nasopharyngeal and throat swabs. Here, the Sino‐nasal Outcome Test 22 (SNOT‐22) was utilized, and the median score of the 130 patients reporting symptoms was four (Spinato et al., 2020). The SNOT‐22 uses a scale of zero to five, with five indicating the symptoms are “as bad as it can be” and the score of four would indicate the OTD is severe (Hopkins, Gillett, Slack, Lund, & Browne, 2009). Importantly, this study presents an objective assessment of OTD where other studies rely on self‐reported questionnaires.
While there is a variance in the severity of OTD in COVID‐19 patients, there has been research into the presence of OTD as a predictor of severe infection. It should be noted that there is still uncertainty in defining a severe infection; however, numerous proxies have been used in literature. Interestingly, multivariable logistic regression of 169 patients in San Diego showed that the presence of normosmia was independently associated with hospitalization with hyposmia or anosmia 10‐fold less likely to be admitted, suggesting alterations in olfaction are associated with milder infections (Yan et al., 2020). However, further work has failed to recreate the same result. Vaira et al. show that there exists no correlation between the severity of disease and the CCCRC score using pneumonia as a marker of severity (Vaira et al., 2020). This is further highlighted in work by Mao et al., while the most common peripheral nervous system symptoms were OTD, the presence of either smell or taste impairment had no bearing on the severity of disease (Mao et al., 2020).
3.7. Recovery
As seen with severity, there is marked variability in the recovery from the OTD (Table 2). In a retrospective analysis of 253 patients with COVID‐19 in Brazil, an online survey indicated that full recovery had a median duration of 12.5 days and with a median follow‐up of 31 days – 121 patients had fully recovered in that time (Kosugi et al., 2020). Similarly, another retrospective analysis of 42 patients in Israel showed a median recovery of 7.6 days in 35.7% of the cohort reporting anosmia (Levinson et al., 2020). However, the follow‐up period was cut short and as such poses a question regarding the true value of recovery. In further studies, the median duration does appear within the range of 1–2 weeks (Lee et al., 2020). Total recovery time among the entire cohort was not well reported among the studies identified; however, Lee et al. noted it took over 3 weeks for a cohort of 3,191 patients in South Korea to fully recover (Lee et al., 2020). The younger population (ages 20–39) were prone to have a longer duration of anosmia than older cohorts (Lee et al., 2020).
3.8. Discussion
A large COVID‐19 case series published by the Chinese Centre of Disease Control and prevention reported that among a total of 72, 314 COVID‐19 cases, a total of 22% of patients were identified as suspected cases and 15% were clinically diagnosed based on clinical symptoms (Wu & McGoogan, 2020). The most common symptoms were reported to be fever and cough, with no detailed description of any other symptoms (Wu & McGoogan, 2020). Increasing evidence shows that symptoms of anosmia and dysgeusia are related to COVID‐19 (Lovato & Filippis, 2020; Zayet et al., 2020). In March 2020, American Academy of Otolaryngology‐Head and Neck Surgery suggested that the symptoms of anosmia and dysgeusia should be included in the list of screening tools for COVID‐19 as these symptoms are frequently reported by patients that ultimately test positive for COVID‐19 (AAO‐HNS: Anosmia, Hyposmia). Symptoms of OTD should prompt self‐isolation and testing for COVID‐19. Suspected COVID‐19 cases should be isolated and health personnel in contact should wear appropriate personal protective equipment such as waterproof gowns, gloves, goggles, and surgical masks or FFP2 masks (AAO‐HNS: Anosmia, Hyposmia; Lovato & Filippis, 2020). This systematic review aimed to investigate the diagnostic value of anosmia and dysgeusia for COVID‐19.
Anosmia and dysgeusia were frequently reported in patients who tested positive for COVID‐19 (Beltrán‐Corbellini et al., 2020; Bénézit et al., 2020; Boscolo‐Rizzo et al., 2020; Carignan et al., 2020; Giacomelli et al., 2020; Hopkins, Surda, & Kumar, 2020; Hopkins, Surda, Whitehead, et al., 2020; Iravani et al., 2020; Kaye et al., 2020; Klopfenstein et al., 2020; Kosugi et al., 2020; Lechien, Cabaraux, & Chiesa‐Estomba et al., 2020a, 2020b; Lechien et al., 2020; Lee et al., 2020; Levinson et al.,2020;Mao et al., 2020;Moein et al., 2020; Spinato et al., 2020; Vaira et al., 2020, 2020; Wee et al., 2020; Yan et al., 2020) High prevalence of these symptoms was reported in both studies with an objective assessment of taste and smell and self‐reported symptom questionnaires (Beltrán‐Corbellini et al., 2020; Bénézit et al., 2020; Boscolo‐Rizzo et al., 2020; Carignan et al., 2020; Giacomelli et al., 2020; Hopkins, Surda, & Kumar, 2020; Hopkins, Surda, Whitehead, et al., 2020; Iravani et al., 2020; Kaye et al., 2020; Klopfenstein et al., 2020; Kosugi et al., 2020; Lechien, Cabaraux, & Chiesa‐Estomba et al., 2020a, 2020b; Lechien et al., 2020; Lee et al., 2020; Levinson et al., 2020; Mao et al., 2020; Moein et al., 2020; Spinato et al., 2020; Vaira et al., 2020, 2020; Wee et al., 2020; Yan et al., 2020) OTD was found to be significantly higher in females and in younger patients (Beltrán‐Corbellini et al., 2020; Bénézit et al., 2020; Boscolo‐Rizzo et al., 2020; Carignan et al., 2020; Giacomelli et al., 2020; Hopkins, Surda, & Kumar, 2020; Hopkins, Surda, Whitehead, et al., 2020; Iravani et al., 2020; Kaye et al., 2020; Klopfenstein et al., 2020; Kosugi et al., 2020; Lechien, Cabaraux, & Chiesa‐Estomba et al., 2020a, 2020b; Lechien et al., 2020; Lee et al., 2020; Levinson et al., 2020; Mao et al., 2020; Moein et al., 2020; Spinato et al., 2020; Vaira et al., 2020, 2020; Wee et al., 2020; Yan et al., 2020). Indeed, neurological manifestations of COVID‐19 such as Guillain–Barré syndrome (GBS) in young people are of great concern (Ahmed et al., 2020). However, so far only a few cases of COVID‐19 associated with GBS have been reported (Webb, Wallace, & Martin‐Lopez, 2020). GBS can rarely present with taste dysfunction (Kogan, Mednick, & Dolgovina, 2011). Although currently there is no literature on the prevalence of OTD in COVID‐19‐positive patients with GBS, we speculate that increased prevalence of taste dysfunction in GBS patients may raise the suspicion of the infection.
