Abstract
COVID-19 and our public health responses to the pandemic may have far-reaching implications for cardiovascular (CV) risk, affecting the general population and not only survivors of COVID-19. In this narrative review, we discuss how the pandemic may affect general CV risk for years to come and explore the mitigating potential of telehealth interventions. From a health care perspective, the shift away from in-person office visits may have led many to defer routine risk- factor management and may have had unforeseen effects on continuity of care and adherence. Fear of COVID-19 has led some patients to forego care for acute CV events. Curtailment of routine outpatient laboratory testing has likely delayed intensification of risk-factor–modifying medical therapy, and drug shortages and misinformation may have negative impacts on adherence to antihypertensive, glucose-lowering, and lipid-lowering agents. From a societal perspective, the unprecedented curtailment of social and economic activities has led to loss of income, unemployment, social isolation, decreased physical activity, and increased frequency of depression and anxiety, all of which are known to be associated with worse CV risk-factor control and outcomes. We must embrace and evaluate measures to mitigate these potential harms to avoid an epidemic of CV morbidity and mortality in the coming years that could dwarf the initial health effects of COVID-19.
Résumé
La pandémie de COVID-19 et les mesures prises par les autorités de santé publique pourraient avoir de lourdes conséquences sur le risque cardiovasculaire (CV) dans l’ensemble de la population, et non seulement pour les personnes qui auront survécu à la COVID-19. Dans cette revue non systématique, nous analysons les répercussions possibles de la pandémie sur le risque CV général au cours des années à venir et nous explorons le potentiel d’atténuation de ces répercussions grâce à la télémédecine. Du point de vue des soins de santé, le délaissement des consultations en personne pourrait en avoir incité plusieurs à reporter la prise en charge courante des facteurs de risque, ce qui pourrait avoir des effets imprévus sur le maintien des soins et l’observance thérapeutique. La peur de la COVID-19 a en outre incité certains patients ayant subi une manifestation CV aiguë à se passer de soins. La limitation de l’exécution des tests de laboratoire de contrôle pour les patients ambulatoires a vraisemblablement retardé l’intensification des traitements médicaux visant à atténuer les facteurs de risques, et les pénuries de médicaments et la mésinformation pourraient avoir des répercussions défavorables sur l’observance des traitements antihypertenseurs, hypoglycémiants et hypolipidémiants. D’un point de vue sociétal, la restriction sans précédent des activités sociales et économiques a entraîné des pertes de revenus, des pertes d’emploi, de l’isolement social, une diminution de l’activité physique et une augmentation des cas de dépression et d’anxiété, qui sont tous associés à une détérioration du contrôle des facteurs de risques et des résultats sur le plan de la santé CV. Nous devons évaluer la situation et adopter des mesures pour atténuer ces méfaits potentiels afin d’éviter au cours des prochaines années une épidémie de morbidité et de mortalité CV qui pourrait bien éclipser les effets initiaux de la COVID-19 sur la santé.
Since first emerging in the Hubei province of China, the novel severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) has spread globally, with 223,297 cases of COVID-19 and 10,023 reported deaths in Canada as of October 28, 2020.1 Acute cardiovascular (CV) complications of COVID-19 are more common than initially thought and can include myocarditis, pericarditis, myocardial infarction, decompensated heart failure, stroke, and pulmonary embolus.2 In addition, a number of the antiviral therapies and immune-response modulators currently being investigated for treatment of COVID-19 have CV side effects and potentially interact with CV medications.3 Nothing is known about the potential long-term CV complications of COVID-19 infection at this early stage of the pandemic. However, the CV implications of COVID-19 will definitely extend beyond direct infection-related CV damage. The public health response to the pandemic, meant to mitigate morbidity and mortality from acute COVID-19, may have the unintended consequence of increasing CV risk in much broader swaths of the general population, including those uninfected with SARS-CoV-2. In this review, we explore potential mechanisms by which COVID-19 and our responses to the pandemic may affect future CV risk. We then discuss some of the factors that might mitigate this risk if successfully harnessed in these challenging circumstances.
Primary Impact of SARS-CoV-2 Infection on CV Risk
SARS-CoV-2 binds to angiotensin converting-enzyme-2 (ACE2) receptors. Physiologically, ACE2 counters renin-angiotensin-aldosterone system activation by degrading angiotensin 2 to angiotensin 1-7. Angiotensin 2 is a potent vasoconstrictor implicated in the pathophysiology of CV disease, whereas angiotensin 1-7 has been shown to have cardioprotective actions, potentially mediated by vasodilatory, antifibrotic, anti-inflammatory, and antithrombotic effects including the inhibition of proinflammatory cytokines and reduced thrombus formation via nitric oxide and prostacyclin pathways. Down-regulation of the ACE2 receptor induced by SARS-CoV-2 binding and endocytosis may lead to an imbalance of angiotensin 2 and angiotensin 1-7, with consequent alterations of normal circulatory homeostasis, particularly in the endothelium of the pulmonary capillaries, where this imbalance may contribute to the immune-thrombotic microvascular coagulopathy associated with respiratory compromise in COVID-19.4, 5, 6, 7 ACE2 and angiotensin 1-7 have been proposed as potential therapies for COVID-19.8 , 9 Other mechanisms of CV injury include direct infection of endothelial cells as well as damage mediated by release of inflammatory mediators and by other aspects of the immune response to COVID-19.7 , 10 , 11 Extensive coronary microvascular thrombi may lead to myocardial infarctions, even in patients with nonobstructive coronary arteries,12 and up to one-third of patients hospitalized with COVID-19 have elevated troponins.13 A recently published cohort study revealed that three-quarters of COVID-19 survivors, most of whom had not even required hospitalization for their illness, had evidence of cardiac involvement on cardiac magnetic resonance imaging, and 60% had ongoing myocardial inflammation more than 2 months after resolution of their COVID-19 symptoms.2 Whether infection with SARS-CoV-2 will lead to long-term CV risk sequelae among COVID-19 survivors is an open question that will require prospective registries of COVID-19 survivors to detect.10 , 14 Certainly, it does appear that COVID-19 may cause diabetes caused by direct viral infection via ACE2 receptors, which are abundant on pancreatic β-cells, and cases of COVID-19 manifesting as new-onset diabetes—and even ketoacidosis—have been reported.15, 16, 17
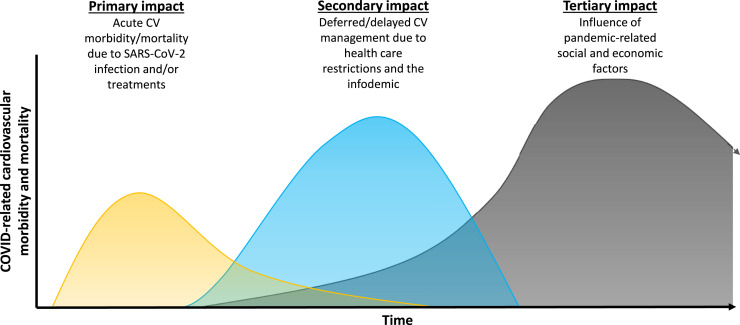
However, COVID-19 may adversely affect CV risk in many more than only those who were infected and survived, by various mechanisms related to our individual and collective pandemic responses as will be outlined as follows. It is probable that the footprint of these secondary and tertiary impacts on CV health may far outweigh that related to primary SARS-CoV-2 infection and its treatment (Fig. 1 and Table 1 ).
Figure 1.
Potential effects of the COVID-19 pandemic on cardiovascular morbidity and mortality. The height and time scale of the 3 waves in this figure are uncertain and not to scale. Waves of cardiovascular mortality and morbidity should be distinguished from “waves” of pandemic COVID-19. We expect each additional “wave” of pandemic COVID-19 to create echoing waves of cardiovascular mortality and morbidity, owing to primary, secondary, and tertiary effects, particularly to the extent that previously relaxed pandemic precautions and curtailments of normal socioeconomic and health care-related activities are reinstated.
Table 1.
Mechanisms for potential effects of COVID-19 pandemic on subsequent cardiovascular mortality and morbidity
| Impacts on CV morbidity or mortality | Direct effects of COVID-19 | Delayed/foregone health care | Social and economic impacts |
|---|---|---|---|
| Primary impact (days-weeks) |
|
|
|
| Secondary impact (weeks-months) |
|
|
|
| Tertiary impact (months-years) |
|
|
|
CV, cardiovascular; MI, myocardial infarction.
