Abstract
Developmental programming alters life-course multi-organ function and significantly affects life-course health. Recently, interest has developed in how programming may influence the rate of aging. This review describes interactions of nutrition and programming-aging interactions in hypothalamo-pituitary-adrenal (HPA) development and function from fetal development to old age. A full picture of these interactions requires data on levels of HPA activity relating to the hypothalamic, adrenal cortical, circulating blood, and peripheral cortisol metabolism. Data are provided from studies on our baboon, nonhuman primate model both across the normal life course and in offspring of maternal baboons who were moderately undernourished by a global 30% diet reduction during pregnancy and lactation. Sex differences in offspring outcomes in response to similar challenges are described. The data clearly show programming of increased HPA axis activity by moderate maternal undernutrition. Increased postnatal circulating cortisol concentrations are related to accelerated aging of the brain and cardiovascular systems. Future studies should address peripheral cortisol production and the influence of aging advantage in females. These data support the view that the HPA is an orchestrator of interactions of programming-aging mechanisms.
Keywords: cardiovascular system, developmental programming: aging, hypothalamo-pituitary-adrenal axis, nonhuman primate
INTRODUCTION
Developmental programming can be defined as “the response to a specific challenge during a critical developmental time window that alters the trajectory of development with a persistent influence on the organism’s structural and functional phenotype.” These changes have been shown to predispose individuals to a wide range of chronic diseases in later life, including cardiovascular, metabolic, and neurological issues.1,2 Strong evidence for developmental programming goes back many years to separate studies such as those by Barraclough3 in the 1950s, who independently showed that exposure of newborn female rats to androgens in the first 5 days of life programs the adult female reproductive cycle to function in an acyclic, male fashion. Classical studies by Widdowson and McCance4 showed that the challenge of undernutrition during rat development has differential effects on life-course growth depending on whether the undernutrition occurs in early or late perinatal development. These observations were given a major impetus by a series of classic human epidemiological studies by Barker et al5 at the University of Southampton, England. As a result of their pioneering studies, many researchers in this field call the concept of developmental programming “the Barker hypothesis.” Barker6 showed, for example, that the likelihood of an individual dying as a result of heart disease is increased by over 50% in men whose birth weight was less than 2.5 kg compared with men with a birth weight of 4.3 kg.
METHODS
All procedures were approved by the Texas Biomedical Research Institute (TBRI) Animal Care and Use Committee and conducted in facilities approved by the Association for Assessment and Accreditation of Laboratory Animal Care. The baboons (Papio species) were housed in 20 × 20 × 15 foot metal and concrete group cages at the Southwest National Primate Research Center, at the TBRI, in San Antonio, Texas. Diet was normal Primate Center laboratory chow (12% energy from fat with 0.29% glucose, 0.32% fructose, and energy content of 3.07 kcal/g protein) Purina Monkey Diet 5038 (Purina, St Louis, MO, USA) ad libitum – a complete life-cycle diet for Old World primates. Water was continuously available in each feeding cage. Animal food consumption, weights, and health status were recorded daily. All animals were given a full veterinary examination prior to recruitment to the study and no obvious cause of ill health was observed.
All methods for hormone quantification, immunohistochemistry, and sources of antibodies used have been published.7
DEFINITION OF AGING
A definition of aging that addresses all its components would be very lengthy and complex as well as being open to considerable differences of opinion. One highly quoted review describes “nine tentative hallmarks that represent common denominators of aging in different organisms, with special emphasis on mammalian aging – genomic instability, telomere attrition, epigenetic alterations, loss of proteostasis, deregulated nutrient sensing, mitochondrial dysfunction, cellular senescence, stem cell exhaustion, and altered intercellular communication.”8 A considerable body of experimental evidence is available from investigations of each of these aging mechanisms. Many of these cellular and molecular systems have been shown to be modified by developmental programming. Thus, it should be anticipated that programming and aging interact.
TEN PRINCIPLES OF DEVELOPMENTAL PROGRAMMING – AGING INTERACTIONS
I. During development, there are critical periods of high vulnerability to suboptimal conditions, especially nutritional challenges. The vulnerable phase occurs at different times in development for different tissues. Cells dividing rapidly at the time of exposure to the challenge are at greatest risk.
II. Programming changes phenotype in ways that emerge at different times in later life. Therefore, it is essential that study of life-course function needs to begin early in life if one is to understand both programming and its effect on life-course health and aging.
III. Antecedents of aging can be seen early in the life course. Tracking these antecedents will enable the development of diagnostic and therapeutic measures that will improve health span.
IV. Programming results in several different structural changes in different organs. These include altered numbers of cells, usually a decrease, and a redistribution of the relative proportions of different types of cells within the organ.
V. While the brain is relatively protected from major adverse, negative aspects of the compensatory responses to developmental programming challenges, it is not completely protected. Thus, programming outcomes may lead to cognitive loss and behavioral changes at an earlier age.
VI. The challenges presented by programming can result in changes at many levels of hierarchical structure and function in neuroendocrine axes such as the hypothalamo-pituitary-adrenal (HPA) axis, including altered hormone production, peripheral hormone receptors, and peripheral hormone production.
VII. Programming challenges may promote compensations with short-term benefits during the developmental period of exposure, which, if they are not reversed, can carry a long-term price in later life and predispose to chronic diseases and accelerated aging. For example, decreased delivery of nutrients to the fetus increases fetal liver gluconeogenesis to compensate for low glucose availability. The resultant altered balance in carbohydrate metabolism that persists into adulthood may predispose undernourished babies to later-life obesity.
VIII. Developmental programming outcomes frequently show sex differences between males and females in severity or form.
IX. Aging processes also differ between males and females both in their mechanisms and rates of development. Females have well-documented slower and less pronounced aging trajectories in many systems – a sex difference sometimes called the female aging advantage.
X. Aging can be accelerated by a second challenge (a second hit) to a physiological system that has already been programmed to function suboptimally. This characteristic is of great interest with regard to the little-understood mechanisms that underlie the phenomenon of resilience.
These principles were first presented in relation to programming only and not in the broader context of programming-aging interactions (see Nathanielsz2 [p30]).
Comorbidities and aging
Aging is accompanied by comorbidities such as increased risk of obesity, diabetes, and cardiovascular disease. It is now clear that the age of onset, rate of progression, and extent of some of these potential comorbidities are often, at least in part, developmentally programmed by the quality and quantity of maternal nutrition in pregnancy and lactation.9 Programming of these and many other disease outcomes may occur following both maternal undernutrition and overnutrition. When considering interactions between comorbidities and aging, it is necessary to determine the extent to which the comorbid condition (eg, obesity) is a result of programming or simply reflects aging itself. Apportioning relative degrees of causation to these closely entwined health origins is extremely difficult as one often feeds the other, setting up a feed-forward system. However, to understand aging mechanisms it is necessary to determine whether the comorbidity influences aging processes or is neutral in this respect since it is always possible that the comorbidity is just an unrelated event occurring in the same aging timeframe.
