Abstract
Background
The COVID-19 pandemic has raised concerns over secondary infections because it has limited treatment options and empiric antimicrobial treatment poses serious risks of aggravating antimicrobial resistance (AMR). Studies have shown that COVID-19 patients are predisposed to develop secondary infections. This study was conducted to ascertain the prevalence and profiles of co- & secondary infections in patients at the COVID-19 facility in North India.
Methods
We studied the profile of pathogens isolated from 290 clinical samples. Bacterial and fungal pathogens were identified, and antimicrobial susceptibility was determined by the Vitek2® system. Additionally, respiratory samples were tested for any viral/atypical bacterial co-infections and the presence of AMR genes by FilmArray test. The clinical and outcome data of these patients were also recorded for demographic and outcome measures analyses.
Results
A total of 151 (13%) patients had secondary infections, and most got infected within the first 14 days of hospital admission. Patients aged >50 years developed severe symptoms (p = 0.0004) and/or had a fatal outcome (p = 0.0005). In-hospital mortality was 33%.K.pneumoniae (33.3%) was the predominant pathogen, followed by A. baumannii (27.1%). The overall resistance was up to 84%.Majority of the organisms were multidrug-resistant (MDR) harbouring MDR genes.
Conclusion
A high rate of secondary infections with resistant pathogens in COVID-19 patients highlights the importance of antimicrobial stewardship programs focussing on supporting the optimal selection of empiric treatment and rapid-de-escalation, based on culture reports.
Keywords: COVID-19, Secondary infections, Film array, Antimicrobial resistance, Antimicrobial stewardship
1. Background
The ongoing COVID-19 pandemic, caused by the Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) virus, has thrust the healthcare systems to a verge of collapse and incapacitated economic activities, globally [1,2]. India alone has reported 1,238,635 COVID-19 cases as of July 23, 2020; the third highest in the world.
In the absence of properly powered, randomized controlled trials of any potential treatment drugs or vaccines, severe COVID-19 may still be considered as essentially untreatable [3].
Hospitalization of COVID-19 cases, especially in the ICUs, predisposes them to undesirable consequences, one of the most serious being healthcare-associated infections (HAIs)/or secondary infections. Table 1 summarizes the current body of evidence surrounding viral/bacterial/fungal co-infection in patients with coronavirus infection, including the SARS-CoV-2 infection. Most of the published reports are from China and only a few from the rest of the world. Although secondary infections appear to be prevalent among COVID-19 patients, it is still an understudiedphenomenon [4].
Table 1.
Summary of published data describing secondary infections in COVID-19 patients.
| Country | Total no. of patients | Secondary infections |
Ref. | ||||
|---|---|---|---|---|---|---|---|
| No. of patients (%) | Identified organisms (No. of patients) |
Clinical Outcome | |||||
| Viral | Bacterial | Fungal | |||||
| USA | 5700 | 42 (2.1%) | Rhinovirus/enterovirus (22), other coronaviridae (7), RSV (4), parainfluenza (3), metapneumovirus (2), and influenza A (1) |
Chlamydophila pneumoniae (2), M. pneumoniae (1) | NA | 21% died | [12] |
| USA | 338 | 19 (5.6%) | NA | NA | [13] | ||
| China | 201 (ARDS) | 1 (0.6%) | Influenza A virus (1) | NA | NA | [14] | |
| China | 191 | 27 (50%) of 54 non-survivors | NA | 96% died | [2] | ||
| USA | 116 | 24 (20.7%) | Rhinovirus/enterovirus (8), RSV (6), other coronaviridae (5), parainfluenza (3), metapneumovirus (2), and influenza A (1) |
NA | NA | [15] | |
| China | 115 | 5 (4.3%) | Influenza A virus (3) Influenza B virus (2) | NA | NA | [16] | |
| China | 99 | 5 (5%) | NA | Acinetobacter baumannii, Klebsiella pneumoniae, and Aspergillus flavus in respiratory samples (1) |
Candida albicans (3) Candida glabrata (1) |
NA | [17] |
| Italy | 73 (ARDS) |
Bacterial pneumonia (9, 17.2%) Secondary bacteremia (27, 37.0%) Other secondary infection (3, 4.1%) |
23.3% died | [18] | |||
| China | 41 | 4 (9.8%) | NA | NA | [19] | ||
| China | 40∗ | 18 (45%) | Influenza A or B virus (3), Adenovirus (1) |
M. pneumoniae (13), Streptococcus pneumoniae (1) | NA | NA | [20] |
| China | 29 | 5 (17.24%) | NA | Enterobacter cloacae (2), Acinetobacter baumannii (1) | Candida albicans (2) | NA | [21] |
| China | 29 | 1 (3.4%) | NA | 3.4% died | [22] | ||
| USA | 21 | 4 (19.0%) | NA | NA | [23] | ||
| China | 11 | 1 (9%) | NA | Mixture seen (1) | NA | NA | [24] |
| China | 7 | 1 (14%) | NA | Legionella pneumophillia (1) | NA | Favorable outcomes, no ICU admission | [25] |
NA; Data not available/Not mentioned, ARDS; acute respiratory distress syndrome, ∗; paediatric population.
