Abstract
Background
In December 2019, a novel pneumonia related to the 2019 coronavirus unexpectedly developed in Wuhan, China. We aimed to review data of the novel Coronavirus (2019-nCoV) by analyzing all the published retrospective studies on the clinical, epidemiological, laboratory, and radiological characteristics of patients with 2019-nCoV.
Methods
We searched in four bibliographic databases PubMed, Scopus, Embase, and Web of Science) for studies March 10, 2020 focused on the clinical, epidemiological, laboratory, and radiological characteristics of patients with 2019-nCoV for meta-analysis. The Newcastle-Ottawa Scale was used to quality assessment, and publication bias was analyzed by Egger's test. In the meta-analysis, a random-effects model with Stata/SE software, v.14.1 (StataCorp, College Station, TX) was used to obtain a pooled incidence rate.
Results
Fifty studies were included in this systematic review and meta-analysis with 8815 patients and the mean age was 46 years and 4647 (52.7%) were male. The pooled incidences rate of clinical symptoms were: fever (83%, 95% CI: 0.77, 0.89), cough (59%, 95% CI: 0.48, 0.69), myalgia or fatigue (31%, 95% CI: 0.23, 0.39), sputum production (29%, 95% CI: 0.21, 0.39), and dyspnea (19%, 95% CI: 0.12, 0.26). The pooled incidence rate of acute respiratory distress syndrome (ARDS) was (22%, 95% CI: 0.00, 0.60).
Conclusion
The results of this systemic review and meta-analysis present a quantitative pooled incidence rate of different characters of 2019-nCoV and has great potential to develop diagnosis and patient's stratification in 2019-nCoV. However, this conclusions of this study still requisite to be warranted by more careful design, larger sample size multivariate studies to corroborate the results of this meta-analysis.
Keywords: 2019-nCoV; Clinical; Epidemiological; Laboratory, and radiological characteristics; Meta-analysis
1. Introduction
In December 8, 2019 a new coronavirus, which was called 2019 novel coronavirus (2019-nCoV), arise the pneumonia epidemic of the severe respiratory disease from Wuhan (Huanan seafood market) across China which now causes the main public health threats worldwide [1,2]. On January 30, 2020, WHO stated that the epidemic of the Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) become as a public health emergency of international concern (PHEIC) [3]. Currently, the number of patients with 2019-nCoV is dramatically increasing to other countries around the world [4,5]. According to worldwide statistics, the death rate is ∼4.6%. Main symptoms of 2019-nCoV include pneumonia, fever, myalgia or fatigue [4,5]. However, some characterizations and conclusions in the published relevant research were varied, limited and controversial. At present, there is no successful vaccine or antiviral drugs has been clinically approved for 2019-nCoV. Therefore, to acquire more exact conclusions on the clinical, epidemiological, laboratory, and radiological characteristics and also to propose significant help for current clinical studies of patients with 2019-nCoV, we performed a systemic review and meta-analysis of all these evidence-based medical epidemiological, clinical, laboratory, and radiological characters.
2. Methods
2.1. Search strategy and study selection
Four bibliographic databases, including international databases (PubMed, Scopus, Embase, and Web of Science) for relevant articles were searched (Until 10th/March/2020) by using the following keywords: (”2019 Novel coronavirus” OR “2019-nCoV” OR “Severe Acute Respiratory Syndrome Coronavirus 2” OR “SARS-CoV-2” OR “COVID-19” OR “Wuhan Coronavirus” OR “Wuhan pneumonia”) in the Title/Abstract/Keywords fields. No limitation regarding ethnicity, language, country, gender, patient age was used while searching databases, but inclusion of the study in our full analysis required at least the abstract to be available in English. The records found through database searching were merged and the duplicates were removed using EndNote X7 (Thomson Reuters, New York, NY, USA).
2.2. Selection criteria and data extraction
One of the team researchers randomly evaluated the search results and reported that no relevant study was ignored. Three authors (Ebrahim Kouhsari, Mohammad Sholeh and Sajad Yaghoubi) independently done all these steps and reviewed the potentially relevant studies to clarify whether they met the predetermined eligibility criteria. Any discrepancies and inconsistencies with article selection were resolved through discussion, and a fourth author (Nourkhoda Sadeghifard) was available to resolve the disagreement. In the first phase, studies obtained from the literature search were precisely screened by titles and abstracts to exclude irrelevant studies. The full text of relevant studies was reviewed in depth conferring to definite criteria. References lists of all related studies were also reviewed for any other related publication.
Studies were excluded if they met the following conditions: reviews, theses, books, conference papers, repeat articles, letters, editorials, expert opinions, animal, in vitro studies, and overlapping, unusable data sets (Fig. 1 ). Information extracted from retrospective studies on the clinical, epidemiological, laboratory, and radiological characteristics of novel Coronavirus (2019-nCoV) infected patients (supplementary data 1).
Fig. 1.

Flow diagram showing the data selection process.
2.3. Outcomes
The main outcome of interest was the clinical, epidemiological, laboratory, and radiological characteristics of 2019-nCoV infected patients.
2.4. Quality assessment
Quality evaluation of the included studies was performed using by two authors (Marzieh Hashemian, Somayeh Karamollahi) independently, using an adapted version of the tool proposed by the Newcastle-Ottawa assessment scale [6]. A score ranging from 0 to 9 points was attributed to each study (≥7 points: high quality, 4–6 points: Moderate quality, ≤ 3 points: low quality). Higher score indicates higher study quality. A third reviewer (Ebrahim Kouhsari) adjudicated in any case of disagreement. Need for arbitration and reason was reported in the data collection tool.
2.5. Publication bias
Publication bias was analyzed using Egger's linear regression test, which measures funnel plot asymmetry.
2.6. Statistical analysis
All statistical analyses were performed using a random-effects model with Stata/SE software, v.14.1 (StataCorp, College Station, TX). A chi-squared test and I2 statistic were used to assess the inter-study heterogeneity. Hence, values above 75% are considered heterogeneity [7]; Thus, DerSimonian and Laird random effects models were used [8]. All statistical interpretations were reported on a 95% confidence interval (CI) basis.
3. Results
3.1. Search results
We evaluated 5 electronic databases and categorized 2095 articles published until 10 March 2020 (Fig. 1). Of these, after initial screening of the title and abstract, 1795 articles were excluded due to their irrelevance and duplication and the full text of remaining 300 articles were reviewed (Fig. 1). Among the 250 articles, were excluded again for specific reasons: case reports, conference papers, repeat articles, letters, editorials, expert opinions, animal, in vitro studies, and unusable data sets. Finally, 50 studies were included in this systematic review and meta-analysis. Supplementary data 1 depicts the main characteristics of 50 included studies.
3.2. Characteristics of studies
A total of 50 articles were included in this meta-analysis [2,4,5,[9], [10], [11], [12], [13], [14], [15], [16], [17], [18], [19], [20]], [[21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40], [41], [42], [43], [44], [45], [46], [47], [48], [49], [50], [51], [52], [53], [54], [55]] including data from 8815 patients. Study size ranged from 4 to 1719 subjects. The methodological quality of the included studies was high for observational studies (Table 1 ). The highest quality of the literature was 8 stars and the lowest 3 stars.
Table 1.