NORMATIVE AGING AND MODIFYING FACTORS
Prenatal and postnatal factors influence the rate of normative aging
As the body ages, our physiological systems (eg, heart, liver, brain, and kidneys) gradually lose functional capacity, giving rise to aging pathophysiology, manifesting as impaired function and frailty. We can ascribe the term normative aging to a large and varied set of physiological processes including those listed above. There are various theories about the causes of normative aging, but one that certainly plays a role is “wear and tear.” In addition, at various times in their lives, humans experience a multitude of challenges that can alter the rate of change in aging. These deviations from the physiological norm vary greatly in incidence and severity between individuals. Challenges in developmental programming are often considered the “first hit” to alter the body’s phenotype. These programmed changes then alter an individual’s response to further changes in the postnatal environment, deemed the “second hit,” as addressed above in Principle X of the programming-aging interactions. The interaction that occurs between programming and these accumulated second hits – what Shakespeare called “the slings and arrows of outrageous fortune” – greatly influences the rate of aging.
Changes that alter the life-course aging trajectory have 2 distinct origins: (1) genetic modifications – variations in the instructions encoded in our genes, and (2) environmental challenges, such as those that give rise to developmental programming and those that occur later in life. These two interlinked categories of factors that affect our health span are often referred to as nature and nurture. In the context of this discussion it is important not to consider these deviations from the norm always as disease processes since it is always possible that variations could have positive effects on health span. To view these influences as disease factors helps in the difficult task of distinguishing pathology and disease from normative aging. Few people would consider aging a disease. However, on many occasions it is difficult to separate normative aging from aging brought about by programming and acquired disease. The ability to make this distinction on firm evidence-based criteria is a central challenge to determining the role of programming-aging interactions when attempting to establish the aging trajectory. The distinction is not just a matter of definition. How we view these two interactive processes affects our attempts to diagnose and modify outcomes, since we cannot prevent the aging process.
Together, the final rate of aging experienced by different individuals (different phenotypes) will depend on the body’s resilience. Some individuals just seem to be more resilient to life’s health challenges. Resilience, defined as the compensatory response to adversity,10 is a fascinating biological phenomenon and may in part be explained by the reserve built up during development in key cellular components such as mitochondria.
The female aging advantage
Females are known to age at a slower rate, with less marked age-related changes in many systems than are seen in males, as stated in Principle IX of the programming-aging interactions. Studies from the San Antonio Barshop Institute in male and female genetically heterogeneous (UM-HET3) mice show that in males, mortality hazard increases dramatically above that for females around postnatal day 100, maximizing around postnatal day 450 (Figure 1A).11,12 This aging advantage observed in the female mouse parallels that observed in women. Understanding the basis of the female advantage in aging is of central importance to developing insights into aging mechanisms.
Figure 1.
(A) The female aging advantage. Mortality hazard in male and female genetically heterogeneous (UM‐HET3) mice.11 Beginning around 100 days of life, the mortality hazard increases at a faster rate in males than in females. B. Twelve studies showing human grip strength decreases from 25-30 years. Modified from Dodds et al (2014).12 The curves represent the 10th, 25th, 50th, 75th, and 90th percentiles. The horizontal solid black line shows the danger that can come from analyses of categorical data at one young age and one old age. In this study, restriction of data to the ages at the extremes of the horizontal line shown would lead to the conclusion that no changes had occurred between these points. It would also miss the important point that grip strength, a major index of aging and frailty, begins to fall at a relatively early age, around 30 years in both males and females. Thus, to obtain a good picture of age-related changes in grip strength, or any other biological variable, studies in humans should begin well before 30 years of age (to cover the aging phase) and continue well into later life (to cover the aged phase).
A WOMB-TO-TOMB APPROACH
There is a fundamental need to study programming-aging interactions from very early stages in the life course, since, as stated in Principles I–III of the programming-aging interactions, there are critical periods of high vulnerability during development, altered phenotypic changes can emerge at different points in life, and antecedents of aging may be seen early in the life course. Whatever their genotype, each individual’s aging trajectory is likely changed to some extent by epigenetic changes in their fetal and immediate neonatal development. A telling example is the demonstration that offspring of obese women live shorter lives.13 Thus, to understand mechanisms that affect later-life aging, it is necessary to take a “womb-to-tomb” approach. Importantly, studies are best performed continuously throughout life using a continuous, serial approach, as some challenges may lead to changes across the life course that are not revealed in single-point, categorical studies (Figure 1B). For this reason, it is greatly preferable to undertake regression analysis over several time points rather than categorical analysis at a few fixed-age time points.
Baboon hypothalamo-pituitary-adrenal function across the life course
The review first discusses the normative development of the HPA axis (Figure 2A)14 through fetal, perinatal, and adult life in our well-established, baboon nonhuman primate model. Next, we discuss how moderate maternal nutrient reduction affects the development of the fetal and postnatal HPA axis. The model described is the only nonhuman primate model of maternal nutrition reduction available for determining the effects on the HPA axis and other organ systems. In this model, baboon mothers experiencing moderate maternal nutrient reduction had a weight-adjusted dietary intake that was 70% of the global diet eaten by control ad libitum–fed baboon mothers in pregnancy and lactation. Male and female offspring are moderately intrauterine growth–restricted (IUGR), with birth weight ∼88% of offspring of control mothers.15,16 Within the first few years of life, IUGR baboons show maladaptation of several organ systems, including cardiovascular,17,18 glucose control,19 and central nervous system.20 Development of all of these systems has been shown to be significantly impacted by the levels of cortisol circulating in blood in the perinatal period and throughout life. Next, the 11beta-hydroxysteroid dehydrogenase system – a powerful peripheral modifier of glucocorticoid function – is discussed. Peripheral synthesis and metabolism of cortisol is increasingly seen to be important in regulating glucocorticoid action in almost every tissue in the body.
Figure 2.
(A) The main levels of activity of the hypothalamo-pituitary-adrenal axis. Stimulatory mechanisms end in arrows; inhibitory mechanisms end in a horizontal bar. (B) Fetal cortisol in five precocial species. Days (d) to delivery (0) in sheep (•), pig (ο), human (▲), guinea pig (□), and horse (Δ). Reproduced from Fowden et al14 with permission. Abbreviations: AVP, arginine vasopressin; ACTH, adrenocorticotropin hormone; CRH, corticotropin-releasing hormone.
The hypothalamus is the source of corticotropin-releasing hormone and arginine vasopressin (AVP) – the major peptides that regulate the release of adrenocorticotropin hormone (ACTH) from the anterior pituitary to stimulate cortisol release from the adrenal cortex (Figure 2). Cortisol then circulates in the blood to regulate cellular metabolism, especially stress responses, in multiple tissues throughout the body, including the brain. When the cortisol level exceeds the upper limits of normal, cortisol exerts negative feedback on the hypothalamus to restore cortisol levels to within normal limits.