Most of the published studies did not stratify the setting-critical care or wards of the patients fromwhere sampling was done. There were also no details on the types of infections, or healthcare settings (whether they were hospital-acquired or community-acquired). Broad-spectrum antibiotic use was reported even for patients with no evidence for bacterial co-infection.
Overall, 72% of COVID-19 patients received antimicrobial therapy, with no description of antimicrobial stewardship interventions, in any of the studies published as of yet. Limited data available suggest that nosocomial infections are associated with increased COVID-19 severity and a higher risk of death [5,6]. Moreover, antibiotic resistance amongst the pathogens causing secondary infections is also a hidden threat lurking behind COVID-19.
It is estimated that many HAIs-like hospital/ventilator-associated pneumonia (HAP/VAP), bloodstream infection (BSIs), and urinary tract infection (UTIs) may be going under-reported, for want of culturing practices/non-availability of culturing facilities in many hospitals. More studies are needed to ascertain if the COVID-19 patients have anincreased risk of infection at specific sites or from specific agents [4].
It is anticipated that during the pandemic, an increased number of patients will require empiric antimicrobial treatment. In India, treatment guidelines for HAIs have been made by the Indian Council of Medical Research, based on our indigenous antimicrobial resistance (AMR) data. Antimicrobial stewardship programs must focus on supporting the optimal selection of empiric treatment and rapid-de-escalation, based on culture reports.
Global, and country-specific clinical data encompassing viral, bacterial, and fungal infections are needed in guiding evidence-based treatment of COVID-19. To fill this major gap in our knowledge, we conducted a prospective study to ascertain the actual burden & molecular profile of pathogens causing secondary viral/bacterial/fungal infections in COVID-19 patients admitted to the JPN Apex Trauma Center, which is a dedicated COVID-19 center of the All India Institute of Medical Sciences, New Delhi, India. TheCOVID-19 facility established at AIIMS is the largest COVID-19 care facility of the Govt. of India for severely ill patients.
2. Methods
Setting: The AIIMS hospital in Delhi is a 3000-bedded, tertiary care, teaching, and referral hospital of North India, with several super-specialty centers. With the onset of the COVID-19 pandemic, the hospital quickly converted its 200-bedded Trauma center to a dedicated COVID-19 facility for moderate to severely ill COVID-19 patients. The first patient was admitted to the hospital on 3rd Aril, 2020. This facility had fully functional ICUs, operation theatres, a dialysis unit, an endoscopy unit, and wards. A total of 13 patients underwent surgeries and 64 underwent dialysis during the study period mentioned below. A further 1500 beds were made available at AIIMS for mild patients. Twenty-five percent of staff from all departments/centerswere made available for the COVID-19 care facilities. The present study was conducted at the Trauma Center (COVID-19 facility for Moderate-severely ill patients) of AIIMS hospital, Delhi.
Patient enrolment and clinical data: From April 3 to July 11, 2020, clinical samples of COVID-19 patients were received in the microbiology laboratory, on clinical suspicion of a secondary infection in patients with initial positive real-time RT-PCR results who were admitted to the JPNA Trauma Center, AIIMS. The clinical and outcome data were obtained from patients’ medical records. Patients whose samples were not sent to the laboratory were excluded from the study.
Specimens collection and processing: A total of 290 clinical samples including blood, urine, respiratory samples,pus, and other samples, of patients, were received during the study period. All clinical specimens were processed in the biosafety cabinets, using recommended personal protective equipment. All samples were discarded as per the biomedical waste management guidelines of India.
Detection of secondary bacterial/fungal infections: The samples were processed as per standard microbiological methods. The identification of bacteria/fungi was done by Vitek2®. Antimicrobial susceptibility test (AST) of the clinical isolates was determined by the Gram-negative, Gram-positive, and yeast Vitek2® AST cards (N235,N280, N281, P628&YST08) (bioMérieux, France), as per manufacturer's instructions. Minimum inhibitory concentrations (MIC) of antimicrobials were determined and interpreted. The antibacterial drugs tested for gram-negative pathogens included amikacin, amoxicillin/clavulanic acid, ampicillin, cefepime cefoperazone/sulbactam, ceftazidime, ciprofloxacin, imipenem, levofloxacin, meropenem, nitrofurantoin, piperacillin/tazobactam, tigecycline, and trimethoprim/sulfamethoxazole. The MIC for colistin was determined by the broth microdilution method. The antibacterial drugs Gram-positive time pathogens included Vancomycin, teicoplanin, tigecycline, linezolid, and daptomycin. Antifungal drugs included caspofungin and fluconazole. Antimicrobial breakpoints were interpreted according to CLSI 2020 guidelines. Multi-drug resistance was defined as resistance to two or more different classes of antimicrobials.
