Abstract
Background
During the ongoing COVID-19 pandemic, healthcare-associated transmission of respiratory viral infections (RVI) is a concern. To reduce the impact of SARS-CoV-2 and other respiratory viruses on patients and healthcare workers (HCWs) we devised and evaluated a multi-tiered infection control strategy with the goal of preventing nosocomial transmission of SARS-CoV2 and other RVIs across a large healthcare campus.
Methods
From January–June 2020, a multi-tiered infection control strategy was implemented across a healthcare campus in Singapore, comprising the largest acute tertiary hospital as well as four other subspecialty centres, with more than 10,000 HCWs. Drawing on our institution's experience with an outbreak of Severe Acute Respiratory Syndrome (SARS) in 2003, this strategy included improved patient segregation and distancing, and heightened infection prevention and control (IPC) measures including universal masking. All symptomatic patients were tested for COVID-19 and common RVIs.
Results
A total of 16,162 admissions campus-wide were screened; 7.1% (1155/16,162) tested positive for COVID-19. Less than 5% of COVID-19 cases (39/1155) were initially detected outside of isolation wards in multi-bedded cohorted wards. Improved distancing and enhanced IPC measures successfully mitigated onward spread even amongst COVID-19 cases detected outside of isolation. COVID-19 rates amongst HCWs were kept low (0.13%, 17/13,066) and reflected community acquisition rather than nosocomial spread. Rates of healthcare-associated-RVI amongst inpatients fell to zero and this decrease was sustained even after the lifting of visitor restrictions.
Conclusion
This multi-tiered infection control strategies can be implemented at-scale to successfully mitigate healthcare-associated transmission of respiratory viral pathogens.
Keywords: Containment, COVID-19, Healthcare associated, Surveillance, Transmission
Introduction
In the current COVID-19 pandemic, healthcare-associated transmission to healthcare workers (HCWs) and patients has been a major concern [1,2]. However, distinguishing cases of COVID-19 can be difficult, as COVID-19 may manifest with non-specific symptoms [3]. During previous outbreaks caused by novel pathogens, various containment strategies were utilised, such as creating “fever wards” to isolate patients with febrile pneumonia [4]. However, transmission in pre-symptomatic or asymptomatic individuals makes containment of COVID-19 challenging [5]. As SARS-CoV-2 may be more transmissible than known respiratory viruses, current infection prevention and control (IPC) measures against respiratory-viral-infections (RVIs) must be strengthened [6]. Given the frequency of inter-institutional movement of patients and HCWs, coordinated containment strategies are necessary [7].
In Singapore, a Southeast Asian city-state, by end-February 2020, the majority of COVID-19 cases were attributed to local community transmission [8]. A subsequent surge attributed to outbreaks in foreign worker dormitories began from April 2020 onwards, with more than 50,000 cases reported nationwide by July 2020 [9]. Containment strategies for COVID-19 have been implemented in acute hospitals [[10], [11], [12]]. However, on the largest healthcare campus in Singapore, a coordinated multi-tiered infection control strategy was necessary, given the presence of a large acute tertiary hospital, multiple large specialised centres, and a community hospital providing step-down care; with frequent inter-institution movements of staff and patients. We aimed to evaluate the effectiveness of this multi-tiered infection control strategy in preventing nosocomial transmission of SARS-CoV2 and secondarily, in the transmission of other RVIs across a large healthcare campus.
Methods
Institutional setting and study period
The Outram campus hosts the Singapore General Hospital (SGH), the largest tertiary hospital in Singapore with 1785 beds, Outram Community Hospital (OCH), a 545-bed community hospital providing step-down care, and other specialist centres, including the National Heart Centre, Singapore (NHCS), National Cancer Centre, Singapore (NCC), Singapore National Eye Centre (SNEC), and the National Neuroscience Institute (NNI). Almost 13,000 HCWs work on-campus. The study period lasted 6 months (7th January 2020 to 7th July 2020).
Campus-wide multi-tiered infection control strategy during COVID-19 pandemic
A multi-tiered approach was adopted campus-wide to contain COVID-19 amongst HCWs and patients. Recognising the need for a campus-level coordinating platform for emergency preparedness in pre-pandemic planning, since 2007, the Outram Campus Command Centre (OCC) was designated the nerve centre for overall campus-wide command-and-control during a disease outbreak situation. As the primary command-and-control centre, the OCC provided instructions and protocols to all identified key hospital command posts for ground implementation and served as the contact-point for external parties (eg. our local Ministry of Health). The Disease Outbreak Taskforce (DOTF) oversaw the OCC, and the SGH Preparedness and Response Department (PRD) acted as Secretariat to the OCC.