Secondary Effects Caused by Pandemic-Related Health Care Restrictions and the Infodemic
Shift from in-person outpatient visits to virtual care
Although the frequency of virtual visits has grown exponentially since the pandemic began, it has not fully made up for the marked decline in outpatient visits owing to restrictions imposed after the onset of the pandemic. A recent study from the US Veterans Health Administration documented that total outpatient visit contacts, both virtual and in-person, were still 30% lower after March 2020, compared with the spring in previous years.18 Less-frequent outpatient visits will undoubtedly translate into less-rigourous risk-factor control, as many patients and clinicians have deferred routine risk-factor management during the pandemic.19 In fact, a recent study from the United States confirmed that although telemedicine visits increased several-fold in the second quarter of 2020, the total number of primary care encounters still decreased by 21%, and new medication starts decreased by 26%.20 Even more concerningly, assessments of blood pressure (BP) decreased by 50% and cholesterol levels by 37%. Moreover, it is unclear whether virtual outpatient visits have the same effect as in-person visits for engaging patients in self-management of CV risk.
The importance of continuity of care in the management of CV risk factors, such as BP, glycemia, or lipids, is well documented. Patients with higher-care continuity report greater satisfaction and exhibit better adherence to prescribed therapies and health-promotion behaviours, fewer visits to emergency departments, or hospitalizations, especially for ambulatory-care sensitive conditions, and even decreased mortality rates in some studies.21, 22, 23, 24 However, all those studies measured continuity of care in terms of face-to-face encounters, and we are not aware of any published studies exploring the impact of virtual care visits on continuity for management of chronic disease or risk-factor control. Physical examination still has a role in medical practice even in 2020. Although it is well known that the accuracy of diagnoses are doubled when clinicians examine patients rather than just hear their histories,25 it is not unreasonable to hypothesize that the therapeutic alliance formed between patient and physician when face to face26 is much stronger than when those visits are merely virtual. As pointed out by Chwistek, “There is no doubt that the virtual visit is a fundamental alteration to the patient-physician encounter…an imposed order commands the in-person visit, and it travels beyond the verbal: body language, rush of emotions, physical proximity, and touch. If it goes well, there can be a sense of peace for the patient that they are cared for.”27 On the other hand, he also argued that “video encounters offer a direct glimpse into the lives of patients, an updated version of the traditional home visit.”27 Thus, determining the impact of virtual visits compared with in-person visits is a clear research priority, as more than half of all visits with physicians in Canada have been virtual since the beginning of the pandemic.28
Acute care avoided or delayed because of COVID-19–related fears
Marked declines in ED and hospital-based clinic visits for non-COVID–related conditions have been observed since the pandemic was declared,29, 30, 31 even for acute CV conditions such as myocardial infarction32 , 33, decompensated heart failure,34, and stroke.35 Correspondingly, there have been reports of up to 3-fold increases in out-of-hospital cardiac arrests during the COVID-19 pandemic, compared with previous years.36 , 37 When patients with acute conditions do present, they have been presenting later than usual, and their treatments have also been delayed after arrival, resulting in poorer outcomes.38 , 39 One study of patients with ST-elevation myocardial infarction (STEMI) documented increases in times from onset of symptoms to first medical contact (over 4 hours), hospital door to percutaneous coronary intervention (PCI) (approximately 30 minutes), and even catheterization laboratory arrival to PCI time (approximately 15 minutes) compared with the same metrics the year before.40 Delays in presentation may occur because of patient fears of contracting COVID-19, public health messages to avoid the emergency department for non–COVID-19 related conditions, or limited access to emergency medical services (because of reduced staffing from illness or isolation requirements). The delays in treatment have been attributed to COVID screening, donning of personal protective equipment, and deep cleaning of catheterization laboratories before and after each case.40 Similar reports have now emerged from Europe, the United Kingdom, and the United States for STEMI39 , 41 , 42 and acute stroke.43 As delays in presentation and treatment become more common, we are likely to see increases in CV-related morbidity caused by increased rates of heart failure, physical disabilities, and cardiac structural sequelae44 in survivors of acute events that some authors have labelled an “impending tsunami.”45 It is important to note that after the 2003 SARS-CoV-1 epidemic, inpatient and outpatient visit rates did not recover to pre-epidemic levels until nearly 4 years later, so this may not be as brief a phenomenon as we may think or wish.46
Curtailment of routine outpatient laboratory testing
Routine laboratory testing is an important part of CV risk reduction. Medical therapy for diabetes, hypertension, and dyslipidemia involves the routine measurement of laboratory parameters, both for safety and to guide appropriate intensification. In March to May 2020, many outpatient laboratories suspended or limited routine or nonessential bloodwork to protect patients and staff from acquiring COVID-19.47 Choosing wisely, Canada recommended delaying nonessential care and laboratory testing when possible.48 Although most outpatient laboratories have since resumed routine bloodwork, albeit at lower volumes—owing to measures put in place for physical distancing and increased personal protective equipment precautions—many patients continue to express anxiety about attending.49 No studies have been published yet on rates of laboratory testing and therapeutic intensification during the COVID-19 pandemic, but we believe it has undoubtedly led to deferred CV risk-factor management for many patients, particularly those with multiple comorbidities, who are both more likely to need routine laboratory work and also more likely to experience severe COVID-19.50
Drug shortages and 30-day prescription refill restrictions
Even before the COVID-19 pandemic, drug shortages were becomingly an increasingly common problem in health care, and the therapeutic turbulence caused by switching among drugs even within the same class has been shown to have a negative impact on patient adherence and outcomes.51 More than 10% of drug shortages documented by the US FDA are for drugs used in the management of CV disease, and antihypertensive agents have remained firmly in the top 5 drug groups subject to shortages in the past decade. Many of these drugs are manufactured in China and India. Given the disruptions to the global supply chain with the pandemic, it should come as no surprise that the monthly number of new drug shortages listed by Health Canada has doubled since March 2020.52 Although not a drug shortage per se, the impact of the recall of several generic valsartan preparations in 2018 clearly illustrates what happens when a prescribed medication is suddenly no longer available: There was a negative impact on persistence with antihypertensive medications of several different drug classes,53 , 54 an increase in health care encounters,53 , 54 and even increased rates of stroke in 1 analysis.54 Although most Canadian pharmacies have restricted prescription refills to 30-day intervals to preserve stocks during the pandemic, the potentially negative consequences for patient adherence (owing to increased costs, inconvenience, and patient fear of going to a pharmacy multiple times over 3 months rather than once55) and clinical outcomes have yet to be revealed. This is a research priority, given that even brief periods of abstinence from antihypertensive or lipid-lowering therapy can be associated with CV events.56, 57, 58
The Infodemic
“Infodemic” is a word coined by Dr Tedres Adhanom Ghebreyesus, Director-General of the World Health Organization, to refer to inaccurate, decontextualized, misleading, biased, or otherwise “fake” information disseminated on social—and sometimes conventional—media.59 , 60 The term may also include legitimate information but accompanied by premature calls to action, as exemplified by the early enthusiasm for hydroxychloroquine, whose benefits were later disproved by higher-quality studies.61 , 62 Regarding CV risk, widespread speculation on social media and in the mainstream media that ACE inhibitors and angiotensin receptor blockers could increase susceptibility to COVID-19 and worsen prognoses created sufficient concern in the public that many organizations, including Hypertension Canada and the Canadian Cardiovascular Society, issued specific policy statements in print and social media, encouraging patients treated with these agents not to discontinue therapy.63 The concerns about these agents were subsequently shown to be unfounded in human studies,3 but the potential adverse effects of this misinformation on patient adherence with antihypertensive therapy remains a concern.
The infodemic is not only a phenomenon in the lay press. In the scientific literature there has also been a proliferation of COVID-related papers on preprint servers that lack the safeguard of peer review.64 , 65 The rush to publication has now been documented in peer-reviewed journals, with articles being published at extraordinary speed in the early phases of the pandemic,65 and several notable instances of articles being later retracted, withdrawn, or having an expression of concern issued.66 The situation is unlikely to change in the near future, as a review of ClinicalTrials.gov has identified a concerningly large proportion of studies with expected low levels of evidence.67 Accordingly, a recent systematic review that examined 42 guidelines related to COVID-19, released in the spring and summer of 2020, found that the quality of all was poor, especially with respect to the rigour of their development.68 The infodemic in scientific literature may result in a churn of studies that capture attention, but do not—and, arguably, should not—contribute meaningfully to clinical practice.