Postviral anosmia has been reported to account for 40% of the anosmia cases and one of the leading causes of anosmia in adults (Hummel et al., 2017). There is variability in OTD dysfunction among patients, and its association with the severity of COVID‐19 infection is currently uncertain. However, a significant correlation between the duration of olfactory and gustatory symptoms and severe COVID‐19 infection has been reported (Vaira et al., 2020). Therefore, hospitalized patients especially younger patients with OTD should be monitored carefully. In terms of other COVID‐19 symptoms, fever and cough were the most common, which is consistent with already published medical literature (Boscolo‐Rizzo et al., 2020; Carignan et al., 2020; Giacomelli et al., 2020; Iravani et al., 2020; Klopfenstein et al., 2020; Kosugi et al., 2020; Lechien, Cabaraux, & Chiesa‐Estomba et al., 2020a, 2020b; Lechien et al., 2020; Lee et al., 2020; Lovato & Filippis, 2020; Mao et al., 2020; Moein et al., 2020; Spinato et al., 2020; Vaira et al., 2020; Wee et al., 2020; Yan et al., 2020).
The presence of anosmia and dysgeusia could help neurologists and otolaryngologists identify COVID‐19 cases early, allowing prompt treatment and reduction in infection transmission. It is possible that symptoms of anosmia and dysgeusia can be used as an effective screening tool and aid diagnosis of COVID‐19. Zayet et al. suggest that the positive predictive value (PPV) for anosmia is 77%, dysgeusia 77% and a combination of anosmia plus dysgeusia 83% for a positive SARS‐CoV‐2 real time‐polymerase chain reaction (RT‐PCR) test on a nasopharyngeal sample (Zayet et al., 2020). However, a key limitation of Zayet et al.'s study is that although RT‐PCR on a nasopharyngeal sample is specific for COVID‐19, it has a sensitivity of 56%‐83% and may be an inaccurate test to diagnose COVID‐19 (AAO‐HNS: Anosmia, Hyposmia; Kokkinakis, Selby, Favrat, Genton, & Cornuz, 2020). Therefore, some patients with anosmia and dysgeusia that tested negative for COVID‐19 may be infected with the disease. Further research is required to confidently determine the utility of OTD dysfunction as an effective screening tool for COVID‐19 infection.
3.9. Mechanisms underlying olfactory and gustatory function in COVID‐19 patients
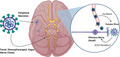
Anosmia associated with COVID‐19 has been well documented by the literature (Beltrán‐Corbellini et al., 2020; Bénézit et al., 2020; Boscolo‐Rizzo et al., 2020; Carignan et al., 2020; Giacomelli et al., 2020; Hopkins, Surda, & Kumar, 2020; Hopkins, Surda, Whitehead, et al., 2020; Iravani et al., 2020; Kaye et al., 2020; Klopfenstein et al., 2020; Kosugi et al., 2020; Lechien, Cabaraux, & Chiesa‐Estomba et al., 2020a, 2020b; Lechien et al., 2020; Levinson et al.,2020;Lovato &Filippis, 2020;Mao et al., 2020;Moein et al., 2020;Spinato et al., 2020; Vaira et al., 2020, 2020; Wee et al., 2020; Whitcroft & Hummel, 2020; Yan et al., 2020) Netland et al. reported that SARS‐CoV can cause neuronal death in mice by invading the brain via the nose, which is close to the olfactory epithelium (Netland, Meyerholz, Moore, Cassell, & Perlman, 2008). Furthermore, the human coronavirus 229E has been isolated in nasal discharge from a patient with postviral olfactory dysfunction (Suzuki et al., 2007). It has been proposed that SARS‐CoV‐2 gains entry into the central nervous systems via several different ways (Ahmed et al., 2020). One of the proposed mechanisms is dissemination and spread from the cribriform plate which is in close contact to the olfactory bulb; this mechanism is postulated to be the underlying cause of olfactory dysfunction in patients (Baig, Khaleeq, Ali, & Syeda, 2020). Furthermore, the general presence of the virus in the circulation can lead to systematic dissemination, allowing the virus to enter the cerebral circulation (Ahmed et al., 2020). The brain has been reported to express ACE2 receptors mainly on glial cells, neurons, and brain vasculature, making these cells susceptible to attacks by the SAR‐CoV‐2 (Ahmed et al., 2020; Turner, Hiscox, & Hooper, 2004). It is well‐established that SARS‐CoV‐2 can exploit the angiotensin‐converting enzyme 2 (ACE2) receptor to gain entry into the cells (Ahmed et al., 2020). Viral interaction with the expression of the ACE2 receptor in neurones can result in significant damage to the neurones without substantial association inflammation previously observed with SARS‐CoV infection (Ahmed et al., 2020; Netland et al., 2008; Wrapp et al., 2020). Therefore, it can be postulated that SARS‐CoV‐2 can cause neuronal damage via the ACE2 receptor leading to OTD (Figure 2) (Ahmed et al., 2020). Binding of the virus to the ACE2 receptors may cause endothelial dysfunction and also lead to serious consequences such as cerebral hemorrhage via unknown mechanisms (Ahmed et al., 2020). Another mechanism by which the neurotropic SARs‐CoV‐2 can disseminate through the central nervous system is by anterograde and retrograde transport with the aid of motor proteins such as kinesins and dynein via sensory and motor nerve endings, the afferent nerve endings from the vagus nerve from the lungs are especially implicated (Ahmed et al., 2020; Li, Bai, & Hashikawa, 2020; Swanson & McGavern, 2015). Through this mechanism, SARS‐CoV‐2 could potentially cause gustatory dysfunction. Besides, other ways in which SARS‐CoV‐2 can cause neurological damage leading to OTD is through a surge of inflammatory cytokines leading to cytokine storm syndrome (Ahmed et al., 2020; Wan, Yi et al., 2020).
Figure 2.
