Abstract
Prostate cancer is the most common solid-organ malignancy among American men. It is currently most commonly diagnosed on random systematic biopsies prompted by elevated serum PSA levels. Multi-parametric MRI (MP-MRI) of the prostate has emerged as an anatomic and functional imaging modality, which offers accurate detection, localization and staging of prostate cancer. Recently, MP-MRI has gained an increasing role in guiding biopsies to sites of abnormality and in monitoring patients on active surveillance. Here, we discuss the historical development, current role, and potential future directions of MP-MRI in the diagnosis of prostate cancer.
Keywords: Cancer Detection, Magnetic Resonance Imaging, Prostate Biopsy, Cancer Imaging
Introduction
Prostate cancer (PCa) is the most common solid-organ malignancy in American men and is most commonly diagnosed on random systematic biopsies prompted by elevated serum PSA levels [1, 2]. Recently, multiparametric-MRI (MP-MRI) of the prostate has emerged as an anatomic and functional imaging modality, which offers accurate detection, localization, and staging of PCa [3-5]. MP-MRI of the prostate typically consists of T2-weighted (T2W), diffusion weighted (DW), and dynamic contrast enhanced (DCE) MRI. At some centers, MR spectroscopy (MRS) is added to the MP-MRI protocol although this is quite costly in terms of time and expertise. Herein, we investigate the historical development, current role, and potential future directions of MP-MRI in the diagnosis of PCa.
Background and History
Prostate MRI: From Staging to Detecting Prostate Cancer
MRI was initially introduced in the late 1980s and early 1990s as a method to locally stage prostate cancer by identifying seminal vesicle invasion and extracapsular extension [6]. Initial MRI studies had relatively low spatial resolution based upon imaging acquisition on medium-field strength (e.g., early 1.5 T) magnets and body coil technologies. The addition of the endorectal coil greatly improved the signal to noise ratio of prostate MRI, allowing higher resolution T2W imaging with better delineation of the prostatic capsule. However, the emphasis remained on staging and not diagnosis. Despite developments in MRI with T2W imaging fast-spin echo (FSE) techniques, phased array surface coils and contrast enhancement, early multicenter trials yielded disappointing results in the assessment of extracapsular extension and seminal vesicle invasion [7, 8]. The detection of primary PCa within the gland, without extension beyond the capsule, was considered limited and unreliable. Indeed, many of the cancers that were “staged” were never actually identified as such. At the time, very little information was available about the location of the cancer as the biopsy cores from systematic samples were usually mixed together without labeling.
By the early 2000s, with continually improving imaging technology, MRI with an endorectal coil was found to be increasingly useful in identifying and characterizing lesions within the prostate as well as detecting local disease recurrence following primary definitive treatment [9, 10]. It was recognized that many Gleason 6 cancers were not visible on MRI and MRI tended to detect larger, more aggressive tumors. Intraprostatic hypointense lesions on T2W MRI scans seemed particularly useful in detecting cancerous foci as these classically correlated to areas of cancer as outlined on corresponding radical prostatectomy specimens. Also, DCE MRI was considered diagnostic in confirming tumors, as tumors tended to enhance preferentially compared with normal prostate tissue.
Development of a Multiparametric Approach to Prostate MRI
In the last decade, there has been a shift of interest in the use of MRI from local staging to the detection and characterization of primary foci of PCa within the prostate gland. This has arisen from increasing frustration with random prostate biopsies that often detect inconsequential posterior tumors while missing significant anterior PCa. Up to this point, intraprostatic lesion characterization was achieved solely with ultrasonography, although results were disappointing. Technological MR improvements employing higher field strength magnets (e.g., 3 T), coupled with multichannel phased-array surface and endorectal coils to augment signal to noise ratio, the development of DWI, faster DCE, and more reliable MR spectroscopic sequences have improved assessment of the prostate [11•, 12, 13]. The rationale for the use of a multiparametric approach has been that any one sequence by itself has considerable overlap between benign and malignant tissue; however, the combination of sequences proves to have more predictive power for cancer. The more parameters used, the higher the accuracy for detecting PCa since each individual MR technique combines the benefits of each and complements the shortcomings of the others [14•, 15, 16]. For example, while T2W imaging offers high sensitivity, the addition of MRS, a functional method that detects relative levels of choline and citrate within the prostate, adds specificity [17]. Also, quantitative evaluation of DWI with calculated apparent diffusion coefficients (ADC) values correlates with Gleason grade as found on tissue histology, allowing for more confident risk stratification of patients [18]. Well-established characteristics for each MRI parameter have been described and allow for identification of suspicious lesions in a standardized manner. A recent development in standardized reporting called “PIRADS” seeks to improve the reproducibility of MP-MRI among different centers [19].