Characteristics and Quality assessment of included studies.
| ID | First Author, Year | Country | Study Design | Selection (4 points) | Comparability (2 points) | Outcome (3 points) | Total (9 points) |
|---|---|---|---|---|---|---|---|
| 1 | Guan W, 2020 | China | retrospective | 3 | 2 | 3 | 8 |
| 2 | Huang Y, 2020 | China | retrospective | 3 | 2 | 2 | 7 |
| 3 | Tang N, 2020 | China | retrospective | 1 | 2 | 2 | 5 |
| 4 | Cai s, 2020 | China | retrospective | 3 | 2 | 2 | 7 |
| 5 | Chen L, 2020 | China | retrospective | 3 | 2 | 2 | 7 |
| 6 | Feng K, 2020 | China | retrospective | 3 | 2 | 2 | 7 |
| 7 | Liu W, 2020 | China | retrospective | 3 | 2 | 2 | 7 |
| 8 | Chen C, 2020 | China | retrospective | 2 | 2 | 2 | 6 |
| 9 | Zhang L, 2020 | China | retrospective | 2 | 2 | 2 | 6 |
| 10 | Tian S, 2020 | China | retrospective | 3 | 2 | 2 | 7 |
| 11 | Bernheim S, 2020 | China | retrospective | 3 | 2 | 2 | 7 |
| 12 | Wu J, 2020 | China | retrospective | 2 | 2 | 2 | 6 |
| 13 | Peng YD 2020 | China | retrospective | 3 | 2 | 2 | 7 |
| 14 | Wang D 2020 | China | retrospective | 3 | 2 | 3 | 8 |
| 15 | Xu H–Y, 2020 | China | retrospective | 3 | 2 | 2 | 7 |
| 16 | Xia W, 2020 | China | retrospective | 3 | 2 | 2 | 7 |
| 17 | Yang W, 2020 | China | retrospective | 2 | 2 | 2 | 6 |
| 18 | Xiong Y,2020 | China | retrospective | 3 | 2 | 2 | 7 |
| 19 | Hu Z,2020 | China | retrospective | 3 | 2 | 2 | 7 |
| 20 | Zhang JJ,2020 | China | retrospective | 2 | 2 | 2 | 6 |
| 21 | Wang D,2020 | China | retrospective | 3 | 2 | 2 | 7 |
| 22 | Walker,2020 | Australia | retrospective | 1 | 2 | 1 | 3 |
| 23 | Liu K,2020 | China | retrospective | 3 | 2 | 2 | 7 |
| 24 | Yang X,2020 | China | retrospective | 2 | 2 | 2 | 6 |
| 25 | Wang X,2020 | China | retrospective | 3 | 2 | 2 | 7 |
| 26 | Chung M,2020 | China | retrospective | 3 | 2 | 2 | 7 |
| 27 | Li Q,2020 | China | retrospective | 3 | 2 | 3 | 8 |
| 28 | Ki M,2020 | Korea | retrospective | 2 | 2 | 3 | 7 |
| 29 | Chen N,2020 | China | retrospective | 2 | 2 | 3 | 7 |
| 30 | Fan BE,2020 | China | retrospective | 3 | 2 | 3 | 8 |
| 31 | Chang D,2020 | China | retrospective | 3 | 2 | 2 | 7 |
| 32 | Yao Y,2020 | China | retrospective | 2 | 2 | 1 | 5 |
| 33 | Cheng J,2020 | China | retrospective | 2 | 2 | 1 | 5 |
| 34 | Song F,2020 | China | retrospective | 3 | 2 | 3 | 8 |
| 35 | Zhou S,2020 | China | retrospective | 3 | 2 | 3 | 8 |
| 36 | Yueying P,2020 | China | retrospective | 4 | 1 | 2 | 7 |
| 37 | Liu C,2020 | China | Retrospectively | 3 | 2 | 2 | 7 |
| 38 | Shi H,2020 | China | retrospectively | 3 | 2 | 2 | 7 |
| 39 | Zhao W,2020 | China | retrospectively | 3 | 2 | 2 | 7 |
| 40 | Pan F,2020 | China | retrospectively | 3 | 2 | 2 | 7 |
| 41 | Huang C,2020 | Chine | retrospectively | 3 | 2 | 2 | 7 |
| 42 | Li YY,2020 | China | retrospectively | 3 | 2 | 2 | 7 |
| 43 | Yang HY,2020 | China | retrospectively | 2 | 1 | 3 | |
| 44 | Zhu ZW,2020 | China | retrospectively | 3 | 2 | 2 | 7 |
| 45 | Ai T,2020 | China | retrospectively | 3 | 2 | 2 | 7 |
| 46 | Ling Y,2020 | China | retrospectively | 3 | 2 | 2 | 7 |
| 47 | Lan L,2020 | China | retrospectively | 3 | 2 | 2 | 7 |
| 48 | Sun,2020 | USA | retrospectively | 2 | 2 | 1 | 5 |
| 49 | Li J,2020 | China | retrospectively | 3 | 2 | 1 | 6 |
| 50 | Xu,2020 | China | retrospectively | 3 | 2 | 1 | 6 |
3.3. Publication bias detection
The results of the Egger test are displayed in Table 3 . There was a publication bias in the meta-analysis of the bilateral pneumonia group (P = 0.004).
Table 3.
Results of Egger test.
| Group | Fever | Cough | Myalgia or fatigue | Acute respiratory distress syndrome | Death | COPD | Multiple mottling and ground-glass opacity | Bilateral patchy shadowing | Bilateral pneumonia |
|---|---|---|---|---|---|---|---|---|---|
| P | 0.103 | 0.054 | 0.592 | 0.868 | 0.197 | 0.127 | 0.155 | 0.238 | 0.004 |
3.4. Epidemiological characteristics
A total of 50 studies including 8815 patients were included in this study, the mean age was 46 years and 4647 (0.54%) were male. Among studies been reported that data on the epidemiological characteristics, evidence of heterogeneity was present in the history contact with another person with respiratory symptoms (I2 = 95.94, P = 0.00), history of travel from China (Wuhan) (I2 = 99.02, P = 0.00), exposure to source of transmission (COVID-19 infected patients, wildlife) within 14 days (I2 = 98.51, P = 0.00), admission to ICU (I2 = 94.70, P = 0.00), smoking history (current or past) (I2 = 99.18, P = 0.00) (Table 2). Among eligible literatures, 26 studies reported that hypertension, diabetes, and cardiovascular illness were more prevalent in patients. Detailed results of Meta-analysis are shown in Table 2.
Table 2.
Meta-analysis results.