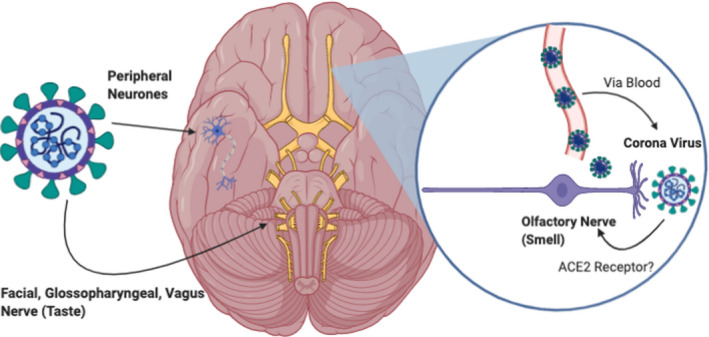
Possible mechanism of interaction between SARs‐CoV‐2 and the cranial nerves. SARS‐CoV‐2 can potentially interact with the cranial nerves (olfactory, vagus, facial, and glossopharyngeal) via the angiotensin‐converting enzyme receptor‐2 (ACE2) leading to olfactory taste dysfunction. SARS‐CoV‐2 can also cause damage to the peripheral nerves leading to several other neurological manifestations. Angiotensin‐converting enzyme 2; ACE2, Original Illustration created using BioRender
COVID‐19 has also been reported to cause other neurological manifestations such as seizures, headache, and dizziness (Figure 3) (Ahmed et al., 2020; Whittaker, Anson, & Harky, 2020). Table 4 shows a summary of neurological manifestations of COVID‐19 reported by literature (Abdelnour, Abdalla, & Babiker, 2020; Alberti et al., 2020; Bernard‐Valnet et al., 2020; Camdessanche et al., 2020; Chen, Zhou, et al., 2020; Chen, Wu, et al., 2020; Coen et al., 2020; Duong, Xu, & Liu, 2020; Guan et al., 2020; Haddadi, Ghasemian, & Shafizad, 2020; Huang et al., 2020; Kaya, Kara, Akinci, & Kocaman, 2020; Klok et al., 2020; Li et al., 2020; Lodigiani et al., 2020; Mao et al., 2020; Moriguchi et al., 2020; Oxley et al., 2020; Padroni et al., 2020; Poyiadji et al., 2020; Sedaghat & Karimi, 2020; Sharifi‐Razavi, Karimi, & Rouhani, 2020; Toscano et al., 2020; Virani et al., 2020; Wan, Xiang et al., 2020; Wan, Cao, Fang, Wang, & Huang, 2020; Wang, Hu et al., 2020; Wang, Yang, Li, Wen, & Zhang, 2020; Yang et al., 2020; Ye, Ren, & Lv, 2020; Zhao, Shen, Zhou, Liu, & Chen, 2020; Zhou et al., 2020). It is unclear whether symptoms of OTD alone can be used to diagnose COVID‐19. However, during this pandemic, COVID‐19 should be considered an important differential diagnosis for patients presenting with OTD dysfunction. Nevertheless, the presence of these symptoms alongside thorough clinical examination, microbiology tests, and the use of diagnostic imaging techniques can help to eliminate differentials and aid the diagnosis of COVID‐19. Chemosensory assessment and treatments used for postviral OTD may also be potentially beneficial in COVID‐19 patients (Whitcroft & Hummel, 2020).
Figure 3.
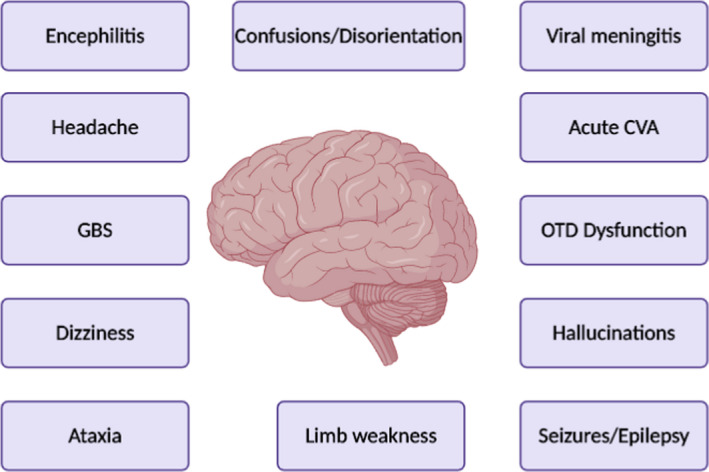
Neurological manifestations of COVID‐19. Guillain–Barré syndrome; GBS, Cerebrovascular Accidents; CVA, Olfactory and taste dysfunction; OTD Original Illustration created using BioRender
Table 4.