Tertiary Effects Caused by Pandemic-Related Social and Economic Restrictions
Loss of income and unemployment
The unprecedented economic upheaval generated by COVID-19 and mitigation measures aimed at reducing spread of disease will likely worsen CV risk-factor control and outcomes in the upcoming years. Canadian companies have had to implement substantial reductions in activity and layoffs in the face of diminished demand for services and products because of stay-at-home orders, fear of COVID-19, and travel restrictions. As a result, unemployment in Canada increased to 13.7% in May 2020, and remained 12.3% in June 2020, more than double the rates for the same months the year before.69 In the United States, 19.6% of respondents in 1 national survey were not working, and one-third described moderate-to-high levels of food insecurity.70
The majority of Canadians (59%) have private health insurance for drug benefits,71 most of which is provided in the form of extended health benefits as part of their employment.72 More than one-half of these people (53%) would not be eligible for public drug benefits, which are accessible only for those older than 65 years of age, on social assistance, or under unique circumstances (eg, catastrophic costs, cancer therapies).73 The loss of employment, for many, will lead to loss of insurance benefits and difficulty affording glucose-lowering, antihypertensive, and lipid-lowering medications.74 , 75
A significant body of literature has examined the effect of reduced income, unemployment, and job stress on CV risk factors. Lower income has been consistently associated with higher rates of diabetes in both cross-sectional76 , 77 and longitudinal studies.78 Lower income has been consistently associated with increased rates of hypertension.79 Unemployment has only sometimes been associated with increased diabetes80 and hypertension;81 most studies have instead found that being employed in a job with a high degree of “job strain” (ie, a combination of high job demands and low job control) increased the risk of hypertension.79 The precise role of unemployment-employment in CV risk-factor control is complicated and may differ by sex and age strata.81 In terms of CV outcomes, the consensus observation across multiple studies has been more consistent; unemployed persons have higher riskS of major adverse CV events.82, 83, 84
The best evidence for increased CV risk following COVID-19 job losses may come from another recent economic upheaval. The Multi-Ethnic Study of Atherosclerosis (MESA) followed a panel of patients from 2000 to 2012. The Great Recession of 2008 to 2010 was associated with a worsening of BP and fasting plasma glucose trajectories, particularly among those aged <65 years and on medications, who may be disproportionately affected by unemployment and loss of health benefits. Onset of the Great Recession was also associated with a decline in medication use and treatment intensity.85 We therefore have good reason to expect the economic fallout from COVID-19 to manifest as worsening CV risk-factor control in years to come.
Physical inactivity caused by stay-at-home orders
A recent study using individual data from 455,404 users of the Argus (Azumio, Palo Alto, CA) smartphone app from 187 countries documented a 27% reduction in step counts worldwide in the month after the WHO declared COVID-19 a pandemic, with a clear link between the degree of local lockdown restrictions and reduced physical activity.86 For example, step counts decreased by 49% in Italy within days of a rigourously enforced lockdown being initiated on March 9, but by only 7% in Sweden, where social distancing, but no lockdown, was implemented. Fitbit (Fitbit, San Francisco, CA) also reported 7% to 38% reductions in average step counts worldwide within the first week after pandemic restrictions came into force, including a 14% reduction in Canada.87 Given the importance of regular physical activity in reducing risk of CV events and stroke,88 there is no question that the pandemic-related lockdown restrictions will have an adverse impact on CV health, particularly as detrimental effects in CV function and increased risk factors can manifest within 1 to 4 weeks of inactivity.89 Although it will be some time before we have empiric data to quantify this impact, modelling studies have estimated that even a 10% decrease in physical activity levels may result in an extra 535,000 all-cause deaths and 42,000 CV deaths.89
Social isolation, depression, and anxiety
The COVID-19 pandemic has been associated with an unprecedented increase in rates of depression and anxiety across the general population. In initial surveys from China during the period of mandatory stay-at-home orders, more than one-half of patients reported a moderate-to-severe psychological impact of the outbreak, with 16.5% reporting moderate-to-severe depressive symptoms, and 28.8% reporting moderate-to-severe anxiety symptoms.90 By the end of May 2020, more than 60 studies had been published, using validated instruments to measure the psychological impact of COVID-19. Pooling the results of these studies, anxiety and depression were identified in 32% and 27% of the general public, respectively.91 Most of these studies took place in China, but recent publications in other countries show similar findings,92 , 93, including 1 large nation-wide US survey showing severe depression symptoms (Center for Epidemiologic Studies Depression Scale [CES-D] score > = 25) in 28% of respondents.70 Reasons for this include social isolation owing to fear of contagion and stay-at-home orders;94 intrinsic fear of COVID-19;95 food insecurity and other economic deprivation owing to job loss;70 and false or misleading information: that is, the “infodemic”60 that has accompanied COVID-19 on media and social media platforms. In addition, even before COVID-19, social isolation and loneliness were recognized risk factors for mortality, with odds ratios of 1.29 and 1.32, consistent with many more traditional “medical” mortality risk factors.96
Many, although not all,97 longitudinal studies have shown a temporal association among depression, anxiety, and diabetes mellitus. Pooling among them, depression appears to increase the risk of diabetes 1.3-fold;98 anxiety appears to increase the risk of diabetes 1.5-fold.99 , 100 Patients with depression may exhibit reduced healthy behaviours,101 worse glycemic control,102 and increased insulin resistance.103 , 104 Depression, prevalent in over one-fifth of adults with hypertension,105 has similarly been associated with a 1.4-fold increased risk of hypertension.106 Anxiety has also been associated with unhealthy behaviours that increase the risk of hypertension,107 and CV disease such as tobacco use, physical inactivity, and unhealthy food choices.107 , 108 Both anxiety and depression have been associated with lower rates of medication adherence.109, 110, 111, 112 Other potential pathways linking depression and anxiety to CV risk have been proposed, including systemic chronic inflammation, hypothalamic-pituitary-adrenal (HPA) axis dysfunction, and autonomic dysregulation as causal mechanisms.113
Moving Forward: Strategies to Mitigate Risk in the Era of COVID-19
We have so far sketched a variety of pathways by which the COVID-19 pandemic and our individual and collective responses to it may unintentionally worsen CV risk in the general population. For health care providers, efforts to continue routine CV risk reduction via virtual means in this challenging landscape will be critical.19 , 114 Virtual visits may create new challenges, yet telehealth technologies offer key means of communicating with and engaging patients during the pandemic. Here, we briefly review some of the evidence for telemonitoring and telecare in CV risk-factor management.
Digital health interventions for the management of CV risk factors
Digital health interventions (DHIs) span a spectrum from generic web-based strategies, to text messaging or e-mail interactions between patients and health care providers, to the use of mobile phone health-related applications, and to telemonitoring with the use of wearable biometric sensors by patients. All have the goal of “watching over the 5000 hours per year when patients are not in direct contact with health care providers … and are deciding whether to take prescribed medications or follow other medical advice, deciding what to eat and drink and whether to smoke, and making other choices about activities that can profoundly affect their health.”115 Numerous systematic reviews have confirmed that DHIs can successfully improve specific CV risk factors such as smoking cessation,116 physical activity,117 and weight loss.118 Importantly, a systematic review of 9 trials in 2263 patients (2 primary prevention trials, 2 in heart failure, and 5 secondary prevention studies) reported a 40% relative reduction in CV disease outcomes (CV events, hospitalizations, and all-cause mortality) from DHI in secondary prevention patients both via risk-factor reduction but also by increasing adherence to evidence-based preventive therapies such as aspirin or statins. The pooled absolute risk reduction of 7.5% implied a number-needed-to-treat of 16 patients.119 Although there was no statistically significant difference in CV outcomes with DHI in the primary prevention studies, there were statistically significant benefits on weight, systolic BP, total and low-density lipoprotein (LDL) cholesterol, and Framingham risk scores.119 However, a Cochrane review of 93 DHI studies highlighted that much of the published evidence is of poor quality, there is evidence of publication bias, and the results are inconsistent across studies with effectiveness influenced by numerous factors, including the type of patients studied, the type and frequency of interactions between patients and health care providers, and the health care system in which the intervention is embedded.120 Further research is clearly needed to determine the most effective DHI modalities in specific populations and to better understand the patient, provider, health system, and program factors that influence effectiveness. Until then, we can expect to see both positive121 and negative122 , 123 trials published in the literature.124
Telehealth for management of BP
Although a number of trials have demonstrated that patient self-monitoring has a small but statistically significant effect on improving control of BP,125 , 126 a Canadian study reported that only 16% of patients complied with all recommended procedures when measuring their BP at home, and less than one-third reported at least 80% of their home measurements to their physicians.127 On the other hand, the addition of telemonitoring, whereby clinicians review BP readings submitted by patients over the internet or via short message service (SMS) and titrate therapy as required, is associated with much larger reductions in BP, in the order of 5 mm Hg for systolic BP,128, 129, 130 and has been shown to be cost effective.129 , 131 However, telemonitoring trials have generally recruited small samples of often highly selected individuals, followed for relatively short periods. There are numerous potential barriers to scaling such interventions up to accommodate large numbers of patients,132 and thus such efforts should be accompanied by robust evaluation plans.