During the COVID-19 pandemic, the DOTF was activated upon detection of the first imported case of COVID-19 in Singapore on the 23rd January 2020 [8]. The DOTF met daily to coordinate the campus-wide pandemic response. The OCC provided a platform for communication and implementation of the multi-tiered infection control strategy, which was devised with specialist input from the DOTF, in particular the Disease Outbreak Response Team (DORT), drawing on our institution's experience with an outbreak of Severe Acute Respiratory Syndrome (SARS) in 2003 [4]. An overview of the organisation of the OCC and DOTF is provided in Supplementary Fig. 1. Each specialist centre had its own IPC lead responsible for implementing the multi-tiered infection control strategy in its own institutional context, with the support of the DOTF and DORT. The first tier involved campus-wide perimeter screening and visitor management, while the second tier involved improved segregation for suspect COVID-19 cases in the hospital emergency department (ED). Enhanced inpatient segregation formed the third tier, and the fourth tier included enhanced campus-wide IPC measures. All four tiers of our containment strategy were implemented by the beginning of February 2020.
Campus-wide perimeter screening and visitor management during COVID-19 pandemic
Screening counters were set up at campus entrances to screen all staff, visitors and patients entering both inpatient and outpatient areas (Supplementary Fig. 2a). All patients and visitors declared symptoms, travel history, contact history and underwent temperature screening. Febrile staff would be directed to SGH Staff Clinic and febrile patients/visitors would be prevented from entering. Waiting areas were also modified for improved safe distancing (Supplementary Fig. 2a), and all visitors were required to wear a face covering. From 7th April 2020 – 1st June 2020, a no-visitor policy was enforced, in-line with the nationwide public lockdown during which all workplaces and schools were closed. The no-visitor policy was lifted from 2nd June 2020 onwards, in conjunction with the lifting of the nationwide lockdown.
Emergency department: improved segregation during COVID-19 pandemic
Our ED's protocols for the safe management of COVID-19 suspects have been previously published elsewhere [[13], [14], [15]]. We evaluated the success of these protocols in the right-siting of COVID-19 patients. In brief, patients with epidemiologic risk factors and patients presenting with clinical syndromes potentially compatible for COVID-19, were strictly segregated in dedicated ‘fever areas’ (Supplementary Fig. 2b). In these ‘fever areas’, improved spacing between ED trolleys (at least 2 m apart) and patient bays separated by partitions were introduced, and HCWs used full personal-protective-equipment (PPE), including disposable gloves, gowns, eye protection, and N95 respirators.
Inpatient wards: improved segregation during COVID-19 pandemic
Pre-pandemic, patients were nursed in open-plan cohorted general wards. A risk-stratified approach was adopted for the right-siting and screening of all inpatient admissions (Supplementary Fig. 2c). High-risk patients who met the criteria for suspected COVID-19 based on suspicious epidemiology, eg. staying in communal settings, high-risk occupations, contact with known COVID-19 clusters or cases, or travel history to areas with high incidence of COVID-19 were admitted to the purpose-built isolation ward (IW) which was equipped with negative-pressure airborne-infection-isolation-rooms (AIIRs) [11]. All HCWs in IW used disposable gloves, gowns, eye protection, and N95 respirators for protection.
From February 2020, given ongoing community transmission, lower-risk individuals with clinical syndromes compatible with COVID-19 (eg. respiratory symptoms, infiltrates on chest imaging, or undifferentiated viral fever) who did not have epidemiologic risk factors were segregated in dedicated general wards, termed as “respiratory surveillance wards” (RSWs) [11,13]. Improved segregation for symptomatic inpatients was hence adopted campus-wide, for general medical and surgical patients in the acute tertiary hospital as well as inpatients of the various specialised centres on campus (cardiology, neurology, and oncology patients). The detailed implementation and specialty-specific protocols have been published elsewhere [11,[16], [17], [18], [19]]. In brief, in these converted RSWs, infrastructural enhancements were implemented (half-height partitions between beds and reduced number of beds per cubicle), and HCWs used disposable gloves, gowns, eye protection, and N95 respirators until COVID-19 was excluded [11,13]. If patients awaiting the results of COVID-19 testing required within-campus movement for clinical care (eg. urgent procedures and imaging, step-down or step-up of care), transfers proceeded with HCWs using disposable gloves, gowns, eye protection, and N95 respirators. Finally, all patients stepped-down to the community hospital were tested for COVID-19 prior to transfer, regardless of symptoms. As all community hospital admissions had to test negative for COVID-19 pre-transfer and the community hospital did not take direct admissions from the community, the community hospital did not have dedicated RSWs. However, isolation rooms and COVID-19 testing were available should patients develop new respiratory symptoms.