Use of MRI to Direct Prostate Biopsies
Recognizing the capability of MP-MRI to detect PCa foci, several centers have investigated the potential role of MRI to guide prostate biopsies [20, 21, 22•, 23, 24, 25•]. Three approaches have emerged to target prostate biopsies and these include: (1) direct “in-bore” MR-guided biopsies, (2) “cognitive fusion” whereby MR findings are used alongside transrectal ultrasound (TRUS) biopsy, and (3) MRI-TRUS fusion which electronically integrates MP-MRI to real-time TRUS imaging via a software-based image co-registration to allow targeting of MRI identified lesions.
Current State of mp-mri in Prostate Cancer Detection
Use of MP-MRI to Identify Suspicious Lesions and Guide Targeted Biopsies
Direct “in-Bore” MRI Guidance
The earliest method to target biopsies based on MRI findings was to perform the biopsy inside the bore of the magnet. For this approach, the patient typically undergoes a diagnostic MP-MRI prior to the biopsy. Once it is ascertained that a biopsy is indicated, the patient returns on another day and is typically placed prone in the MRI scanner. A limited diagnostic MRI is first obtained to co-localize lesions found previously. Using either a transrectal or transperineal approach, core biopsy needles are introduced into the visible lesions using a rapid MR technique such as MR fluoroscopy and samples are obtained while serial MR images are acquired to confirm biopsy needle placement. Typically, only lesions visible on MR are targeted reducing time in the bore of the scanner but also reducing the number of biopsy cores obtained, whilst allowing for precise documentation of biopsy locations [20, 26]. Disadvantages to this approach include the cumbersome nature of the intervention given the limited space inside an MRI scanner, specialized MR compatible equipment required, and associated costs and availability of the scanner and skilled MRI personnel. These challenges have limited the use of this approach from becoming generally practiced.
Cognitive Fusion Guidance
The cognitive fusion method is conceptually the simplest of the MRI-guided biopsy techniques. This system uses diagnostic MP-MRI to identify intraprostatic lesions suspicious for PCa and these are targeted under TRUS by estimating the location of the MRI defined lesion on ultrasound. While this method does not require additional equipment, it relies on the spatial abilities of the operator to mentally co-register the MRI and TRUS and is, thus, subject to inter-operator variability. Either the transrectal or transperineal approach can be used although the former is more common. Because this operator-dependent technique requires a “mental map” of suspicious lesions, it can be inaccurate. Hence, the primary disadvantage of this technique is that it places an inordinate burden on the practitioner to translate the MRI findings onto the real-time ultrasound. However, in the absence of resources to perform MR guided or fusion-guided biopsies, this is a viable alternative.
MRI-TRUS Fusion Guidance
The next step in the evolution of MRI guided prostate biopsies is the MR-TRUS fusion biopsy system. There are three important components to this technology: a high quality diagnostic MRI, a robust software fusion algorithm that allows the MRI to be fused to the TRUS image, and a method of tracking the ultrasound probe in three-dimensional space.
After the patient undergoes an MP-MRI, lesions suspicious for PCa are identified and a contour of the prostate gland is drawn. Increasingly, this can be done automatically with software. This “segmented” prostate image, along with the coordinates of the lesions within the prostate, is sent to the fusion software platform. This device need not be in the same location as the MRI; indeed, it might not even be in the same city. In the ultrasound suite, a 3D TRUS is obtained through the prostate and the prostate gland is similarly segmented. The MR and TRUS segmented contours are then electronically “fused” using either a rigid semi-rigid or deformable model. Since the gland may be deformed during the MRI (due to the presence or absence of an endorectal coil), it may not readily “fuse” to a TRUS in which a probe is placed in the rectum and variable pressure is applied. Thus, deformable models are preferred. The final component of this method is a tracking method that fixes the prostate in a 3D coordinate system, so that motion within that system can be accurately detected and, therefore, reflected on the MRI. This allows the operator to move the TRUS probe in any direction while simultaneously displaying the comparable fused MR image. Two approaches to probe tracking have been championed: an articulated arm attached to the TRUS probe that mechanically records the location and a radiofrequency or optical “global positioning device”, (GPS) that attaches to the probe and permits the probe to be detected. Multiple MRI-TRUS fusion biopsy systems have been developed which employ different fusion software methods and varying mechanisms of tracking the TRUS probe. Although it would appear to be less operator dependent, there is still a human element involved as the MRI-TRUS registration needs to be assessed and manually adjusted, thus, compensating for altered gland contours or misregistration artifacts. During the fusion biopsy, approaches differ with regard to what the operator sees; some units present side-by-side displays of the MRI and TRUS while others present a single fused image. An MR-TRUS fusion guided biopsy system does require the purchase of an additional device with tracking capabilities, but it can be performed in an outpatient setting with local anesthesia, not significantly altering the workflow compared to the standard-of-care, systematic ultrasound-guided biopsies. Thus, as the technology has become more widely available, this route is gaining popularity.
