Abstract
Objective
Testosterone has been postulated to be involved in ALS causation.
Materials and methods
CSF levels of free testosterone and dihydrotestosterone were measured in 13 ALS patients [7 males, 6 females] and 22 controls [12 males, 10 females].
Results
CSF free testosterone levels did not show any significant differences but CSF dihydrotestosterone levels were significantly decreased in all male and female ALS patients.
Conclusions
DHT is probably integral to survival of motor neurons. In patients predisposed to develop ALS, there is possibly a sort of “testosterone resistance” at level of blood–brain barrier [BBB] existing right from birth and is likely the result of dysfunctional transport protein involved in testosterone transfer across the BBB. In these patients, lesser amount of testosterone is able to breach the BBB and enter the central neural axis. Lesser amount of testosterone is available to 5 α reductase in the anterior pituitary to be converted to DHT and lesser amount of DHT is generated. There is inadequate negative feedback suppression of LH at the level of anterior pituitary by DHT. As a result of higher LH levels, testosterone levels rise in the peripheral testosterone fraction [the fraction outside the BBB] and this explains the various physical attributes of ALS patients like lower Ratio of the index and ring finger lengths (2D:4D ratio), increased incidence of early onset alopecia etc. This deficiency of DHT leads to motor neuron death causing ALS.
Keywords: Amyotrophic Lateral Sclerosis, blood–brain barrier, Cerebrospinal fluid, dihydrotestosterone, Luteinizing hormone, sex hormone binding globulin
CSF levels of dihydrotestosterone were measured in 13 ALS patients [7 males and 6 females] and in 22 controls [12 males and 10 females], and we found that CSF dihydrotestosterone levels were significantly decreased in all the male and female ALS patients dihydrotestosterone probably plays a key role in ALS pathogenesis.
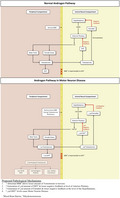
1. INTRODUCTION
Amyotrophic lateral sclerosis is a progressive neurodegenerative disorder characterized by affliction of both the upper and lower motor neurons.(Chio et al., 2009; Cozzolino, Ferri, & Teresa Carrì, 2008; Manjaly et al., 2010; Worms, 2001) It has been postulated that androgens/testosterone may play some role in causation of ALS (Weiner, 1980 ; Wicks, 2012) based on the following observations—Male‐to‐female ratio. There have been frequent reports of preponderance of male to female patients with ALS (Manjaly et al., 2010). Sparing of neurons of cranial nerves III, IV, and VI and Onuf's nucleus in ALS that lack androgen receptors and are not involved even in advanced MND (Weiner, 1980). A type of MND—X‐linked spinobulbar muscular atrophy (Kennedy's disease), results from a trinucleotide repeat expansion in the androgen receptor gene (La Spada, Wilson, Lubahn, Harding, & Fischbeck, 1991). Athletes especially those engaging in contact sports like Soccer/baseball which require a high degree of endurance have an increased susceptibility to ALS. Studies have found that both male and female athletes in aggressive contact sports have higher testosterone levels (Pillay, 2006). Some authors have hypothesized that individuals with high prenatal testosterone exposure self‐select into aggressive sports and occupations which require endurance (Wicks, 2012). Fondell, Fitzgerald, Falcone, O’Reilly, & Ascherio (2013) found an increased risk of ALS in men with early‐onset androgenic alopecia. Gargiulo‐Monachelli (2014) found that postmenopausal female ALS patients had significantly higher serum total testosterone and serum free testosterone concentrations than age‐matched postmenopausal controls. Studies have found a link between head trauma and ALS Chen, Richard, Sandler, Umbach, & Kamel (2007). This can be explained by the simple fact that many of these injuries occurred in those engaging in contact/collision sports and as mentioned in point 4, probably individuals with high testosterone self‐select into aggressive sports/jobs requiring physical exercise. Vivekananda et al. (2011) found that ALS patients had lower Ratio of the index and ring finger lengths (2D:4D ratio) in comparison with controls. 2D:4D ratio is dependent on prenatal testosterone levels. A study (Militello et al., 2002) of male and female patients of ALS found that serum free testosterone levels were significantly lower in both male and female ALS patients.
However, there also have been observations which did not directly implicate testosterone Bruson et al. (2012) analyzed Androgen Receptor CAG expansions in 336 patients with ALS and 100 controls and found no significant difference between the 2 groups. Jones, Riley, & Antel (1982) treated male ALS patients with high dose testosterone and found that exogenous high dose testosterone therapy caused a predictable decrease in basal LH and FSH levels and expected dampening of LH and FSH response to GnRH stimulation.
In our study, we measured concentrations of testosterone and its principal metabolite dihydrotestosterone in cerebrospinal fluid (CSF) of ALS patients and compared it with those of normal controls.
2. METHODS
CSF levels of free testosterone and dihydrotestosterone were measured in 13 ALS patients [ 7 males and 6 females] and in 22 controls [12 males and 10 females]. All CSF samples were collected in the morning between 9:00 a.m. and 11:00 a.m. as some studies (Goodman, Hotchkiss, Karsch, & Knobil, 1974) have shown that testosterone concentrations vary during the day. Due clearances from Institutional Research and Ethical Committees was obtained. Written informed consent was taken from patients and controls for CSF and clinical data collection.
Inclusion criteria used for patient selection:
a) Patients fulfilling the diagnosis of clinically definite ALS and clinically probable ALS as per the El Escorial Criteria (EEC) (Brooks, Miller, Swash, & Munsat, 2000).
Inclusion criteria used for control selection:
a) Controls were enrolled from surgery, gynecology and obstetrics and from orthopedics wards. CSF was obtained from male and female controls undergoing lumbar puncture for spinal anesthesia for surgery.
Exclusion criteria used for both patient and control selection:
(a) Subjects not giving consent for study. (b) Subjects suffering from cryptorchidism, testicular malignancy or any other testicular pathology. (c) Subjects taking anabolic hormones. (d) Subjects suffering from pituitary or adrenal disease. (e) Female subjects with history of PCOD or history of hirsuitism. (f) Subjects with family history of ALS or any form of motor neuron disease or frontotemporal dementia [FTD].
CSF concentrations of free testosterone and dihydrotestosterone were measured using solid phase enzyme‐linked immunosorbent assay (ELISA) based kits sourced from IBL International.