Enhanced campus-wide IPC measures during COVID-19 pandemic
From February 2020 onwards, a universal masking policy was introduced, mandating that HCWs wear surgical masks in all clinical areas as a mandatory minimum [20]. Additionally, all aerosol-generating-procedures (AGPs) in clinical areas were performed using disposable gloves, gowns, eye protection, and N95 respirators, regardless of patients' COVID-19 status. Prior to the pandemic, AGPs were performed using surgical masks, disposable gloves and apron as the mandatory minimum, with N95 respirators used for patients isolated under airborne precautions. Safe distancing measures were introduced campus-wide [21]. A centralised campus-wide staff surveillance strategy was adopted, with temperature monitoring twice-daily in an electronic surveillance system. HCWs with fever or symptoms of acute respiratory illness (ARI) were required to report to the Staff Clinic for COVID-19 testing, and were placed on mandatory 5-day medical leave [10]. Our institution encourages all HCWs to receive yearly influenza vaccination at the Staff Clinic; this was also reinforced during the pandemic. As part of general IPC measures, pre-pandemic all patient areas were disinfected with 1:1000 hypochlorite-based solution at least twice a day; during the pandemic, disinfection frequency was stepped up to thrice a day. Cleaners in high-risk areas, such as the IW and ‘fever areas’ in the ED, were required to wear disposable gloves, gowns, eye protection, and N95 respirators. Additionally, hand hygiene compliance with the World Health Organisation (WHO) “Your 5 Moments for Hand Hygiene” was re-emphasised during the COVID-19 pandemic and alcohol handrub/wipes were provided in all clinical areas.
Evaluation of effectiveness of campus-wide containment strategy in preventing healthcare-associated transmission of respiratory viral infections
Prevention of healthcare-associated transmission of SARS-CoV-2 was the primary outcome assessed. When cases of COVID-19 were detected amongst HCWs, or in patients housed outside of IW, contact tracing was done within 24-h to identify close-contacts [[10], [11], [12]]. All patient and HCW close-contacts were tested if symptoms developed within 14-days post-exposure. Epidemiological investigations were conducted to determine if cases arose from nosocomial transmission (patient-to-patient, patient-to-HCW, or HCW-HCW). As a secondary outcome, we also evaluated the impact of the multi-tiered infection control strategy on the transmission of other RVIs amongst hospitalised inpatients. All patients with respiratory symptoms were tested for both COVID-19 as well as a panel of 16 common RVI via multiplex PCR [24]. PCR-positive cases of RVI were categorized as healthcare-associated if the respiratory virus was identified beyond the maximum incubation period from the time of admission [20]. Comparisons of RVI rates pre-pandemic and during the pandemic were made using the incidence-rate-ratio method, with the null hypothesis being that the proportion of RVIs would be proportional to the number of at-risk inpatient days for each period. A p-value of ≤0.05 was considered significant.
The objective of improved segregation was to ensure that all COVID-19 cases were managed in areas with heightened infection prevention precautions (ED ‘fever areas’, IW, RSW). To estimate the relative success of improved segregation, descriptive statistics were computed for the number of COVID-19 tests done campus-wide, and at which tier positive cases were detected-at perimeter screening, ED, IW, RSW, or general ward. The goal of enhanced campus-wide IPC measures was to prevent nosocomial transmission of SARS-CoV-2 in cases of COVID-19 detected in the general ward setting. We assessed, through epidemiological investigations, the possibility of onward transmission to patients and HCWs when cases of COVID-19 were encountered outside of isolation. Finally, to assess compliance with enhanced campus-wide IPC measures, we compared the following measures, pre-pandemic and during the pandemic period: campus-wide uptake of influenza vaccination and report-sick rates for acute respiratory illness at Staff Clinic; campus-wide consumption of alcohol handrub and cleaning wipes; and results of campus-wide regular environmental cleaning audit using fluorescent markers (Glogerm) and hand-hygiene audit.