Diagnostic Yield of Image Guided Biopsies Compared to Systematic Biopsy Techniques
The diagnostic yield of PCa on MRI-guided prostate biopsies has been compared to that of systematic biopsy for each of the various systems of biopsy guidance: in-bore, cognitive fusion, and MRI/US fusion. Hambrock and colleagues reported that in-bore biopsies targeting lesions identified on MP-MRI were significantly more efficient in detecting PCa than a systematic 10-core TRUS-guided biopsy (88 % versus 55 %, p=0.001) for PCa detection when compared to the final post prostatectomy specimens[22•]. Results of cognitive fusion guidance compared to systematic 10-core to 12-core TRUS-guided biopsy demonstrate that this form of biopsy targeting also increases PCa detection and more accurately depicts overall disease burden in higher-grade disease [27, 28]. Specifically, one study by Puech and colleagues, evaluating the efficacy of cognitive biopsy targeting of lesions, demonstrated PCa detection rates up to 10 % higher (15 % for high-grade disease) compared with systematic biopsies in similar populations of patients[29]. MRI/US fusion biopsies were equal to or superior to the detection of PCa with systematic biopsies, particularly for higher-grade disease [30, 31]. A study by Siddiqui and colleagues demonstrated that MRI-TRUS fusion guided targeted biopsies often upgraded the amount of Gleason 4 pattern detected at biopsy compared to standard-of-care systematic 12-core biopsies inpatients who underwent both biopsy techniques during the same biopsy session [32].
The utility of MRI-guided biopsies has been best appreciated in patients with previously negative systematic biopsies whose PSA continues to rise [33-36]. This patient population represents a group for which there is a high clinical concern for cancer and they often undergo repeated biopsies despite prior negative biopsy findings. Employing MP-MRI of the prostate and targeted biopsy of suspicious lesions in such patients leads to high positive diagnostic rates of PCa in the range of 34 % to 52 % of cases [34, 35]. Interestingly, the number of prior negative biopsy sessions was not predictive of cancer detection on followup MRI-guided biopsy, suggesting that imaging can be used early in the course of PSA monitoring without diminished sensitivity.
For patients with no prior biopsies, MP-MRI followed by image-guided biopsy can be useful. Park et al., showed that men undergoing MRI prior to initial biopsy had a significantly higher cancer detection rate (29.5 % versus 9.8 %, respectively) and higher positive core rate (9.9 % versus 2.5 %, respectively) than those undergoing systematic biopsy.
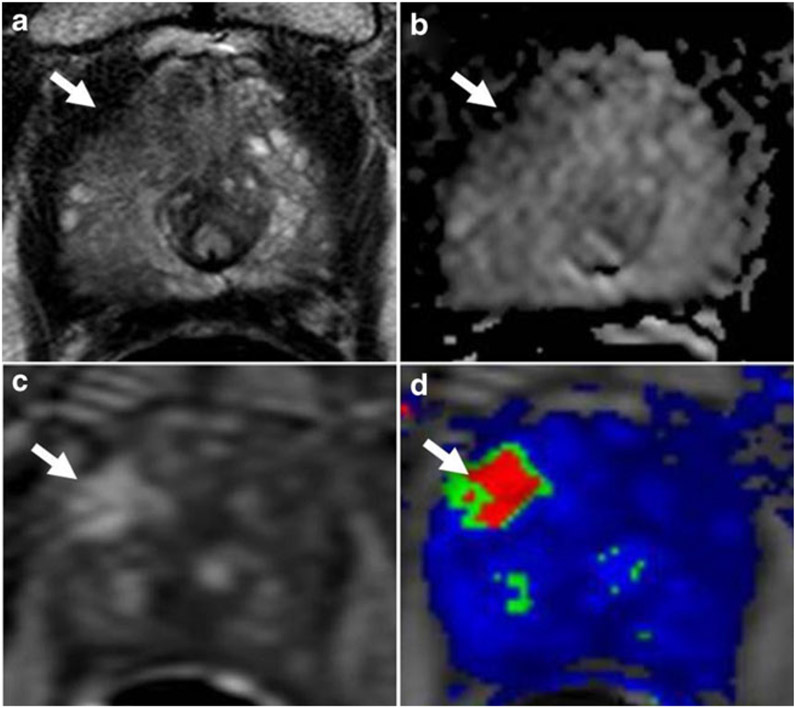
MR guided biopsies are particularly important in detecting central and anterior PCa which are often large by the time they are detected. These tumors cannot be detected by digital rectal examination and are often missed or undersampled by template biopsies of the prostate [37]. Anteriorly located PCa lesions were reportedly missed in up to 46 % of 12-core systematic biopsies [38]. Furthermore, among those detected on systematic biopsy, MRI guided biopsies often resulted in an increase in the Gleason score; Gleason score upgrading was seen in 44 % of those undergoing targeted compared with systematic biopsies (Fig. 1).