Detection of co-infections with respiratory pathogens using Film Array: The BioFire® FilmArray® Respiratory (RP) Panel was used for detection of co-infection with 15 bacteria &20 other respiratory pathogens including 17 viruses; Adenovirus, Coronaviruses (HCoV-HKU, HCoV-NL63, HCoV-229E, HCoV−OC43), Human Metapneumovirus (HMPV), Human Rhinovirus/Enterovirus (HRV), Influenza viruses (FluA, FluA/H, FluA/H3, FluA/H1-2009, and FluB), Parainfluenza types (PIV 1, 2, 3, & 4), and Respiratory Syncytial Virus (RSV), and 3 atypical bacteria including Bordetella pertussis, Chlamydia pneumoniae, and Mycoplasma pneumoniae, only in the respiratory samples of COVID-19 patients. The tests were conducted as per the manufacturer's instructions. The RP panel also detected the presence of antimicrobial resistance (AMR) genes including- CTX-M, IMP, KPC, mecA/C & MREJ, NDM, OXA-48-like, and VIM in the bacteria detected in representative respiratory samples.
Statistical analysis: Number of patients and the number of resistant organisms were presented as n (%), and age was presented as mean ± standard deviation (SD), and range. Categorical variables were expressed as number (%) and compared by the Chi-square test or Fisher's exact test among multiple groups. A p-value of P < 0.05 was considered to be statistically significant. SPSS 19.0 software and GraphPad 7.0 were used for statistical analysis.
Ethical clearance: The study was approved by the AIIMS Institute Ethics Committee (IEC-556/June 19, 2020).
3. Results
Demographics and Outcome Measures: The main outcomes measured in this study admission to the intensive care unit (ICU) and in-hospital mortality. Only those patients who had positive COVID-19 RT-PCR results were admitted to the trauma center during the study period and subsequently included in this study; therefore, the mortalities of the patients were all attributable to COVID-19 infection.
A total of 1179 patients were admitted during the study period. Among these, 375 (32%) had a severe illness and were admitted to the ICUs, while 804 (68%) were admitted to the wards. Overall, 151/1179 (13%) patients whose clinical samples were culture positive, were included for demographic and outcome measures analyses. Thus, a total of 56/375 (15%) ICU patients and 95/804 (12%) non-ICU patients developed secondary infections.
Patients who required ICU admission, were 51.73 ± 0.004 (mean ± SD) years old, (range 1–91 years), while the mean age of patients who did not need ICU admission was 42.6 years, and this difference was found to be statistically significant (p=0.0044). Most of the patients (95/151, 63%) did not need ICU admission, only 56/151 (37%) patients had critical illness and were admitted to the ICU (p=0.0004). This difference was found to be statistically significant.
At the study's cut-off date, most of the patients in our cohort were still alive (101; 67%). Of these, 55/101 (54.5%) were discharged from the hospital and 46/101 (45.5%) were active-inpatients. Fifty (33%) patientssuccumbed to the disease. A significant finding was that majority of the patients, 45% (25/56), who succumbed to COVID-19 were admitted to the ICU, while non-ICU in-hospital mortality remained lowerat 26% (25/95) (p=0.0001).
As shown in Table 2 , the overall in-hospital mortality was found to be 18% (209/1179); 50 of these 209 (23.9%) fatal patients also developed secondary infections (p=0.0001). The most common causes of death were septic shock with respiratory failure and cardiac arrest.
Table 2.
Demographic characteristics of COVID-19 patients who had secondary infections.
| Demographics | Admission |
p-value | Clinical Outcome |
p-value | ||||
|---|---|---|---|---|---|---|---|---|
| Non-ICU | ICU | Alive | Dead | |||||
| Total admissions# | 1179 | 804 (68%) | 375 (32%) | p=0.1969 | 970 (82%) | 209 (18%) | p = 0.0001∗ | |
| Patients having Secondary Infections | 151 | 95 (63%) | 56 (37%) | 101 (67%) | 50 (33%) | |||
| Age (years) n (%) | Mean ± SD [range] | 46.01 ± 19.03 [0.6–93] | 42.638 ± 17.87 [0.6–93] | 51.73 ± 0.004 [1–91] | p = 0.0044∗ | 41.610 ± 18.34 [0.6–93] | 57.5 ± 17.58 [12–91] | p = 0.0001∗ |
| 0–49 | 80 (53%) | 61 (64%) | 19 (34%) | p = 0.0004∗ | 64 (63%) | 16 (32%) | p = 0.0005∗ | |
| > 50 | 71 (47%) | 34 (36%) | 37 (66%) | 37 (37%) | 34 (68%) | |||
| Gender n (%) | Female | 53 (35%) | 32 (34%) | 21 (37.5%) | p=0.7245 | 35 (35%) | 18 (36%) | p=1.0000 |
| Male | 98 (65%) | 63 (66%) | 35 (62.5%) | 66 (65%) | 32 (64%) | |||
| Length of stay (days) | 12.21 ± 6.90 | 11.75 ± 6.94 | 13.00 ± 6.77 | p=0.3999 | 12.56 ± 6.13 | 11.90 ± 7.66 | p = 0.6488 | |
#Instudy duration, ∗statistically significant.