Telehealth for diabetes care
Telehealth interventions in diabetes care have usually involved self-monitoring of blood glucose and communication back to the clinician via a variety of means (text message, web portal, smartphone apps, and telephone), with feedback offered to the patient ranging from none; to generic messages generated by automated processes; to nurse, pharmacist, or physician feedback with or without concrete medication changes; with or without diet and lifestyle coaching, communicated either synchronously or asynchronously.133 , 134 Critical appraisal of the field is complicated by the high degree of heterogeneity of interventions, but a recent systematic review of 111 randomized trials identified a pooled mean hemoglobin (Hb) A1c reduction of 0.57% (95% confidence interval [CI], 0.40%-0.74%),135 consistent with other reviews.136, 137, 138 Other reported benefits of telehealth in diabetes include increased patient satisfaction, knowledge, and self-efficacy outcomes.139 However, 1 broad theme appears consistently: Interventions involving personal feedback from a health care provider—whether a specialist nurse, pharmacist, or physician does not appear to matter—with the ability to make medication changes appear to be critical to achieving reductions in HbA1c.135 , 139 In recognition of this, the current Diabetes Canada guidelines have assigned telehealth a grade A recommendation, as a technology that may help facilitate many of the quality improvement strategies derived from the Chronic Care Model.140
Thus, although the evidence for DHI and telehealth is heterogeneous in CV risk-factor management, a key determinant of success appears to be clinician review of patient-generated data linked to actual changes in medical management and the involvement of a broader health care team than merely a single physician.114 The very best examples of telehealth in the literature improved patient satisfaction, knowledge, and self-efficacy in addition to CV risk factors such as BP levels and HbA1c. Many barriers remain: for example, devising means of transmitting clinical data accessible to patients of varying ages and skill; appropriate remuneration for telemedicine activities; availability of technical support; and navigating new workflows. These barriers are not insurmountable.141 Although there is no question that implementing and scaling up these new models of care is challenging, the COVID-19 pandemic offers an opportunity to rethink how we deliver care and to embrace new technologies for reaching out to our patients—and allowing our patients to reach out to us—to remodel how we optimize reduction of CV risk in Canada and improve the accessibility and efficiency of health care.
Conclusions
The COVID-19 pandemic is likely to have a wide and long-lasting impact on CV risk-factor control and outcomes for the general population and not only for survivors of COVID-19. The threat of an “impending tsunami”45 of CV morbidity and mortality in the coming years is real. Many of the challenges to CV risk management caused by the pandemic will require forward-looking social and economic policies to address. New messaging from public health authorities, who have until now been focused on preventing acute COVID-19 infections, will be needed: messaging that considers the overall health needs of the population, including primary and secondary prevention of CV disease, as the pandemic stretches out over time.19 , 114 As clinicians, we also need to rise to this challenge by finding ways of remodelling care delivery and improving the effectiveness and efficiency of CV risk management during the COVID-19 pandemic. In doing so, we are likely to find ourselves innovating during the most unlikely of times.
Acknowledgements
The authors thank Dr Leiah Luoma for her assistance in creating the Figure.
Funding Sources
Dr McAlister is supported by the Alberta Health Services Chair in Cardiovascular Outcomes Research. There was no project-specific funding for this study.
Disclosures
The authors have no conflicts of interest to disclose.
Footnotes
See page 728 for disclosure information.
References
- 1.Government of Canada Coronavirus disease (COVID-19): outbreak update. August 6, 2020. Ottawa, ON: Government of Canada; 2020. https://www.canada.ca/en/public-health/services/diseases/2019-novel-coronavirus-infection.html Available at:
- 2.Puntmann V.O., Carerj M.L., Wieters I. Outcomes of cardiovascular magnetic resonance imaging in patients recently recovered from coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020;5:1265–1273. doi: 10.1001/jamacardio.2020.3557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Driggin E., Madhavan M.V., Bikdeli B. Cardiovascular considerations for patients, health care workers, and health systems during the COVID-19 pandemic. J Am Coll Cardiol. 2020;75:2352–2371. doi: 10.1016/j.jacc.2020.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nicolai L., Leunig A., Brambs S. Immunothrombotic dysregulation in Covid-19 pneumonia is associated with respiratory failure and coagulopathy. Circulation. 2020;142:1176–1189. doi: 10.1161/CIRCULATIONAHA.120.048488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Verdecchia P., Cavallini C., Spanevello A., Angeli F. COVID-19: ACE2-centric infective disease? Hypertension. 2020;76:294–299. doi: 10.1161/HYPERTENSIONAHA.120.15353. [DOI] [PubMed] [Google Scholar]
- 6.Shete A. Urgent need for evaluating agonists of angiotensin-(1-7)/Mas receptor axis for treating patients with COVID-19. Int J Infect Dis. 2020;96:348–351. doi: 10.1016/j.ijid.2020.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mansueto G., Niola M., Napoli C. Can COVID 2019 disease induce a specific cardiovascular damage or it exacerbates pre-existing cardiovascular diseases? Pathol Res Pract. 2020;216:153086. doi: 10.1016/j.prp.2020.153086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zoufaly A., Poglitsch M., Aberle J.H. Human recombinant soluble ACE2 in severe COVID-19. Lancet Respir Med. 2020;8:1154–1158. doi: 10.1016/S2213-2600(20)30418-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Magalhaes G.S., Rodrigues-Machado M.D.G., Motta-Santos D., Campagnole-Santos M.J., Santos R.A.S. Activation of Ang-(1-7)/Mas receptor is a possible strategy to treat coronavirus (SARS-CoV-2) infection. Front Physiol. 2020;11:730. doi: 10.3389/fphys.2020.00730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Madjid M., Safavi-Naeini P., Solomon S.D., Vardeny O. Potential effects of coronaviruses on the cardiovascular system: a review. JAMA Cardiol. 2020;5:831–840. doi: 10.1001/jamacardio.2020.1286. [DOI] [PubMed] [Google Scholar]
- 11.Boukhris M., Hillani A., Moroni F. Cardiovascular implications of the COVID-19 pandemic: a global perspective. Can J Cardiol. 2020;36:1068–1080. doi: 10.1016/j.cjca.2020.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guagliumi G., Sonzogni A., Pescetelli I., Pellegrini D., Finn A.V. Microthrombi and ST-segment-elevation myocardial infarction in COVID-19. Circulation. 2020;142:804–809. doi: 10.1161/CIRCULATIONAHA.120.049294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lala A., Johnson K.W., Januzzi J.L. Prevalence and impact of myocardial injury in patients hospitalized with COVID-19 infection. J Am Coll Cardiol. 2020;76:533–546. doi: 10.1016/j.jacc.2020.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mitrani R.D., Dabas N., Goldberger J.J. COVID-19 cardiac injury: implications for long-term surveillance and outcomes in survivors. Heart Rhythm. 2020;17:1984–1990. doi: 10.1016/j.hrthm.2020.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chee Y.J., Ng S.J.H., Yeoh E. Diabetic ketoacidosis precipitated by Covid-19 in a patient with newly diagnosed diabetes mellitus. Diabetes Res Clin Pract. 