| Characteristic | Value | (-CL, +CL) | I2 | P | Positive | Number of patients |
|---|---|---|---|---|---|---|
| Epidemiology | ||||||
| Male | 0.54 | (0.51, 0.56) | 68.34 | 0.00 | 4647 | 8815 |
| Female | 0.46 | (0.44, 0.49) | 68.34 | 0.00 | 4168 | 8815 |
| Contact with another person with respiratory symptoms | 0.27 | (0.15, 0.42) | 95.94 | 0.00 | 328 | 1289 |
| History of travel from china (Wuhan, and …) | 0.58 | (0.41, 0.73) | 99.02 | 0.00 | 1917 | 4208 |
| Exposure to source of transmission | 0.30 | (0.16, 0.45) | 98.51 | 0.00 | 719 | 3583 |
| Smoking history | 0.17 | (0.00, 0.53) | 99.18 | 0.00 | 1001 | 1559 |
| Admission to ICU | 0.16 | (0.08, 0.27) | 94.70 | 0.00 | 175 | 1843 |
| Diabetes | 0.11 | (0.08, 0.14) | 68.64 | 0.00 | 250 | 2505 |
| Hypertension | 0.19 | (0.12, 0.27) | 94.35 | 0.00 | 484 | 2403 |
| Malignancy | 0.05 | (0.02, 0.08) | 82.12 | 0.00 | 72 | 2250 |
| Cardiovascular | 0.12 | (0.06, 0.20) | 95.16 | 0.00 | 207 | 2301 |
| Other comorbidity | 0.16 | (0.11, 0.22) | 75.84 | 0.00 | 598 | 2897 |
| COPD | 0.03 | (0.01, 0.06) | 75.84 | 0.00 | 48 | 1900 |
| Clinical symptoms | ||||||
| Fever | 0.83 | (0.77, 0.89) | 95.15 | 0.00 | 3273 | 4370 |
| Cough | 0.59 | (0.48, 0.69) | 97.33 | 0.00 | 2100 | 4308 |
| Myalgia or fatigue | 0.31 | (0.23, 0.39) | 94.28 | 0.00 | 1051 | 3029 |
| Sputum production | 0.29 | (0.21, 0.39) | 84.96 | 0.00 | 478 | 1497 |
| Headache | 0.10 | (0.06, 0.14) | 70.94 | 0.00 | 306 | 3557 |
| Hemoptysis | 0.02 | (0.00, 0.05) | 70.94 | 0.00 | 21 | 1370 |
| Diarrhea | 0.08 | (0.06, 0.11) | 80.05 | 0.00 | 203 | 3690 |
| Dyspnea | 0.19 | (0.12, 0.26) | 93.99 | 0.00 | 495 | 2651 |
| Acute respiratory distress syndrome (ARDS) | 0.22 | (0.00, 0.60) | 96.19 | 0.00 | 49 | 173 |
| Vomiting | 0.03 | (0.02, 0.05) | 65.12 | 0.00 | 105 | 2961 |
| Sore throat | 0.12 | (0.07, 0.18) | 91.85 | 0.00 | 287 | 2996 |
| Rhinorrhea | 0.09 | (0.03, 0.17) | 87.72 | 0.00 | 64 | 1455 |
| Chest pain | 0.11 | (0.04, 0.21) | 95.44 | 0.00 | 108 | 1834 |
| Laboratory | ||||||
| WBC(Normal) | 0.81 | (0.69, 0.91) | 95.56 | 0.00 | 958 | 1260 |
| WBC (Decrease) | 0.21 | (0.16, 0.27) | 70.65 | 0.00 | 180 | 785 |
| WBC(Increase) | 0.14 | (0.08, 0.21) | 84.85 | 0.00 | 109 | 760 |
| Neutrophil (Normal) | 0.95 | (0.87, 1.00) | 93.15 | 0.00 | 721 | 797 |
| Neutrophil (Decrease) | 0.16 | (0.12, 0.21) | ∗ | ∗ | 39 | 229 |
| Neutrophil (Increase) | 0.17 | (0.05, 0.34) | 92.34 | 0.00 | 67 | 380 |
| Albumin (Normal) | 0.95 | (0.84, 1.00) | 86.35 | 0.00 | 243 | 259 |
| Albumin (Decrease) | 0.54 | (0.00, 1.00) | 99.38 | 0.00 | 121 | 277 |
| Albumin (Increase) | 0.03 | (0.01, 0.06) | ∗ | ∗ | 7 | 181 |
| Serum Creatinine (Normal) | 1.00 | (0.99, 1.00) | ∗ | ∗ | 190 | 190 |
| Serum Creatinine (Decrease) | 0.17 | (0.10, 0.26) | ∗ | ∗ | 17 | 99 |
| Serum Creatinine (Increase) | 0.03 | (0.01, 0.08) | ∗ | ∗ | 5 | 128 |
| D-Dimer (Normal) | 0.94 | (0.82, 1.00) | 93.22 | 0.00 | 440 | 475 |
| D-Dimer (Increase) | 0.48 | (0.04, 0.94) | 99.24 | 0.00 | 254 | 479 |
| Procalcitonin (Normal) | 0.88 | (0.67, 1.00) | 95.57 | 0.00 | 313 | 368 |
| Procalcitonin (Increase) | 0.60 | (0.15, 0.96) | 99.00 | 0.00 | 276 | 461 |
| Blood Urea nitrogen (Normal) | 0.98 | (0.88, 1.00) | 94.23 | 0.00 | 382 | 399 |
| Blood Urea nitrogen (Decrease) | 0.14 | (0.10, 0.18) | ∗ | ∗ | 34 | 248 |
| Blood Urea nitrogen (Increase) | 0.08 | (0.04, 0.13) | ∗ | ∗ | 11 | 128 |
| Thromboplastin time (Normal) | 0.98 | (0.87, 1.00) | 96.48 | 0.00 | 559 | 599 |
| Thromboplastin time (Decrease) | 0.05 | (0.02, 0.09) | ∗ | ∗ | 16 | 99 |
| Thromboplastin time (Increase) | 0.20 | (0.04, 0.45) | 94.02 | 0.00 | 72 | 298 |
| C-reactive protein (Normal) | 0.48 | (0.26, 0.69) | 96.21 | 0.00 | 211 | 599 |
| C-reactive protein (Increase) | 0.72 | (0.54, 0.87) | 97.46 | 0.00 | 865 | 1159 |
| Total Bilirubin (Normal) | 0.95 | (0.85, 1.00) | 92.38 | 0.00 | 514 | 541 |
| Total Bilirubin (Decrease) | 0.05 | (0.02, 0.09) | ∗ | ∗ | 7 | 149 |
| Total Bilirubin (Increase) | 0.20 | (0.04, 0.45) | 94.02 | 0.00 | 38 | 297 |
| Prothrombin time (Normal) | 0.95 | (0.83, 1.00) | 95.40 | 0.00 | 532 | 571 |
| Prothrombin time (Decrease) | 0.10 | (0.00, 0.33) | 95.30 | 0.00 | 35 | 282 |
| Prothrombin time (Increase) | 0.44 | (0.00, 0.97) | 99.44 | 0.00 | 177 | 420 |
| Creatinine (Normal) | 0.98 | (0.88, 1.00) | 95.81 | 0.00 | 502 | 546 |
| Creatinine (Decrease) | 0.53 | (0.47, 0.59) | ∗ | ∗ | 151 | 299 |
| Creatinine (Increase) | 0.24 | (0.18, 0.31) | ∗ | ∗ | 47 | 190 |
| Platelet count (Normal) | 0.96 | (0.87, 1.00) | 93.01 | 0.00 | 524 | 563 |
| Platelet count (Decrease) | 0.27 | (0.12, 0.45) | 93.13 | 0.00 | 83 | 436 |
| Platelet count (Increase) | 0.05 | (0.02, 0.08) | 0.00 | 0.79 | 14 | 279 |
| Aspartate Aminotransferase (Normal) | 0.90 | (0.71, 1.00) | 96.86 | 0.00 | 528 | 608 |
| Aspartate Aminotransferase (Increase) | 0.29 | (0.18, 0.41) | 84.10 | 0.00 | 135 | 449 |
| Lactate Dehydrogenase (Normal) | 0.95 | (0.72, 1.00) | 97.02 | 0.00 | 321 | 370 |
| Lactate Dehydrogenase (Increase) | 0.69 | (0.36, 0.95) | 98.04 | 0.00 | 300 | 478 |
| Erythrocyte Sedimentation rate (Increase) | 0.80 | (0.73, 0.85) | ∗ | ∗ | 126 | 177 |
| Alanine Aminotransferase (Normal) | 0.90 | (0.77, 0.98) | 92.20 | 0.00 | 459 | 500 |
| Alanine Aminotransferase (Decrease) | 0.01 | (0.00, 0.05) | ∗ | ∗ | 2 | 149 |
| Alanine Aminotransferase (Increase) | 0.18 | (0.12, 0.25) | 54.18 | 0.00 | 65 | 358 |
| Creatine kinase (Normal) | 0.94 | (0.81, 1.00) | 93.92 | 0.00 | 427 | 467 |
| Creatine kinase (Decrease) | 0.17 | (0.12, 0.22) | ∗ | ∗ | 42 | 248 |
| Creatine kinase (Increase) | 0.12 | (0.03, 0.24) | 85.74 | 0.00 | 32 | 320 |
| Lymphocyte (Normal) | 0.61 | (0.46, 0.75) | 93.25 | 0.00 | 385 | 701 |
| Lymphocyte (Decrease) | 0.58 | (0.40, 0.75) | 97.86 | 0.00 | 826 | 1431 |
| Lymphocyte (Increase) | 0.14 | (0.06, 0.24) | 0.00 | 0.00 | 9 | 63 |
| Hemoglobin (Normal) | 1.00 | (0.98, 1.00) | ∗ | ∗ | 69 | 69 |
| Hemoglobin (Decrease) | 0.98 | (0.95, 1.00) | ∗ | ∗ | 162 | 179 |
| Radiology | ||||||
| Multiple mottling and ground-glass opacity | 0.60 | (0.50, 0.70) | 95.37 | 0.00 | 1399 | 2951 |
| Bilateral patchy shadowing | 0.50 | (0.44, 0.57) | 40.60 | 0.17 | 592 | 1257 |
| Crazy paving | 0.16 | (0.06, 0.29) | 85.09 | 0.00 | 47 | 324 |