Exponential increase in cortisol in late fetal life prepares the fetus for an independent life and plays a central role in programming
The exponential rise in cortisol levels in fetal sheep in late gestation has been shown to initiate parturition by several investigators. The key evidence is the demonstration of a gradual rise in fetal cortisol beginning ∼20 days before delivery21 and the demonstration that both fetal hypophysectomy22 and bilateral lesions of the fetal hypothalamic paraventricular nuclei23 will delay delivery. The rise in cortisol is also responsible for terminal differentiation of the fetal lung,24 thyroid,25 and many other physiological systems. This makes the rise in cortisol a critical period of high vulnerability in perinatal development, which can result in structural changes to organs with long-lasting consequences, as stated in Principles I and IV of the programming-aging interactions. This exponential increase in cortisol in the final days or weeks of gestation has been demonstrated in a variety of other species in addition to sheep (Figure 2B). Given the multiple and pronounced effects that cortisol exerts on development, it is not surprising that this powerful regulatory hormone plays an important role in developmental programming.
Normal development of the fetal baboon hypothalamo-pituitary-adrenal axis
In published studies in this model of baboon pregnancy, no changes were observed in levels of maternal baboon cortisol during pregnancy.26 In fetuses of normally fed mothers, ACTH increased from 65% gestation (0.65 G) to 0.9 G, and remained elevated until 0.95 G (Figure 3A). Fetal cortisol showed the same profile, indicating an increase in fetal HPA axis activity in late gestation, as shown previously (Figure 3B). As mentioned above, this increased circulating fetal cortisol has been shown to play a central role in initiating and maintaining terminal differentiation of multiple fetal organs in preparation for an independent extrauterine existence.27
Figure 3.
(A) Plasma ACTH (n = 4–15) and (B) cortisol (n = 7–22) in the fetal baboon from 50% of gestation (0.5 G) to term (0.95 G). Data are mean + SEM. The percentages in A and B show the amount of change between two age points. *P < 0.05 between bracketed time points. Fetal baboon (C) ACTH and (D) cortisol over the second half of gestation in fetuses of control well-fed mothers (filled histograms, n = 4-25) and IUGR fetuses of undernourished mothers (open histograms, n = 5-13). Data are mean + SEM. *P < 0.05 values in fetuses of undernourished compared to fetuses of control, well-fed mothers. Reproduced from Zambrano et al.66 Abbreviations: ACTH, adrenocorticotropin hormone; IUGR, intrauterine growth restricted; SEM, standard error of the mean.
Programming of fetal baboon hypothalamo-pituitary-adrenal axis by moderate maternal undernutrition
Moderate maternal undernutrition (30% reduction in global intake) throughout pregnancy increased fetal ACTH (Figure 3C) and cortisol above the levels found in well-fed mothers (Figure 3D). These data demonstrate Principle VI of the programming-aging interactions, which states that challenges during critical periods of development can result in changes at many levels of hierarchical structure and function in neuroendocrine axes. Baboons born IUGR have shorter renal tubules and hence have less reserve for regulating modification of the glomerular filtrate and are thus predisposed to be less resilient to renal disease.28 Kidneys of adult who were growth restricted at birth have substantial variation in nephron endowment.29 These changes to organ structure and function (Principle IV) have lifelong costs, since early-life malnutrition results in structural alterations in the kidney, predisposing offspring to later-life renal dysfunction.
Mitochondrial bioenergetics plays a key role in energy metabolism, growth, and function in all tissues. In our model of moderate maternal nutrient reduction, despite the smaller overall fetal weight, the ratio of fetal kidney to body weight was not affected. In response to exposure to nutrient reduction, there was a fetal gender-specific differential messenger RNA (mRNA) expression of genes encoding proteins responsible for mitochondrial metabolite transport, which was greater in females than in males,28 demonstrating Principle VIII (sex-based differences) of the programming-aging interactions. These changes were accompanied by a decrease in mitochondrial protein COX6C – a cytochrome c oxidase subunit key to functioning of the electron transfer chain.
Corticotropin-releasing hormone, but not AVP, peptide abundance in the fetal hypothalamic paraventricular nucleus and median eminence was increased in our IUGR baboon fetuses.7 Several investigators have shown adverse consequences of fetal and postnatal exposure to levels of cortisol higher than those appropriate for the current stage of gestation.30–35 At term, plasma cortisol was elevated in baboon fetuses of nutrient-restricted mothers in the absence of any significant change in ACTH (Figure 3D).
Effect of nutritional programming of peripheral fetal cortisol production
Cortisol is produced in peripheral tissues by conversion of cortisone to cortisol by the reductase function of the bidirectional enzyme 11beta-hydroxysteroid dehydrogenase (11β-HSD1) (Figure 4). Hexose-6-phosphate-dehydrogenase, which generates nicotinamide adenine dinucleotide phosphate, the cofactor responsible for 11β-HSD1 reductase activity, is an essential coenzyme for this reaction. A productive hypothesis is that the ACTH-independent increase in fetal cortisol in IUGR fetuses in late fetal life is, at least in part, due to increased cortisol production in peripheral tissues as a result of this conversion of cortisone to cortisol by 11β-HSD1. Figure 5 shows that the cortisol concentration in fetal adipose tissue and liver is increased in a fetal sex-dependent manner. Cortisol concentration is increased in the liver in male but not female IUGR fetuses and increased in adipose tissue of female but not male fetuses. These changes are accompanied by the appropriate increases in mRNA and protein for 11β-HSD1 and hexose-6-phosphate-dehydrogenase in the perirenal adipose tissue of term IUGR female baboon fetuses and in the liver of term IUGR male baboon fetuses. Figure 6 shows that both mRNA and protein for 11β-HSD1 and hexose-6-phosphate-dehydrogenase are increased in adipose tissue but not in liver in IUGR females. In contrast, mRNA and protein for 11β-HSD1 and hexose-6-phosphate-dehydrogenase are increased in fetal liver but not in adipose tissue in IUGR males. These molecular changes explain the sex difference in cortisol concentrations in the liver and adipose tissue,36 once again emphasizing the importance of Principle VIII, which states the importance of sex-based differences in programming-aging interactions.
Figure 4.

Peripheral tissue cortisol production from cortisone by the enzyme 11ß hydroxysteroid dehydrogenase type 1. Abbreviations: 11ß-HSD1, 11ßhydroxysteroid dehydrogenase type 1; G6P, glucose-6-phosphate; H6PD, hexose-6-phosphate dehydrogenase; NADP, nicotinamide adenine dinucleotide phosphate; NADPH, nicotinamide adenine dinucleotide phosphate (reduced form); 6PG, 6-phosphogluconate.
Figure 5.
Effect of IUGR on cortisol in term fetal baboon perirenal adipose tissue and liver. Solid black histograms are data from male fetuses of well-fed mothers, solid white male IUGR fetuses of undernourished mothers, solid grey female fetuses of well-fed mothers, striped grey female IUGR fetuses of undernourished mothers. Data are mean + SEM; n = 5–7; *P < 0.05 vs. controls. Modified from Guo et al.36 Abbreviations: IUGR, intrauterine growth restricted; SEM, standard error of the mean.