2020;164:108166. doi: 10.1016/j.diabres.2020.108166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li J., Wang X., Chen J., Zuo X., Zhang H., Deng A. COVID-19 infection may cause ketosis and ketoacidosis. Diabetes Obes Metab. 2020;22:1935–1941. doi: 10.1111/dom.14057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rubino F., Amiel S.A., Zimmet P. New-onset diabetes in Covid-19. N Engl J Med. 2020;383:789–790. doi: 10.1056/NEJMc2018688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baum A., Kaboli P.J., Schwartz M.D. Reduced in-person and increased telehealth outpatient visits during the COVID-19 pandemic. Ann Intern Med. 2021;174:129–131. doi: 10.7326/M20-3026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carter P., Anderson M., Mossialos E. Health system, public health, and economic implications of managing COVID-19 from a cardiovascular perspective. Eur Heart J. 2020;41:2516–2518. doi: 10.1093/eurheartj/ehaa342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alexander G.C., Tajanlangit M., Heyward J., Mansour O., Qato D.M., Stafford R.S. Use and content of primary care office-based vs telemedicine care visits during the COVID-19 pandemic in the US. JAMA Netw Open. 2020;3 doi: 10.1001/jamanetworkopen.2020.21476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barker I., Steventon A., Deeny S.R. Association between continuity of care in general practice and hospital admissions for ambulatory care sensitive conditions: cross sectional study of routinely collected, person level data. BMJ. 2017;356:j84. doi: 10.1136/bmj.j84. [DOI] [PubMed] [Google Scholar]
- 22.Bentler S.E., Morgan R.O., Virnig B.A., Wolinsky F.D. The association of longitudinal and interpersonal continuity of care with emergency department use, hospitalization, and mortality among Medicare beneficiaries. PLoS One. 2014;9 doi: 10.1371/journal.pone.0115088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weir D.L., McAlister F.A., Majumdar S.R., Eurich D.T. The interplay between continuity of care, multimorbidity, and adverse events in patients with diabetes. Med Care. 2016;54:386–393. doi: 10.1097/MLR.0000000000000493. [DOI] [PubMed] [Google Scholar]
- 24.Pereira Gray D.J., Sidaway-Lee K., White E., Thorne A., Evans P.H. Continuity of care with doctors-a matter of life and death? A systematic review of continuity of care and mortality. BMJ Open. 2018;8 doi: 10.1136/bmjopen-2017-021161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reilly B.M. Physical examination in the care of medical inpatients: an observational study. Lancet. 2003;362:1100–1105. doi: 10.1016/S0140-6736(03)14464-9. [DOI] [PubMed] [Google Scholar]
- 26.Costanzo C., Verghese A. The physical examination as ritual: social sciences and embodiment in the context of the physical examination. Med Clin North Am. 2018;102:425–431. doi: 10.1016/j.mcna.2017.12.004. [DOI] [PubMed] [Google Scholar]
- 27.Chwistek M. Are You wearing your white coat? Telemedicine in the time of pandemic. JAMA. 2020;324:149–150. doi: 10.1001/jama.2020.10619. [DOI] [PubMed] [Google Scholar]
- 28.Canada Health Infoway Experiences of Health Care During COVID-19 Reported by Canadians. Toronto, ON: Canada Health Infoway; 2020. https://www.infoway-inforoute.ca/en/component/edocman/3828-experiences-of-health-care-during-covid-19-reported-by-canadians/view-document?Itemid=101 Available at:
- 29.Hartnett K.P., Kite-Powell A., DeVies J. Impact of the COVID-19 pandemic on emergency department visits: United States, January 1, 2019-May 30, 2020. MMWR. 2020;69:699–704. doi: 10.15585/mmwr.mm6923e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rosenbaum L. The untold toll: the pandemic's effects on patients without Covid-19. N Engl J Med. 2020;382:2368–2371. doi: 10.1056/NEJMms2009984. [DOI] [PubMed] [Google Scholar]
- 31.Jeffery M.M., D'Onofrio G., Paek H. Trends in emergency department visits and hospital admissions in health care systems in 5 states in the first months of the COVID-19 pandemic in the US. JAMA Intern Med. 2020;180:1328–1333. doi: 10.1001/jamainternmed.2020.3288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mafham M.M., Spata E., Goldacre R. COVID-19 pandemic and admission rates for and management of acute coronary syndromes in England. Lancet. 2020;396:381–389. doi: 10.1016/S0140-6736(20)31356-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Solomon M.D., McNulty E.J., Rana J.S. The Covid-19 pandemic and the incidence of acute myocardial infarction. N Engl J Med. 2020;383:691–693. doi: 10.1056/NEJMc2015630. [DOI] [PubMed] [Google Scholar]
- 34.Frankfurter C., Buchan T.A., Kobulnik J. Reduced rate of hospital presentations for heart failure during the Covid-19 pandemic in Toronto, Canada. Can J Cardiol. 2020;36:S74. doi: 10.1016/j.cjca.2020.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Baum A., Schwartz M.D. Admissions to Veterans Affairs hospitals for emergency conditions during the COVID-19 pandemic. JAMA. 2020;324:96–99. doi: 10.1001/jama.2020.9972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Baldi E., Sechi G.M., Mare C. COVID-19 kills at home: the close relationship between the epidemic and the increase of out-of-hospital cardiac arrests. Eur Heart J. 2020;32:3045–3054. doi: 10.1093/eurheartj/ehaa508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Marijon E., Karam N., Jost D. Out-of-hospital cardiac arrest during the COVID-19 pandemic in Paris, France: a population-based, observational study. Lancet Public Health. 2020;5:e437–e443. doi: 10.1016/S2468-2667(20)30117-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Secco G.G., Zocchi C., Parisi R. Decrease and delay in hospitalization for acute coronary syndromes during the 2020 SARS-CoV-2 pandemic. Can J Cardiol. 2020;36:1152–1155. doi: 10.1016/j.cjca.2020.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gluckman T.J., Wilson M.A., Chiu S.T. Case rates, treatment approaches, and outcomes in acute myocardial infarction during the coronavirus disease 2019 pandemic. JAMA Cardiol. 2020;5:1419–1424. doi: 10.1001/jamacardio.2020.3629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tam C.F., Cheung K.S., Lam S. Impact of coronavirus disease 2019 (COVID-19) outbreak on st-segment-elevation myocardial infarction care in Hong Kong, China. Circ Cardiovasc Qual Outcomes. 2020;13 doi: 10.1161/CIRCOUTCOMES.120.006631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Roffi M., Guagliumi G., Ibanez B. The obstacle course of reperfusion for ST-segment- elevation myocardial infarction in the COVID-19 pandemic. Circulation. 2020;141:1951–1953. doi: 10.1161/CIRCULATIONAHA.120.047523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wilson S.J., Connolly M.J., Elghamry Z. Effect of the COVID-19 pandemic on ST-segment-elevation myocardial infarction presentations and in-hospital outcomes. Circ Cardiovasc Interv. 2020;13 doi: 10.1161/CIRCINTERVENTIONS.120.009438. [DOI] [PubMed] [Google Scholar]
- 43.Montaner J., Barragán-Prieto A., Pérez-Sánchez S. Break in the stroke chain of survival due to COVID-19. Stroke. 2020;51:2307–2314. doi: 10.1161/STROKEAHA.120.030106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jacques F., Voisine P., Perrault L. Cardiovascular collateral damages at the time of COVID-19. Can J Cardiol. 2020;36:1327–e1327. doi: 10.1016/j.cjca.2020.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Allahwala U.K., Denniss A.R., Zaman S., Bhindi R. Cardiovascular disease in the post-COVID-19 era: the impending tsunami? Heart Lung Circ. 2020;29:809–811. doi: 10.1016/j.hlc.2020.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chu D., Chen R.C., Ku C.Y., Chou P. The impact of SARS on hospital performance. BMC Health Serv Res. 2008;8:228. doi: 10.1186/1472-6963-8-228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Alberta Precision Laboratories, Dynalife Medical Laboratories Memorandum re: Cessation of Routine Laboratory & Fecal Immunochemical Testing During COVID-19 Pandemic. Edmonton, AB: Alberta Health Services; March 25, 2020. http://www.albertahealthservices.ca/assets/info/ppih/if-ppih-apl-memo-physicians-cease-non-essential.pdf Available at:
- 48.Choosing Wisely Campaigns COVID-19: Recommendations for the Public (#1-4); Recommendations for Clinicians (#5-9). 2020 Apr 30: Choosing Wisely Canada; 2020. http://www.choosingwiselycanada.org/covid-19/ Available at:
- 49.Ellis E.G. What if you can’t avoid the hospital as Covid-19 spreads? San Francisco, CA: Wired, Conde Nast Publications; 2020. https://www.wired.com/story/coronavirus-covid-19-hospital-visits/ Available at:
- 50.Guan W.J., Liang W.H., Zhao Y. Comorbidity and its impact on 1590 patients with COVID-19 in China: a nationwide analysis. Eur Respir J. 2020;55:2000547. doi: 10.1183/13993003.00547-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Reed B.N., Fox E.R., Konig M. The impact of drug shortages on patients with cardiovascular disease: causes, consequences, and a call to action. Am Heart J. 2016;175:130–141. doi: 10.1016/j.ahj.2016.02.004. [DOI] [PubMed] [Google Scholar]
- 52.Drug Shortages Canada Summary Report. www.drugshortagescanada.ca. https://www.drugshortagescanada.ca/rws-search?perform=1 July 16, 2020: Bell Canada; 2020. Available at:
- 53.McAlister F.A., Youngson E. Impact of the generic valsartan recall in Alberta, Canada. J Am Coll Cardiol. 2020;75:1860–1862. doi: 10.1016/j.jacc.2020.02.034. [DOI] [PubMed] [Google Scholar]
- 54.Jackevicius C.A., Krumholz H.M., Chong A. Population impact of generic valsartan recall. Circulation. 2020;141:411–413. doi: 10.1161/CIRCULATIONAHA.119.044494. [DOI] [PubMed] [Google Scholar]
- 55.Armstrong P.W., McAlister F.A. Searching for adherence: can we fulfill the promise of evidence-based medicines? J Am Coll Cardiol. 2016;68:802–804. doi: 10.1016/j.jacc.2016.06.006. [DOI] [PubMed] [Google Scholar]
- 56.Beeftink M.M., van der Sande N.G., Bots M.L. Safety of temporary discontinuation of antihypertensive medication in patients with difficult-to-control hypertension. Hypertension. 2017;69:927–932. doi: 10.1161/HYPERTENSIONAHA.116.08793. [DOI] [PubMed] [Google Scholar]
- 57.DeFelice A., Willard J., Lawrence J. The risks associated with short-term placebo-controlled antihypertensive clinical trials: a descriptive meta-analysis. J Hum Hypertens. 2008;22:659–668. doi: 10.1038/jhh.2008.51. [DOI] [PubMed] [Google Scholar]
- 58.Heeschen C., Hamm C.W., Laufs U., Snapinn S., Böhm M., White H.D. Withdrawal of statins increases event rates in patients with acute coronary syndromes. Circulation. 2002;105:1446–1452. doi: 10.1161/01.cir.0000012530.68333.c8. [DOI] [PubMed] [Google Scholar]
- 59.Department of Global Communications UN tackles ‘infodemic’ of misinformation and cybercrime in COVID-19 crisis. United Nations, COVID-19 Response, March 31, 2020. New York, NY: United Nations; 2020. https://www.un.org/en/un-coronavirus-communications-team/un-tackling-%E2%80%98infodemic%E2%80%99-misinformation-and-cybercrime-covid-19 Available at:
- 60.Love J.S., Blumenberg A., Horowitz Z. The parallel pandemic: medical misinformation and COVID-19: primum non nocere. J Gen Intern Med. 2020;35:2435–2436. doi: 10.1007/s11606-020-05897-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Boulware D.R., Pullen M.F., Bangdiwala A.S. A randomized trial of hydroxychloroquine as postexposure prophylaxis for Covid-19. N Engl J Med. 2020;383:517–525. doi: 10.1056/NEJMoa2016638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.National Instiotues of Health Press Office NIH Halts Clinical Trial of Hydroxychloroquine (press release). Bethesda, MD: National Institutes of Health; 2020. https://www.nih.gov/news-events/news-releases/nih-halts-clinical-trial-hydroxychloroquine Available at:
- 63.Vervoort D., Ma X., Luc J.G.Y., Zieroth S. Rapid scholarly dissemination and cardiovascular community engagement to combat the infodemic of the COVID-19 Pandemic. Can J Cardiol. 2020;36 doi: 10.1016/j.cjca.2020.03.042. 969.e961-969.e962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Majumder M.S., Mandl K.D. Early in the epidemic: impact of preprints on global discourse about COVID-19 transmissibility. Lancet Glob Health. 2020;8:e627–e630. doi: 10.1016/S2214-109X(20)30113-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Palayew A., Norgaard O., Safreed-Harmon K., Andersen T.H., Rasmussen L.N., Lazarus J.V. Pandemic publishing poses a new COVID-19 challenge. Nat Hum Behav. 2020;4:666–669. doi: 10.1038/s41562-020-0911-0. [DOI] [PubMed] [Google Scholar]
- 66.Bramstedt K.A. The carnage of substandard research during the COVID-19 pandemic: a call for quality. J Med Ethics. 2020;46:803–807. doi: 10.1136/medethics-2020-106494. [DOI] [PubMed] [Google Scholar]
- 67.Pundi K., Perino A.C., Harrington R.A., Krumholz H.M., Turakhia M.P. Characteristics and strength of evidence of COVID-19 studies registered on ClinicalTrials.gov. JAMA Intern Med. 2020;180:1398–1400. doi: 10.1001/jamainternmed.2020.2904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Dagens A., Sigfrid L., Cai E. Scope, quality, and inclusivity of clinical guidelines produced early in the Covid-19 pandemic: rapid review. BMJ. 2020;369:m1936. doi: 10.1136/bmj.m1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Statistics Canada. Table 14-10-0287-03. In: Labour Force Characteristics by Province, Monthly, Seasonally Adjusted. Ottawa, ON: Statistics Canada; 2020. Available at: 10.25318/1410028701-eng. Accessed July 22, 2020. [DOI]
- 70.Fitzpatrick K.M., Harris C., Drawve G. Living in the midst of fear: depressive symptomatology among US adults during the COVID-19 pandemic. Depress Anxiety. 2020;37:957–964. doi: 10.1002/da.23080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Law M.R., Cheng L., Kolhatkar A. The consequences of patient charges for prescription drugs in Canada: a cross-sectional survey. CMAJ Open. 2018;6:E63–70. doi: 10.9778/cmajo.20180008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Brandt J., Shearer B., Morgan S.G. Prescription drug coverage in Canada: a review of the economic, policy and political considerations for universal pharmacare. J Pharm Policy Pract. 2018;11:28. doi: 10.1186/s40545-018-0154-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Dinh T., Sutherland G. Conference Board of Canada; Ottawa, ON: 2017. Understanding the Gap: A Pan-Canadian Analysis of Prescription Drug Insurance Coverage. [Google Scholar]
- 74.Law M.R., Cheng L., Dhalla I.A., Heard D., Morgan S.G. The effect of cost on adherence to prescription medications in Canada. CMAJ. 2012;184:297–302. doi: 10.1503/cmaj.111270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Barnes S., Anderson L. Wellesley Institute; Toronto, ON: 2015. Low Earnings, Unfilled Prescriptions: Employer-Provided Health Benefit Coverage in Canada. [Google Scholar]
- 76.Rabi D.M., Edwards A.L., Southern D.A. Association of socio-economic status with diabetes prevalence and utilization of diabetes care services. BMC Health Serv Res. 2006;6:124. doi: 10.1186/1472-6963-6-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Dinca-Panaitescu S., Dinca-Panaitescu M., Bryant T., Daiski I., Pilkington B., Raphael D. Diabetes prevalence and income: results of the Canadian Community Health Survey. Health Policy. 2011;99:116–123. doi: 10.1016/j.healthpol.2010.07.018. [DOI] [PubMed] [Google Scholar]
- 78.Dinca-Panaitescu M., Dinca-Panaitescu S., Raphael D., Bryant T., Pilkington B., Daiski I. The dynamics of the relationship between diabetes incidence and low income: longitudinal results from Canada's National Population Health Survey. Maturitas. 2012;72:229–235. doi: 10.1016/j.maturitas.2012.03.017. [DOI] [PubMed] [Google Scholar]
- 79.Leng B., Jin Y., Li G., Chen L., Jin N. Socioeconomic status and hypertension: a meta-analysis. J Hypertens. 2015;33:221–229. doi: 10.1097/HJH.0000000000000428. [DOI] [PubMed] [Google Scholar]