Neurological manifestations of COVID‐19
| Author | Type of publication | Number of COVID−19‐Positive Patients | Results |
|---|---|---|---|
| Neuropathy | |||
| Abdelnour et al. (2020) | Case Report | 1 | Bilateral lower limb weakness in 69‐year‐old male |
| Wan et al., 2020 | Case Report | 1 | Bell's palsy in a 65‐year‐old female. |
| Stroke | |||
| Klok et al. (2020) | Retrospective observational study | 184 | 1.6% develop an ischemic or hemorrhagic stroke |
| Lodigiani et al. (2020) | Retrospective observational study | 338 | 2.5% developed an ischemic or hemorrhagic stroke |
| Mao et al. (2020) | Retrospective observational study | 214 | 2.8% developed an ischemic or hemorrhagic stroke |
| Li et al. (2020a) | Retrospective observational study | 219 | 6.0% developed an ischemic or hemorrhagic stroke |
| Sharifi‐Razavi et al. (2020) | Case Report | 1 | Intracerebral hemorrhage in 79‐year‐old male with |
| Haddadi et al. (2020) | Case Report | 1 | Intracerebral hemorrhage in 54‐year‐old female |
| Oxley et al. (2020) | Case Report | 5 | Ischemic stroke in patients younger than 50 years of age |
| Guillain–Barré Syndrome (GBS) | |||
| Alberti et al. (2020) | Case report | 1 | 71‐year‐old male patient presented with symptoms of subacute onset of paresthesia at limb extremities, distal weakness rapidly evolving to severe, flaccid tetra paresis over 3 days consistent with the diagnosis of GBS |
| Toscano et al. (2020) | Case report | 5 | Symptoms of GBS including lower limb weakness and paresthesia in 4 patients and facial diplegia followed by ataxia and paresthesia in 1 patient. Generalized, flaccid tetra paresis/tetraplegia over a period of 36 hr to 4 days in 4 patients. |
| Coen et al. (2020) | Case report | 1 | Male patient in his 70’s presented with paraparesis, distal allodynia, difficulties in voiding and constipation preceding symptoms of myalgia, fatigue, and a dry cough. |
| Camdessanche et al. (2020) | Case report | 1 | Hospitalized 64‐year‐old man COVID−19‐positive patients developed paresthesia in feet and hands and later flaccid severe tetra paresis. Patient was diagnosed with GBS on the basis of neurological examination and investigations. |
| Virani et al. (2020) | Case report | 1 | 54‐year‐old male presented with numbness and weakness and was diagnosed with GBS and COVID−19 positive. |
| Zhao et al. (2020) | Case Report | 1 | 61‐year‐old female presented with weakness in both legs and severe fatigue, progressing over a day, was diagnosed with GBS. Later, she developed respiratory symptoms of COVID−19 |
| Padroni et al. (2020) | Case Report | 1 |
70‐year‐old female was presented to the emergency department complaining of asthenia, hands and feet paresthesia and gait difficulties progressing within 1 day. Diagnosed with GBS, later developed respiratory symptoms of COVID−19 |
| Sedaghat et al. (2020) | Case Report | 1 | 65‐years‐ old male patient presented to emergency department, with symptoms of acute progressive symmetric ascending quadriparesis. 2 weeks prior to onset of neurological symptoms, he was diagnosed with COVID−19. |
| Headache | |||
| Wan et al., 2020 | Case series |
135 |
33% of patients reported a headache |
| Wang et al. (2020) | Case series | 138 | 7% of patients reported a headache |
| Wang et al. (2020) | Retrospective review | 69 | 14% of patients reported a headache |
| Yang et al. (2020) | Retrospective review | 52 | 6% of patients reported a headache |
| Mao et al. (2020) | Retrospective study | 214 | 13% patients reported headache |
| Chen, Wu, et al. (2020) | Retrospective study | 99 | 8% patients reported headache |
| Dizziness | |||
| Mao et al. (2020) | Retrospective review | 214 | 17% of patients reported dizziness |
| Chen, Wu, et al. (2020) | Retrospective review | 113 | 8% of patients reported dizziness |
| Wang et al. (2020) | Retrospective review | 138 | 6.5% of patients reported dizziness |
| Myalgia | |||
| Wang et al. (2020) | Retrospective review | 138 | 34.8% of patients reported myalgia |
| Zhou et al. (2020) | Retrospective review | 191 | 15.2% of patients reported myalgia |
| Chen, Wu, et al. (2020) | Retrospective review | 99 | 11% of patients reported myalgia |
| Huang et al. (2020) | Retrospective review | 41 | 44% of patients reported myalgia or arthralgia |
| Guan et al. (2020) | Retrospective review | 1,099 | 15% of patients reported myalgia or arthralgia |
| Encephalitis | |||
| Ye et al. (2020) | Case report | 1 | Fever, shortness of breath and myalgia with diminished consciousness |
| Bernard‐Valnet et al. (2020) | Case report | 2 | 1 patient developed tonic‐clonic seizures and lumbar puncture consistent with viral encephalitis 1 other patient developed intense headache with confirmed SARS‐CoV−2 swab |
| Poyiadji et al. (2020) | Case report | 1 | 58‐year‐old female presented with symptoms of COVID−19 and altered mental state. |
| Confusion | |||
| Chen, Wu, et al. (2020) | Retrospective review | 99 | 9.10% of patients reported confusions |
| Kaya et al. (2020) | Case report | 1 | Patient reported confusion and visual agnosia |
| Meningitis | |||
| Moriguchi et al. (2020) | Case report | 1 | 24‐year‐old male developed symptoms of meningitis/encephalitis |
| Status epilepticus/Seizures | |||
| Mao et al. (2020) | Retrospective review | 214 | 0.5% of patients presented with seizures |
| Doug et al. (2020) | Case Report | 1 | 41‐year‐old female presented with headache, confusion fever, and new‐onset seizure |
| Moriguchi et al. (2020) | Case Report | 1 | 24‐year‐old male bought to the emergency department due to convulsions with impaired consciousness. The patient had symptoms and imaging was consistent with a diagnosis of meningitis and patient was COVID−19 positive. |
3.10. Limitations
Our review, only focussed on COVID‐19‐positive patients, however, these symptoms may also be present in patients that were COVID‐19‐negative. Therefore, to fully establish the diagnostic value of OTD comparison of symptoms between both COVID‐19‐positive and COVID‐19‐negative patients should be investigated. The risk versus benefit of researching COVID‐19 and OTD also needs to be highlighted. In studies with an objective assessment of OTD, there is possible risk of infection transmission due to physician–patient contact. Although telephone interviews and online surveys can help to minimize this risk, there is a potential bias associated with subjective assessment of symptoms. Another key limitation of our review is that we did not consider the limitations of the method of assessment of OTD in the studies. Studies used either individualized online surveys or specific tests such as CCCRC and SNOT‐22. The differences between the questionnaires and reliability of the objective tests in reporting OTD were not taken into consideration. Currently, in clinical practice, subjective assessment of chemosensory function is relied upon as there is a limited correlation with objective measures. Furthermore, key confounding factors such as underlying respiratory disease or hay fever were not discussed. Moreover, the follow‐up duration of the recovery of patients with OTD is not well documented. Finally, it is possible that some studies would have rushed to publish presenting incomplete data.
4. IMPLICATIONS FOR FURTHER RESEARCH
Currently, the literature on COVID‐19 and OTD is limited and is mainly confined to reports of self‐reported symptoms. Further studies with large cohort sizes and global collaborations with an objective assessment of OTD are required to fully establish the merit of these symptoms in the diagnosis of COVID‐19. OTD can also be present in non‐COVID‐19 patients (Kosugi et al., 2020). Kosugi et al. reported OTD in patients which subsequently tested negative for COVID‐19 (Kosugi et al., 2020). Future studies with larger cohort sizes comparing the difference in duration and prevalence of OTD in COVID‐19‐positive patients compared to non‐COVID‐19 patients would help elucidate the true diagnostic value of these symptoms in COVID‐19. OTD tends to significantly affect patients who are female and from younger age groups; however, the reasons are unknown. Furthermore, there is uncertainty regarding the utility of these symptoms as a predictor of severe COVID‐19 infection. Therefore, further research is required to clarify the association between OTD and the severity of COVID‐19 infection. The pathophysiology of OTD in COVID‐19 remains to be determined.