Figure 6.
Perirenal A) mRNA and B) protein for 11β-HSD1 and H6PD. Liver C) mRNA and D) protein for 11β-HSD1 and H6PD. Solid black histograms are data from male fetuses of well-fed mothers, solid white male IUGR fetuses of undernourished mothers, solid grey female fetuses of well-fed mothers, striped grey female IUGR fetuses of undernourished mothers. Data are mean + SEM; n = 4-7; *P < 0.05 vs. controls. Modified from Guo et al.36 Abbreviations: 11b-HSD1, 11bhydroxysteroid dehydrogenase type 1; H6PD, hexose-6-phosphate dehydrogenase; IUGR, intrauterine growth restricted; mRNA, messenger ribonucleic acid; SEM, standard error of the mean.
Hepatic developmental programming changes driven by the increase in liver cortisol
The increased local concentration of cortisol within the liver – both circulating cortisol and locally produced cortisol – can have important fetal programming consequences. Principle VII of our list of developmental programming-aging interactions states that compensations occur in response to developmental programming challenges. These compensations may have short-term benefits while the fetus is exposed to the challenge, but if the compensation results in persistent structural or epigenetic changes, such changes can carry a long-term price in later life and affect the rate of aging. For example, poor delivery of nutrients to the fetus induces increased fetal liver gluconeogenesis – an attempt to compensate for low glucose availability.37 This gives rise to the “thrifty” metabolic phenotype in which calories are guarded and stored in liver and adipose tissue. As a result, this altered balance in carbohydrate metabolism may predispose undernourished babies to later-life obesity and dysfunctional carbohydrate metabolism.38,39 In our IUGR baboon fetuses, plasma cortisol levels were elevated at term (0.9 G; Figure 3). Phosphoenolpyruvate carboxykinase (PEPCK) is the rate-limiting enzyme in gluconeogenesis and is known to be stimulated by cortisol. When fetal liver PEPCK1 gene (PCK1) and its protein product (PEPCK1) at 0.9 G were measured in control and IUGR fetuses, immunoreactive PEPCK1 rose to 63% of adult levels in IUGR fetuses by term.37 PCK1 promoter methylation analysis using bisulfite sequencing was significantly reduced in 6 out of 9 CpG-dinucleotides in the PCK1 promoter in IUGR, compared with control liver, samples (Figure 7). In keeping with these changes, PEPCK1 protein levels increased in hepatocytes isolated from IUGR fetuses, and PEPCK1 mRNA expression was stimulated by glucocorticoids in vitro.40
Figure 7.
Percentage of overall methylation (using bisulfite sequencing) was significantly reduced in the IUGR liver PCK1 promoter in both sexes at term. Solid black histograms are data from male fetuses of well-fed mothers, solid white male IUGR fetuses of undernourished mothers, solid grey female fetuses of well-fed mothers, striped grey female IUGR fetuses of undernourished mothers. Data are mean + SEM; n = 3-5 per group; **P < 0.01; ***P < 0.001. Modified from Nijland et al.37 Abbreviation: IUGR, intrauterine growth restricted; SEM, standard error of the mean.
As young adults, IUGR baboons show increased subcutaneous, pericardial, and serum lipids, all indicative of accelerated aging, with distinct changes in male and female lipid profiles (Figure 8).41 Glucocorticoids are known to increase fat deposition and alter lipid metabolism, and these changes may well reflect the changes in local production of lipids discussed above.
Figure 8.
Apical pericardial fat deposition and serum LDL measurements in IUGR and age-matched control (CTL). 41 (A) Apical pericardial fat normalized for body surface area at 5-6 years (human equivalent ∼20-24 years). Data are mean + SEM; *P < 0.05. (B) Normalized apical pericardial fat thickness of IUGR males (closed squares, n = 8) against the normal LC cohort (open circles, n = 18) with (C) corresponding data for female normal life course (LC) cohort (open circles, N=18) and female IUGR (closed squares, N=7). In B and C solid regression lines are for the LC cohort with dotted 95% Confidence Interval bands. (D) LDL cholesterol concentrations at 8-9 years (human equivalent ∼32-36 years). Data are mean + SEM; *P < 0.05. Modified from Kuo et al.41 Abbreviations: CTL, control; LC life course; LDL, low density lipoprotein; SEM, standard error of the mean.
Thus, the challenge of maternal undernutrition during a critical period of development (Principle I) results in changes that emerge at different points during the life course (Principle II), with antecedents visible at very young ages (Principle III). Changes to organ structure and function (Principle IV) and to neuroendocrine axes (Principle VI) result from compensations to the programming challenge, with long-term consequences (Principle VII) that occur in a sex-dimorphic manner (Principles VIII and IX).
Further illustration of the important role glucocorticoids play in programming-aging interactions in metabolism can be seen in baboon studies of exposure to synthetic glucocorticoids in late gestation. One study investigated the common clinical treatment for threatened preterm birth in pregnant women by administering betamethasone phosphate at weight-adjusted human clinical doses to pregnant baboons during the second half of gestation. This treatment exposes the developing fetus to glucocorticoid concentrations higher than those normally experienced at the equivalent stage of maturation. This synthetic glucocorticoid treatment resulted in increased maternal and fetal blood pressure,42 altered fetal neuronal cytoskeleton and synapses,43 and functional changes to musculoskeletal insulin signaling pathways.44 As middle-age adults (10 years old, human equivalent ∼40 years), the baboons exposed to synthetic glucocorticoids during fetal life exhibited a 150% increase in pericardial fat thickness and 432% increase in liver lipids compared with age-matched controls (Figure 9),45 as well as increased subcutaneous fat deposition and increased serum lipids.46 When compared with the normal baboon life-course increase in low-density lipoprotein cholesterol, the baboons exposed to synthetic glucocorticoids were found to show an accelerated aging phenotype, with biological age advanced 3 years beyond chronological age, equivalent in humans to being about a decade older than expected.46 These findings underscore the necessity of considering environmental influences on aging processes from the earliest stages of life through the entire life course, with particular emphasis placed on the actions of glucocorticoids.
Figure 9.
MRI transverse section of abdomen showing abdominal fat in baboons at 10 years of age (human equivalent ∼40 years). Fat is represented in white. Left: Offspring of CTL vehicle-treated mother. Right: Baboon exposed to ßM. (Unpublished observations Kuo A, Clarke G Nathanielsz P) Abbreviations: CTL, control, βM, betamethasone.
Life-course studies of normative aging and programming of the nonhuman primate hypothalamo-pituitary-adrenal axis
As discussed above, whatever the variable, to establish the normative life-course aging baseline, data are needed from early stages of life. In the absence of an early-life baseline, determination of progression through the aging process cannot be firmly established.47–49 The earlier and more continuous the baseline established and the greater the number of age time points determined, the firmer the conclusions. Thus, early-life baseline data on circulating cortisol concentrations must be obtained prior to emergence of a clear aged phenotype. The best normative aging data are obtained when homogeneous, well-characterized subjects are evaluated. Variability in the life-course history of subjects studied will significantly affect the HPA axis aging trajectory (Figure 10). This is particularly true for the programming challenges each individual has faced during development.