3. RESULTS
Clinical and demographic data and CSF concentrations of free testosterone and dihydrotestosterone in ALS patients and controls are summarized in Tables 1, 2, 3, 4, 5 and Figure 1.
Table 1.
Age distribution of ALS patients and controls
| ALS patients | Controls | ||
|---|---|---|---|
| Mean Age and range(years) | Mean Age and range(years) | ||
| Males | 51.57 (30–60) | Males | 44.75 (19–62) |
| Females | 59.33 (50–68) | Females | 46.50 (25–71) |
Table 2.
Clinical details of Male ALS patients
|
Age (years) |
Clinical Symptoms | Comorbidities, Drugs being taken | Symptom duration at presentation to our institute | CSF | MRI Brain and Cervical spine |
|---|---|---|---|---|---|
| 58 |
right upper limb weakness f/b left UL weakness f/b right LL weakness f/b bulbar symptoms |
Bronchial Asthma. Salbutamol inhaler, Riluzole |
1 years 3 months |
P−44 G−36 Cells‐ 2 L |
Normal |
| 30 |
right upper limb weakness f/b right LL weakness f/b left LL weakness, no bulbar symptoms. Tongue fasciculations present. |
None Riluzole |
2 years 5 months |
P−40 G−56 Cells‐Acellular |
Mild Fronto‐parietal atrophy |
| 45 |
left UL weakness with atrophy f/b right UL weakness |
COPD, alcoholism,old treated PTB 6 years back. Riluzole |
1 year |
P−32.6 G−57 Cells−0 |
Frontal atrophy |
| 54 |
Right lower limb weakness f/b left LL weakness f/b right UL weakness for 1 ½ year with bulbar symptoms for 4 months. |
HTN,DCM Beta blockers, ACE inhibitors,Diuretics, Riluzole, Edaravone |
1 ½ year. |
P−48 G−51 Cells−0 |
White matter periventricular hyperintensities. |
| 60 |
florid fasciculations f/b right UL wasting f/b left UL wasting f/b left LL wasting. |
Riluzole | 8 months |
P−32 G−44 Cells−3L |
Frontal atrophy |
| 59 |
bulbar onset weakness f/b UL weakness f/b LL weakness |
HTN Diuertics, Riluzole |
1 ½ year |
P−26.2 G−51 Cells−0 |
normal |
| 55 | fasciculations over proximal arms, shoulders f/b dysarthria for 3 months, right UL weakness for 3 months, left UL weakness for 2 months. | none | 6 months |
P−28.9 G−62 Cells‐Acellular |
normal |
P‐Total CSF protein, G‐Glucose, L‐lymphocytes.
Table 3.
Clinical details of Male Controls
|
Age (years) |
Surgical Procedure Undergone | Comorbidities |
|---|---|---|
| 50 | Stapler hemorroidectomy for hemorrhoids | COPD, reformed Alcoholic. |
| 61 | Ureteric stricture reconstructive surgery | HTN x 5 years |
| 60 | Right tibia fracture | — |
| 24 | Right ACL tear arthroscopic surgery | — |
| 26 | Fistula‐in‐ano surgery | — |
| 19 | ACL tear arthroscopic surgery plus bucket handle tear of medial meniscus | — |
| 22 | Right ACL tear arthroscopic surgery | — |
| 35 | Fournier Gangrene | None |
| 60 | TURBT – Transurethral resection of bladder tumor | — |
| 60 | Cholecystectomy for Cholelithiasis | Hyperlipidemia with HTN |
| 62 | Ureteroscopy for urolithiasis | — |
| 58 | Exploratory laprotomy | HTN |
Table 4.
Clinical details of female ALS patients
|
Age (years) |
Clinical Symptoms | Comorbidities,Drugs being taken | Symptom duration at presentation to our institute | CSF | MRI Brain and Cervical spine |
|---|---|---|---|---|---|
| 50 | Proximal weakness in right lower limb for 11 months f/b dysarthria and left lower limb weakness for 7 months. | Riluzole | 11 months |
P−46 G−68 Cells −0 |
T2/FLAIR HI post limb IC with decreased fractional isotropy on DTI in B/L CST. |
| 68 | Distal weakness in B/L upper limb for 1 ½ year f/b neck drop for 6 months f/b bulbar symptoms for 4 months. |
Hypothyroidism for 30 years. Thyroxine, Riluzole |
1 ½ year |
P−32 G−57 Cells−2L |
normal |
| 65 | Left UL weakness for 6 months f/b left LL weakness for 4 months | Riluzole | 6 months |
P−30 G−64 Cells −0 |
Mild frontal atrophy |
| 60 | Slurring of speech for 6 months with neurogenic dysphagia. |
HTN. ACE inhibitors,Riluzole |
6 months |
P−42 G−62 Cells−4L |
white matter arteriosclerotic Hyperintensities. |
| 62 | B/L proximal lower limb weakness with wasting for 1 year f/b distal lower limb weakness for 7 months f/b development of florid fasciculations and bulbar symptoms for 4 months. |
B/L mal rotated kidney on abdominal imaging. Riluzole |
8 months |
P−34 G−51 Cells−0 |
Fronto‐parietal atrophy |
| 51 | Difficulty in speaking with nasal regurgitation of liquids for 5 months, no limb weakness. |
DM. MetforminglimepirideRiluzole. |
5 months |
P−44 G−60 Acellular |
Normal |
P‐Total CSF protein, G‐Glucose, L‐lymphocytes
Table 5.
Clinical details of female controls
|
Age (years) |
Surgical Procedure | Comorbidities |
|---|---|---|
| 56 | Left Acetabular Fx Surgery | |
| 40 | Recto‐vaginal fistula repair | |
| 71 | Right total hip replacement. | HTN |
| 45 | Left ACL tear repair. | |
| 35 | Tubal Surgery | VSD |
| 57 | Exploratory Laprotomy | |
| 35 | Uterine surgery | Hypothyroidism |
| 36 | Uterine surgery | DM with Hypothyroidism |
| 25 | Arthroscopic right lateral parameniscal cyst excision | |
| 65 | Vaginal hysterectomy |
Figure 1.