Fig. 1.
A 70 year old man with serum PSA of 51 ng/mL and four prior negative biopsies. (a) Axial T2W MRI, (b) ADC map from DW MRI, (c) raw DCE MRI , and (d) Ktrans map of DCE MRI depict a right apical-mid anterior transitional zone lesion (arrow). TRUS/MRI fusion-guided biopsy of the lesion revealed Gleason 4+ 4 tumor (100 % core involvement)
In addition to missing tumors in the anterior gland, standard-of-care systematic biopsies may also undersample the prostatic apex. Nix et al., reported the use of MP-MRI in detecting and guiding biopsies to very distal apical lesions demonstrating that targeted biopsies detected more cancers in this area than systematic biopsy cores and tended to diagnose higher grade disease [39]. In enlarged prostates, the standard 12-core biopsy also tends to undersample the gland. In this setting, MR guidance has also proven useful in increasing the yield of biopsy compared to standard-of-care biopsies [40].
Future Directions
Dissemination of MP-MRI for Prostate Cancer Biopsy
In light of the recent recommendations by the US Preventive Services Task Force against widespread PSA screening, an imaging modality with the potential to detect PCa with higher specificity coupled with a more precise biopsy method could be the key in identifying men with curable PCa [41]. Although MP-MRI has been suggested as a possible substitute for PSA screening, this is probably years away. MP-MRI provides an anatomic and functional evaluation of the prostate gland resulting in an increased detection rate of significant tumors compared with standard-of-care biopsy, thus reducing over-treatment. This could address some of the concerns related to screening with PSA, which results in blind systematic biopsies and the detection of numerous inconsequential tumors that, nonetheless, require invasive and expensive medical treatment [42-44].
Recent work by Villers and Puech stated that “with time and education, an increase in the use of MP-MRI in the diagnostic pathway of prostate cancer, such as before the first biopsy, would result in the enhanced detection of clinically significant disease, fewer men diagnosed with clinically insignificant disease, fewer men biopsied overall and fewer needle deployments in those who do undergo biopsy,” nicely summarizing the future direction for MP-MRI in this arena [45].
Perhaps the most important effect of implementing MRI is a higher rate of clinically significant PCa detection compared with standard-of-care biopsies. Coupled with the potential to minimize the number of biopsy cores obtained and the number of repeat biopsy sessions required to render a definitive, actionable diagnosis, this added value of MRI not only has the potential to improve patient quality of life but could affect mortality outcomes for men with intermediate to high-risk disease with earlier diagnoses in a cost effective manner.
To date, both the MRI technology and the expertise to interpret prostate MRIs have not been uniformly available. Barriers to implementation of routine MP-MRI include scarcity of the resource in some communities and the cost/interest of training radiologists to interpret MP-MRI. For now, MP-MRI is confined to specialized medical facilities. However, proponents of MRI suggest that a “stripped down” study utilizing only T2W and diffusion weighted sequences without an endorectal coil could be done quickly and at low cost. Such advocates point to reductions in biopsy numbers and frequency as a balance to the increased cost of a screening MRI [46].
Often lost in the discussion of prostate cancer detection is the value of MP-MRI in staging, which was the original indication for prostate MRI. MRI is considered to be the most accurate imaging modality for the local staging of PCa [47]. Local and regional staging using MP-MRI has been reported to be effective for identifying extracapsular extension, seminal vesicle invasion (SVI), and regional lymph node involvement [48]. MP-MRI is particularly important for SVI and correlates well with final pathology results [49]. As an extension, MRI findings have been integrated into statistical nomograms for clinical prognostication along with classically used clinical parameters (age, PSA, Gleason score, etc.) to predict pathologic outcomes and disease recurrence following RP [50].
MP-MRI of the Prostate in Managing Patients on Active Surveillance
Recognizing that many cancers are currently overtreated, there has been increased use of active surveillance (AS) to manage patients with low grade PCa. MP-MRI can be used for serial followup of patients on AS, monitoring for growth of index lesions. Lesions identified on MP-MRI, but deemed to have low suspicion for harboring PCa based on imaging characteristics, have been shown to mostly represent either benign tissue or low-grade PCa when targeted biopsies were performed [51]. Also, Somford and colleagues reported that the high negative predictive value of MRI in low and intermediate risk patients is an asset in counseling patients to utilize AS to manage their PCa [52].
Suitability for AS based upon MP-MRI findings was compared to other validated risk-stratification tools including the D’Amico, Epstein, and CAPRA guidelines and was found to be superior for predicting active surveillance candidacy (defined as a dominant tumor measuring less than 0.5 mL without Gleason pattern 4 or 5 and without evidence of extracapsular or seminal vesicle invasion) in a cohort of 133 patients who underwent RP [53]. The number of lesions identified on MP-MRI, lesion density (defined as volume of lesions divided by total prostate volume), and highest MRI suspicion score were then used to formulate a nomogram to confirm AS candidacy in a cohort of 85 patients who underwent confirmatory MP-MRI and MRI-TRUS fusion guided biopsy following referral with a diagnosis of PCa, which met Epstein criteria based on a previous systematic 12-core biopsy [54].