5. CONCLUSION
Clinical examination of the olfactory nerve is often neglected in routine clinical practice. The COVID‐19 pandemic has highlighted the importance of this much forgotten cranial nerve. COVID‐19 patients frequently report symptoms of anosmia and dysgeusia, and therefore, these symptoms should raise a high index of suspicion for COVID‐19 infection especially in young and female patients. The presence of these symptoms alongside objective clinical assessment would help to make a diagnosis. Further research is warranted as currently both the performance of these symptoms as predictors of COVID‐19 infection and their diagnostic value is uncertain.
DISCLOSURES
All authors have no conflicts of interest to declare.
AUTHOR CONTRIBUTIONS
Syeda Anum Zahra planned and drafted the manuscript, created figures, and is responsible for the final draft of the manuscript. Kiran Pillai and Sashini Iddawela researched and helped with drafting and formatting the final manuscript. Rozina Choudhury helped with drafting and formatting the final manuscript. Amer Harky provided supervision and edited the final manuscript.
Peer Review
The peer review history for this article is available at https://publons.com/publon/10.1002/brb3.1839.
Zahra SA, Iddawela S, Pillai K, Choudhury RY, Harky A. Can symptoms of anosmia and dysgeusia be diagnostic for COVID‐19?. Brain Behav. 2020;10:e01839 10.1002/brb3.1839
Funding
No funding has been obtained for this work.
REFERENCES
- AAO‐HNS: Anosmia, Hyposmia, and Dysgeusia Symptoms of Coronavirus Disease [Internet]. American Academy of Otolaryngology‐Head and Neck Surgery, 2020. Retrieved from https://www.entnet.org/content/aao-hns-anosmia-hyposmia-and-dysgeusia-symptoms-coronavirus-disease [Google Scholar]
- Abdelnour, L. , Abdalla, M. , & Babiker, S. (2020). COVID 19 infection presenting as motor peripheral neuropathy. Journal of the Formosan Medical Association, 119, 1119–1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed, M. , Hanif, M. , Ali, M. , Haider, M. , Kherani, D. , Memon, G. , … Sattar, A. (2020). Neurological manifestations of COVID‐19 (SARS‐CoV‐2): A review. Frontiers in Neurology, 11, 518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alberti, P. , Beretta, S. , Piatti, M. , Karantzoulis, A. , Piatti, M. L. , Santoro, P. , … Ferrarese, C. (2020). Guillain‐Barré syndrome related to COVID‐19 infection. Neurol Neuroimmunol Neuroinflamm., 7(4), e741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baig, A. , Khaleeq, A. , Ali, U. , & Syeda, H. (2020). Evidence of the COVID‐19 virus targeting the CNS: Tissue distribution, host‐virus interaction, and proposed neurotropic mechanisms. ACS Chemical Neuroscience, 11(7), 995–998. [DOI] [PubMed] [Google Scholar]
- Beltrán‐Corbellini, Á. , Chico‐García, J. , Martínez‐Poles, J. , Rodríguez‐Jorge, F. , Natera‐Villalba, E. , Gómez‐Corral, J. , … Alonso‐Cánovas, A. (2020). Acute‐onset smell and taste disorders in the context of COVID‐19: A pilot multicentre polymerase chain reaction based case–control study. European Journal of Neurology, 27(9), 1738–1741. 10.1111/ene.14273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bénézit, F. , Le Turnier, P. , Declerck, C. , Paillé, C. , Revest, M. , Dubée, V. , … RAN COVID Study Group (2020). Utility of hyposmia and hypogeusia for the diagnosis of COVID‐19. The Lancet Infectious Diseases, 20, 1014–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard‐Valnet, R. , Pizzarotti, B. , Anichini, A. , Demars, Y. , Russo, E. , Schmidhauser, M. , … Du Pasquier, R. (2020). Two patients with acute meningo‐encephalitis concomitant to SARS‐CoV‐2 infection. medRxiv. 2020 Apr 21;2020.04.17.20060251. [DOI] [PMC free article] [PubMed]
- Boscolo‐Rizzo, P. , Borsetto, D. , Spinato, G. , Fabbris, C. , Menegaldo, A. , Gaudioso, P. , … Polesel, J. (2020). New onset of loss of smell or taste in household contacts of home‐isolated SARS‐CoV‐2‐positive subjects. European Archives of Oto‐Rhino‐Laryngology, 277, 2637–2640, 10.1007/s00405-020-06066-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camdessanche, J.‐P. , Morel, J. , Pozzetto, B. , Paul, S. , Tholance, Y. , & Botelho‐Nevers, E. (2020). COVID‐19 may induce Guillain‐Barré syndrome. Revue Neurologique, 176(6), 516–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carignan, A. , Valiquette, L. , Grenier, C. , Musonera, J. B. , Nkengurutse, D. , Marcil‐Héguy, A. , … Fortier, P. H. (2020). Anosmia and dysgeusia associated with SARS‐CoV‐2 infection: An age‐matched case‐control study. CMAJ, 192, E702–E707. 10.1503/cmaj.200869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrillo‐Larco, R. , & Altez‐Fernandez, C. (2020). Anosmia and dysgeusia in COVID‐19: A systematic review. Wellcome Open Research, 5, 94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, N. , Zhou, M. , Dong, X. , Qu, J. , Gong, F. , Han, Y. , … Yu, T. (2020). Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: A descriptive study. Lancet, 395(10223), 507–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, T. , Wu, D. , Chen, H. , Yan, W. , Yang, D. , Chen, G. , … Wang, T. (2020). Clinical characteristics of 113 deceased patients with coronavirus disease 2019: Retrospective study. BMJ, 368, m1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, Y. , Liu, Q. , & Guo, D. (2020). Emerging coronaviruses: Genome structure, replication, and pathogenesis. Journal of Medical Virology, 92(4), 418–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coen, M. , Jeanson, G. , Culebras Almeida, L. A. , Hübers, A. , Stierlin, F. , Najjar, I. , … Kronig, I. (2020). Guillain‐Barré syndrome as a complication of SARS‐CoV‐2 infection. Brain, Behavior, and Immunity, 87, 111–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duong, L. , Xu, P. , & Liu, A. (2020). Meningoencephalitis without respiratory failure in a young female patient with COVID‐19 infection in Downtown Los Angeles, early April 2020. Brain, Behavior, and Immunity, 87, 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Entuk.org. 2020. [Internet]. Retrieved from https://www.entuk.org/sites/default/files/files/Loss%20of%20sense%20of%20smell%20as%20marker%20of%20COVID.pdf