| Discrete nodules | 0.10 | (0.00, 0.30) | 93.19 | 0.00 | 15 | 305 |
| Peripheral distribution | 0.61 | (0.45, 0.75) | 91.16 | 0.00 | 327 | 517 |
| Unilateral Pneumonia | 0.61 | (0.45, 0.75) | 91.16 | 0.00 | 61 | 249 |
| Local patchy shadowing | 0.36 | (0.34, 0.39) | ∗ | ∗ | 411 | 1114 |
| Consolidation | 0.37 | (0.24, 0.51) | 94.74 | 0.00 | 650 | 1594 |
| Cavitation | 0.00 | (0.00, 0.02) | 4 | 141 | ||
| Lymphadenopathy | 0.02 | (0.00, 0.05) | 59.24 | 0.02 | 18 | 523 |
| Bilateral pneumonia | 0.70 | (0.59, 0.79) | 90.99 | 0.00 | 1330 | 1644 |
| Pneumothorax | 0.01 | (0.00, 0.05) | ∗ | ∗ | 1 | 99 |
| Interstitial abnormalities | 0.13 | (0.11, 0.15) | ∗ | ∗ | 143 | 1099 |
| Linear | 0.08 | (0.04, 0.13) | ∗ | ∗ | 12 | 142 |
| Pleural effusion | 0.05 | (0.02, 0.09) | 69.66 | 0.00 | 39 | 615 |
| Supportive treatment | ||||||
| Antiviral therapy | 0.90 | (0.74, 0.99) | 98.61 | 0.00 | 1374 | 2205 |
| Antibiotic therapy | 0.68 | (0.49, 0.84) | 97.80 | 0.00 | 1094 | 1806 |
| Use of corticosteroid | 0.32 | (0.19, 0.47) | 96.97 | 0.00 | 498 | 2028 |
| Immunotherapy | 0.39 | (0.13, 0.69) | 98.92 | 0.00 | 428 | 1674 |
| Oxygen support | 0.56 | (0.32, 0.78) | 98.95 | 0.00 | 1003 | 2141 |
| Non-invasive ventilation or high-flow nasal canula | 0.11 | (0.05, 0.19) | 93.91 | 0.00 | 163 | 1858 |
| Invasive mechanical ventilation | 0.08 | (0.01, 0.19) | 96.06 | 0.00 | 88 | 1643 |
| Invasive mechanical ventilation and ECMO | 0.02 | (0.00, 0.05) | 71.69 | 0.00 | 15 | 576 |
| Nasal cannula | 0.55 | (0.24, 0.84) | 97.00 | 0.00 | 218 | 339 |
| Continuous renal replacement therapy | 0.06 | (0.01, 0.13) | 79.86 | 0.00 | 23 | 361 |
| Clinical outcomes | ||||||
| Recovered | 0.53 | 98.63 | 0.00 | 788 | 2952 | |
| Staying in hospital | 0.67 | 97.93 | 0.00 | 1791 | 2355 | |
| Death | 0.05 | 89.08 | 0.00 | 151 | 3054 | |
3.5. Clinical characteristics
There were 13 symptoms of 2019-nCoV in infected patients which were reported. Among studies been reported that data on the clinical symptoms, evidence of heterogeneity was present in the symptoms of fever (I2 = 95.15, P = 0.00), cough (I2 = 97.33, P = 0.00), myalgia or fatigue (I2 = 94.28, P = 0.00), sputum production (I2 = 84.96, P = 0.00), headache or hemoptysis (I2 = 70.94, P = 0.00), and diarrhea (I2 = 80.05, P = 0.00) (Table 2). Among been reported clinical symptoms, the pooled incidence rate was calculated for four symptoms: acute respiratory distress syndrome (ARDS) (22%, 95% CI: 0.00, 0.60), dyspnea (19%, 95% CI: 0.12, 0.26), sore throat (12%, 95% CI: 0.07, 0.18), chest pain (11%, 95% CI: 0.04, 0.21), rhinorrhea (9%, 95% CI: 0.03, 0.17), vomiting (3%, 95% CI: 0.02, 0.05) (Table 2).
3.6. Laboratory characteristics
Among been reported laboratory characteristics, white blood cells were decreased in 180 patients (the pooled incidence rate was 21%, I2 = 70.65, P = 0.00) and increased in 109 patients (the pooled incidence rate was 14%, I2 = 84.85, P = 0.00) (Table 3). Lymphocyte were decreased in 826 patients (the pooled incidence rate was 58%, I2 = 97.86, P = 0.00) and increased in 9 patients (the pooled incidence rate was 14%, I2 = 0.00, P = 0.00) (Table 2). The increased neutrophils observed in 67 patients, evidence of heterogeneity was present in it (I2 = 92.34%, P = 0.00). Albumin were decreased in 121 patients (the pooled incidence rate was 54%, I2 = 99.38, P = 0.00). The D-Dimer and thromboplastin time were increased in 254 and 72 patients (the pooled incidence rates were 48%; 20%, I2 = 99.24; 94.02, P = 0.00). Procalcitonin, C-reactive protein, alanine aminotransferase, aspartate aminotransferase, Lactate Dehydrogenase and creatine kinase were increased in 276, 865, 65, 135, 300 and 32 patients (the pooled incidence rates were 60%, 72%, 18%, 29%, 69% and 12%, P = 0.00) (Table 3). Prothrombin time were decreased in 35 patients (the pooled incidence rate was 10%, I2 = 95.30, P = 0.00) and increased in 177 patients (the pooled incidence rate was 44%, I2 = 99.44, P = 0.00) (Table 2).
3.7. Radiological characteristics
The radiological characteristics of 2019-nCoV infected patients were described differently. By reviewing the literature, there are different common manifestations as follows: multiple mottling and ground-glass opacity, bilateral pneumonia, consolidation, and bilateral or local patchy shadowing. Among been reported radiological characteristics, evidence of heterogeneity were reported in the multiple mottling and ground-glass opacity (60%, I2 = 95.37, P = 0.00), bilateral pneumonia (70%, I2 = 90.99, P = 0.00), consolidation (37%, I2 = 94.74, P = 0.00), and bilateral patchy shadowing (50%, I2 = 40.60, P = 0.17). Additionally, pneumothorax happened in one patient [13].
3.8. Treatment
Among been reported treatment, 1374, 1094 patients were treated with antiviral and antimicrobial agents (the pooled incidence rates and heterogeneities were 90%; 68%, I2 = 98.61; 97.80). The pooled incidence rates were 32% and 39% in use of corticosteroids and immunotherapy. Totally, 1510 patients used oxygen therapy. Among these studies, there were 218 patients who used nasal cannula, the pooled incidence was 55% (95% CI: 0.24, 0.84) for five studies. 11% (95% CI: 0.32, 0.78) patients used non-invasive ventilation or high-flow nasal cannula. Additionally, 88 and 15 patients were treated with invasive mechanical ventilation and invasive mechanical ventilation or extra-corporeal membrane oxygenation (ECMO), the pooled incidence were 8% and 2% (Table 2). Three articles had no detailed data on oxygen therapy [12,55]. There were 23 patients who used continuous renal replacement therapy, the pooled incidence was 6% (95% CI: 0.01, 0.13) for five studies.
3.9. Clinical outcomes
Among been reported clinical outcomes, unfortunately, 151 died cases were reported, the pooled incidence of mortality was 53% with significant heterogeneity (I2 = 89.08%, P = 0.00). Subsequently the course of treatment of patients is about several weeks until some articles published, some patients still staying in the hospital, the statistics on mortality may be inaccurate. Incidence rate correlation is shown in Table 4 . In addition, 1791 and 788 cases were reported as staying in hospital and recovered with significant heterogeneity (I2 = 97.93%; 98.63, P = 0.00) (Table 2).