The difference between the average age (±SD) of patients who had a fatal outcome (57.5 ± 17.58 years) and those who were still alive (41.610 ± 18.34 years) was found to be statistically significant (p=0.0001).
Overall, patients above 50 years of age needed ICU admission (p=0.0004) and/or had a fatal outcome (p=0.0005). Patients admitted to the hospital were predominantly males (65%). The mean (±SD) length of stay (LOS) for the patients was 12.21 (±6.90) days. A breakdown by age of patients based on ICU admission and clinical outcomes is outlined in Table 2.
Profile of secondary infections: The highest and lowest rates ofsecondary infections were found in patients aged between 38 and 60 years, and below 20 years, respectively. Most co-infections occurred within the first two weeks of hospital admission, with an average of 10.43 days.
A total of 290 samples were received for microbiological culture, of which 115 (40%) samples were culture-negative/sterile (Fig. 1 ). Overall, 175 (60%) samples were found to be culture positive, including those categorized as contaminants. The detailed profile of secondary infection pathogens is given inTable 3.
Fig. 1.
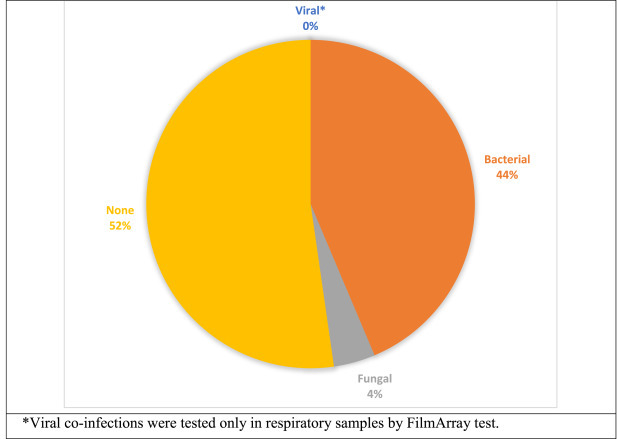
Distribution of secondary infections in COVID-19 patients.
Table 3.
Profile of secondary infections in COVID-19 patients.
| Sample Type N (%) |
Blood 126 (42.8%) |
Urine 62 (21.8%) |
ET/BAL 53 (18.6%) |
Pus 27 (9.1%) |
Others 22 (7.7%) |
Total 290 |
|
|---|---|---|---|---|---|---|---|
| Organisms | |||||||
| Bacterial | Acinetobacter baumannii | 12 | 1 | 12 | 0 | 1 | 26 |
| Citrobacter koseri | 0 | 1 | 0 | 0 | 0 | 1 | |
| Escherichia coli | 6 | 2 | 1 | 5 | 2 | 16 | |
| Enterococcus faecium | 3 | 1 | 0 | 0 | 0 | 4 | |
| Klebsiella oxytoca | 1 | 0 | 0 | 0 | 0 | 1 | |
| Klebsiella pneumoniae | 13 | 2 | 13 | 3 | 1 | 32 | |
| Proteus mirabilis | 0 | 3 | 0 | 0 | 0 | 3 | |
| Pseudomonas aeruginosa | 3 | 2 | 5 | 1 | 0 | 11 | |
| Stenotrophomonas maltophilia | 1 | 0 | 1 | 0 | 0 | 2 | |
| Fungal | Candida albicans | 0 | 1 | 0 | 0 | 0 | 1 |
| Candida parapolosis | 0 | 3 | 0 | 0 | 0 | 3 | |
| Candida spp | 0 | 5 | 0 | 0 | 0 | 5 | |
| Contaminants∗ | 19 | 24 | 12 | 10 | 5 | 70 | |
| Sterile | 68 | 20 | 9 | 5 | 13 | 115 | |
| Viral# | Adenovirus | NA | NA | 0 | NA | NA | 0 |
| Coronaviruses HCoV-HKU HCoV-NL63 HCoV-229E HCoV−OC43 |
0 | 0 | |||||
| Human Metapneumovirus | 0 | 0 | |||||
| Human Rhinovirus/Enterovirus | 0 | 0 | |||||
| Influenza viruses FluA FluA/H FluA/H3 FluA/H1-2009 FluB |
0 | 0 | |||||
| Parainfluenza types (PIV 1, 2, 3, 4) | 0 | 0 | |||||
| Respiratory Syncytial Virus | 0 | 0 | |||||
| Atypical bacteria# | Bordetella pertussis | 0 | 0 | ||||
| Chlamydia pneumoniae | 0 | 0 | |||||
| Mycoplasma pneumoniae | 1 | 1 | |||||
ET; Endotracheal aspirate samples, BAL; Bronchoalveolar lavage samples, Others; CSF, Ascitic fluid, Bile, ICD fluid, and CVP tip.