- Flahault, A. (2020). Has China faced only a herald wave of SARS‐CoV‐2? The Lancet, 395(10228), 947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giacomelli, A. , Pezzati, L. , Conti, F. , Bernacchia, D. , Siano, M. , & Oreni, L. (2020). Self‐reported olfactory and taste disorders in SARS‐CoV‐2 patients: A cross‐sectional study. Clinical Infectious Diseases, 71, 889–890. 10.1093/cid/ciaa330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan, W. , Ni, Z. , Hu, Y. , Liang, W. , Ou, C. , He, J. , … Du, B. (2020). Clinical characteristics of coronavirus disease 2019 in China. New England Journal of Medicine, 382(18), 1708–1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddadi, K. , Ghasemian, R. , & Shafizad, M. (2020). Basal Ganglia involvement and altered mental status: A unique neurological manifestation of coronavirus disease 2019. Cureus, 12, e7869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins, C. , Gillett, S. , Slack, R. , Lund, V. , & Browne, J. (2009). Psychometric validity of the 22‐item Sinonasal Outcome Test. Clinical Otolaryngology, 34(5), 447–454. [DOI] [PubMed] [Google Scholar]
- Hopkins, C. , Surda, P. , & Kumar, N. (2020). Presentation of new onset anosmia during the COVID‐19 pandemic. Rhinology, 58(3), 295–298. 10.4193/Rhin20.116 [DOI] [PubMed] [Google Scholar]
- Hopkins, C. , Surda, P. , Whitehead, E. , & Kumar, B. (2020). Early recovery following new onset anosmia during the COVID‐19 pandemic – An observational cohort study. Journal of Otolaryngology ‐ Head & Neck Surgery, 49(1), 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, C. , Wang, Y. , Li, X. , Ren, L. , Zhao, J. , Hu, Y. , … Cheng, Z. (2020). Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. The Lancet, 395(10223), 497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hummel, T. , Whitcroft, K. , Andrews, P. , Altundag, A. , Cinghi, C. , Costanzo, R. , … Haehner, A. (2017). Position paper on olfactory dysfunction. Rhinology Journal, 54, 1–30. [DOI] [PubMed] [Google Scholar]
- Important Info On COVID‐19 [Internet]. Mailchi.mp. 2020. Retrieved from https://mailchi.mp/4c241a511c2d/important-info-on-covid-19?e=d610a2d247.
- Iravani, B. , Arshamian, A. , Ravia, A. , Mishor, E. , Snitz, K. , Shushan, S. , … Karagach, S. (2020). Relationship between odor intensity estimates and COVID‐19 prevalence prediction in a Swedish population. Chemical Senses, bjaa034. 10.1093/chemse/bjaa034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang, Y. , Cho, J. , Lee, M. , Kim, Y. , & Park, C. (2020). The diagnostic value of detecting sudden smell loss among asymptomatic COVID‐19 patients in early stage: The possible early sign of COVID‐19. Auris, Nasus, Larynx, 47, 565–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaya, Y. , Kara, S. , Akinci, C. , & Kocaman, A. (2020). Transient cortical blindness in COVID‐19 pneumonia; a PRES‐like syndrome: Case report. Journal of the Neurological Sciences, 413, 116858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaye, R. , Chang, C. W. D. , Kazahaya, K. , Brereton, J. , & Denneny, J. C. (2020). COVID‐19 anosmia reporting tool: Initial findings. Otolaryngology ‐ Head and Neck Surgery, 163, 132–134. 10.1177/0194599820922992 [DOI] [PubMed] [Google Scholar]
- Klok, F. A. , Kruip, M. J. H. A. , van der Meer, N. J. M. , Arbous, M. S. , Gommers, D. A. M. P. J. , Kant, K. M. , … Endeman, H. (2020). Incidence of thrombotic complications in critically ill ICU patients with COVID‐19. Thrombosis Research, 1(191), 145–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klopfenstein, T. , Kadiane‐Oussou, N. J. , Toko, L. , Royer, P. Y. , Lepiller, Q. , Gendrin, V. , Zayet, S. (2020). Features of anosmia in COVID‐19. Médecine Et Maladies Infectieuses, 50, 436–439. 10.1016/j.medmal.2020.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kogan, A. , Mednick, T. , & Dolgovina, M. (2011). Loss of taste as the only cranial nerve finding in Guillain‐Barre syndrome. Journal of Clinical Neuromuscular Disease, 13(1), 56. [DOI] [PubMed] [Google Scholar]
- Kokkinakis, I. , Selby, K. , Favrat, B. , Genton, B. , & Cornuz, J. (2020). Performance du frottis nasopharyngé‐PCR pour le diagnostic du Covid‐19 ‐ Recommandations pratiques sur la base des premières données scientifiques [Covid‐19 diagnosis: Clinical recommendations and performance of nasopharyngeal swab‐PCR]. Revue Médicale Suisse, 16(689), 699–701. [PubMed] [Google Scholar]
- Kosugi, E. , Lavinsky, J. , Romano, F. , Fornazieri, M. , Luz‐Matsumoto, G. , Lessa, M. , … Sant’Anna, G. D. (2020). Incomplete and late recovery of sudden olfactory dysfunction in COVID‐19. Brazilian Journal of Otorhinolaryngology, 86, 490–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lechien, J. R. , Cabaraux, P. , Chiesa‐Estomba, C. M. , … Saussez, S. (2020). Objective olfactory evaluation of self‐reported loss of smell in a case series of 86 COVID‐19 patients. Head and Neck, 42, 1583–1590. 10.1002/hed.26279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lechien, J. R. , Cabaraux, P. , Chiesa‐Estomba, C. M. , … Saussez, S. (2020). Psychophysical olfactory tests and detection of COVID‐19 in patients with sudden onset olfactory dysfunction: A prospective study. Ear, Nose, and Throat Journal, 145561320929169. 10.1177/0145561320929169 [DOI] [PubMed] [Google Scholar]