Table 4.
Summary of Pearson Correlation Coefficient Values between deaths with other variable.
| Pearson r | Death vs. Age mean | Death vs. History of travel from china (Wuhan and …) | Death vs. Fever (≥37·3 °C) or (≥38 °C) | Death vs. Cough | Death vs. Diarrhea | Death vs. thromboplastin time (Increase) | Death vs. lymphocyte (Normal) | Death vs. Cavitation | Death vs. Linear | Death vs. Antiviral therapy |
|---|---|---|---|---|---|---|---|---|---|---|
| r | 0.5376 | −0.5368 | 0.5784 | 0.3752 | 0.5155 | −0.7788 | 0.7483 | 0.7989 | 0.8951 | 0.8801 |
| 95% confidence interval | 0.2383 to 0.7437 | −0.8228 to −0.03390 | 0.3037 to 0.7643 | 0.01724 to 0.6479 | 0.09454 to 0.7801 | −0.9578 to −0.1643 | 0.4456 to 0.8975 | 0.1152 to 0.9690 | 0.5158 to 0.9810 | 0.5938 to 0.9686 |
| R squared | 0.289 | 0.2882 | 0.3345 | 0.1408 | 0.2657 | 0.6065 | 0.56 | 0.6383 | 0.8012 | 0.7747 |
| P value | ||||||||||
| P (two-tailed) | 0.0013 | 0.0391 | 0.0003 | 0.0411 | 0.02 | 0.0228 | 0.0002 | 0.0311 | 0.0027 | 0.0004 |
| P value summary | ∗∗ | ∗ | ∗∗∗ | ∗ | ∗ | ∗ | ∗∗∗ | ∗ | ∗∗ | ∗∗∗ |
| Significant? (alpha = 0.05) | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Number of XY Pairs | 33 | 15 | 35 | 30 | 20 | 8 | 19 | 7 | 8 | 11 |
Pooled incidence rate for characters is shown in Fig. 2 .
Fig. 2.


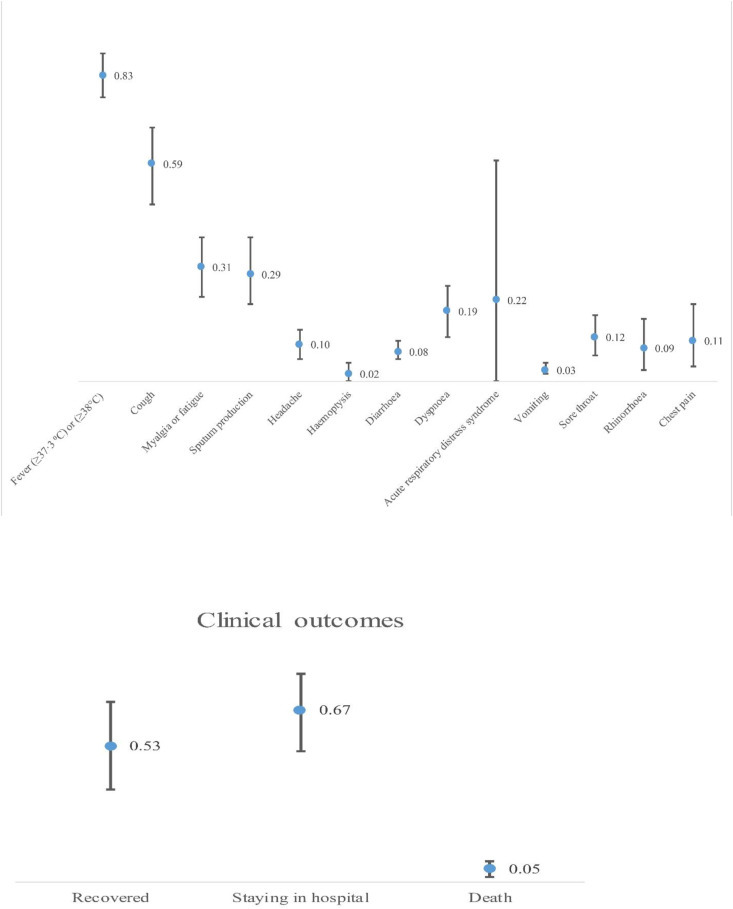
Pooled incidence rate for characters in the study.
4. Discussion
2019-nCoV is one type of coronaviruses are enveloped non-segmented positive-sense RNA viruses belonging to the β-coronavirus cluster like SARS and Middle East respiratory syndrome (MERS) and now it had diseased more than half millions of people worldwide [12,13,55,56]. It is assumed that 2019-nCoV to be a recombinant virus between bat coronavirus and coronavirus of another unknown origin [57]. Up to now, unfortunately, there is no detailed and precise treatments presented for 2019-nCoV. Symptomatic and supportive treatment is the basis of therapy for patients infected by 2019-nCoV. Our meta-analysis was based on data from 50 retrospective studies in 8815 patients of 2019-nCoV. The Most of the cases were from hospitals in China. Several clinical predictors of mortality were found including increased age, male sex and underlying illness, including hypertension, diabetes, renal disease, heart disease and respiratory disease. In our meta-analysis, the frequency of males more than females (52.7% vs 47.3%). The similar findings with the gender distribution have been reported in MERS and SARS [13,15]. It may be related to the occupational risk factors for males [4]. There are some possible reasons in the reduced susceptibility of females to 2019-nCoV such as Gender-specific effects and X chromosome in infectious disease susceptibility, and their more strong immune responses [58,59]. Although, a recent study that revealed there was no divergence with the gender distribution of males and females between ICU patients and non-ICU patients [34]. However, we suggest that more investigations are required in order to identify potential risk factors, their relation to different populations, and their mechanisms involved. Older adults and severe patients with comorbidities are as high-risk group to 2019-nCoV [45]. A study performed on influenza illness demonstrated the higher risk of mortality for severe patients with chronic obstructive pulmonary disease (COPD) (OR 1.49, 95% CI: 1.10–2.01), cardiovascular disease (OR 2.92, 95% CI: 1.76–4.86), hypertension (OR 1.49, 95% CI: 1.10–2.10) [60]. The comorbidities effect had also been observed to have similar effects in 2019-nCoV and MERS [61]. Age and comorbidities are major predictors of numerous adverse outcomes in SARS [62]. SARS cases were mostly occurred in younger people; while half of the cases of MERS infection seen in people older than 50 years [63]. Compared with SARS patients, comorbidities, such as diabetes, hypertension, chronic heart disease and chronic pulmonary disease, were more common in MERS cases [64]. Based on to the outcomes of meta-analysis, incidence rates of clinical characteristic includes fever, cough, myalgia or fatigue, and sputum production were 83, 59, 31, and 29% respectively. The incidence of ARDS was 22%, and the case mortality rate of patients with 2019-nCoV infection was 5% which is lower than to SARS and MERS [65]. Several reports propose that pulmonary fibrosis will become one of the severe problems in cases with 2019-nCoV infection [[66], [67], [68]]. How to stop and decrease the incidence of pulmonary fibrosis in cases with 2019-nCoV infection are crucial complications in the treatment of 2019-nCoV [[66], [67], [68]]. Additionally, we observed that hemoptysis, vomiting, diarrhea rhinorrhea, headache chest pain and sore throat are less than occurred in patients with 2019-nCoV. Air-space opacities (unilateral focal and both unilateral multifocal or bilateral involvement) are the key radiological characters in SARS cases [69,70]. Although, ground–glass opacities and consolidation were the most frequent radiological characters in MERS patients [71,72]. Guan W and colleagues [17] observed that the frequent radiographic features were ground-glass opacity (50%) and bilateral patchy shadowing (46%) in 1099 cases with 2019-nCoV infection. Huang C and colleagues [4] reported that the normal radiographic feature of severe patients with 2019-nCoV were bilateral multiple lobular and subsegmental areas of consolidation. The pooled incidences of the bilateral pneumonia multiple mottling and ground-glass opacity bilateral patchy shadowing and consolidation were 70%, 60%, 50%, and 37%. Based on the laboratory characters, the pooled incidence rate of lymphocytes decrease and increase were 58% and 14%. Otherwise, the pooled incidence rate of increasing and decreasing Neutrophils was 17% and 16%. These defects are comparable to those previously detected in cases with MERS and SARS infection [73]. These outcomes more endorse that lymphocytes decrease along with increasing neutrophils was a characteristic of SARS, and 2019-nCoV might primarily effect on lymphocytes, especially T lymphocytes [74]. Additionally, the administration of glucocorticosteroids cause immunosuppression, decreasing the function and/or numbers of lymphocytes, and deregulated lymphocyte responses. Therefore, treatment with glucocorticoids difficult the concern about Lymphopenia [75]. On the other hand, immune insufficiency may be also a risk factor for poor outcome in patients with 2019-nCoV. Currently, outcomes on the death of 2019-nCoV are varying. The recent four reports include 138, 41, 507 and 41 cases, the mortality was 4.3%, 15%, 7.9% and 14.6% respectively [4,34,52,56]. However, the mortality rates of SARS (10%) and MERS (35%) are higher than to 2019-nCoV [76]. In our meta-analysis, the pooled incidence death was 5% respectively. Although, this result higher than the death reported by the previous reports [52,56]. The cause for this occurrence may be related with the absence of identifying information on data, and also deficient data on diagnosis approaches and treatment practices about 2019-nCoV. However, there were also some limitations of our meta-analysis: (1) all reports included had retrospective designed with high statistic heterogeneity (large variation in the sample size among studies; (2) often cases in this meta-analysis are Chinese; (3) large variation in lengths of follow-up led to some cases may be still stating in hospital in the included studies. In conclusion, the outcomes of our systemic review and meta-analysis provide a quantitative pooled incidence rate of clinical, epidemiological, laboratory, and radiological features of 2019-nCoV and has great potential to develop diagnosis and patient's stratification in 2019-nCoV. However, this conclusions of this study still requisite to be warranted by more careful design, larger sample size multivariate studies to corroborate the results of this meta-analysis.