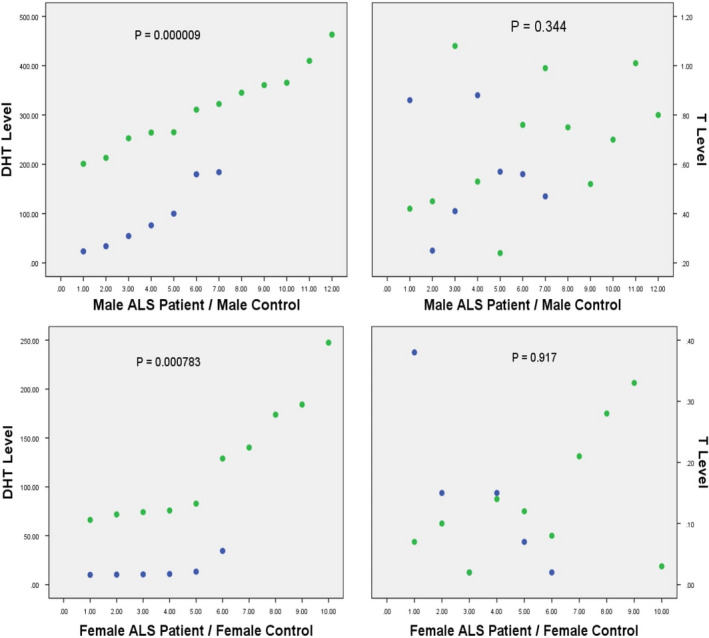
CSF Dihydrotestosterone and CSF Testosterone concentrations in male and female ALS patients and controls compared. DHT‐Dihydrotestosterone, T‐ Testosterone, ALS – Amyotrophic lateral sclerosis. Blue dots indicate male and female ALS patients, green dots indicate male and female controls. Plotted on Y axis are levels of Dihydrotestosterone and Testosterone, on X‐ axis are plotted the study groups‐ ALS patients and controls
The data sets were found to have a normal distribution using Kolmogorov–Smirnov [K‐S] goodness‐of‐fit test. Thus, we applied students t test to evaluate whether there was any significant difference between the 2 groups.
Results are summarized as
CSF testosterone values in Male ALS patients and controls ‐No significant difference [p = .344].
CSF dihydrotestosterone values in Male ALS patients and controls—Significant difference was found with values significantly less in ALS patients.[p = .000009]. CSF testosterone values in female ALS patients and controls‐No significant difference [p = .917]. CSF dihydrotestosterone values in female ALS patients and controls—significant difference was found with values significantly less in ALS patients [p = .000783].
Thus, we see that CSF free testosterone levels did not show any significant differences in both male and female ALS patients as compared to those of male and female controls. However, CSF dihydrotestosterone levels were significantly decreased both in male and female ALS patients as compared with those of male and female controls.
4. DISCUSSION
In our study, we have demonstrated significantly decreased CSF dihydrotestosterone levels in both male and female ALS patients. More importantly, all ALS patients studied had decreased CSF dihydrotestosterone pointing to this being an important contributing factor to ALS pathogenesis.
There have been few studies done on CSF penetration and CSF metabolism of testosterone and dihydrotestosterone and how testosterone and its metabolites exert a negative influence on LH release. These studies have demonstrated the following observations.
Radioactively labeled dihydrotestosterone was given intravenously to six adult male rhesus monkeys (Macaca mulata), and it was found that that almost no radioactively labeled DHT could be found in CSF postinjection thus demonstrating that dihydrotestosterone has minimal penetration across the blood–brain barrier [BBB] (Marynick, Havens, Ebert, & Loriaux, 1976). A study (Abbott, Batty, Dubey, Herbert, & Shiers, 1985) on castrated male monkeys found that even in presence of very high, supraphysiological serum levels of DHT, CSF concentrations of DHT stayed very low. Also, there was no correlation between levels of unbound fraction of DHT in serum and DHT in CSF despite the fact that in CSF, the entire DHT fraction is unbound. The authors hypothesized that some special mechanism or carrier protein limits the influx of DHT in CSF from serum even in presence of very high supraphysiological DHT serum concentrations (Abbott et al., 1985). In another study (Schaison, Renoir, Lagoguey, & Mowszowicz, 1980), it was found that administration of DHT was unable to suppress LH in either normal men or agonadal patients. DHT was administered through the percutaneous route as a hydrosoluble gel for 3 months to all subjects and plasma DHT levels 8–10 times the normal plasma range were reached and maintained without any reduction in circulating LH levels. This study demonstrated that DHT is unable to enter the CSF compartment despite the presence of sustained high, supraphysiological serum levels. The above findings are in contrast to CSF penetration dynamics of testosterone. Studies (Backstrom, Carstensen, & Sodergard, 1976; Dubey, Herbert, Abbott, & Martensz, 1984) have shown that CSF levels of testosterone are equal to those of unbound serum testosterone levels with the entire CSF testosterone being unbound and that there is a predictable relationship between serum total testosterone levels, serum unbound testosterone levels and testosterone levels in the CSF. Also, it is known that testosterone crosses the BBB (blood brain barrier) only in the unbound state. These studies also found that serum testosterone concentrations show a pronounced diurnal variation and that in castrated animals, concentrations similar to the high early morning serum concentrations in the precastrated state could only be achieved with much higher doses of testosterone. The study (Dubey et al., 1984) also found that a sharp increase in serum total testosterone, unbound serum testosterone and CSF testosterone coincided with an abrupt fall in serum LH (Luteinising hormone) levels. Abbott et al. (1985) demonstrated in their study that DHT can suppress serum LH levels and also concurred with a previous study by Sholl, Goy, & Uno (1982) that a majority of DHT within the brain comes from the precursor testosterone. A study (Martini, Celotti, & Melcangi, 1996) using fetal and neonatal rat brain cells found that formation of DHT takes place preferentially in the neurons in the nervous system although type‐2 astrocytes and oligodendrocytes also possess some 5 alpha‐reductase activity. Other studies (Celotti, Melcangi, Negri‐Cesi, & Poletti, 1991) have also echoed similar findings. Pardridge, Moeller, Mietus, & Oldendorf (1980) in their study found that DHT is sequestered to a greater degree in mammalian brains than is testosterone. Moreover, it is a known fact that DHT is a much more potent hormone than testosterone because its binding affinity to the androgen receptor is two times that of testosterone and it has a dissociation rate about a fifth of that of testosterone (Marchetti & Barth, 2013). Sholl, Robinson, & Goy (1975) from their study concluded that DHT is the primary androgen for activation of neural mediated effects, at least in the guinea pig. In a study done by Zoppi et al. (1988) on rats, it was found that when testosterone was given along with an inhibitor of 5 alpha‐reductase thereby leading to lesser conversion of testosterone to DHT, LH levels were reduced to a lesser extent than they were when testosterone was given alone. Also, it has been found that patients with 5 alpha‐reductase type 2 deficiency have high circulating LH levels despite having normal or elevated serum levels of testosterone. However, these patients have reduced serum DHT levels (Martini, Celotti, & Serio, 1979). Various other analyses (Zanisi, Motta, & Martini, 1973) have also shown that DHT and its metabolite, 3‐alpha diol are much more effective that testosterone in suppressing LH secretion. A study evaluating effects of testosterone,DHT and its metabolites in cultured pituitary cells (Denef, 1983) provided further evidence for a specific physiological role of 5‐DHT and 3 ‐alpha diol. This study concluded that at least at the gonadotroph cell level, DHT and possibly 3‐alpha diol are the active androgens which depress LH release. Another study (Kennedy, Rawlings, & Cook, 1985) done on bull calves also found that LH serum concentrations were suppressed by administration of sialistic implants