- Lechien, J. R. , Chiesa‐Estomba, C. M. , De Siati, D. R. , Horoi, M. , Le Bon, S. D. , Rodriguez, A. , … & Chekkoury‐Idrissi, Y. (2020). Olfactory and gustatory dysfunctions as a clinical presentation of mild‐to‐moderate forms of the coronavirus disease (COVID‐19): A multicenter European study. European Archives of Oto‐Rhino‐Laryngology, 277, 2251–2261. 10.1007/s00405-020-05965-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, Y. , Min, P. , Lee, S. , & Kim, S. (2020). Prevalence and duration of acute loss of smell or taste in COVID‐19 patients. Journal of Korean Medical Science, 35(18), e174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levinson, R. , Elbaz, M. , Ben‐Ami, R. , Shasha, D. , Levinson, T. , Choshen, G. , … Paran, Y. (2020). Anosmia and dysgeusia in patients with mild SARS‐CoV‐2 infection. 10.1101/2020.04.11.20055483 [DOI] [PubMed]
- Li, Y. , Bai, W. , & Hashikawa, T. (2020). The neuroinvasive potential of SARS‐CoV2 may play a role in the respiratory failure of COVID‐19 patients. Journal of Medical Virology, 92(6), 552–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, Y. , Li, M. , Wang, M. , Zhou, Y. , Chang, J. , Xian, Y. , … Hu, B. (2020). Acute cerebrovascular disease following COVID‐19: a single center, retrospective, observational study. Stroke Vasc Neurol, 2020 10.1136/svn-2020-000431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodigiani, C. , Iapichino, G. , Carenzo, L. , Cecconi, M. , Ferrazzi, P. , Sebastian, T. , … Sandri, M. T. (2020). Venous and arterial thromboembolic complications in COVID‐19 patients admitted to an academic hospital in Milan, Italy. Thrombosis Research, 1(191), 9–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovato, A. , & de Filippis, C. (2020). Clinical presentation of COVID‐19: A systematic review focusing on upper airway symptoms. Ear, Nose & Throat Journal. 10.1177/0145561320920762. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Mao, L. , Jin, H. , Wang, M. , Hu, Y. , Chen, S. , He, Q. , … Hu, B. (2020). Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurology, 77(6), 683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moein, S. , Hashemian, S. , Mansourafshar, B. , Khorram‐Tousi, A. , Tabarsi, P. , & Doty, R. (2020). Smell dysfunction: A biomarker for COVID‐19. International Forum of Allergy & Rhinology, 10, 944–950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moher, D. , Liberati, A. , Tetzlaff, J. , & Altman, D. (2009). Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. BMJ, 339, b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriguchi, T. , Harii, N. , Goto, J. , Harada, D. , Sugawara, H. , Takamino, J. , … Nakao, A. (2020). A first case of meningitis/encephalitis associated with SARS‐Coronavirus‐2. International Journal of Infectious Diseases: IJID: Official Publication of the International Society for Infectious Diseases., 94, 55–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Netland, J. , Meyerholz, D. , Moore, S. , Cassell, M. , & Perlman, S. (2008). Severe acute respiratory syndrome coronavirus infection causes neuronal death in the absence of encephalitis in mice transgenic for human ACE2. Journal of Virology, 82(15), 7264–7275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oxley, T. , Mocco, J. , Majidi, S. , Kellner, C. , Shoirah, H. , Singh, I. , … Skliut, M. (2020). Large‐vessel stroke as a presenting feature of Covid‐19 in the young. New England Journal of Medicine., 382(20), e60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padroni, M. , Mastrangelo, V. , Asioli, G. , Pavolucci, L. , Abu‐Rumeileh, S. , Piscaglia, M. , … Foschi, M. (2020). Guillain‐Barré syndrome following COVID‐19: New infection, old complication? Journal of Neurology, 267(7), 1877–1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poyiadji, N. , Shahin, G. , Noujaim, D. , Stone, M. , Patel, S. , & Griffith, B. (2020). COVID‐19–associated acute hemorrhagic necrotizing encephalopathy: Imaging features. Radiology, 296(2), E119–E120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren, L. , Wang, Y. , Wu, Z. , Xiang, Z. C. , Guo, L. , Xu, T. , … & Li, H. (2020). Identification of a novel coronaviruscausing severe pneumonia in human: a descriptive study. Chinese Medical Journal, 133, 1015–1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedaghat, Z. , & Karimi, N. (2020). Guillain Barre syndrome associated with COVID‐19 infection: A case report. Journal of Clinical Neuroscience, 76, 233–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharifi‐Razavi, A. , Karimi, N. , & Rouhani, N. (2020). COVID‐19 and intracerebral haemorrhage: Causative or coincidental? New Microbes and New Infections., 35, 100669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spinato, G. , Fabbris, C. , Polesel, J. , Cazzador, D. , Borsetto, D. , Hopkins, C. , & Boscolo‐Rizzo, P. (2020). Alterations in smell or taste in mildly symptomatic outpatients with SARS‐CoV‐2 infection. JAMA, 323(20), 2089–2090. 10.1001/jama.2020.6771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Statement from the UK Chief Medical Officers on an update to coronavirus symptoms: 18 May 2020 [Internet]. GOV.UK. 2020. Retrieved from https://www.gov.uk/government/news/statement-from-the-uk-chief-medical-officers-on-an-update-to-coronavirus-symptoms-18-may-2020.