- 80.Varanka-Ruuska T., Rautio N., Lehtiniemi H. The association of unemployment with glucose metabolism: a systematic review and meta-analysis. Int J Public Health. 2018;63:435–446. doi: 10.1007/s00038-017-1040-z. [DOI] [PubMed] [Google Scholar]
- 81.Levenstein S., Smith M.W., Kaplan G.A. Psychosocial predictors of hypertension in men and women. Arch Intern Med. 2001;161:1341–1346. doi: 10.1001/archinte.161.10.1341. [DOI] [PubMed] [Google Scholar]
- 82.Meneton P., Kesse-Guyot E., Méjean C. Unemployment is associated with high cardiovascular event rate and increased all-cause mortality in middle-aged socially privileged individuals. Int Arch Occup Environ Health. 2015;88:707–716. doi: 10.1007/s00420-014-0997-7. [DOI] [PubMed] [Google Scholar]
- 83.Dupre M.E., George L.K., Liu G., Peterson E.D. The cumulative effect of unemployment on risks for acute myocardial infarction. Arch Intern Med. 2012;172:1731–1737. doi: 10.1001/2013.jamainternmed.447. [DOI] [PubMed] [Google Scholar]
- 84.Schultz W.M., Kelli H.M., Lisko J.C. Socioeconomic status and cardiovascular outcomes: challenges and interventions. Circulation. 2018;137:2166–2178. doi: 10.1161/CIRCULATIONAHA.117.029652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Seeman T., Thomas D., Merkin S.S., Moore K., Watson K., Karlamangla A. The Great Recession worsened blood pressure and blood glucose levels in American adults. Proc Natl Acad Sci USA. 2018;115:3296–3301. doi: 10.1073/pnas.1710502115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tison G.H., Avram R., Kuhar P. Worldwide Effect of COVID-19 on physical activity: a descriptive study. Ann Intern Med. 2020:173. doi: 10.7326/M20-2665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Fitbit Staff The Impact of Coronavirus on Global Activity. Fitbit News. March 23, 2020. San Francisco, CA: Fitbit Inc.; 2020. https://blog.fitbit.com/covid-19-global-activity/ Available at:
- 88.Lee I.M., Shiroma E.J., Lobelo F., Puska P., Blair S.N., Katzmarzyk P.T. Effect of physical inactivity on major non-communicable diseases worldwide: an analysis of burden of disease and life expectancy. Lancet. 2012;380:219–229. doi: 10.1016/S0140-6736(12)61031-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Peçanha T., Goessler K.F., Roschel H., Gualano B. Social isolation during the COVID-19 pandemic can increase physical inactivity and the global burden of cardiovascular disease. Am J Physiol Heart Circ Physiol. 2020;318:H1441–H1446. doi: 10.1152/ajpheart.00268.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wang C., Pan R., Wan X. Immediate psychological responses and associated factors during the initial stage of the 2019 coronavirus disease (COVID-19) epidemic among the general population in China. Int J Environ Res Public Health. 2020;17:1729. doi: 10.3390/ijerph17051729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Luo M., Guo L., Yu M., Jiang W., Wang H. The psychological and mental impact of coronavirus disease 2019 (COVID-19) on medical staff and general public: a systematic review and meta-analysis. Psychiatry Res. 2020;291:113190. doi: 10.1016/j.psychres.2020.113190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Liu C.H., Zhang E., Wong G.T.F., Hyun S., Hahm H.C. Factors associated with depression, anxiety, and PTSD symptomatology during the COVID-19 pandemic: clinical implications for US young adult mental health. Psychiatry Res. 2020;290:113172. doi: 10.1016/j.psychres.2020.113172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Li L.Z., Wang S. Prevalence and predictors of general psychiatric disorders and loneliness during COVID-19 in the United Kingdom. Psychiatry Res. 2020;291:113267. doi: 10.1016/j.psychres.2020.113267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Dubey S., Biswas P., Ghosh R. Psychosocial impact of COVID-19. Diabetes Metab Syndr. 2020;14:779–788. doi: 10.1016/j.dsx.2020.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Vindegaard N., Benros M.E. COVID-19 pandemic and mental health consequences: systematic review of the current evidence. Brain Behav Immun. 2020;89:531–542. doi: 10.1016/j.bbi.2020.05.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Holt-Lunstad J., Smith T.B., Baker M., Harris T., Stephenson D. Loneliness and social isolation as risk factors for mortality: a meta-analytic review. Perspect Psychol Sci. 2015;10:227–237. doi: 10.1177/1745691614568352. [DOI] [PubMed] [Google Scholar]
- 97.Brown L.C., Majumdar S.R., Newman S.C., Johnson J.A. Type 2 diabetes does not increase risk of depression. CMAJ. 2006;175:42–46. doi: 10.1503/cmaj.051429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Yu M., Zhang X., Lu F., Fang L. Depression and risk for diabetes: a meta-analysis. Can J Diabetes. 2015;39:266–272. doi: 10.1016/j.jcjd.2014.11.006. [DOI] [PubMed] [Google Scholar]
- 99.Smith K.J., Deschênes S.S., Schmitz N. Investigating the longitudinal association between diabetes and anxiety: a systematic review and meta-analysis. Diabet Med. 2018;35:677–693. doi: 10.1111/dme.13606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Khambaty T., Callahan C.M., Perkins A.J., Stewart J.C. Depression and anxiety screens as simultaneous predictors of 10-year incidence of diabetes mellitus in older adults in primary care. J Am Geriatr Soc. 2017;65:294–300. doi: 10.1111/jgs.14454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Egede L.E. Effect of depression on self-management behaviors and health outcomes in adults with type 2 diabetes. Curr Diabetes Rev. 2005;1:235–243. doi: 10.2174/157339905774574356. [DOI] [PubMed] [Google Scholar]
- 102.Naicker K., Øverland S., Johnson J.A. Symptoms of anxiety and depression in type 2 diabetes: associations with clinical diabetes measures and self-management outcomes in the Norwegian HUNT study. Psychoneuroendocrinology. 2017;84:116–123. doi: 10.1016/j.psyneuen.2017.07.002. [DOI] [PubMed] [Google Scholar]
- 103.Everson-Rose S.A., Meyer P.M., Powell L.H. Depressive symptoms, insulin resistance, and risk of diabetes in women at midlife. Diabetes Care. 2004;27:2856–2862. doi: 10.2337/diacare.27.12.2856. [DOI] [PubMed] [Google Scholar]
- 104.Semenkovich K., Brown M.E., Svrakic D.M., Lustman P.J. Depression in type 2 diabetes mellitus: prevalence, impact, and treatment. Drugs. 2015;75:577–587. doi: 10.1007/s40265-015-0347-4. [DOI] [PubMed] [Google Scholar]
- 105.Li Z., Li Y., Chen L., Chen P., Hu Y. Prevalence of depression in patients with hypertension: a systematic review and meta-analysis. Medicine (Baltimore) 2015;94:e1317. doi: 10.1097/MD.0000000000001317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Meng L., Chen D., Yang Y., Zheng Y., Hui R. Depression increases the risk of hypertension incidence: a meta-analysis of prospective cohort studies. J Hypertens. 2012;30:842–851. doi: 10.1097/HJH.0b013e32835080b7. [DOI] [PubMed] [Google Scholar]
- 107.Johnson H.M. Anxiety and hypertension: is there a link? A literature review of the comorbidity relationship between anxiety and hypertension. Curr Hypertens Rep. 2019;21:66. doi: 10.1007/s11906-019-0972-5. [DOI] [PubMed] [Google Scholar]
- 108.Strine T.W., Mokdad A.H., Dube S.R. The association of depression and anxiety with obesity and unhealthy behaviors among community-dwelling US adults. Gen Hosp Psychiatry. 2008;30:127–137. doi: 10.1016/j.genhosppsych.2007.12.008. [DOI] [PubMed] [Google Scholar]
- 109.Mendes R., Martins S., Fernandes L. Adherence to medication, physical activity and diet in older adults with diabetes: its association with cognition, anxiety and depression. J Clin Med Res. 2019;11:583–592. doi: 10.14740/jocmr3894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Eze-Nliam C.M., Thombs B.D., Lima B.B., Smith C.G., Ziegelstein R.C. The association of depression with adherence to antihypertensive medications: a systematic review. J Hypertens. 2010;28:1785–1795. doi: 10.1097/HJH.0b013e32833b4a6f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Abreu-Silva E.O., Todeschini A.B. Depression and its relation with uncontrolled hypertension and increased cardiovascular risk. Curr Hypertens Rev. 2014;10:8–13. doi: 10.2174/157340211001141111144533. [DOI] [PubMed] [Google Scholar]