Source(s) of support
None.
Declaration of competing interest
The authors declare that there are no conflict of interests.
Acknowledgements
We thank the health workers, nurses, clinical staff and all the people who fight with 2019-nCoV.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijmmb.2020.10.004.
Appendix A. Supplementary data
The following are the supplementary data to this article:
References
- 1.Hui D.S., I Azhar E., Madani T.A., Ntoumi F., Kock R., Dar O. The continuing 2019-nCoV epidemic threat of novel coronaviruses to global health—the latest 2019 novel coronavirus outbreak in Wuhan, China. Int J Infect Dis. 2020;91:264–266. doi: 10.1016/j.ijid.2020.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chang D., Lin M., Wei L., Xie L., Zhu G., Cruz C.S.D. Epidemiologic and clinical characteristics of novel coronavirus infections involving 13 patients outside Wuhan, China. Jama. 2020;323(11):1092–1093. doi: 10.1001/jama.2020.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Balassiano I.T., dos Santos-Filho J., Vital-Brazil J.M., Nouér S.A., Souza C.R., Brazier J.S. Detection of cross-infection associated to a Brazilian PCR-ribotype in a university hospital in Rio de Janeiro, Brazil. Antonie Leeuwenhoek. 2011;99(2):249–255. doi: 10.1007/s10482-010-9483-8. [DOI] [PubMed] [Google Scholar]
- 4.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhao S., Lin Q., Ran J., Musa S.S., Yang G., Wang W. Preliminary estimation of the basic reproduction number of novel coronavirus (2019-nCoV) in China, from 2019 to 2020: a data-driven analysis in the early phase of the outbreak. Int J Infect Dis. 2020;92:214–217. doi: 10.1016/j.ijid.2020.01.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25(9):603–605. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- 7.Higgins J.P., Thompson S.G., Deeks J.J., Altman D.G. Measuring inconsistency in meta-analyses. Bmj. 2003;327(7414):557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.DerSimonian R., Laird N. Meta-analysis in clinical trials. Contr Clin Trials. 1986;7(3):177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 9.Ai T., Yang Z., Hou H., Zhan C., Chen C., Lv W. Correlation of chest CT and RT-PCR testing in coronavirus disease 2019 (COVID-19) in China: a report of 1014 cases. Radiology. 2020:200642. doi: 10.1148/radiol.2020200642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bernheim A., Mei X., Huang M., Yang Y., Fayad Z.A., Zhang N. Chest CT findings in coronavirus disease-19 (COVID-19): relationship to duration of infection. Radiology. 2020:200463. doi: 10.1148/radiol.2020200463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cai S., Wu L., Chen D., Li Y., Liu Y., Fan Y. Analysis of bronchoscope-guided tracheal intubation in 12 cases with COVID-19 under the personal protective equipment with positive pressure protective hood. Zhonghua jie he he hu xi za zhi= Zhonghua jiehe he huxi zazhi= Chin J Tubercul Respir Dis. 2020;43:E033. doi: 10.3760/cma.j.cn112147-20200222-00153. [DOI] [PubMed] [Google Scholar]
- 12.Chen C., Chen C., Yan J., Zhou N., Zhao J., Wang D. Analysis of myocardial injury in patients with COVID-19 and association between concomitant cardiovascular diseases and severity of COVID-19. Zhonghua Xinxueguanbing Zazhi. 2020;48:E008. doi: 10.3760/cma.j.cn112148-20200225-00123. [DOI] [PubMed] [Google Scholar]
- 13.Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cheng J., Huang C., Zhang G., Liu D., Li P., Lu C. Epidemiological characteristics of novel coronavirus pneumonia in Henan. Zhonghua jie he he hu xi za zhi= Zhonghua jiehe he huxi zazhi= Chin J Tubercul Respir Dis. 2020;43:E027. doi: 10.3760/cma.j.cn112147-20200222-00148. [DOI] [PubMed] [Google Scholar]
- 15.Chung M., Bernheim A., Mei X., Zhang N., Huang M., Zeng X. CT imaging features of 2019 novel coronavirus (2019-nCoV) Radiology. 2020:200230. doi: 10.1148/radiol.2020200230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fan B.E., Chong V.C.L., Chan S.S.W., Lim G.H., Lim K.G.E., Tan G.B. Hematologic parameters in patients with COVID-19 infection. Am J Hematol. 2020;95(6):E131–E134. doi: 10.1002/ajh.25774. [DOI] [PubMed] [Google Scholar]
- 17.Guan W-j, Ni Z-y, Hu Y., Liang W-h, Ou C-q, He J-x. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hu Z., Song C., Xu C., Jin G., Chen Y., Xu X. Clinical characteristics of 24 asymptomatic infections with COVID-19 screened among close contacts in Nanjing, China. Sci China Life Sci. 2020:1–6. doi: 10.1007/s11427-020-1661-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang Y., Tu M., Wang S., Chen S., Zhou W., Chen D. Clinical characteristics of laboratory confirmed positive cases of SARS-CoV-2 infection in Wuhan, China: a retrospective single center analysis. Diabetes. 2020;4:11–18. doi: 10.1016/j.tmaid.2020.101606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ki M. Epidemiologic characteristics of early cases with 2019 novel coronavirus (2019-nCoV) disease in Republic of Korea. Epidemiol Health 2020:e2020007. [DOI] [PMC free article] [PubMed]
- 21.Kui L., Fang Y.-Y., Deng Y., Liu W., Wang M.-F., Ma J.-P. Clinical characteristics of novel coronavirus cases in tertiary hospitals in Hubei Province. Chin Med J. 2020;133(9):1025–1031. doi: 10.1097/CM9.0000000000000744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lan L., Xu D., Ye G., Xia C., Wang S., Li Y. Positive RT-PCR test results in patients recovered from COVID-19. Jama. 2020;323(15):1502–1503. doi: 10.1001/jama.2020.2783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li Q., Guan X., Wu P., Wang X., Zhou L., Tong Y. Early transmission dynamics in Wuhan, China, of novel coronavirus–infected pneumonia. N Engl J Med. 2020;382(13):1199–1207. doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li Y., Wang W., Lei Y., Zhang B., Yang J., Hu J. Comparison of the clinical characteristics between RNA positive and negative patients clinically diagnosed with 2019 novel coronavirus pneumonia. Zhonghua jie he he hu xi za zhi= Zhonghua Jiehe he Huxi Zazhi= Chin J Tubercul Respir Dis. 2020;43 doi: 10.3760/cma.j.cn112147-20200214-00095. E023-E023. [DOI] [PubMed] [Google Scholar]
- 25.Ling Y., Xu S.-B., Lin Y.-X., Tian D., Zhu Z.-Q., Dai F.-H. Persistence and clearance of viral RNA in 2019 novel coronavirus disease rehabilitation patients. Chinese Med J. 2020;133(9):1039–1043. doi: 10.1097/CM9.0000000000000774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu C., Jiang Z., Shao C., Zhang H., Yue H., Chen Z. Preliminary study of the relationship between novel coronavirus pneumonia and liver function damage: a multicenter study. Zhonghua gan zang bing za zhi= Zhonghua ganzangbing zazhi= Chin J Hepatol. 2020;28(2):148. doi: 10.3760/cma.j.issn.1007-3418.2020.02.003. [DOI] [PubMed] [Google Scholar]