Figure 10.
Fasting baboon morning plasma cortisol declines linearly with age. A) Data from 24 females in the Texas Pregnancy and Life-Course Health colony in San Antonio at Southwest National Primate Research Center 7; white dots represent animals for which hypothalamic peptide data are presented below in Figure 11. B) 31 Oklahoma University Health Sciences Center male and female baboons.55
Most aging studies on the HPA axis and other physiological systems have evaluated life-course changes using comparison between data obtained in limited categorical groups of ages, generally only 2: one in early life and one in later life. A more robust approach is to measure as many time points as possible across as large a section of the life course as possible (for review, see Zambrano et al50). In addition, as described in our programming-aging principles above, outcomes of developmental programming by early-life factors may lay dormant to emerge considerably later in the life course. Existing reviews discuss the role of glucocorticoids as well as adrenal dehydroepiandrosterone sulfate in programming life-course health and suggest a potential key role in aging7,51 life-course glucocorticoid profiles.
The HPA axis is highly labile in its responses to short- and long-term physiological and pathophysiological challenges. There is thus a need for a consistent and homogeneous set of subjects to determine changes in baseline HPA axis activity across the life course. Some studies that attempt to plot the life-course trajectory of plasma cortisol include data from subjects with chronic diseases, such as Alzheimer’s, hypertension, or diabetes.52 These conditions themselves alter cortisol production. Another report included subjects with high blood pressure and heart disease; young subjects were all men to avoid differences due to the menstrual cycle, while the older population was composed of males and females since it was considered that sex differences no longer exist late in life – a big assumption.53 Showing the weakness of limited time points, problems are compounded in single-sex studies, such as one that restricted the sample to eight young (18–35 years) and eight elderly (60–72 years) men.53
The mean baboon life span is around 21 years in most colonies, including the one at the Southwest National Primate Research Center (SNPRC).54 In 24 female baboons in this colony, aged 6–21 years (∼18–70 years human equivalent), there was a linear fall in fasting morning cortisol (Figure 10A).7 Investigators from the University of Oklahoma Primate Center have produced remarkably similar data (Figure 10B).55 The differences in absolute cortisol values may reflect either or both the different environmental conditions at the 2 primate centers and/or assay methodology. However, the similarity between the life-course changes is remarkable. The slope of the cortisol fall in the Oklahoma data was 23.7 ng/mL cortisol/year, while at the SNPRC it was 24.7 ng/mL cortisol/year. The remarkable similarity of the data in these 2 completely independent studies conducted at different locations shows that when homogeneous populations of well-characterized subjects are studied, data on the fall in life-course cortisol are robust. It is of interest that although the slopes of the life-course changes are almost identical, the absolute values are almost double in the Oklahoma study. This is not surprising and does not detract from the similarity in the slope. The colonies are maintained under different conditions. In addition, the Oklahoma study was conducted with both males and females, while the SNPRC study was conducted among females only.
Studies on the mechanisms responsible for the life-course fall in cortisol in the baboon
One study quantified hypothalamic paraventricular nuclear (PVN) AVP, corticotropin-releasing hormone, steroid receptors, and pituitary proopiomelanocortin immunohistochemically in 14 of the females from Figure 10 at 6–13 years of age7 and identified a significant age-related linear fall in PVN AVP immunoreactivity concurrent with the fall in circulating cortisol from as early as 6 years of age (human equivalent ∼24 years). Since AVP is a major stimulator of pituitary proopiomelanocortin, this fall in AVP would decrease the drive from the PVN to the pituitary (Figure 11). There was also a rise in PVN glucocorticoid receptor immunoreactivity that would increase glucocorticoid negative feedback, dampening the PVN drive (Figure 11). In addition, immunoreactivity of PVN 11β-HSD1 and -2, regulators of local cortisol production, was increased (data not shown). Depending on the balance of the change in the 2 enzymes (Figure 4), these increases would result in greater local PVN cortisol production that would further increase age-related negative feedback and act as a potential mechanism for decreasing PVN drive to HPA axis activity, with a resultant fall in age-related circulating cortisol. Finally, pituitary proopiomelanocortin immunoreactivity decreased with age, which would also reduce the drive to ACTH secretion.
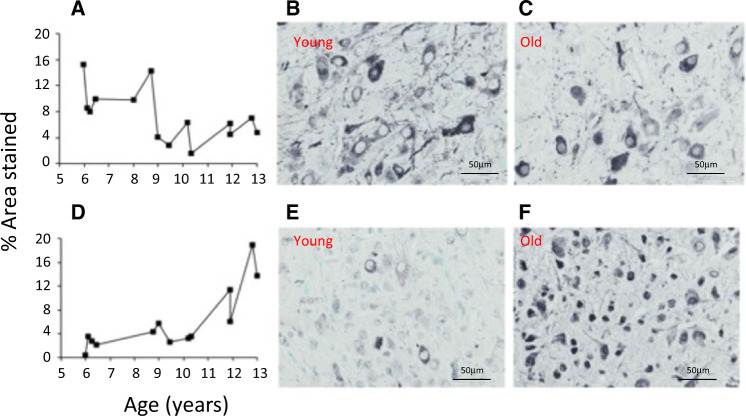
Figure 11.
Immunohistochemical staining for baboon paraventricular nucleus. A) Arginine vasopressin in female baboons from 6-13 years; B) AVP in a female baboon at 6 years; and C) AVP in a female baboon at 23 years. D) Glucocorticoid receptor in female baboons from 6-13 years; E) Glucocorticoid receptor in a female baboon at 6 years; and F) Glucocorticoid receptor in a female baboon at 23 years.7 Magnification 40x. Reproduced with permission from Yang et al.7
Weaning increases circulating cortisol concentrations at a critical stage in early-life development
Figure 12A shows that cortisol increases significantly with weaning. The metabolic changes responsible for this major increase merit investigation. Weaning is known to be a stressful process in nonhuman primates,56 and is therefore important to consider since it occurs at a critical stage of early-life development and plays a role in setting the normative profile (Principle I). This finding shows the importance of obtaining data from an early stage of life.
Figure 12.
(A) Average postnatal cortisol before (1–9 months) and after (12–36 months) weaning. No differences were found between males and females, so data were pooled. Data are mean + SEM; Controls (filled, n = 5–12) and IUGR (open, n = 3–8). **P < 0.01 compared to 1–9 month period. Reproduced from Li et al (2017)26 with permission. (B) Increased morning fasting plasma cortisol in female IUGR baboons. Closed histogram represents cortisol in 10 female controls; open histogram represents cortisol in 8 IUGR females. Controls (7.7 ± 0.44 years; mean ± SEM) and IUGR baboons (6.9 ± 0.48 y; n.s.) were similar in age. *P < 0.05 control vs IUGR. (Unpublished observations Huber H, Li C, Nathanielsz P) Abbreviations: IUGR, intrauterine growth restricted; SEM, standard error of the mean.