Contaminants∗- Coagulase-negative Staphylococcus aureus (CONS), mixture of >3 types of Gram-negative/Gram-positive organisms, and Upper respiratory flora.
NA; Not applicable, #Tested by film array respiratory panel.
Of the total 290 clinical samples, the most predominant were blood samples (126, 42.8%) followed by urine (62, 21.8%), Endotracheal aspirate (ET)/Bronchoalveolar lavage (BAL) samples (53, 18.6%), pus (27, 9.1%), and others (22, 7.7%).
Among the bacterial (n = 96) isolates, Klebsiella pneumoniae (32, 33.3%), was the most common organism, followed by Acinetobacter baumannii (26, 27.1%) Escherichia coli (16, 16.7%), and Pseudomonas aeruginosa (11, 11.5%).
Positive blood cultureswere identified in 58/126 (46%) samples. Among these, Klebsiella pneumoniae and Acinetobacter baumanniiwere the most common isolates. Of the total 58 organisms isolated 19 were classified as contaminants; 12/19 (63%)Coagulase-negativeStaphylococcus aureus (CONS) (4/12Methicillin-resistantMR-CONS, 8/12Methicillin-sensitive MS-CONS).
Significant growth was seen in 18/62 urine cultures, with Candidaspp (9/18, 50%) being the predominant pathogen followed by Proteus mirabilis in 3/18 (16.7%). Contaminants (a mixture of Gram-negative/positive organisms)/insignificant growth was seen in 24/62 (38%).
A positive ET/BAL sample culture was found in 44/53 (83%) samples including endotracheal aspirates and bronchoalveolar lavage samples, and 12/44 were classified as contaminants (a mixture of Gram-negative/positive organisms or upper respiratory flora). Klebsiella pneumoniae and Acinetobacter baumanniiwere the most commonly isolated pathogens. From the sputum sample of one of the patients, Ascaris lumbricoides was also isolated.
Of the total 27 pus samples, 22 (81.5%) were culture positive, including 10 contaminants (a mixture of Gram-negative/positive organisms). Escherichia coli (5/22, 23%) was the predominant organism isolated from pus samples.
Antimicrobial resistance(AMR) profile of pathogens causing secondary infections: The AMR profiles of pathogens isolated from the clinical samples of COVID-19 patients are given in Table 4 . The overall resistance ranged from 9% to 84% amongst all organisms. The highest resistance observed was to amoxicillin/clavulanic acid (84%), followed by levofloxacin (83%), ciprofloxacin (79%), piperacillin/tazobactam (77%), and trimethoprim/sulfamethoxazole (75%). Overall resistance tothird-generation cephalosporins and carbapenems was found to be 64%–69%. All isolates were sensitive to colistin.
Table 4.
Resistance profile of clinical isolates causing secondary infections in COVID-19 patients.
| Sample Type Number of samples (%) |
Blood 39 (37.1%) |
Urine 18 (17.1) |
Respiratory samples 32 (30.5%) |
Pus 12 (11.4%) |
Others 4 (3.8%) |
Total 105 |
|
|---|---|---|---|---|---|---|---|
| Antimicrobials | Amikacin | 29 (74.4%) | 0 (0%) | 16 (50%) | 2 (16.7%) | 1 (25%) | 48 (46%) |
| Amoxicillin/Clavulanic Acid | 39 (100.0%) | 18 (100%) | 21 (65.6%) | 6 (50%) | 4 (100%) | 88 (84%) | |
| Ampicillin | 39 (100.0%) | 18 (100%) | 0 | 9 (75%) | 4 (100%) | 70 (67%) | |
| Caspofungin# | NT | 0 | NT | NT | NT | 0 | |
| Cefepime | 37 (94.9%) | 0 | 26 (81%) | 5 (41.7%) | 4 (100%) | 72 (69%) | |
| Cefoperazone/Sulbactam | 36 (92.3%) | 5 (25%) | 22 (69%) | 5 (41.7%) | 4 (100%) | 72 (69%) | |
| Ceftazidime | 36 (92.3%) | 0 | 27 (84.4%) | 0 | 4 (100%) | 67 (64%) | |
| Ciprofloxacin | 38 (97.4%) | 3 (16.70%) | 28 (88%) | 10 (83.3%) | 4 (100%) | 83 (79%) | |
| Colistin∗ | 3 (7.7%) | 0 | 3 (9.37%) | 3 (25%) | 0 |
9 (9%) | |
| Fluconazole# | NT | 0 | NT | NT | NT | 0 | |
| Imipenem | 36 (92.3%) | 0 | 24 (75%) | 5 (41.7%) | 2 (50%) | 67 (64%) | |
| Levofloxacin | 36 (92.3%) | 5 (25%) | 30 (94%) | 12 (100%) | 4 (100%) | 87 (83%) | |
| Meropenem | 37 (94.9%) | 3 (14.30%) | 26 (81%) | 4 (33.3%) | 2 (50%) | 72 (69%) | |
| Nitrofurantoin | 28 (71.8%) | 9 (50%) | 0 | 8 (66.7%) | 4 (100%) | 49 (47%) | |
| Piperacillin/Tazobactam | 38 (97.4%) | 3 (16.70%) | 29 (91%) | 7 (58.3%) | 4 (100%) | 81 (77%) | |
| Tigecycline | 14 (35.9%) | 0 | 17 (53%) | 3 (25%) | 1 (25%) | 35 (33%) | |
| Trimethoprim/Sulfamethoxazole | 37.00% (94.9%) | 6 (33.30%) | 24 (75%) | 8 (66.7%) | 4 (100%) | 79 (75%) | |
#; Antifungal., NT; Not Tested, ∗; The minimum inhibitor concentration for colistin was tested by the broth microdilution method as per the joint guidelines of EUCAST-CLSI. The resistance profile is depicted as the number of resistant isolates and percentages.