- 27.Liu W., Tao Z.-W., Lei W., Ming-Li Y., Kui L., Ling Z. Analysis of factors associated with disease outcomes in hospitalized patients with 2019 novel coronavirus disease. Chin Med J. 2020;133(9):1032–1038. doi: 10.1097/CM9.0000000000000775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pan F, Ye T, Sun P, Gui S, Liang B, Li L, et al. Time course of lung changes on chest CT during recovery from 2019 novel coronavirus (COVID-19) pneumonia. Radiology 2020:200370. [DOI] [PMC free article] [PubMed]
- 29.Peng Y., Meng K., Guan H., Leng L., Zhu R., Wang B. Clinical characteristics and outcomes of 112 cardiovascular disease patients infected by 2019-nCoV. Zhonghua Xinxueguanbing Zazhi. 2020;48:E004. doi: 10.3760/cma.j.cn112148-20200220-00105. [DOI] [PubMed] [Google Scholar]
- 30.Shi H., Han X., Jiang N., Cao Y., Alwalid O., Gu J. Radiological findings from 81 patients with COVID-19 pneumonia in Wuhan, China: a descriptive study. Lancet Infect Dis. 2020;20(4):425–434. doi: 10.1016/S1473-3099(20)30086-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Song F, Shi N, Shan F, Zhang Z, Shen J, Lu H, et al. Emerging 2019 novel coronavirus (2019-nCoV) pneumonia. Radiology 2020:200274. [DOI] [PMC free article] [PubMed]
- 32.Tang N., Li D., Wang X., Sun Z. Abnormal Coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemostasis. 2020 Apr;18(4):844–847. doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Team -nNIRS . Communicable diseases intelligence; 2018. 2019-nCoV acute respiratory disease, Australia: epidemiology report 1 (reporting week 26 January-1 February 2020) 2020; 44. [DOI] [PubMed] [Google Scholar]
- 34.Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. Jama. 2020 Mar 17;323(11):1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang D., Ju X., Xie F., Lu Y., Li F., Huang H. Clinical analysis of 31 cases of 2019 novel coronavirus infection in children from six provinces (autonomous region) of northern China. Zhonghua er ke za zhi= Chin J Pediatr. 2020;58(4) doi: 10.3760/cma.j.cn112140-20200225-00138. E011-E011. [DOI] [PubMed] [Google Scholar]
- 36.Wang L., Gao Y-h, Zhang G.-J. The clinical dynamics of 18 cases of COVID-19 outside of Wuhan, China. Eur Respir J. 2020;55(4) doi: 10.1183/13993003.00398-2020. 2000398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wu J, Wu X, Zeng W, Guo D, Fang Z, Chen L, et al. Chest CT findings in patients with corona virus disease 2019 and its relationship with clinical features. Invest Radiol https://doi.org/101097/RLI2020; 670. [DOI] [PMC free article] [PubMed]
- 38.Xia W., Shao J., Guo Y., Peng X., Li Z., Hu D. Clinical and CT features in pediatric patients with COVID-19 infection: different points from adults. Pediatr Pulmonol. 2020;55(5):1169–1174. doi: 10.1002/ppul.24718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xiong Y., Sun D., Liu Y., Fan Y., Zhao L., Li X. Clinical and high-resolution CT features of the COVID-19 infection: comparison of the initial and follow-up changes. Invest Radiol. 2020 doi: 10.1097/RLI.0000000000000674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xu Y.-H., Dong J.-H., An W.-M., Lv X.-Y., Yin X.-P., Zhang J.-Z. Clinical and computed tomographic imaging features of Novel Coronavirus Pneumonia caused by SARS-CoV-2. J Infect. 2020;80(4):394–400. doi: 10.1016/j.jinf.2020.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yang H., Xu J., Li Y., Liang X., Jin Y., Chen S. The preliminary analysis on the characteristics of the cluster for the Corona Virus Disease. Zhonghua liu xing bing xue za zhi= Zhonghua liuxingbingxue zazhi. 2020;41:623. doi: 10.3760/cma.j.cn112338-20200223-00153. [DOI] [PubMed] [Google Scholar]
- 42.Yang W., Cao Q., Qin L., Wang X., Cheng Z., Pan A. Clinical characteristics and imaging manifestations of the 2019 novel coronavirus disease (COVID-19): a multi-center study in Wenzhou city, Zhejiang, China. J Infect. 2020;80(4):388–393. doi: 10.1016/j.jinf.2020.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yang X., Yu Y., Xu J., Shu H., Liu H., Wu Y. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8(5):475–481. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yao Y., Tian Y., Zhou J., Ma X., Yang M., Wang S. Epidemiological characteristics of 2019-ncoV infections in Shaanxi, China by February 8, 2020. Eur Respir J. 2020;55(4) doi: 10.1183/13993003.00310-2020. 2000310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang Jj, Dong X., Cao Y.Y., Yuan Yd, Yang Yb, Yan Yq. Clinical characteristics of 140 patients infected by SARS-CoV-2 in Wuhan, China. Allergy. 2020;75(7):1730–1741. doi: 10.1111/all.14238. [DOI] [PubMed] [Google Scholar]
- 46.Zhang L., Jiang Y., Wei M., Cheng B., Zhou X., Li J. Analysis of the pregnancy outcomes in pregnant women with COVID-19 in Hubei Province. Zhonghua Fu Chan Ke Za Zhi. 2020;55 doi: 10.3760/cma.j.cn112141-20200218-00111. E009-E009. [DOI] [PubMed] [Google Scholar]
- 47.Zhao W., Zhong Z., Xie X., Yu Q., Liu J. Relation between chest CT findings and clinical conditions of coronavirus disease (COVID-19) pneumonia: a multicenter study. Am J Roentgenol. 2020:1–6. doi: 10.2214/AJR.20.22976. [DOI] [PubMed] [Google Scholar]
- 48.Zhu Z., Tang J., Chai X., Fang Z., Liu Q., Hu X. Comparison of heart failure and 2019 novel coronavirus pneumonia in chest CT features and clinical characteristics. Zhonghua Xinxueguanbing Zazhi. 2020;48:E007. doi: 10.3760/cma.j.cn112148-20200218-00093. [DOI] [PubMed] [Google Scholar]
- 49.Cheng L.T.-E., Chan L.P., Tan B.H., Chen R.C., Tay K.H., Ling M.L. Déjà Vu or Jamais Vu? How the severe acute respiratory syndrome experience influenced a Singapore radiology Department's response to the coronavirus disease (COVID-19) epidemic. Am J Roentgenol. 2020:1–5. doi: 10.2214/AJR.20.22927. [DOI] [PubMed] [Google Scholar]
- 50.Feng K., Yun Y., Wang X., Yang G., Zheng Y., Lin C. Analysis of CT features of 15 Children with 2019 novel coronavirus infection. Zhonghua er ke za zhi= Chin J Pediatr. 2020;58:E007. doi: 10.3760/cma.j.issn.0578-1310.2020.0007. [DOI] [PubMed] [Google Scholar]
- 51.Li J., Li S., Cai Y., Liu Q., Li X., Zeng Z. 2020. Epidemiological and Clinical Characteristics of 17 Hospitalized Patients with 2019 Novel Coronavirus Infections Outside Wuhan, China. medRxiv. 02.11.20022053. [Google Scholar]