Thus, MRI can be used to identify tumors that can be monitored during AS. It has been suggested that the high NPV of MP-MRI could potentially serve as a “digital biopsy” in cases where the lesion remained stable, hence increase,ing the interval between biopsies sessions for men on AS [55].
Conclusion
Multi-parametric MRI of the prostate is an established non-invasive technique to localize and locally stage prostate cancer. The movement to utilize MP-MRI in the management of patients known or suspected of PCa is gaining momentum worldwide both for treatment and monitoring decisions. Finally, MP-MRI can be used to guide biopsy using direct ingantry methods, “cognitive fusion” or MR-TRUS fusion. MP-MRI, along with MR guided biopsies, is predicted to assume a larger role in the future management of prostate cancer.
Acknowledgments
This research was supported by the Intramural Research Program of the National Institutes of Health, National Cancer Institute, Center for Cancer Research, and the Center for Interventional Oncology. NIH and Philips Healthcare have a cooperative research and development agreement. NIH and Philips share intellectual property in the field.
We also thank the administrative support staff of the Urologic Oncology Branch, Center for Cancer Research for assisting with the manuscript review and submission process.
Abbreviations
- bGS
Biopsy Gleason Score
- B-MRI
Biparametric MRI
- DWI
Diffusion Weighted Imaging
- DRE
Digital Rectal Examination
- MRI
Magnetic Resonance Imaging
- MR/US
Magnetic Resonance/Ultrasound
- MP-MRI
Multiparametric MRI
- NPV
Negative Predictive Value
- PPV
Positive Predictive Value
- PCa
Prostate Cancer
- PSA
Prostate Specific Antigen
- PSAD
PSA Density
- SPL
Screen Positive Lesions
- T2W
T2 Weighted
Footnotes
Conflict of Interest Dr. Soroush Rais-Bahrami, Dr. Baris Turkbey, Dr. Kinzya B. Grant, Dr. Peter A. Pinto, and Dr. Peter L. Choyke each declare no potential conflicts of interest relevant to this article.
Human and Animal Rights and Informed Consent This article does not contain any studies with human or animal subjects performed by any of the authors.
Contributor Information
Soroush Rais-Bahrami, Urologic Oncology Branch, National Cancer Institute, National Institutes of Health, 10 Center Drive, Building 10 - CRC, Bethesda, MD 20892, USA.
Baris Turkbey, Molecular Imaging Program, National Cancer Institute, National Institutes of Health, 10 Center Drive, Building 10 - CRC, Bethesda, MD 20892, USA.
Kinzya B. Grant, Molecular Imaging Program, National Cancer Institute, National Institutes of Health, 10 Center Drive, Building 10 - CRC, Bethesda, MD 20892, USA
Peter A. Pinto, Urologic Oncology Branch, National Cancer Institute, National Institutes of Health, 10 Center Drive, Building 10 - CRC, Bethesda, MD 20892, USA; Center for Interventional Oncology, National Cancer Institute, National Institutes of Health, 10 Center Drive, Building 10 - CRC, Bethesda, MD 20892, USA
Peter L. Choyke, Molecular Imaging Program, National Cancer Institute, National Institutes of Health, 10 Center Drive, Building 10 - CRC, Bethesda, MD 20892, USA
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63(1):11–30. [DOI] [PubMed] [Google Scholar]
- 2.Derweesh IH, Kupelian PA, Zippe C, Levin HS, Brainard J, Magi-Galluzzi C, et al. Continuing trends in pathological stage migration in radical prostatectomy specimens. Urol Oncol. 2004;22(4):300–6. [DOI] [PubMed] [Google Scholar]
- 3.Pinto PA, Chung PH, Rastinehad AR, Baccala AA Jr, Kruecker J, Benjamin CJ, et al. Magnetic resonance imaging/ultrasound fusion guided prostate biopsy improves cancer detection following transrectal ultrasound biopsy and correlates with multiparametric magnetic resonance imaging. J Urol. 2011;186(4):1281–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hegde JV, Mulkern RV, Panych LP, Fennessy FM, Fedorov A, Maier SE, et al. Multiparametric MRI of prostate cancer: an update on state-of-the-art techniques and their performance in detecting and localizing prostate cancer. J Magn Reson Imaging. 2013;37(5):1035–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bloch BN, Genega EM, Costa DN, Pedrosa I, Smith MP, Kressel HY, et al. Prediction of prostate cancer extracapsular extension with high spatial resolution dynamic contrast-enhanced 3-T MRI. Eur Radiol. 2012;22(10):2201–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Poon PY, McCallum RW, Henkelman MM, Bronskill MJ, Sutcliffe SB, Jewett MA, et al. Magnetic resonance imaging of the prostate. Radiology. 1985;154(1):143–9. [DOI] [PubMed] [Google Scholar]
- 7.Dahms SE, Hohenfellner M, Linn JF, Eggersmann C, Haupt G, Thüroff JW. Retrovesical mass in men: pitfalls of differential diagnosis. J Urol. 1999;161:1244–8. [DOI] [PubMed] [Google Scholar]