Amongst Gram-negativepathogens isolated from blood and urine, all were highly resistant toamoxicillin/clavulanic acid and ampicillin. Those isolated form ET/BAL& pus samples were least susceptible to levofloxacin. Bacteria and fungi isolated from all sample types were most susceptible to colistin and tigecycline. High rates of multi-drug resistance were seen (60%).
Amongst Gram-positive pathogens isolated were MR-CONS and MS-CONS and were sensitive to vancomycin, teicoplanin, tigecycline, linezolid, and daptomycin.
Co-infections with respiratory pathogens using Film Array:The BioFire® FilmArray® Respiratory (RP) Panel used for detection of co-infection with 20 respiratory pathogens. This panel was used in the respiratory (ET/BAL) samples of 31 patients. The bacteria detected in these samples were by and large concordant to those reported by Vitek2®. FilmArray could also detectorganisms with very low Copies/mL i.e. which could not be cultured. The Gram-negative bacteria (n = 70) detected were Klebsiella pneumoniae group (19, 27.1%), Acinetobacter calcoaceticus-baumanniicomplex (19, 27.1%), Pseudomonas aeruginosa (15, 21.4%), Escherichia coli (6, 8.6%), Enterobacter cloacae complex (3, 4.3%), Haemophilus influenzae (3, 4.3%), Proteus spp (3, 4.3%), Serratia marcescens (1,1.4%), and Acinetobacter baumanniicomplex (1, 1.4%). The Gram-positive bacteria (n = 2)detected by FilmArray included one eachof Streptococcus pneumoniae. The isolates of S. pneumoniae and H influenzae were not detected by conventional microbiological culture methods.
In the samples tested, the predominant AMR gene detected was NDM (22, 71%), followed by OXA-48-like (19, 61%), CTX-M (19, 61%), and IMP (6, 19%), KPC and VIM were also detected in one sample each. None of the ET/BAL samples was co-infected with any of the 17 viruses tested (Table 3). Among the atypical organisms; Mycoplasma pneumoniae was found to be present in one patient's sputum sample.
Antimicrobial therapy & stewardship interventions: All patients who showed clinical symptoms of secondary infection were sampled. The laboratory findings were communicated to the clinicians at indigenous digital platforms. Culture-based antimicrobial treatment was administered to all patients. Subsequent samples were sent on the discretion of the treating clinicians for guided & rapid de-escalation.
4. Discussion
The current body of evidence from high-burden COVID-19 areas globally suggests that superinfections are common, particularly in severe cases. In a study conducted in Wuhan, of the total 41 patients, secondary infections were reported in 31% of ICU patients and 10% of patients overall. In another study from Wuhan, of 68 patients who died, 11/68 (16%) were found to have secondary infections, although further details were not provided [7].In the present study, we found that of the total 151 patients having secondary infections, 56 (37%) were ICU patients and in-hospital mortality of 33% was observed among these patients.
Similar to our findings, the majority of the pathogenic organisms reported in patients with COVID-19 are multidrug-resistant (MDR) nosocomial organisms. At our center, MDR was found in 60% isolates, AMR genes were also detected in bacteria coinfecting COVID-19 patients. The published data from ICMR indicated a high prevalence of AMR in Indian hospitals in the pre-COVID times. High antimicrobial pressure in the ICUs for treating COVID-19 patients with empiric antimicrobials will further aggravate the problem of AMR. This is particularly true for COVID-19 centers which do not have an adequate microbiological back-up for culturing, or the culture of culturing is not there (due to fear of taking samples or lack of policies/lack of resources). Published data suggest that apart from the risk factors for secondary bacterial and fungal nosocomial infections in COVID-19 patients including diabetes, hypertension, indwelling catheters, and corticosteroid therapy, combination antibiotic therapy may further predispose COVID-19 patients to these secondary infections [8]. A single-center study from Wuhan has also reported that 75% of COVID-19 patients having secondary HAIs, received prophylactic antibiotics [9].