releasing DHT and also by 3‐alpha diol implants. Studies (Martini, 1982) have found that the anterior pituitary very efficiently metabolises testosterone to DHT and 3α‐diol. Yields of DHT generated are second only to yields seen in the prostate gland. Also, in the anterior pituitary, around 25% of testosterone is converted into 3‐alpha diol while in the prostrate, only 7.6% of testosterone is converted into 3‐alpha diol. It has also been observed that the anterior pituitary of adult mammals does not have significant aromatizing ability while the hypothalamus has potent aromatizing abilities. Studies (Chimento, Sirianni, Casaburi, & Pezzi, 2014) suggest that estradiol derived from intracerebral testosterone is the main hormone that provides negative feedback at the hypothalamic level while at the level of the anterior pituitary, both DHT and estradiol, both derived from intracerebral testosterone, are required for negative LH feedback. Numerous animal studies (Abdelgadir et al., 1994; Scott, Kuehl, Ferreira, & Jackson, 1997) also support that estradiol derived from intracerebral testosterone is the main hormone that provides negative feedback at the hypothalamic level while at the anterior pituitary level, DHT also plays a very important role in suppressing LH. Interventions like reducing DHT levels with either a reductase inhibitor or by using antibodies to estradiol or through administration of aromatase inhibitors—all these maneuvers compromise the ability of T to suppress LH.
Interestingly, the anterior pituitary and the hypothalamic/preoptic area possess 5 α reductase type 2 isoenzyme whereas the glial cells and the neurons predominantly express the 5 α reductase type 1 isoenzyme. It was also found that in the rat pituitary, 5 α reductase type 2 isoenzyme is located mainly in the gonadotropes. Various authors have concluded that type 1 5 α reductase is constitutively expressed in the mammalian brain and appears to have a neuroprotective role and mutations in type 1 5 α reductase lead to death of the organism in gestation itself (Mahendroo, Cala, Landrum, & Russell, 1997; Poletti et al., 1998). As mentioned above, patients with 5 alpha‐reductase type 2 deficiency have high circulating LH levels, normal or elevated serum levels of testosterone and reduced serum DHT levels. However, these patients do not have an increased risk of ALS. Total testosterone and free non‐SHBG bound testosterone have been found to have a diurnal circadian variation with highest levels being seen in the morning and lowest in the evening (Bremner, Vitiello, & Prinz, 1983; Plymate, Tenover, & Bremner, 1989; Tenover, Matsumoto, Clifton, & Bremner, 1988). A study (Bremner et al., 1983) documented that elderly males have reduced testosterone levels as compared to younger males but more importantly, there was a greater, more pronounced difference between the circadian excursion of total serum testosterone levels between the two groups. Plymate et al. (1989) found that elderly males have a reduced significant circadian rhythm in free non‐SHBG bound testosterone as compared to younger males [60% versus 100%]. This study further found that even in 60% of older males showing circadian variation in free non‐SHBG bound testosterone, the circadian excursion was only 26% of what was seen in the younger males. Tenover et al. (1988) found that the circadian pattern of pulsatile LH secretion was blunted in healthy, elderly males as compared to young males. Studies (Gapstur et al., 2002; Leifke, Gorenoi, Wichers, Von Zur, & Brabant, 2000) have consistently shown that both total testosterone and free testosterone concentrations decrease with ageing while SHBG levels increase with ageing. Studies (Laaksonen et al., 2004) have shown that obesity, insulin resistance, metabolic syndrome and dyslipidemia have a strong association with low serum levels of total testosterone, free testosterone and sex hormone binding globulin (SHBG). In addition, it was found (Laaksonen et al., 2004) that the association between low levels of free testosterone and diabetes was abolished and that between low levels of total testosterone and diabetes was attenuated after adjusting for BMI (Body Mass Index) while in case of SHBG, the association was unaffected. This study suggested that SHBG levels plays a more important role in the development of insulin resistance/metabolic syndrome than total or free testosterone does. Many studies (Scarmeas, Shih, Stern, Ottman, & Rowland, 2002) have suggested that ALS patients have had a lower premorbid body mass index as compared to controls and have been leaner, fitter in their life as compared to controls. In other words, being obese or having a higher BMI is protective against development of ALS in later life. Studies (Reich‐Slotky et al., 2013) have found that having a higher premorbid BMI predicts a better result on the ALS Functional Rating Scale (ALSFRS‐R) meaning that overweight/obese persons even after developing symptoms and signs of ALS fare better than their leaner counterparts ALS patients having higher subcutaneous fat had increased duration of survival after developing the disease. There is emerging evidence that high carbohydrate/high fat hypercaloric diets may improve survival in ALS patients. In a clinical trial (Wills, Hubbard, & Macklin, 2014) on 24 ALS patients utilizing hypercaloric high carbohydrate diet, it was found that the high carbohydrate diet group had a longer survival than the placebo group (0% death versus 43% death, respectively, after five months of follow‐up). Studies in animal models of ALS have demonstrated improved survival with high fat high calorie diets. Breedlove & Arthur (1983) in their study on motor neurons found that following systemic administration of radioactive androgens, DHT accumulated to a greater degree in the spinal motor neuron nuclei than testosterone and unlike testosterone, DHT concentrations in spinal motor neurons did not show any sex difference. This shows that DHT is an essential, integral component of sex steroid machinery in the motor neurons regardless of sex of the organism.It was earlier widely believed that the hypothalamic–pituitary–gonadal axis in humans remains quiescent after birth for around 10 years until the onset of pubertal activation and that LH and FSH night‐day rhythms begin just before the onset of puberty, triggering its onset. This erroneous assumption probably stemmed from the higher detection limits of the hormonal assays used in the previous studies (Mitamura et al., 1999). However, studies using ultra‐sensitive assay methods have shown that circulating gonadotrophin concentrations and diurnal Rhythms of Luteinizing Hormone, Follicle‐Stimulating Hormone, Testosterone, and Estradiol Secretion exist and are well established in prepubertal children as young as 4.6 years One is justified in making an assumption based on these findings that probably biorhythms of these hormones exist in younger children too. Studies on animal models of ALS (McLeod et al., 2019; Yoo & Ko, 2012) have also shown androgens deficiency/receptor antagonism may contribute to ALS.