Results
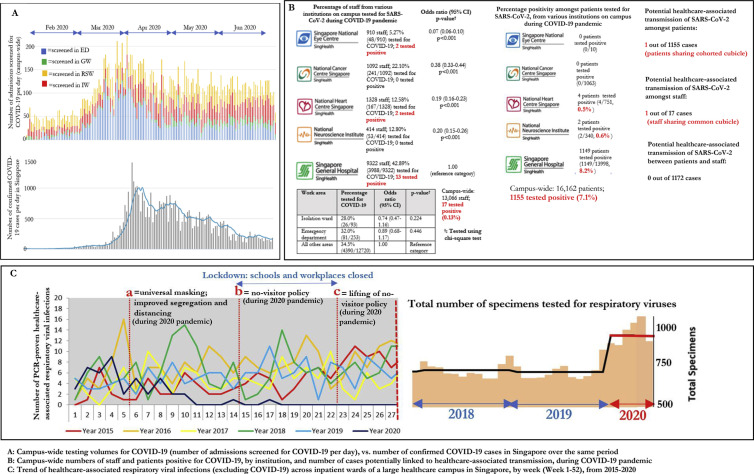
Over a 6-month period during the COVID-19 pandemic, nosocomial transmission of SARS-CoV-2 was limited across a large healthcare campus, despite managing a significant volume of COVID-19 cases. A total of 1525 cases of COVID-19 were managed on-campus; of which the majority (75.7%, 1155/1525) were undiagnosed at the time of presentation. Over the study period, an average of 130 patients-a-day were tested for COVID-19, with a peak of more than 200 patients in April 2020, corresponding to the nationwide surge in cases (Fig. 1 a). A total of 16,162 patient admissions campus-wide were screened for COVID-19, with a positive rate of 7.2% (1155/16,162) (Fig. 1b). Epidemiological investigations revealed only one potential case of healthcare-associated transmission of SARS-CoV-2 between patients, with potential overlapping contact in a RSW [12].
Fig. 1.
Effect of campus-wide multi-tiered infection control strategy on healthcare-associated transmission of SARS-CoV-2 as well as common respiratory viral infections during 2020 COVID-19 pandemic.
Less than 1% of the campus-wide workforce (0.1%, 17/13,066) were diagnosed with COVID-19 (Fig. 1b). Over the study period, one-third (34.4%, 4497/13,066) of HCWs had been tested for SARS-CoV-2; an average of 30 HCWs were tested for COVID-19 at the Staff Clinic daily. Staff working in high-risk areas, such as the IW and the ED, did not have higher odds of COVID-19 testing, compared to staff working in other areas (Fig. 1b). Epidemiology investigations indicated that the majority of COVID-19 cases in HCWs were linked to known community cases outside of hospital (70.6%, 12/17). Four remaining HCW cases were unlinked, but did not have patient contact in high-risk areas and did not have contact with each other; one HCW shared an office cubicle with a known COVID-19 case [10]. There was no evidence of patient-HCW transmission [10].
Infection control measures were also remarkably successful in containing healthcare-associated-RVI across a large healthcare campus. In the six months pre-pandemic, the campus-wide incidence of healthcare-associated-RVI was 7.8 cases per-10,000 patient-days (195 cases; 250,596 patient-days). After introduction of the campus-wide containment strategy, the incidence of healthcare-associated-RVI was 1.5 cases per-10,000 patient-days (31 cases; 203,711 patient-days). The incidence-rate-ratio of healthcare-associated-RVI per 10,000-patient-days between the two periods (pre- and post-pandemic) was 0.2 (95% confidence interval, 95%CI = 0.13–0.29, p < 0.001) (Fig. 1c). The marked decrease in healthcare-associated-RVI was observed despite increased testing. Prior to the pandemic, an average of 701 RVI-panels/month were ordered; during the pandemic, this rose to 970 tests/month.
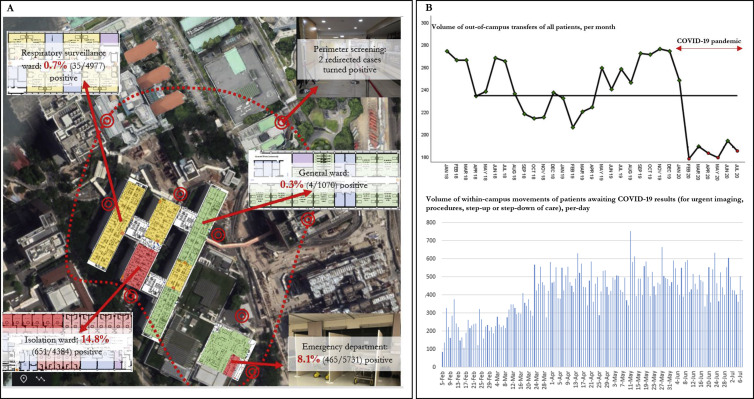
Improved segregation, a crucial part of our containment strategy, was successful in ensuring that most COVID-19 cases were managed in areas with heightened infection prevention precautions (ED ‘fever areas’, IW, RSW), prior to diagnosis. Only 4 cases of newly-diagnosed COVID-19 (0.3%, 4/1155) were picked up in the general ward; the remainder had been managed in areas with heightened precautions while awaiting the return of diagnostic testing (Fig. 2 a). COVID-19 suspects were assigned to IW or RSW based on risk-stratification; not unexpectedly, the testing yield of patients assigned to the IW was much higher compared to those assigned to the RSW (14.8%, 651/4384 in IW, vs 0.7%, 35/4977 in the RSW) (Fig. 2a). One-third of admissions were first tested in ED ‘fever areas’ (35.5%, 5731/16,162), with a positive rate of 8.1% (465/5731). Testing yield outside of ED ‘fever areas’, IW or the RSW was low. Amongst patients screened on the general ward, only 4 tested positive (0.4%, 4/1070) (Fig. 2a). A total of 3080 patients were swabbed prior to community hospital stepdown; none tested positive. Despite segregation, in order not to compromise on patient care, within-campus movement for clinical care of patients with pending COVID-19 results was continued. During the pandemic, while out-of-campus transfers were significantly curtailed, within-campus transfers of patients awaiting the results of COVID-19 testing were maintained at an average of 388 patient movements per-day (Fig. 2b). No nosocomial transmission of COVID-19 occurred as a result of these transfers; all HCWs involved in these transfers used PPE.