Unlike normal circumstances, in COVID-19 patients, various invasive modalities otherwise employed to help diagnose secondary infections are restricted as part of infection control measures. Thus, clinicians often resort to empiric broad-spectrum antimicrobial therapy. Given that empiric prophylactic antibiotics may instead select for MDR pathogens, if the clinicians lower their threshold to obtain culture data and choose targeted antibiotic therapy over empiric prophylaxis, it would allow later de-escalation or retarget of treatment. Itwould also result in lesser secondary infections and favorable patient outcomes in COVID-19 patients.
Limited data suggest that nosocomial infections are associated with increased COVID-19 severity, long ICU stays, and a higher risk of death [10]. In our study, although, there was not much difference between the length of stay of patients in wards and ICUs, the in-hospital mortality was observed in 50/151 (33%) COVID-19 patients with secondary infections. It was found to be statistically significant that in 24% of the total in-hospital mortalities were attributable to a subsequent secondary infection.
As reported previously, in the ICMR report, NDM was the most prevalent carbapenemases across the Indian AMR network [11]. In our present study, the AMR genes encoding for carbapenemases- NDM (71%), OXA-48-like (61%), and Extended-spectrumbeta-lactamases- CTX-M (61%) were highly prevalent in the respiratory samples tested by FilmArray. This was inconcordance with the high resistance to third-generation cephalosporins (up to 84%) and carbapenems (up to 81%), observed among these respiratory pathogens.
Strengths of our study: Most of the studies published so far had a sample size of fewer than 50 patients having secondary infections. While the majority of them did not mention the pathogen profile and outcome measures, we described in detail the antimicrobial profile of pathogens causing these secondary infections, drew the associations with the severity of disease; based on the requirement of ICU admission, as well as the hospital outcome of these patients. We also employed FilmArray based multiplex identification on respiratory specimens, for identification of other viruses/atypical bacteria and antimicrobial resistance genes.
Limitations: The FilmArray based test used in our study gave very rapid results. However, it is costly and larger studies are needed to correlate the bin/copy numbers in respiratory samples with the semiquantitative bacterial culture counts, since the isolation of a pathogen from the respiratory tract does not always signify true infection.
5. Conclusion
We found an overall high rate of secondary infection (13%) and 15% in ICU patients; up to 84% AMR, as well as 33% in-hospital mortalityamong COVID-19 patients who developed secondary infections. However, little is known about the mechanism by which a virus can predispose the patient to develop a secondary infection which results in longer ICU stays and in-hospital mortalities.
Thus, in the immediacy of this problem, existing healthcare facilities, infection control, and antimicrobial stewardship programs have to be promptly pivoted to cater to this rapidly evolving pandemic situation.
Declaration of competing interest
None declared.
References
- 1.Zhu N., Zhang D., Wang W., Li X., Yang B., Song J. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382(8):727–733. doi: 10.1056/NEJMoa2001017. http://www.nejm.org/doi/10.1056/NEJMoa2001017 [Internet] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet [Internet] 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. https://linkinghub.elsevier.com/retrieve/pii/S0140673620305663 Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Phelan A.L., Katz R., Gostin L.O. The novel coronavirus originating in wuhan, China. JAMA [Internet] 2020;323(8):709. doi: 10.1001/jama.2020.1097. https://jamanetwork.com/journals/jama/fullarticle/2760500 Available from: [DOI] [PubMed] [Google Scholar]
- 4.Jancin B. Secondary infections common in COVID-19, implications unclear. MDEdge-Internal Med. News2020;
- 5.Rawson T.M., Moore L.S.P., Zhu N., Ranganathan N., Skolimowska K., Gilchrist M. Bacterial and fungal co-infection in individuals with coronavirus: a rapid review to support COVID-19 antimicrobial prescribing. Clin Infect Dis [Internet] 2020;(478) doi: 10.1093/cid/ciaa530. http://www.ncbi.nlm.nih.gov/pubmed/32358954 1–4. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lai C.C., Wang C.Y., Hsueh P.R. Co-infections among patients with COVID-19: the need for combination therapy with non-anti-SARS-CoV-2 agents? J Microbiol Immunol Infect [Internet] 2020;(xxxx) doi: 10.1016/j.jmii.2020.05.013. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kwon Woo Joo, Li Gabrielle, Zheng Matthew, Kaur Harleen, Noah Magbual, Ds Superinfections and coinfections in COVID-19 — separating the signal from the noise. Intern Med. News Free C. Online. 2020 https://www.medpagetoday.com/infectiousdisease/covid19/86192 Available from: [Google Scholar]
- 8.Alison C. Massachusetts Gen. Hosp; 2020. Incidence of Co-infection nosocomial infection.https://advances.massgeneral.org/research-and-innovation/article.aspx?id=1193 [cited 2020 Jul 10];Available from: [Google Scholar]
- 9.He Y., Li W., Wang Z., Chen H., Tian L., Liu D. Nosocomial infection among patients with COVID-19: a retrospective data analysis of 918 cases from a single center in Wuhan, China. Infect Control Hosp Epidemiol [Internet] 2020:1–2. doi: 10.1017/ice.2020.126. https://www.cambridge.org/core/product/identifier/S0899823X20001269/type/journal_article Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Almazeedi S., Youha S Al, Jamal M.H., Al-Haddad M., Al-Muhaini A., Al-Ghimlas F. Clinical characteristics, risk factors and outcomes among the first consecutive 1,096 patients diagnosed with COVID-19: the Kuwait experience. medRxiv. 2020. 000:2020.05.09.20096495. Available from: http://medrxiv.org/lookup/doi/10.1101/2020.05.09.20096495. [DOI] [PMC free article] [PubMed]
- 11.Indian Council of Medical Research Annual report: antimicrobial resistance surveillance network. 2018. [Internet]. 2018. Available from: https://main.icmr.nic.in/sites/default/files/reports/AMRSN_Annual_Report_2018_0.pdf.