Based on data from the above studies, we postulate the following hypothesis‐DHT or one of its metabolites is probably integral to survival of motor neurons and in ALS, it is lack of DHT in the motor neurons which leads to their death. CSF levels of DHT are probably the final arbiter of LH release at the level of anterior pituitary. Entire DHT fraction in the central neural axis is derived from the testosterone fraction which penetrates the BBB since DHT itself cannot penetrate BBB. Dubey et al. (1984) had found that a sharp increase in serum total testosterone, unbound serum testosterone and CSF testosterone had coincided with an abrupt fall in serum LH (Luteinising hormone) levels. We postulate that when high testosterone concentrations are reached and they breach a certain “critical” threshold, the binding capacity of SHBG (Sex hormone binding globulin) is exceeded and only then does the levels of free, unbound serum testosterone rise sufficiently to cause the rise in CSF testosterone [ in CSF, all of testosterone is free and unbound]. This CSF testosterone is converted to dihydrotestosterone and the resultant increase in dihydrotestosterone suppresses LH levels by exerting a negative feedback on LH release. We postulate that in patients who are predisposed to develop ALS, there is a sort of “testosterone resistance” at the level of BBB. This “testosterone resistance” exists right from birth. This “testosterone resistance” is likely to be the result of a faulty, mutated transport protein involved in testosterone transfer across the BBB. In these patients, lesser amount of testosterone is able to breach the BBB and enter the central neural axis. As a result, lesser amount of testosterone is available to 5 α reductase type 2 isoenzyme in the anterior pituitary to be converted to DHT and lesser amount of DHT is generated. As a result, there is inadequate negative feedback suppression of LH at the level of anterior pituitary by DHT or its metabolites like 3‐alpha diol. As a result of higher LH levels, testosterone levels rise in the peripheral testosterone fraction [the fraction outside the BBB] and this explains the various physical attributes of ALS patients like the lower Ratio of the index and ring finger lengths (2D:4D ratio), increased incidence of early‐onset androgenic alopecia, the increased athleticism in the premorbid years, the lower BMI in the premorbid years (the “always been lean”) phenomenon etc. It is also possible that the higher peripheral concentrations of testosterone in ALS patients are a sort of compensatory mechanism of the human body to protect the motor neurons against DHT deprivation. Higher concentrations of testosterone in the peripheral pool would translate in higher amounts of testosterone being available at the “testosterone resistant” BBB as compared to normal individuals with normal BBB and even with less than normal levels entering the central neural axis as compared to normal individuals with normal BBB [ Although this would exactly depend on the morning peak testosterone levels], these high peripheral free testosterone levels would in some degree compensate for the “testosterone resistance” at the level of BBB and maintain the DHT levels in the central neural axis and motor neurons essential to their survival. As long as these compensatory mechanisms worked, those with the BBB “testosterone resistance” would not develop signs of ALS. However, when these compensatory mechanisms would start failing and the high peripheral pool testosterone concentrations would start decreasing, the amounts of testosterone traversing the “testosterone resistant” BBB would decrease, the free testosterone entering the central neural axis would decrease, and as a result, the concentration of DHT in central neural axis and in the motor neurons would fall leading to their death and ALS would then set in. This fail in the compensatory mechanisms can be because of many reasons. For example, with advancing age, the circadian excursion in free non‐SHBG bound testosterone would decrease leading to lesser free testosterone being available at the BBB for intracerebral transport and this in a person having BBB “testosterone resistance” would translate in ALS initiation as in their case, the amount of testosterone crossing the BBB in proportion to their highest morning testosterone levels would be already low as compared to normal persons and any decrease in free testosterone levels in the central neural axis would probably also make the DHT levels in the central neural axis go below the critical threshold limit.Probably this is the reason ALS incidence increases with increasing age. We also hypothesise that persons developing ALS at younger age have a greater degree of “testosterone resistance” at the BBB and in this subset, the compensatory mechanisms fail sooner as compared to those developing ALS at a later age who would probably have a lesser degree of “testosterone resistance” at the BBB. Some evidence for the above proposed theory already exists in observations of Gargiulo‐Monachelli (2014) who found higher total and free testosterone levels in ALS patients who had a more rapid decline in their clinical status. Probably these patients had higher “testosterone resistance” at the BBB, lower DHT levels in anterior pituitary, higher LH release and resultant higher total and free testosterone levels. Our hypothesis would also justify some studies which have proposed that high carbohydrate/high fat hypercaloric diets improve survival in ALS patients. Probably these dietary interventions lead to lowering of serum SHBG levels. This in turn increases serum free testosterone levels and more free testosterone was available at the “testosterone resistant” BBB leading to higher DHT levels in the central neural axis and improved motor neuron survival. The same explanation suffices for the fact that overweight/obese persons even after developing symptoms and signs of ALS fare better than their leaner counterparts owing to their lower SHBG levels and higher free testosterone levels. We have proposed that reduced levels of DHT or its metabolites secondary to a dysfunctional BBB which has “testosterone resistance” is the primary cause of ALS. Reduced levels of DHT can be either because of substrate deficiency, enzyme deficiency or dysfunction and receptor dysfunction. However, we have kept substrate deficiency, that is decreased amount of free testosterone in the central neural axial compartment as the first probability owing to these reasons. The substrate deficiency in ALS is age dependent and is dependent on the degree of “testosterone resistance” of the faulty BBB. Till the high peripheral testosterone levels are maintained, substrate deficiency does not set in but as soon as the peripheral testosterone levels fall, there ensues deficiency of free testosterone in the central neural axis for conversion to DHT. Both 5 α reductase isoenzymes are apparently normal in ALS patients. In addition to reasons already mentioned, it is unlikely that enzyme dysfunction is at fault because of usual late age of onset of ALS and that ALS symptoms stay confined to the neural axis while the enzymes are widespread across many systems of the human body. For the same reasons, receptor dysfunction is unlikely to be the culprit. In addition, a study (Bruson et al., 2012) found normal androgen receptor function in ALS. More studies evaluating these parameters and also concurrent serum testosterone, serum dihydrotestosterone and LH levels would shed more light on ALS pathogenesis.