Fig. 2.
Campus-wide testing yield for COVID-19, broken down according to individual tiers of containment response; and continuation of campus-wide patient movement during COVID-19 pandemic.
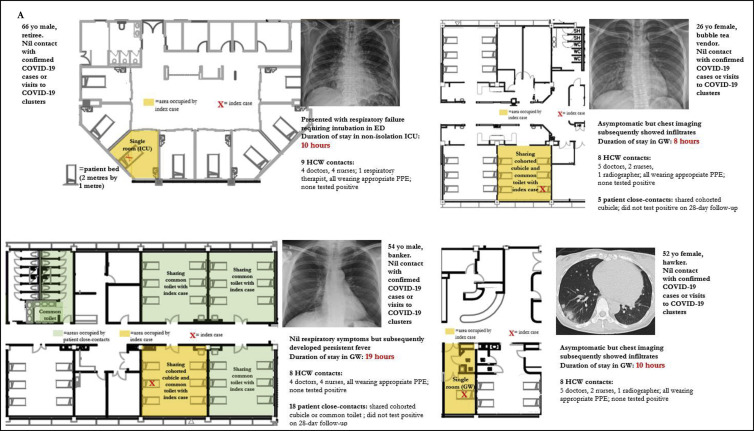
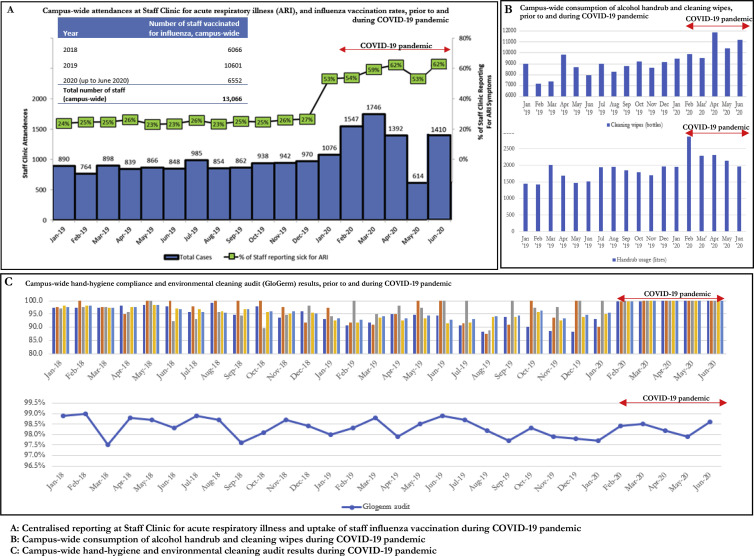
Amongst the small number of COVID-19 cases picked up outside of isolation in the general ward (N = 4), a total of 23 patient close-contacts and 33 HCW close-contacts were identified (Fig. 3 ). None tested positive on 28-day follow-up. Enhanced campus-wide IPC measures, including a universal masking policy, ensured that all HCWs had used a surgical mask, at the minimum, when coming into contact with these unsuspected COVID-19 cases prior to diagnosis. Although one case required intubation and was in a non-isolation ICU room for 10 h prior to diagnosis, all HCWs performing AGPs had used disposable gloves, gowns, eye protection, and N95 respirators (Fig. 3). To evaluate compliance with enhanced campus-wide IPC measures, comparisons of Staff Clinic attendances for acute respiratory illness and influenza vaccination, as well as consumption of handrub/cleaning wipes and results of hand hygiene and environmental cleaning audits pre- and post-pandemic are provided in Fig. 4 . Campus-wide compliance to yearly influenza vaccination was high pre-pandemic, with 81.1% of HCWs receiving influenza vaccination in 2019 (Fig. 4a). During the pandemic, there was an increase in Staff Clinic ARI attendances, likely due to mandatory centralised reporting to facilitate contact tracing as well as staff advisories discouraging presenteeism. Campus-wide consumption of alcohol handrub rose from 1746 L/month in the preceding year (2019) to 2313 L/month during the pandemic period (Fig. 4b). Similarly, consumption of cleaning wipes rose from 8669 bottles/month pre-pandemic to 10,391 bottles/month, during the pandemic period (Fig. 4b). Pre-pandemic, campus-wide hand hygiene rates were high (95.0%; min 87.1%, max 100%); during the pandemic period, hand hygiene rates were maintained at close to 100.0% across all 5 hand hygiene moments audited (Fig. 4c). Standards of environmental cleaning pre-pandemic on regular audit using fluorescent markers (Glogerm) were high, and these standards were maintained throughout the pandemic (Fig. 4c).