Nutritional programming-aging interactions in the hypothalamo-pituitary-adrenal system
Figure 12B shows that at ∼7.3 years of life, cortisol is elevated by 48.5% in the IUGR female offspring of nutrient-reduced mothers, compared to controls. At the same age as they show increased cortisol, the IUGR baboons also show accelerated aging of the brain57 and heart.17 These findings of higher cortisol and accelerated aging in the same animals in 2 organ systems, although only associations, support the hypothesis that developmental programming by elevated cortisol across significant periods of the life course accelerates aging.
SIGNIFICANCE OF THE NORMATIVE LIFE-COURSE FALL IN CORTISOL ON THE AGING TRAJECTORY
There are 4 functional hypotheses to explain the causes and consequences of the role of cortisol in aging. The fall in cortisol across the life course may (1) protect against aging, (2) accelerate aging, (3) be a response to aging, or (4) be an aging-related epiphenomenon. There is a need for an intervention study to determine whether raising life-course cortisol concentration shortens life. If increasing cortisol decreases life span, that would be compatible with our first hypothesis – namely, that the normal fall in cortisol across the life course is beneficial to longevity. It is also necessary to determine whether this conclusion applies to aging of function in all tissues, as differences between tissues are likely in the start and rate of aging. There is much support for the view that cortisol accelerates aging processes,53 as well as for the view that both cortisol resistance and cortisol-enhanced aging develop in different metabolic pathways.53 An added complication is that cortisol may have concentration-dependent effects; both low and high levels have been connected with age-related pathology. Both excess activation of the HPA axis (Cushing’s disease) and impaired activation of the HPA axis (Addison’s disease) produce age-related pathology,58 indicating a bimodal dose-response relationship.59 A metanalysis of 28 articles on the relationship of HPA axis function to body mass index concluded, “peripheral cortisol levels decline with aging. However, since increased peripheral cortisol output aggravates consequences of both obesity and aging, investigation of other determining factors of hypothalamo-pituitary-adrenal axis activity is of outstanding importance.”60
Although the role of glucocorticoids in our 10 principles of programming and aging interactions has been considered, Principles V and X have not yet been considered. Principle V states that “while the brain is relatively protected from major adverse, negative aspects of the compensatory responses to developmental programming challenges, it is not completely protected.” Thus, programming outcomes may lead to acceleration of cognitive loss. An ultrasound study on IUGR baboon offspring and their age-matched controls at ∼9 years of age demonstrated that the femoral and external iliac, but not the brachial or common carotid, arteries were smaller in IUGR offspring.61 In addition, distensibility was decreased in the iliac but not the carotid arteries in IUGR offspring. Moreover, in IUGR offspring there was increased carotid arterial blood flow velocity during late systole and diastole, with a trend toward increased overall blood flow. While further experiments are required to determine the extent to which the elevated fetal cortisol in IUGR fetuses may have contributed to these vascular changes, these observations do indicate attempts to develop structural changes that improve cerebral perfusion at the expense of other vascular beds.61
Principle X states that aging can be accelerated by a second challenge (a second hit) during life to a physiological system that has already been programmed to function suboptimally. During an intravenous glucose tolerance test at 3.5 years of age, IUGR baboon offspring showed increased fasting glucose, fasting insulin, and insulin area under the curve compared with offspring of control well-fed mothers.19 Insulin area under the curve also increased following an arginine challenge. Baseline homeostatic model assessment showed insulin β-cell sensitivity was greater in 3.5-year-old IUGR offspring than in controls. This increased pancreatic sensitivity in early life has been shown to be a marker of later-life decreased β-cell function in the rat.62,63 In a hyperinsulinemic, euglycemic clamp, IUGR offspring showed a 26% decreased glucose disposal rate, indicating that a degree of insulin resistance was already present by 3.5 years of age. When exposed to a second hit of later-life dietary challenges such as a westernized diet, these IUGR offspring that had been exposed to high levels of glucocorticoids at critical periods of development are likely predisposed to later-life type 2 diabetes. Clearly, other factors that play a role in IUGR may also predispose to diabetes. However, rat studies have shown that the direct administration of glucocorticoids in pregnancy impairs postnatal pancreatic function and peripheral insulin sensitivity.63 In addition, we have demonstrated increased maternal corticosterone in 19-day gestation fetuses and in 2-day-old neonates of undernourished rat mothers.64
SUMMARY AND FUTURE APPROACHES
Data presented in this review strongly support the need for studies on aging to obtain data over an adequate period of the life course for the purpose of undertaking regression analysis, beginning as early in life as possible. This approach is preferred to categorical analysis of a few isolated time points across the life course. Categorical analysis can miss important watersheds in the physiology of life-course aging such as puberty or menopause. As shown above, using the regression analysis strategy, we and the group from the Oklahoma Primate Center have shown that baboon circulating cortisol concentrations fall from as early as 26% of the life course with very similar slopes. The age-related factors determining changes in this normative decreasing trajectory of circulating cortisol are likely to be multiple and complex. In addition to genetic predisposition, outcomes in response to developmental programming challenges such as poor maternal nutrition or stress must be evaluated. We have presented data showing that as a result of an extensively studied developmental programming challenge, moderate global maternal undernutrition, activity of the female baboon postnatal HPA axis is increased by nearly 50% as early as ∼7.3 years of life – equivalent to ∼30 years of human life. Importantly, in the same baboons, brain and cardiac aging are accelerated. Given the importance of cortisol in early development, glucocorticoids have been called the “gatekeepers”65 or “orchestrators”66 of developmental programming. The data presented here lead us to propose the HPA axis performs a similar role as an orchestrator of interactions of programming-aging mechanisms and studies are needed to determine the role of this important neuroendocrine axis in aging.
CONCLUSIONS
Developmental programming interacts with the aging process. Thus, an individual’s aging trajectory will be influenced by nutritional and other challenges in early development. There is now compelling evidence from multiple laboratories in a variety of species, including nonhuman primates, that the HPA axis is an orchestrator of interactions of programming-aging mechanisms.
Future studies should address the effects of both adrenal and peripheral cortisol production on the sex-specific rates of aging in different organs.
Acknowledgments
Author contributions. P.W.N., H.F.H., C.L., G.D.C., A.K, and E.Z. participated in the study design and conduct of the studies and analysis of the data. All authors also participated in the writing of the manuscript.
Funding. Our own work presented in this review was supported by the National Institutes of Health NICHD P01 HD21350 and NIA U19 AG057758-01A1.
Declaration of interest. The authors have no interests to declare.