- 52.Sun K, Chen J, Viboud C. Early Epidemiological Analysis of the 2019-nCoV Outbreak Based on a Crowdsourced Data. medRxiv2020.01.31.20019935. [DOI] [PMC free article] [PubMed]
- 53.Xu X.-W., Wu X.-X., Jiang X.-G., Xu K.-J., Ying L.-J., Ma C.-L. Clinical findings in a group of patients infected with the 2019 novel coronavirus (SARS-Cov-2) outside of Wuhan, China: retrospective case series. Bmj. 2020;368 doi: 10.1136/bmj.m606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pan Y., Guan H., Zhou S., Wang Y., Li Q., Zhu T. Initial CT findings and temporal changes in patients with the novel coronavirus pneumonia (2019-nCoV): a study of 63 patients in Wuhan, China. Eur Radiol. 2020:1–4. doi: 10.1007/s00330-020-06731-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tian S., Hu N., Lou J., Chen K., Kang X., Xiang Z. Characteristics of COVID-19 infection in Beijing. J Infect. 2020;80(4):401–406. doi: 10.1016/j.jinf.2020.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chen L., Liu H., Liu W., Liu J., Liu K., Shang J. Analysis of clinical features of 29 patients with 2019 novel coronavirus pneumonia. Zhonghua jie he he hu xi za zhi= Zhonghua jiehe he huxi zazhi= Chin J Tubercul Respir Dis. 2020;43:E005. doi: 10.3760/cma.j.issn.1001-0939.2020.0005. [DOI] [PubMed] [Google Scholar]
- 57.Ji W., Wang W., Zhao X., Zai J., Li X. Cross-species transmission of the newly identified coronavirus 2019-nCoV. J Med Virol. 2020;92(4):433–440. doi: 10.1002/jmv.25682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jaillon S., Berthenet K., Garlanda C. Sexual dimorphism in innate immunity. Clin Rev Allergy Immunol. 2017:1–14. doi: 10.1007/s12016-017-8648-x. [DOI] [PubMed] [Google Scholar]
- 59.Schurz H., Salie M., Tromp G., Hoal E.G., Kinnear C.J., Möller M. The X chromosome and sex-specific effects in infectious disease susceptibility. Hum Genom. 2019;13(1):2. doi: 10.1186/s40246-018-0185-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mertz D., Kim T.H., Johnstone J., Lam P.-P., Kuster S.P., Fadel S.A. Populations at risk for severe or complicated influenza illness: systematic review and meta-analysis. Bmj. 2013;347:f5061. doi: 10.1136/bmj.f5061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Badawi A., Ryoo S.G. Prevalence of comorbidities in the Middle East respiratory syndrome coronavirus (MERS-CoV): a systematic review and meta-analysis. Int J Infect Dis. 2016;49:129–133. doi: 10.1016/j.ijid.2016.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chan K., Zheng J., Mok Y., Li Y., Liu Y.N., Chu C. SARS: prognosis, outcome and sequelae. Respirology. 2003;8:S36–S40. doi: 10.1046/j.1440-1843.2003.00522.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Assiri A., Al-Tawfiq J.A., Al-Rabeeah A.A., Al-Rabiah F.A., Al-Hajjar S., Al-Barrak A. Epidemiological, demographic, and clinical characteristics of 47 cases of Middle East respiratory syndrome coronavirus disease from Saudi Arabia: a descriptive study. Lancet Infect Dis. 2013;13(9):752–761. doi: 10.1016/S1473-3099(13)70204-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yin Y., Wunderink R.G. MERS, SARS and other coronaviruses as causes of pneumonia. Respirology. 2018;23(2):130–137. doi: 10.1111/resp.13196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hui D.S., Zumla A. Severe acute respiratory syndrome: historical, epidemiologic, and clinical features. Infect Dis Clin. 2019;33(4):869–889. doi: 10.1016/j.idc.2019.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hui D., Joynt G., Wong K., Gomersall C., Li T., Antonio G. Impact of severe acute respiratory syndrome (SARS) on pulmonary function, functional capacity and quality of life in a cohort of survivors. Thorax. 2005;60(5):401–409. doi: 10.1136/thx.2004.030205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sheng G., Chen P., Wei Y., Yue H., Chu J., Zhao J. Viral infection increases the risk of idiopathic pulmonary fibrosis: a meta-analysis. Chest. 2019;80(4):401–406. doi: 10.1016/j.chest.2019.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Xie L., Liu Y., Xiao Y., Tian Q., Fan B., Zhao H. Follow-up study on pulmonary function and lung radiographic changes in rehabilitating severe acute respiratory syndrome patients after discharge. Chest. 2005;127(6):2119–2124. doi: 10.1378/chest.127.6.2119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Booth C.M., Matukas L.M., Tomlinson G.A., Rachlis A.R., Rose D.B., Dwosh H.A. Clinical features and short-term outcomes of 144 patients with SARS in the greater Toronto area. Jama. 2003;289(21):2801–2809. doi: 10.1001/jama.289.21.JOC30885. [DOI] [PubMed] [Google Scholar]
- 70.Wong K., Antonio G.E., Hui D.S., Lee N., Yuen E.H., Wu A. Severe acute respiratory syndrome: radiographic appearances and pattern of progression in 138 patients. Radiology. 2003;228(2):401–406. doi: 10.1148/radiol.2282030593. [DOI] [PubMed] [Google Scholar]
- 71.Choi W.S., Kang C.-I., Kim Y., Choi J.-P., Joh J.S., Shin H.-S. Clinical presentation and outcomes of Middle East respiratory syndrome in the Republic of Korea. Infect Chemother. 2016;48(2):118–126. doi: 10.3947/ic.2016.48.2.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Das K.M., Lee E.Y., Jawder S.E.A., Enani M.A., Singh R., Skakni L. Acute Middle East respiratory syndrome coronavirus: temporal lung changes observed on the chest radiographs of 55 patients. Am J Roentgenol. 2015;205(3):W267–S274. doi: 10.2214/AJR.15.14445. [DOI] [PubMed] [Google Scholar]
- 73.Leist S.R., Jensen K.L., Baric R.S., Sheahan T.P. Increasing the translation of mouse models of MERS coronavirus pathogenesis through kinetic hematological analysis. PloS One. 2019;14(7) doi: 10.1371/journal.pone.0220126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Panesar N. What caused lymphopenia in SARS and how reliable is the lymphokine status in glucocorticoid-treated patients? Med Hypotheses. 2008;71(2):298–301. doi: 10.1016/j.mehy.2008.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Roe M., Bloxham D., White D., Ross-Russell R., Tasker R., O'Donnell D. Lymphocyte apoptosis in acute respiratory syncytial virus bronchiolitis. Clin Exp Immunol. 2004;137(1):139–145. doi: 10.1111/j.1365-2249.2004.02512.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Nkengasong J. China's response to a novel coronavirus stands in Stark contrast to the 2002 SARS outbreak response. Nat Med. 2020:1–2. doi: 10.1038/s41591-020-0771-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