Conclusions—Our study implicates dihydrotestosterone deficiency in the neural axis as an important component of ALS pathogenesis. More studies evaluating these parameters and also concurrent evaluation of serum testosterone, serum dihydrotestosterone and LH levels would shed more light on ALS pathogenesis. Long‐term serial studies on asymptomatic familial ALS gene carriers would be invaluable in delineating the pathogenesis of this dreaded disease.
CONFLICTS OF INTEREST
None.
AUTHORS CONTRIBUTION
Dr Nishit Sawal: Conceived the entire idea , recruited all patients , wrote the manuscript and uploaded it. Dr Jasbinder Kaur: Did the CSF ELISA testosterone and dihydrotestosterone evaluation of all patients and controls and co‐authored the manuscript. Kamaljeet Kaur: Did the CSF ELISA testosterone and dihydrotestosterone evaluation of all patients and controls and co‐authored the methods portion of the manuscript. Dr Satinder Gombar: Recruited Controls for the study. There is no conflict of interest for any of the authors. The authors take full responsibility for the data, the analyses and interpretation, and the conduct of the research; full access to all of the data; and the right to publish any and all data.
Supporting information
Appendix S1
ACKNOWLEDGEMENTS
Prof. Narinder Kumar, Professor and Manish Goyal , Research Fellow, Department of Statistics, Panjab University ,Chandigarh for performing the statistical analysis in the study.
Sawal N, Kaur J, Kaur K, Gombar S. Dihydrotestosterone in Amyotrophic lateral sclerosis—The missing link?. Brain Behav. 2020;10:e01645 10.1002/brb3.1645
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
REFERENCES
- Abbott, D. A. , Batty, K. A. , Dubey, A. K. , Herbert, J. , & Shiers, H. M. (1985). The passage of 5a‐dihydrotestosterone from serum into cerebrospinal fluid and LH negative feedback in castrated rhesus monkeys. Journal of Endocrinology, 104, 325–330. [DOI] [PubMed] [Google Scholar]
- Abdelgadir, S. E. , Resko, J. A. , Ojeda, S. R. , Lephart, E. D. , McPhaul, M. J. , & Roselli, C. E. (1994). Androgens regulate aromatase cytochrome P450 messenger ribonucleic acid in rat brain. Endocrinology, 135, 395–401. 10.1210/endo.135.1.8013375 [DOI] [PubMed] [Google Scholar]
- Backstrom, T. , Carstensen, H. , & Sodergard, R. (1976). Concentration of estradiol, testosterone and progesterone in cerebrospinal fluid compared to plasma unbound and total concentrations. Journal of Steroid Biochemistry, 7, 469–472. 10.1016/0022-4731(76)90114-X [DOI] [PubMed] [Google Scholar]
- Breedlove, S. M. , & Arthur, A. P. (1983). Differences in the pattern of steroid accumulation by motoneurons of the rat lumbar spinal cord. Journal of Comparative Neurology, 215, 211‐216. [DOI] [PubMed] [Google Scholar]
- Bremner, W. J. , Vitiello, M. V. , & Prinz, P. N. (1983). Loss of circadian rhythmicity in blood testosterone levels with aging in normal men. Journal of Clinical Endocrinology and Metabolism, 56, 1278–1281. 10.1210/jcem-56-6-1278 [DOI] [PubMed] [Google Scholar]
- Brooks, B. R. , Miller, R. G. , Swash, M. , Munsat, T. L. . (2000). El Escorial revisited: Revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotrophic Lateral Sclerosis and Other Motor Neuron Disorders, 1, 293–299. 10.1080/146608200300079536 [DOI] [PubMed] [Google Scholar]
- Bruson, A. , Sambataro, F. , Querin, G. , D’Ascenzo, C. , Palmieri, A. , Agostini, J. , … Soraru, G. (2012). CAG repeat length in androgen receptor gene is not associated with amyotrophic lateral sclerosis. European Journal of Neurology, 19(10), 1373–1375. 10.1111/j.1468-1331.2011.03646.x [DOI] [PubMed] [Google Scholar]
- Celotti, F. , Melcangi, R. C. , Negri‐Cesi, P. , & Poletti, A. (1991). Testosterone metabolism in brain cells and membranes. . The Journal of Steroid Biochemistry and Molecular Biology, 40(4–6), 673–678. 10.1016/0960-0760(91)90289-H [DOI] [PubMed] [Google Scholar]
- Chen, H. , Richard, M. , Sandler, D. P. , Umbach, D. M. , & Kamel, F. (2007). Head injury and amyotrophic lateral sclerosis. American Journal of Epidemiology, 166(7), 810–816. 10.1093/aje/kwm153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chimento, A. , Sirianni, R. , Casaburi, I. , & Pezzi, V. (2014). Role of estrogen receptors and G protein‐coupled estrogen receptor in regulation of hypothalamus–pituitary–testis axis and spermatogenesis. Frontiers in Endocrinology, 5, 1 10.3389/fendo.2014.00001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chio, A. , Logroscino, G. , Hardiman, O. , Swingler, R. , Mitchell, D. , Beghi, E. , & Traynor, B. G. (2009). Prognostic factors in ALS: A critical review. Amyotrophic Lateral Sclerosis, 10, 310–323. 10.3109/17482960802566824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cozzolino, M. , Ferri, A. , & Teresa Carrì, M. (2008). Amyotrophic lateral sclerosis: From current developments in the laboratory to clinical implications. Antioxidants & Redox Signaling, 10(3), 405–444. 10.1089/ars.2007.1760 [DOI] [PubMed] [Google Scholar]
- Denef, C. (1983). 5 alpha ‐Dihydrotestosterone formation and its functional significance in rat anterior pituitary, subpopulations of gonadotrophs and cell cultures. Journal of Steroid Biochemistry, 19, 235–239. [PubMed] [Google Scholar]
- Dubey, A. K. , Herbert, J. , Abbott, D. , & Martensz, N. D. (1984). Serum and CSF concentrations of testosterone and LH related to negative feedback in male rhesus monkeys. Neuroendocrinology, 39, 176–185. 10.1159/000123975 [DOI] [PubMed] [Google Scholar]