- Suzuki, M. , Saito, K. , Min, W. , Vladau, C. , Toida, K. , Itoh, H. , … Murakami, S. (2007). Identification of viruses in patients with postviral olfactory dysfunction. The Laryngoscope, 117(2), 272–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson, P. , & McGavern, D. (2015). Viral diseases of the central nervous system. Current Opinion in Virology, 11, 44–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toscano, G. , Palmerini, F. , Ravaglia, S. , Ruiz, L. , Invernizzi, P. , Cuzzoni, M. G. , … Cavallini, A. (2020). Guillain‐Barré syndrome associated with SARS‐CoV‐2. New England Journal of Medicine, 382(26), 2574–2576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner, A. , Hiscox, J. , & Hooper, N. (2004). ACE2: From vasopeptidase to SARS virus receptor. Trends in Pharmacological Sciences, 25(6), 291–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaira, L. A. , Hopkins, C. , Salzano, G. , Petrocelli, M. , Melis, A. , Cucurullo, M. , … Fiore, V. (2020). Olfactory and gustatory function impairment in COVID‐19 patients: Italian objective multicenter‐study. Head and Neck, 42, 1560–1569. 10.1002/hed.26269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaira, L. A. , Salzano, G. , Deiana, G. , & De Riu, G. (2020). Anosmia and ageusia: Common findings in COVID‐19 patients. Laryngoscope, 130, 1787 10.1002/lary.28692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virani, A. , Rabold, E. , Hanson, T. , Haag, A. , Elrufay, R. , & Cheema, T. (2020). Guillain‐Barré Syndrome associated with SARS‐CoV‐2 infection. IDCases, 20, e00771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker, H. K. 1990Cranial nerve I: The olfactory nerve In: Walker H. K., Hall W. D., Hurst J. W. (Eds.) Clinical methods: The history, physical, and laboratory examinations. 3rd edn Boston: Butterworths; Chapter 59. Retrieved from: https://www.ncbi.nlm.nih.gov/books/NBK382/ [PubMed] [Google Scholar]
- Wan, S. , Xiang, Y. , Fang, W. , Zheng, Y. , Li, B. , Hu, Y. , … Huang, X. (2020). Clinical features and treatment of COVID‐19 patients in northeast Chongqing. Journal of Medical Virology., 92(7), 797–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan, S. , Yi, Q. , Fan, S. , Lv, J. , Zhang, X. , Guo, L. , … Qiang, M. (2020). Characteristics of lymphocyte subsets and cytokines in peripheral blood of 123 hospitalized patients with 2019 novel coronavirus pneumonia (NCP). 10.1101/2020.02 [DOI]
- Wan, Y. , Cao, S. , Fang, Q. , Wang, M. , & Huang, Y. (2020). Coronavirus disease 2019 complicated with Bell’s palsy: a case report. 10.21203/rs.3.rs-23216/v1 [DOI]
- Wang, D. , Hu, B. , Hu, C. , Zhu, F. , Liu, X. , & Zhang, J. (2020). Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus‐infected pneumonia in Wuhan, China. JAMA, 323, 1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, Z. , Yang, B. , Li, Q. , Wen, L. , & Zhang, R. (2020). Clinical features of 69 cases with coronavirus disease 2019 in Wuhan, China. Clinical Infectious Diseases, 71, 769–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb, S. , Wallace, V. C. , Martin‐Lopez, D. . & Yogarajah, M. (2020). Guillain‐Barré syndrome following COVID‐19: A newly emerging post‐infectious complication. BMJ Case Reports CP, 13, e236182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wee, L. E. , Chan, Y. F. Z. , Teo, N. W. Y. , Cherng, B. P. Z. , Thien, S. Y. , Wong, H. M. , … Tan, T. T. (2020). The role of self‐reported olfactory and gustatory dysfunction as a screening criterion for suspected COVID‐19. European Archives of Oto‐Rhino‐Laryngology, 277, 2389–2390. 10.1007/s00405-020-05999-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells, G. A. , Shea, B. , O’Connell, D. , Peterson, J. , Welch, V. , Losos, M. & Tugwell, P. J. (2020). The Newcastle‐Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta‐analyses. Retrieved from http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp
- Whitcroft, K. , & Hummel, T. (2020). Olfactory dysfunction in COVID‐19. JAMA, 323(24), 2512. [DOI] [PubMed] [Google Scholar]
- Whittaker, A. , Anson, M. , & Harky, A. (2020). Neurological Manifestations of COVID‐19: A systematic review and current update. Acta Neurologica Scandinavica, 142(1), 14–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organisation . (2020). Coronavirus disease 2019 (COVID‐19) situation report‐209 [Internet]. Retrieved from https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200816-covid-19-sitrep-209.pdf?sfvrsn=5dde1ca2_2
- World Health Organization . (2020). WHO Director‐General's opening remarks at the media briefing on COVID‐19‐11 March 2020. Retrieved from https://www.who.int/dg/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19–-11-march-2020
- Wrapp, D. , Wang, N. , Corbett, K. , Goldsmith, J. , Hsieh, C. , Abiona, O. , … McLellan, J. S. (2020). Cryo‐EM structure of the 2019‐nCoV spike in the prefusion conformation. Science, 367(6483), 1260–1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, Z. , & McGoogan, J. (2020). Characteristics of and important lessons from the coronavirus disease 2019 (COVID‐19) outbreak in China. JAMA, 323(13), 1239. [DOI] [PubMed] [Google Scholar]
- Yan, C. H. , Faraji, F. , Prajapati, D. P. , Ostrander, B. T. , & DeConde, A. S. (2020). Self‐reported olfactory loss associates with outpatient clinical course in Covid‐19. International Forum of Allergy & Rhinology, 10, 821–831. 10.1002/alr.22592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, X. , Yu, Y. , Xu, J. , Shu, H. , Xia, J. , & Liu, H. (2020). Clinical course and outcomes of critically ill patients with SARS‐CoV‐2 pneumonia in Wuhan, China: A single‐centered, retrospective, observational study. Lancet, 8, 475–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye, M. , Ren, Y. , & Lv, T. (2020). Encephalitis as a clinical manifestation of COVID‐19. Brain, Behavior, and Immunity, 1(88), 945–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuki, K. , Fujiogi, M. , & Koutsogiannaki, S. (2020). COVID‐19 pathophysiology: A review. Clinical Immunology, 215, 108427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zayet, S. , Klopfenstein, T. , Mercier, J. , Kadiane‐Oussou, N. , Lan Cheong Wah, L. , Royer, P. , … Gendrin, V. (2020). Contribution of anosmia and dysgeusia for diagnostic of COVID‐19 in outpatients. Infection. 10.1007/s15010-020-01442-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao, H. , Shen, D. , Zhou, H. , Liu, J. , & Chen, S. (2020). Guillain‐Barré syndrome associated with SARS‐CoV‐2 infection: Causality or coincidence? The Lancet Neurology, 19(5), 383–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, F. , Yu, T. , Du, R. , Fan, G. , Liu, Y. , Liu, Z. , …, Guan, L. (2020). Clinical course and risk factors for mortality of adult inpatients with COVID‐19 in Wuhan, China: A retrospective cohort study. Lancet, 395(10229), 1054–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]