References
- 1. Barker DJ. Childhood causes of adult diseases. Arch Dis Child. 1988;63:867–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Nathanielsz PW. Life in the Womb: the Origin of Health and Disease. Ithaca, NY: Promethean Press; 1999. [Google Scholar]
- 3. Barraclough CA. Production of anovulatory, sterile rats by single injections of testosterone propionate. Endocrinology. 1961;68:62–67. [DOI] [PubMed] [Google Scholar]
- 4. Widdowson EM, McCance RA. The effect of finite periods of undernutrition at different ages on the composition and subsequent development of the rat. Proc R Soc Lond B Biol Sci. 1963;158:329–342. [DOI] [PubMed] [Google Scholar]
- 5. Barker DJP, Eriksson JG, Forsen T, et al. Fetal origins of adult disease: strength of effects and biological basis. Int J Epidemiol. 2002;31:1235–1239. [DOI] [PubMed] [Google Scholar]
- 6. Barker D. Mothers, Babies and Disease in Later Life. 2nd ed. London, England: Churchill Livingstone; 1998. [Google Scholar]
- 7. Yang S, Gerow KG, Huber HF, et al. A decline in female baboon hypothalamo-pituitary-adrenal axis activity anticipates aging. Aging. 2017;9:1375–1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. López-Otín C, Blasco MA, Partridge L, et al. The hallmarks of aging. Cell. 2013;153:1194–1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zambrano E, Nathanielsz PW. Mechanisms by which maternal obesity programs offspring for obesity: evidence from animal studies. Nutr Rev. 2013;71(suppl 1):S42–S54. [DOI] [PubMed] [Google Scholar]
- 10. Pruchno R, Carr D. Successful aging 2.0: resilience and beyond. J Gerontol B Psychol Sci Soc Sci. 2017;72:201–203. [DOI] [PubMed] [Google Scholar]
- 11. Cheng CJ, Gelfond JAL, Strong R, et al. Genetically heterogeneous mice exhibit a female survival advantage that is age- and site-specific: results from a large multi-site study. Aging Cell. 2019;18:e12905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dodds RM, Syddall HE, Cooper R, et al. Grip strength across the life course: normative data from twelve British studies. PLoS One. 2014;9:e113637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Reynolds RM, Allan KM, Raja EA, et al. Maternal obesity during pregnancy and premature mortality from cardiovascular event in adult offspring: follow-up of 1 323 275 person years. BMJ. 2013;347:f4539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fowden AL, Li J, Forhead AJ. Glucocorticoids and the preparation for life after birth: are there long-term consequences of the life insurance? Proc Nutr Soc. 1998;57:113–122. [DOI] [PubMed] [Google Scholar]
- 15. Li C, Ramahi E, Nijland MJ, et al. Up-regulation of the fetal baboon hypothalamo-pituitary-adrenal axis in intrauterine growth restriction: coincidence with hypothalamic glucocorticoid receptor insensitivity and leptin receptor down-regulation. Endocrinology. 2013;154:2365–2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Li C, McDonald TJ, Wu G, et al. Intrauterine growth restriction alters term fetal baboon hypothalamic appetitive peptide balance. J Endocrinol. 2013;217:275–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kuo AH, Li C, Li J, et al. Cardiac remodelling in a baboon model of intrauterine growth restriction mimics accelerated ageing. J Physiol. 2017;595:1093–1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kuo AH, Li C, Huber HF, et al. Ageing changes in biventricular cardiac function in male and female baboons (Papio spp.). J Physiol. 2018;596:5083–5098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Choi J, Li C, McDonald TJ, et al. Emergence of insulin resistance in juvenile baboon offspring of mothers exposed to moderate maternal nutrient reduction. Am J Physiol Regul Integr Comp Physiol. 2011;301:R757–R762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Antonow-Schlorke I, Schwab M, Cox LA, et al. Vulnerability of the fetal primate brain to moderate reduction in maternal global nutrient availability. Proc Natl Acad Sci USA. 2011;108:3011–3016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Magyar DM, Fridshal D, Elsner CW, et al. Time-trend analysis of plasma cortisol concentrations in the fetal sheep in relation to parturition. Endocrinology. 1980;107:155–159. [DOI] [PubMed] [Google Scholar]
- 22. Liggins GC, Fairclough RJ, Grieves SA, et al. The mechanism of initiation of parturition in the ewe. Recent Prog Horm Res. 1973;29:111–159. [DOI] [PubMed] [Google Scholar]
- 23. McDonald TJ, Nathanielsz PW. Bilateral destruction of the fetal paraventricular nuclei prolongs gestation in sheep. Am J Obs Gynecol. 1991;165:764–770. [DOI] [PubMed] [Google Scholar]
- 24. Liggins GC, Howie RN. A controlled trial of antepartum glucocorticoid treatment for prevention of the respiratory distress syndrome in premature infants. Pediatrics. 1972;50:515–525. [PubMed] [Google Scholar]
- 25. Thomas AL, Krane EJ, Nathanielsz PW. Changes in the fetal thyroid axis after induction of premature parturition by low dose continuous intravascular cortisol infusion to the fetal sheep at 130 days of gestation. Endocrinology. 1978;103:17–23. [DOI] [PubMed] [Google Scholar]
- 26. Li C, Jenkins S, Mattern V, et al. Effect of moderate, 30 percent global maternal nutrient reduction on fetal and postnatal baboon phenotype. J Med Primatol. 2017;46:293–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Fowden AL, Forhead AJ. Glucocorticoids as regulatory signals during intrauterine development. Exp Physiol. 2015;100:1477–1487. [DOI] [PubMed] [Google Scholar]
- 28. Pereira SP, Oliveira PJ, Tavares LC, et al. Effects of moderate global maternal nutrient reduction on fetal baboon renal mitochondrial gene expression at 0.9 gestation. Am J Physiol Renal Physiol. 2015;308:F1217–F1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Luyckx VA, Brenner BM. The clinical importance of nephron mass. J Am Soc Néphron 2010;21:898–910. [DOI] [PubMed] [Google Scholar]
- 30. Crudo A, Suderman M, Moisiadis VG, et al. Glucocorticoid programming of the fetal male hippocampal epigenome. Endocrinology. 2013;154:1168–1180. [DOI] [PubMed] [Google Scholar]
- 31. Matthews SG. Antenatal glucocorticoids and programming of the developing CNS. Pediatr Res. 2000;47:291–300. [DOI] [PubMed] [Google Scholar]
- 32. Long NM, Ford SP, Nathanielsz PW. Multigenerational effects of fetal dexamethasone exposure on the hypothalamic-pituitary-adrenal axis of first- and second-generation female offspring. Am J Obstet Gynecol. 2013;208:217.e1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Long NM, Smith DT, Ford SP, et al. Elevated glucocorticoids during ovine pregnancy increase appetite and produce glucose dysregulation and adiposity in their granddaughters in response to ad libitum feeding at 1 year of age. Am J Obstet Gynecol. 2013;209:353.e1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lee J-H, Zhang J, Massmann GA, et al. Antenatal betamethasone increases vascular reactivity to endothelin-1 by upregulation of CD38/cADPR signaling. J Dev Orig Health Dis. 2014;5:56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Moss TJ, Sloboda DM, Gurrin LC, et al. Programming effects in sheep of prenatal growth restriction and glucocorticoid exposure. Am J Physiol Regul Integr Comp Physiol. 2001;281:R960–R970. [DOI] [PubMed] [Google Scholar]