- 12.Richardson S., Hirsch J.S., Narasimhan M., Crawford J.M., McGinn T., Davidson K.W. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York city area. JAMA [Internet] 2020;323(20) doi: 10.1001/jama.2020.6775. https://jamanetwork.com/journals/jama/fullarticle/2765184 2052. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goyal P., Choi J.J., Pinheiro L.C., Schenck E.J., Chen R., Jabri A. Clinical characteristics of covid-19 in New York city. N Engl J Med [Internet] 2020;382(24):2372–2374. doi: 10.1056/NEJMc2010419. Available from: http://www.nejm.org/doi/10.1056/NEJMc2010419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu C., Chen X., Cai Y., Xia J., Zhou X., Xu S. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in wuhan, China. JAMA Intern Med [Internet] 2020;180(7):934. doi: 10.1001/jamainternmed.2020.0994. https://jamanetwork.com/journals/jamainternalmedicine/fullarticle/2763184 Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim D., Quinn J., Pinsky B., Shah N.H., Brown I. Rates of Co-infection between SARS-CoV-2 and other respiratory pathogens. JAMA [Internet] 2020;323(20):2085. doi: 10.1001/jama.2020.6266. Available from: https://jamanetwork.com/journals/jama/fullarticle/2764787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ding Q., Lu P., Fan Y., Xia Y., Liu M. The clinical characteristics of pneumonia patients coinfected with 2019 novel coronavirus and influenza virus in Wuhan, China. J Med Virol [Internet] 2020 doi: 10.1002/jmv.25781. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet [Internet] 2020;395(10223):507–513. doi: 10.1016/S0140-6736(20)30211-7. https://linkinghub.elsevier.com/retrieve/pii/S0140673620302117 Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zangrillo A., Beretta L., Scandroglio A.M., Monti G., Fominskiy E., Colombo S., Morselli F., Belletti A., Silvani P., Crivellari M.M.F. Characteristics, treatment, outcomes and cause of death of invasively ventilated patients with COVID-19 ARDS in Milan, Italy. Crit Care Resusc. 2020 doi: 10.1016/S1441-2772(23)00387-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet [Internet] 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0140673620301835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li H., Chen K., Liu M., Xu H., Xu Q. The profile of peripheral blood lymphocyte subsets and serum cytokines in children with 2019 novel coronavirus pneumonia. J Infect [Internet] 2020;81(1):115–120. doi: 10.1016/j.jinf.2020.04.001. https://linkinghub.elsevier.com/retrieve/pii/S0163445320302073 Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in wuhan, China. JAMA [Internet] 2020;323(11):1061. doi: 10.1001/jama.2020.1585. https://jamanetwork.com/journals/jama/fullarticle/2761044 Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen L., Liu H.G., Liu W., Liu J., Liu K., Shang J. [Analysis of clinical features of 29 patients with 2019 novel coronavirus pneumonia] Chinese J Tuberc Respir Dis [Internet] 2020;43:E005. doi: 10.3760/cma.j.issn.1001-0939.2020.0005. http://www.ncbi.nlm.nih.gov/pubmed/32026671 Available from: [DOI] [PubMed] [Google Scholar]
- 23.Arentz M., Yim E., Klaff L., Lokhandwala S., Riedo F.X., Chong M. Characteristics and outcomes of 21 critically ill patients with COVID-19 in Washington state. JAMA [Internet] 2020;323(16):1612. doi: 10.1001/jama.2020.4326. https://jamanetwork.com/journals/jama/fullarticle/2763485 Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dong X., Cao Y., Lu X., Zhang J., Du H., Yan Y. Eleven faces of coronavirus disease 2019. Allergy [Internet] 2020;75(7):1699–1709. doi: 10.1111/all.14289. https://onlinelibrary.wiley.com/doi/abs/10.1111/all.14289 Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yu N., Li W., Kang Q., Xiong Z., Wang S., Lin X. Clinical features and obstetric and neonatal outcomes of pregnant patients with COVID-19 in Wuhan, China: a retrospective, single-centre, descriptive study. Lancet Infect Dis [Internet] 2020;20(5):559–564. doi: 10.1016/S1473-3099(20)30176-6. https://linkinghub.elsevier.com/retrieve/pii/S1473309920301766 Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]