Fig. 3.
Containment of unsuspected COVID-19 cases in general ward setting (N = 4) = patient bed (2 m by 1 m).
Fig. 4.
Compliance with campus-wide enhanced infection prevention and control measures during COVID-19 pandemic Campus-wide consumption of alcohol handrub and cleaning wipes, prior to and during COVID-19 pandemic.
Discussion
The key finding of this study is that a multi-tiered infection control strategy was successful in mitigating healthcare-associated transmission of COVID-19 as well as common RVIs across a large healthcare campus, over a sustained duration. Over a 6-month period, no documented patient-HCW transmission of COVID-19 occurred, despite caring for more than 1500 cases of COVID-19 campus-wide. While healthcare institutions in Hong Kong and Taiwan introduced similar strategies [22], ongoing community transmission, with more than 50,000 cases reported nationwide as of July 2020, meant that our containment strategy has been tested over time. In the initial phases of the pandemic, various healthcare systems progressively adopted more aggressive measures [23,24]. However, the key components of our containment strategy were all implemented by early February 2020 and sustained over a six-month period.
Several factors were key to our success. Firstly, one-third of admissions were first tested in ED, allowing for faster turnaround of results and determination of COVID-19 status from arrival. Furthermore, due to improved segregation, less than 1% of COVID-19 cases campus-wide were picked up in multi-bedded cohorted general wards, outside of designated areas for the management of COVID-19 cases [12]. Given that a single unsuspected COVID-19 patient in a cohorted general ward could cause significant disruption through ward closures [22], our institution's strategy minimised this possibility. The mutually reinforcing nature of our multi-tiered infection control strategy meant that enhanced IPC measures, such as universal masking, hand hygiene and environmental cleaning, could prevent onward transmission even when COVID-19 cases presented outside of containment in the general ward, or if minimally symptomatic HCWs with COVID-19 had prolonged staff/patient contact prior to diagnosis [25].
As an unintended consequence, implementation of our COVID-19 containment strategy effectively curtailed transmission of healthcare-associated-RVIs across an entire healthcare system. Though community surveillance demonstrated a reduction in influenza-like activity [26], there was no corresponding decrease in the number of patients presenting with community-acquired-RVI to our institution [27]. Given that healthcare-associated-RVI remains an underappreciated cause of morbidity and mortality in adult inpatients [28], IPC measures that potentially mitigate healthcare-associated-RVI have clear value. Although it was not possible to ascertain the individual contribution of each tier of our infection control strategy, careful comparison of audit results pre- and post-pandemic provided some insights. General IPC measures, such as hand hygiene, environmental cleaning standards, and compliance to yearly influenza vaccination were already at high levels pre-pandemic. The marginal changes observed during the pandemic are unlikely to have accounted for the substantial drop in healthcare-associated-RVI alone. While visitor restrictions formed a key tier of measures introduced during the pandemic, the reduction in healthcare-associated-RVI persisted even after the rollback of visitor restrictions. Improved campus-wide segregation of symptomatic patients and reductions in presenteeism amongst staff with ARI symptoms are likely to have contributed significantly to the campus-wide decrease in healthcare-associated-RVI. Indeed, on our institution's haematology wards, which had implemented a universal masking policy pre-pandemic, outbreaks of RVI persisted despite the universal masking policy and only subsided after the introduction of improved segregation as part of pandemic measures [29].
The limitations of our study were as follows. Only symptomatic patient and staff close-contacts were tested for COVID-19, similar to the practice in other large healthcare systems [22,24]. To improve detection, centralised free testing was available at the Staff Clinic [20]. Ongoing national serological surveys of HCWs have not detected additional unsuspected cases of COVID-19 to-date [30].
Conclusion
A multi-tiered infection control strategy was successful in mitigating healthcare-associated transmission of COVID-19 and other common RVIs across a large healthcare campus over a sustained period. Multi-tiered infection control strategies to mitigate healthcare-associated transmission of respiratory viral pathogens can be feasibly implemented, even in large and complex healthcare systems.