- 8.D’Amico AV, Schnall M, Whittington R, et al. Endorectal coil magnetic resonance imaging identifies locally advanced prostate cancer in select patients with clinically localized disease. Urology. 1998;51:449–54. [DOI] [PubMed] [Google Scholar]
- 9.Sella T, Schwartz LH, Swindle PW, et al. Suspected local recurrence after radical prostatectomy: endorectal coil MR imaging. Radiology. 2004;231:379–85. [DOI] [PubMed] [Google Scholar]
- 10.Tatli S, Mortele KJ, Breen EL, Bleday R, Silverman SG. Local staging of rectal cancer using combined pelvic phased-array and endorectal coil MRI. J Magn Reson Imaging. 2006;23:534–40. [DOI] [PubMed] [Google Scholar]
- 11.•.Hricak H, Choyke PL, Eberhardt SC, Leibel SA, Scardino PT. Imaging prostate cancer: a multidisciplinary perspective. Radiology 2007;243(1):28–53.Excellent review on multi-disciplinary imaging approach for prostate cancer.
- 12.Hambrock T, Somford DM, Huisman HJ, van Oort IM, Witjes JA. Hulsbergen-van de Kaa CA, Scheenen T, Barentsz JO. Relationship between apparent diffusion coefficients at 3.0-T MR imaging and Gleason grade in peripheral zone prostate cancer. Radiology. 2011;259(2):453–61. [DOI] [PubMed] [Google Scholar]
- 13.Hoeks CM, Barentsz JO, Hambrock T, Yakar D, Somford DM, Heijmink SW, et al. Prostate cancer: multiparametric MR imaging for detection, localization, and staging. Radiology. 2011;261(1):46–66. [DOI] [PubMed] [Google Scholar]
- 14.•.Turkbey B, Mani H, Shah V, et al. Multiparametric 3T prostate magnetic resonance imaging to detect cancer: histopathological correlation using prostatectomy specimens processed in customized magnetic resonance imaging based molds. J Urol. 2011;186:1818–24.Important correlative study for multi-parametric prostate MRI since a patient specific customized mold is used for processing radical prostatectomy specimens.
- 15.Hegde JV, Mulkern RV, Panych LP, et al. Multiparametric MRI of prostate cancer: an update on state-of-the-art techniques and their performance in detecting and localizing prostate cancer. J Magn Reson Imaging. 2013;37:1035–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Engelbrecht MR, Puech P, Colin P, Akin O, Lemaître L, Villers A. Multimodality magnetic resonance imaging of prostate cancer. J Endourol. 2010;24(5):677–84. [DOI] [PubMed] [Google Scholar]
- 17.Zakian KL, Eberhardt S, Hricak H, et al. Transition zone prostate cancer: metabolic characteristics at 1H MR spectroscopic imaging–initial results. Radiology. 2003;229:241–7. [DOI] [PubMed] [Google Scholar]
- 18.Turkbey B, Choyke PL. Multiparametric MRI and prostate cancer diagnosis and risk stratification. Curr Opin Urol. 2012;22:310–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Turkbey B, Mani H, Aras O, Ho J, Hoang A, Rastinehad A, Agarwal H, Shah V, Bernardo M, Pang Y, Daar D, McKinney YL, Linehan WM, Kaushal A, Merino MJ, Wood BJ, Pinto PA, Choyke PL. Can Multi-parametric MRI Identity Prostate Cancer Patients who are Candidates for Active Surveillance? Radiology. 2013;268:144–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Engelhard K, Hollenbach HP, Kiefer B, Winkel A, Goeb K, Engehausen D. Prostate biopsy in the supine position in a standard 1.5-T scanner under real time MR-imaging control using a MR-compatible endorectal biopsy device. Eur Radiol. 2006;16:1237–43. [DOI] [PubMed] [Google Scholar]
- 21.Zangos S, Melzer A, Eichler K, et al. MR-compatible assistance system for biopsy in a high-field-strength system: initial results in patients with suspicious prostate lesions. Radiology. 2011;259:903–10. [DOI] [PubMed] [Google Scholar]
- 22.•.Hambrock T, Hoeks C. Hulsbergen-van de Kaa C, et al. Prospective assessment of prostate cancer aggressiveness using 3-T diffusion-weighted magnetic resonance imaging-guided biopsies versus a systematic 10-core transrectal ultrasound prostate biopsy cohort. Eur Urol. 2012;61:177–84. [DOI] [PubMed] [Google Scholar]
- 23.Kasivisvanathan V, Dufour R, Moore CM, et al. Transperineal magnetic resonance image targeted prostate biopsy versus transperineal template prostate biopsy in the detection of clinically significant prostate cancer. J Urol. 2013;189:860–6. [DOI] [PubMed] [Google Scholar]
- 24.Delongchamps NB, Peyromaure M, Schull A, et al. Prebiopsy magnetic resonance imaging and prostate cancer detection: comparison of random and targeted biopsies. J Urol. 2013;189:493–9. [DOI] [PubMed] [Google Scholar]
- 25.•.Pinto PA, Chung PH, Rastinehad AR, et al. Magnetic resonance imaging/ultrasound fusion guided prostate biopsy improves cancer detection following transrectal ultrasound biopsy and correlates with multiparametric magnetic resonance imaging. J Urol. 2011;186:1281–5.One of the early studies, which compare TRUS/MRI fusion guided biopsy with systemic biopsy.