- Fondell, E. , Fitzgerald, K. , Falcone, G. J. , O’Reilly, E. , & Ascherio, A. Early‐onset alopecia and amyotrophic lateral sclerosis: A cohort study. American Journal of Epidemiology 2013;178(7):1146–1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gapstur, S. M. , Gann, P. H. , Kopp, P. , Colangelo, L. , Longcope, C. , & Liu, K. (2002). Serum androgen concentrations in young men: A longitudinal analysis of associations with age, obesity, and race. The CARDIA male hormone study. Cancer Epidemiology, Biomarkers & Prevention, 11, 1041–1047. [PubMed] [Google Scholar]
- Gargiulo‐Monachelli, G. M. et al (2014). Circulating steroids in amyotrophic lateral sclerosis: Possible markers of susceptibility and outcome. Hormone and Metabolic Research, 46, 433–439. [DOI] [PubMed] [Google Scholar]
- Goodman, R. L. , & Hotchkiss, J. , Karsch, F. J. , & Knobil, E. (1974). Diurnal variations in serum testosterone concentration in the adult male rhesus monkey. Biology of Reproduction, 11, 624–630. [DOI] [PubMed] [Google Scholar]
- Jones, T. M. , Riley, Y. , & Antel, J. P. (1982). Response of patients with amyotrophic lateral sclerosis to testosterone therapy. Archives of Neurology, 39, 721–722. 10.1001/archneur.1982.00510230047014 [DOI] [PubMed] [Google Scholar]
- Kennedy, R. I. , Rawlings, N. C. , & Cook, S. J. (1985). Effects of dihydrotestosterone and 5α‐ androstane‐3α l7 β‐diol on serum concentrations of LH and FSH in the prepubertal bull. Canadian Journal of Animal Science, 65, 95–99. [Google Scholar]
- La Spada, A. R. , Wilson, E. M. , Lubahn, D. B. , Harding, A. E. , & Fischbeck, K. H. (1991). Androgen receptor gene mutations in X‐linked spinal and bulbar muscular atrophy. Nature, 352, 77–79. 10.1038/352077a0 [DOI] [PubMed] [Google Scholar]
- Laaksonen, D. E. , Niskanen, L. , Punnonen, K. , Nyyssonen, K. , Tuomainen, T. P. , Valkonen, V. P. , … Salonen, J. T. (2004). Testosterone and sex hormone‐binding globulin predict the metabolic syndrome and diabetes in middle‐aged men. Diabetes Care, 27, 1036–1041. 10.2337/diacare.27.5.1036 [DOI] [PubMed] [Google Scholar]
- Leifke, E. , Gorenoi, V. , Wichers, C. , Von Zur, M. A. , & Brabant, G. (2000). Age‐related changes of serum sex hormones, insulin‐like growth factor‐1 and sex hormone binding globulin levels in men: Cross‐sectional data from a healthy male cohort. Clinical Endocrinology ‐ Oxford, 53, 689–695. 10.1046/j.1365-2265.2000.01159.x [DOI] [PubMed] [Google Scholar]
- Mahendroo, M. S. , Cala, K. M. , Landrum, C. P. , & Russell, D. W. (1997). Fetal death in mice lacking 5α‐reductase type 1 caused by estrogen excess. Molecular Endocrinology, 11, 917–927. [DOI] [PubMed] [Google Scholar]
- Manjaly, Z. R. , Scott, K. M. , Abhinav, K. , Wijesekera, L. , Ganesalingam, J. , Goldstein, L. H. , … Al‐Chalabi, A. (2010). The sex ratio in amyotrophic lateral sclerosis: A population based study. Amyotroph Lateral Scler, 11, 439–442. 10.3109/17482961003610853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchetti, P. M. , & Barth, J. H. (2013). Clinical biochemistry of dihydrotestosterone. Annals of Clinical Biochemistry, 50, 95–107. 10.1258/acb.2012.012159 [DOI] [PubMed] [Google Scholar]
- Martini, L. (1982). The 5α‐reduction of testosterone in the neuroendocrine structures. Biochemical and Physiological Implications. Endocrine Reviews, 3, 1–25. 10.1210/edrv-3-1-1 [DOI] [PubMed] [Google Scholar]
- Martini, L. , Celotti, F. , & Melcangi, R. C. (1996). Testosterone and progesterone metabolism in the central nervous system: Cellular localization and mechanism of control of the enzymes involved. Cellular and Molecular Neurobiology, 16(3), 271–282. 10.1007/BF02088095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martini, L. , Celotti, F. , & Serio, M. (1979). 5α‐Reductase deficiency in humans: Support to the theory that 5α‐reduction of testosterone is an essential step in the control of LH secretion. Journal of Endocrinological Investigation, 2(4), 463–464. 10.1007/bf03349351 [DOI] [PubMed] [Google Scholar]
- Marynick, S. P. , Havens, W. W. , Ebert, M. H. , & Loriaux, D. L. (1976). Studies on the transfer of steroid hormones across the blood‐cerebrospinal fluid barrier in the rhesus monkey. Endocrinology (Baltimore), 99, 400–405. 10.1210/endo-99-2-400 [DOI] [PubMed] [Google Scholar]
- McLeod, V. M. , Lau, C. L. , Chiam, M. D. , Rupasinghe, T. W. , Roessner, U. , Djouma, E. , … Turner, B. J. (2019). Androgen receptor antagonism accelerates disease onset in the SOD1G93A mouse model of ALS. British Journal of Pharmacology, 176, 2111–2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Militello, A. , Vitello, G. , Lunetta, C. , Toscano, A. , Maiorana, G. , Piccoli, T. , & La Bella, V. (2002). The serum level of free testosterone is reduced in amyotrophic lateral sclerosis. Journal of the Neurological Sciences, 195, 67–70. [DOI] [PubMed] [Google Scholar]
- Mitamura, R. , Yano, K. , Suzuki, N. , Ito, Y. , Makita, Y. , & Okuno, A. (1999). Diurnal rhythms of luteinizing hormone, follicle‐stimulating hormone, and testosterone secretion before the onset of male puberty. Journal of Clinical Endocrinology and Metabolism, 84, 29–37. 10.1210/jc.84.1.29 [DOI] [PubMed] [Google Scholar]
- Pardridge, W. M. , Moeller, T. L. , Mietus, L. J. , & Oldendorf, W. H. (1980). Blood brain barrier transport and brain sequestration of steroid hormones. American Journal of Physiology, 239, E96‐EI02 10.1152/ajpendo.1980.239.1.E96 [DOI] [PubMed] [Google Scholar]
- Pillay, R. M. (2006). Athletes’ Testosterone Levels by Sports Team: An Exploratory Analysis. Simon Fraser University.