- 112.Gentil L., Vasiliadis H.M., Berbiche D., Préville M. Impact of depression and anxiety disorders on adherence to oral hypoglycemics in older adults with diabetes mellitus in Canada. Eur J Ageing. 2017;14:111–121. doi: 10.1007/s10433-016-0390-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Cohen B.E., Edmondson D., Kronish I.M. State of the art review: depression, stress, anxiety, and cardiovascular disease. Am J Hypertens. 2015;28:1295–1302. doi: 10.1093/ajh/hpv047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Khera A., Baum S.J., Gluckman T.J. Continuity of care and outpatient management for patients with and at high risk for cardiovascular disease during the COVID-19 pandemic: a scientific statement from the American Society for Preventive Cardiology. Am J Prev Cardiol. 2020;1:100009. doi: 10.1016/j.ajpc.2020.100009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Asch D.A., Muller R.W., Volpp K.G. Automated hovering in health care: watching over the 5000 hours. N Engl J Med. 2012;367:1–3. doi: 10.1056/NEJMp1203869. [DOI] [PubMed] [Google Scholar]
- 116.Whittaker R., McRobbie H., Bullen C., Rodgers A., Gu Y. Mobile phone-based interventions for smoking cessation. Cochrane Database Syst Rev. 2016;4:CD006611. doi: 10.1002/14651858.CD006611.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Fanning J., Mullen S.P., McAuley E. Increasing physical activity with mobile devices: a meta-analysis. J Med Internet Res. 2012;14:e161. doi: 10.2196/jmir.2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Stephens J., Allen J. Mobile phone interventions to increase physical activity and reduce weight: a systematic review. J Cardiovasc Nurs. 2013;28:320–329. doi: 10.1097/JCN.0b013e318250a3e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Widmer R.J., Collins N.M., Collins C.S., West C.P., Lerman L.O., Lerman A. Digital health interventions for the prevention of cardiovascular disease: a systematic review and meta-analysis. Mayo Clin Proc. 2015;90:469–480. doi: 10.1016/j.mayocp.2014.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Flodgren G., Rachas A., Farmer A.J., Inzitari M., Shepperd S. Interactive telemedicine: effects on professional practice and health care outcomes. Cochrane Database Syst Rev. 2015;9:CD002098. doi: 10.1002/14651858.CD002098.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Chow C.K., Redfern J., Hillis G.S. Effect of lifestyle-focused text messaging on risk factor modification in patients with coronary heart disease: a randomized clinical trial. JAMA. 2015;314:1255–1263. doi: 10.1001/jama.2015.10945. [DOI] [PubMed] [Google Scholar]
- 122.Anand S.S., Samaan Z., Middleton C. A digital health intervention to lower cardiovascular risk: a randomized clinical trial. JAMA Cardiol. 2016;1:601–606. doi: 10.1001/jamacardio.2016.1035. [DOI] [PubMed] [Google Scholar]
- 123.Salisbury C., O'Cathain A., Thomas C. Telehealth for patients at high risk of cardiovascular disease: pragmatic randomised controlled trial. BMJ. 2016;353:i2647. doi: 10.1136/bmj.i2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Nittas V., Mütsch M., Ehrler F., Puhan M.A. Electronic patient-generated health data to facilitate prevention and health promotion: a scoping review protocol. BMJ Open. 2018;8 doi: 10.1136/bmjopen-2017-021245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Agarwal R., Bills J.E., Hecht T.J., Light R.P. Role of home blood pressure monitoring in overcoming therapeutic inertia and improving hypertension control: a systematic review and meta-analysis. Hypertension. 2011;57:29–38. doi: 10.1161/HYPERTENSIONAHA.110.160911. [DOI] [PubMed] [Google Scholar]
- 126.Uhlig K., Patel K., Ip S., Kitsios G.D., Balk E.M. Self-measured blood pressure monitoring in the management of hypertension: a systematic review and meta-analysis. Ann Intern Med. 2013;159:185–194. doi: 10.7326/0003-4819-159-3-201308060-00008. [DOI] [PubMed] [Google Scholar]
- 127.Milot J.P., Birnbaum L., Larochelle P. Unreliability of home blood pressure measurement and the effect of a patient-oriented intervention. Can J Cardiol. 2015;31:658–663. doi: 10.1016/j.cjca.2015.03.006. [DOI] [PubMed] [Google Scholar]
- 128.Tucker K.L., Sheppard J.P., Stevens R. Self-monitoring of blood pressure in hypertension: a systematic review and individual patient data meta-analysis. PLoS Med. 2017;14 doi: 10.1371/journal.pmed.1002389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Omboni S., Gazzola T., Carabelli G., Parati G. Clinical usefulness and cost effectiveness of home blood pressure telemonitoring: meta-analysis of randomized controlled studies. J Hypertens. 2013;31:455–467. doi: 10.1097/HJH.0b013e32835ca8dd. [DOI] [PubMed] [Google Scholar]
- 130.McLean G., Band R., Saunderson K. Digital interventions to promote self-management in adults with hypertension systematic review and meta-analysis. J Hypertens. 2016;34:600–612. doi: 10.1097/HJH.0000000000000859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Monahan M., Jowett S., Nickless A. Cost-effectiveness of telemonitoring and self-monitoring of blood pressure for antihypertensive titration in primary care (TASMINH4) Hypertension. 2019;73:1231–1239. doi: 10.1161/HYPERTENSIONAHA.118.12415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Scott Kruse C., Karem P., Shifflett K., Vegi L., Ravi K., Brooks M. Evaluating barriers to adopting telemedicine worldwide: a systematic review. J Telemed Telecare. 2018;24:4–12. doi: 10.1177/1357633X16674087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Wu C., Wu Z., Yang L. Evaluation of the clinical outcomes of telehealth for managing diabetes: a PRISMA-compliant meta-analysis. Medicine (Baltimore) 2018;97 doi: 10.1097/MD.0000000000012962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Andrès E., Meyer L., Zulfiqar A.A. Telemonitoring in diabetes: evolution of concepts and technologies, with a focus on results of the more recent studies. J Med Life. 2019;12:203–214. doi: 10.25122/jml-2019-0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Faruque L.I., Wiebe N., Ehteshami-Afshar A. Effect of telemedicine on glycated hemoglobin in diabetes: a systematic review and meta-analysis of randomized trials. CMAJ. 2017;189:E341–E364. doi: 10.1503/cmaj.150885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Su D., Zhou J., Kelley M.S. Does telemedicine improve treatment outcomes for diabetes? A meta-analysis of results from 55 randomized controlled trials. Diabetes Res Clin Pract. 2016;116:136–148. doi: 10.1016/j.diabres.2016.04.019. [DOI] [PubMed] [Google Scholar]
- 137.So C.F., Chung J.W. Telehealth for diabetes self-management in primary healthcare: a systematic review and meta-analysis. J Telemed Telecare. 2018;24:356–364. doi: 10.1177/1357633X17700552. [DOI] [PubMed] [Google Scholar]
- 138.Lee P.A., Greenfield G., Pappas Y. The impact of telehealth remote patient monitoring on glycemic control in type 2 diabetes: a systematic review and meta-analysis of systematic reviews of randomised controlled trials. BMC Health Serv Res. 2018;18:495. doi: 10.1186/s12913-018-3274-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Mignerat M., Lapointe L., Vedel I. Using telecare for diabetic patients: a mixed systematic review. Health Policy Technol. 2014;3:90–112. [Google Scholar]
- 140.Clement M., Filteau P., Harvey B. Organization of diabetes care. Can J Diabetes. 2018;42(suppl 1):S27–35. doi: 10.1016/j.jcjd.2017.10.005. [DOI] [PubMed] [Google Scholar]
- 141.Ahn D.T. The COVID-19 pandemic: a "tech"-tonic shift toward virtual diabetes care. J Diabetes Sci Technol. 2020;14:708–709. doi: 10.1177/1932296820929719. [DOI] [PMC free article] [PubMed] [Google Scholar]