- 26.Beyersdorff D, Winkel A, Hamm B, Lenk S, Loening SA, Taupitz M. MR imaging-guided prostate biopsy with a closed MR unit at 1.5 T: initial results. Radiology. 2005;234:576–81. [DOI] [PubMed] [Google Scholar]
- 27.Haffner J, Lemaitre L, Puech P, et al. Role of magnetic resonance imaging before initial biopsy: comparison of magnetic resonance imaging-targeted and systematic biopsy for significant prostate cancer detection. BJU Int. 2011;108:E171–8. [DOI] [PubMed] [Google Scholar]
- 28.Park BK, Park JW, Park SY, et al. Prospective evaluation of 3-T MRI performed before initial transrectal ultrasound-guided prostate biopsy in patients with high prostate-specific antigen and no previous biopsy. AJR Am J Roentgenol. 2011;197:W876–81. [DOI] [PubMed] [Google Scholar]
- 29.Puech P, Rouvière O, Renard-Penna R, Villers A, Devos P, Colombel M, et al. Prostate Cancer Diagnosis: Multiparametric MR-targeted Biopsy with Cognitive and Transrectal US-MR Fusion Guidance versus Systematic Biopsy–Prospective Multicenter Study. Radiology. 2013;268(2):461–9. [DOI] [PubMed] [Google Scholar]
- 30.Rais-Bahrami S, Siddiqui MM, Turkbey B, Stamatakis L, Logan J, Hoang AN, et al. Usefulness of Multiparametric Magnetic Resonance Imaging Suspicion Levels in Detecting Prostate Cancer. J Urol. 2013. doi: 10.1016/j.juro.2013.05.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sonn GA, Natarajan S, Margolis DJ, et al. Targeted biopsy in the detection of prostate cancer using an office based magnetic resonance ultrasound fusion device. J Urol. 2013;189:86–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Siddiqui MM, Rais-Bahrami S, Truong H, Stamatakis L, Vourganti S, Nix J, et al. Magnetic Resonance Imaging/Ultrasound-Fusion Biopsy Significantly Upgrades Prostate Cancer Versus Systematic 12-core Transrectal Ultrasound Biopsy. Eur Urol. 2013. doi: 10.1016/j.eururo.2013.05.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vourganti S, Rastinehad A, Yerram NK, Nix J, Volkin D, Hoang A, et al. Multiparametric magnetic resonance imaging and ultrasound fusion biopsy detect prostate cancer in patients with prior negative transrectal ultrasound biopsies. J Urol. 2012;188(6):2152–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sonn GA, Chang E, Natarajan S, Margolis DJ, Macairan M, Lieu P, Huang J, Dorey FJ, Reiter RE, Marks LS. Value of Targeted Prostate Biopsy Using Magnetic Resonance-Ultrasound Fusion in Men with Prior Negative Biopsy and Elevated Prostate-specific Antigen. Eur Urol. 2013. doi: 10.1016/j.eururo.2013.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Roethke M, Anastasiadis AG, Lichy M, et al. MRI-guided prostate biopsy detects clinically significant cancer: analysis of a cohort of 100 patients after previous negative TRUS biopsy. World J Urol. 2012;30:213–8. [DOI] [PubMed] [Google Scholar]
- 36.Franiel T, Stephan C, Erbersdobler A, et al. Areas suspicious for prostate cancer: MR-guided biopsy in patients with at least one transrectal US-guided biopsy with a negative finding–multiparametric MR imaging for detection and biopsy planning. Radiology. 2011;259:162–72. [DOI] [PubMed] [Google Scholar]
- 37.Komai Y, Numao N, Yoshida S, et al. High Diagnostic Ability of Multiparametric Magnetic Resonance Imaging to Detect Anterior Prostate Cancer Missed by Transrectal 12-Core Biopsy. J Urol. 2013;190:867–73 [DOI] [PubMed] [Google Scholar]
- 38.Ouzzane A, Puech P, Lemaitre L, Leroy X, Nevoux P, Betrouni N, et al. Combined multiparametric MRI and targeted biopsies improve anterior prostate cancer detection, staging, and grading. Urology. 2011;78(6):1356–62. [DOI] [PubMed] [Google Scholar]