Ethics
Waiver of informed consent was approved by our hospital's Institutional Review Board (CIRB Ref 2020/2436).
Authorship statement
Conceptualisation - EW, IV, XS, LW; Manuscript review - IV, XS, KT, RW, CT, WG, KK, WH, GC, EC, CS, XN, JO, JC, YC, ML, TT, LW; Analysis - EW; Manuscript preparation - EW; Project administration - KT, RW, CT, WG, KK; Data curation - KK, WH, GC, EC, CS, XN, JO, JC; Supervision - YC, ML, TT, LW.
Conflict of interest
The authors report no conflicts of interest.
Funding
This study was not grant-funded.
Provenance and peer review
Not commissioned; externally peer reviewed.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.idh.2020.11.004.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Vanhems P., Saadatian-Elahi M., Chuzeville M., Marion E., Favrelle L., Hilliquin D. Fast nosocomial spread of SARS-CoV2 in a French geriatric unit. Infect Contr Hosp Epidemiol. 2020 doi: 10.1017/ice.2020.99. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang X., Zhou Q., He Y., Liu L., Ma X., Wei X. Nosocomial outbreak of COVID-19 pneumonia in Wuhan, China. Eur Respir J. 2020;55(6):2000544. doi: 10.1183/13993003.00544-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020 doi: 10.1016/S0140-6736(20)30183-5. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tan Y.M., Chow P.K., Tan B.H., Kurup A., Tan B.K., Tan F.L. Management of inpatients exposed to an outbreak of severe acute respiratory syndrome (SARS) J Hosp Infect. 2004;58(3):210–215. doi: 10.1016/j.jhin.2004.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gandhi M., Yokoe D.S., Havlir D.V. Asymptomatic transmission, the Achilles' heel of current strategies to control COVID-19. N Engl J Med. 2020 doi: 10.1056/NEJMe2009758. Apr 24 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Klompas M. Coronavirus disease 2019 (COVID-19): protecting hospitals from the invisible. Ann Intern Med. 2020 doi: 10.7326/M20-0751. Mar 11 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Heinzerling A., Stuckey M.J., Scheuer T., Xu K., Perkins K.M., Resseger H. Transmission of COVID-19 to Health care personnel during exposures to a hospitalized patient - solano county, California, february 2020. MMWR Morb Mortal Wkly Rep. 2020;69(15):472–476. doi: 10.15585/mmwr.mm6915e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wong J.E.L., Leo Y.S., Tan C.C. COVID-19 in Singapore-current experience: critical global issues that require attention and action. J Am Med Assoc. 2020 Feb 20 doi: 10.1001/jama.2020.2467. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 9.Chew M.H., Koh F.H., Wu J.T., Ngaserin S., Ng A., Ong B.C. Clinical assessment of COVID-19 outbreak among migrant workers residing in a large dormitory in Singapore. J Hosp Infect. 2020 May 31;S0195–6701(20):30274–30277. doi: 10.1016/j.jhin.2020.05.034. Epub ahead of print. PMID: 32492454; PMCID: PMC7261446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wee L.E., Sim X.Y.J., Conceicao E.P., Aung M.K., Goh J.Q., Yeo D.W.T. Containment of COVID-19 cases among healthcare workers: the role of surveillance, early detection, and outbreak management. Infect Control Hosp Epidemiol. 2020:1–7. doi: 10.1017/ice.2020.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wee L.E., Hsieh J.Y.C., Phua G.C., Tan Y., Conceicao E.P., Wijaya Respiratory surveillance wards as a strategy to reduce nosocomial transmission of COVID-19 through early detection: the experience of a tertiary-care hospital in Singapore. Infect Control Hosp Epidemiol. 2020:1–6. doi: 10.1017/ice.2020.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wee L.E.I., Sim X.Y.J., Conceicao E.P., Aung M.K., Tan K.Y., Ko K.K.K. Containing COVID-19 outside the isolation ward: the impact of an infection control bundle on environmental contamination and transmission in a cohorted general ward. Am J Infect Contr. 2020;S0196–6553(20):30569. doi: 10.1016/j.ajic.2020.06.188. 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wee L.E., Fua T.P., Chua Y.Y., Ho A.F.W., Sim X.Y.J., Conceicao E.P. Containing COVID-19 in the emergency department: the role of improved case detection and segregation of suspect cases. Acad Emerg Med. 2020 May;27(5):379–387. doi: 10.1111/acem.13984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Quah L.J.J., Tan B.K.K., Fua T.P., Wee C.P.J., Lim C.S., Nadarajan G. Reorganising the emergency department to manage the COVID-19 outbreak. Int J Emerg Med. 2020;13(1):32. doi: 10.1186/s12245-020-00294-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thangayah J.R., Tan K.B.K., Lim C.S., Fua T.P. Disease outbreak surge response: how a Singapore tertiary hospital converted a multi-storey carpark into a flu screening area to respond to the COVID-19 pandemic. Disaster Med Public Health Prep. 2020:1–19. doi: 10.1017/dmp.2020.