- Plymate, S. R. , Tenover, J. S. , & Bremner, W. J. (1989). Circadian variation in testosterone, sex hormone‐binding globulin, and calculated non‐sex hormone‐binding globulin bound testosterone in healthy young and elderly men. Journal of Andrology, 10, 366–437. 10.1002/j.1939-4640.1989.tb00120.x [DOI] [PubMed] [Google Scholar]
- Poletti, A. , Coscarella, A. , Negri‐Cesi, P. , Colciago, A. , Celotti, F. , & Martini, L. (1998). The 5 alpha‐reductase isozymes in the central nervous system. Steroids, 63, 246–251. [DOI] [PubMed] [Google Scholar]
- Reich‐Slotky, R. , Andrews, J. , Cheng, B. , Buchsbaum, R. , Levy, D. , Kaufmann, P. , Thompson, J. L. (2013). Body mass index (BMI) as predictor of ALSFRS‐R score decline in ALS patients. Amyotrophic Lateral Sclerosis and Frontotemporal Degeneration, 14(3), 212–216. 10.3109/21678421.2013.770028 [DOI] [PubMed] [Google Scholar]
- Scarmeas, N. , Shih, T. , Stern, Y. , Ottman, R. , & Rowland, L. P. (2002). Premorbid weight, body mass, and university athletics in ALS. Neurology, 59, 773–775. [DOI] [PubMed] [Google Scholar]
- Schaison, G. , Renoir, M. , & Lagoguey, M. , Mowszowicz, I. (1980). On the role of dihydrotestosterone in regulating luteinizing hormone secretion in man. Journal Clinical Endo and Metabolism, 51, 1133–1137. 10.1210/jcem-51-5-1133 [DOI] [PubMed] [Google Scholar]
- Scott, C. J. , Kuehl, D. E. , Ferreira, S. A. , & Jackson, G. L. (1997). Hypothalamic sites of action for testosterone, dihydrotestosterone, and estrogen in the regulation of luteinizing hormone secretion in male sheep. Endocrinology, 138, 3686–3694. [DOI] [PubMed] [Google Scholar]
- Sholl, S. , Goy, R. W. & Uno, H. (1982). Differences in brain uptake and metabolism of testosterone in gonadectomized, adrenalectomized and female rhesus monkeys. Endocrinology, 11, 806–813. [DOI] [PubMed] [Google Scholar]
- Sholl, S. , Robinson, J. A. , & Goy, R. W. (1975). Dihydrotestosterone in the guinea pig. Steroids, 15, 203–215. [DOI] [PubMed] [Google Scholar]
- Tenover, J. S. , Matsumoto, A. M. , Clifton, D. K. , & Bremner, W. J. (1988). Age‐related alterations in the circadian rhythms of pulsatile luteinizing hormone and testosterone secretion in healthy men. The Journal of Gerontology, 43:M163–M169. 10.1093/geronj/43.6.M163 [DOI] [PubMed] [Google Scholar]
- Vivekananda, U. , Manjalay, Z. , Ganesalingham, J. , Simms, J. , Shaw, E. , Leigh, P. , … Al‐Chalabi, A. (2011). Low index‐to‐ring finger length ratio in sporadic ALS supports prenatally defined motor neuronal vulnerability. Journal of Neurology, Neurosurgery and Psychiatry, 82, 6 10.1136/jnnp.2010.237412 [DOI] [PubMed] [Google Scholar]
- Weiner, L. P. (1980). Possible role of androgen receptors in amyotrophic lateral sclerosis: a Hypothesis. Archives of Neurology, 37(3), 129–131. 10.1001/archneur.1980.00500520027002 [DOI] [PubMed] [Google Scholar]
- Wicks, P. (2012). Hypothesis: Higher prenatal testosterone predisposes ALS patients to improved athletic performance and manual professions. Amyotrophic Lateral Sclerosis, 13(3), 251–253. 10.3109/17482968.2011.634009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wills, A.‐M. , Hubbard, J. , & Macklin, E. A. (2014). Hypercaloric enteral nutrition in patients with amyotrophic lateral sclerosis: A randomised, double‐blind, placebo‐controlled phase 2 trial. Te Lancet, 383(9934), 2065–2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worms, P. M. (2001). The epidemiology of motor neuron diseases: A review of recent studies. Journal of the Neurological Sciences, 191(1‐2), 3–9. 10.1016/S0022-510X(01)00630-X [DOI] [PubMed] [Google Scholar]
- Yoo, Y.‐E. , & Ko, C.‐P. (2012). Dihydrotestosterone ameliorates degeneration in muscle, axons and motoneurons and improves motor function in amyotrophic lateral sclerosis model mice. PLoS ONE, 7, e37258 10.1371/journal.pone.0037258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanisi, M. , Motta, M. , & Martini, L. (1973). Inhibitory effect of 5 α‐reduced metabolites of testosterone on gonadotrophin secretion. Journal of Endocrinology, 56, 315 10.1677/joe.0.0560315 [DOI] [PubMed] [Google Scholar]
- Zoppi, S. , Cocconi, M. , Lechuga, M. J. , Messi, E. , Zanisi, M. , & Motta, M. (1988). Antihormonal activities of 5 α reductase and aromatase inhibitors. Journal of Steroid Biochemistry, 31, 677–683. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.