- 39.Nix JW, Turkbey B, Hoang A, Volkin D, Yerram N, Chua C, et al. Very distal apical prostate tumours: identification on multiparametric MRI at 3 Tesla. BJU Int. 2012;110(11 Pt B):E694–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Walton-Diaz A, Hoang AN, Turkbey B, Hong CW, Truong H, Sterling T, et al. Can MR-US Fusion Biopsy Improve Cancer Detection in Enlarged Prostates? J Urol. 2013. doi: 10.1016/j.juro.2013.05.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Moyer VA. Screening for prostate cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2012;157:120. [DOI] [PubMed] [Google Scholar]
- 42.Andriole GL, Crawford ED, Grubb RL 3rd, Buys SS, Chia D, Church TR, et al. Mortality results from a randomized prostate-cancer screening trial. N Engl J Med. 2009;360(13):1310–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schröder FH, Hugosson J, Roobol MJ, Tammela TL, Ciatto S, Nelen V, et al. ERSPC Investigators. Prostate-cancer mortality at 11 years of follow-up. N Engl J Med. 2012;366(11):981–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wilt TJ, Brawer MK, Jones KM, Barry MJ, Aronson WJ, Fox S, et al. Radical prostatectomy versus observation for localized prostate cancer. N Engl J Med. 2012;367(3):203–13. doi: 10.1056/NEJMoa1113162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Villers A, Puech P. When and How Should Magnetic Resonance Imaging be Used in Evaluation of the Patient with Prostate Cancer or Increased Prostate Specific Antigen? J Urol. 2013. doi: 10.1016/j.juro.2013.08.014. [DOI] [PubMed] [Google Scholar]
- 46.Villers A, Marliere F, Ouzzane A, Puech P, Lemaître L. MRI in addition to or as a substitute for prostate biopsy: the clinician’s point of view. Diagn Interv Imaging. 2012;93(4):262–7. [DOI] [PubMed] [Google Scholar]
- 47.Fütterer JJ. MR imaging in local staging of prostate cancer. Eur J Radiol. 2007;63:328. [DOI] [PubMed] [Google Scholar]
- 48.Soylu FN, Eggener S, Oto A. Local staging of prostate cancer with MRI. Diagn Interv Radiol. 2012;18(4):365–73. [DOI] [PubMed] [Google Scholar]
- 49.Soylu FN, Peng Y, Jiang Y, Wang S, Schmid-Tannwald C, Sethi I, et al. Seminal vesicle invasion in prostate cancer: evaluation by using multiparametric endorectal MR imaging. Radiology. 2013;267(3):797–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jeong IG, Lim JH, You D, Kim MH, Choi HJ, Kim JK, et al. Incremental Value of Magnetic Resonance Imaging for Clinically High Risk Prostate Cancer: A Large Single institution Experience of 922 Radical Prostatectomies. J Urol. 2013. doi: 10.1016/j.juro.2013.06.035. [DOI] [PubMed] [Google Scholar]
- 51.Yerram NK, Volkin D, Turkbey B, Nix J, Hoang AN, Vourganti S, et al. Low suspicion lesions on multiparametric magnetic resonance imaging predict for the absence of high-risk prostate cancer. BJU Int. 2012;110(11 PtB):E783–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Somford DM, Hamoen EH, Fütterer JJ, van Basten JP. Hulsbergen-van de Kaa CA, Vreuls W, van Oort IM, Vergunst H, Kiemeney LA, Barentsz JO, Witjes JA. The Predictive Value of Endorectal 3 Tesla Multiparametric Magnetic Resonance Imaging for Extraprostatic Extension in Patients with Low, Intermediate and High Risk Prostate Cancer. J Urol. 2013;190:1728. [DOI] [PubMed] [Google Scholar]
- 53.Turkbey B, Mani H, Aras O, Ho J, Hoang A, Rastinehad AR, et al. Prostate cancer: can multiparametric MR imaging help identify patients who are candidates for active surveillance? Radiology. 2013;268(1):144–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stamatakis L, Siddiqui MM, Nix JW, Logan J, Rais-Bahrami S, Walton-Diaz A, et al. Accuracy of multiparametric magnetic resonance imaging in confirming eligibility for active surveillance for men with prostate cancer. Cancer. 2013. doi: 10.1002/cncr.28216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ouzzane A, Puech P, Villers A. MRI and surveillance. Curr Opin Urol. 2012;22(3):231–6. [DOI] [PubMed] [Google Scholar]