- 36. Guo C, Li C, Myatt L, et al. Sexually dimorphic effects of maternal nutrient reduction on expression of genes regulating cortisol metabolism in fetal baboon adipose and liver tissues. Diabetes. 2013;62:1175–1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Nijland MJ, Mitsuya K, Li C, et al. Epigenetic modification of fetal baboon hepatic phosphoenolpyruvate carboxykinase following exposure to moderately reduced nutrient availability. J Physiol. 2010;588:1349–1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hales CN, Barker DJ. Type 2 (non-insulin-dependent) diabetes mellitus: the thrifty phenotype hypothesis. Diabetologia. 1992;35:595–601. [DOI] [PubMed] [Google Scholar]
- 39. Hales CN, Ozanne SE. The dangerous road of catch-up growth. J Physiol. 2003;547:5–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Li C, Shu Z-J, Lee S, et al. Effects of maternal nutrient restriction, intrauterine growth restriction, and glucocorticoid exposure on phosphoenolpyruvate carboxykinase-1 expression in fetal baboon hepatocytes in vitro. J Med Primatol. 2013;42:211–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kuo AH, Li C, Mattern V, et al. Sex-dimorphic acceleration of pericardial, subcutaneous, and plasma lipid increase in offspring of poorly nourished baboons. Int J Obes. 2018;42:1092–1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Koenen SV, Mecenas CA, Smith GS, et al. Effects of maternal betamethasone administration on fetal and maternal blood pressure and heart rate in the baboon at 0.7 of gestation. Am J Obs Gynecol. 2002;186:812–817. [DOI] [PubMed] [Google Scholar]
- 43. Antonow-Schlorke I, Schwab M, Li C, et al. Glucocorticoid exposure at the dose used clinically alters cytoskeletal proteins and presynaptic terminals in the fetal baboon brain. J Physiol. 2003;547:117–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Blanco CL, Moreira AG, McGill-Vargas LL, et al. Antenatal corticosteroids alter insulin signaling pathways in fetal baboon skeletal muscle. J Endocrinol. 2014;221:253–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kuo AH, Li J, Li C, et al. Prenatal steroid administration leads to adult pericardial and hepatic steatosis in male baboons. Int J Obes. 2017;41:1299–1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Huber HF, Kuo AH, Li C, et al. Antenatal synthetic glucocorticoid exposure at human therapeutic equivalent doses predisposes middle-age male offspring baboons to an obese phenotype that emerges with aging. Reprod Sci. 2019;26:591–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Bowman RE, Maclusky NJ, Diaz SE, et al. Aged rats: sex differences and responses to chronic stress. Brain Res. 2006;1126:156–166. [DOI] [PubMed] [Google Scholar]
- 48. Ferrari E, Cravello L, Muzzoni B, et al. Age-related changes of the hypothalamic-pituitary-adrenal axis: pathophysiological correlates. Eur J Endocrinol. 2001;144:319–329. [DOI] [PubMed] [Google Scholar]
- 49. Peeters G, van Schoor NM, Visser M, et al. Relationship between cortisol and physical performance in older persons. Clin Endocrinol. 2007;67:398–406. [DOI] [PubMed] [Google Scholar]
- 50. Zambrano E, Reyes-Castro LA, Nathanielsz PW. Aging, glucocorticoids and developmental programming. Age (Dordr). 2015;37:9774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Maestripieri D, Hoffman CL, Anderson GM, et al. Mother-infant interactions in free-ranging rhesus macaques: relationships between physiological and behavioral variables. Physiol Behav. 2009;96:613–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Huang C-W, Lui C-C, Chang W-N, et al. Elevated basal cortisol level predicts lower hippocampal volume and cognitive decline in Alzheimer’s disease. J Clin Neurosci. 2009;16:1283–1286. [DOI] [PubMed] [Google Scholar]
- 53. Zhao Z-Y, Lu F-H, Xie Y, et al. Cortisol secretion in the elderly: influence of age, sex and cardiovascular disease in a Chinese population. Steroids. 2003;68:551–555. [DOI] [PubMed] [Google Scholar]
- 54. Bronikowski AM, Alberts SC, Altmann J, et al. The aging baboon: comparative demography in a non-human primate. Proc Natl Acad Sci USA. 2002;99:959–955. www.pnas.orgcgidoi10.1073pnas.142675599. Accessed June 17, 2019. [DOI] [PMC free article] [PubMed]
- 55. Willis EL, Eberle R, Wolf RF, et al. The effects of age and cytomegalovirus on markers of inflammation and lymphocyte populations in captive baboons. PLoS One. 2014;9:e107167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Mandalaywala TM, Higham JP, Heistermann M, et al. Physiological and behavioural responses to weaning conflict in free-ranging primate infants. Anim Behav. 2014;97:241–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Franke K, Clarke GD, Dahnke R, et al. Premature brain aging in baboons resulting from moderate fetal undernutrition. Front Aging Neurosci. 2017;9:92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. McEwen BS. Protective and damaging effects of stress mediators. N Engl J Med. 1998;338:171–179. [DOI] [PubMed] [Google Scholar]
- 59. Yeager MP, Pioli PA, Guyre PM. Cortisol exerts bi-phasic regulation of inflammation in humans. Dose Response. 2011;9:332–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Tenk J, Mátrai P, Hegyi P, et al. In obesity, HPA axis activity does not increase with BMI, but declines with aging: a meta-analysis of clinical studies. PLoS One. 2016;11:e0166842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Kuo AH, Li C, Huber HF, et al. Intrauterine growth restriction results in persistent vascular mismatch in adulthood. J Physiol. 2018;596:5777–5790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Morimoto S, Calzada L, Sosa TC, et al. Emergence of ageing-related changes in insulin secretion by pancreatic islets of male rat offspring of mothers fed a low-protein diet. Br J Nutr. 2012;107:1562–1565. [DOI] [PubMed] [Google Scholar]
- 63. Morimoto S, Sosa TC, Calzada L, et al. Developmental programming of aging of isolated pancreatic islet glucose-stimulated insulin secretion in female offspring of mothers fed low-protein diets in pregnancy and/or lactation. J Dev Orig Health Dis. 2012;3:483–488. [DOI] [PubMed] [Google Scholar]
- 64. Zambrano E, Rodríguez-González GL, Guzmán C, et al. A maternal low protein diet during pregnancy and lactation in the rat impairs male reproductive development. J Physiol. 2005;563:275–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. McMullen S, Langley-Evans SC, Gambling L, et al. A common cause for a common phenotype: the gatekeeper hypothesis in fetal programming. Med Hypotheses. 2012;78:88–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Zambrano E, Tuersunjiang N, Long N, et al. Increased central and peripheral glucocorticoid synthesis act as an orchestrator of developmental programming In: Zhang L, Longo LD, eds. Stress and Developmental Programming of Health and Disease: Beyond Phenomenology.United Kingdom: Nova Science Publishers, Inc; 2014:463–485. [Google Scholar]