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wee L.E., Sim X.Y.J., Conceicao E.P., Aung M.K., Wong H.M., Teh Y.E. Surgical Infections; 2020. Early recognition of COVID-19 infection in surgical inpatients: the importance of a risk-stratified approach for early testing and isolation. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 17.Wee L.E., Ruan W., Sim X.Y.J., Ng K., Phoon P.C., Conceicao E.P. Surveillance for COVID-19 in cardiac inpatients: containing COVID-19 in a specialised cardiac centre. Can J Cardiol. 2020 Aug;36(8):1327.e3–1327.e4. doi: 10.1016/j.cjca.2020.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wee L.E.I., Thien S.Y., Singh S.R., Ling M.L., Venkatachalam I. Neurological Sciences; 2020. Containing COVID-19 in a specialised neurology centre: the risks of pre-symptomatic transmission. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chiang J., Yang V.S., Han S., Zhuang Q., Ooi G., Sin I.H. Minimising transmission of COVID-19 while delivering optimal cancer care in a National Cancer Centre. J Can Pol. 2020 Sep;25:100241. doi: 10.1016/j.jcpo.2020.100241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wee L.E., Conceicao E.P., Sim X.Y.J., Ko K.K.K., Ling M.L., Venkatachalam I. Reduction in healthcare-associated respiratory viral infections during a COVID-19 outbreak. Clin Microbiol Infect. 2020 Nov;26(11):1579–1581. doi: 10.1016/j.cmi.2020.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wee L.E., Conceicao E.P., Sim X.Y.J., Aung M.K., Tan K.Y., Wong H.M. Minimising intra-hospital transmission of COVID-19: the role of social distancing. J Hosp Infect. 2020;105(2):113–115. doi: 10.1016/j.jhin.2020.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wong S.C.Y., Kwong R.T., Wu T.C., Chan J.W.M., Chu M.Y., Lee S.Y. Risk of nosocomial transmission of coronavirus disease 2019: an experience in a general ward setting in Hong Kong. J Hosp Infect. 2020;105(2):119–127. doi: 10.1016/j.jhin.2020.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rhee C., Baker M.A., Klompas M. The COVID-19 infection control arms race. Infect Control Hosp Epidemiol. 2020:1–3. doi: 10.1017/ice.2020.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang X., Ferro E.G., Zhou G., Hashimoto D., Bhatt D.L. Association between universal masking in a Health care system and SARS-CoV-2 positivity among Health care workers. J Am Med Assoc. 2020 doi: 10.1001/jama.2020.12897. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wee L.E., Sim X.Y.J., Conceicao E.P., Aung M.K., Tan J.Y., Venkatachalam I. Containment of COVID-19 amongst ancillary healthcare workers: an integral component of infection control. J Hosp Infect. 2020 Oct;106(2):392–396. doi: 10.1016/j.jhin.2020.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Soo R.J.J., Chiew C.J., Ma S., Pung R., Lee V. Decreased influenza incidence under COVID-19 control measures, Singapore. Emerg Infect Dis. 2020 Apr 27;(8):26. doi: 10.3201/eid2608.201229. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wee L.E., Ko K.K.K., Ho W.Q., Kwek G.T.C., Tan T.T., Wijaya L. Community-acquired viral respiratory infections amongst hospitalized inpatients during a COVID-19 outbreak in Singapore: co-infection and clinical outcomes. J Clin Virol. 2020;128:104436. doi: 10.1016/j.jcv.2020.104436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chow E.J., Mermel L.A. Hospital-acquired respiratory viral infections: incidence, morbidity, and mortality in pediatric and adult patients. Open Forum Infect Dis. 2017;4(1):ofx006. doi: 10.1093/ofid/ofx006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wee L.E., Conceicao E.P., Tan J.Y., Venkatachalam I., Ling M.L. Zero healthcare-associated respiratory viral infections amongst haematology inpatients: unexpected consequence of heightened infection control during COVID-19 outbreak. J Hosp Infect. 2021 Jan;107(1):1–4. doi: 10.1016/j.jhin.2020.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.National Centre for Infectious Diseases . National Healthcare Group; Singapore: 2020. Singapore. Three national-level seroepidemiological studies to determine level of COVID-19 infection in Singapore.https://www.ncid.sg/News-Events/News/Pages/Three-national-level-seroepidemiological-studies-to-determine-level-of-COVID-19-infection-in-Singapore.aspx Published 29th April 2020; accessed 17th July. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.